A Review on Laser-Induced Graphene-Based Electrocatalysts for the Oxygen Reduction Reaction in Electrochemical Energy Storage and Conversion
Abstract
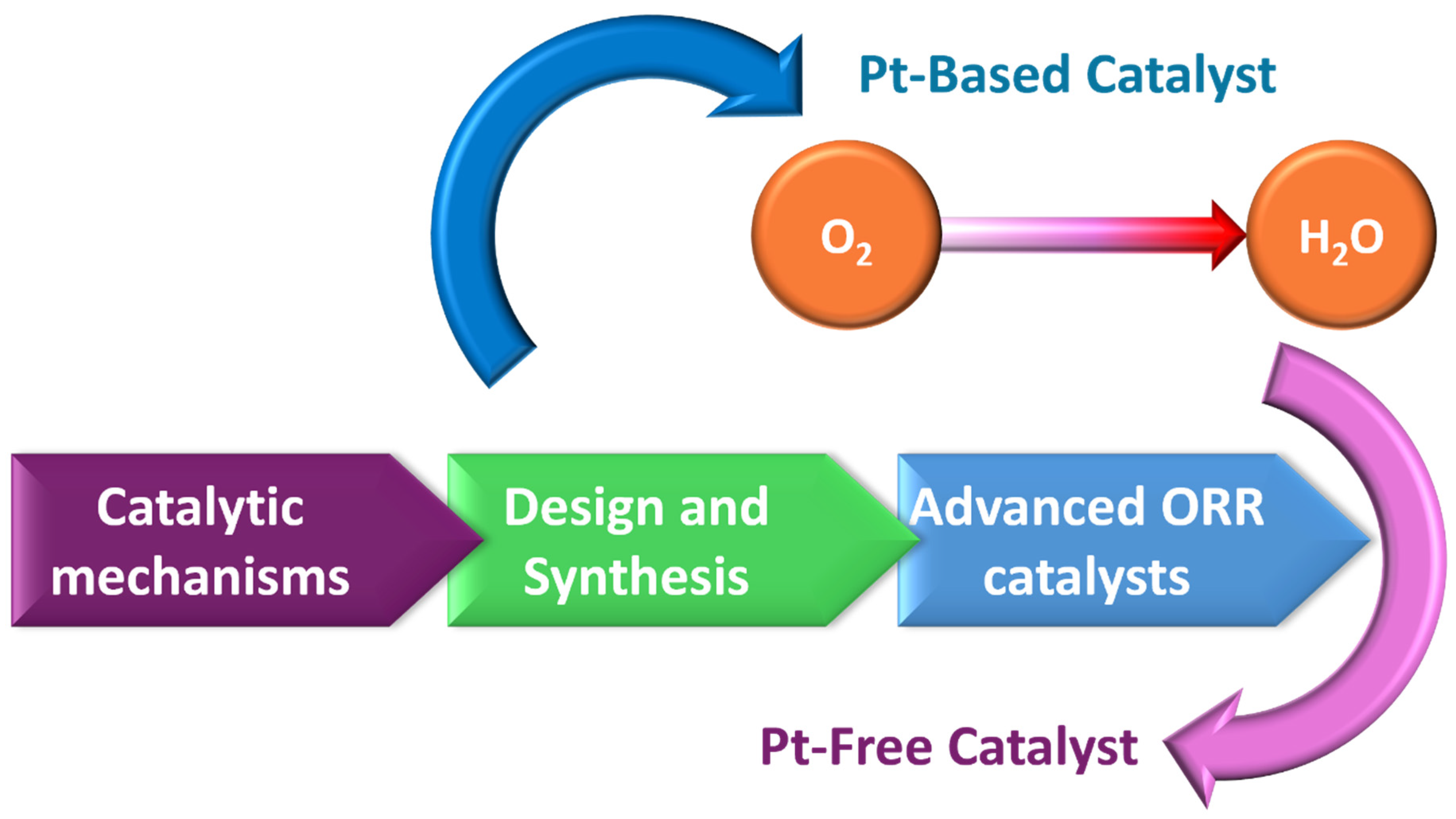
1. Introduction
2. Synthesis and Properties of Laser-Induced-Graphene
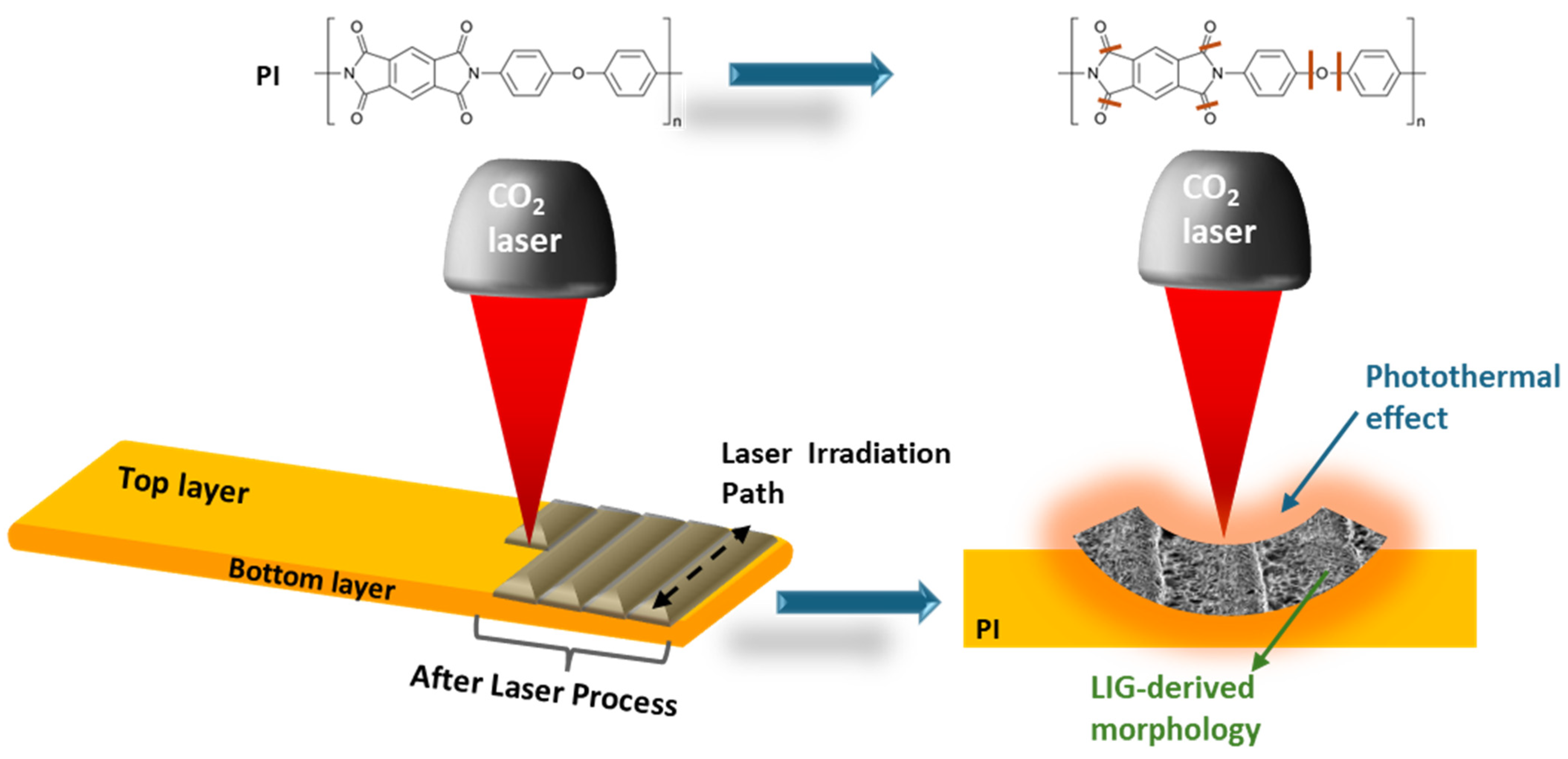
