Corrosion Resistance Study of Cyclocarboxypropyl Oleic Acid-Doped Polyaniline/Epoxy Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of CCHOA-Doped Polyaniline
2.3. Preparation of Epoxy Composite Coatings
2.4. Characterization
3. Results
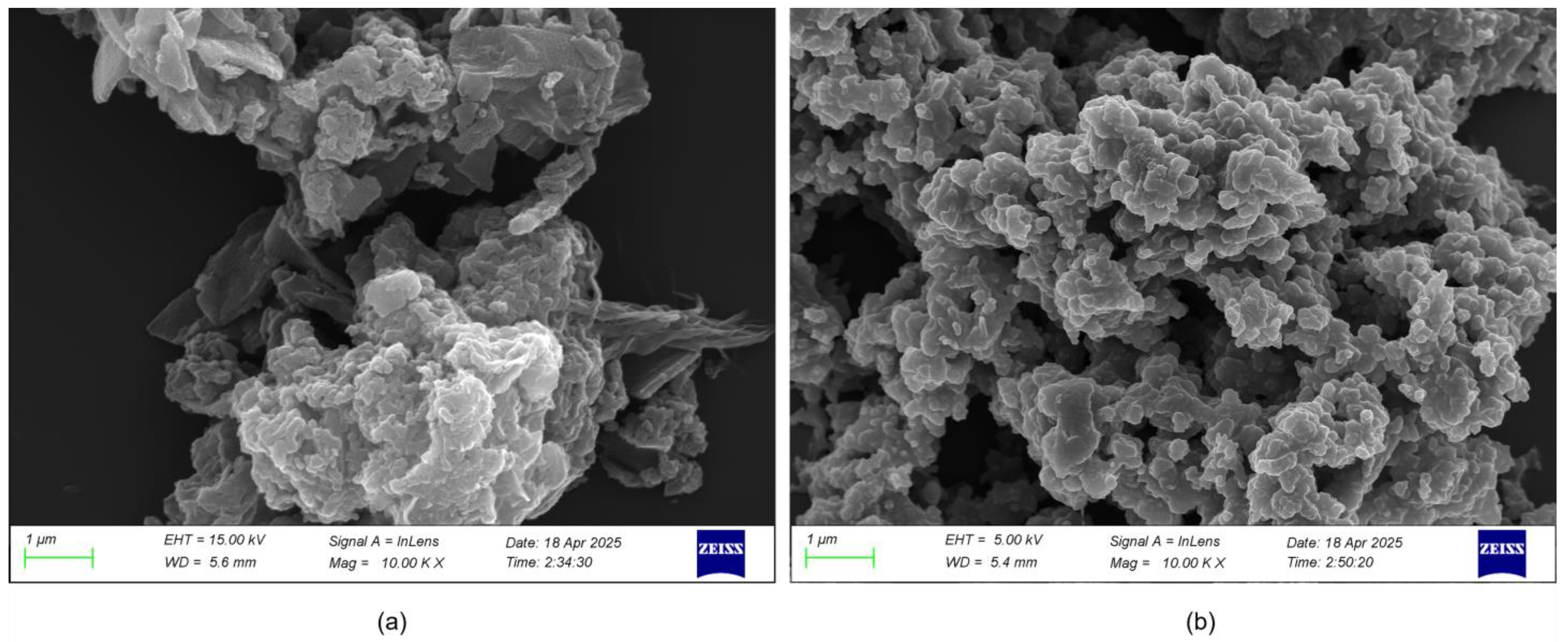
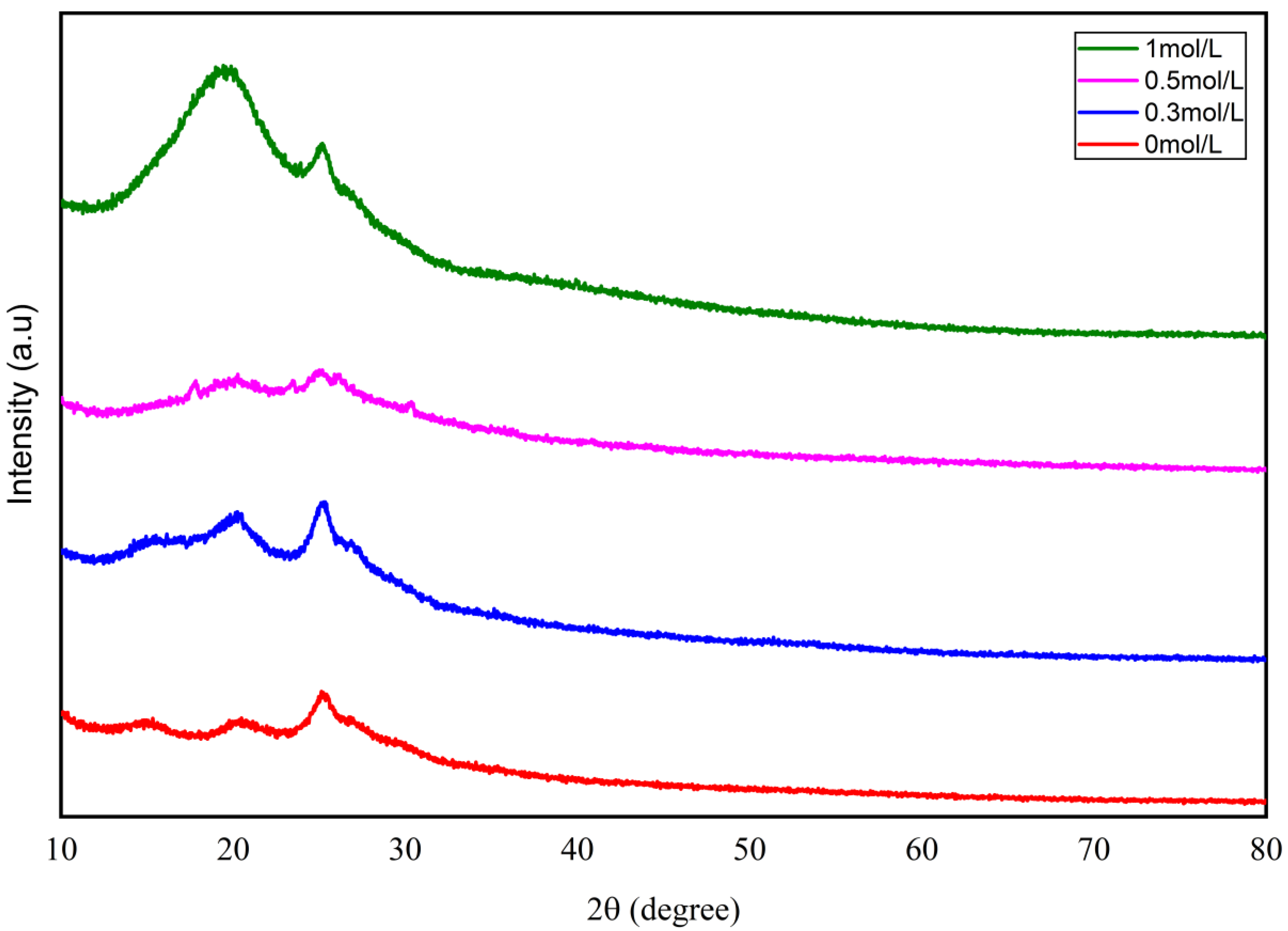
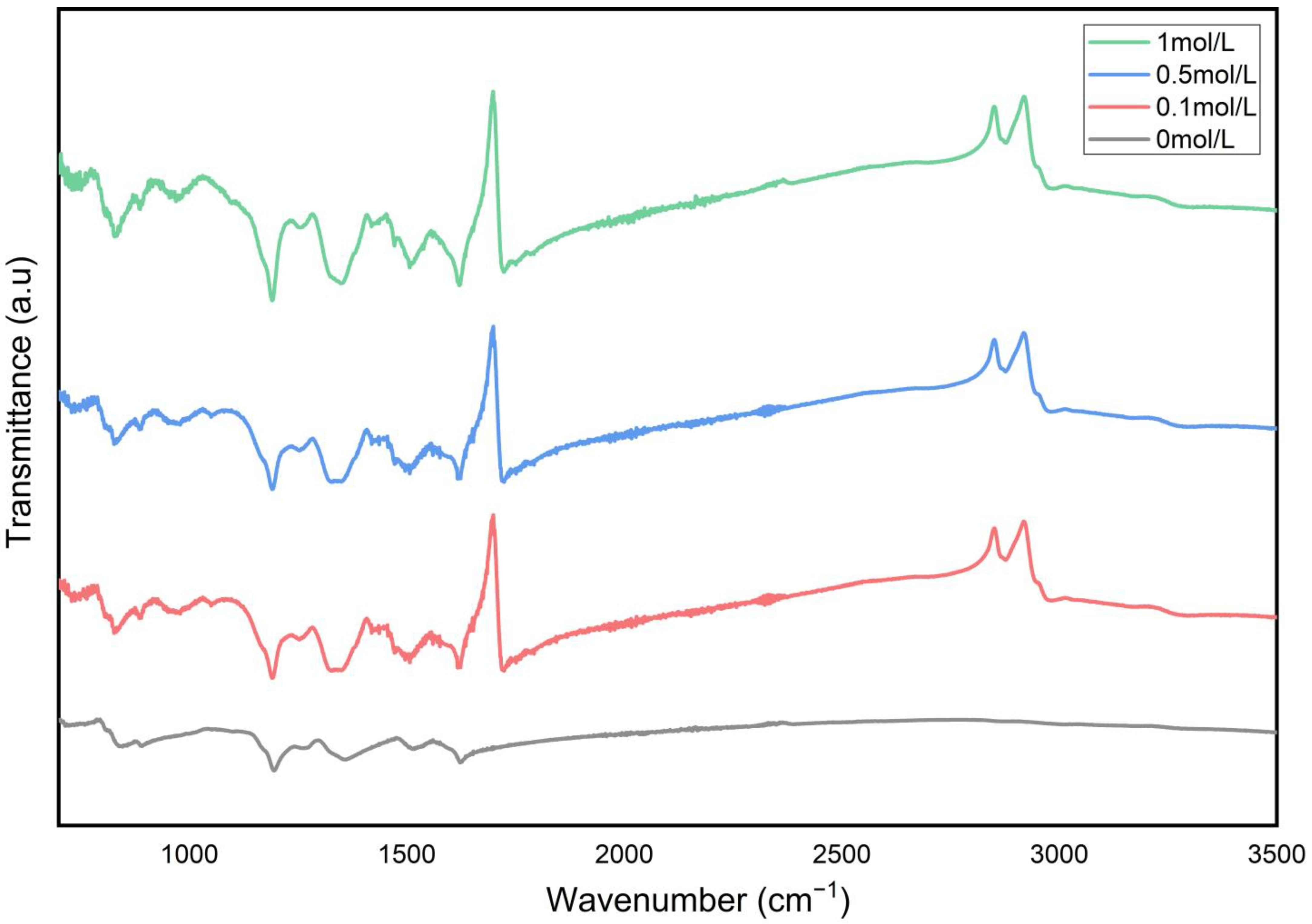
3.1. Morphology and Structure Analysis
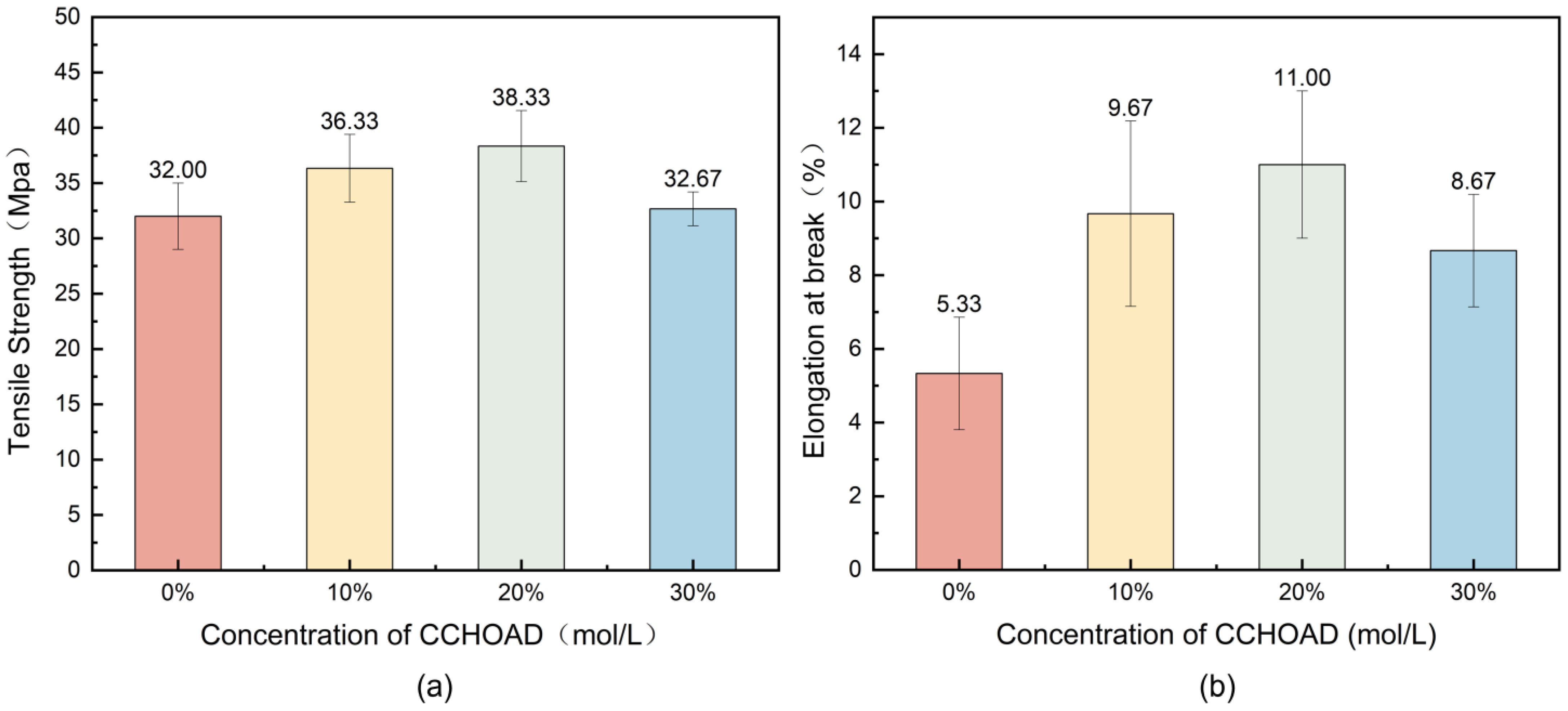
3.2. Mechanical Property Analysis
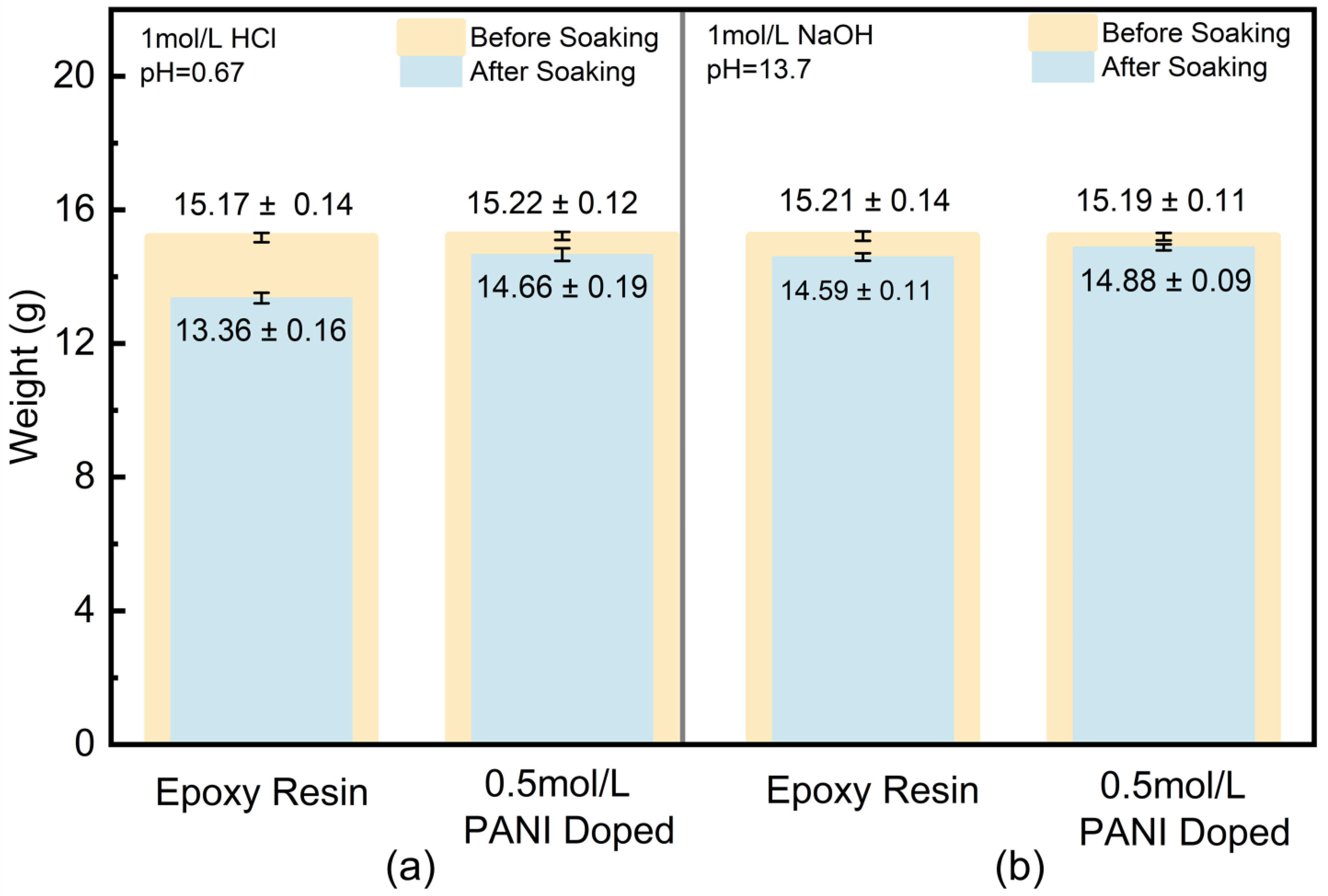
3.3. Corrosion Resistance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCHOA | Cyclocarboxypropyl Oleic Acid |
| EB | Emeraldine Base |
| EIS | Electrochemical Impedance Spectroscopy |
| ES | Emeraldine Salt |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MD | Molecular Dynamics |
| MXene | Two-dimensional transition metal carbides/nitrides |
| PANI | Polyaniline |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| XRD | X-ray Diffraction |
