MoS2-PtX2 Vertical Heterostructures
Abstract
1. Introduction
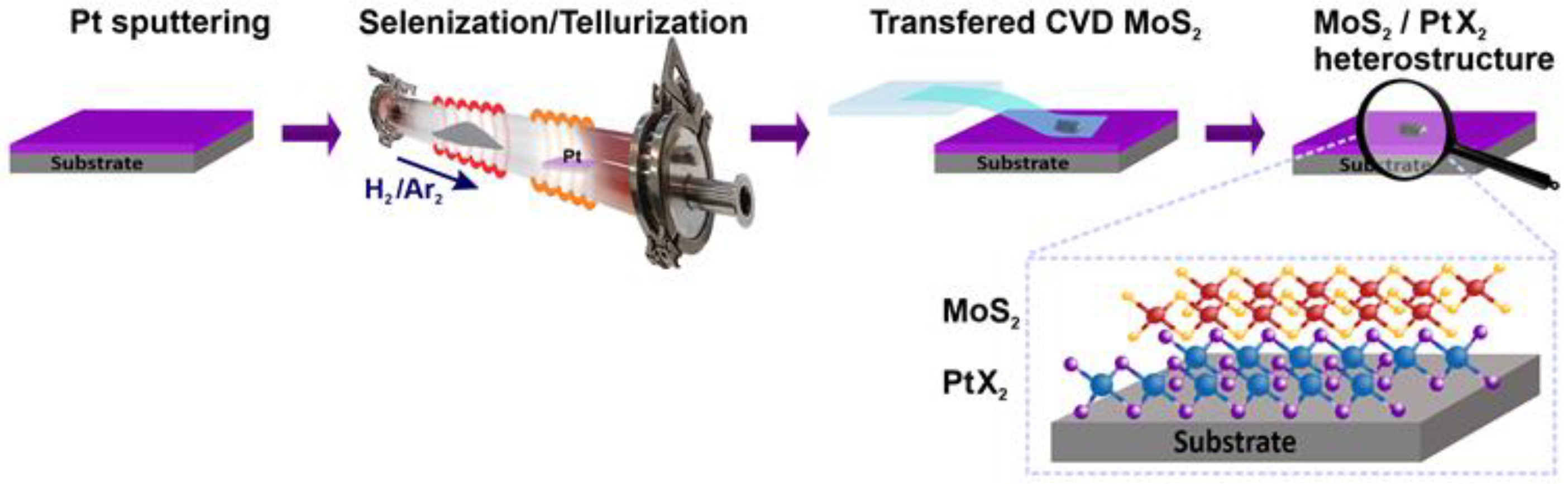
2. Materials and Methods
3. Results
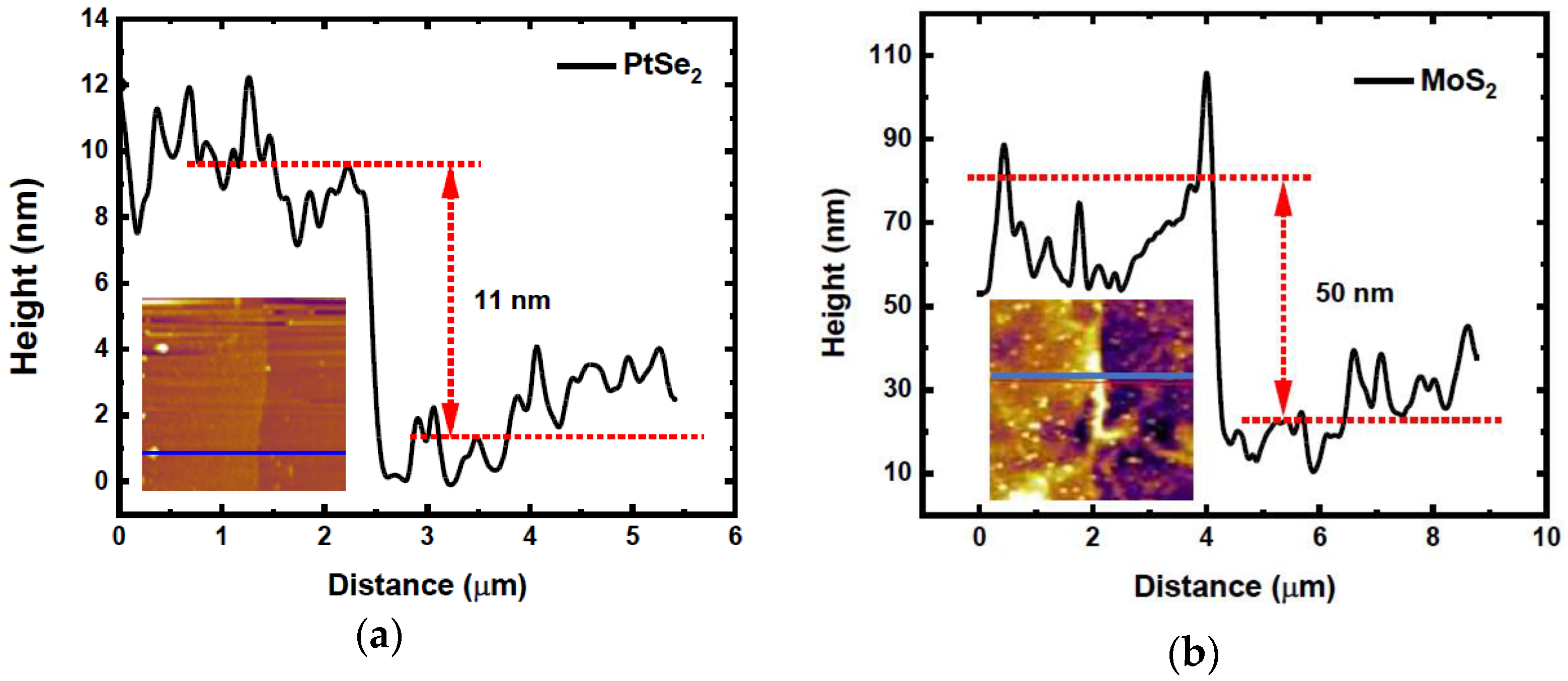
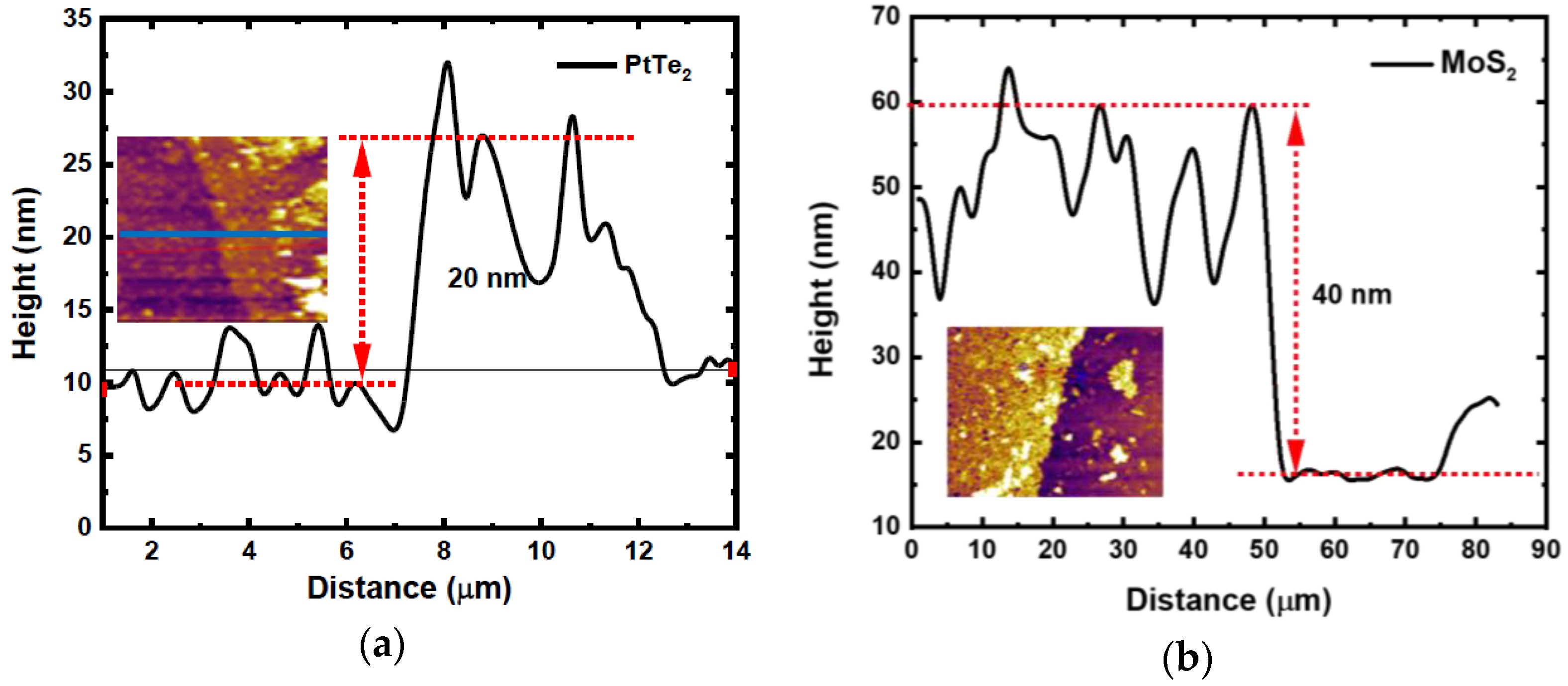
3.1. AFM Analysis
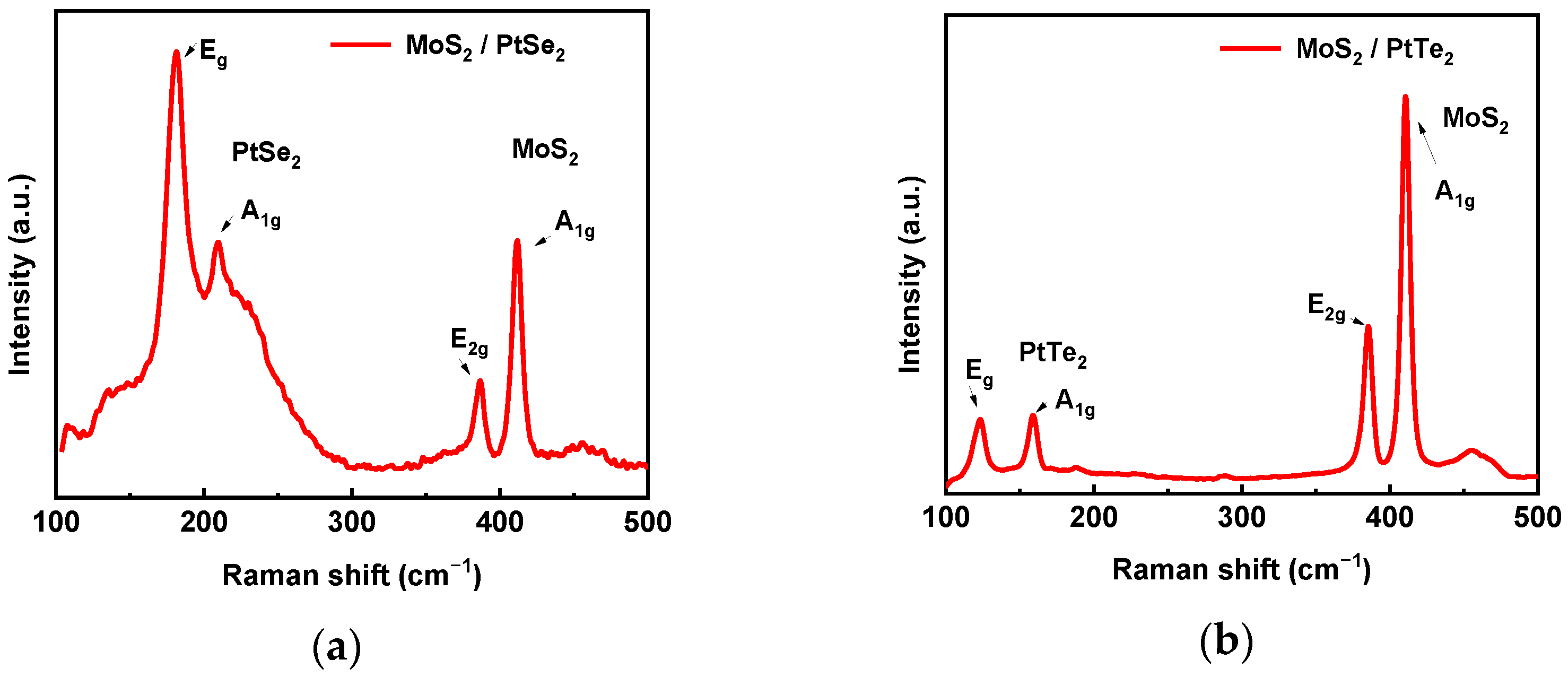
3.2. Raman Spectroscopy
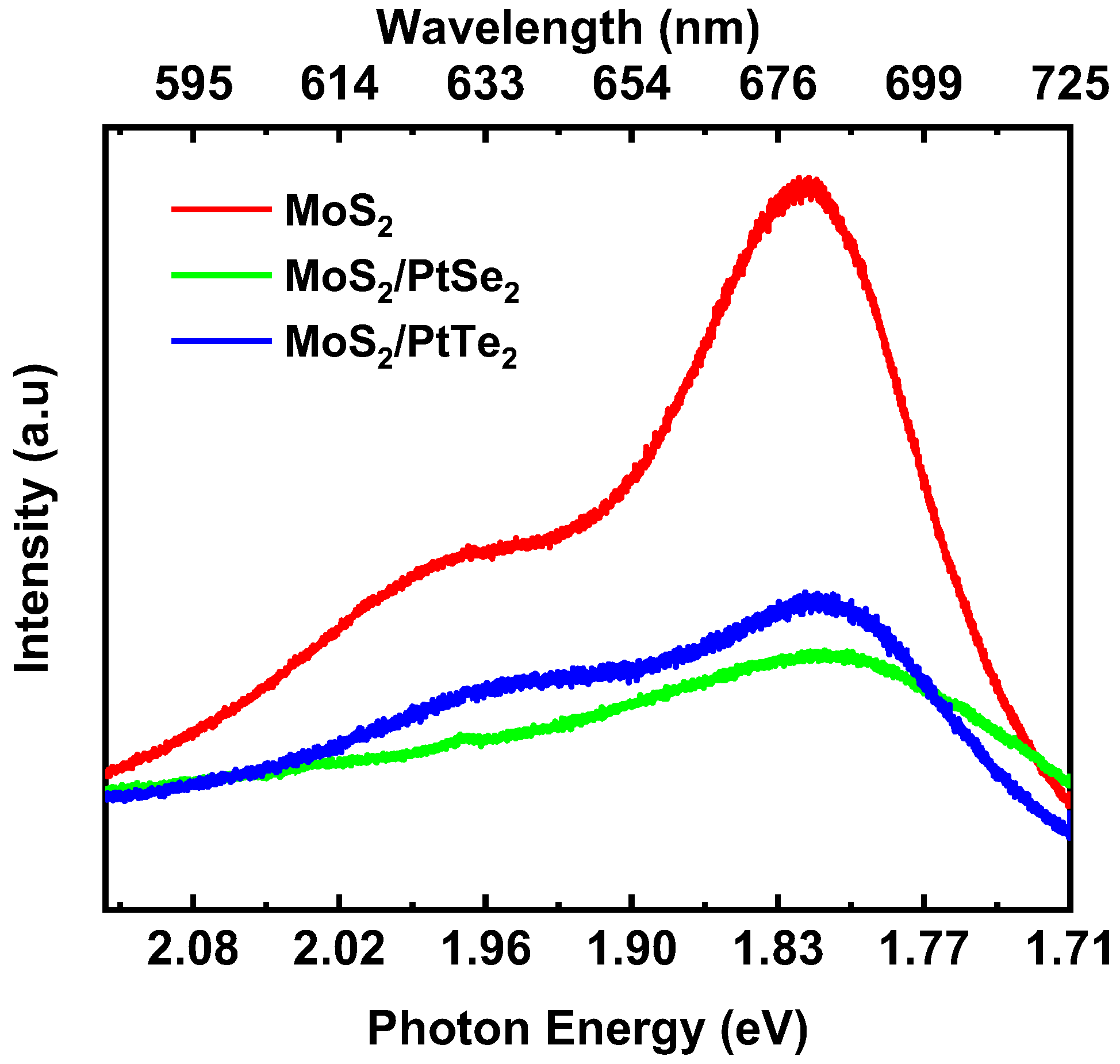
3.3. Photoluminescence
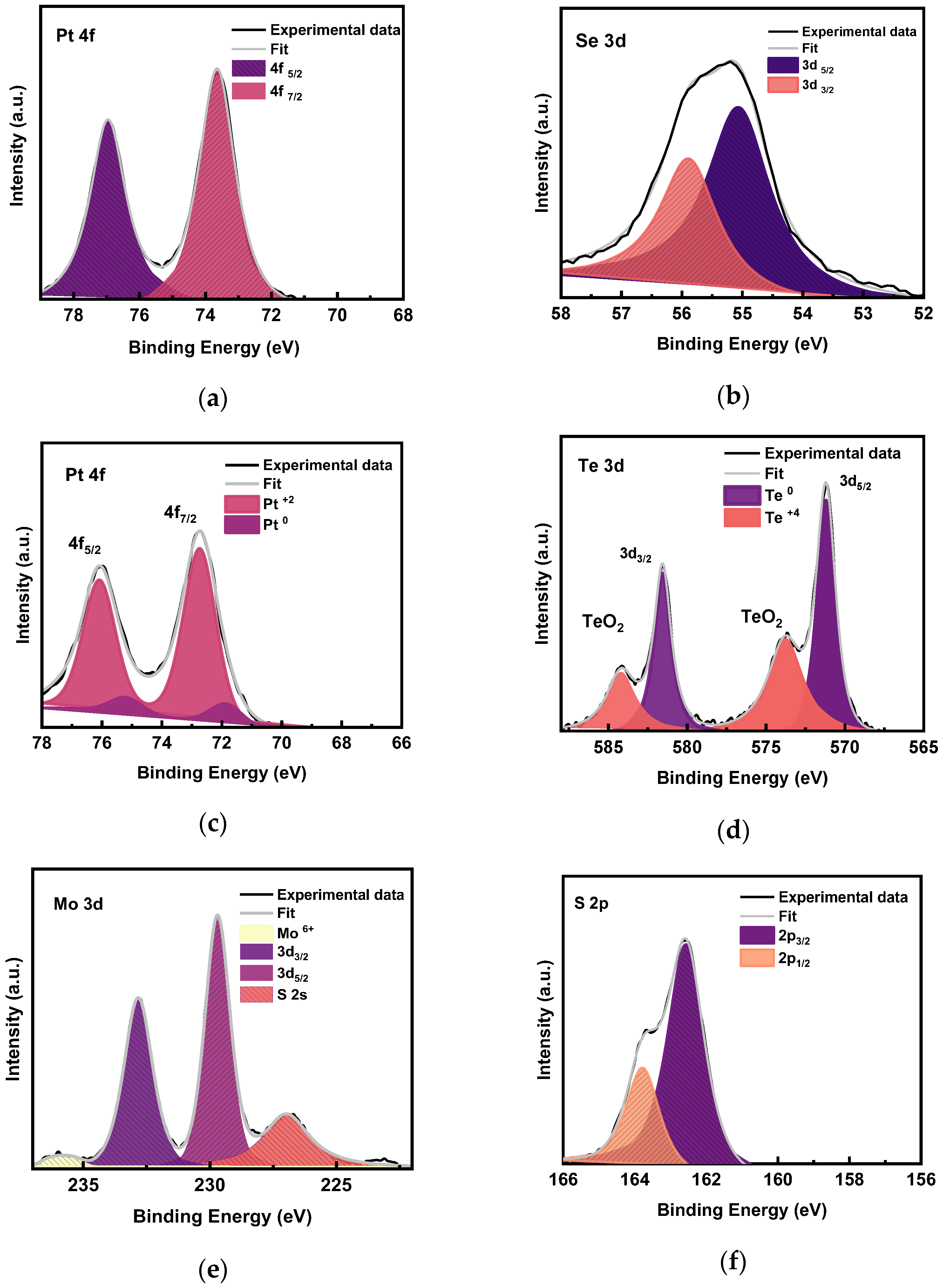
3.4. XPS Analysis
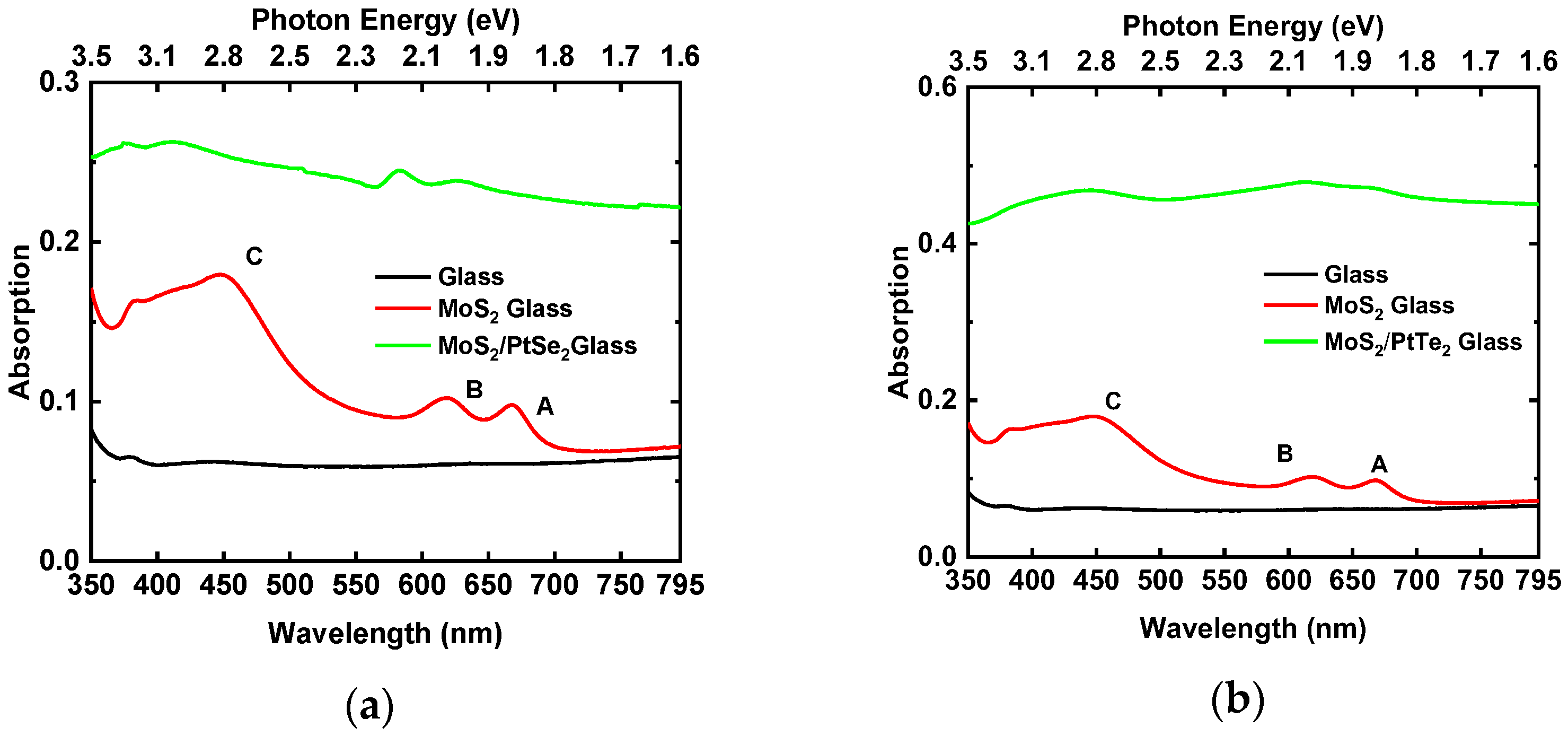
3.5. Optical Properties (Transmittance and Absorbance)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| AFM | Atomic force microscopy |
| FWHM | Full width at half-maximum |
| MoS2 | Molybdenum disulfide |
| PL | Photoluminescence |
| PtSe2 | Platinum diselenide |
| PtTe2 | Platinum ditelluride |
| TMDCs | Transition metal dichalcogenides |
| TRT | Thermal release tape |
| XPS | X-ray photoelectron spectroscopy |
References
- Joseph, S.; Mohan, J.; Lakshmy, S.; Thomas, S.; Chakraborty, B.; Thomas, S.; Kalarikkal, N. A Review of the Synthesis, Properties, and Applications of 2D Transition Metal Dichalcogenides and Their Heterostructures. Mater. Chem. Phys. 2023, 297, 127332. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, Y.; Gao, F.; Wu, X.; Hao, S.; Qiu, M.; Zheng, X.; Wei, Y.; Hao, G. Controllable Growth of Wafer-Scale Two-Dimensional WS2 with Outstanding Optoelectronic Properties. 2D Mater. 2023, 11, 015007. [Google Scholar] [CrossRef]
