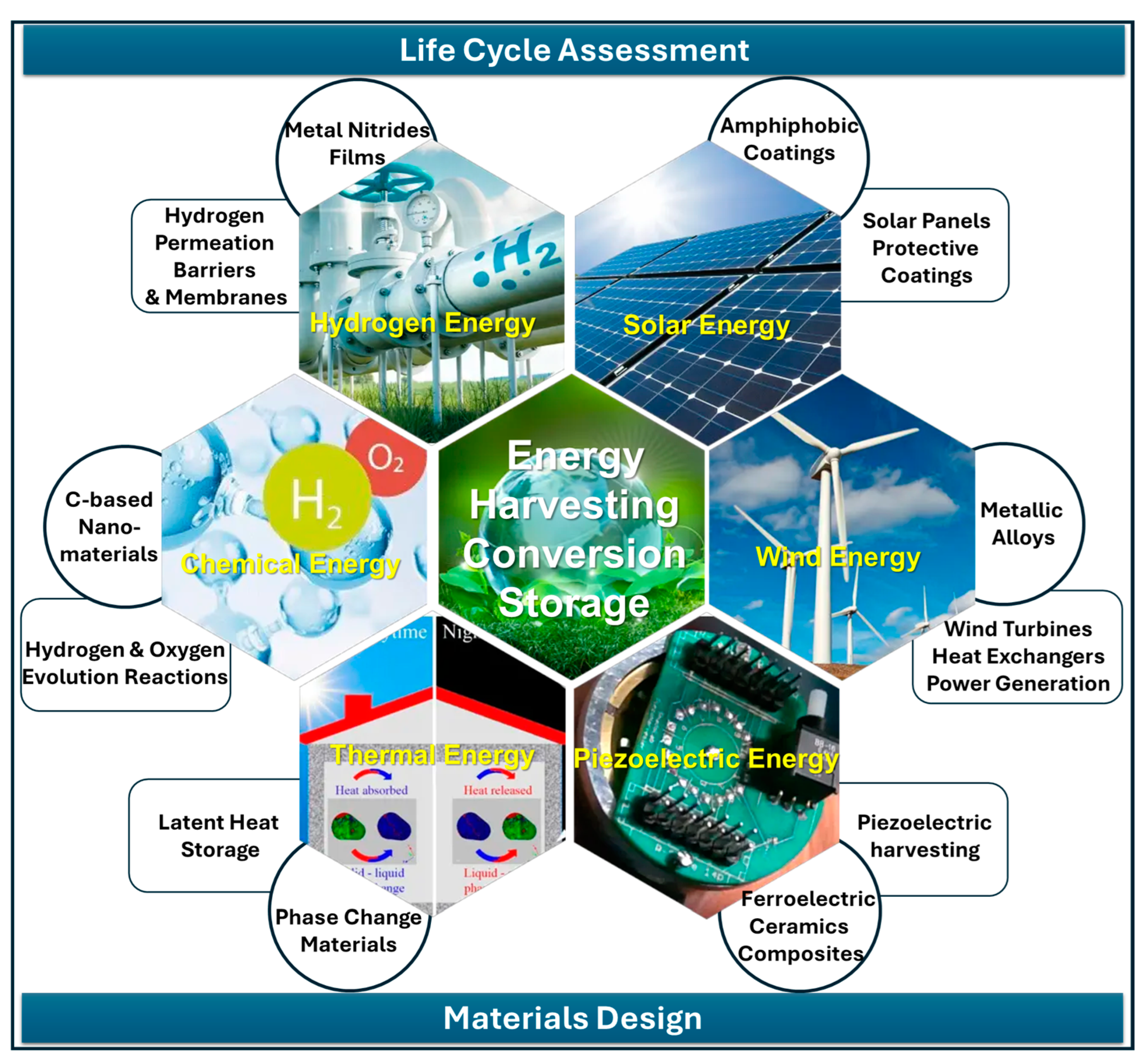
Sustainable Materials for Energy
Abstract
1. Introduction
2. Carbon-Based Nanomaterials for Chemical Energy Conversion
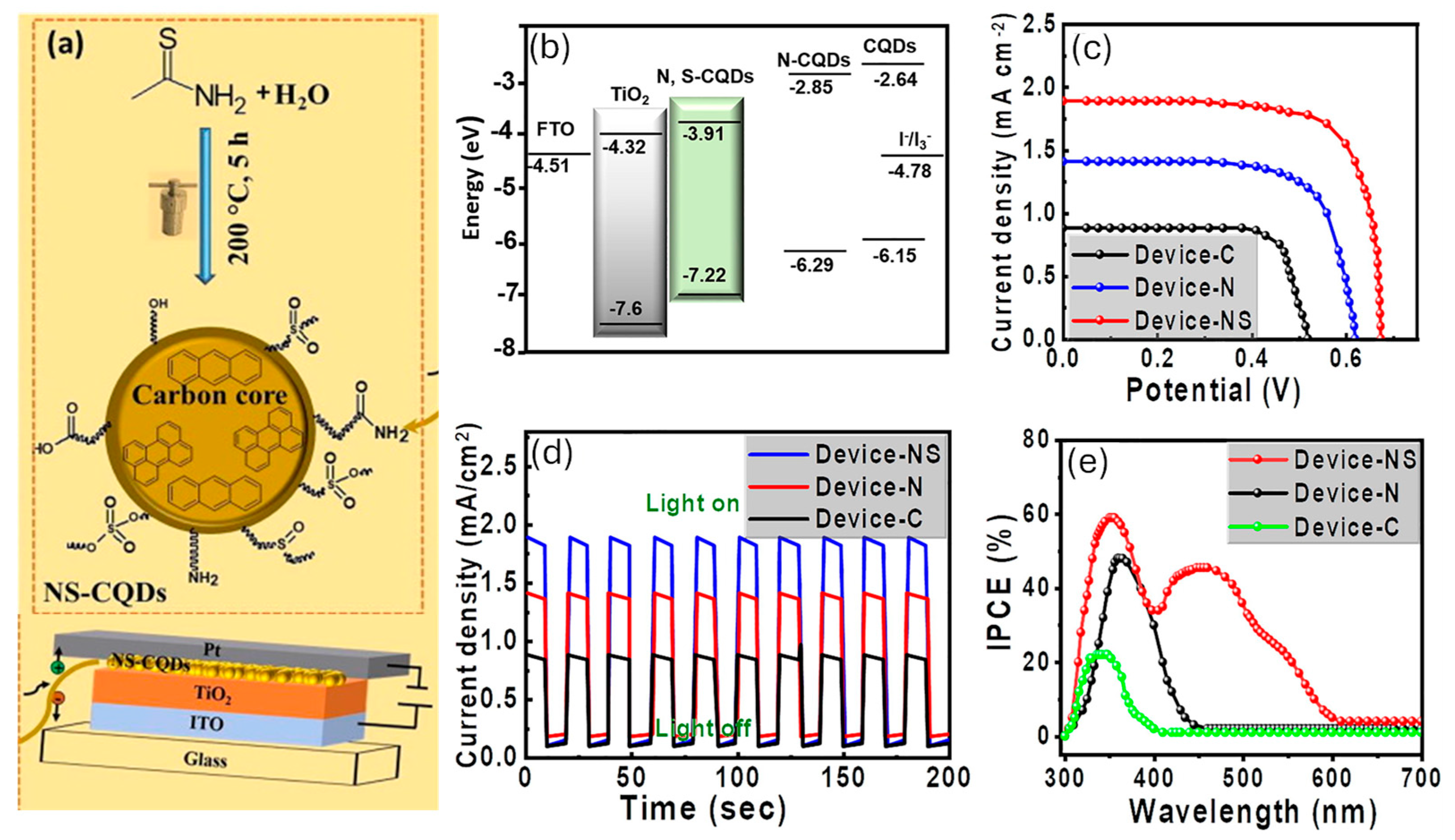
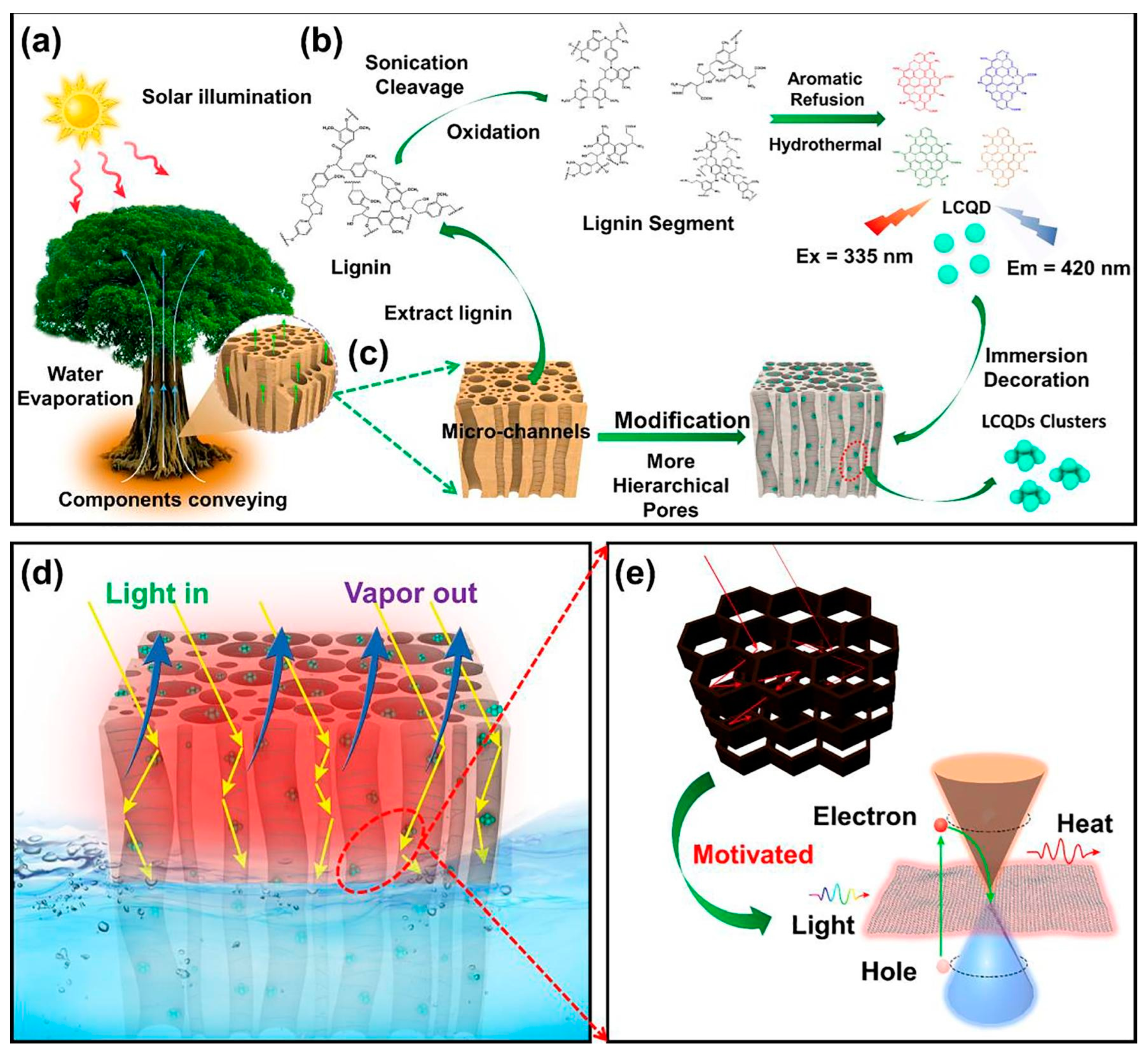
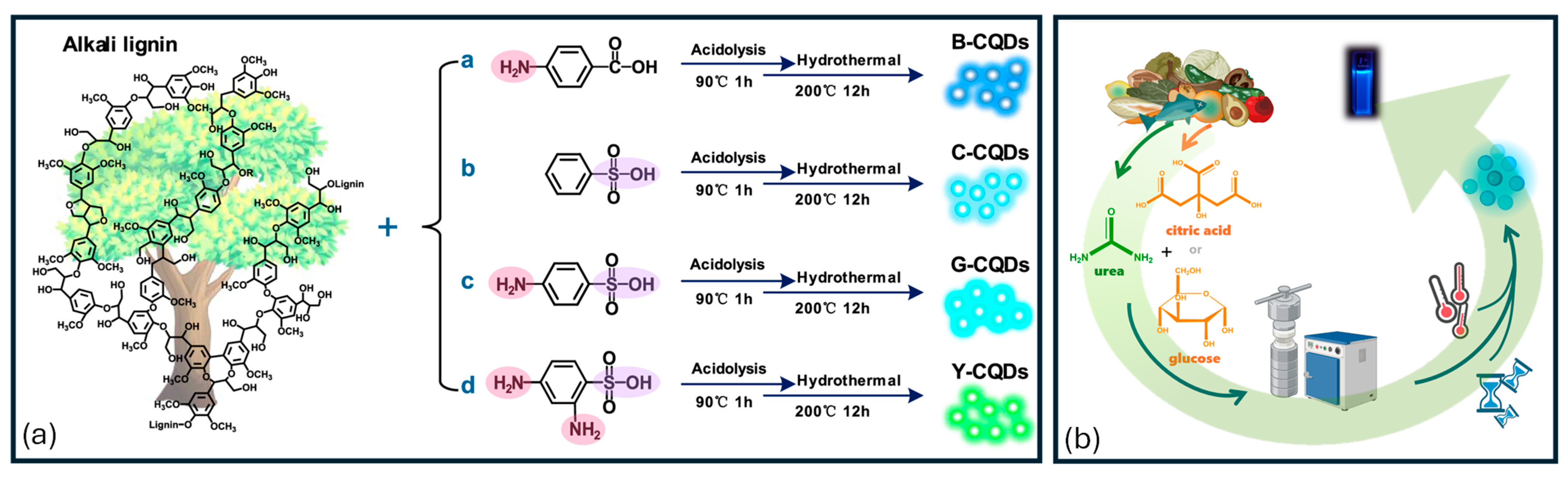
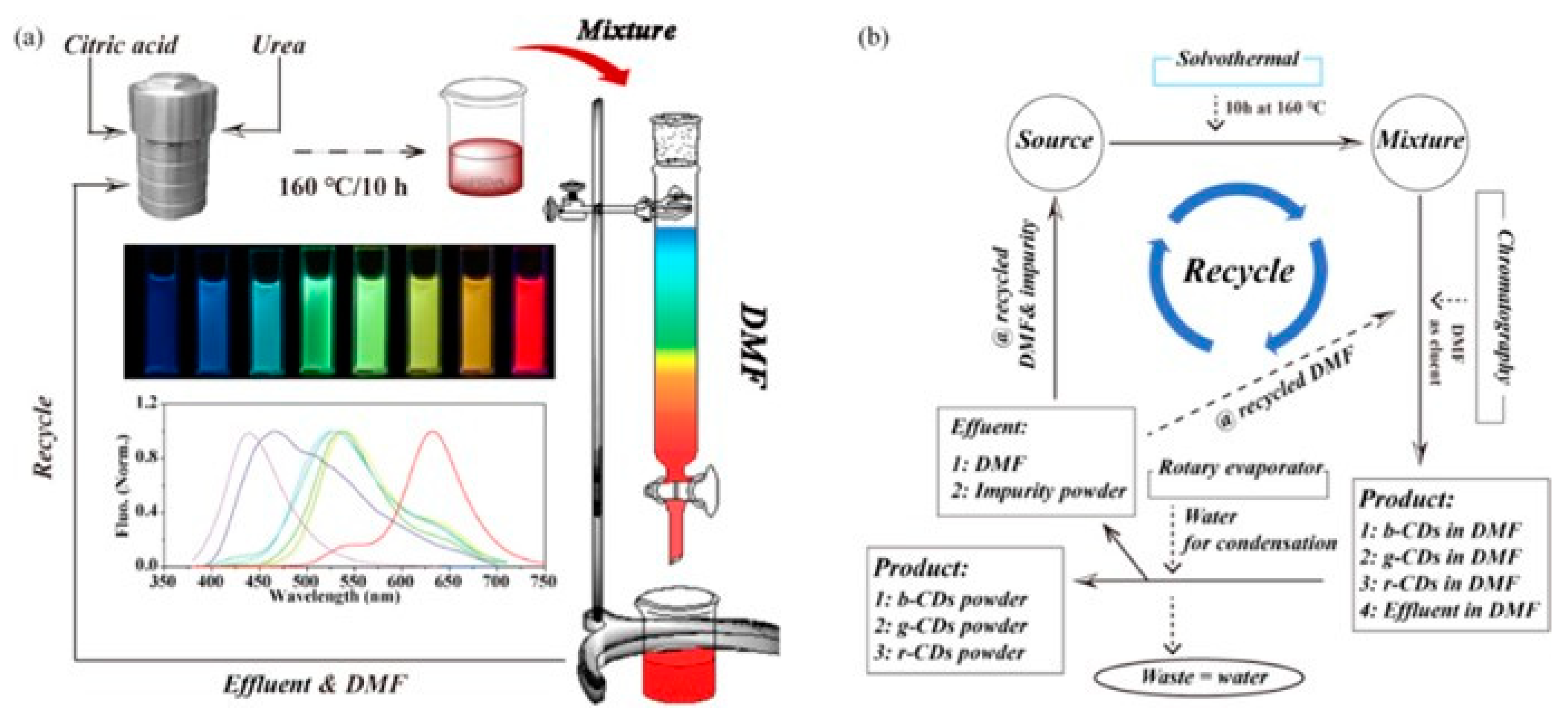
2.1. Biomass-Derived Carbon Dots for Solar Energy, Thermal Conversion, and Hydrogen Evolution Reaction
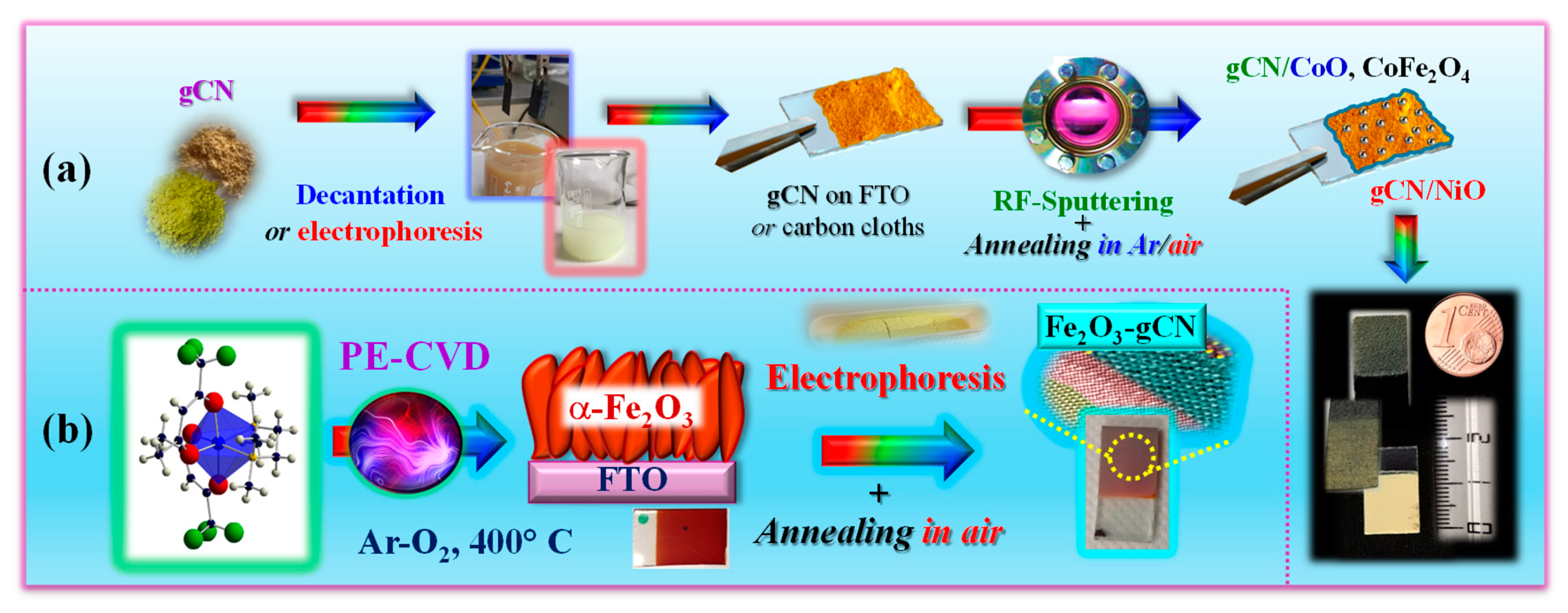
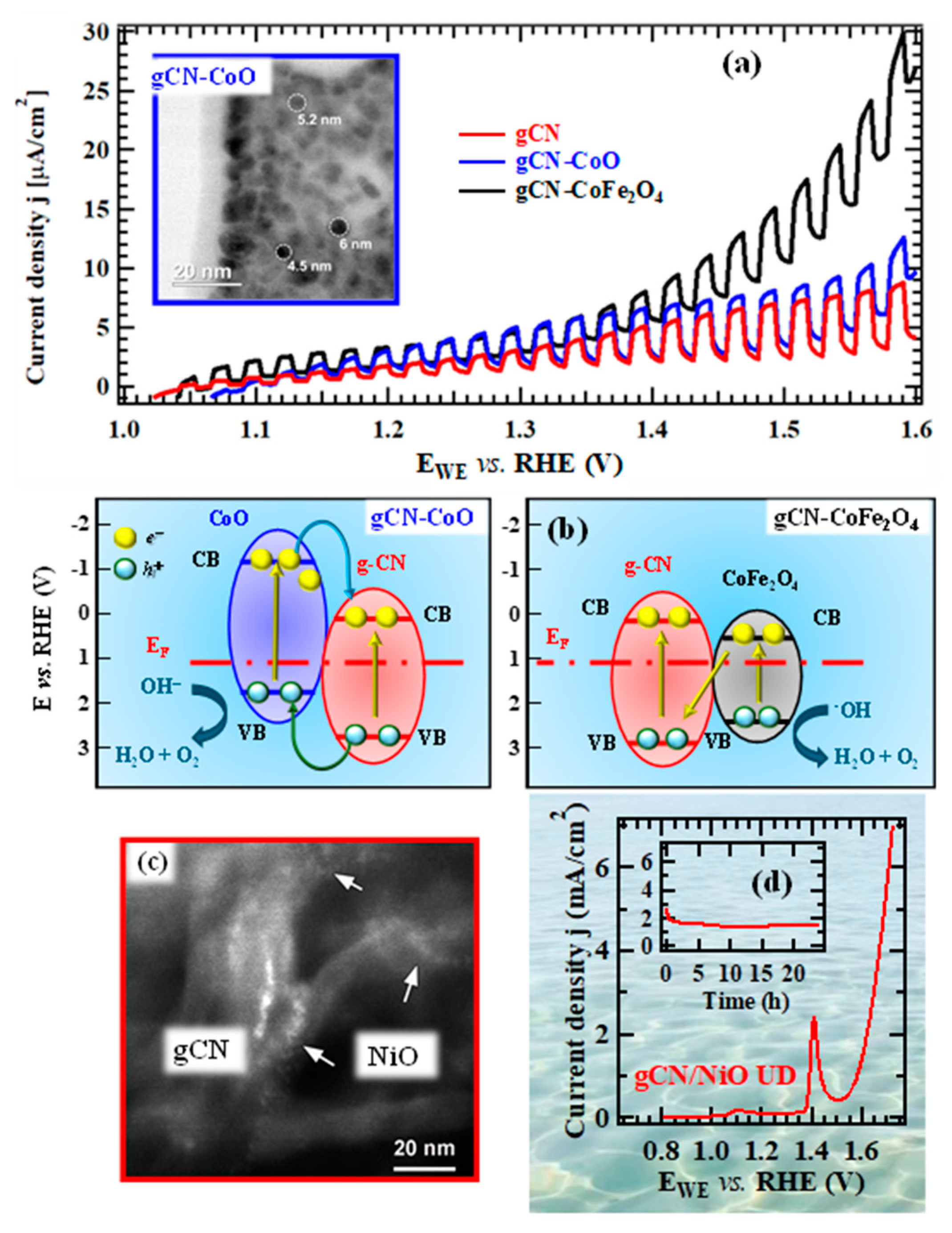
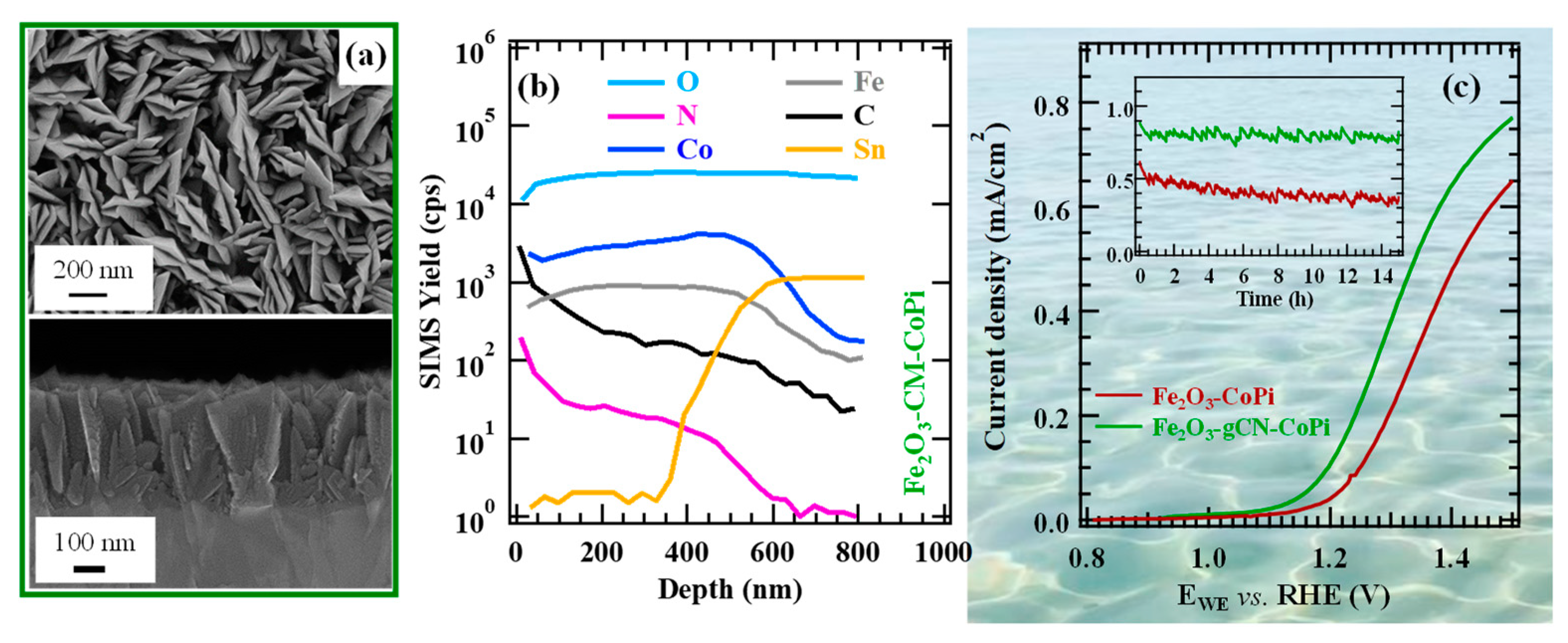
2.2. Nanoarchitectures Based on gCN for Sustainable Energy Production
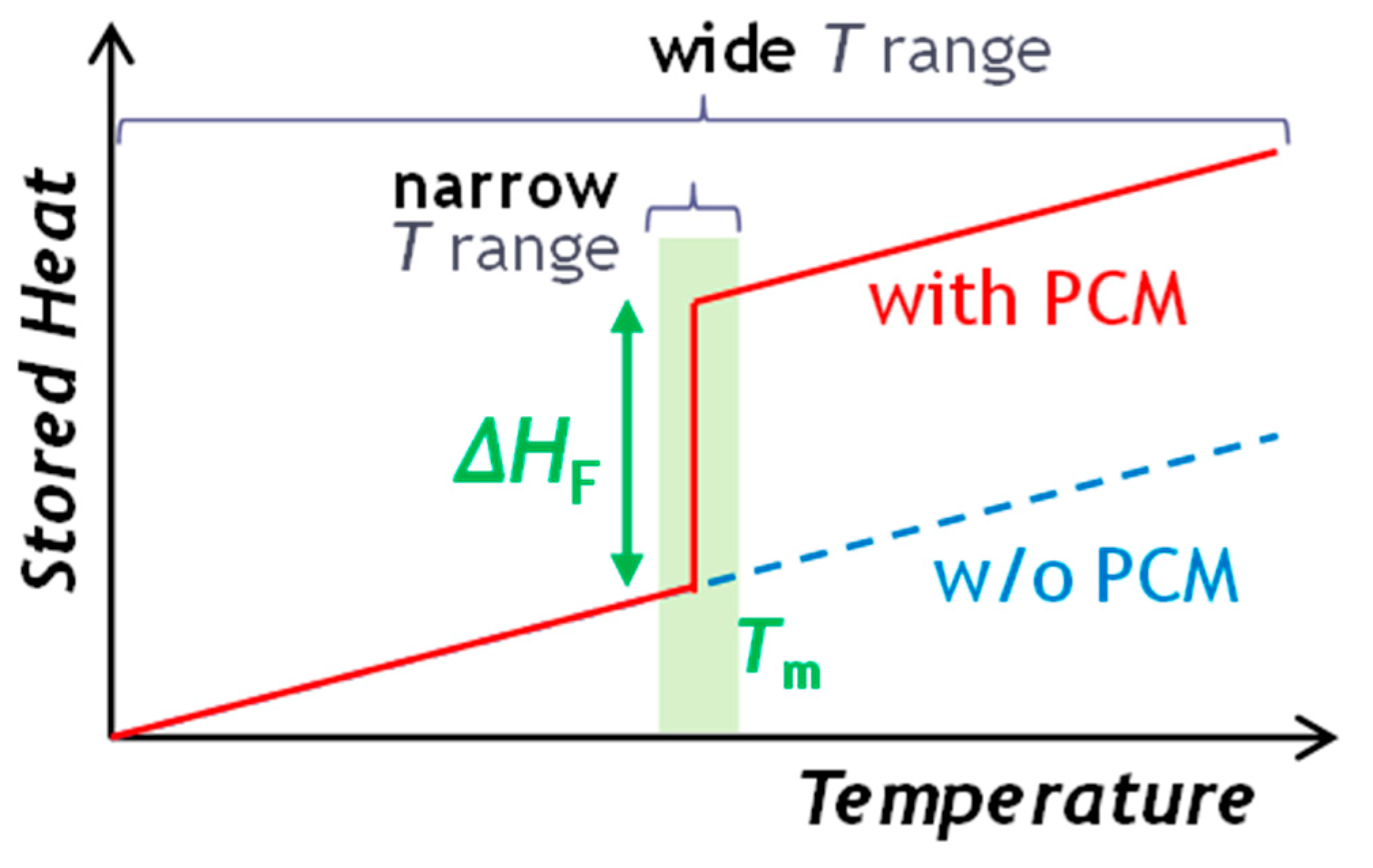
3. Phase-Change Materials as Innovative Approach to Thermal Energy Storage
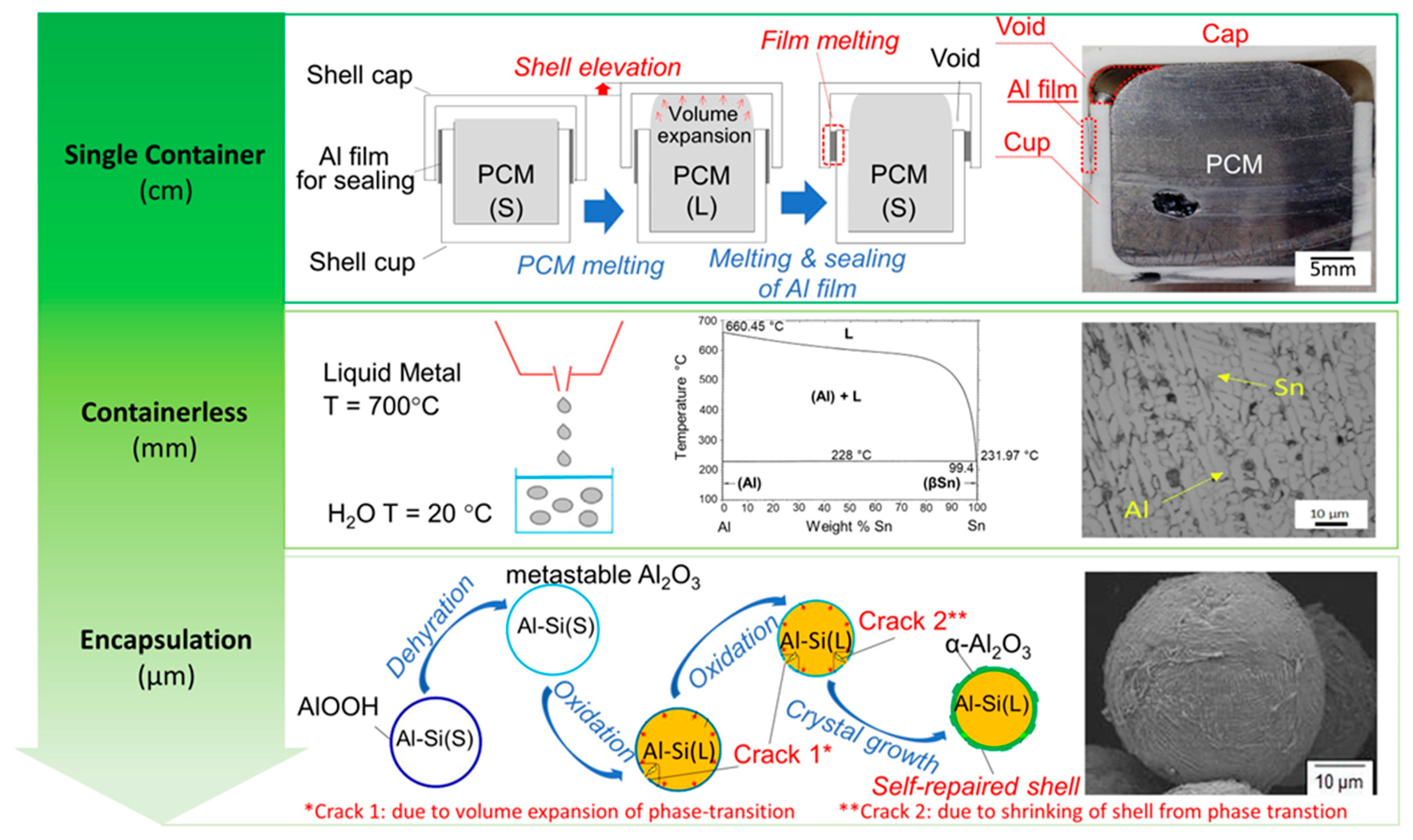
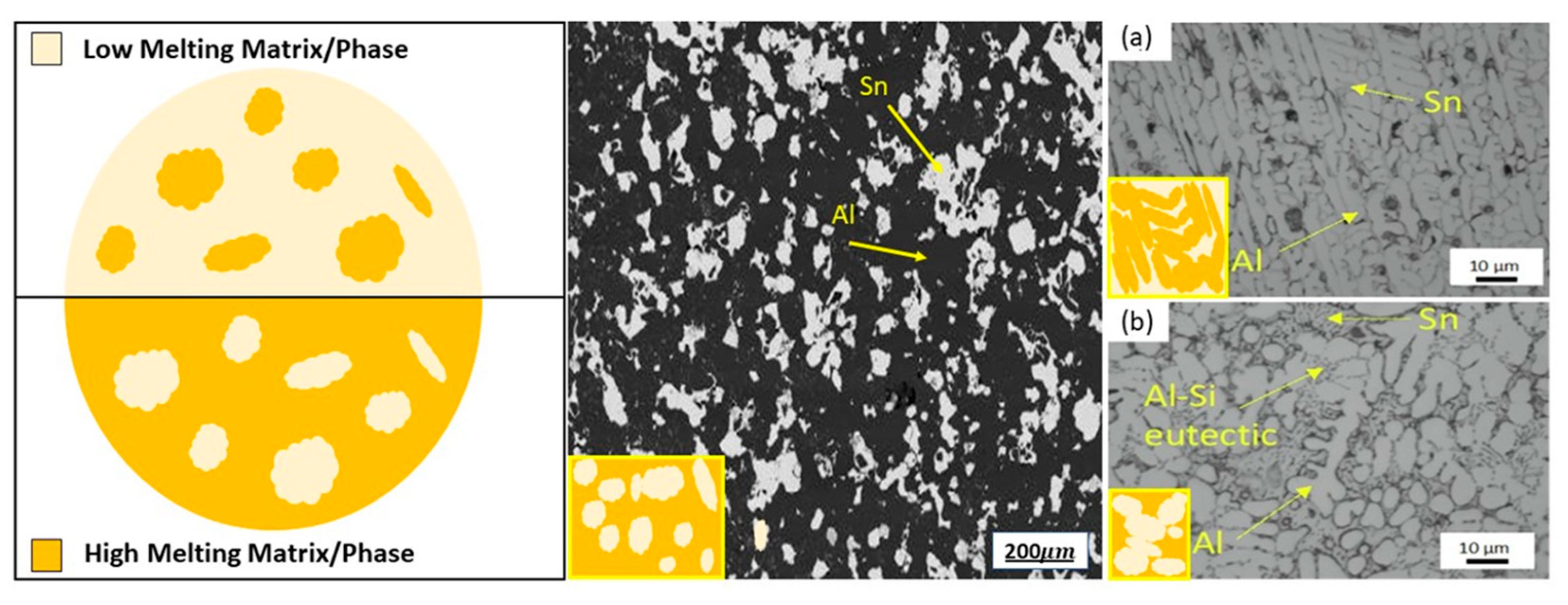
3.1. New Frontiers by Moderate-to-High-Temperature Metallic Phase-Change Materials
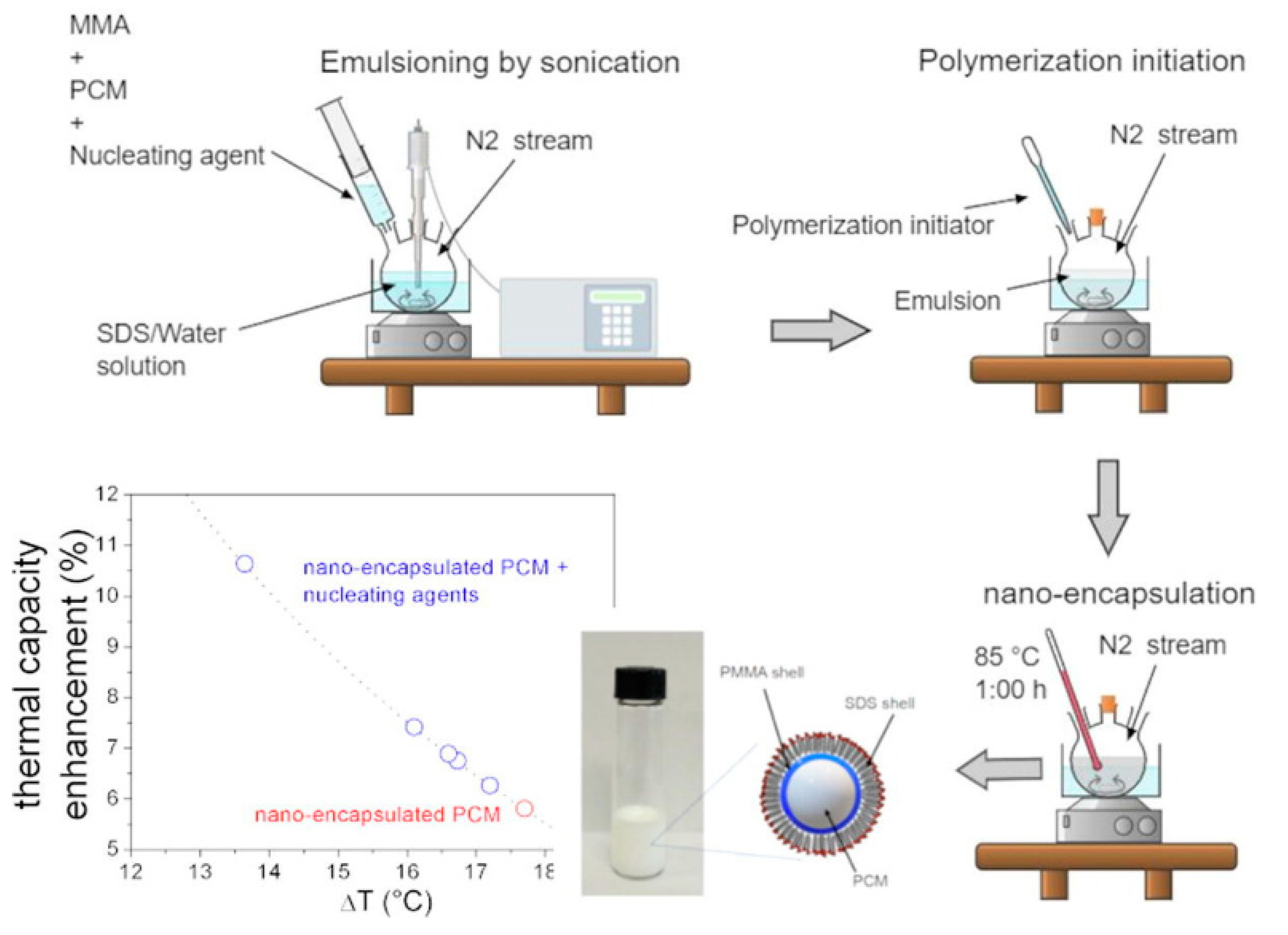
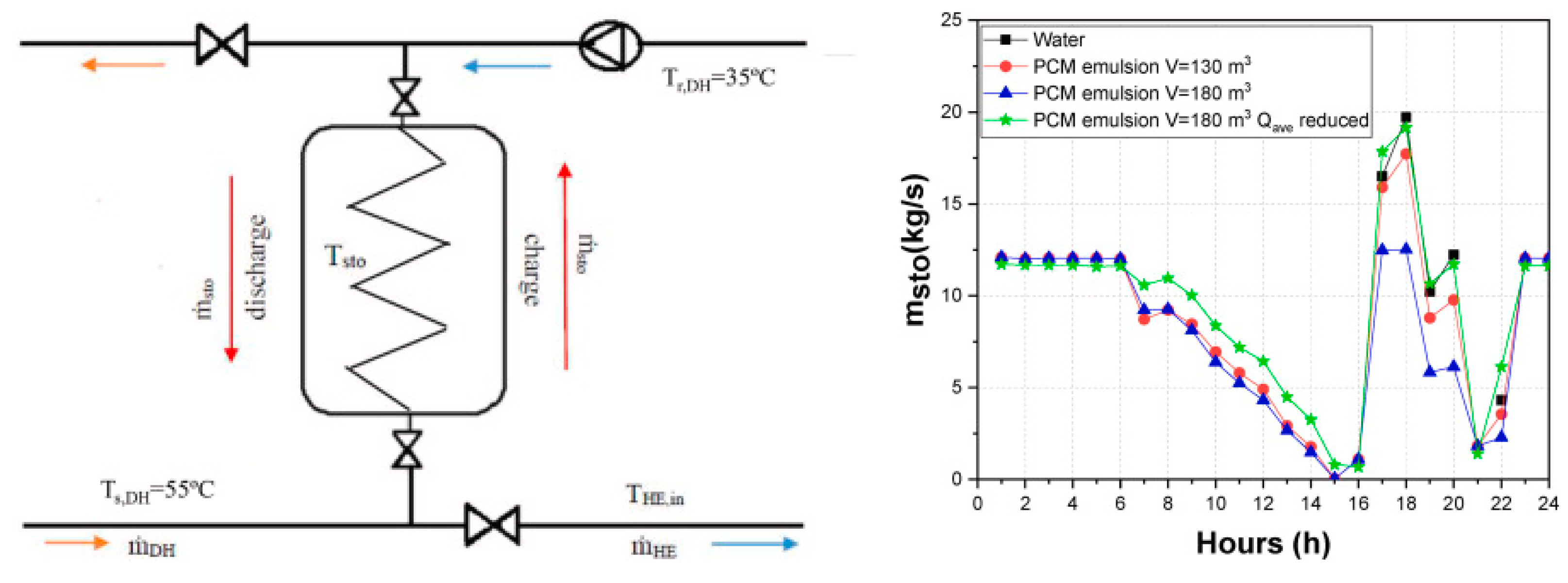
3.2. Phase-Change Material Emulsions for Thermal Energy Storage and Phase Change Materials in Buildings
4. Thin Films for Hydrogen Energy Technologies
4.1. Nitride Thin Films as Hydrogen Permeation Barriers: An Advanced Solution for Hydrogen Embrittlement Prevention
