Chemical Mechanical Polishing of Zerodur® Using Silica and Ceria Nanoparticles: Toward Ultra-Smooth Optical Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Silica Oxide Nanoparticle
2.2. Synthesis of Cerium Oxide Nanoparticle
2.3. Pretreatment Polishing of Zerodur® Mirrors
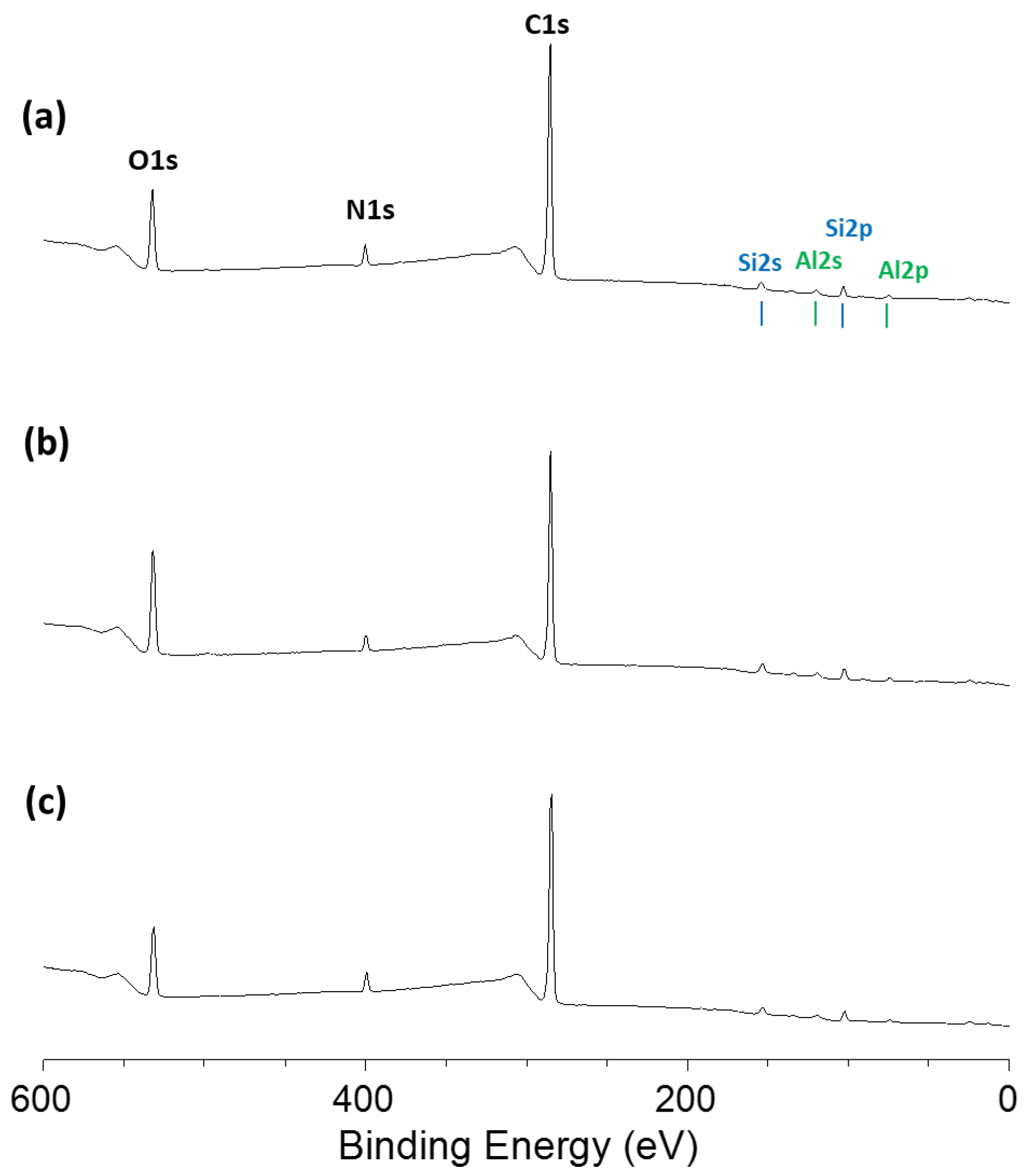
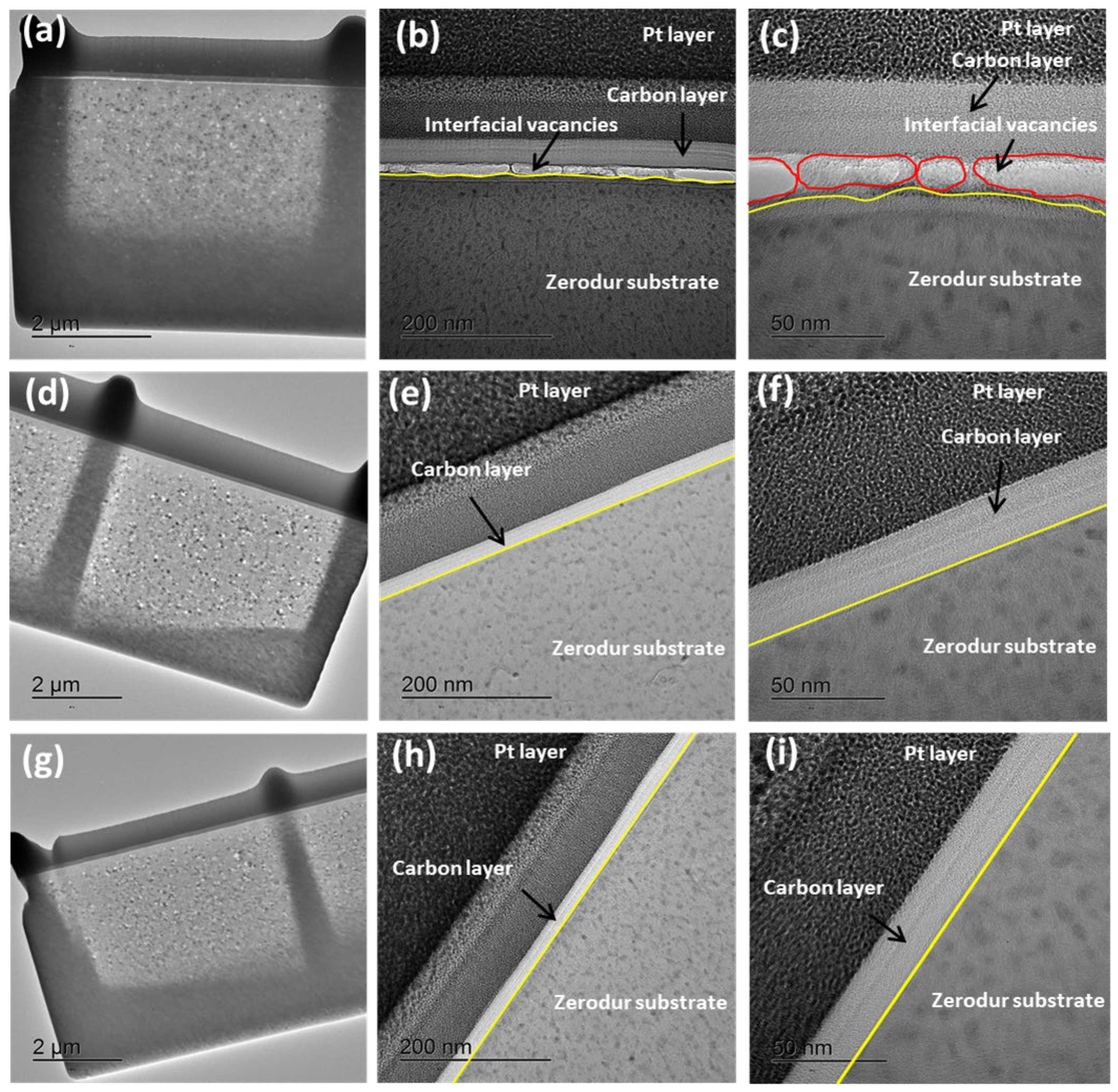
2.4. Characterizations
3. Results and Discussion
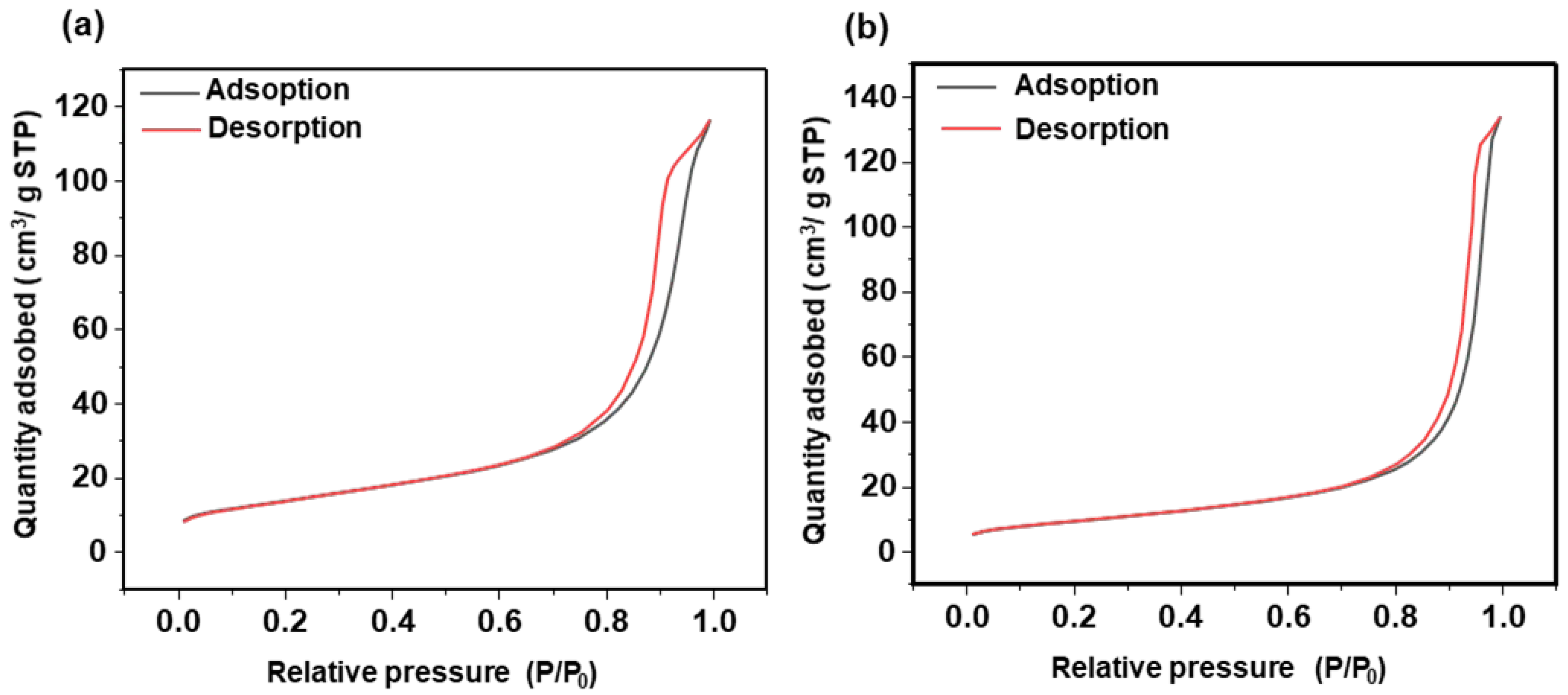
3.1. Synthesis and Characterizations
3.1.1. SiO2 Nanoparticles
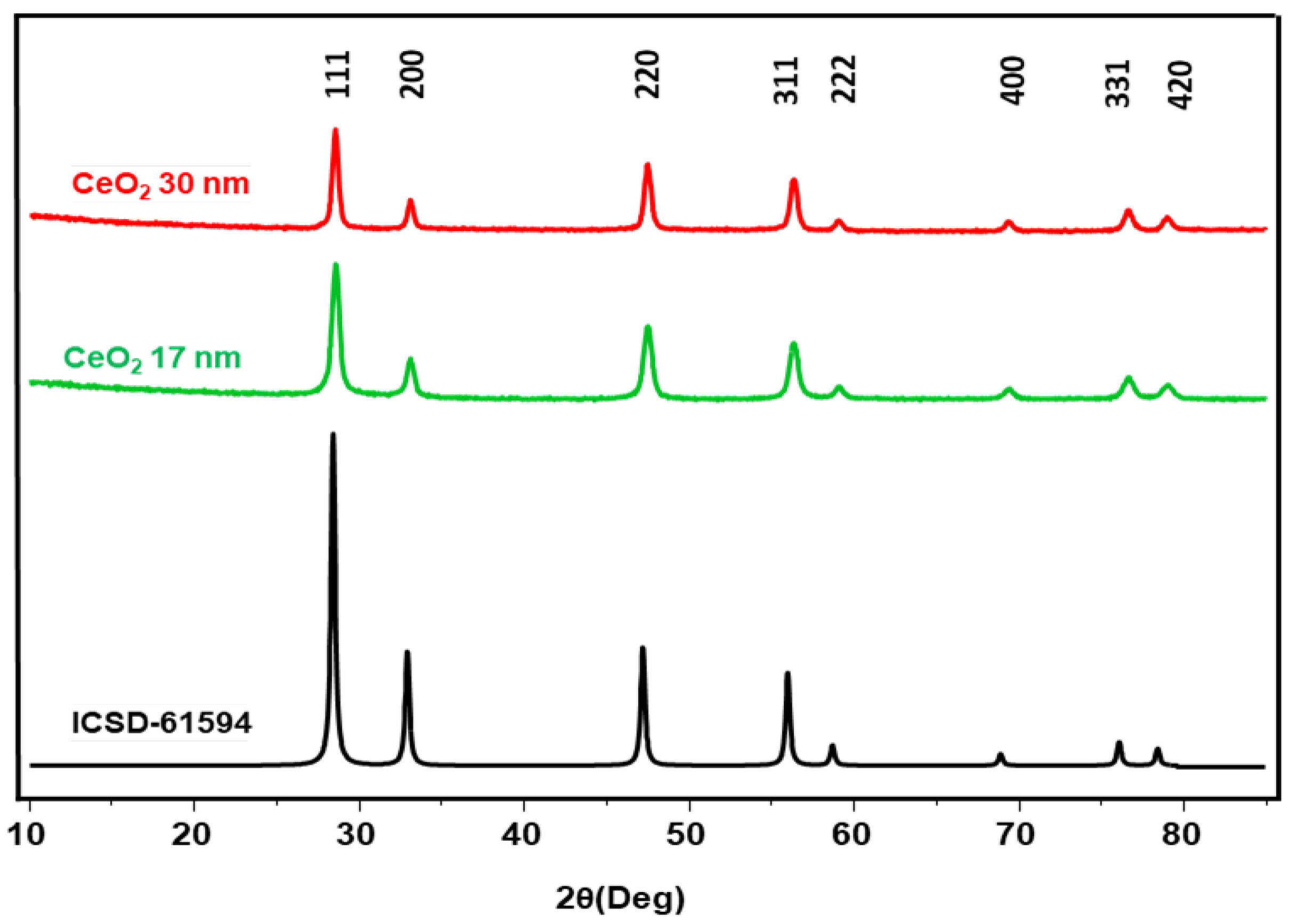
3.1.2. CeO2 Nanoparticles
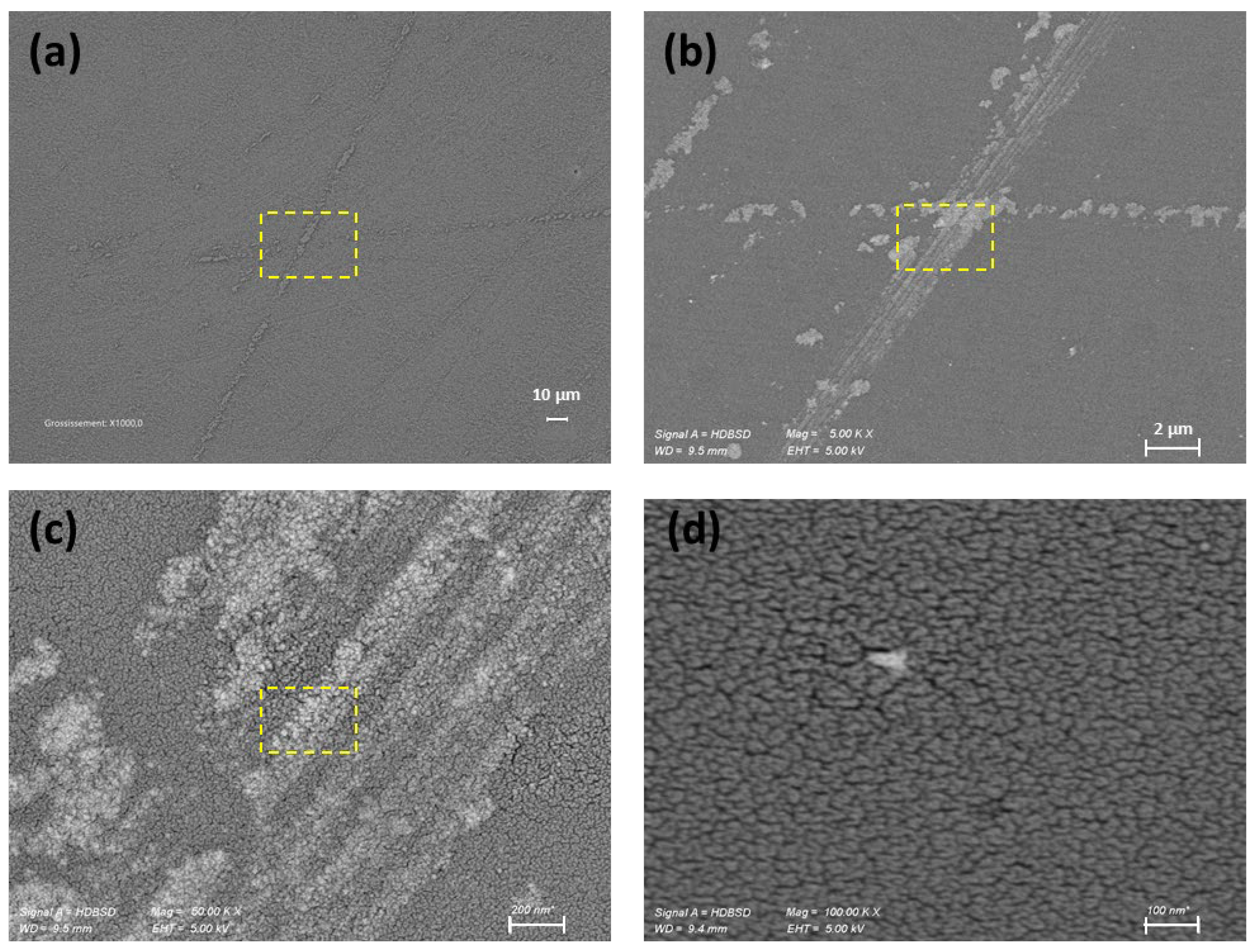
3.2. Surface Roughness Evolution During Nanoparticle-Assisted Hyperpolishing of Hexagonal Small-Scale Zerodur Mirrors
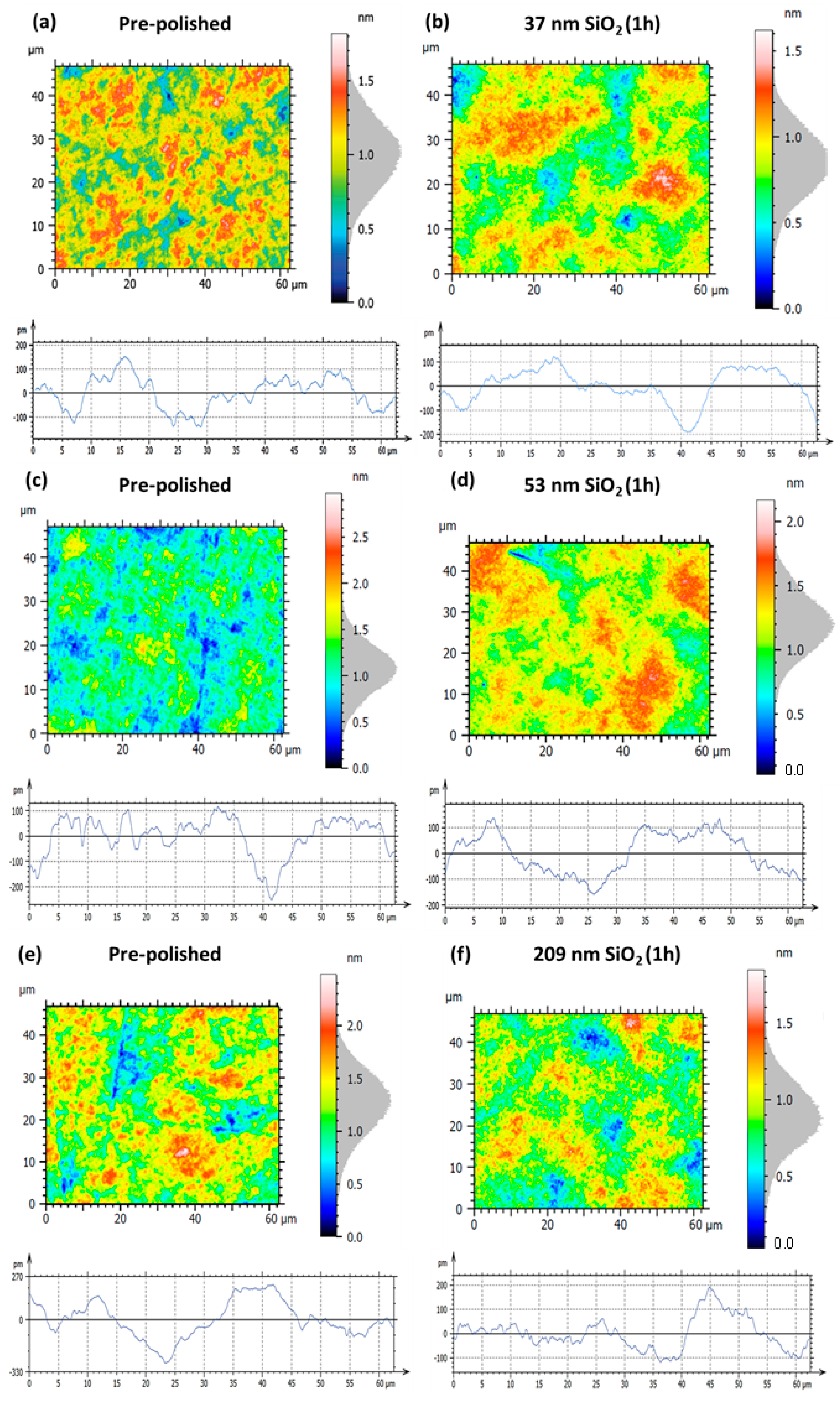
3.2.1. Surface Roughness Evolution via SiO2 Nanoparticle-Assisted Hyperpolishing
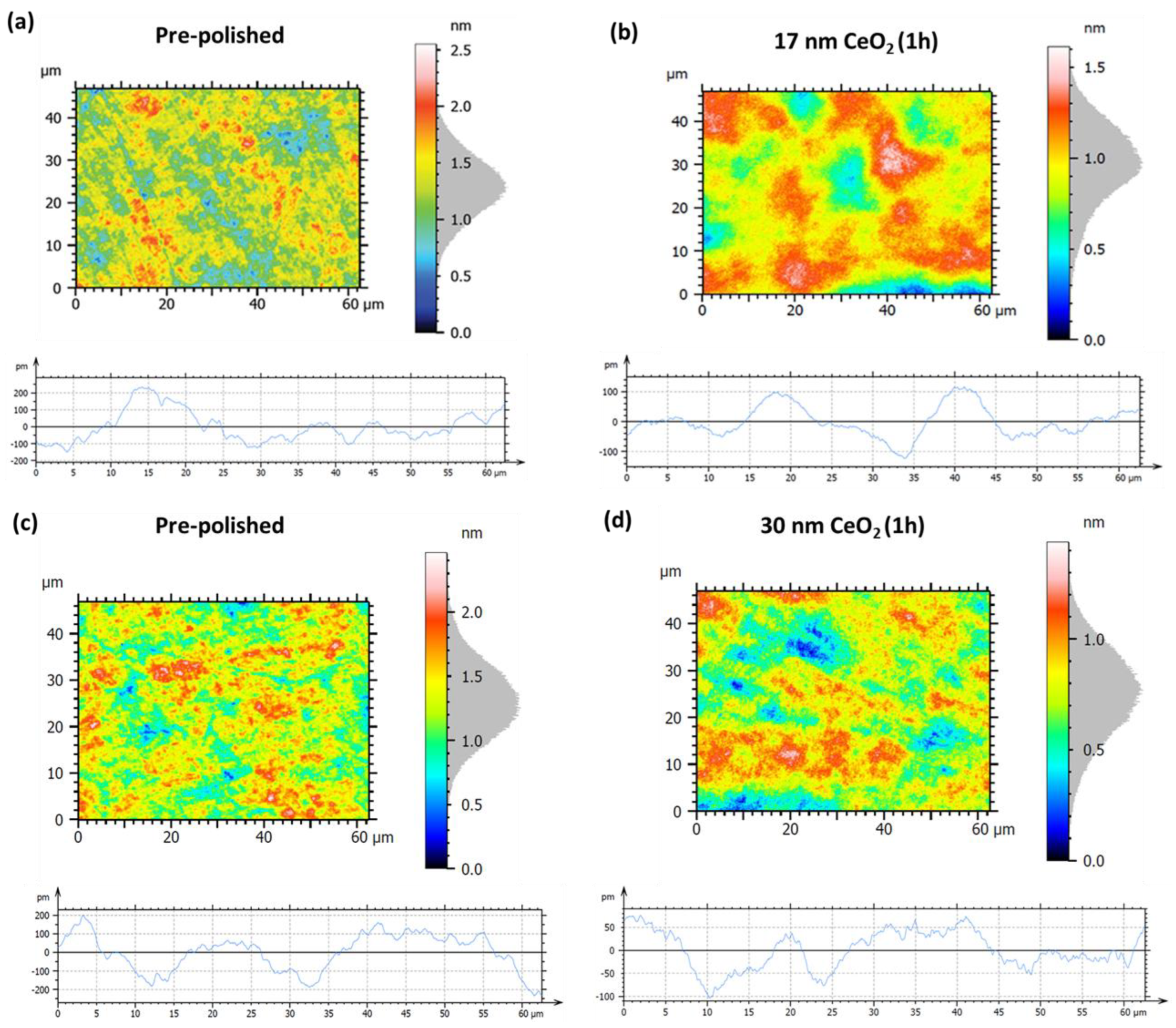
3.2.2. Surface Roughness Evolution via CeO2 Nanoparticle-Assisted Hyperpolishing
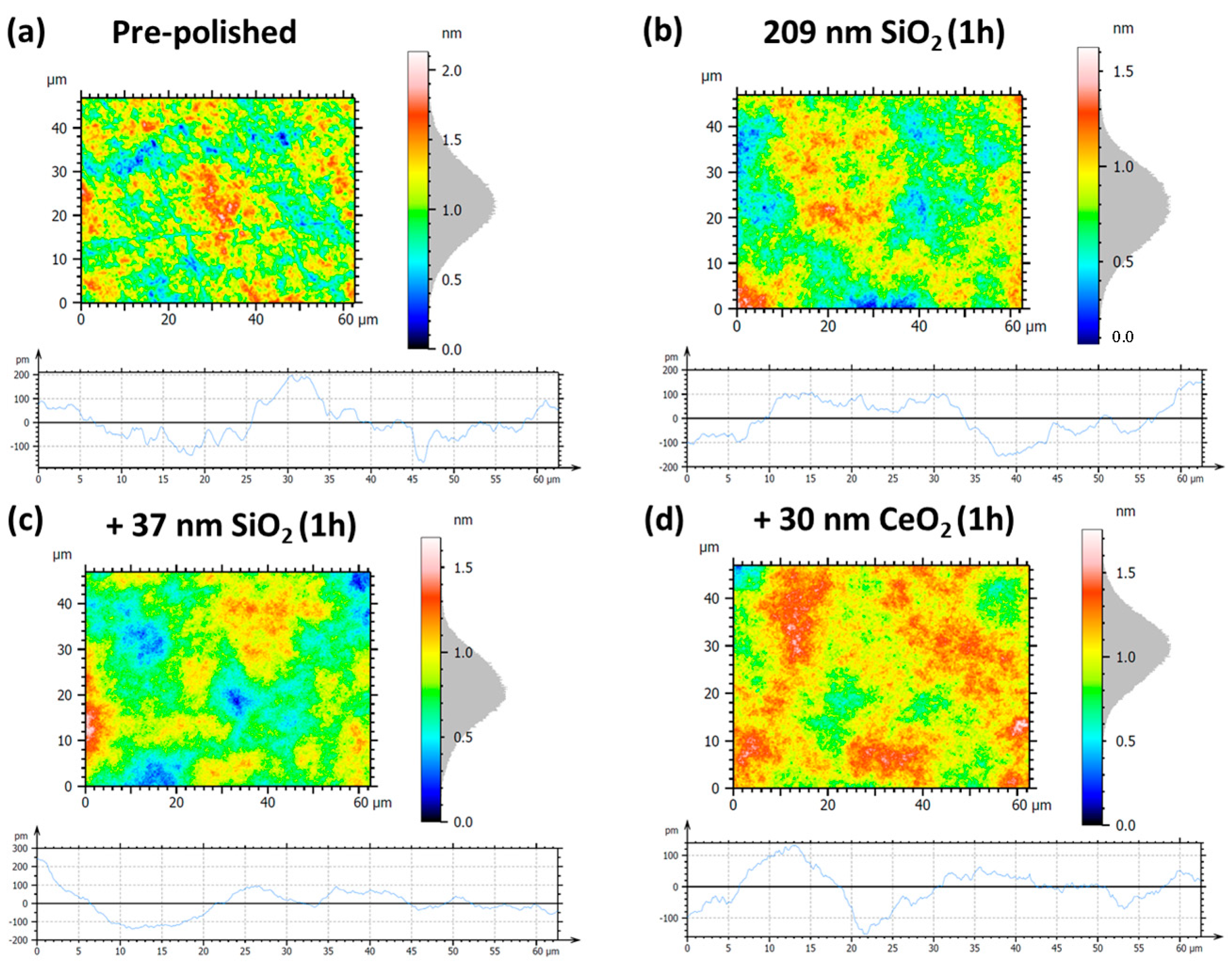
3.2.3. Surface Roughness Evolution via Synergic Effect of Nanoparticle
3.3. Surface Roughness Evolution During Nanoparticle-Assisted Hyperpolishing of Spherical Large-Scale Zerodur Mirrors
4. Conclusions
- (i)
- This first experimental phase allowed us to demonstrate that all tested nanoparticle sizes led to a significant improvement in surface quality. More specifically, both the largest (209 nm) and smallest (37 nm) SiO2 nanoparticles produced very similar final surface states, with a performance advantage for the larger particles. However, this advantage could easily be compensated for by extending the polishing time for the smaller nanoparticles; achieving better results after two hours of polishing instead of one.
- (ii)
- The slurry containing CeO2 nanoparticles of 30 nm showed a noticeably better surface quality and higher hyperpolishing efficiency in comparison to the results obtained with the 37 nm SiO2 particles.
- (iii)
- We then evaluated a sequential polishing approach using different SiO2 nanoparticle sizes (209 nm followed by 37 nm), and then CeO2 nanoparticles (30 nm). This sequential strategy further confirmed the trends observed in the single-slurry tests.
- (iv)