- Hao, S.; Hao, Y.; Li, J.; Wang, K.; Fan, C.; Zhang, S.; Wei, Y.; Hao, G. Controllable Growth of Two-Dimensional Wrinkled WSe2 Nanostructures via Chemical Vapor Deposition Based on Thermal Mismatch Strategy. Appl. Phys. Lett. 2024, 125, 072102. [Google Scholar] [CrossRef]
- McCreary, A.; Kazakova, O.; Jariwala, D.; Al Balushi, Z.Y. An Outlook into the Flat Land of 2D Materials beyond Graphene: Synthesis, Properties and Device Applications. 2D Mater. 2021, 8, 013001. [Google Scholar] [CrossRef]
- Napoleonov, B.; Petrova, D.; Minev, N.; Rafailov, P.; Videva, V.; Karashanova, D.; Ranguelov, B.; Atanasova-Vladimirova, S.; Strijkova, V.; Dimov, D.; et al. Growth of Monolayer MoS2 Flakes via Close Proximity Re-Evaporation. Nanomaterials 2024, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Minev, N.; Buchkov, K.; Todorova, N.; Todorov, R.; Videva, V.; Stefanova, M.; Rafailov, P.; Karashanova, D.; Dikov, H.; Strijkova, V.; et al. Synthesis of 2D PtSe2 Nanolayers on Glass Substrates and Their Integration in Near-Infrared Light Shutters. ACS Omega 2024, 9, 14874–14886. [Google Scholar] [CrossRef]
