Highly Efficient Tribocatalysis of Superhard SiC for Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SiC Particle Synthesis
2.3. Material Characterization
2.4. Tribocatalytic Decomposition of RhB Using SiC Particles
2.5. Activity Detection
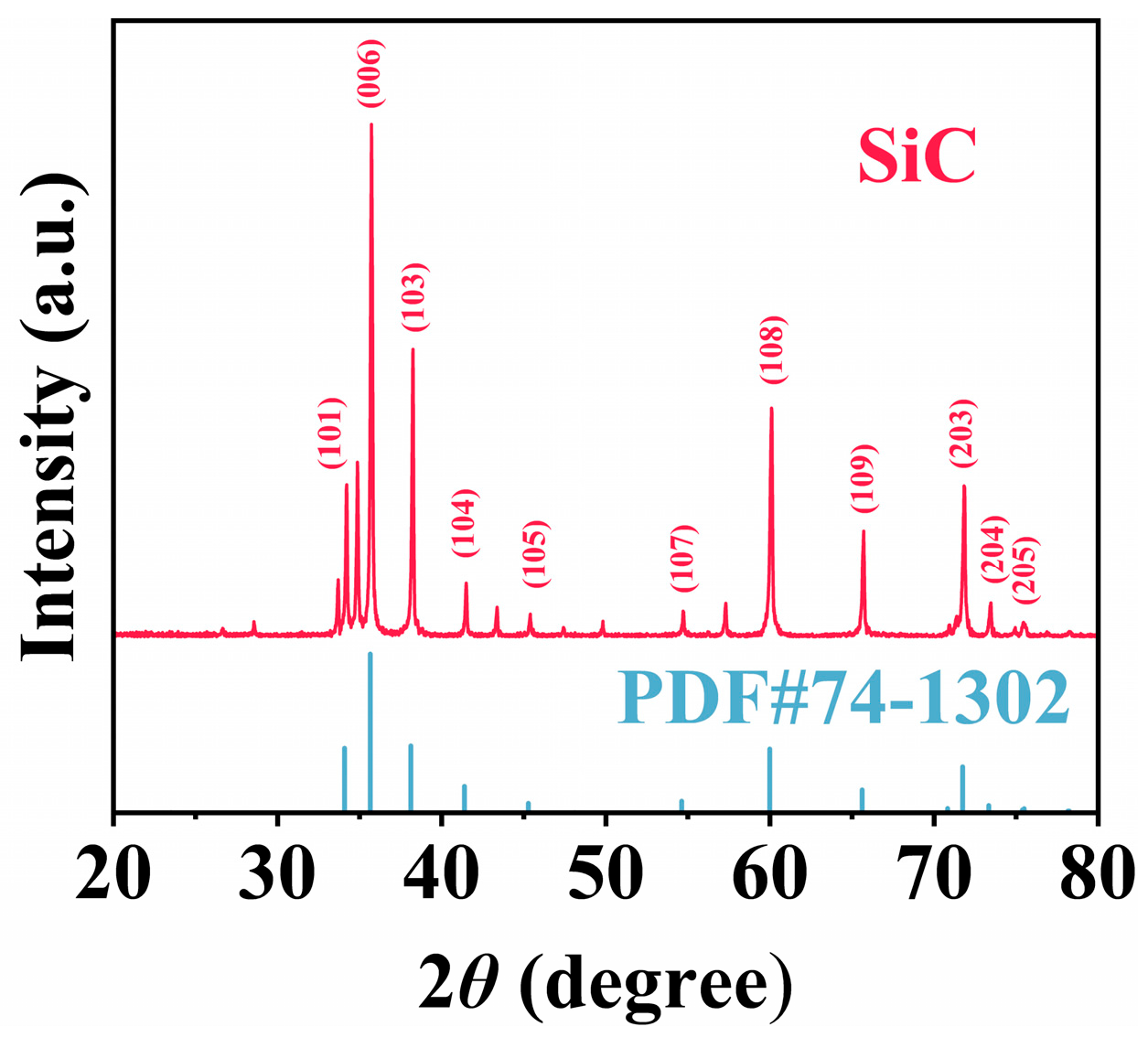
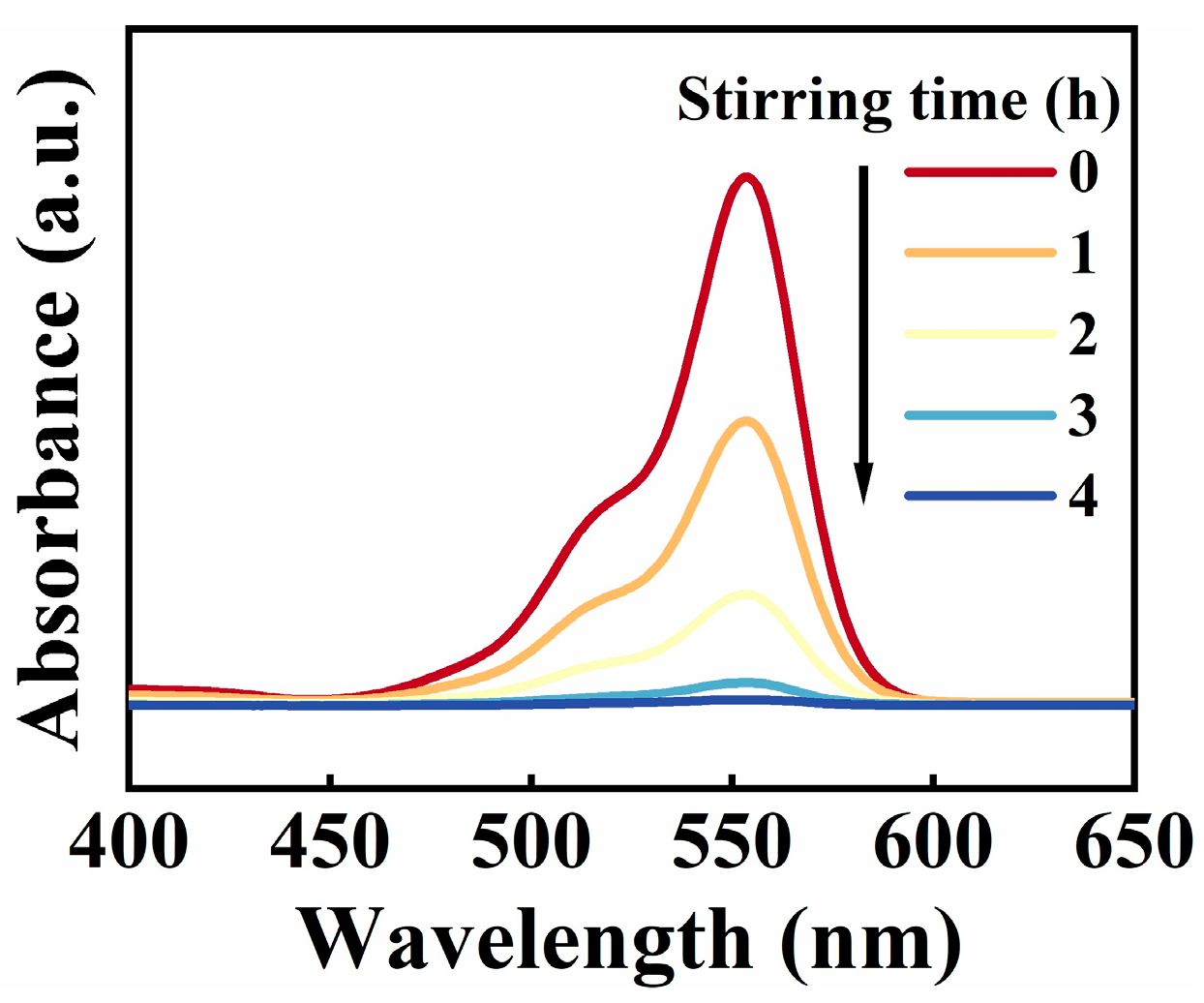
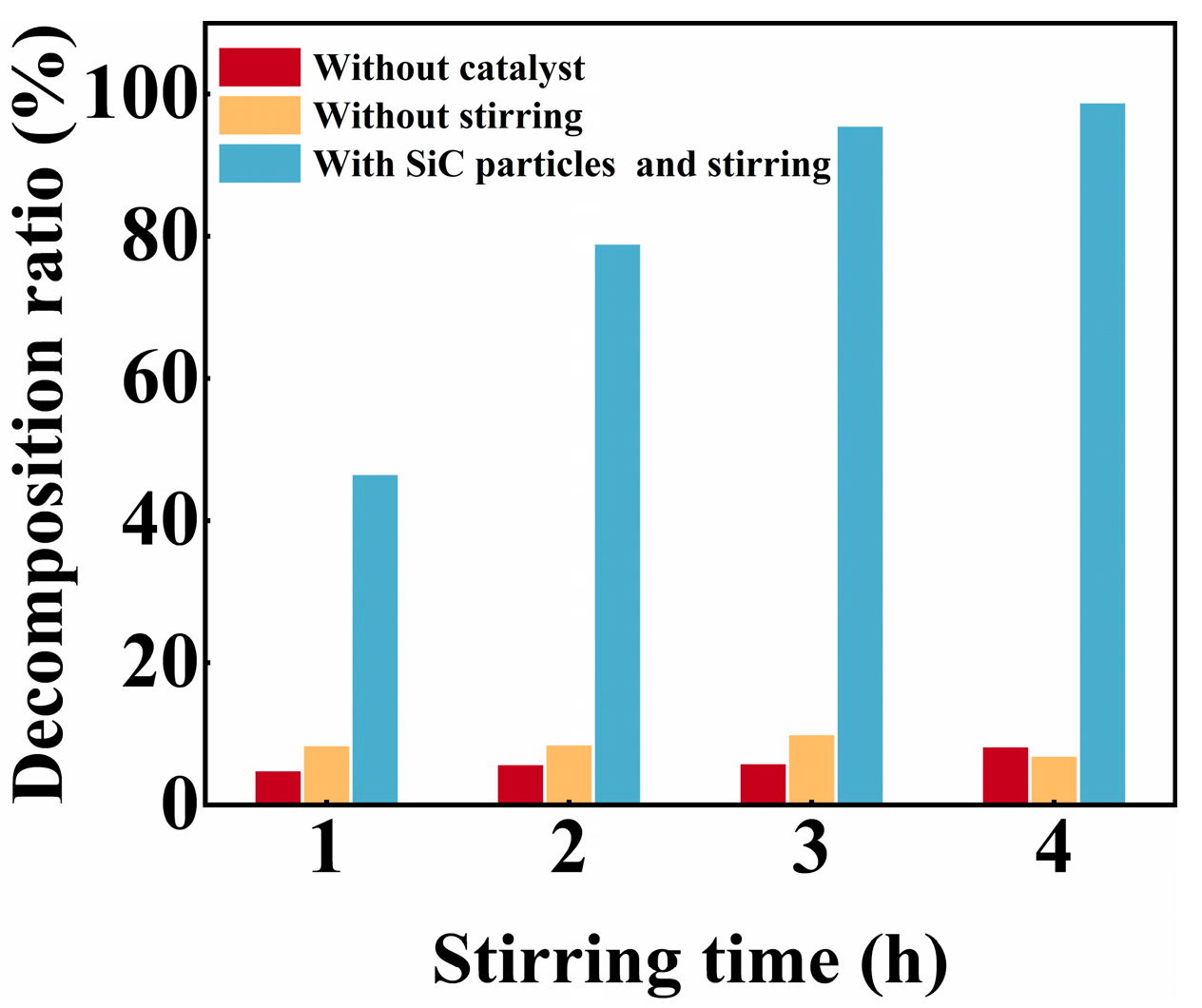
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yap, P.L.; Nine, M.J.; Hassan, K.; Tung, T.T.; Tran, D.N.; Losic, D. Graphene-based sorbents for multipollutants removal in water: A review of recent progress. Adv. Funct. Mater. 2021, 31, 2007356. [Google Scholar] [CrossRef]
- Remanan, S.; Padmavathy, N.; Ghosh, S.; Mondal, S.; Bose, S.; Das, N.C. Porous graphene-based membranes: Preparation and properties of a unique two-dimensional nanomaterial membrane for water purification. Sep. Purif. Rev. 2021, 50, 262–282. [Google Scholar] [CrossRef]
- Kumari, P.; Tripathi, K.M.; Jangir, L.K.; Gupta, R.; Awasthi, K. Recent advances in application of the graphene-based membrane for water purification. Mater. Today Chem. 2021, 22, 100597. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, Z.; Gu, Y.; Bing, L.; Xie, Y.; Shen, Z.; Chen, W. Powerful tribocatalytic degradation of methyl orange solutions with concentrations as high as 100 mg/L by BaTiO3 nanoparticles. Nanomaterials 2025, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Chava, R.K.; Yu, Y.-T.; Kang, M. Layer-by-Layer Deposition of Hollow TiO2 Spheres with enhanced photoelectric conversion efficiency for dye-sensitized solar cell applications. Nanomaterials 2024, 14, 1782. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Ge, H.; Liu, X.; He, Q.; Wang, W.; Ma, W.; Guo, F. Assembly of chitosan/caragana fibers to construct an underwater superelastic 2D layer-supported 3D architecture for rapid congo red removal. Nanomaterials 2024, 14, 1510. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Zhang, L.; Wu, Z.; Hong, S.; Chen, B.; Zhu, G.; Jia, Y. Low-frequency contact-electro-catalysis driven by friction between the PTFE-coated fabric and dye solution. J. Alloys Compd. 2024, 957, 177440. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Wang, D.; Zhang, Y.; Li, F.; Zhang, Y.; Jiao, T. Fabrication of self-assembled BiFeO3/CeO2 nanocatalytic materials for efficient catalytic dye degradation. Nanomaterials 2023, 13, 2545. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Xu, T.; Wu, Z.; Chen, B.; Zhang, L.; Zhu, G.; Jia, Y. Piezoelectric potential activated interfacial electric field in BiFeO3@BaTiO3 heterojunction for rapid and round-the-clock photocatalytic degradation of organic pollutants. J. Adv. Ceram. 2024, 13, 2030–2042. [Google Scholar]
- Wen, L.; Cheng, F.; Zhao, X.; Han, L.; Zhao, D.; Wang, S. Crystal facet engineering of 2D SnSe2 photocatalysts for efficient degradation of malachite green organic dyes. Nanomaterials 2025, 15, 850. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, Y.; Xiang, X.; Tang, Q.; Zhang, H.; Liu, L.; Gao, J.; Xu, B.; Liang, R.; Shu, L.; et al. Modulating low-frequency tribocatalytic performance through defects in uni-doped and bi-doped SrTiO3. J. Adv. Ceram. 2024, 13, 1153–1163. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, X.; Yu, H.; Zhang, Y.; Ye, W. Efficient piezocatalytic properties of Na0.5Bi0.5TiO3 nanoparticles for dye degradation and hydrogen peroxide production. J. Adv. Dielectr. 2025, 15, 2450006. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Br. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.L.; Zhang, Y.Q.; Li, W.Z.; Li, J.; Luan, J. Fabrication of carbon-based materials derived from a cobalt-based organic framework for enhancing photocatalytic degradation of dyes. Dalton Trans. 2024, 53, 4314–4324. [Google Scholar] [CrossRef]
- Loukopoulos, S.; Sakellis, E.; Tsipas, P.; Gardelis, S.; Psycharis, V.; Kostakis, M.G.; Thomaidis, N.S.; Likodimos, V. Visible-light-responsive Ag(Au)/MoS2-TiO2 inverse opals: Synergistic plasmonic, photonic, and charge transfer effects for photoelectrocatalytic water remediation. Nanomaterials 2025, 15, 1076. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Niculescu, A.-G.; Bîrcă, A.C.; Mihaiescu, D.E.; Rădulescu, M.; Grumezescu, A.M. Nanostructured aerogels for water decontamination: Advances, challenges, and future perspectives. Nanomaterials 2025, 15, 901. [Google Scholar] [CrossRef]