4.2. Metallic Thin Films as Separation Membranes for Hydrogen Energy
5. Ferroelectric Ceramics and Composites as Transformative Approach to Energy Storage and Harvesting
6. Metallic Materials for Energy Applications
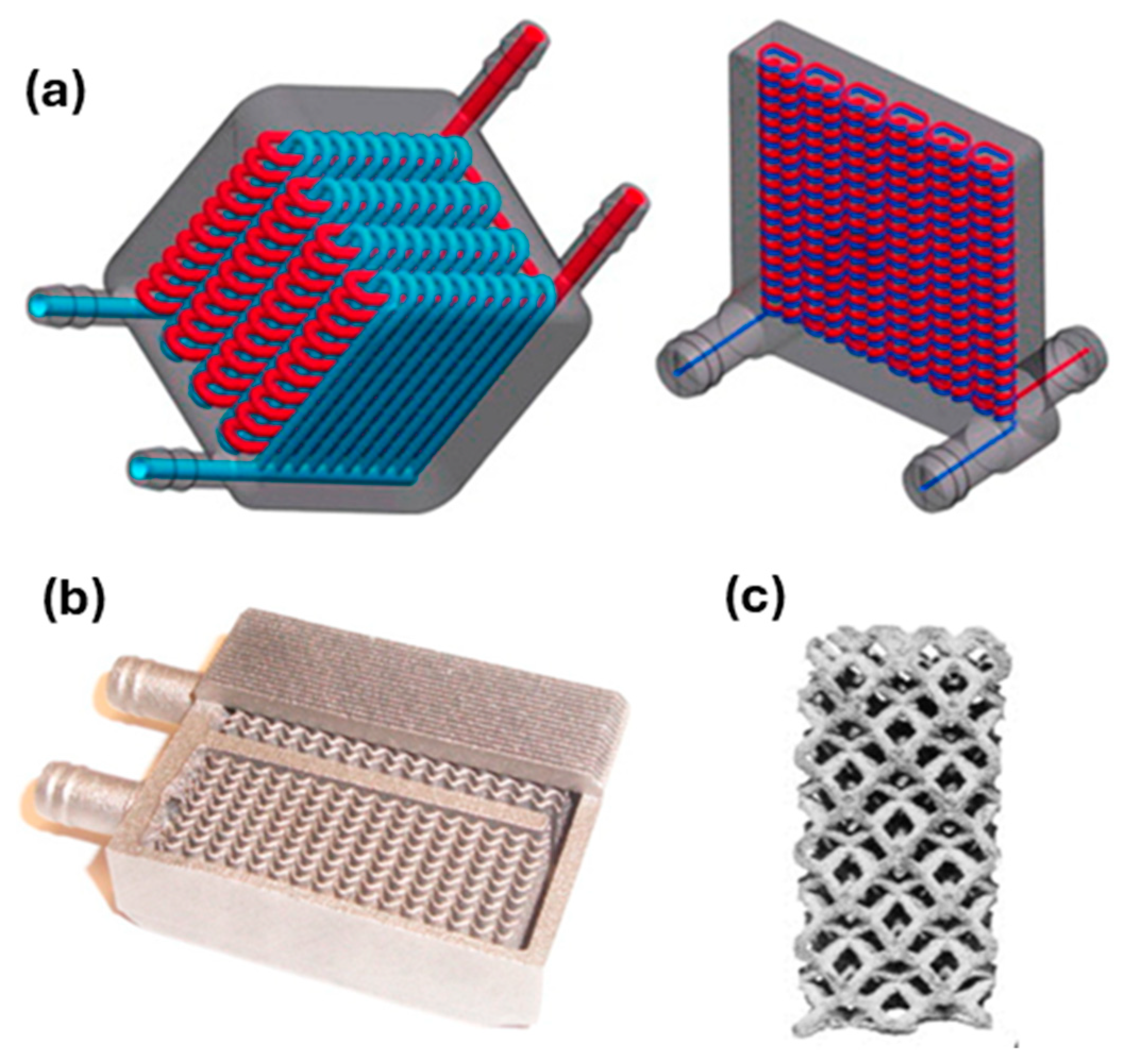
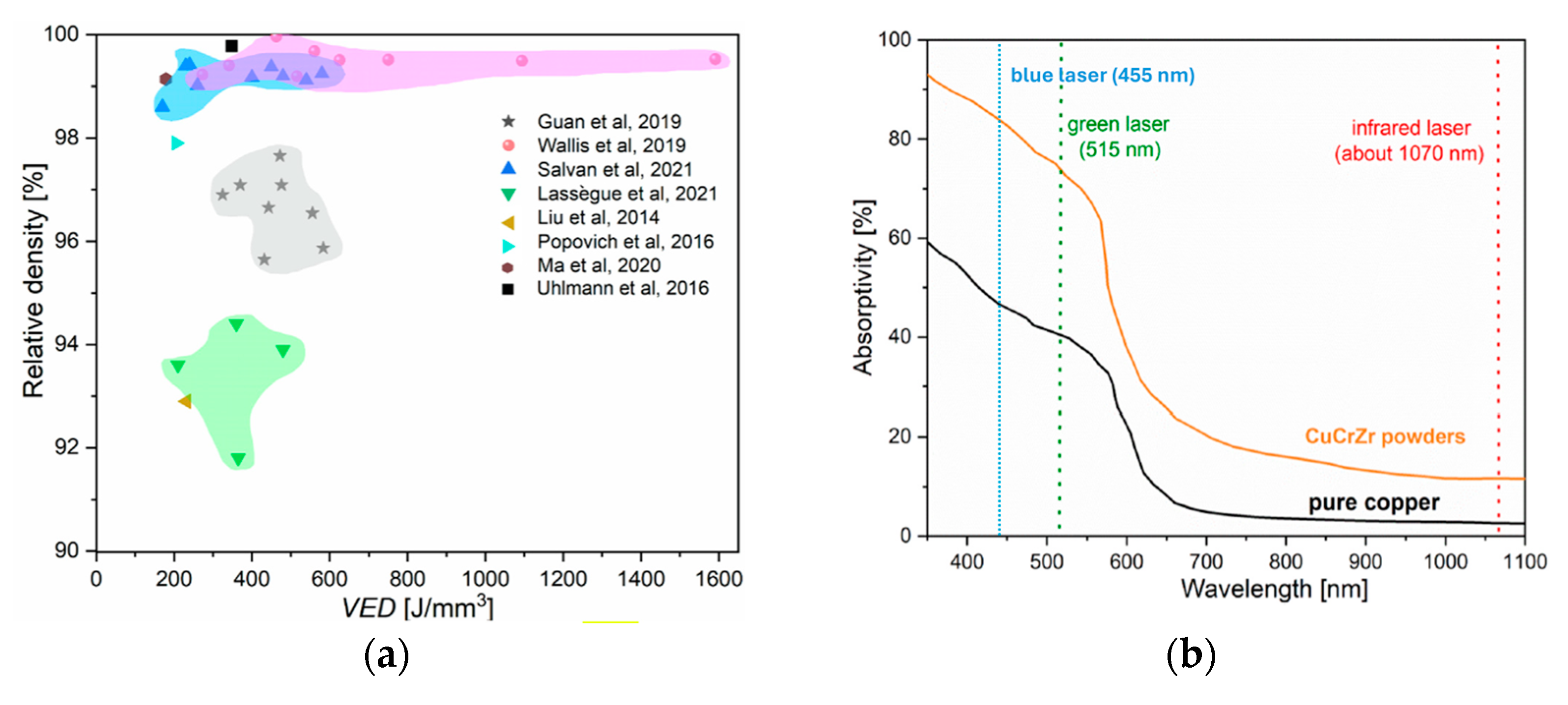
6.1. Cu-Based Alloys Manufactured by Additive Manufacturing for Advanced Heat Exchangers
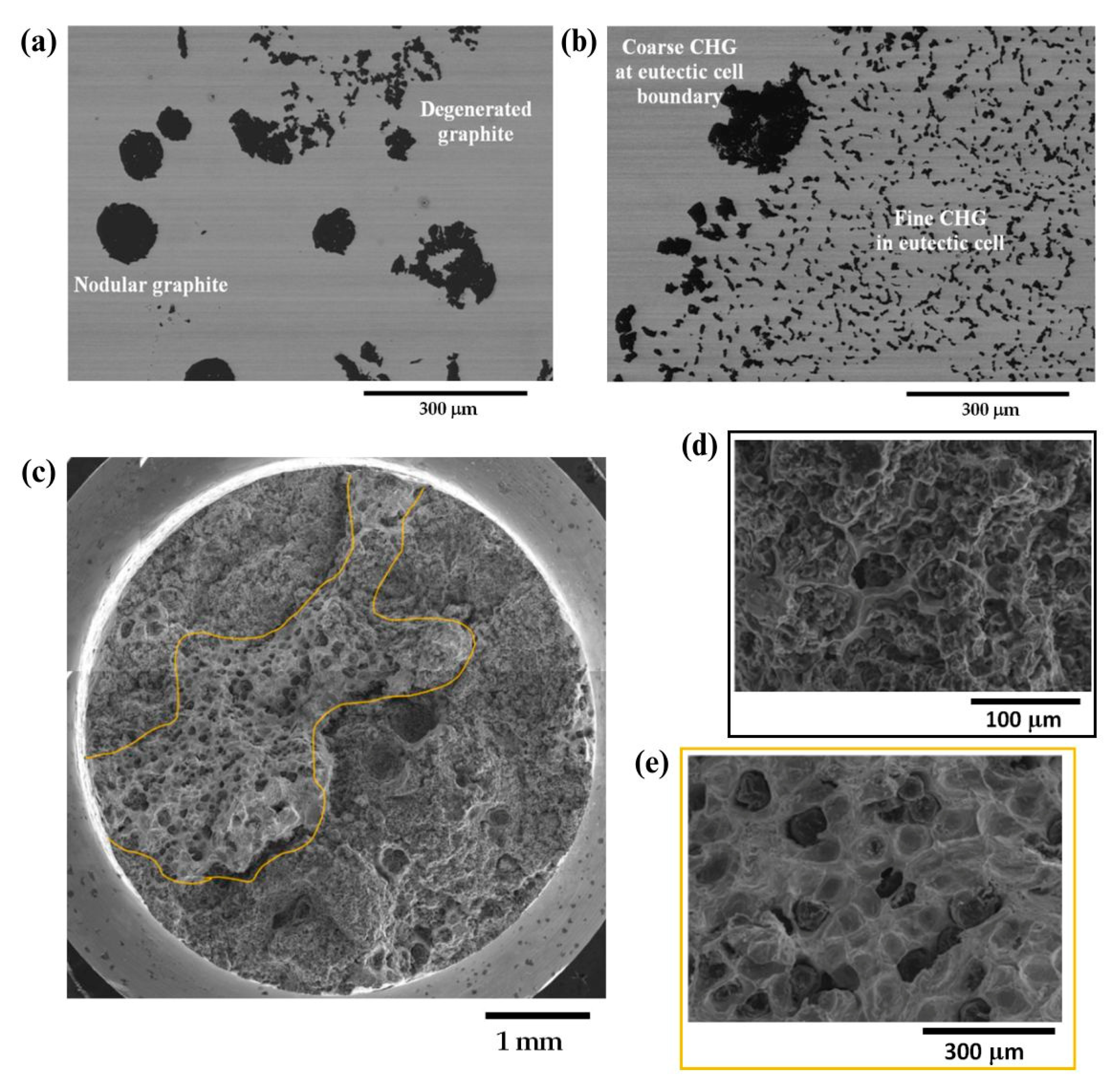
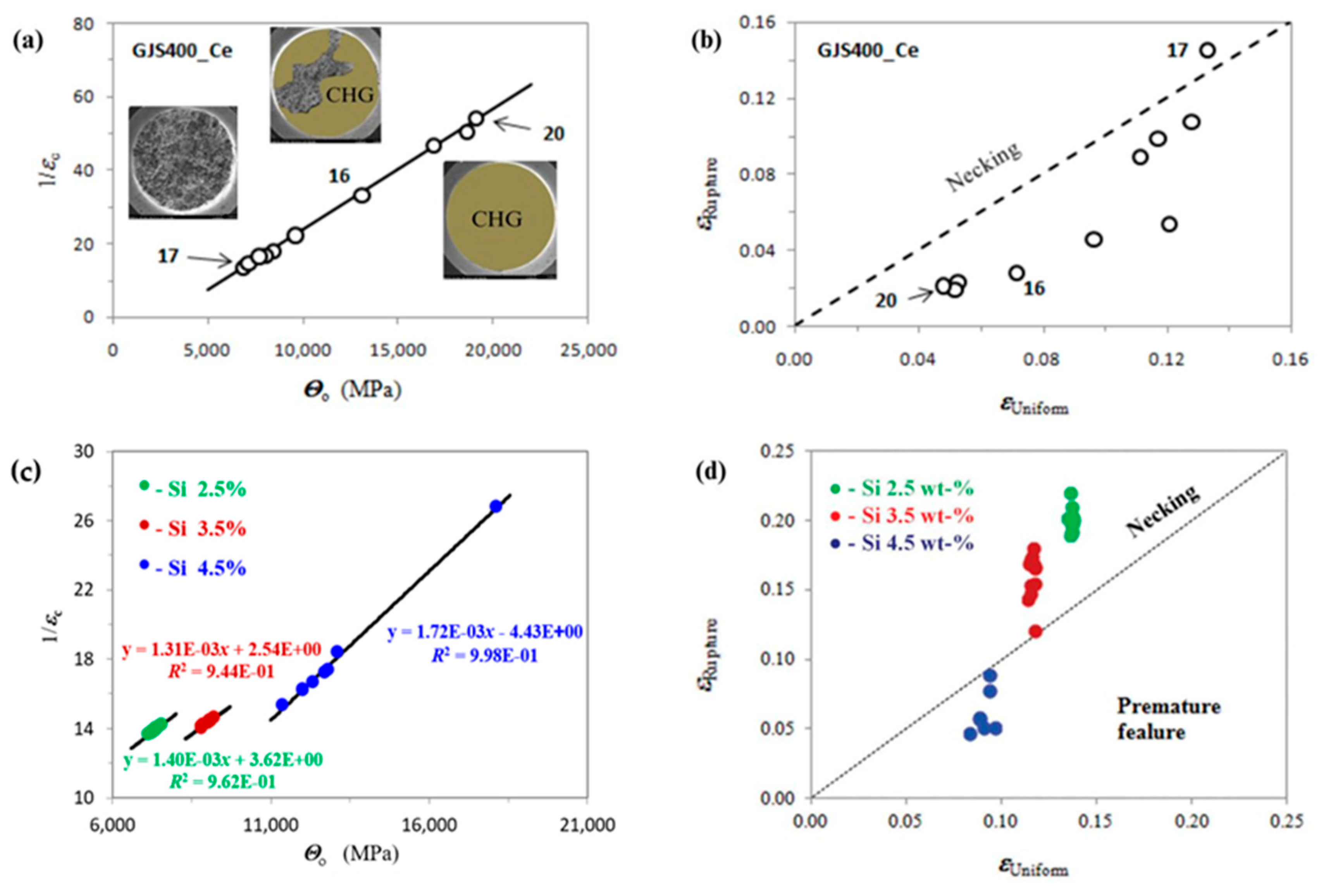
6.2. Ductile Iron Castings as an Opportunity for Wind Energy
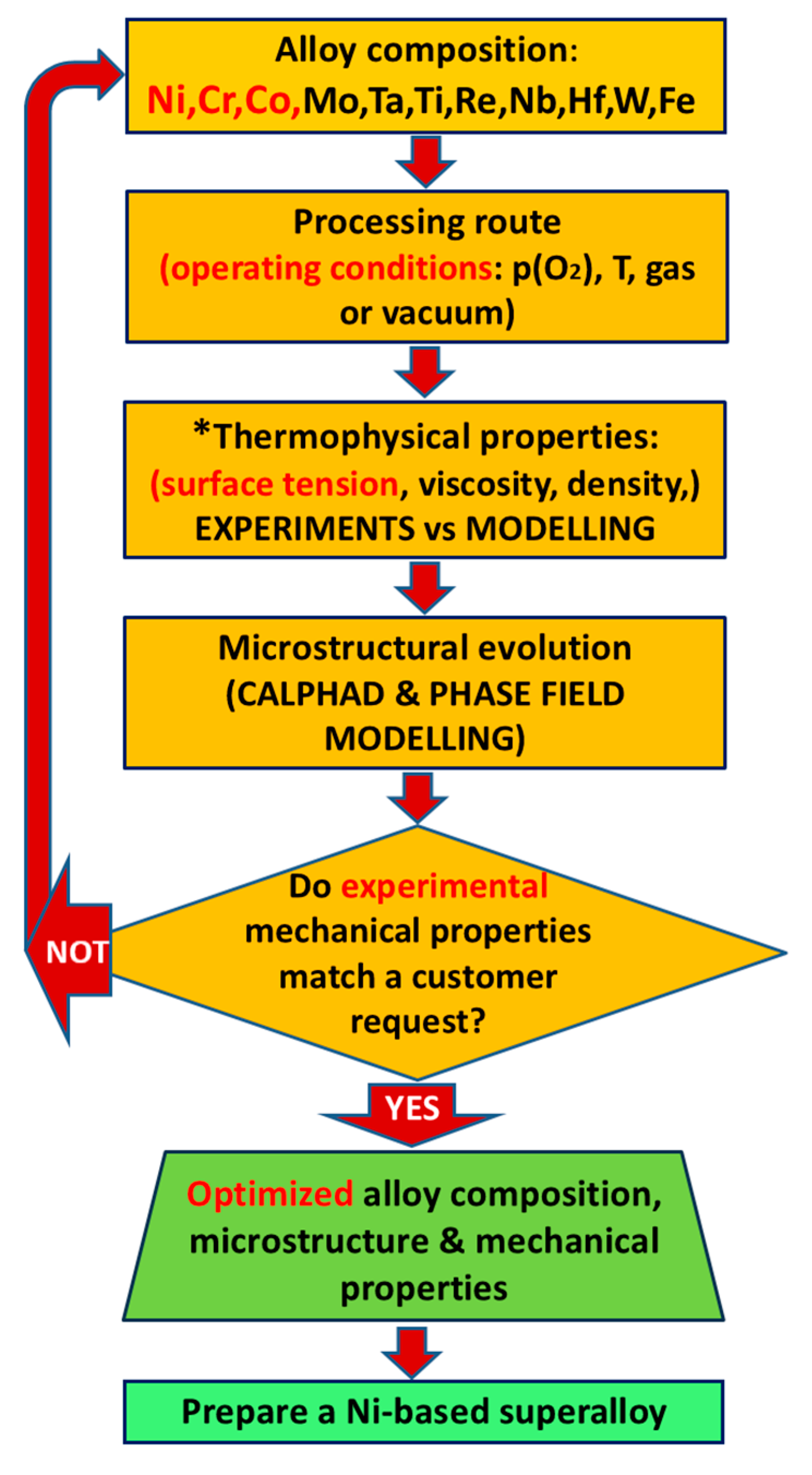
6.3. Relevance of Material Design for Power Generation—Case Study of Ni-Based Alloys by Casting
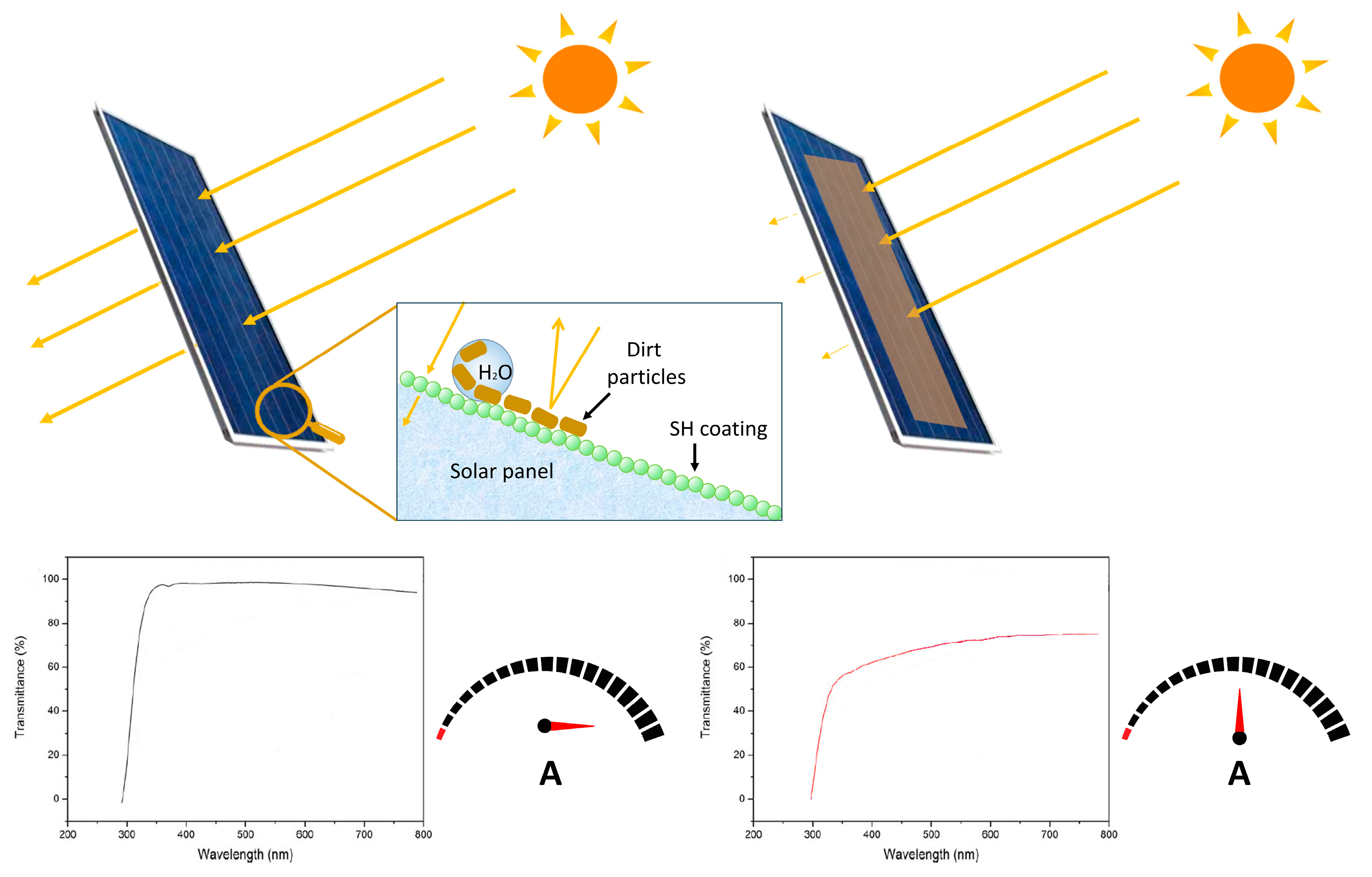
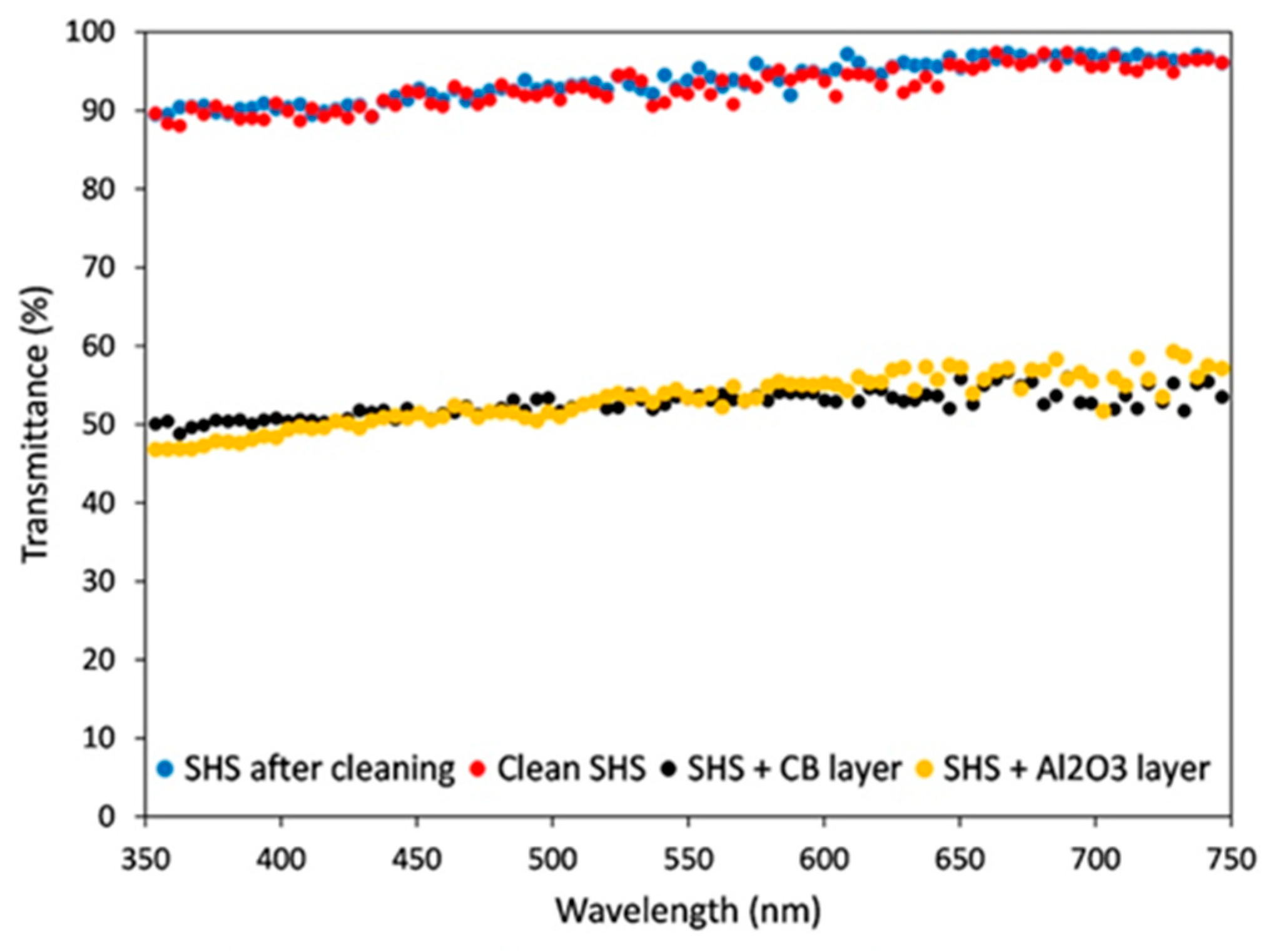
7. Amphiphobic Coatings for Environmental Protection of Solar Panels
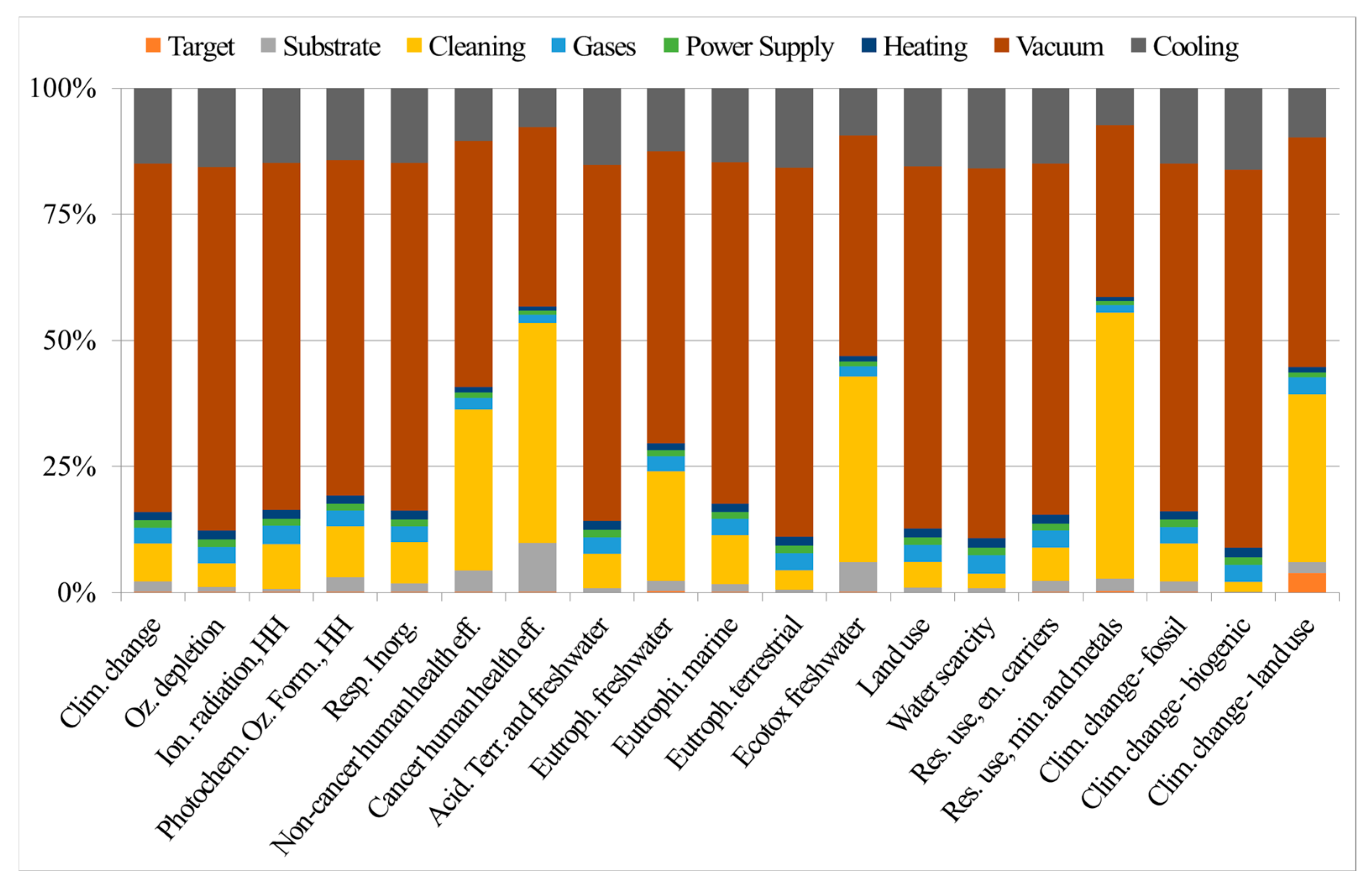
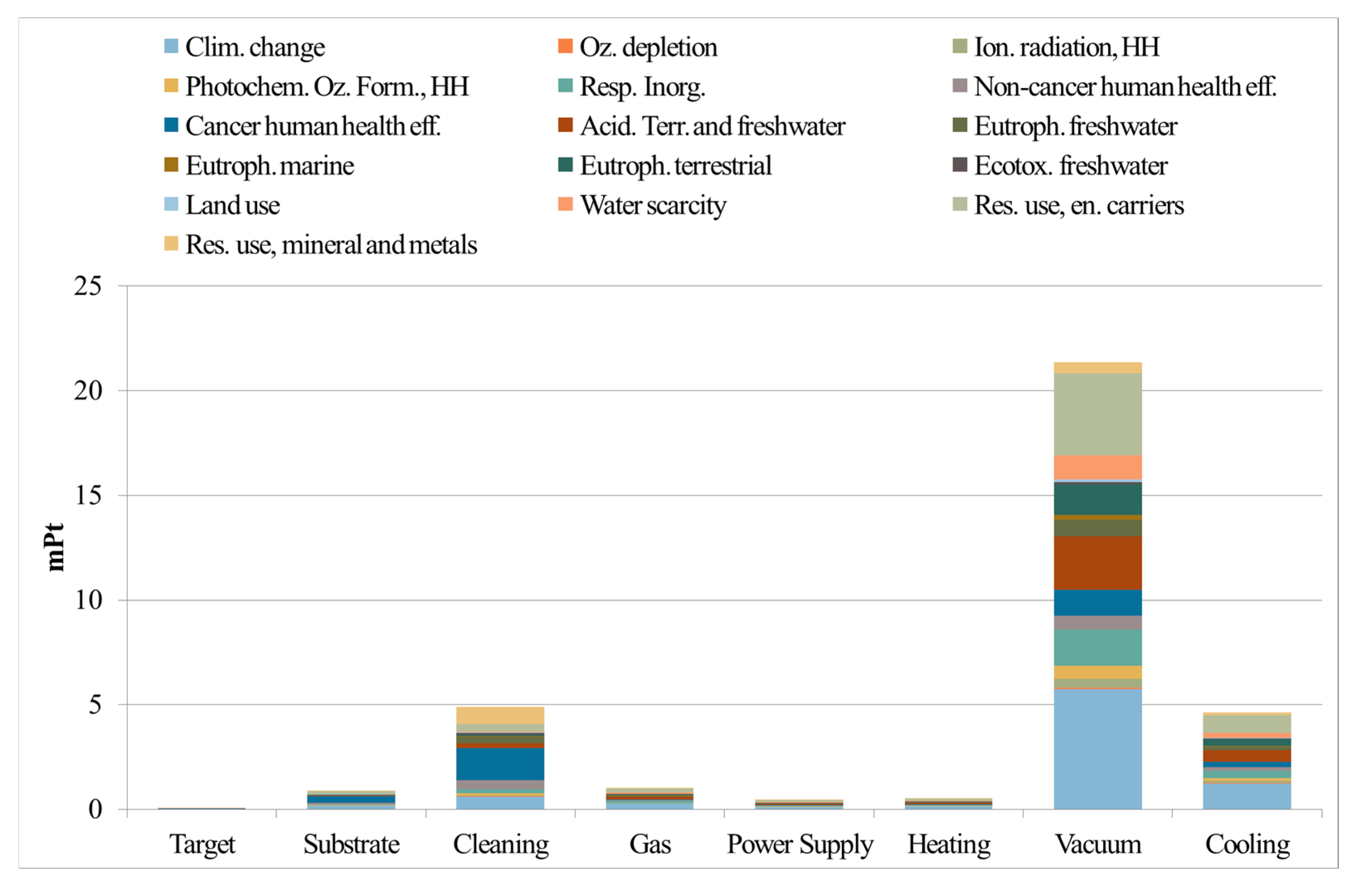
8. Life Cycle Assessment Applied to the AlTiN Case Study
9. Recycle and Reuse of Materials for Energy
10. Conclusions and Perspectives
- The synthetic approaches of C-based nanostructures are still limited to the laboratory scale; consequently, the commercialization of CDs is limited to few companies that offer small amounts of customized CDs for laboratory applications in their catalogues.