- We focused next on validating one of the most promising polishing protocols on larger optics. Based on the results obtained with the small 30 mm mirrors, where sequential polishing with SiO2 nanoparticles (209 nm followed by 37 nm) proved effective, we scaled up the process to larger Zerodur® mirrors (135 mm in diameter) with both spherical and plano surface profiles. These geometries are representative of optics intended for high-precision applications such as exoplanet detection and imaging. For this scale-up, we retained the sequential SiO2-based polishing protocol, applying two hours of polishing with 209 nm particles followed by two additional hours with 37 nm particles. This process yielded optics with excellent surface quality, achieving area roughness (Sa) of 1 Å for the spherical mirrors and 0.9 Å for the plano mirrors, a remarkable result considering the total hyperpolishing time was limited to just 4 h.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Attenuated Total Reflectance |
| BET | Brunauer–Emmett–Teller |
| CMP | Chemical Mechanical Polishing |
| DLS | Dynamic Light Scattering |
| DTG | Derivative Thermogravimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HRSEM | High-Resolution Scanning Electron Microscopy |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| IR | Infrared Spectroscopy |
| MAS | Magic Angle Spinning |
| NASA | National Aeronautics and Space Administration |
| NMR | Nuclear Magnetic Resonance |
| NPs | Nanoparticles |
| Pa | Arithmetic Mean Height |
| Pq | Root Mean Square Height |
| Sa | Areal Arithmetic Mean Height |
| SEM | Scanning Electron Microscopy |
| Sq | Root Mean Square Roughness |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| TGA | Thermogravimetric Analysis |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
References
- Rouan, D.; Lagrange, A.-M. Detection of exoplanets: Exploiting each property of light. Comptes Rendus Phys. 2023, 24, 11–25. [Google Scholar] [CrossRef]
- Caillat, A.; Ferrari, M.; Marcos, M.; Pariès, C.; Bonnefoi, A.; Vitrac, V.; Roulet, M.; Hugot, E. Super polished mirrors for the Roman Space Telescope CoronaGraphic Instrument: Design, manufacturing, and optical performances. In Proceedings of the Space Telescopes and Instrumentation 2022: Optical, Infrared, and Millimeter Wave, Montréal, QC, Canada, 17–23 July 2022; SPIE: Bellingham, WA, USA, 2022; Volume 12180, pp. 720–728. [Google Scholar]