- Liu, T.; Cao, L.; Yan, M.; Hu, Y.; Ren, T.; Wu, Z. Efficient photocatalytic degradation of statin via optimization on ZnIn2S4/Bi2WO6 Z-scheme heterostructure. J. Adv. Dielectr. 2024, 14, 2440020. [Google Scholar] [CrossRef]
- Li, R.; Wei, D.; Wang, Z. Synergizing machine learning algorithm with triboelectric nanogenerators for advanced self-powered sensing systems. Nanomaterials 2024, 14, 165. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Ji, R.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Strong tribocatalytic dye degradation by tungsten bronze Ba4Nd2Fe2Nb8O30. Ceram. Int. 2021, 47, 5038–5043. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J.; Yang, Y.; Liu, Q.; Du, Y.; Lou, X. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Li, P.; Tang, C.; Xiao, X.; Jia, Y.; Chen, W. Flammable gases produced by TiO2 nanoparticles under magnetic stirring in water. Friction 2021, 10, 1127–1133. [Google Scholar] [CrossRef]
- Huo, X.; Li, S.; Sun, B.; Wang, Z.; Wei, D. Revealing the Role of Interfacial Charge Transfer in Mechanoluminescence. Nanomaterials 2025, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Khade, V.; Wuppulluri, M. A comparative study on rigid and flexible magnetoelectric composites: Review. J. Adv. Dielectr. 2024, 14, 2340001. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J.; et al. Strong tribo-catalysis of zinc oxide nanorods via triboelectrically-harvesting friction energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic degradation of organic pollutants through bismuth oxyiodate triboelectrically harvesting mechanical energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; He, Q.; Sun, P.; Weng, X.; Dong, X. Tribocatalysis of homogeneous material with multi-size granular distribution for degradation of organic pollutants. J. Colloid Interface Sci. 2022, 622, 602–611. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, R.; Zhang, Y.; Zhou, B.; Sun, P.; Dong, X. Unveiling the mechanism of frictional catalysis in water by Bi12TiO20: A charge transfer and contaminant decomposition path study. Langmuir 2022, 38, 14153–14161. [Google Scholar] [CrossRef]