- Todorova, N.; Minev, N.; Marinova, V.; Buchkov, K.; Videva, V.; Todorov, R.; Rafailov, P.; Strijkova, V.; Psycharis, V.; Giannakopoulou, T.; et al. Two-Dimensional PtSe2 Coatings with Antibacterial Activity. Appl. Surf. Sci. 2023, 611, 155534. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, Y.; Hao, S.; Lu, X.; Zhou, J.; Fan, C.; Liu, J.; Hao, G. Wafer-Scale Synthesis of Transition Metal Dichalcogenides and van Der Waals Heterojunctions. Nanotechnology 2025, 36, 232004. [Google Scholar] [CrossRef] [PubMed]
- Taghinejad, H.; Eftekhar, A.A.; Adibi, A. Lateral and Vertical Heterostructures in Two-Dimensional Transition-Metal Dichalcogenides [Invited]. Opt. Mater. Express 2019, 9, 1590. [Google Scholar] [CrossRef]
- Minev, N.; Dimitrov, D.; Dimov, D.; Rafailov, P.; Napoleonov, B.; Videva, V.; Petrova, D.; Strijkova, V.; Avramova, I.; Marinova, V. Direct Synthesis of WSe2/PtSe2 Heterostructures. J. Phys. Conf. Ser. 2024, 2710, 012008. [Google Scholar] [CrossRef]
- Babu, R.S.; Georgiadou, D.G. 2D Transition Metal Dichalcogenides for Energy-Efficient Two-Terminal Optoelectronic Synaptic Devices. Device 2025, 100805. [Google Scholar] [CrossRef]
- Jiao, C.; Pei, S.; Wu, S.; Wang, Z.; Xia, J. Tuning and Exploiting Interlayer Coupling in Two-Dimensional van Der Waals Heterostructures. In Reports on Progress in Physics; IOP Publishing: Bristol, England, 2023; Volume 86. [Google Scholar]