- Nancy Grace Roman Space Telescope. Available online: https://roman.gsfc.nasa.gov/ (accessed on 21 May 2025).
- Gaudi, B.S.; Mennesson, B.; Seager, S.; Cahoy, K.; Clarke, J.; Domagal-Goldman, S.; Feinberg, L.; Guyon, O.; Kasdin, J.; Marois, C.; et al. The Habitable Exoplanet Observatory (HabEx). In Proceedings of the Space Telescopes and Instrumentation 2018: Optical, Infrared, and Millimeter Wave, Austin, TX, USA, 10–15 June 2018; SPIE: Bellingham, WA, USA, 2018; Volume 10698, pp. 245–253. [Google Scholar]
- Stahl, H.P. Advanced ultraviolet, optical, and infrared mirror technology development for very large space telescopes. J. Astron. Telesc. Instrum. Syst. 2020, 6, 025001. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Wen, D.; Long, Y.; Wang, B.; Li, C.; Ding, Y.; Yang, Q. Surface Integrity and Material Removal Mechanisms of Zerodur Glass-Ceramics by Gallium Infiltration in High-Pressure Polishing. Ceram. Int. 2023, 49, 40091–40104. [Google Scholar] [CrossRef]
- Fabris, D.C.N.; Miguel, E.H.; Vargas, R.; Canto, R.B.; Villas-Boas, M.d.O.C.; Peitl, O.; Sglavo, V.M.; Zanotto, E.D. Microstructure, Residual Stresses, and Mechanical Performance of Surface Crystallized Translucent Glass-Ceramics. J. Eur. Ceram. Soc. 2022, 42, 4631–4642. [Google Scholar] [CrossRef]
- Lu, K.; He, Q.; Xie, J.; Yang, H.; Chen, Z.; Ge, D.; Zhou, C.; Yin, L. Nano-to-Microscale Ductile-to-Brittle Transitions for Edge Cracking Suppression in Single-Diamond Grinding of Lithium Metasilicate/Disilicate Glass-Ceramics. J. Eur. Ceram. Soc. 2023, 43, 1698–1713. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Mu, D.; Lawn, B.R. Science and Art of Ductile Grinding of Brittle Solids. Int. J. Mach. Tools Manuf. 2021, 161, 103675. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Z.; Zhao, F.; Fan, C.; Feng, J.; Zhou, H.; Meng, F.; Zhuang, X.; Wang, J. Advanced Polishing Methods for Atomic-Scale Surfaces: A Review. Mater. Today Sustain. 2024, 27, 100841. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Jeong, H. Mechanical Aspects of the Chemical Mechanical Polishing Process: A Review. Int. J. Precis. Eng. Manuf. 2016, 17, 525–536. [Google Scholar] [CrossRef]