- Wu, M.; Lei, H.; Chen, J.; Dong, X. Friction energy harvesting on bismuth tungstate catalyst for tribocatalytic degradation of organic pollutants. J. Colloid Interface Sci. 2021, 587, 883–890. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Tribocatalytic degradation of dyes by tungsten bronze ferroelectric Ba2.5Sr2.5Nb8Ta2O30 submicron particles. RSC Adv. 2021, 11, 13386–13395. [Google Scholar] [CrossRef]
- Cao, J.; Jia, Y.; Wan, X.; Li, B.; Zhang, Y.; Huang, S.; Yang, H.; Yuan, G.; Li, G.; Cui, X. Strong tribocatalysis of strontium titanate nanofibers through harvesting friction energy for dye decomposition. Ceram. Int. 2022, 48, 9651–9657. [Google Scholar] [CrossRef]
- Liu, S.; Wang, E.H.; Liu, S.C.; Guo, C.Y.; Wang, H.L.; Yang, T.; Hou, X.M. Mild fabrication of SiC/C nanosheets with prolonged cycling stability as supercapacitor. J. Mater. Sci. Technol. 2022, 110, 178. [Google Scholar] [CrossRef]
- He, R.J.; Zhou, N.P.; Zhang, K.Q.; Zhang, X.Q.; Zhang, L.; Wang, W.Q.; Fang, D.N. Progress and challenges towards additive manufacturing of SiC ceramic. J. Adv. Ceram. 2021, 10, 637. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, F.L.; Gao, F.M.; Wang, L.; Teng, J.; Fu, D.F.; Zhang, H.; Yang, W.Y.; Chen, S.L. Single-crystal integrated photoanodes based on 4H-SiC nanohole arrays for boosting photoelectrochemical water splitting activity. ACS Appl. Mater. Interfaces 2020, 12, 20469. [Google Scholar] [CrossRef]
- Zhou, L.L.; Yang, T.; Wang, E.H.; Zhou, G.Z.; Hou, X.M. Integrated photoanode based on silicon carbide nanowire arrays for efficient water splitting. Chin. J. Eng. 2023, 45, 1149. [Google Scholar]
- Nguyen, T.M.H.; Shin, S.G.; Choi, H.W.; Bark, C.W. Recent advances in self-powered and flexible UVC photodetectors. Exploration 2022, 2, 20210078. [Google Scholar] [CrossRef]
- Du, B.; Zhang, D.Y.; Qian, J.J.; Cai, M.; He, C.; Zhou, P.; Shui, A.Z. Multifunctional carbon nanofiber-SiC nanowire aerogel films with superior microwave absorbing performance. Adv. Compos. Hybrid. Mater. 2021, 4, 1281. [Google Scholar] [CrossRef]