- Applications of PCMs are traditionally dominated by the use of organic compounds and inorganic salts. However, the recent commercial developments and industrial applications of metallic PCMs are focused on specific sectors, where their unique thermal properties could provide significant advantages. As an example, they can be employed in the energy storage at high temperature in concentrated solar power plants or for the recovery of waste industrial heat generated by metallurgical or cement plants. Moreover, metallic PCMs can be integrated into power-to-heat technologies that store the excess electricity by transforming it into thermal energy.
- As for ferroelectric materials, although they offer valuable properties for both commercial and domestic applications, their target market faces significant challenges, primarily due to the high costs of raw materials and complex production processes that hinder widespread adoption. Additionally, strict environmental regulations, especially concerning lead-based compounds, are driving the shift toward more environmentally sustainable alternatives, influencing market dynamics. To address current challenges, ongoing research is also increasingly focused on cost-effective, eco-friendly solutions, such as ferroelectric composites, for next-generation applications in flexible electronics, sensors, and energy-efficient systems, promoting a more scalable and sustainable evolution within this emerging market segment.
- Geopolitical instability, fluctuations in raw material prices, and logistics bottlenecks can affect the timely delivery and cost of cast iron components. The trend toward ever-larger wind turbines drives demand for correspondingly larger castings, pushing the technical limits of foundry capabilities.
- As for commercialization pathways of the metallic-based systems for hydrogen barriers and membranes, the key factors to be considered are that barrier layers produced by PVD or CVD require that these processes are scaled for larger components and more complex geometries; cost considerations could also be perceived as a barrier to commercial exploitation; however, while initial costs may be higher than for traditional coatings, the durability and performance benefits are proving attractive in demanding environments. Furthermore, standardization and certification have to be addressed to certify intermetallic-based materials for use in critical infrastructure, ensuring reliability and interoperability.