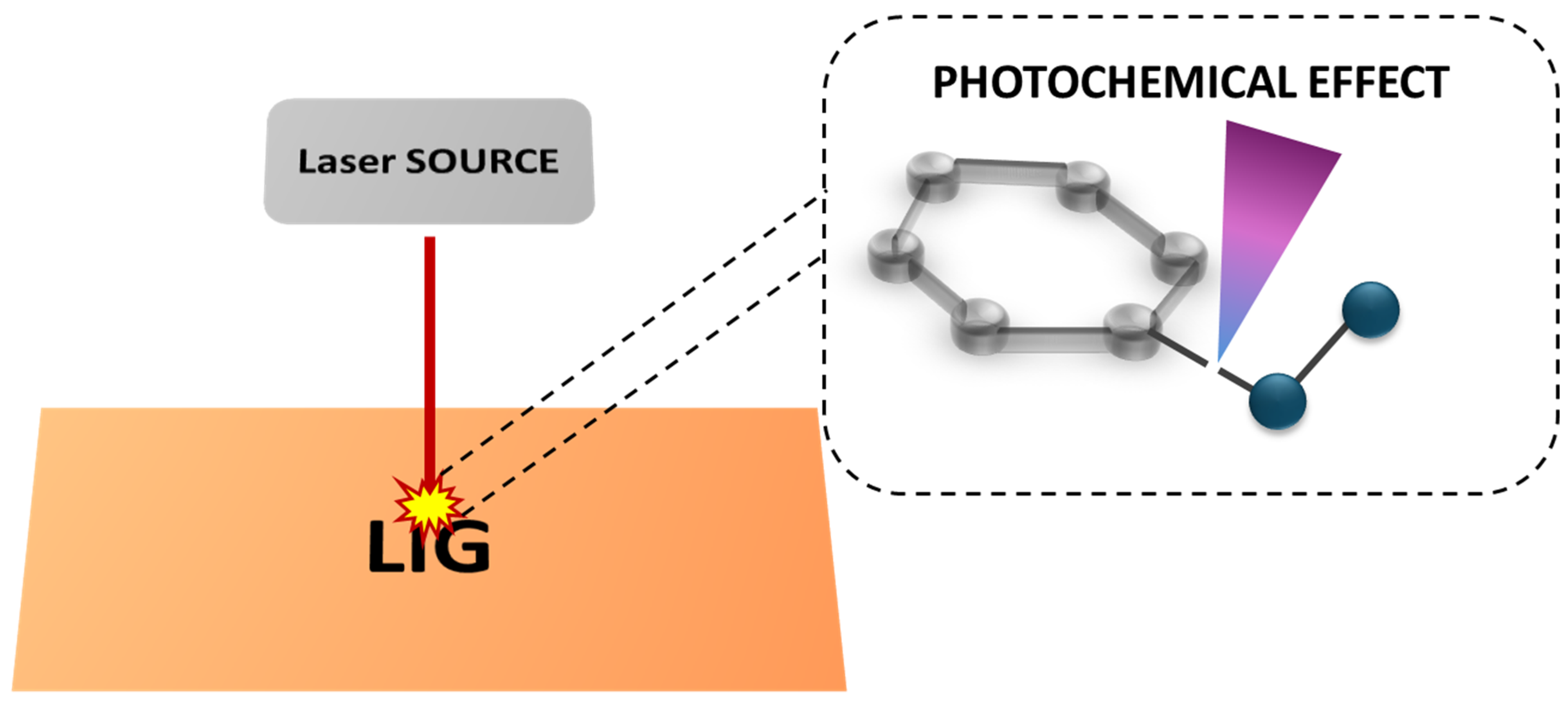
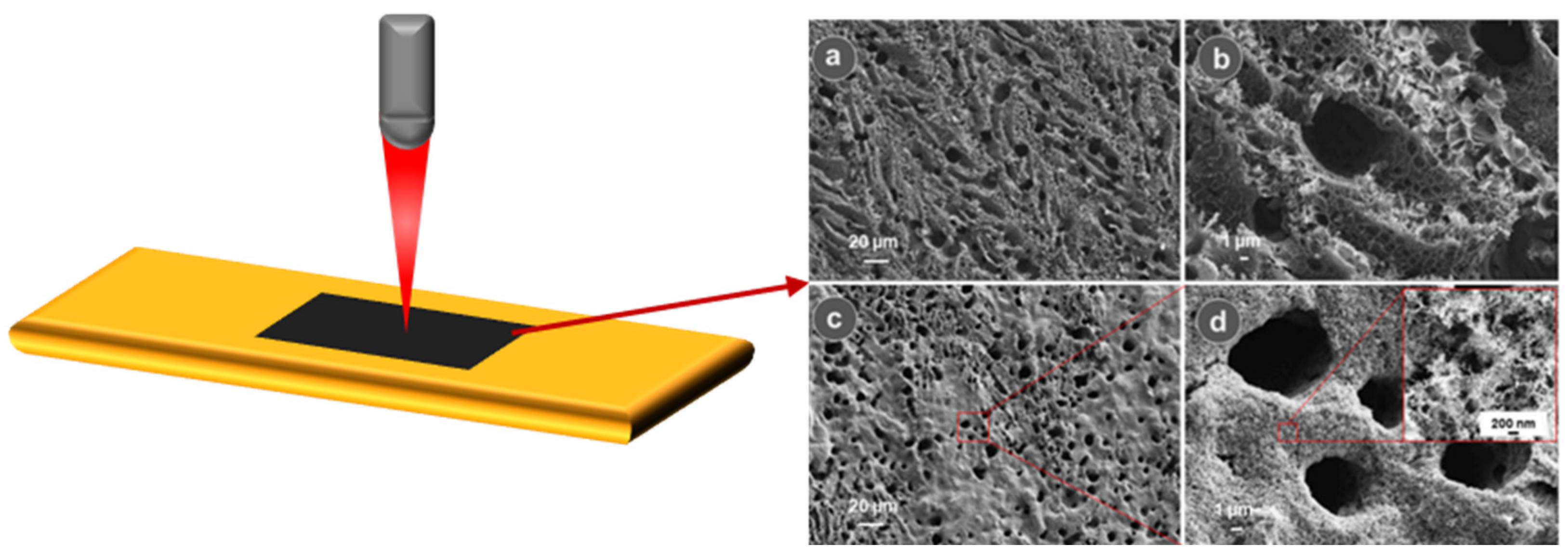
2.1. Laser Processing Techniques for LIG Fabrication