- Medveď, D.; Andrejovská, J.; Zhou, Y.-Z.; Liu, Y.; Guo, W.-M.; Vojtko, M.; Petruš, O.; Lin, H.-T.; Dusza, J. Exceptionally wear resistant (W0.2Mo0.2Ta0.2Nb0.2Ti0.2)(C0.75N0.25) high entropy carbonitride. J. Adv. Ceram. 2025, 14, 9221127. [Google Scholar]
- Hou, G.; Chen, W.; Sun, Q.; Chen, J.; Chen, J.; Tan, H.; Cheng, J.; Zhu, S.; Yang, J.; Liu, W. Super wear-resistant WB4–B super-hard ceramic by in-situ formed lubrication film in high moisture. J. Adv. Ceram. 2024, 13, 1955–1964. [Google Scholar] [CrossRef]
- Fidan, S.; Korkusuz, O.B.; Toker, P.O.; Gulturk, E.; Ates, B.H.; Sinmazgelik, T. Effect of filling materials on the tribological performance of polytetrafluoroethylene in different wear modes. Polym. Compos. 2024, 45, 13561–13577. [Google Scholar] [CrossRef]
- Hu, T.; Li, X.; Wang, X.; Sheng, H.; Yin, J.; Guo, W. Assessing the mechanical-to-electrical energy conversion process of a droplet sliding on the poly(tetrafluoroethylene) surface. ACS Appl. Mater. Interfaces 2024, 16, 1892–1898. [Google Scholar] [CrossRef]
- Nie, J.; Ren, Z.; Xu, L.; Lin, S.; Zhan, F.; Chen, X.; Wang, Z.L. Probing contact-electrification-induced electron and ion transfers at a liquid-solid interface. Adv. Mater. 2020, 32, 1905696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zou, J.; Li, Y.; Hansen, E.J.; Sun, D.; Wang, H.; Wang, L.; Liu, J. Regulating zinc nucleation sites and electric field distribution to achieve high-performance zinc metal anode via surface texturing. Small 2023, 19, 2206634. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Li, W.; Tu, J.; Fan, F.R. Mechanically induced contact-electro-catalysis: Free radical generation, reaction pathways, and catalytic applications. ChemCatChem 2025, 17, e202500282. [Google Scholar] [CrossRef]
- He, W.; Liu, W.; Yang, H.; Guo, H.; Chen, J.; Wang, Z.; Liu, Y.; Pu, X.; Yang, H.; Tang, Q.; et al. Boosting output performance of sliding mode triboelectric nanogenerator by charge space-accumulation effect. Nat. Commun. 2020, 11, 4277. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical Organic Synthesis. Chem. Soc. Rev. 2013, 42, 7668. [Google Scholar] [CrossRef]