- Zhao, D.; Lu, X. Chemical Mechanical Polishing: Theory and Experiment. Friction 2013, 1, 306–326. [Google Scholar] [CrossRef]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of Silicon Surface with a Single Atomic Layer Precision via Mechanochemical Reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef]
- Bahar Basim, G.; Brown, S.C.; Vakarelski, I.U.; Moudgil, B.M. Strategies for Optimal Chemical Mechanical Polishing (CMP) Slurry Design. J. Dispers. Sci. Technol. 2003, 24, 499–515. [Google Scholar] [CrossRef]
- Vazquez Bengochea, L.; Sampurno, Y.; Kavaljer, M.; Johnston, R.; Philipossian, A. Characterization of CMP Slurries Using Densitometry and Refractive Index Measurements. Micromachines 2018, 9, 542. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, Z.; Gao, P.; Liu, T.; Boyjoo, Y.; Guo, D. Design of Composite Abrasives and Substrate Materials for Chemical Mechanical Polishing Applications. Appl. Nanosci. 2020, 10, 1379–1393. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Li, M.; Xie, G. Synthesis of Core-Shell Micro/Nanoparticles and Their Tribological Application: A Review. Materials 2020, 13, 4590. [Google Scholar] [CrossRef]
- Lee, G.; Lee, K.; Sun, S.; Song, T.; Paik, U. Engineering SiO2 Nanoparticles: A Perspective on Chemical Mechanical Planarization Slurry for Advanced Semiconductor Processing. KONA Powder Part. J. 2025, 42, 79–99. [Google Scholar] [CrossRef]
- Ren, G.; Wang, L.; Wang, S. Innovative Synthesis of CeO2 Nanoparticles for Advanced Chemical Mechanical Polishing. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135764. [Google Scholar] [CrossRef]
- Ma, J.; Xu, N.; Cheng, J.; Pu, Y. A Review on the Development of Ceria for Chemical Mechanical Polishing. Powder Technol. 2024, 444, 119989. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Wu, X.; Peng, Y.; Lin, S.; Huang, L.; Huang, X.; Zhu, L. Effect of Dispersion Performance of Polishing Slurry on the Polishing Quality of Glass-Ceramics in Bonnet Polishing. Int. J. Adv. Manuf. Technol. 2023, 127, 107–121. [Google Scholar] [CrossRef]
- Super-Polishing of Zerodur Aspheres by Means of Conventional Polishing Technology. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/9442/944212/Super-polishing-of-Zerodur-aspheres-by-means-of-conventional-polishing/10.1117/12.2175899.short (accessed on 21 May 2025).