2.2. Carbon Sources for LIG-Based Electrodes
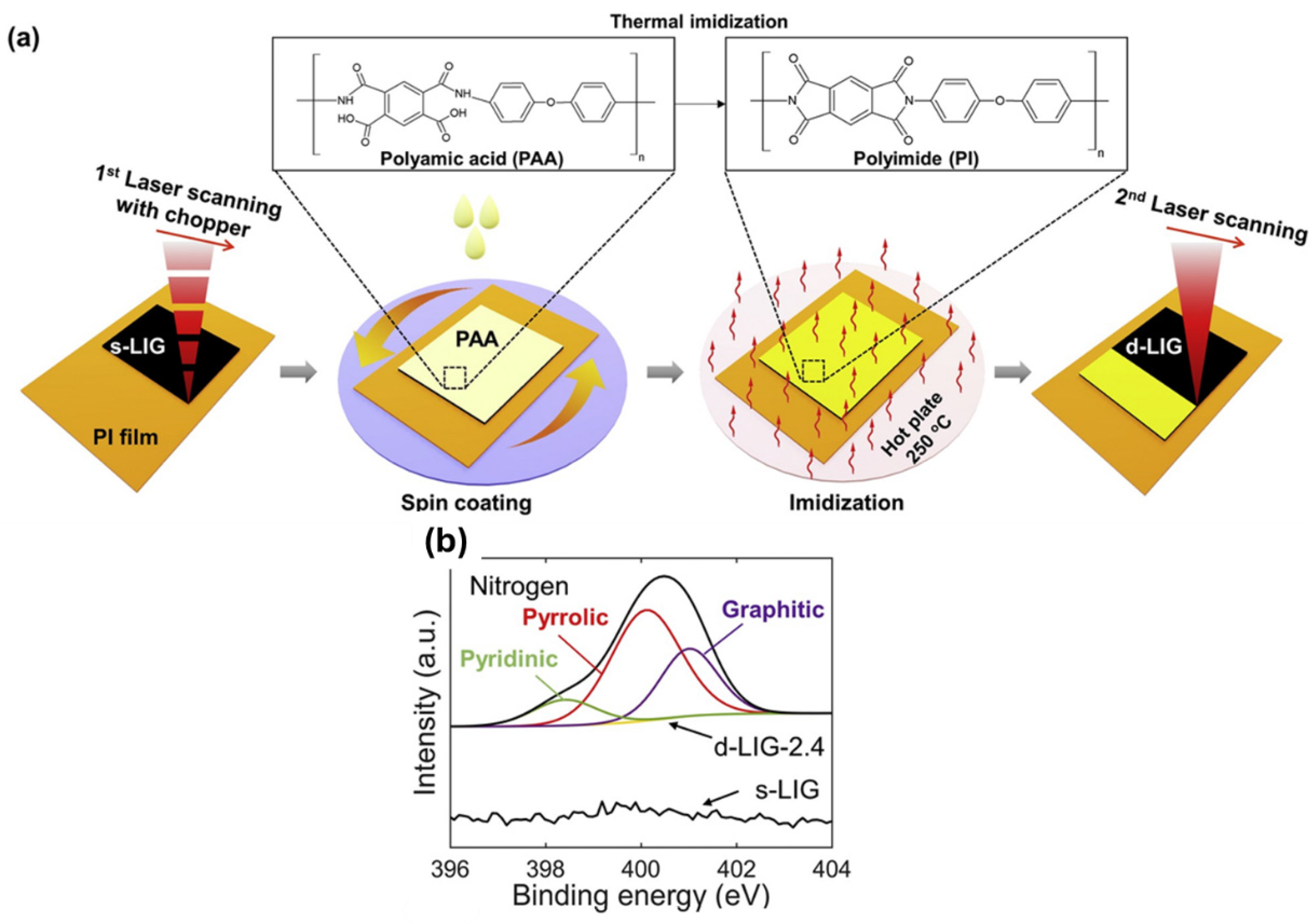
2.2.1. Aromatic Polymers Transformed into LIG Materials