- Ralphs, K.; Hardacre, C.; James, S.L. Application of heterogeneous catalysts prepared by mechanochemical synthesis. Chem. Soc. Rev. 2013, 42, 7701. [Google Scholar] [CrossRef]
- Xu, L.; Yang, N.; Xu, T.; Yang, Y.; Lv, Y. Enhancing visible-light photocatalytic activity of AgCl photocatalyst by CeO2 modification for degrading multiple organic pollutants. Nanomaterials 2025, 15, 537. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Y.; Dong, Y.; Jin, Q.; Lin, H. A critical review on reliability of quenching experiment in advanced oxidation processes. Chem. Eng. J. 2023, 466, 143161. [Google Scholar] [CrossRef]
- Adarsha, J.R.; Ravishankar, T.N.; Manjunatha, C.R.; Ramakrishnappa, T. Green synthesis of nanostructured calcium ferrite particles and its application to photocatalytic degradation of Evans blue dye. Mater. Today Proc. 2022, 49, 777–788. [Google Scholar] [CrossRef]
- Hristu, R.; Stanciu, S.G.; Tranca, D.E.; Polychroniadis, E.K.; Stanciu, G.A. Identification of stacking faults in silicon carbide by polarization-resolved second harmonic generation microscopy. Sci. Rep. 2017, 7, 4874. [Google Scholar] [CrossRef]
- Omote, K. Crystal defects in SiC wafers and a new X-ray topography ssystem. Rigaku J. 2013, 29, 9–14. [Google Scholar]
- Shen, G.; Liang, R.; Wang, Z.; Liu, Z.; Shu, L. Flexoelectricity in oxide thin films. J. Adv. Dielectr. 2024, 14, 2330001. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, M.; Zhang, H.; Gao, J.; Kwok, K.W.; Kong, L.-B.; Jia, Y.; Liu, L.; Peng, B. Enhanced tribocatalytic degradation of dye pollutants through governing the charge accumulations on the surface of ferroelectric barium zirconium titanate particles. Nano Energy 2022, 101, 107519. [Google Scholar] [CrossRef]
- Wu, M.; Chen, R.; Xu, Y.; Zhang, Y.; Sun, P.; Dong, X. The Friction pair composed of polymers and Bi12TiO20 facilitates the tribocatalytic degradation of organic pollutants. Mater. Today Sustain. 2024, 25, 100850. [Google Scholar]
- Lu, C.X.; Han, C.B.; Gu, G.Q.; Chen, J.; Yang, Z.W.; Jiang, T.; He, C.; Wang, Z.L. Temperature effect on performance of triboelectric nanogenerator. Adv. Eng. Mater. 2017, 19, 1700275. [Google Scholar] [CrossRef]