- Esmaeilzare, A.; Rahimi, A.; Rezaei, S.M. Investigation of Subsurface Damages and Surface Roughness in Grinding Process of Zerodur® Glass–Ceramic. Appl. Surf. Sci. 2014, 313, 67–75. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Z.; Yu, S.; Chen, X.; Shi, C.; Zhou, H.; Meng, F.; Yu, J.; Wen, W. Unprecedented Atomic Surface of Silicon Induced by Environmentally Friendly Chemical Mechanical Polishing. Nanoscale 2023, 15, 9304–9314. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, W.; Liu, W.; Song, Z. Enhancing Slurry Stability and Surface Flatness of Silicon Wafers through Organic Amine-Catalyzed Synthesis Silica Sol. Nanomaterials 2024, 14, 1371. [Google Scholar] [CrossRef]
- Willinger, M.; Felhofer, M.; Reimhult, E.; Zirbs, R. Method for High-Yield Hydrothermal Growth of Silica Shells on Nanoparticles. Materials 2021, 14, 6646. [Google Scholar] [CrossRef]
- Sakthiraj, K.; Karthikeyan, B. Synthesis and Characterization of Cerium Oxide Nanoparticles Using Different Solvents for Electrochemical Applications. Appl. Phys. A 2019, 126, 52. [Google Scholar] [CrossRef]
- Wang, J.-F.; Sthuraman, A.; Wang, H.-M.; Cook, L.M. Polishing Slurries Comprising Two Abrasive Components and Methods for Their Use. Patent US08541898, 2 December 1997. [Google Scholar]
- Stober, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1928123 (accessed on 15 October 2024). [CrossRef]
- Davies, G.-L.; Barry, A.; Gun’ko, Y.K. Preparation and Size Optimisation of Silica Nanoparticles Using Statistical Analyses. Chem. Phys. Lett. 2009, 468, 239–244. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Raimundo, I.M.; Pimentel, M.F. Revising the Synthesis of Stöber Silica Nanoparticles: A Multivariate Assessment Study on the Effects of Reaction Parameters on the Particle Size. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 1–7. [Google Scholar] [CrossRef]
- Fouilloux, S.; Daillant, J.; Thill, A. Single Step Synthesis of 5–30 nm Monodisperse Silica Nanoparticles: Important Experimental Parameters and Modeling. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 122–127. [Google Scholar] [CrossRef]
- Synthesis of TiO2-SiO2 Powder and Thin Film Photocatalysts by Sol-Gel Method. Available online: https://www.researchgate.net/publication/288256868_Synthesis_of_TiO2-SiO2_powder_and_thin_film_photocatalysts_by_sol-gel_method (accessed on 22 May 2025).
- Arun Kumar, D.; Merline Shyla, J.; Xavier, F.P. Synthesis and Characterization of TiO2/SiO2 Nano Composites for Solar Cell Applications. Appl. Nanosci. 2012, 2, 429–436. [Google Scholar] [CrossRef]
- Gholami, T.; Salavati-Niasari, M.; Bazarganipour, M.; Noori, E. Synthesis and Characterization of Spherical Silica Nanoparticles by Modified Stöber Process Assisted by Organic Ligand. Superlattices Microstruct. 2013, 61, 33–41. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Jaafar, M.Z.; Yusof, M.A.M.; Shye, C.A.; Idris, A.K. Influence of Size and Surface Charge on the Adsorption Behaviour of Silicon Dioxide Nanoparticles on Sand Particles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131943. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Rahman, I.A.; Sipaut, C.S. Synthesis of Silica Nanoparticles by Modified Sol–Gel Process: The Effect of Mixing Modes of the Reactants and Drying Techniques. J. Sol-Gel Sci. Technol. 2009, 50, 328–336. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-08-057103-4. [Google Scholar]
- Hassanzadeh-Tabrizi, S.A. Precise Calculation of Crystallite Size of Nanomaterials: A Review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Hellenbrandt, M. The Inorganic Crystal Structure Database (ICSD)—Present and Future. Crystallogr. Rev. 2004, 10, 17–22. [Google Scholar] [CrossRef]
- Miguel-García, I.; Parres-Esclapez, S.; Lozano-Castelló, D.; Bueno-López, A. H2 Assisted Decomposition of Cerium Nitrate to Ceria with Enhanced Catalytic Properties. Catal. Commun. 2010, 11, 848–852. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yang, Y.Q.; Shen, J.M.; Wang, J.A. Mesostructured CeO2 and Pd/CeO2 Nanophases: Templated Synthesis, Crystalline Structure and Catalytic Properties. J. Mol. Catal. A Chem. 2005, 237, 182–190. [Google Scholar] [CrossRef]
- Knoblauch, N.; Dörrer, L.; Fielitz, P.; Schmücker, M.; Borchardt, G. Surface Controlled Reduction Kinetics of Nominally Undoped Polycrystalline CeO2. Phys. Chem. Chem. Phys. 2015, 17, 5849–5860. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.; Yoko, A.; Tomai, T.; Naka, T.; Wang, H.; Frenkel, A.I.; Adschiri, T. Effect of Exposed Facets and Oxidation State of CeO2 Nanoparticles on CO2 Adsorption and Desorption. ACS Sustain. Chem. Eng. 2024, 12, 7532–7540. [Google Scholar] [CrossRef]
- Schneider, M.; Gasparov, V.A.; Richter, W.; Deckwerth, M.; Rüssel, C. XPS Studies on Oxynitride Glasses in the System Si Al O N. J. Non-Cryst. Solids 1997, 215, 201–207. [Google Scholar] [CrossRef]
- Conger, C.P.; Suzer, S. Response of Polyelectrolyte Layers to the SiO2 Substrate Charging as Probed by XPS. Langmuir 2009, 25, 1757–1760. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Song, Z. Particle Size and Surfactant Effects on Chemical Mechanical Polishing of Glass Using Silica-Based Slurry. Appl. Opt. 2010, 49, 5480–5485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shan, L.; Hight, J.R.; Danyluk, S.; Ng, S.H.; Paszkowski, A.J. Influence of Colloidal Abrasive Size on Material Removal Rate and Surface Finish in SiO2 Chemical Mechanical Polishing. Tribol. Trans. 2002, 45, 232–238. [Google Scholar] [CrossRef]
- Lei, H.; Luo, J. CMP of Hard Disk Substrate Using a Colloidal SiO2 Slurry: Preliminary Experimental Investigation. Wear 2004, 257, 461–470. [Google Scholar] [CrossRef]
- Levert, J.A.; Korach, C.S. CMP Friction as a Function of Slurry Silica Nanoparticle Concentration and Diameter. Tribol. Trans. 2009, 52, 256–261. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Wang, F.; Di, W. Investigation on the Final Polishing Slurry and Technique of Silicon Substrate in ULSI. Microelectron. Eng. 2003, 66, 438–444. [Google Scholar] [CrossRef]
- Alfonsetti, R.; De Simone, G.; Lozzi, L.; Passacantando, M.; Picozzi, P.; Santucci, S. SiOx Surface Stoichiometry by XPS: A Comparison of Various Methods. Surf. Interface Anal. 1994, 22, 89–92. [Google Scholar] [CrossRef]
- Datta, D.; Rai, H.; Singh, S.; Srivastava, M.; Sharma, R.K.; Gosvami, N.N. Nanoscale Tribological Aspects of Chemical Mechanical Polishing: A Review. Appl. Surf. Sci. Adv. 2022, 11, 100286. [Google Scholar] [CrossRef]
- Suratwala, T.; Steele, R.; Feit, M.D.; Wong, L.; Miller, P.; Menapace, J.; Davis, P. Effect of Rogue Particles on the Sub-Surface Damage of Fused Silica during Grinding/Polishing. J. Non-Cryst. Solids 2008, 354, 2023–2037. [Google Scholar] [CrossRef]
- Boripatkosol, S.; Bun-Athuek, N.; Khajornrungruang, P.; Suzuki, K.; Chanthawong, N.; Phaisalpanumas, P. Study on Relationship of the Material Removal Amount and the Increase in Abrasive Nanoparticle Size during Si-CMP. In Proceedings of the 2018 Third International Conference on Engineering Science and Innovative Technology (ESIT), North Bangkok, Thailand, 19–22 April 2018; pp. 1–3. [Google Scholar]
- Bellahsene, H.; Sene, S.; Félix, G.; Larionova, J.; Ferrari, M.; Guari, Y. A Comprehensive Review of the Nano-Abrasives Key Parameters Influencing the Performance in Chemical Mechanical Polishing. Nanomaterials 2025, 15, 1366. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, W. Characterization of Ceria Nanoparticles as Abrasives Applied with Defoaming Polymers for CMP (Chemical Mechanical Polishing) Applications. Polymers 2024, 16, 844. [Google Scholar] [CrossRef] [PubMed]
- Ceria Based Slurry—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/engineering/ceria-based-slurry (accessed on 9 April 2025).