References
- Wu, Y.; Zhao, W.; Wang, L. State of the art and current trends on the metal corrosion and protection strategies in deep sea. J. Mater. Sci. Technol. 2025, 215, 192–213. [Google Scholar] [CrossRef]
- Akpan, E.D.; Kumar Singh, A.; Lgaz, H.; Quadri, T.W.; Kumar Shukla, S.; Mangla, B.; Dwivedi, A.; Dagdag, O.; Sheetal; Edem Inyang, E.; et al. Coordination compounds as corrosion inhibitors of metals: A review. Coord. Chem. Rev. 2024, 499, 215503. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Singh Chauhan, D.; Lgaz, H.; Chung, I.-M. Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Verma, C.; Singh, A.; Singh, P.; Yop Rhee, K.; Alfantazi, A. Regioisomeric effect of heteroatoms and functional groups of organic ligands: Impacts on coordination bonding and corrosion protection performance. Coord. Chem. Rev. 2024, 515, 215966. [Google Scholar] [CrossRef]
- Li, F.-T.; Wang, Z.-K.; Zhou, Y.; Li, L.-Z.; Liu, Y.-Q.; Wang, L.; Li, C.-L.; Zhu, H.-F.; Sun, S.-Q.; Hu, S.-Q. Improvement of carbon dots corrosion inhibition by ionic liquid modification: Experimental and computational investigations. Corros. Sci. 2023, 224, 111541. [Google Scholar] [CrossRef]
- Fang, J.; Xu, K.; Zhu, L.; Zhou, Z.; Tang, H. A study on mechanism of corrosion protection of polyaniline coating and its failure. Corros. Sci. 2007, 49, 4232–4242. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Chen, S.-A.; Hwang, G.-W. Water-Soluble Self-Acid-Doped Conducting Polyaniline: Structure and Properties. J. Am. Chem. Soc. 1995, 117, 10055–10062. [Google Scholar] [CrossRef]
- Neoh, K.G.; Kang, E.T.; Khor, S.H.; Tan, K.L. Stability studies of polyaniline. Polym. Degrad. Stab. 1990, 27, 107–117. [Google Scholar] [CrossRef]
- Qi, F.; Chen, J.; Zeng, Z.; Huang, Z.; Niu, Y. Periodate activation with polyaniline-derived carbon for bisphenol A degradation: Insight into the roles of nitrogen dopants and non-radical species formation. Chem. Eng. J. 2024, 499, 156077. [Google Scholar] [CrossRef]