- Saisnith, V.; Fridrici, V. A Study of the wear damage of a PTFE coating: The effects of temperature and environment on its mechanical and tribological properties. Wear 2021, 480–481, 203946. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, G.; Guo, T.; Chen, J.; Ding, Z.; Cheng, N.; Ding, R.; Jiang, M.; Jiao, W.; Liu, Y. Deoxidation regulation of SiC surface and its effect on enhancing photocatalytic performance. Appl. Surf. Sci. 2022, 601, 155181. [Google Scholar] [CrossRef]
- Hao, J.-Y.; Wang, Y.-Y.; Tong, X.-L.; Jin, G.-Q.; Guo, X.-Y. Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 18904–18910. [Google Scholar] [CrossRef]
- Sanromán, M.Á.; Pazos, M.; Cameselle, C. Optimisation of electrochemical decolourisation process of an azo dye, methyl orange. J. Chem. Technol. Biotechnol. 2004, 79, 1349–1353. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of chitosan microspheres for efficient adsorption of methyl orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, J.; Zhang, R.; Han, P.; Ren, P.; Liu, M.; Wong, N.H.; Sunarso, J. Integrated MnO2 nanosheet ultrafiltration ceramic membrane with micro-nano bubbles for catalytic treatment of dye wastewater. Sep. Purif. Technol. 2022, 300, 121786. [Google Scholar] [CrossRef]
- Qi, L.; Wang, S.; Liu, Y.; Zhao, P.; Tian, J.; Zhu, B.; Zhang, S.; Xie, W.; Yu, H. Facile preparation of magnetically separable Fe3O4/ZnO nanocomposite with enhanced photocatalytic activity for degradation of Rhodamine B. Nanomaterials 2024, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, R. Formic acid enhanced effective degradation of methyl orange dye in aqueous solutions under UV–Vis irradiation. Water Res. 2016, 98, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Jia, Y.; Gao, Y.; Wang, X. Review on tribocatalysis through harvesting friction energy for mechanically-driven dye decomposition. J. Alloys Compd. 2024, 957, 175413. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, L.-Z.; Fu, Z.-C.; Li, C.; Yang, Z.; Chen, Y.; Fu, W.-F. Photocatalytic oxidation of arylalcohols to aromatic aldehydes promoted by hydroxyl radicals over a CoP/CdS photocatalyst in water with hydrogen evolution. Catal. Sci. Technol. 2018, 8, 2540–2545. [Google Scholar] [CrossRef]