- Given the worldwide spread of large solar plants, the potential commercial upscale such self-cleaning of transparent coatings is highly demanding. Their comparatively low cost and easy-to-maintain features make them good candidates, provided that the present limitations, like durability data, can be overcome in the long term.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADI | Austempered Ductile Iron |
| AM | Additive manufacturing |
| BDS | Breakdown strength |
| CA | Contact angle |
| CALPHAD | Calculation of Phase Diagrams |
| CDs | Carbon-based dots |
| CVD | Chemical vapor deposition |
| CHG | Chunky graphite |
| DCMS | Direct-Current Magnetron Sputtering |
| DI | Ductile iron |
| DSC | Differential scanning calorimetry |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| FE | Ferroelectric |
| FTO | Fluorine-doped tin oxide |
| GHG | Greenhouse gas |
| HE | Hydrogen embrittlement |
| HER | Hydrogen evolution reaction |
| HiPIMS | High-power impulse magnetron sputtering |
| HPB | Hydrogen permeation barriers |
| LCA | Life cycle assessment |
| LHS | Latent heat storage |
| LPBF | Laser Powder Bed Fusion |
| LSVs | Linear sweep voltammetries |
| MWCNT | Multi-wall carbon nanotube |
| OER | Oxygen evolution reaction |
| PCM | Phase-change material |
| PE | Photothermal evaporation |
| PE-CVD | Plasma-enhanced chemical vapor deposition |
| PHE | Photocatalytic hydrogen evolution |
| PV | Photovoltaic |
| RFE | Relaxor ferroelectric |
| SEM | Scanning electron microscopy |
| SIMS | Secondary ion mass spectrometry |
| TEM | Transmission electron microscopy |
| TES | Thermal energy storage |
| XRD | X-ray diffraction |
References
- Oni, A.M.; Mohsin, A.S.M.; Belal, M.; Bhuian, H. A comprehensive evaluation of solar cell technologies, associated loss mechanisms, and efficiency enhancement strategies for photovoltaic cells. Energy Rep. 2024, 11, 3345–3366. [Google Scholar] [CrossRef]
- Rehman, F.; Hussain Syed, I.; Khanam, S.; Ijaz, S.; Mehmood, S.; Zubair, M.; Massoud, Y.; Qasim Mehmood, M. Fourth-generation solar cells: A review. Energy Adv. 2023, 2, 1239. [Google Scholar] [CrossRef]
- Martulli, L.M.; Diani, M.; Sabetta, G.; Bontumasi, S.; Colledani, M.; Bernasconi, A. Critical review of current wind turbine blades’ design and materials and their influence on the end-of-life management of wind turbines. Eng. Struct. 2025, 327, 119625. [Google Scholar] [CrossRef]
- Mahajan, P.; Sharma, M. A comprehensive review on developments and future perspectives of biopolymer-based materials for energy storage. Energy Storage 2024, 6, e634. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Peng, X.; Chen, J.; Kang, L.; Yin, X.; Yusuke, Y.; Ding, B. Emerging Issues and Opportunities of 2D Layered Transition Metal Dichalcogenide Architectures for Supercapacitors. ACS Nano 2025, 19, 13591–13636. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sajjad, M.; Zhang, J.; Li, D.; Mao, Z. The rise of novel 2D materials beyond graphene: A comprehensive review of their potential as supercapacitor electrodes. Surf. Interfaces 2023, 42, 103334. [Google Scholar] [CrossRef]
- Murali, G.; Rawal, J.; Reddy Modigunta, J.R.; Park, Y.H.; Lee, J.H.; Lee, S.Y.; Park, S.J.; In, I. A review on MXenes: New-generation 2D materials for supercapacitors. Sustain. Energy Fuels 2021, 5, 5672–5693. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Yang, F.; Li, H.; Chen, H.; Chen, Y.; Liu, Z.; Qiu, Z.P.; et al. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967. [Google Scholar] [CrossRef]
- White, M.A.; Kahwaji, S.; Noël, J.A. Recent advances in phase change materials for thermal energy storage. Chem. Commun. 2024, 60, 1690–1706. [Google Scholar] [CrossRef]
- Clementi, G.; Cottone, F.; Mattarelli, M.; Perna, G.; Di Michele, A.; Gammaitoni, L.; López-Suárez, M.; Baglio, S.; Trigona, C.; Neri, I. Review on Innovative Piezoelectric Materials for Mechanical Energy Harvesting. Energies 2022, 15, 6227. [Google Scholar] [CrossRef]
- Anand, C.; Chandraja, B.; Nithiya, P.; Akshaya, M.; Tamizhdurai, P.; Shoba, G.; Subramani, A.; Kumaran, R.; Yadav, K.K.; Gacem, A.; et al. Green hydrogen for a sustainable future: A review of production methods, innovations, and applications. Int. J. Hydrog. Energy 2025, 111, 319–341. [Google Scholar] [CrossRef]
- Roy, R.; Antonini, G.; Hayibo, K.S.; Rahman, M.; Khan, S.; Tian, W.; Boutilier, M.S.; Zhang, W.; Zheng, Y.; Bassi, A.; et al. Comparative techno-environmental analysis of grey, blue, green/yellow and pale-blue hydrogen production. Int. J. Hydrog. Energy 2025, 116, 200–210. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Liu, G.; Zavelani-Rossi, M.; Han, G.; Zhao, H.; Vomiero, A. Red-emissive carbon quantum dots enable high efficiency luminescent solar concentrators. J. Mater. Chem. A 2023, 11, 8950–8960. [Google Scholar] [CrossRef]
- Sreekumar, S.; Shaji, J.; Cherian, G.; Thomas, S.; Deb Mondol, J.; Shah, N. Corrosion analysis and performance investigation of hybrid MXene/C-dot Nanofluid-Based direct absorption solar collector. Sol. Energy 2024, 269, 112317. [Google Scholar] [CrossRef]
- Vercelli, B. The Role of Carbon Quantum Dots in Organic Photovoltaics: A Short Overview. Coatings 2021, 11, 232. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. Thioacetamide-derived nitrogen and sulfur co-doped carbon quantum dots for “green” quantum dot solar cells. J. Ind. Eng. Chem. 2022, 105, 111–120. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, S.; Kang, X.; ·He, C.; Zhao, P.; Meng, X.; Arthur, J.; Ragauskas, A.J.; Pang, J.; Song, X. Gradient synthesis of carbon quantum dots and activated carbon from pulp black liquor for photocatalytic hydrogen evolution and supercapacitor. Adv. Compos. Hybrid Mater. 2023, 6, 131. [Google Scholar] [CrossRef]
- Vercelli, B.; Donnini, R.; Ghezzi, F.; Sansonetti, A.; Giovanella, U.; La Ferla, B. Nitrogen-doped carbon quantum dots obtained hydrothermally from citric acid and urea: The role of the specific nitrogen centers in their electrochemical and optical responses. Electrochim. Acta 2021, 387, 138557. [Google Scholar] [CrossRef]
- Kumar, P.; Dua, S.; Kaur, R.; Kumarde, M.; Bhatt, G. A review on advancements in carbon quantum dots and their application in photovoltaics. RSC Adv. 2022, 12, 4714. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Huang, C. Development of nitrogen and sulfur-doped carbon dots for cellular imaging. J. Pharm. Anal. 2019, 9, 127–132. [Google Scholar] [CrossRef]
- Singh, V.; Kashyap, S.; Yadav, U.; Srivastava, A.; Singh, A.V.; Singh, R.K.; Singh, S.K.; Saxena, P.S. Nitrogen doped carbon quantum dots demonstrate no toxicity under in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol. Res. 2019, 8, 395–406. [Google Scholar] [CrossRef]
- Xiao, Q.; Liang, Y.; Zhu, F.W.; Lu, S.Y.; Huang, S. Microwave-assisted one-pot synthesis of highly luminescent N-doped carbon dots for cellular imaging and multi-ion probing. Microchim. Acta 2017, 184, 2429–2438. [Google Scholar] [CrossRef]
- Fan, R.J.; Sun, Q.; Zhang, L.; Zhang, Y.; Lu, A.H. Photoluminescent carbon dots directly derived from polyethylene glycol and their application for cellular imaging. Carbon 2014, 71, 87–93. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Z.; Chen, M.; Zhou, P. Ultra-stable carbon quantum dot nanofluids for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2022, 240, 111720. [Google Scholar] [CrossRef]
- Seifikar, F.; Azizian, S. Super-stable carbon quantum dots nanofluid for efficient solar-thermal conversion. Energy Convers. Manag. 2021, 228, 113675. [Google Scholar] [CrossRef]
- Chao, W.; Li, Y.; Sun, X.; Cao, G.; Wang, C.; Ho, S.-H. Enhanced wood-derived photothermal evaporation system by in-situ incorporated lignin carbon quantum dots. Chem. Eng. J. 2021, 405, 126703. [Google Scholar] [CrossRef]
- Wu, H.; Lu, S.; Yang, B. Carbon-Dot-Enhanced Electrocatalytic Hydrogen Evolution. Acc. Mater. Res. 2022, 3, 319–330. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Biplab Bag, B. Carbon Quantum Dot Electrocatalysts in Hydrogen Evolution Reaction. Energy Technol. 2024, 12, 2300913. [Google Scholar] [CrossRef]
- Zhuang, J.; Ren, S.; Zhu, B.; Han, C.; Li, Y.Y.; Zhang, X.; Gao, H.; Fan, M.; Tian, Q. Lignin-based carbon dots as high-performance support of Pt single atoms for photocatalytic H2 evolution. Chem. Eng. J. 2022, 446, 136873. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef]
- Rajamanikandan, S.; Biruntha, M.; Ramalingam, G. Blue Emissive Carbon Quantum Dots (CQDs) from Bio-waste Peels and Its Antioxidant Activity. J. Clust. Sci. 2022, 33, 1045. [Google Scholar] [CrossRef]
- Tao, X.Y.; Liao, M.; Wu, F.X.; Jiang, Y.H.; Sun, J.P.; Shi, S.H. Designing of biomass-derived carbon quantum dots@polyvinyl alcohol film with excellent fluorescent performance and pH-responsiveness for intelligent detection. Chem. Eng. J. 2022, 443, 136442. [Google Scholar] [CrossRef]
- Vercelli, B.; De Micheli, E.; Donnini, R.; Losurdo, M.; Lange, H.; La Ferla, B.; Pavan, A.; Saibene, M.; Capitani, G.; Ghezzi, F.; et al. Hydrothermal Approach for the Preparation of Blue-Emitting Carbon Quantum Dots: An Insight into the Influence of the Reaction Parameters. Small Struct. 2024, 6, 2400481. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Tejwan, N.; Saha, S.K.; Das, J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020, 275, 102046. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, T.Y.; Ge, S.B.; Fan, W.; Wang, H.; Zhang, Z.F.; Lichtfouse, E.; Van Tran, T.; Liew, R.K.; Rezakazemi, M.; et al. Synthesis and applications of carbon quantum dots derived from biomass waste: A review. Environ. Chem. Lett. 2023, 21, 3393–3424. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Wang, Q.; Hong Luo, K.H. Green Synthesis of Tunable Fluorescent Carbon Quantum Dots from Lignin and Their Application in Anti-Counterfeit Printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Mei, F.; Zhang, J.; Liang, C.; Dai, K. Fabrication of novel CoO/porous graphitic carbon nitride S-scheme heterojunction for efficient CO2 photoreduction. Mater. Lett. 2021, 282, 128722. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Mullins, C.B. Understanding charge transport in carbon nitride for enhanced photocatalytic solar fuel production. Acc. Chem. Res. 2019, 52, 248–257. [Google Scholar] [CrossRef]
- Benedet, M.; Rizzi, G.A.; Gasparotto, A.; Gauquelin, N.; Orekhov, A.; Verbeeck, J.; Maccato, C.; Barreca, D. Functionalization of graphitic carbon nitride systems by cobalt and cobalt-iron oxides boosts solar water oxidation performances. Appl. Surf. Sci. 2023, 618, 156652. [Google Scholar] [CrossRef]
- Benedoue, S.; Benedet, M.; Gasparotto, A.; Gauquelin, N.; Orekhov, A.; Verbeeck, J.; Seraglia, R.; Pagot, G.; Rizzi, G.A.; Balzano, V.; et al. Insights into the Photoelectrocatalytic Behavior of gCN-Based Anode Materials Supported on Ni Foams. Nanomaterials 2023, 13, 1035. [Google Scholar] [CrossRef]
- Scattolin, E.; Benedet, M.; Rizzi, G.A.; Gasparotto, A.; Lebedev, O.I.; Barreca, D.; Maccato, C. Graphitic Carbon Nitride Structures on Carbon Cloth Containing Ultra- and Nano-Dispersed NiO for Photoactivated Oxygen Evolution. ChemSusChem 2024, 17, e202400948. [Google Scholar] [CrossRef]
- Benedet, M.; Rizzi, G.A.; Lebedev, O.I.; Roddatis, V.; Sada, C.; Wree, J.-L.; Devi, A.; Maccato, C.; Gasparotto, A.; Barreca, D. Advances in photo-assisted seawater splitting promoted by green iron oxide-carbon nitride photoelectrocatalysts. J. Mater. Chem. A 2023, 11, 21595–21609. [Google Scholar] [CrossRef]
- Benedet, M.; Gallo, A.; Maccato, C.; Rizzi, G.A.; Barreca, D.; Lebedev, O.I.; Modin, E.; McGlynn, R.; Mariotti, D.; Gasparotto, A. Controllable Anchoring of Graphitic Carbon Nitride on MnO2 Nanoarchitectures for Oxygen Evolution Electrocatalysis. ACS Appl. Mater. Interfaces 2023, 15, 47368–47380. [Google Scholar] [CrossRef]
- Han, M.; Wang, H.; Zhao, S.; Hu, L.; Huang, H.; Liu, Y. One-step synthesis of CoO/g-C3N4 composites by thermal decomposition for overall water splitting without sacrificial reagents. Inorg. Chem. Front. 2017, 4, 1691–1696. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, R.; Zhang, N.; Zhu, B.; Yang, J. High-performance photocatalysis afforded by g-C3N4/NiCo2O4-decorated carbon cloth. Appl. Surf. Sci. 2020, 532, 147410. [Google Scholar] [CrossRef]
- Bigiani, L.; Barreca, D.; Gasparotto, A.; Andreu, T.; Verbeeck, J.; Sada, C.; Modin, E.; Lebedev, O.I.; Morante, J.R.; Maccato, C. Selective anodes for seawater splitting via functionalization of manganese oxides by a plasma-assisted process. Appl. Catal. B 2021, 284, 119684. [Google Scholar] [CrossRef]
- Yang, Z.L.; Walvekar, R.; Wong, W.P.; Sharma, R.K.; Dharaskar, S.; Khalid, M. Advances in phase change materials, heat transfer enhancement techniques, and their applications in thermal energy storage: A comprehensive review. J. Energy Storage 2024, 87, 111329. [Google Scholar] [CrossRef]
- Jankowski, N.R.; McCluskey, F.P. A review of phase change materials for vehicle component thermal buffering. Appl. Energy 2014, 113, 1525–1561. [Google Scholar] [CrossRef]
- Bianco, V.; De Rosa, M.; Vafai, K. Phase-change materials for thermal management of electronic devices. Appl. Therm. Eng. 2022, 214, 118839. [Google Scholar] [CrossRef]
- Tamme, R.; Bauer, T.; Buschle, J.; Laing, D.; Müller-Steinhagen, H.; Steinmann, W.-D. Latent heat storage above 120 °C for applications in the industrial process heat sector and solar power generation. Int. J. Energy Res. 2008, 32, 264–271. [Google Scholar] [CrossRef]
- Shamberger, P.J.; Bruno, N.M. Review of metallic phase change materials for high heat flux transient thermal management applications. Appl. Energy 2020, 258, 113955. [Google Scholar] [CrossRef]
- Kazaz, O.; Abu-Nada, E. Thermal performance of nano-architected phase change energetic materials for a next-generation solar harvesting system. Energy Convers. Manag. 2025, 327, 119541. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Chen, Y.; Xiang, H.; Ma, B.; Xu, Z.; Ma, X. Encapsulation of copper-based phase change materials for high temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 128, 131–137. [Google Scholar] [CrossRef]
- Yao, Y.; Li, W.; Liu, J.; Deng, Z. Liquid metal phase change materials for thermal management of electronics. Adv. Phys. X 2024, 9, 2324910. [Google Scholar] [CrossRef]
- Cárdenas, B.; León, N. High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques. Renew. Sustain. Energy Rev. 2013, 27, 724–737. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Mehling, H.; Hiebler, S.; Ziegler, F. Heat Transfer Enhancement in Water When Used as PCM in Thermal Energy Storage. Appl. Therm. Eng. 2002, 22, 1141–1151. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Thermal analysis of Al–Si alloys as high-temperature phase-change material and their corrosion properties with ceramic materials. Appl. Energy 2016, 163, 1–8. [Google Scholar] [CrossRef]
- Sun, J.Q.; Zhang, R.Y.; Liu, Z.P.; Lu, G.H. Thermal reliability test of Al–34%Mg–6%Zn alloy as latent heat storage material and corrosion of metal with respect to thermal cycling. Energy Convers. Manag. 2007, 48, 619–624. [Google Scholar] [CrossRef]