- Rahman, S.U.; Dan, X.; Farooq, S.; Sajid, M.; Tao, F.-Y.; Kitchamsetti, N.; Liu, C.; Xu, W.-J.; Zhang, J.-P. Tailoring polyaniline with dual dopant engineering as a high efficiency cathode material for aqueous zinc ion batteries. J. Colloid Interface Sci. 2025, 700, 138600. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, J.; Wang, H.; Wang, J.; Lu, G.; Huang, J.; Zhao, C.; Liu, S. Corrosion Protection of Q235 Steel Using Epoxy Coatings Based on Polyaniline Loaded with Benzotriazole. Coatings 2024, 14, 456. [Google Scholar] [CrossRef]
- Nazari, H.; Arefinia, R. Corrosion inhibition potential of polyaniline nanoparticles: Electrochemical and computational evaluation. J. Mol. Struct. 2025, 1330, 141491. [Google Scholar] [CrossRef]
- Luchkina, V.A.; Min’Kin, M.S.; Luchkin, A.Y.; Kuznetsov, Y.I. Protective and water-repellent properties of alkylcarboxylic and alkylphosphonic acid films on technically pure magnesium. J. Magnes. Alloys 2023, 11, 3272–3286. [Google Scholar] [CrossRef]
- Wang, S.; Meng, D.; Ma, Y.; Li, C.; Chen, W.; Zheng, Y.; Pan, L.; Li, B.; Huang, W. Carboxyl-functionalized TiO2 as a highly efficient adsorbent for separation, enrichment, and immobilization of Cu(II), Pb(II), and Hg(II). Chem. Eng. J. 2025, 520, 165774. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and emerging trends of inorganic, organic and eco-friendly corrosion inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Beyond graphene and boron nitride: Why MXene can be used in composite for corrosion protection on metals? Compos. Part B Eng. 2024, 271, 111168. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Y.; Ou, Y.; Shi, X.; Zhang, Y.; Chen, Q.; Li, L.; Zhou, F.; Liu, W. Synergistic anti-corrosion and anti-wear of epoxy coating functionalized with inhibitor-loaded graphene oxide nanoribbons. J. Mater. Sci. Technol. 2025, 220, 140–149. [Google Scholar] [CrossRef]