- Ferraro, C.; Garcia-Tunon, E.; Barg, S.; Miranda, M.; Ni, N.; Bell, R.; Saiz, E. SiC porous structures obtained with innovative shaping technologies. J. Eur. Ceram. Soc. 2018, 38, 861–875. [Google Scholar] [CrossRef]
- Chae, S.-H.; Kim, Y.-W.; Song, I.-H.; Kim, H.-D.; Narisawa, M. Porosity control of porous silicon carbide ceramics. J. Eur. Ceram. Soc. 2009, 29, 2921–2927. [Google Scholar] [CrossRef]

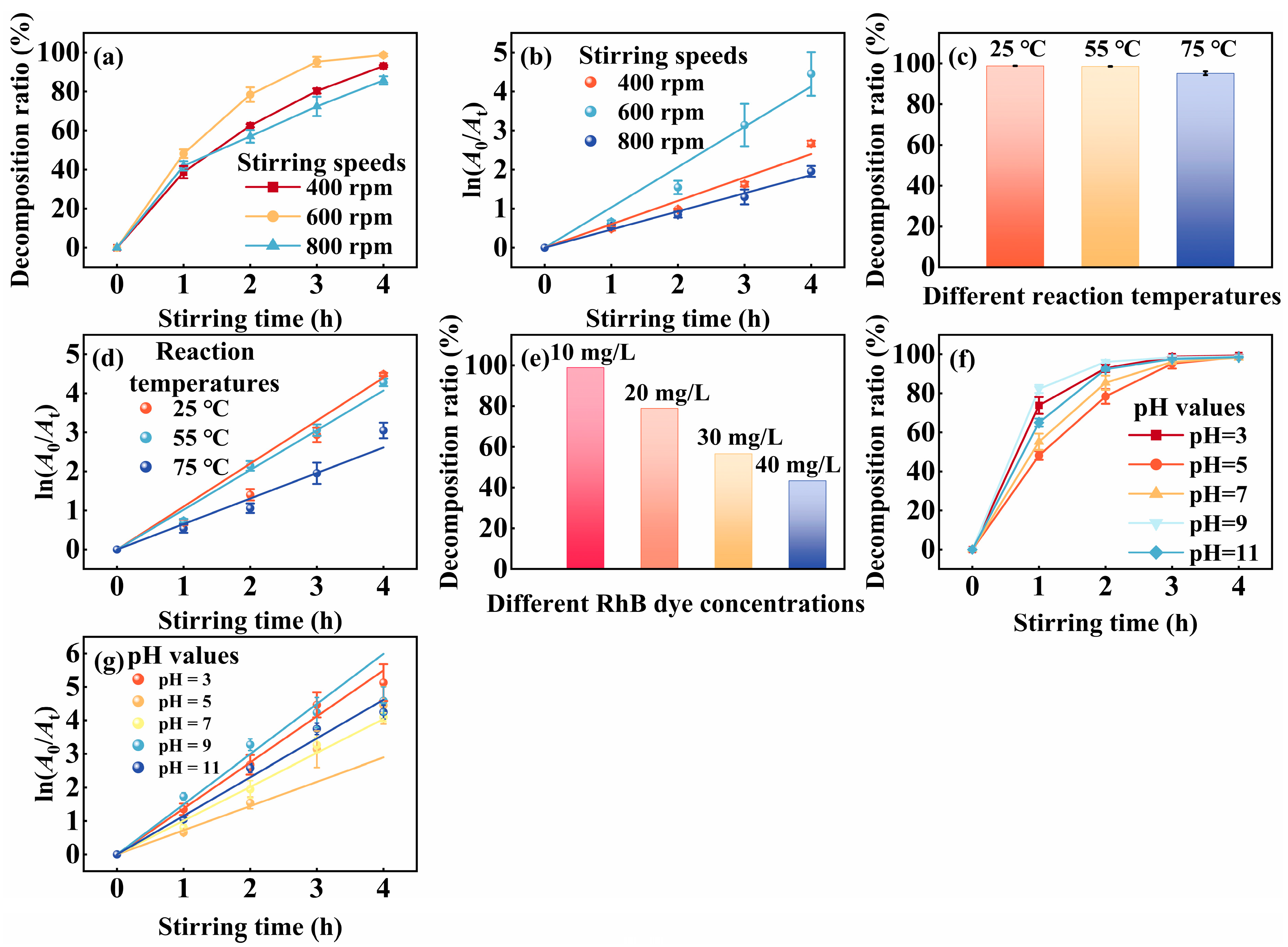
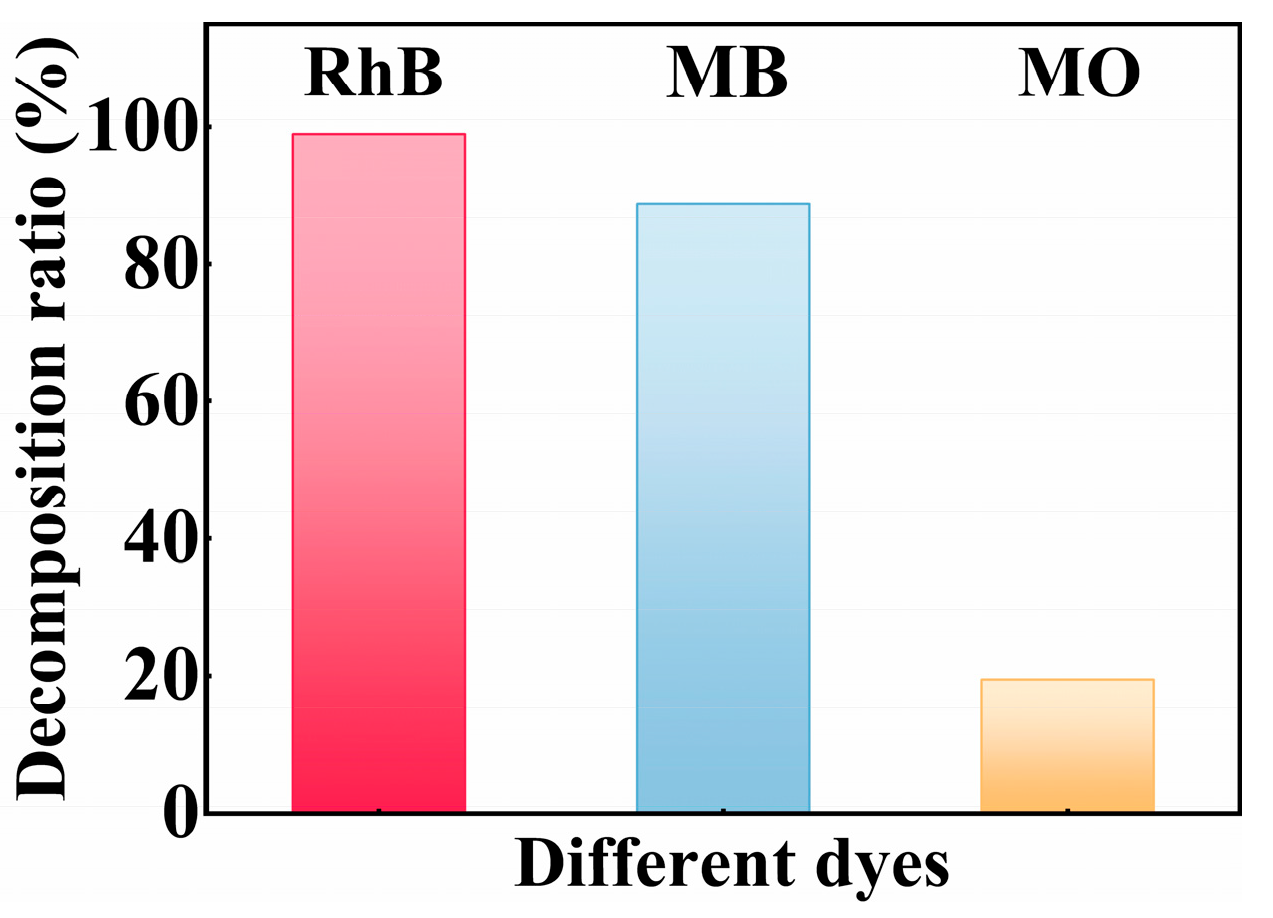

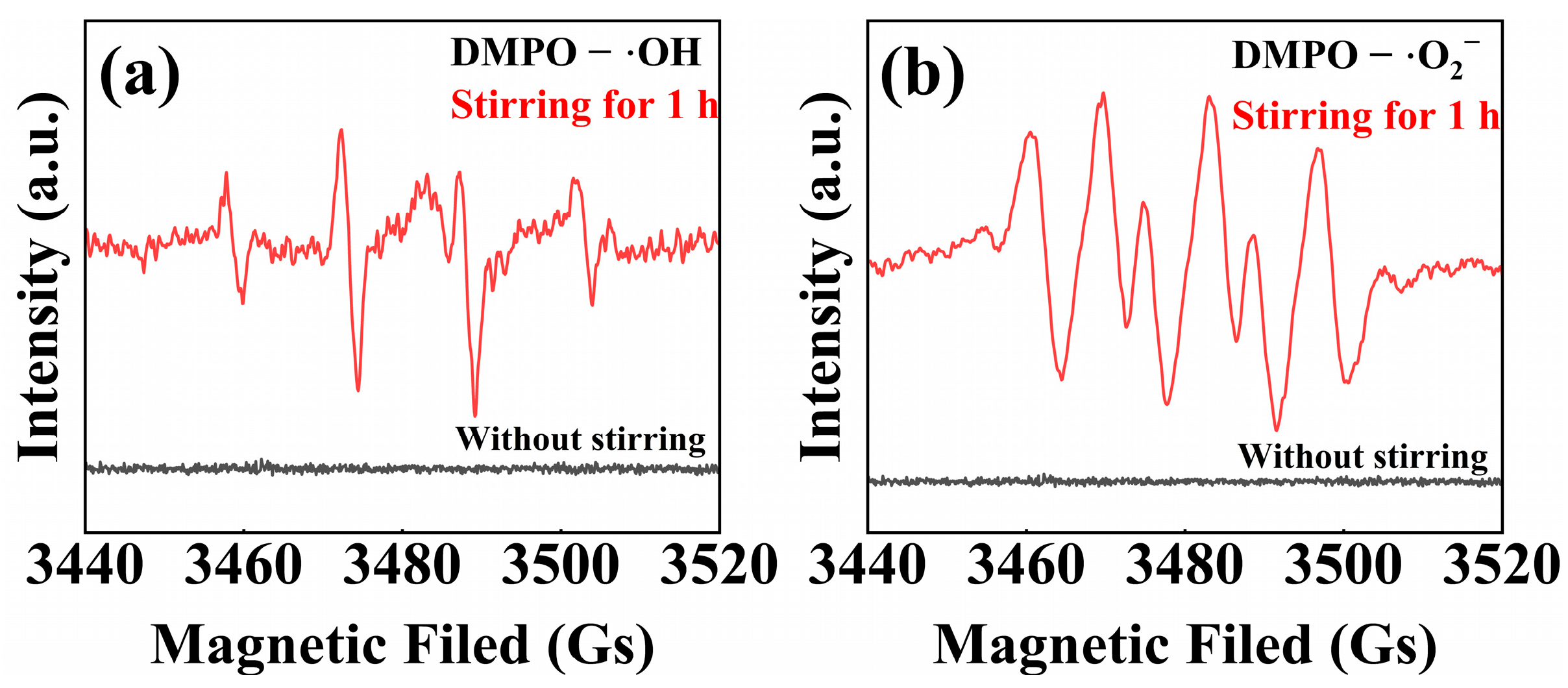
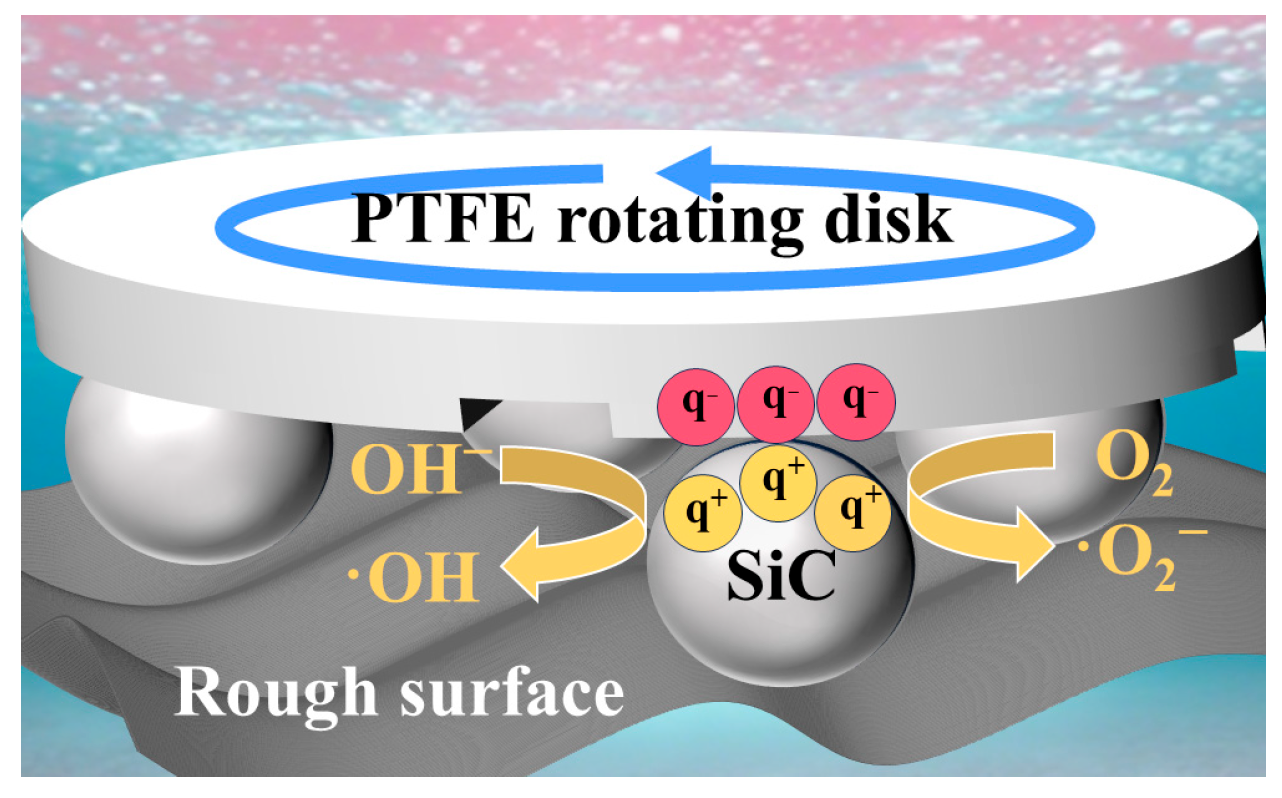

| Condition Type | Parameter | Decomposition (%) | K (h−1) |
|---|---|---|---|
| Stirring Speeds (rpm) | 400 | 93.05 ± 0.50 | 0.60 ± 0.04 |
| 600 | 98.70 ± 0.73 | 1.03 ± 0.07 | |
| 800 | 85.74 ± 2.05 | 0.47 ± 0.02 | |
| Reaction Temperatures (°C) | 25 | 98.87 ± 0.05 | 1.10 ± 0.04 |
| 55 | 98.61 ± 0.13 | 1.02 ± 0.06 | |
| 75 | 95.20 ± 0.90 | 0.65 ± 0.05 | |
| pH Values | 3 | 99.35 ± 0.30 | 1.37 ± 0.04 |
| 5 | 98.70 ± 0.73 | 0.72 ± 0.07 | |
| 7 | 98.33 ± 0.24 | 1.01 ± 0.04 | |
| 9 | 98.92 ± 0.46 | 1.50 ± 0.12 | |
| 11 | 98.56 ± 0.29 | 1.15 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Z.; Hong, S.; Zhu, Z.; Wu, S.; Chen, B.; Jia, Y. Highly Efficient Tribocatalysis of Superhard SiC for Water Purification. Nanomaterials 2025, 15, 1206. https://doi.org/10.3390/nano15151206
Wang Y, Wu Z, Hong S, Zhu Z, Wu S, Chen B, Jia Y. Highly Efficient Tribocatalysis of Superhard SiC for Water Purification. Nanomaterials. 2025; 15(15):1206. https://doi.org/10.3390/nano15151206
Chicago/Turabian StyleWang, Yuanfang, Zheng Wu, Siqi Hong, Ziqi Zhu, Siqi Wu, Biao Chen, and Yanmin Jia. 2025. "Highly Efficient Tribocatalysis of Superhard SiC for Water Purification" Nanomaterials 15, no. 15: 1206. https://doi.org/10.3390/nano15151206
APA StyleWang, Y., Wu, Z., Hong, S., Zhu, Z., Wu, S., Chen, B., & Jia, Y. (2025). Highly Efficient Tribocatalysis of Superhard SiC for Water Purification. Nanomaterials, 15(15), 1206. https://doi.org/10.3390/nano15151206