- Wrobel, R.; Suchorski, Y.; Becker, S.; Weiss, H. Cerium Oxide Layers on the Cu(1 1 1) Surface: Substrate-Mediated Redox Properties. Surf. Sci. 2008, 602, 436–442. [Google Scholar] [CrossRef]
- Chen, G.; Ni, Z.; Xu, L.; Li, Q.; Zhao, Y. Performance of Colloidal Silica and Ceria Based Slurries on CMP of Si-Face 6H-SiC Substrates. Appl. Surf. Sci. 2015, 359, 664–668. [Google Scholar] [CrossRef]

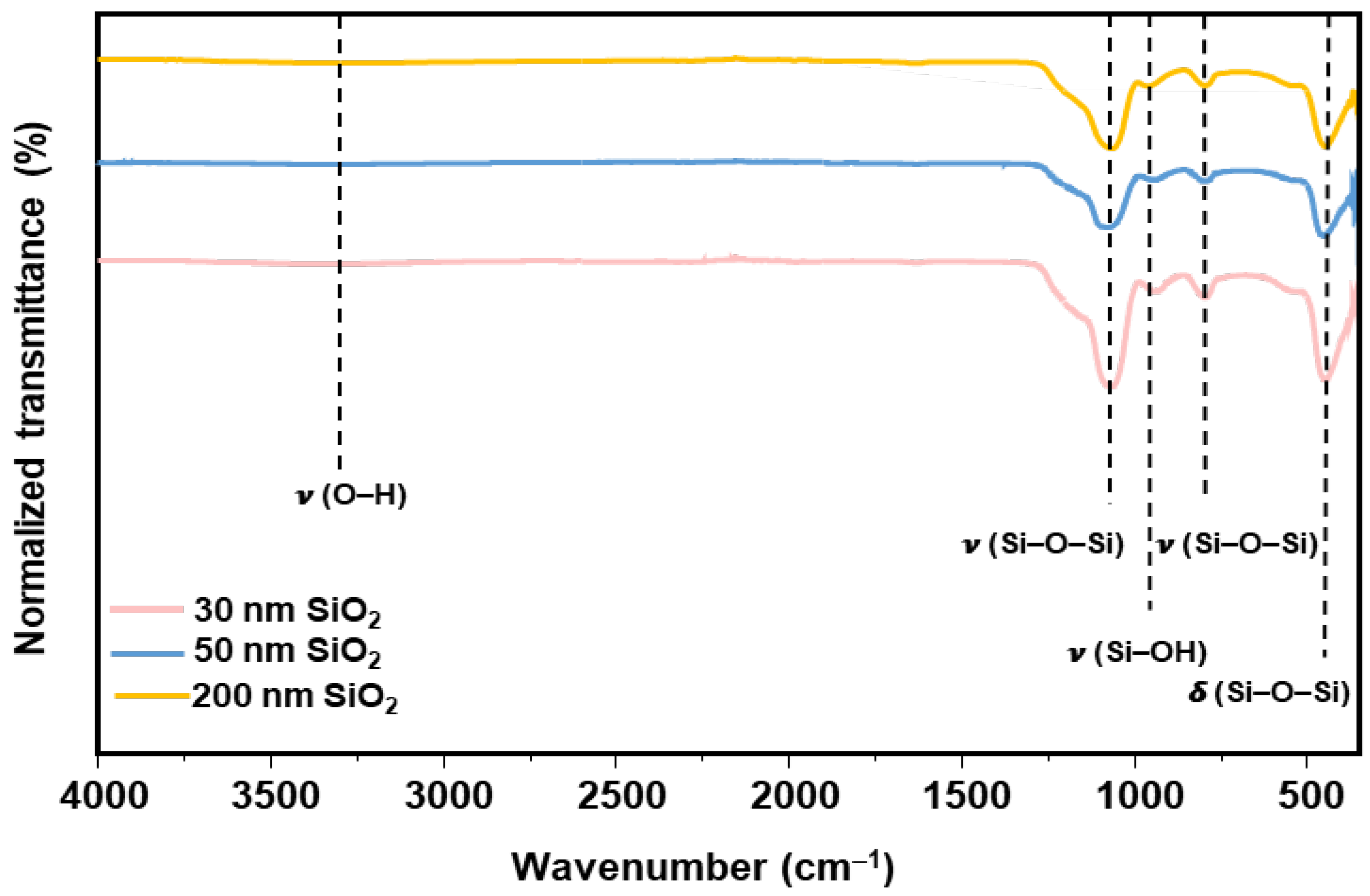

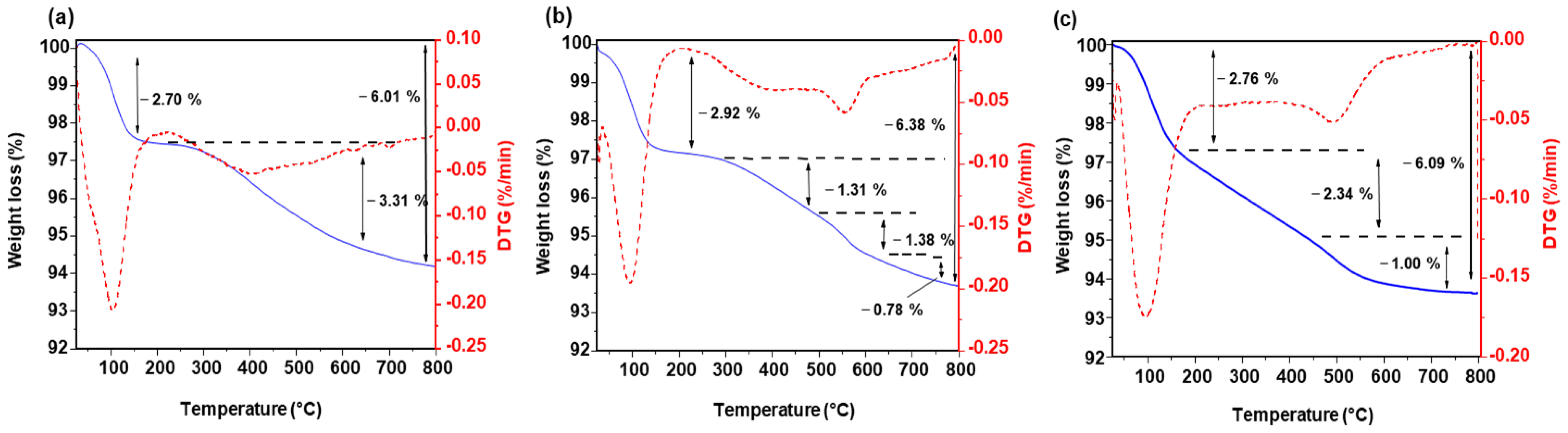
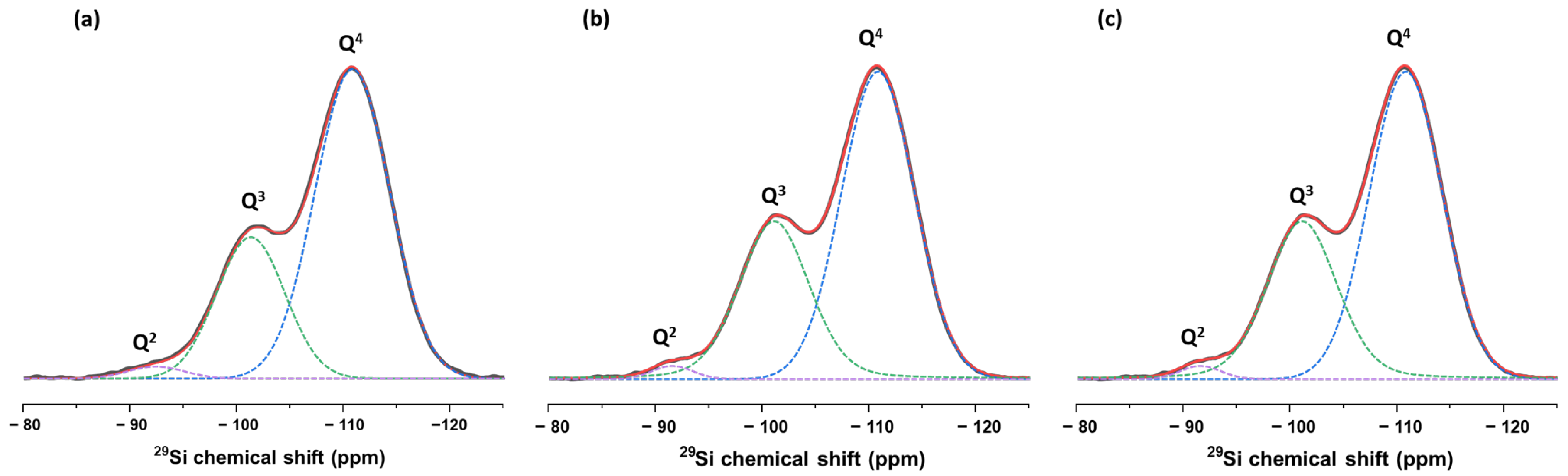

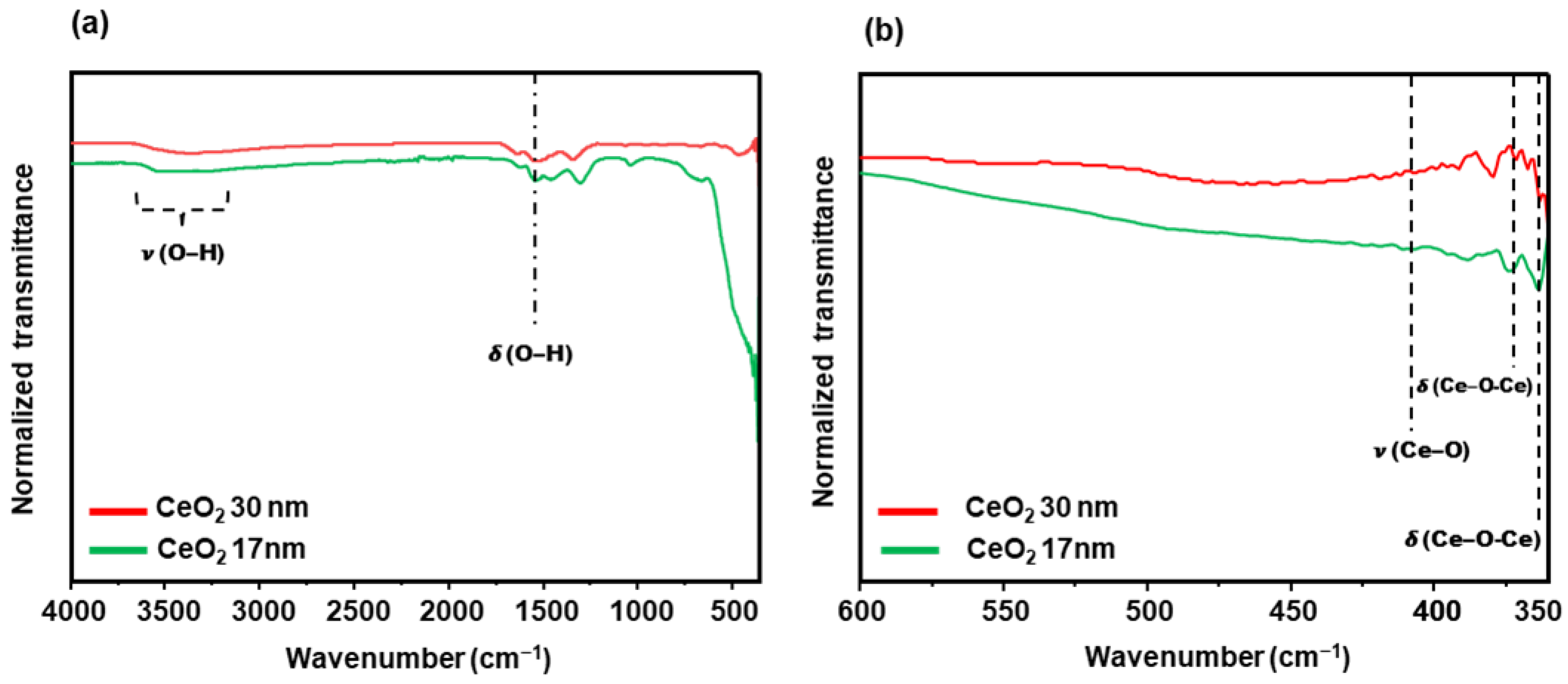



| Size | VEtOH | VH2O | VTEOS | Temperature |
|---|---|---|---|---|
| 209 ± 11 nm | 400 mL | 8 mL | 12 mL | 20 °C |
| 53 ± 6 nm | 200 mL | 200 mL | 12 mL | 30 °C |
| 37 ± 5 nm | 400 mL | 8 mL | 12 mL | 75 °C |
| Figure 11 Panels | Sa | Sq | Pa | Pq |
|---|---|---|---|---|
| (a) pre-polished | 0.18 nm | 0.22 nm | 55.5 pm | 66.9 pm |
| (b) 37 nm SiO2, 1 h | 0.15 nm (−16.7%) | 0.18 nm (−18.2%) | 56.1 pm (+1.10%) | 71.2 pm (+6.40%) |
| (c) pre-polished | 0.20 nm | 0.25 nm | 79.0 pm | 62.5 pm |
| (d) 53 nm SiO2, 1 h | 0.17 nm (−15.0%) | 0.22 nm (−12.0%) | 77.8 pm (−1.50%) | 70.8 pm (+13.3%) |
| (e) pre-polished | 0.23 nm | 0.29 nm | 92.1 pm | 115.9 pm |
| (f) 209 nm SiO2, 1 h | 0.16 nm (−30.4%) | 0.20 nm (−31.0%) | 52.0 pm (−43.5%) | 66.9 pm (−42.3%) |
| Figure 15 Panels | Sa | Sq | Pa | Pq |
|---|---|---|---|---|
| (a) pre-polished | 0.21 nm | 0.26 nm | 71.8 pm | 90.3 pm |