- El Karim, Y.; Grosu, Y.; Faik, A.; Lbibb, R. Investigation of magnesium-copper eutectic alloys with high thermal conductivity as a new PCM for latent heat thermal energy storage at intermediate-high temperature. J. Energy Storage 2019, 26, 100974. [Google Scholar] [CrossRef]
- Gokon, N.; Jie, C.S.; Nakano, Y.; Okazaki, S.; Kodama, T.; Hatamachi, T.; Bellan, S. Phase Change Material of Copper–Germanium Alloy as Solar Latent Heat Storage at High Temperatures. Front. Energy Res. 2021, 9, 696213. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Macro-encapsulation of metallic phase change material using cylindrical-type ceramic containers for high-temperature thermal energy storage. Appl. Energy 2016, 170, 324–328. [Google Scholar] [CrossRef]
- Sun, Z.; Zou, L.; Cheng, X.; Zhu, J.; Li, Y.; Zhou, W. Fabrication, Structure, and Thermal Properties of Mg-Cu Alloys as High Temperature PCM for Thermal Energy Storage. Materials 2021, 14, 4246. [Google Scholar] [CrossRef]
- Yagi, J.; Akiyama, T. Storage of Thermal Energy for Effective Use of Waste Heat from Industries. J. Mater. Process. Technol. 1995, 48, 793–804. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, Y.; Di, H.; Jiang, Y. Experimental research on a kind of novel high temperature phase change storage heater. Energy Convers. Manag. 2006, 47, 2211–2222. [Google Scholar] [CrossRef]
- Nardin, G.; Meneghetti, A.; Dal Magro, F.; Benedetti, N. PCM-based energy recovery from electric arc furnaces. Appl. Energy 2014, 136, 947–955. [Google Scholar] [CrossRef]
- Navarrete, N.; La Zara, D.; Goulas, A.; Valdesueiro, D.; Hernández, L.; van Ommen, J.R.; Mondragón, R. Improved thermal energy storage of nanoencapsulated phase change materials by atomic layer deposition. Sol. Energy Mater. Sol. Cells 2020, 206, 110322. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, Y.; Yang, X.; Lin, J.; Zhu, Z. Encapsulation of metal-based phase change materials using ceramic shells prepared by spouted bed CVD method. Sol. Energy Mater. Sol. Cells 2017, 170, 137–142. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sheng, N.; Saito, G.; Akiyama, T. Microencapsulation of metal-based phase change material for high-temperature thermal energy storage. Sci. Rep. 2015, 5, 9117. [Google Scholar] [CrossRef]
- Maruoka, N.; Sato, K.; Yagi, J.; Akiyama, T. Development of PCM for Recovering High Temperature Waste Heat and Utilization for Producing Hydrogen by Reforming Reaction of Methane. ISIJ Int. 2002, 42, 215–219. [Google Scholar] [CrossRef]
- Ma, B.; Li, J.; Xu, Z.; Peng, Z. Fe-shell/Cu-core encapsulated metallic phase change materials prepared by aerodynamic levitation method. Appl. Energy 2014, 132, 568–574. [Google Scholar] [CrossRef]
- Qian, R.; Weng, K.; Liu, M.; Huang, L.; Zou, D. Development of a novel Al-Si microcapsules with heat-resistant, high latent heat, and efficient solar heat absorption. Chem. Eng. J. 2024, 496, 1539. [Google Scholar] [CrossRef]
- Sugo, H.; Kisi, E.; Cuskelly, D. Miscibility gap alloys with inverse microstructures and high thermal conductivity for high energy density thermal storage applications. Appl. Therm. Eng. 2013, 51, 1345–1350. [Google Scholar] [CrossRef]
- Bassani, P.; Molteni, M.; Gariboldi, E. Microstructural features and thermal response of granulated Al and A356 alloy with relevant Sn additions. Mater. Des. 2023, 229, 111879. [Google Scholar] [CrossRef]
- Confalonieri, C.; Gariboldi, E. Al-Sn Miscibility Gap Alloy produced by Power Bed Laser Melting for application as Phase Change Material. J. Alloys Compd. 2021, 881, 160596. [Google Scholar] [CrossRef]
- Confalonieri, C.; Perrin, M.; Gariboldi, E. Combined powder metallurgy routes to improve thermal and mechanical response of Al−Sn composite phase change materials. Trans. Nonferrous Met. Soc. China 2020, 30, 3226–3239. [Google Scholar] [CrossRef]
- Gariboldi, E.; Perrin, M. Metallic Composites as Form-Stable Phase Change Alloys. Mater. Sci. Forum 2018, 941, 1966–1971. [Google Scholar] [CrossRef]
- Jie, J.; Zheng, Z.; Liu, S.; Yue, S.; Li, T. Solidification structure evolution of immiscible Al–Bi–Sn alloys at different cooling rates. J. Mater. Res. 2019, 34, 2563–2571. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Lane, G.A.; Shamsundar, N. Solar Heat Storage: Latent Heat Materials, Vol. I: Background and Scientific Principles. J. Sol. Energy Eng. 1983, 105, 467. [Google Scholar] [CrossRef]
- European Commission. An EU Strategy on Heating and Cooling; COM/2016/051 Final; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Wang, F.; Rao, Z.; Zhang, G.; Ma, X.; Wang, L. Experimental and simulative investigations on a phase change material nano-emulsion-based liquid cooling thermal management system for a lithium-ion battery pack. Energy 2020, 207, 118215. [Google Scholar] [CrossRef]
- Wang, F.; Rao, Z.; Wang, L.; Ma, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Fedele, L.; Bobbo, S.; Barison, S.; Agresti, F. Review on phase change material emulsions for advanced thermal management: Design, characterization and thermal performance. Renew. Sustain. Energy Rev. 2022, 159, 112238. [Google Scholar] [CrossRef]
- Agresti, F.; Fedele, L.; Rossi, S.; Cabaleiro, D.; Bobbo, S.; Ischia, G.; Barison, S. Nano-encapsulated PCM emulsions prepared by a solvent-assisted method for solar applications. Sol. Energy Mater. Sol. Cells 2019, 194, 268–275. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulated phase change materials: Preparation, characterization and heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Agresti, F.; Cabaleiro, D.; Fedele, L.; Rossi, S.; Barison, S. PMMA nano-encapsulated phase change material colloids for heat management applications. J. Mol. Liq. 2023, 377, 121576. [Google Scholar] [CrossRef]
- Huang, L.; Günther, E.; Doetsch, C.; Mehling, H. Subcooling in PCM emulsions—Part 1: Experimental. Thermochim. Acta 2010, 509, 93. [Google Scholar] [CrossRef]
- Nagar, S.; Sharma, K. Modern solar systems driven by nanoparticles-based fatty acids and paraffin wax phase change materials. J. Mater. Sci. 2021, 56, 4941. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Zhu, C.; Zhao, J.; Rao, Z. 3D printing of phase change material-based Pickering emulsion gel for solar-thermal-electric conversion. Chem. Eng. J. 2024, 499, 155940. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Li, P.; Zhao, X.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and building applications. Renew. Sustain. Energy Rev. 2017, 77, 246–262. [Google Scholar] [CrossRef]
- Shijina, S.S.; Akbar, S.; Sajith, V. Graphene functionalized nano-encapsulated composite phase change material based nanofluid for battery cooling: An experimental investigation. Appl. Therm. Eng. 2025, 259, 124893. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Losada-Barreiro, S.; Agresti, F.; Hermida-Merino, C.; Fedele, L.; Lugo, L.; Barison, S.; Pineiro, M.M. Development and thermophysical profile of cetyl alcohol-in-water nanoemulsions for thermal management. Fluids 2022, 7, 11. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Liang, H.; Niu, J.; Wu, J.Y. Cooling storage performance of a novel phase change material nano-emulsion for room air-conditioning in a self-designed pilot thermal storage unit. Appl. Energy 2022, 308, 118405. [Google Scholar] [CrossRef]
- Rinaldi, G.; Lazaro, A.; Delgado, M.; Marin, J.M.; Verda, V. Use of a low-cost phase change material emulsion in decentralized thermal energy storage for district heating network enlargement. Energy 2024, 306, 132517. [Google Scholar] [CrossRef]
- Romdhane, S.B.; Jmal, A.; Guizani, A.; Al-Sulaiman, F.A. A review on thermal energy storage using phase change materials in passive building applications. J. Build. Eng. 2020, 32, 101563. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Lin, K.; Zhang, Q.; Di, H. Application of latent heat thermal energy storage in buildings: State-of-the-art and outlook. Build. Environ. 2007, 42, 2197. [Google Scholar] [CrossRef]
- Al-Yasiri, Q.; Szabò, M. Incorporation of phase change materials into building envelope for thermal comfort and energy saving: A comprehensive analysis. J. Build. Eng. 2021, 36, 102122. [Google Scholar] [CrossRef]
- Murathan, E.K.; Manioglu, G. Evaluation of phase change materials used in building components for conservation of energy in buildings in hot dry climatic regions. Renew. Energy 2020, 162, 1919. [Google Scholar] [CrossRef]
- Pisello, A.L.; Cotana, F.; Petrozzi, A.; Rossi, F. PCM for improving polyurethane-based cool roof membranes durability. Sol. Energy Mater. Sol. Cells 2017, 160, 34. [Google Scholar] [CrossRef]
- Galvagnini, F.; Cesaro, S.N.; Giovannini, A.; Rosace, G. Multifunctional polyurethane foams with thermal energy storage/release capability. J. Therm. Anal. Calorim. 2022, 147, 297–313. [Google Scholar] [CrossRef]
- Lach, M.; Srebowata, A.; Paradowska, K.; Jasiński, P. Review of solutions for the use of phase change materials in geopolymers. Materials 2021, 14, 6044. [Google Scholar] [CrossRef]
- Laadel, N.E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation barriers for hydrogen embrittlement prevention in metals—A review on mechanisms, materials suitability and efficiency. Int. J. Hydrog. Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Tamura, M.; Noma, M.; Yamashita, M. Characteristic change of hydrogen permeation in stainless steel plate by BN coating. Surf. Coat. Technol. 2014, 260, 148–154. [Google Scholar] [CrossRef]
- Nemanič, V.; McGuiness, P.J.; Daneu, N.; Zajec, B.; Siketić, Z.; Waldhauser, W. Hydrogen permeation through silicon nitride films. J. Alloys Compd. 2012, 539, 184–189. [Google Scholar] [CrossRef]
- Matějíček, J.; Veverka, J.; Nemanič, V.; Cvrček, L.; Lukáč, F.; Havránek, V.; Illková, K. Characterization of less common nitrides as potential permeation barriers. Fusion Eng. Des. 2019, 139, 74–80. [Google Scholar] [CrossRef]
- Liu, L.; Ruan, Q.; Xiao, S.; Meng, X.; Huang, C.; Wu, Y.; Fu, R.K.Y.; Chu, P.K. Fabrication and hydrogen permeation resistance of dense CrN coatings. Surf. Coat. Technol. 2022, 437, 128326. [Google Scholar] [CrossRef]
- Daub, K.; van Nieuwenhove, R.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Obrosov, A.; Sutygina, A.N.; Uludintceva, E.; Mitrofanov, A.; Weiß, S. Hydrogen Permeation, and Mechanical and Tribological Behavior, of CrNx Coatings Deposited at Various Bias Voltages on IN718 by Direct Current Reactive Sputtering. Coatings 2018, 8, 66. [Google Scholar] [CrossRef]
- Ronnebro, E.C.E.; Oelrich, R.L.; Gates, R.O. Recent advances and prospects in design of hydrogen permeation barrier materials for energy applications-A review. Molecules 2022, 27, 6528. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, D.; Zhang, Y.; Ouyang, T.; Suo, J. Microstructure and hydrogen impermeability of titanium nitride thin films deposited by direct current reactive magnetron sputtering. J. Alloys Compd. 2016, 688, 44–50. [Google Scholar] [CrossRef]
- Kura, C.; Kunisada, Y.; Tsuji, E.; Zhu, C.; Habazaki, H.; Nagata, S.; Müller, M.P.; De Souza, R.A.; Aoki, Y. Hydrogen separation by nanocrystalline titanium nitride membranes with high hydride ion conductivity. Nat. Energy 2017, 2, 786–794. [Google Scholar] [CrossRef]
- Tamura, M.; Eguchi, T. Nanostructured thin films for hydrogen-permeation barrier. J. Vac. Sci. Technol. A 2015, 33, 041503. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Lundin, D.; Lundin, T.; Minea, J.T.G. (Eds.) High Power Impulse Magnetron Sputtering-Fundamentals, Technologies, Challenges and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128124543. [Google Scholar]
- Deambrosis, S.M.; Montagner, F.; Zin, V.; Fabrizio, M.; Badini, C.; Padovano, E.; Sebastiani, M.; Bemporad, E.; Brunelli, K.; Miorin, E. Ti1−xAlxN coatings by Reactive High Power Impulse Magnetron Sputtering: Film/substrate interface effect on residual stress and high temperature oxidation. Surf. Coat. Technol. 2018, 354, 56–65. [Google Scholar] [CrossRef]
- Xiao, S.; Meng, X.; Shi, K.; Liu, L.; Wu, H.; Lian, W.; Zhou, C.; Lyu, Y.; Chu, P.K. Hydrogen permeation barriers and preparation techniques: A review. J. Vac. Sci. Technol. A 2022, 40, 060803. [Google Scholar] [CrossRef]
- Li, J.; Hallil, A.; Metsue, A.; Oudriss, A.; Bouhattate, J.; Feaugas, X. Antagonist effects of grain boundaries between the trapping process and the fast diffusion path in nickel bicrystals. Sci. Rep. 2021, 11, 15533. [Google Scholar] [CrossRef]
- Ostrovskaya, O.; Badini, C.; Deambrosis, S.M.; Miorin, E.; Biamino, S.; Padovano, E. Protection from oxidation of second and third generation TiAl intermetallic alloys by magnetron sputtering deposition of a TiAl/TiAlN coating. Mater. Des. 2021, 208, 109905. [Google Scholar] [CrossRef]
- Miorin, E.; Montagner, F.; Zin, V.; Giuranno, D.; Ricci, E.; Pedroni, M.; Spampinato, V.; Vassallo, E.; Deambrosis, S.M. Al rich PVD protective coatings: A promising approach to prevent T91 steel corrosion in stagnant liquid lead. Surf. Coat. Technol. 2019, 377, 124890. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membrane. Renew. Sustain. Energ. Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. Palladium Membrane Technology for Hydrogen Production, Carbon Capture and Other Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Polina, P.; Georgy, A. Hydrogen Permeability of Composite Pd–Au/Pd–Cu Membranes and Methods for Their Preparation. Membranes 2023, 13, 649. [Google Scholar]
- Barison, S.; Fasolin, S.; Boldrini, S.; Ferrario, A.; Romano, M.; Montagner, F.; Deambrosis, S.M.; Fabrizio, M.; Armelao, L. PdAg/alumina membranes prepared by high power impulse magnetron sputtering for hydrogen separation. Int. J. Hydrog. Energy 2018, 43, 7982–7989. [Google Scholar] [CrossRef]
- Phair, J.W.; Donelson, R. Developments and Design of Novel (Non-Palladium-Based) Metal Membranes for Hydrogen Separation. Ind. Eng. Chem. Res. 2006, 45, 5657–5674. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, H.C.; Lee, J.; Cho, E.; Lee, S.M. Vanadium alloy membranes for high hydrogen permeability and suppressed hydrogen embrittlement. Scr. Mater. 2013, 68, 905–908. [Google Scholar] [CrossRef]
- Moss, T.S.; Peachey, N.M.; Snow, R.C.; Dye, R.C. Multilayer metal membranes for hydrogen separation. Int. J. Hydrog. Energy 1998, 23, 99–106. [Google Scholar] [CrossRef]
- Yan, E.; Zhang, S.; Liu, W.; Zhang, K.; Huang, G.; Jia, L.; Tao, L.; Chen, H.; Bai, J.; Chu, H.; et al. Development of Nb-based alloy membranes with improved resistance to corrosion by H2S. J. Membr. Sci. 2024, 700, 122714. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Liang, X.; Fu, H.; Guo, J.; Liu, D. Reactive magnetron sputtering to enhance the hydrogen permeation performance of NbC/V composite membranes. Mater. Chem Phys. 2023, 302, 127769. [Google Scholar] [CrossRef]
- Park, S.B.; Nam, G.H.; Park, Y. Layer-structured hydrogen permeable membranes and their application in hydrogen membrane fuel cells. Thin Solid Film. 2022, 757, 139391. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Liu, D.; Nagaumi, H.; Li, X. Magnetron sputtering deposition of Mo2N catalytic films on vanadium foils for high temperature hydrogen separation. Vacuum 2022, 2023, 111232. [Google Scholar] [CrossRef]