- Komsa, H.P.; Krasheninnikov, A.V. Electronic Structures and Optical Properties of Realistic Transition Metal Dichalcogenide Heterostructures from First Principles. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 88, 085318. [Google Scholar] [CrossRef]
- Hong, X.; Kim, J.; Shi, S.F.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast Charge Transfer in Atomically Thin MoS2/WS2 Heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, J.; Che, M.; Liu, M.; Zou, Y.; An, J.; Tan, F.; Shi, Y.; Zhang, N.; Qi, L.; et al. High-Performance Broadband Photodetector Based on PtSe2/MoS2 Heterojunction from Visible to near-Infrared Region. Sci. China Inf. Sci. 2024, 67, 132401. [Google Scholar] [CrossRef]
- Rao, G.; Wang, X.; Wang, Y.; Wangyang, P.; Yan, C.; Chu, J.; Xue, L.; Gong, C.; Huang, J.; Xiong, J.; et al. Two-Dimensional Heterostructure Promoted Infrared Photodetection Devices. InfoMat 2019, 1, 272–288. [Google Scholar] [CrossRef]
- Xu, H.; Han, X.; Dai, X.; Liu, W.; Wu, J.; Zhu, J.; Kim, D.; Zou, G.; Sablon, K.A.; Sergeev, A.; et al. High Detectivity and Transparent Few-Layer MoS2/Glassy-Graphene Heterostructure Photodetectors. Adv. Mater. 2018, 30, e1706561. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, Y.; Yang, Z.; Lu, W.; Chen, S.; Zhang, X.; Ma, H.; Sun, T.; Huo, P.; Cui, X.; et al. Near-Ideal Schottky Junction Photodetectors Based on Semimetal-Semiconductor Van Der Waals Heterostructures. Adv. Funct. Mater. 2024, 34, 2316267. [Google Scholar] [CrossRef]