| (b) 17 nm CeO2, 1 h | 0.16 nm (−23.8%) | 0.21 nm (−19.2%) | 39.6 pm (−44.8%) | 51.1 pm (−43.4%) |
| (c) pre-polished | 0.23 nm | 0.28 nm | 84.2 pm | 100.8 pm |
| (d) 30 nm CeO2, 1 h | 0.15 nm (−34.8%) | 0.19 nm (−32.1%) | 35.6 pm (−57.7%) | 41.9 pm (−58.4%) |
| Figure 17 Panels | Sa | Sq | Pa | Pq |
|---|---|---|---|---|
| (a) pre-polished | 0.20 nm | 0.25 nm | 60.6 pm | 76.4 pm |
| (b) 209 nm SiO2 | 0.15 nm (−25.0%) | 0.20 nm (−20.0%) | 68.7 pm (+13.4%) | 78.8 pm (+3.10%) |
| (c) +37 nm SiO2 | 0.15 nm (−25.0%) | 0.19 nm (−24.0%) | 57.4 pm (−5.30%) | 74.7 pm (−2.20%) |
| (d) +30 nm CeO2 | 0.14 nm (−30.0%) | 0.18 nm (−28.0%) | 46.3 pm (−23.6%) | 59.0 pm (−22.7%) |
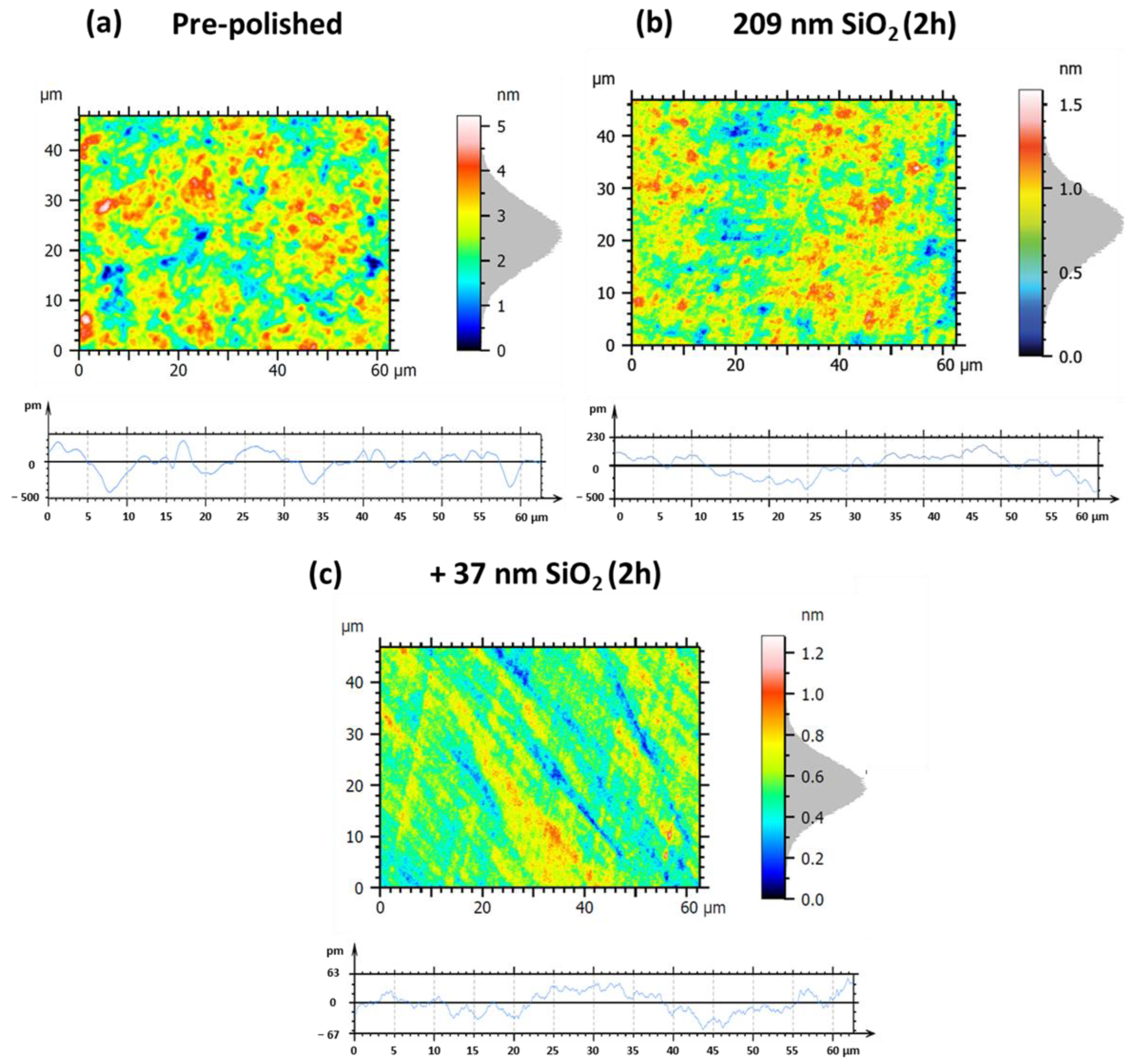
| Figure 19 Panels | Sa | Sq | Pa | Pq |
|---|---|---|---|---|
| (a) pre-polished | 0.53 nm | 0.67 nm | 0.11 nm | 0.14 nm |
| (b) 209 nm SiO2, 2 h | 0.15 nm (−71.7%) | 0.19 nm (−71.6%) | 74.0 pm (−32.7%) | 87.0 pm (−37.9%) |
| (c) +37 nm SiO2, 2 h | 0.10 nm (−81.1%) | 0.13 nm (−80.6%) | 19.3 pm (−82.5%) | 23.0 pm (−83.6%) |
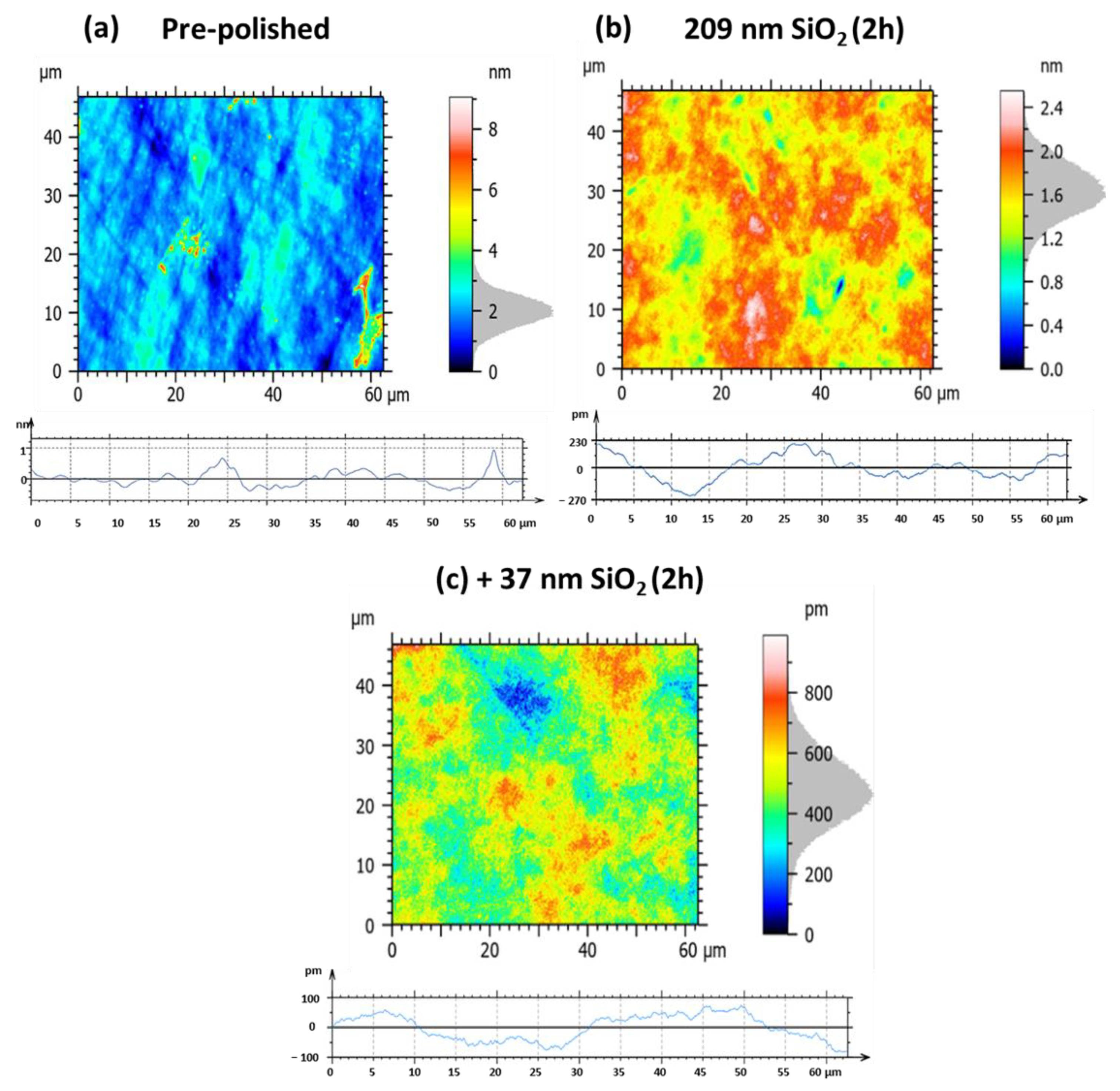
| Figure 20 Panels | Sa | Sq | Pa | Pq |
|---|---|---|---|---|
| (a) pre-polished | 0.46 nm | 0.67 nm | 0.17 nm | 0.22 nm |
| (b) 209 nm SiO2, 2 h | 0.19 nm (−58.7%) | 0.24 nm (−64.2%) | 83.2 pm (−51.1%) | 103 pm (−53.2%) |