2.2.2. Lignocellulose as Initial Substrates to Obtain LIG
2.3. Heteroatom Doping LIG
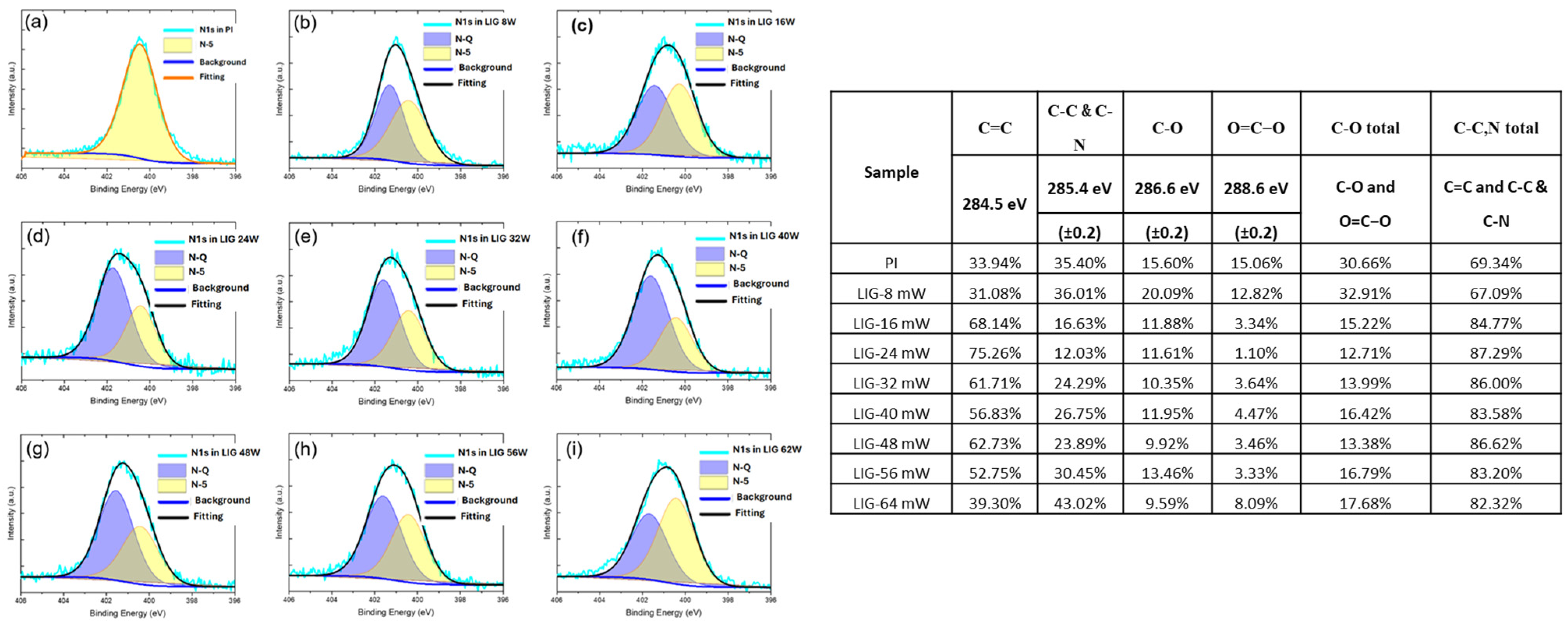
2.3.1. N-Doped LIG