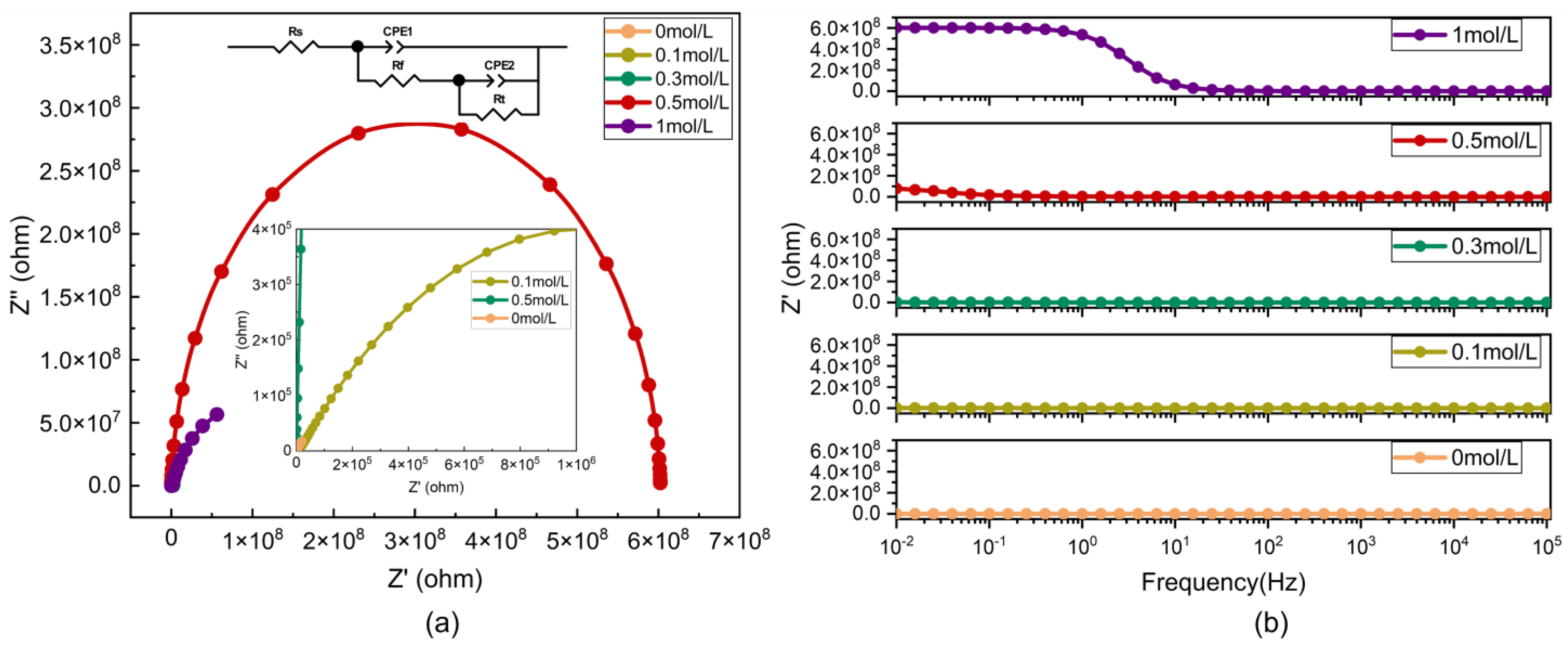
| 0 mol/L | 0.1 mol/L | 0.3 mol/L | 0.5 mol/L | 1 mol/L | |
|---|---|---|---|---|---|
| Rs (Solution resistance)/Ω cm2 | 6.122 | 1310 | 186.5 | 1588 | 61.79 |
| CPE1 (Constant phase element 1)/F cm−2 | 1.116 × 10−4 | 1.089 × 10−10 | 4.953 × 10−10 | 6.800 × 10−11 | 1.036 × 10−10 |
| n1 (Exponent of CPE1) | 0.7556 | 1 | 0.8968 | 1 | 0.9775 |
| Rf (Film resistance)/Ω cm2 | 1.159 × 104 | 1.205 × 104 | 5.600 × 104 | 2.416 × 107 | 9.386 × 105 |
| CPE2 (Constant phase element 2)/F cm−2 | 2.333 × 10−4 | 4.732 × 10−7 | 5.698 × 10−7 | 1.368 × 10−11 | 4.040 × 10−8 |
| n2 (Exponent of CPE2) | 0.8663 | 0.4778 | 0.6441 | 1 | 0.7773 |
| Rt (Charge transfer resistance)/Ω cm2 | 4.060 × 104 | 2.030 × 106 | 2.730 × 106 | 5.710 × 108 | 1.810 