| (c) +37 nm SiO2, 2 h | 90.1 pm (−80.4%) | 114.5 pm (−82.9%) | 39.0 pm (−77.1%) | 42.8 pm (−80.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellahsene, H.; Sene, S.; Félix, G.; Fabregue, N.; Marcos, M.; Uhart, A.; Dupin, J.-C.; Oliviero, E.; Larionova, J.; Ferrari, M.; et al. Chemical Mechanical Polishing of Zerodur® Using Silica and Ceria Nanoparticles: Toward Ultra-Smooth Optical Surfaces. Nanomaterials 2025, 15, 1391. https://doi.org/10.3390/nano15181391
Bellahsene H, Sene S, Félix G, Fabregue N, Marcos M, Uhart A, Dupin J-C, Oliviero E, Larionova J, Ferrari M, et al. Chemical Mechanical Polishing of Zerodur® Using Silica and Ceria Nanoparticles: Toward Ultra-Smooth Optical Surfaces. Nanomaterials. 2025; 15(18):1391. https://doi.org/10.3390/nano15181391
Chicago/Turabian StyleBellahsene, Houda, Saad Sene, Gautier Félix, Nicolas Fabregue, Michel Marcos, Arnaud Uhart, Jean-Charles Dupin, Erwan Oliviero, Joulia Larionova, Marc Ferrari, and et al. 2025. "Chemical Mechanical Polishing of Zerodur® Using Silica and Ceria Nanoparticles: Toward Ultra-Smooth Optical Surfaces" Nanomaterials 15, no. 18: 1391. https://doi.org/10.3390/nano15181391
APA StyleBellahsene, H., Sene, S., Félix, G., Fabregue, N., Marcos, M., Uhart, A., Dupin, J.-C., Oliviero, E., Larionova, J., Ferrari, M., & Guari, Y. (2025). Chemical Mechanical Polishing of Zerodur® Using Silica and Ceria Nanoparticles: Toward Ultra-Smooth Optical Surfaces. Nanomaterials, 15(18), 1391. https://doi.org/10.3390/nano15181391