- O’Brien, M.; McEvoy, N.; Motta, C.; Zheng, J.Y.; Berner, N.C.; Kotakoski, J.; Elibol, K.; Pennycook, T.J.; Meyer, J.C.; Yim, C.; et al. Raman Characterization of Platinum Diselenide Thin Films. 2D Mater. 2016, 3, 021004. [Google Scholar] [CrossRef]
- Parhizkar, S.; Prechtl, M.; Giesecke, A.L.; Suckow, S.; Wahl, S.; Lukas, S.; Hartwig, O.; Negm, N.; Quellmalz, A.; Gylfason, K.; et al. Two-Dimensional Platinum Diselenide Waveguide-Integrated Infrared Photodetectors. ACS Photonics 2022, 9, 859–867. [Google Scholar] [CrossRef]
- Szydłowska, B.M.; Hartwig, O.; Tywoniuk, B.; Hartman, T.; Stimpel-Lindner, T.; Sofer, Z.; McEvoy, N.; Duesberg, G.S.; Backes, C. Spectroscopic Thickness and Quality Metrics for PtSe2 Layers Produced by Top-down and Bottom-up Techniques. 2D Mater. 2020, 7, 045027. [Google Scholar] [CrossRef]
- Lukas, S.; Hartwig, O.; Prechtl, M.; Capraro, G.; Bolten, J.; Meledin, A.; Mayer, J.; Neumaier, D.; Kataria, S.; Duesberg, G.S.; et al. Correlating Nanocrystalline Structure with Electronic Properties in 2D Platinum Diselenide. Adv. Funct. Mater. 2021, 31, 2102929. [Google Scholar] [CrossRef]