- Fasolin, S.; Barison, S.; Boldrini, S.; Ferrario, A.; Romano, M.; Montagner, F.; Miorin, E.; Fabrizio, M.; Armelao, L. Hydrogen separation by thin vanadium-based multi-layered membranes. Int. J. Hydrog. Energy 2018, 43, 3235–3243. [Google Scholar] [CrossRef]
- Fasolin, S.; Barison, S.; Agresti, F.; Battiston, S.; Fiameni, S.; Isopi, J.; Armelao, L. New Sustainable Multilayered Membranes Based on ZrVTi for Hydrogen Purification. Membranes 2022, 12, 722. [Google Scholar] [CrossRef]
- Lei, C.; Bokov, A.A.; Ye, Z.-G. Ferroelectric to Relaxor Crossover and Dielectric Phase Diagram in the BaTiO3–BaSnO3 System. J. Appl. Phys. 2007, 101, 084105. [Google Scholar] [CrossRef]
- Petzelt, J.; Bovtun, V.; Nuzhnyy, D.; Kempa, M.; Savinov, M.; Paściak, M.; Kamba, S.; Canu, G.; Buscaglia, V. Broadband Dielectric, Terahertz, and Infrared Spectroscopy of BaTiO3–BaZrO3 Solid Solution: From Proper Ferroelectric over Diffuse and Relaxor Ferroelectrics and Dipolar Glass to Normal Dielectric. Phys. Status Solidi B 2021, 258, 2100259. [Google Scholar] [CrossRef]
- Canu, G.; Confalonieri, G.; Deluca, M.; Curecheriu, L.; Buscaglia, M.T.; Asandulesa, M.; Horchidan, N.; Dapiaggi, M.; Mitoseriu, L.; Buscaglia, V. Structure-Property Correlations and Origin of Relaxor Behaviour in BaCexTi1-XO3. Acta Mater. 2018, 152, 258–268. [Google Scholar] [CrossRef]
- Buscaglia, V.; Buscaglia, M.T.; Canu, G. BaTiO3-Based Ceramics: Fundamentals, Properties and Applications. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 311–344. ISBN 978-0-12-822233-1. [Google Scholar]
- Veerapandiyan, V.; Popov, M.N.; Mayer, F.; Spitaler, J.; Svirskas, S.; Kalendra, V.; Lins, J.; Canu, G.; Buscaglia, M.T.; Pasciak, M.; et al. Origin of Relaxor Behavior in Barium-Titanate-Based Lead-Free Perovskites. Adv. Electron. Mater. 2022, 8, 2100812. [Google Scholar] [CrossRef]
- Yuan, R.; Tian, Y.; Xue, D.; Xue, D.; Zhou, Y.; Ding, X.; Sun, J.; Lookman, T. Accelerated Search for BaTiO3-Based Ceramics with Large Energy Storage at Low Fields Using Machine Learning and Experimental Design. Adv. Sci. 2019, 6, 1901395. [Google Scholar] [CrossRef]
- Dai, Z.; Xie, J.; Liu, W.; Wang, X.; Zhang, L.; Zhou, Z.; Li, J.; Ren, X. Effective Strategy to Achieve Excellent Energy Storage Properties in Lead-Free BaTiO3-Based Bulk Ceramics. ACS Appl. Mater. Interfaces 2020, 12, 30289–30296. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, T.; Zhang, Q.; Ma, W.; Fan, P.; Salamon, D.; Zhang, S.-T.; Nan, B.; Tan, H.; Ye, Z.-G. A Review on the Development of Lead-Free Ferroelectric Energy-Storage Ceramics and Multilayer Capacitors. J. Mater. Chem. C 2020, 8, 16648–16667. [Google Scholar] [CrossRef]
- Long, C.; Zhou, W.; Song, H.; Zheng, K.; Ren, W.; Wu, H.; Ding, X.; Liu, L. Simultaneously Realizing Ultrahigh Energy Storage Density and Efficiency in BaTiO3-Based Dielectric Ceramics by Creating Highly Dynamic Polar Nanoregions and Intrinsic Conduction. Acta Mater. 2023, 256, 119135. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, H.; Zhang, J.; da Silva, L.L.; Sheng, Z.; Yao, Y.; Wang, G.; Hinterstein, M.; Zhang, S.; Chen, J. Ultrahigh Energy-Storage in Dual-Phase Relaxor Ferroelectric Ceramics. Adv. Mater. 2024, 36, 2410088. [Google Scholar] [CrossRef]
- Canu, G.; Valenza, F. Effect of Zr Content on the Wetting of BaTi1−xZrxO3 Perovskite Ceramics by Ag-Based Liquids. J. Eur. Ceram. Soc. 2022, 42, 3886–3891. [Google Scholar] [CrossRef]
- Wu, H.; Zhuo, F.; Qiao, H.; Kodumudi Venkataraman, L.; Zheng, M.; Wang, S.; Huang, H.; Li, B.; Mao, X.; Zhang, Q. Polymer-/Ceramic-Based Dielectric Composites for Energy Storage and Conversion. Energy Environ. Mater. 2022, 5, 486–514. [Google Scholar] [CrossRef]
- Bouhamed, A.; Missaoui, S.; Ben Ayed, A.; Attaoui, A.; Missaoui, D.; Jeder, K.; Guesmi, N.; Njeh, A.; Khemakhem, H.; Kanoun, O. A Comprehensive Review of Strategies toward Efficient Flexible Piezoelectric Polymer Composites Based on BaTiO3 for Next-Generation Energy Harvesting. Energies 2024, 17, 4066. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, F.; Tu, Z.; Jiang, Q.; Wang, Y.; Liu, X.; Sheng, S. Microstructure-Property Relationships in Piezoelectric-Polymer Composites: A Review. J. Polym. Res. 2025, 32, 41. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, J.; Shen, Z.; Lin, Y.; Nan, C.W.; Shen, Y. High-Energy-Density Ferroelectric Polymer Nanocomposites for Capacitive Energy Storage: Enhanced Breakdown Strength and Improved Discharge Efficiency. Mater. Today 2019, 29, 49–67. [Google Scholar] [CrossRef]
- He, Q.; Briscoe, J. Piezoelectric Energy Harvester Technologies: Synthesis, Mechanisms, and Multifunctional Applications. ACS Appl. Mater. Interfaces 2024, 16, 29491–29520. [Google Scholar] [CrossRef]
- Prateek; Thakur, V.K.; Gupta, R.K. Recent Progress on Ferroelectric Polymer-Based Nanocomposites for High Energy Density Capacitors: Synthesis, Dielectric Properties, and Future Aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef]
- Craciun, F.; Cordero, F.; Mercadelli, E.; Ilic, N.; Galassi, C.; Baldisserri, C.; Bobic, J.; Stagnaro, P.; Canu, G.; Buscaglia, M.T.; et al. Flexible Composite Films with Enhanced Piezoelectric Properties for Energy Harvesting and Wireless Ultrasound-Powered Technology. Compos. B Eng. 2023, 263, 110835. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, D.; Cheng, S.; Zhang, J.; Chen, W.; Zhou, L.; Zhang, P. Recent Advances in Dispersion and Alignment of Fillers in PVDF-Based Composites for High-Performance Dielectric Energy Storage. Mater Today Energy 2023, 31, 101208. [Google Scholar] [CrossRef]
- Jin, Y.; Gwak, Y.; Gerhardt, R.A. Effects of Nanoparticles Size and Interactions on Dielectric Properties of Polymer Matrix Flexible Dielectric Nanocomposites. Adv. Compos. Mater. 2020, 29, 235–246. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.; Wang, S.; Guo, J.; Hong, J.; Yang, S. Improving the Performance of Flexible Composites Based on 3-3 Interconnected Skeletons for Piezoelectric Energy Harvesting. Ceram. Int. 2023, 49, 23349–23357. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Zhu, Y.; Li, S.; Jiang, P. High-k Polymer Nanocomposites with 1D Filler for Dielectric and Energy Storage Applications. Prog. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Padurariu, L.; Brunengo, E.; Canu, G.; Curecheriu, L.P.; Conzatti, L.; Buscaglia, M.T.; Stagnaro, P.; Mitoseriu, L.; Buscaglia, V. Role of Microstructures in the Dielectric Properties of PVDF-Based Nanocomposites Containing High-Permittivity Fillers for Energy Storage. ACS Appl. Mater. Interfaces 2023, 15, 13535–13544. [Google Scholar] [CrossRef]
- Shee, C.; Banerjee, S.; Alagirusamy, R.; Ali, S.W. Deployment of Piezoelectric Transducers for Diverse Technical Applications: A Comprehensive Review. Results Eng. 2025, 25, 103881. [Google Scholar] [CrossRef]
- Tomer, V.; Randall, C.A.; Polizos, G.; Kostelnick, J.; Manias, E. High- and Low-Field Dielectric Characteristics of Dielectrophoretically Aligned Ceramic/Polymer Nanocomposites. J. Appl. Phys. 2008, 103, 034115. [Google Scholar] [CrossRef]
- Purusothaman, Y.; Leng, H.; Nanda, A.; Levine, I.; Priya, S. Textured Lead-Free Piezoelectric Ceramics for Flexible Energy Harvesters. ACS Appl. Mater. Interfaces 2023, 15, 6584–6593. [Google Scholar] [CrossRef]
- Kim, H.; Hong, S.-K.; Ryu, J.-K.; Park, S.-H. Effect of Filler Alignment on Piezo-Resistive and Mechanical Properties of Carbon Nanotube Composites. Materials 2020, 13, 2598. [Google Scholar] [CrossRef]
- Athira, B.S.; George, A.; Vaishna Priya, K.; Hareesh, U.S.; Gowd, E.B.; Surendran, K.P.; Chandran, A. High-Performance Flexible Piezoelectric Nanogenerator Based on Electrospun PVDF-BaTiO3 Nanofibers for Self-Powered Vibration Sensing Applications. ACS Appl. Mater. Interfaces 2022, 14, 44239–44250. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Ruan, K.; Guo, H.; He, M.; Shi, X.; Guo, Y.; Kong, J.; Gu, J. Advances in 3D Printing for Polymer Composites: A Review. InfoMat 2024, 6, e12568. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, D.Z.; Liu, F.; Jiang, J.; Zhao, M.; Zhang, T. Lattice structures of Cu-Cr-Zr copper alloy by selective laser melting: Microstructures, mechanical properties and energy absorption. Mat. Des. 2020, 187, 108406. [Google Scholar] [CrossRef]
- Tran, T.Q.; Chinnappan, A.; Lee, J.K.Y.; Loc, N.H.; Tran, L.T.; Wang, G.; Kumar, V.V.; Jayathilaka, W.A.D.M.; Ji, D.; Doddamani, M.; et al. 3D Printing of Highly Pure Copper. Metals 2019, 9, 756. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Sun, F.; Li, L.; Liu, P.; Zhou, H.; Fu, S.; Li, A. A study on the mechanical and electrical properties of high-strength CuCrZr alloy fabricated using laser powder bed fusion. J. Alloys Compd. 2022, 924, 166627. [Google Scholar] [CrossRef]
- Khandpur, M.S.; Giubilini, A.; Iuliano, L.; Minetola, P. On the use of green and blue laser sources for powder bed fusion: State of the art review for additive manufacturing of copper and its alloys. Metals 2024, 14, 1464. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Sun, F.; Liu, P.; Zhou, H.; Fu, S. The current state of CuCrZr and CuCrNb alloys manufactured by additive manufacturing: A review. Mat. Des. 2022, 224, 111419. [Google Scholar] [CrossRef]
- Fiocchi, J.; Zarini, S.; Kurtay, T.; Tuissi, A.; Biffi, C.A. Dissimilar Cu/Al welding by high power blue laser for E-mobility. J. Laser Appl. 2025, 37, 012032. [Google Scholar] [CrossRef]
- Biffi, C.A.; Fiocchi, J.; Boldrini, S.; Tuissi, A. CuCrZr Alloy Manufactured by LPBF Process: Correlation Between Microstructure, Mechanical and Thermal Properties. Lasers Manuf. Mater. Process. 2024, 11, 143–153. [Google Scholar] [CrossRef]
- Wegener, T.; Koopmann, J.; Richter, J.; Krooss, P.; Niendorf, T. CuCrZr processed by laser powder bed fusion-Processability and influence of heat treatment on electrical conductivity, microstructure and mechanical properties. Fatigue Frat. Eng. Mat. Struct. 2021, 44, 2570–2590. [Google Scholar] [CrossRef]
- Nordet, G.; Gorny, C.; Mayi, Y.; Daligault, J. Absorptivity measurements during laser powder bed fusion of pure copper with a 1 kW cw green laser. Opt. Laser Technol. 2022, 147, 107612. [Google Scholar] [CrossRef]
- Yang, X.; Qi, Y.; Zhang, W.; Wang, Y.; Zhu, W. Laser powder bed fusion of C18150 copper alloy with excellent comprehensive properties. Mater. Sci. Eng. A 2023, 862, 144512. [Google Scholar] [CrossRef]
- Olawale, J.O.; Ibitoye, S.A.; Oluwasegun, K.M. Processing Techniques and Productions of Ductile Iron: A Review. Int. J. Sci. Eng. Res. 2016, 7, 397–423. [Google Scholar]
- Labrecque, C.; Gagne, M. Ductile Iron: Fifty Years of Continuous Development. Can. Metall. Q. 1998, 37, 343–378. [Google Scholar]
- Jenkins, L.; Ruff, G.; Dube, F. Ductile Iron Data for Design Engineers; Rio Tinto Iron & Titanium Inc.: Montreal, QC, Canada, 1990. [Google Scholar]
- Zanardi, F.; Bonollo, F.; Bonora, N.; Ruggiero, A.; Angella, G. A contribution to new material standards for Ductile Irons and Austempered Ductile Irons. Int. J. Met. 2017, 11, 136–147. [Google Scholar] [CrossRef]
- Kocks, U.F.; Mecking, H. Physics and phenomenology of strain hardening: The FCC case. Prog. Mater. Sci. 2003, 48, 171–273. [Google Scholar] [CrossRef]
- Mecking, H.; Kocks, U.F. Kinetics of flow and strain-hardening. Acta Metall. 1981, 29, 1865–1875. [Google Scholar] [CrossRef]
- Angella, G.; Taloni, M.; Górny, M.; Tarasiuk, J.; Wronski, S.; Montanari, R.; Pedranz, M.; Benedetti, M.; Fontanari, V.; Lusuardi, D. An Insight into the Defects-Driven Plasticity in Ductile Cast Irons. Materials 2023, 16, 3748. [Google Scholar] [CrossRef]
- Angella, G.; Cova, M.; Bertuzzi, G.; Zanardi, F. Soundness Discrimination in Ferrite Ductile Irons through Tensile Data Analysis. Int. J. Met. 2020, 14, 816–826. [Google Scholar] [CrossRef]
- Angella, G.; Zanardi, F. Validation of a New Quality Assessment Procedure for Ductile Irons Production Based on Strain Hardening Analysis. Metals 2019, 9, 837. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Schafrik, R.E.; Sprague, R. Saga of gas turbine materials: Part III. Adv. Mater. Process. 2004, 162, 29–34. [Google Scholar]
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815. [Google Scholar] [CrossRef]
- Selvaraj, S.K.; Sundaramali, G.; Dev, S.J.; Swathish, R.S.; Karthikeyan, R.; Vishaal, K.E.V.; Paramasivam, V. Recent Advancements in the Field of Ni-Based Superalloys. Adv. Mater. Sci. Eng. 2021, 9723450, 1–60. [Google Scholar] [CrossRef]
- Kothari, D.P.; Ranjan, R.; Singal, K.C. Renewable Energy Sources and Emerging Technologies, 3rd ed.; Ghosh, A.K., Ed.; PHI Learning Private Limited: Delhi, India, 2021; pp. 1–496. [Google Scholar]
- Bassford, T.H.; Hosier, J. Nickel and its alloys. In Handbook of Materials Selection; Kutz, M., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2002; pp. 235–258. [Google Scholar]
- Rao, K.A. Nickel Based Superalloys—Properties and Their Applications. Int. J. Manag. Eng. 2018, 8, 268–277. [Google Scholar]
- Griffiths, M. Ni-Based Alloys for Reactor Internals and Steam Generator Applications. In Structural Alloys for Nuclear Energy Applications, 1st ed.; Odette, G.R., Zinkle, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–409. [Google Scholar]
- Rowcliffe, A.F.; Mansur, L.K.; Hoelzer, D.T.; Nanstad, R.K. Perspectives on radiation effects in nickel-base alloys for applications in advanced reactors. J. Nucl. Mater. 2009, 392, 341–352. [Google Scholar] [CrossRef]
- Raney, M. Catalysts from Alloys. Ind. Eng. Chem. 1940, 32, 1199–1203. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Demura, M.; Hirano, T.; Matsushita, Y.; Tanaka, M.; Katsuya, Y. Catalytic performance of Ni–Al nanoparticles fabricated by arc plasma evaporation for methanol decomposition. Int. J. Hydrog. Energy 2014, 39, 13156–13163. [Google Scholar] [CrossRef]
- Sarvghad, M.; Maher, S.D.; Collard, D.; Tassan, M.; Will, G.; Steinberg, T.A. Materials compatibility for the next generation of Concentrated Solar Power plants. Energy Storage Mater. 2018, 14, 179–198. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Goh, K.; Yu, F.-D.; Wang, Z.-B. High-stability Mn–Co–Ni ternary metal oxide microspheres as conversion-type anodes for sodium-ion batteries. Ceram. Int. 2021, 47, 17540–17549. [Google Scholar] [CrossRef]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef]
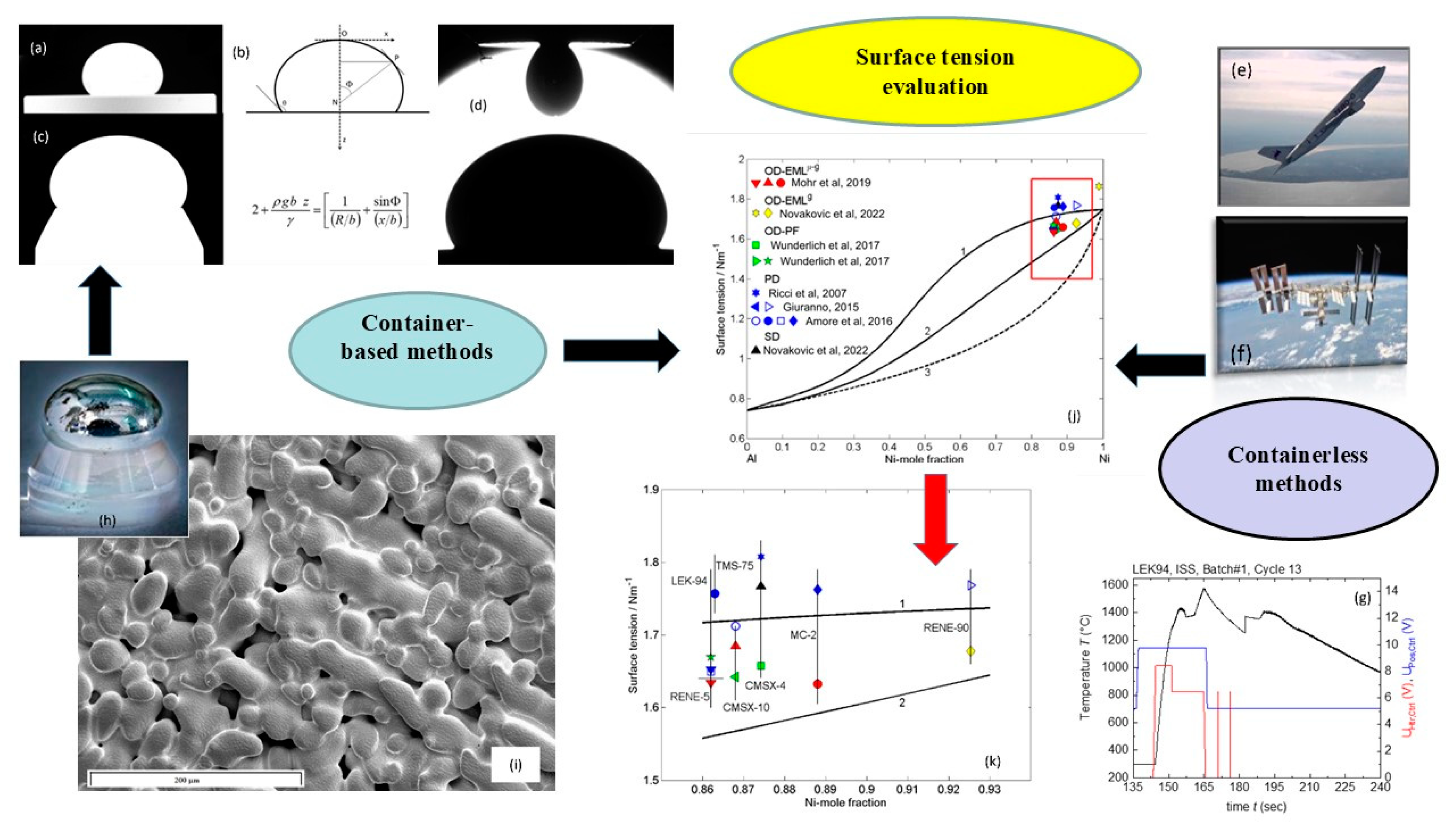
- Mills, K.C. Recommended Values of Thermophysical Properties for Selected Commercial Alloys, 1st ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2002; pp. 1–244. [Google Scholar]
- Novakovic, R.; Giuranno, D.; Mohr, M.; Brillo, J.; Fecht, H.-J. Thermophysical Properties of Ni-based superalloys. In Metallurgy in Space—Recent Results from ISS, 1st ed.; Fecht, H.-J., Mohr, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 315–355. [Google Scholar]
- Ashby, M.F.; Bréchet, Y.J.M.; Cebon, D.; Salvo, L. Selection strategies for materials and processes. Mater. Des. 2004, 25, 51–67. [Google Scholar] [CrossRef]
- Cann, J.L.; De Luca, A.; Dunand, D.C.; Dye, D.; Miracle, D.B.; Oh, H.S.; Olivetti, E.A.; Pollock, T.M.; Poole, W.J.; Yang, R.; et al. Sustainability through alloy design: Challenges and opportunities. Prog. Mater. Sci. 2021, 117, 100722. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Ma, D.; Song, M.; Ludwig, A.; Kharicha, A.; Wu, M. Digital twin for directional solidification of a single-crystal turbine blade. Acta Mater. 2023, 244, 118579. [Google Scholar] [CrossRef]