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Li, X.; Zhang, T.; Guo, W.; An, Y.; Liu, T. Corrosion Resistance Study of Cyclocarboxypropyl Oleic Acid-Doped Polyaniline/Epoxy Composite Coatings. Nanomaterials 2025, 15, 1416. https://doi.org/10.3390/nano15181416
Xu X, Li X, Zhang T, Guo W, An Y, Liu T. Corrosion Resistance Study of Cyclocarboxypropyl Oleic Acid-Doped Polyaniline/Epoxy Composite Coatings. Nanomaterials. 2025; 15(18):1416. https://doi.org/10.3390/nano15181416
Chicago/Turabian StyleXu, Xinning, Xiaofeng Li, Taihua Zhang, Wei Guo, Yan An, and Tao Liu. 2025. "Corrosion Resistance Study of Cyclocarboxypropyl Oleic Acid-Doped Polyaniline/Epoxy Composite Coatings" Nanomaterials 15, no. 18: 1416. https://doi.org/10.3390/nano15181416
APA StyleXu, X., Li, X., Zhang, T., Guo, W., An, Y., & Liu, T. (2025). Corrosion Resistance Study of Cyclocarboxypropyl Oleic Acid-Doped Polyaniline/Epoxy Composite Coatings. Nanomaterials, 15(18), 1416. https://doi.org/10.3390/nano15181416