- Mxakaza, L.F.; Mashindi, V.; Linganiso, C.E.; Moloto, N.; Tetana, Z.N. Evaluating the Hydrogen Evolution Reaction Activity of Colloidally Prepared PtSe2 and PtTe2 Catalysts in an Alkaline Medium. ChemistryOpen 2024, 13, e202400146. [Google Scholar] [CrossRef] [PubMed]
- Gyeon, M.; Seo, J.E.; Oh, S.; Noh, G.; Lee, C.; Choi, M.; Kwon, S.; Kim, T.S.; Jeong, H.Y.; Song, S.; et al. Wafer-Scale Growth of Ultrauniform 2D PtSe2 Films with Spatial and Thickness Control through Multi-Step Metal Conversion. ACS Nano 2024, 18, 33977–33987. [Google Scholar] [CrossRef]
- Cullen, C.P.; Hartwig, O.; Coileáin, C.Ó.; McManus, J.B.; Peters, L.; Ilhan, C.; Duesberg, G.S.; McEvoy, N. Synthesis and Thermal Stability of TMD Thin Films: A Comprehensive XPS and Raman Study. arXiv 2021, arXiv:2106.07366. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Yim, C.; Lee, K.; McEvoy, N.; O’Brien, M.; Riazimehr, S.; Berner, N.C.; Cullen, C.P.; Kotakoski, J.; Meyer, J.C.; Lemme, M.C.; et al. High-Performance Hybrid Electronic Devices from Layered PtSe2 Films Grown at Low Temperature. ACS Nano 2016, 10, 9550–9558. [Google Scholar] [CrossRef]
- Dhakal, K.P.; Duong, D.L.; Lee, J.; Nam, H.; Kim, M.; Kan, M.; Lee, Y.H.; Kim, J. Confocal Absorption Spectral Imaging of MoS2: Optical Transitions Depending on the Atomic Thickness of Intrinsic and Chemically Doped MoS2. Nanoscale 2014, 6, 13028–13035. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, X.; Cui, X. Probing Excitonic States in Suspended Two-Dimensional Semiconductors by Photocurrent Spectroscopy. Sci. Rep. 2015, 5, 9218. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Zhang, L.; Hong, G.; Chen, C.; Jing, H.; Lu, J.; Wang, P.; Chen, X.; Wang, L.; et al. Controllable Growth of Type-II Dirac Semimetal PtTe2 Atomic Layer on Au Substrate for Sensitive Room Temperature Terahertz Photodetection. InfoMat 2021, 3, 705–715. [Google Scholar] [CrossRef]
- Bampoulis, P.; Van Bremen, R.; Yao, Q.; Poelsema, B.; Zandvliet, H.J.W.; Sotthewes, K. Defect Dominated Charge Transport and Fermi Level Pinning in MoS2/Metal Contacts. ACS Appl. Mater. Interfaces 2017, 9, 19278–19286. [Google Scholar] [CrossRef]
- Song, S.; Yoon, A.; Ha, J.K.; Yang, J.; Jang, S.; Leblanc, C.; Wang, J.; Sim, Y.; Jariwala, D.; Min, S.K.; et al. Atomic Transistors Based on Seamless Lateral Metal-Semiconductor Junctions with a Sub-1-Nm Transfer Length. Nat. Commun. 2022, 13, 4916. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Wang, Y.; Jiang, W.; Liu, X.; Qi, H. Investigation of the Band Alignment at MoS2/PtSe2 Heterojunctions. Appl. Phys. Lett. 2019, 114, 201601. [Google Scholar] [CrossRef]
- Chen, F.X.R.; Kawakami, N.; Lee, C.T.; Shih, P.Y.; Wu, Z.C.; Yang, Y.C.; Tu, H.W.; Jian, W.B.; Hu, C.; Lin, C.L. Visualizing Correlation between Carrier Mobility and Defect Density in MoS2 FET. Appl. Phys. Lett. 2022, 121, 151601. [Google Scholar] [CrossRef]