- Keller, T.; Lindwall, G.; Ghosh, S.; Ma, L.; Lane, B.M.; Zhang, F.; Kattner, U.R.; Lass, E.A.; Heigel, J.C.; Idell, Y.; et al. Application of finite element, phase-field, and CALPHAD-based methods to additive manufacturing of Ni-based superalloys. Acta Mater. 2017, 139, 244–253. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, C.; Zhang, H.; Yan, X.; Tang, N.; Liu, B. Multiscale Modeling and Simulation of Directional Solidification Process of Ni-Based Superalloy Turbine Blade Casting. Metals 2018, 8, 632. [Google Scholar] [CrossRef]
- Novakovic, R.; Mohr, M.; Giuranno, D.; Ricci, E.; Brillo, J.; Wunderlich, R.; Egry, I.; Plevachuk, Y.; Fecht, H.-J. Surface Proper-ties of Liquid Al-Ni Alloys: Experiments Vs Theory. Microgravity Sci. Technol. 2020, 32, 1049–1064. [Google Scholar] [CrossRef]
- Novakovic, R.; Giuranno, D.; Mohr, M.; Brillo, J.; Fecht, H.-J. Viscosity of Liquid Ni-based Industrial Alloys: Experiments versus Theory. Int. Mater. Rev. 2024, 69, 63–79. [Google Scholar] [CrossRef]
- Ricci, E.; Giuranno, D.; Novakovic, R.; Matsushita, T.; Seetharaman, S.; Brooks, R.; Chapman, L.; Quested, P. Density, surface tension, and viscosity of CMSX-4 superalloy. Int. J. Thermophys. 2007, 28, 1304–1321. [Google Scholar] [CrossRef]
- Giuranno, D.; Amore, S.; Novakovic, R.; Ricci, E. Surface tension and density of RENE N5® and RENE 90® Ni-based superalloys. J. Mater. Sci. 2015, 50, 3763–3771. [Google Scholar] [CrossRef]
- Amore, S.; Valenza, F.; Giuranno, D.; Novakovic, R.; Dalla Fontana, G.; Battezzati, L.; Ricci, E. Thermophysical properties of some Ni-based superalloys in the liquid state relevant for solidification processing. J. Mater. Sci. 2016, 51, 1680–1688. [Google Scholar] [CrossRef]
- Fecht, H.J. The ThermoLab project: High-precision thermophysical property data of liquid metals for modelling of industrial solidification processes. High Temp. Mater. 2008, 27, 385–388. [Google Scholar] [CrossRef]
- Egry, I.; Brillo, J.; Holland-Moritz, D.; Plevachuk, Y. The surface tension of liquid aluminium-based alloys. Mater. Sci. Eng. A 2008, 495, 14–18. [Google Scholar] [CrossRef]
- Wunderlich, R.K.; Fecht, H.-J.; Lohöfer, G. Surface Tension and Viscosity of the Ni-Based Superalloys LEK94 and CMSX-10 Measured by the Oscillating Drop Method on Board a Parabolic Flight. Metall. Mater. Trans. 2017, B48, 237–246. [Google Scholar] [CrossRef]
- Mohr, M.; Wunderlich, R.; Dong, Y.; Furrer, D.; Fecht, H.-J. Thermophysical Properties of Advanced Ni-Based Superalloys in the Liquid State Measured on Board the International Space Station (ISS). Adv. Eng. Mater. 2019, 22, 1901228. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Prelas, M.A. Energy Resources and Systems Volume 2: Renewable Resources; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Grossmann, W.; Steininger, K.W.; Schmid, C.; Grossmann, I. Investment and Employment from Large-Scale Photovoltaics up to 2050. Empirica 2012, 39, 165–189. [Google Scholar] [CrossRef]
- Nomeir, B.; Lakhouil, S.; Boukheir, S.; Ali, M.A.; Naamane, S. Recent Progress on Transparent and Self-Cleaning Surfaces by Superhydrophobic Coatings Deposition to Optimize the Cleaning Process of Solar Panels. Sol. Energy Mater. Sol. Cells 2023, 257, 112347. [Google Scholar] [CrossRef]
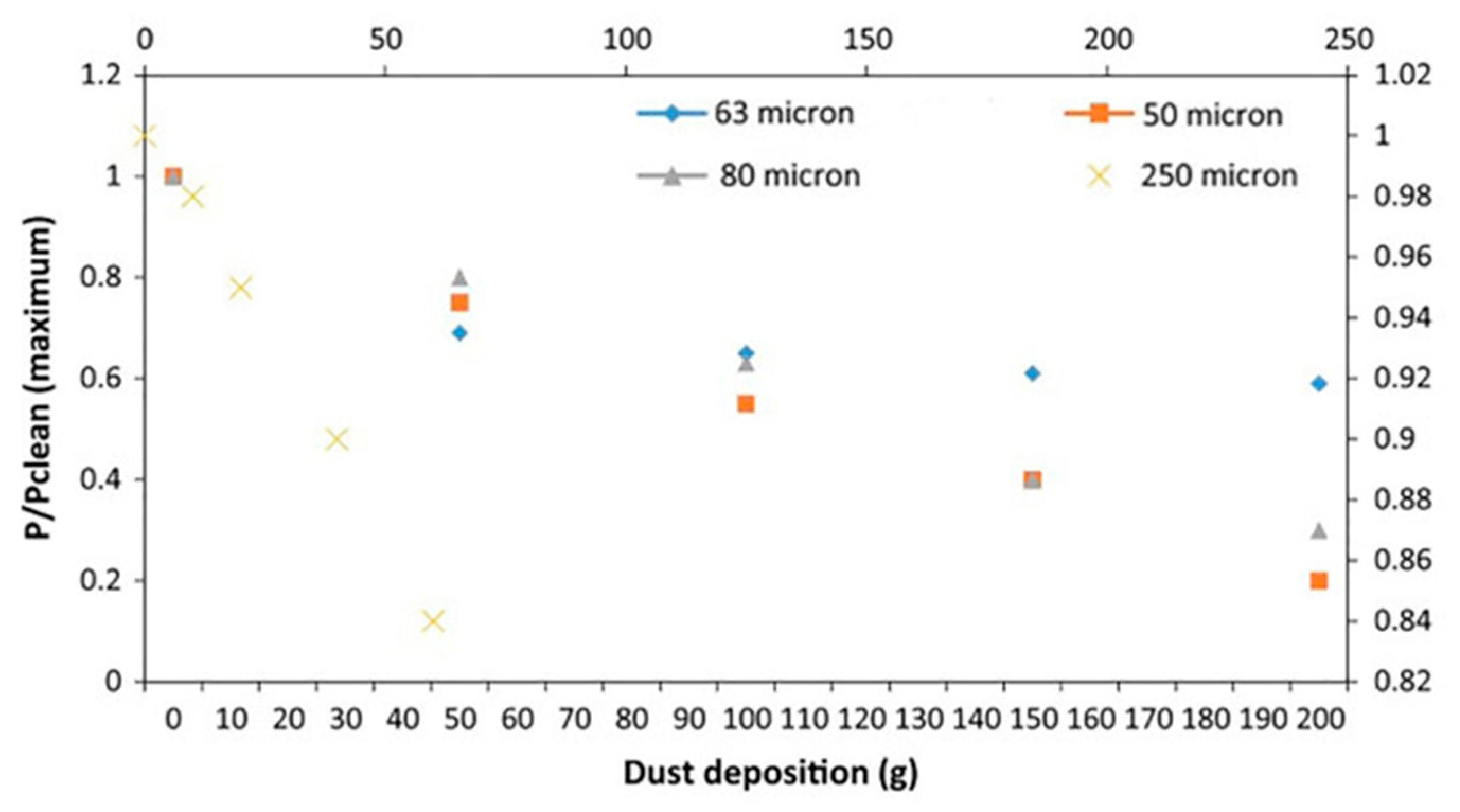
- Saidan, M.; Albaali, A.G.; Alasis, E.; Kaldellis, J.K. Experimental Study on the Effect of Dust Deposition on Solar Photovoltaic Panels in Desert Environment. Renew Energy 2016, 92, 499–505. [Google Scholar] [CrossRef]
- Kazem, H.A.; Chaichan, M.T. Experimental Analysis of the Effect of Dust’s Physical Properties on Photovoltaic Modules in Northern Oman. Sol. Energy 2016, 139, 68–80. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F. High Transmittance and Highly Amphiphobic Coatings for Environmental Protection of Solar Panels. Adv. Colloid Interface Sci. 2020, 286, 102309. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Sun, X.; Zheng, Y.; Cui, X.; Ma, W.; Han, S.; Zhou, L.; Wei, X. Non-Fluorinated Superhydrophobic Film with High Transparency for Photovoltaic Glass Covers. Appl. Surf. Sci. 2023, 609, 155299. [Google Scholar] [CrossRef]
- Zhuang, A.; Liao, R.; Dixon, S.C.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Transparent Superhydrophobic PTFE Films via One-Step Aerosol Assisted Chemical Vapor Deposition. RSC Adv. 2017, 7, 29275–29283. [Google Scholar] [CrossRef]
- Jeong, E.; Zhao, G.; Song, M.; Yu, S.M.; Rha, J.; Shin, J.; Cho, Y.R.; Yun, J. Simultaneous Improvements in Self-Cleaning and Light-Trapping Abilities of Polymer Substrates for Flexible Organic Solar Cells. J. Mater. Chem. A Mater. 2018, 6, 2379–2387. [Google Scholar] [CrossRef]
- Mazumder, P.; Jiang, Y.; Baker, D.; Carrilero, A.; Tulli, D.; Infante, D.; Hunt, A.T.; Pruneri, V. Superomniphobic, Transparent, and Antireflection Surfaces Based on Hierarchical Nanostructures. Nano. Lett. 2014, 14, 4677–4681. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, B.; Zhao, W. Experimental Study on the Super-Hydrophobic Coating Performance for Solar Photovoltaic Modules at Different Wind Directions. Sol. Energy 2023, 249, 725–733. [Google Scholar] [CrossRef]
- Ahmed, A.; Elsakka, M.; Elhenawy, Y.; Amer, A.; Mansi, A.; Bassyouni, M.; Gadalla, M.; Refaat, A. Experimental Investigation of a Nano Coating Efficiency for Dust Mitigation on Photovoltaic Panels in Harsh Climatic Conditions. Sci. Rep. 2024, 14, 23013. [Google Scholar] [CrossRef]
- Ferrari, M.; Piccardo, P.; Vernet, J.; Cirisano, F. High Transmittance Superhydrophobic Coatings with Durable Self-Cleaning Properties. Coatings 2021, 11, 493. [Google Scholar] [CrossRef]
- Jeswiet, J.; Hauschild, M. EcoDesign and future environmental impacts. Mater. Des. 2005, 26, 629–634. [Google Scholar] [CrossRef]
- Batuecas, E.; Martinez-Cisneros, C.S.; Serrano, D.; Varez, A. Life cycle assessment of lab-scale solid sodium-ion batteries: A sustainable alternative to liquid lithium-ion batteries. J. Energy Storage 2024, 80, 110355. [Google Scholar] [CrossRef]
- Erakca, M.; Baumann, M.; Helbig, C.; Weil, M. Systematic review of scale-up methods for prospective life cycle assessment of emerging technologies. J. Clean. Prod. 2024, 451, 142161. [Google Scholar] [CrossRef]
- Bergerson, J.A.; Brandt, A.; Cresko, J.; Carbajales-Dale, M.; MacLean, H.L.; Matthews, H.S.; McCoy, S.; McManus, M.; Miller, S.A.; Morrow, W.R.; et al. Life cycle assessment of emerging technologies: Evaluation techniques at different stages of market and technical maturity. J. Ind. Ecol. 2020, 24, 11–25. [Google Scholar] [CrossRef]
- Audenaert, B.; Boonen, B.; Passel, V. The Future of Ex-Ante LCA? Lessons Learned and Practical Recommendations. Sustainability 2019, 11, 5456. [Google Scholar] [CrossRef]
- Cucurachi, S.; Van Der Giesen, C.; Guinée, J. Ex-ante LCA of Emerging Technologies. Procedia CIRP 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Hetherington, A.C.; Borrion, A.L.; Griffiths, O.G.; McManus, M.C. Use of LCA as a development tool within early research: Challenges and issues across different sectors. Int. J. Life Cycle Assess. 2014, 19, 130–143. [Google Scholar] [CrossRef]
- Moni, S.M.; Mahmud, R.; High, K.; Carbajales-Dale, M. Life cycle assessment of emerging technologies: A review. J. Ind. Ecol. 2020, 24, 52–63. [Google Scholar] [CrossRef]
- Battiston, S.; Fiameni, S.; Fasolin, S.; Barison, S.; Armelao, L. Life cycle environmental impact assessment of lab-scale preparation of porous alumina pellets as substrate for hydrogen separation metal layer-based membranes. LCA Chem. 2023, 28, 1117–1131. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Xing, X.; Zhang, X.; Yang, Y.; Ciu, G. A comprehensive review on hydrogen pearmeation barrier in the hydrogen transportation pipeline: Mechanism, application, preparation, and recent advances. Int. J. Hydrog. Energy. 2025, 101, 504–528. [Google Scholar] [CrossRef]
- Battiston, S.; Montagner, F.; Fiameni, S.; Famengo, A.; Boldrini, S.; Ferrario, A.; Fanciulli, C.; Agresti, F.; Fabrizio, M. AlTiN based thin films for degradation protection of tetrahedrite thermoelectric material. J. Alloys Compd. 2019, 792, 953–959. [Google Scholar] [CrossRef]
- Fiameni, S.; Battiston, S.; Castellani, V.; Barison, S.; Armelao, L. Implementing sustainability in laboratory activities: A case study on aluminum titanium nitride based thin film magnetron sputtering deposition onto commercial laminated steel. J. Clean Prod. 2021, 285, 124869. [Google Scholar] [CrossRef]
- Corona, A.; Ambye-Jensen, M.; Vega, G.C.; Hauschild, M.Z.; Birkved, M. Techno-environmental assessment of the green biorefinery concept: Combining process simulation and life cycle assessment at an early design stage. Sci. Total Environ. 2018, 635, 100–111. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Predicting the environmental impact of a future nanocellulose production at industrial scale: Application of the life cycle assessment scale-up framework. J. Clean Prod. 2018, 174, 283–295. [Google Scholar] [CrossRef]
- Elginoz, N.; Atasoy, M.; Finnveden, G.; Cetecioglu, Z. Ex-ante life cycle assessment of volatile fatty acid production from dairy wastewater. J. Clean Prod. 2020, 269, 122267. [Google Scholar] [CrossRef]
- Shen, C.-L.; Lou, Q.; Zheng, G.-S.; Wu, M.-Y.; Zang, J.-H.; Liu, K.-K.; Dong, L.; Shan, C.-X. Recycling synthetic route to full-color fluorescent carbon nanodots. ACS Sustain. Chem. Eng. 2022, 10, 1624–1632. [Google Scholar] [CrossRef]

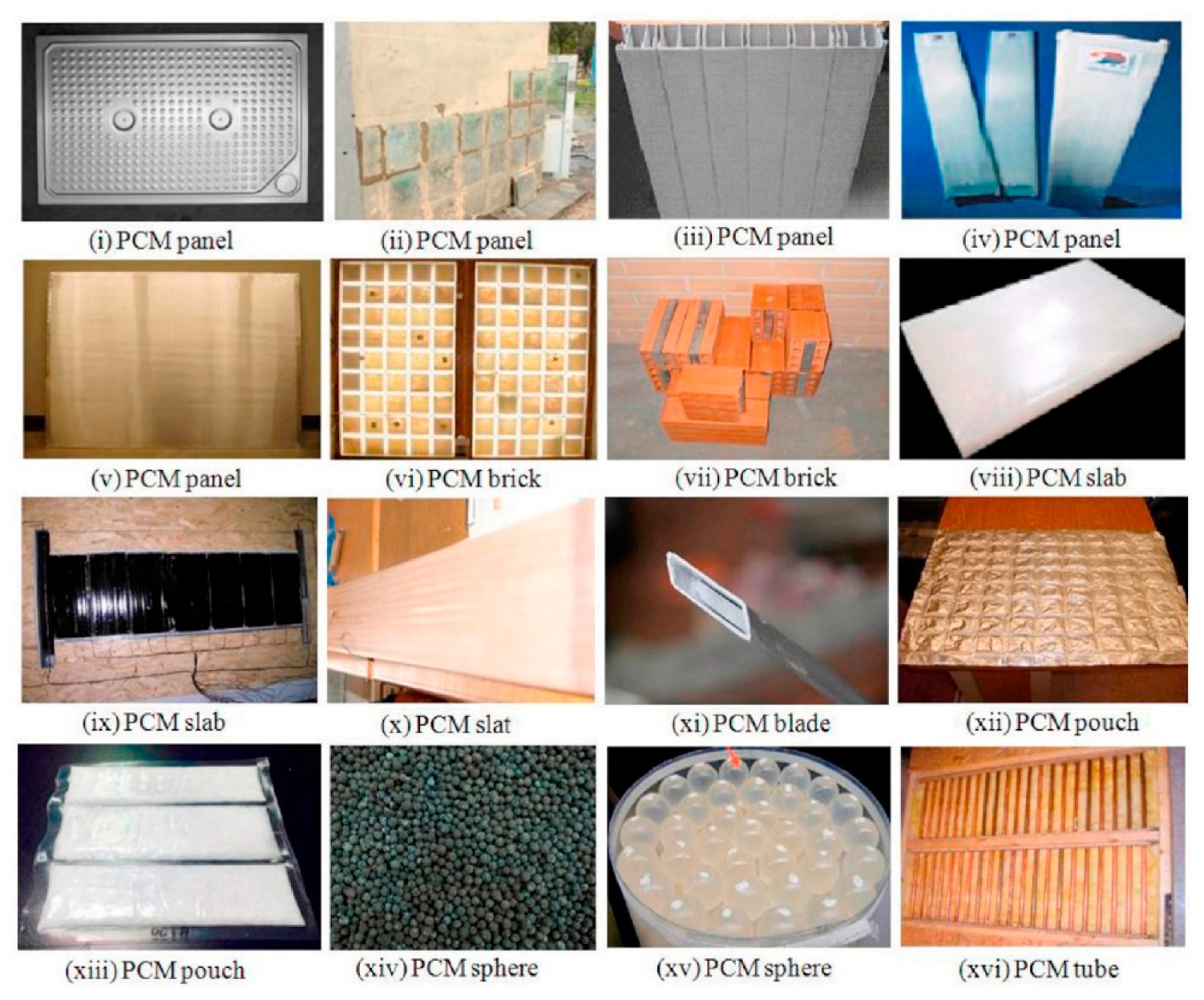






| Type of PCM | Paraffin | Non-Paraffin | Sugar Alcohols | Hydrated Salts | Molten Salts | Metal-Based |
|---|---|---|---|---|---|---|
| Latent heat (J g−1) | 140–260 | 45–200 | 0–350 | 100–300 | 60–1044 | 16–1800 |
| Thermal conductivity (W m−1 K−1) | 0.22–0.82 | 0.1–0.2 | 0.37–1.4 | 0.15–1.0 | <1.0 | 1–430 |
| Melting point (°C) | −10–70 | −5–70 | 0–250 | 8–90 | 200–1000 | −40–1400 |
| Advantages |
|
|
|
|
|
|
| Disadvantages |
|
|
|
|
|
|
| Approach | Strategy | Processing State * | Materials PCM/Container | PC Temperature | Ref |
|---|---|---|---|---|---|
| Single container | Induction melting | L | Al34%Mg6%Zn alloys/SS, CS | 450 °C | [69] |
| Furnace melting | L | Mg84Cu16 and Mg59Cu41 (% at.) eutectic alloys/SS | 488 °C 550 °C | [70] | |
| Furnace melting | L | Cu–Ge alloy/steels and ceramics | 644 °C | [71] | |
| Furnace melting | L | Al-(0–25% wt) Si/Al2O3 | 577 °C | [68,72] | |
| Furnace melting | L | Mg–Cu alloys/SS | 485 °C | [73] | |
| Melting | L | Pb/SS Al-(0, 12.6, 25.1) %Si/SS | 230 °C 660–570 °C | [74] | |
| N.A | N.A. | Al–Si alloy/ceramic container | 585 °C | [75] | |
| Sim | L | Al, Pb, Sn/SS | 660 °C Al, 327 °C Pb, 231 °C Sn | [76] | |
| Encapsulation | ALD | S | encapsulated Sn nanoparticles/oxide layers (SiO2, Al2O3) | 232 °C | [77] |
| CVD | S | Fe particles/SiC/C-shells | 1136 °C | [78] | |
| Micro-encapsulation | M | Al-Si alloy particles/Al2O3 | 573 °C | [79] | |
| Electroplating method | S | Cu ball/other metals | 1083 °C | [64,80] | |
| ADL | L | Fe–Cu alloys/Fe oxide | 1083 °C | [81] | |
| Self-encapsulation | Self-encapsulation (Sim) | M | Al–Si shot/Si | 577 °C | [82] |
| Containerless | Simple powder metallurgy | S | Al-Sn, Fe-Cu | 232 °C Al-Sn, 1085 °C Cu-Fe | [83] |
| Casting-water granulation | L | Al, A356, Al-(40% wt) Sn, A356-(40% wt) Sn alloys | 231 °C | [84] | |
| Powder bed laser melting | L | Al-40% wt Sn Miscibility Gap Alloy | 231 °C | [85] | |
| Powder metallurgy and ball milling | S | Al−Sn alloy | 231 °C | [86] | |
| Powder metallurgy | S | Al-Sn system | 230 °C | [87] | |
| Casting | L | Al70Bi10Sn20 alloy | 200 °C | [88] |
| Assembly | DI Parts | Average Weight (ton/MW) |
|---|---|---|
| Rotor system | Hub, blade, adapter, bearings | 6.44 (hub: 4.5 ton/MW) |
| Shaft | Shaft, bearings | 0.44 |
| Turbine Frame | Nacelle, bed plate, yaw ring | 3.85 (nacelle: up to 10 ton) |
| Gear Box | Housing, support, bearings | 1.71 |
| Others | 1.00 | |
| TOTAL | 13.44 |
| Property | Metric | Imperial |
|---|---|---|
| Tensile Strength | 400 MPa | 58 ksi |
| Yield Strength | 240 MPa | 35 ksi |
| Elongation | 18% | 18% |
| Hardness | 160−170 BHN | 160−170 BHN |
| Impact Energy at 20 °C (−4 °F) Average of 3 tests | 12 J | 8.8 ft∙lb |
| Minimum (1 specimen) | 9 J | 6.6 ft∙lb |
| Alloy Ref. | Ni | Cr | Co | Mo | W | Nb | Ti | Al | Ta | Re | Hf | Other Elements | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMSX-10 [224] | 69.57 | 2.0 | 3.0 | 0.4 | 5.0 | 0.1 | 0.2 | 5.7 | 8.0 | 6.0 | 0.03 | − | |
| CMSX-10 [220] | 69.70 | 2.0 | 3.0 | 0.4 | 5.0 | 0.1 | 0.2 | 5.7 | 8.0 | 6.0 | 0.03 | Bal = −0.03 | |
| Liquidus Temp. , | Specific Heat Capacity , | Measurement Temperature Range, K | Method | Exp. Error % | |||||||||
| CMSX-10 [224] | 1706 ± 5 | 0.71 ± 0.05 | 1650−1775 | MC- | 1.0 | ||||||||
| CMSX-10 [220] | 1702 ± 4 | 0.7 | 1690−1730 | DSC | ±10.0 | ||||||||
| Liquidus temp. , | Density at , · | Temperature coefficient , · | Measurement temperature range, | Method | Exp. Error % | ||||||||
| CMSX-10 [224] | 1706 ± 5 | 8.21 ± 0.15 | −0.535 ± 0.05 | 1525−1875 | OD- | 1.6 | |||||||
| CMSX-10 [220] | 1702 | 7.19 ± 0.03 | −1.6 | 1715−1773 | PD | ±5.0 | |||||||
| CMSX-10 [209] | 1676 | 8.08 | −0.661 | 1676−1825 | MSDM | ±0.88 | |||||||
| Liquidus temp. , | Surface tension at , | Temperature coefficient , | Measurement temperature range, | Method | Exp. error % | ||||||||
| CMSX-10 [220] | 1702 | 1.758 ± 0.025 | −4.7 ± 0.4 | 1715−1773 | PD | <3 | |||||||
| CMSX-10 [223] | 1683 | 1.71 ± 0.02 | −5.80 | 1575−1925 | <1 | ||||||||
| CMSX-10 [224] | 1706 ± 5 | 1.698 ± 0.002 | −1.438 ± 0.294 | 1575−1825 | <±1 | ||||||||
| Liquidus temp. , | Viscosity , | Viscosity , | Activation energy , | Measurement temperature range, | Method | Exp. error % | |||||||
| CMSX-10 [223] | 1683 | 7.7 ± 1.2 | 0.23 | 0.16 | 1600−1840 | ±15 | |||||||
| CMSX-10 [224] | 1706 ± 5 | 8.6 | 0.406 | 0.449 | 1600−1780 | 1.9 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agresti, F.; Angella, G.; Arshad, H.; Barison, S.; Barreca, D.; Bassani, P.; Battiston, S.; Biffi, C.A.; Buscaglia, M.T.; Canu, G.; et al. Sustainable Materials for Energy. Nanomaterials 2025, 15, 1388. https://doi.org/10.3390/nano15181388
Agresti F, Angella G, Arshad H, Barison S, Barreca D, Bassani P, Battiston S, Biffi CA, Buscaglia MT, Canu G, et al. Sustainable Materials for Energy. Nanomaterials. 2025; 15(18):1388. https://doi.org/10.3390/nano15181388
Chicago/Turabian StyleAgresti, Filippo, Giuliano Angella, Humaira Arshad, Simona Barison, Davide Barreca, Paola Bassani, Simone Battiston, Carlo Alberto Biffi, Maria Teresa Buscaglia, Giovanna Canu, and et al. 2025. "Sustainable Materials for Energy" Nanomaterials 15, no. 18: 1388. https://doi.org/10.3390/nano15181388
APA StyleAgresti, F., Angella, G., Arshad, H., Barison, S., Barreca, D., Bassani, P., Battiston, S., Biffi, C. A., Buscaglia, M. T., Canu, G., Cirisano, F., Deambrosis, S. M., Fasan, A., Fasolin, S., Favaro, M., Ferrari, M., Fiameni, S., Fiocchi, J., Fortunato, M., ... Losurdo, M. (2025). Sustainable Materials for Energy. Nanomaterials, 15(18), 1388. https://doi.org/10.3390/nano15181388



