- Saadati, M.; Akhavan, O.; Fazli, H. Single-Layer MoS2-MoO3-x Heterojunction Nanosheets with Simultaneous Photoluminescence and Co-Photocatalytic Features. Catalysts 2021, 11, 1445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minev, N.; Napoleonov, B.; Dimitrov, D.; Videva, V.; Strijkova, V.; Nicheva, D.; Avramova, I.; Petkova, T.; Marinova, V. MoS2-PtX2 Vertical Heterostructures. Nanomaterials 2025, 15, 1415. https://doi.org/10.3390/nano15181415
Minev N, Napoleonov B, Dimitrov D, Videva V, Strijkova V, Nicheva D, Avramova I, Petkova T, Marinova V. MoS2-PtX2 Vertical Heterostructures. Nanomaterials. 2025; 15(18):1415. https://doi.org/10.3390/nano15181415
Chicago/Turabian StyleMinev, Nikolay, Blagovest Napoleonov, Dimitre Dimitrov, Vladimira Videva, Velichka Strijkova, Denitsa Nicheva, Ivalina Avramova, Tamara Petkova, and Vera Marinova. 2025. "MoS2-PtX2 Vertical Heterostructures" Nanomaterials 15, no. 18: 1415. https://doi.org/10.3390/nano15181415
APA StyleMinev, N., Napoleonov, B., Dimitrov, D., Videva, V., Strijkova, V., Nicheva, D., Avramova, I., Petkova, T., & Marinova, V. (2025). MoS2-PtX2 Vertical Heterostructures. Nanomaterials, 15(18), 1415. https://doi.org/10.3390/nano15181415