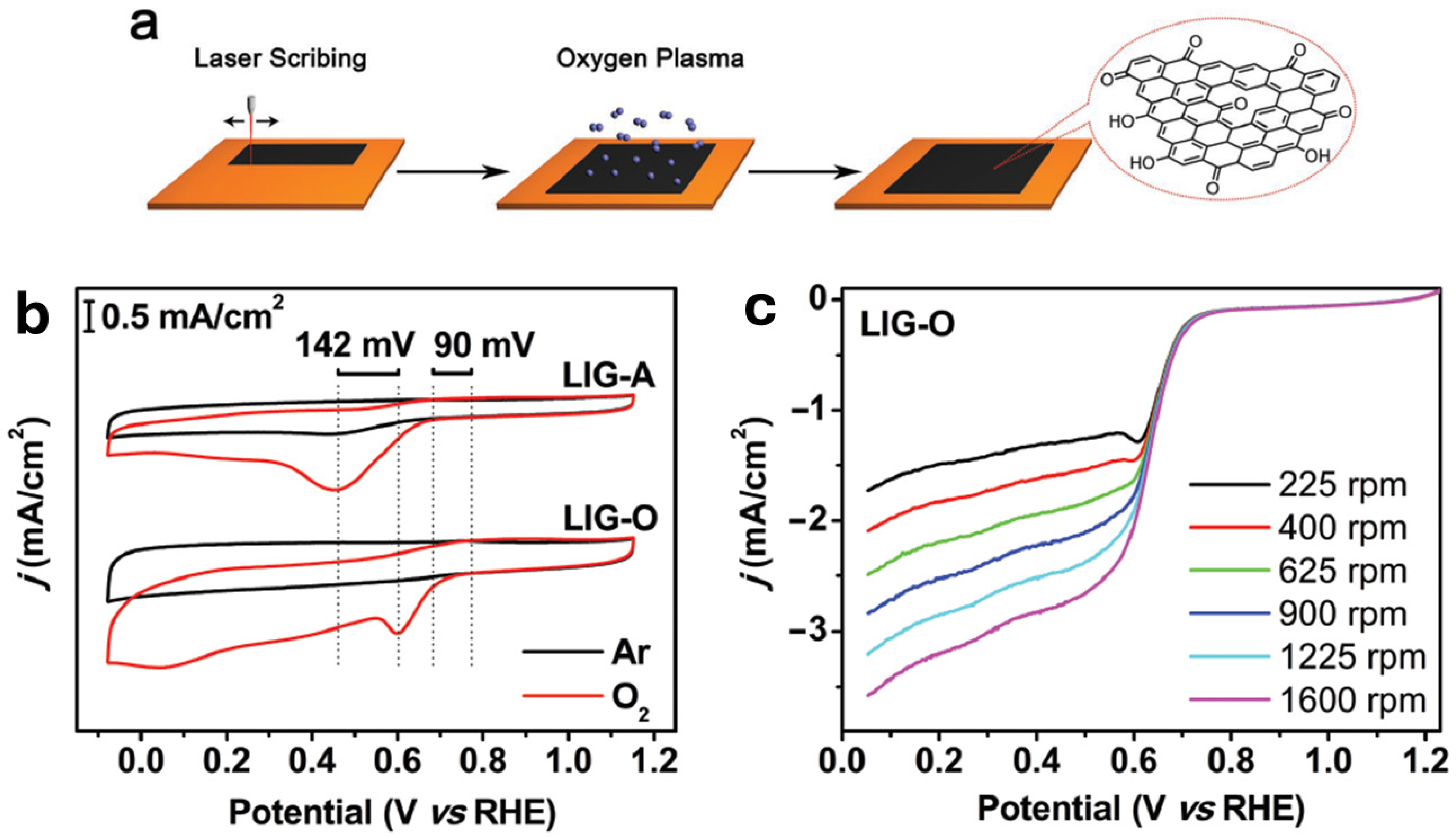
2.3.2. Oxidized LIG (LIG-O)
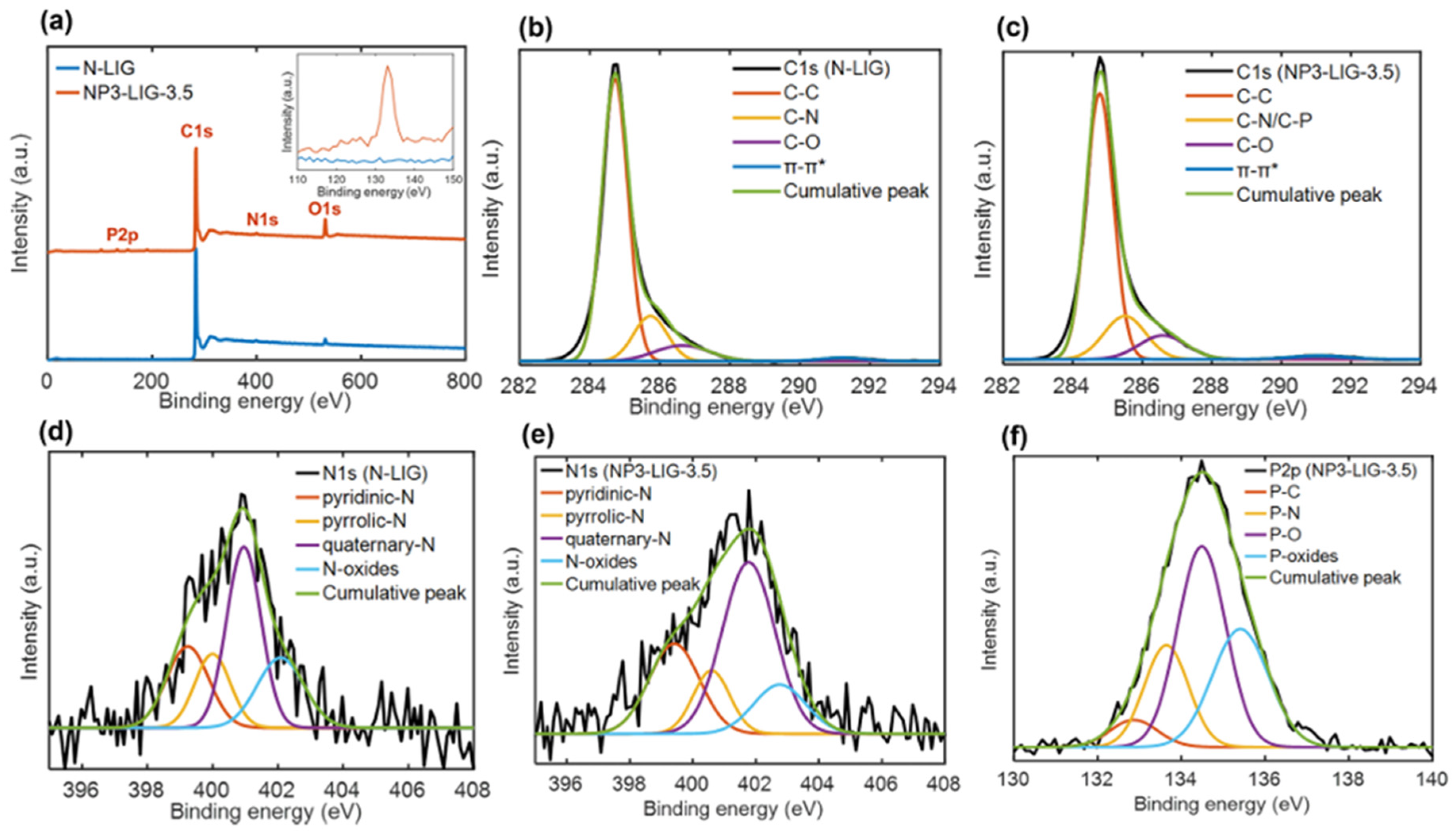
2.3.3. Co-Doped LIG
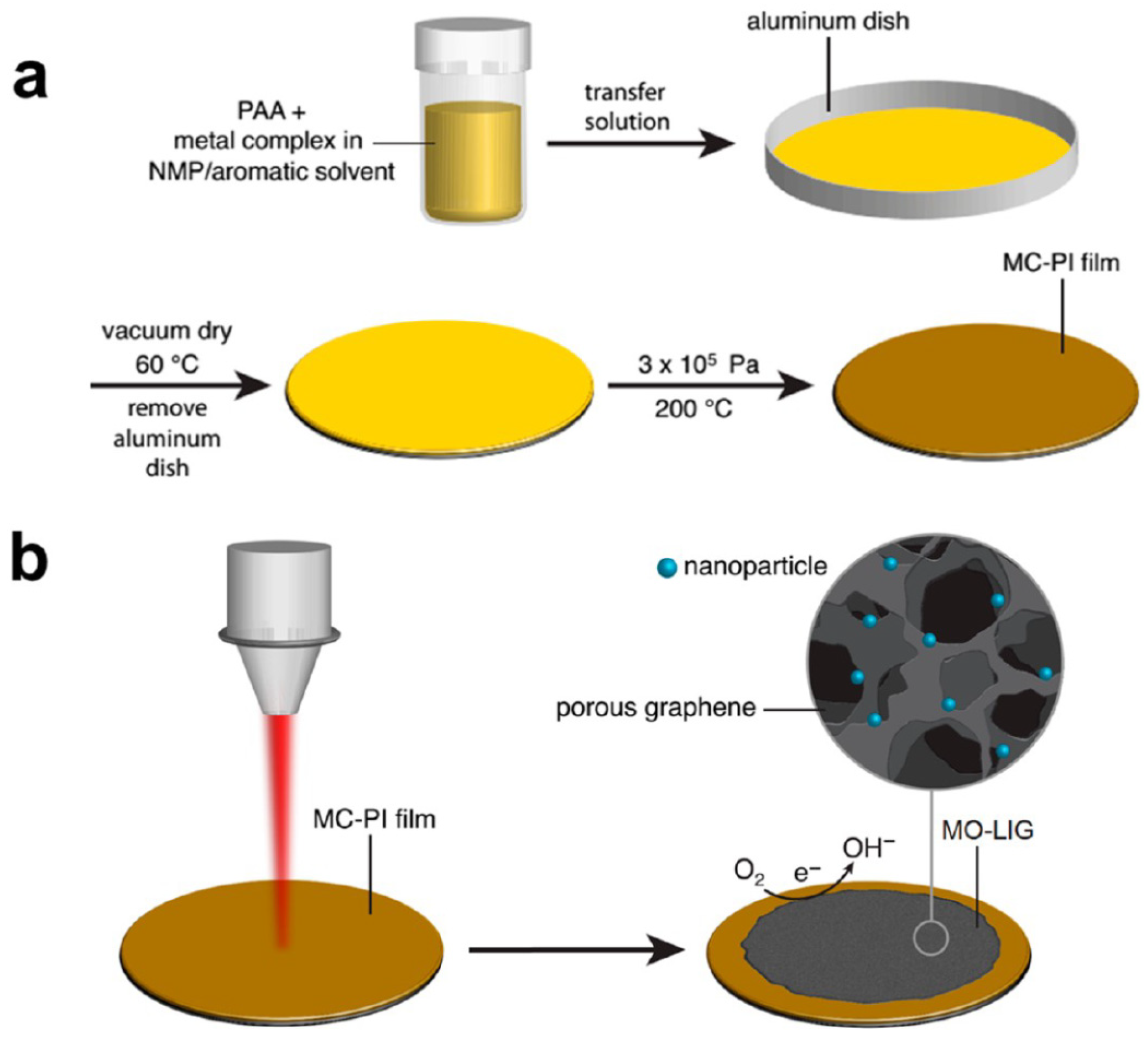
2.3.4. In Situ Formation of Metal Oxide Nanoparticles
3. Application of LIG-Based Electrocatalysts for Renewable Energy Sources
3.1. Renewable Energy Storage and Conversion Systems
3.2. LIG as Sustainable Catalysts for the Oxygen Reduction Reaction in Renewable Energy Devices
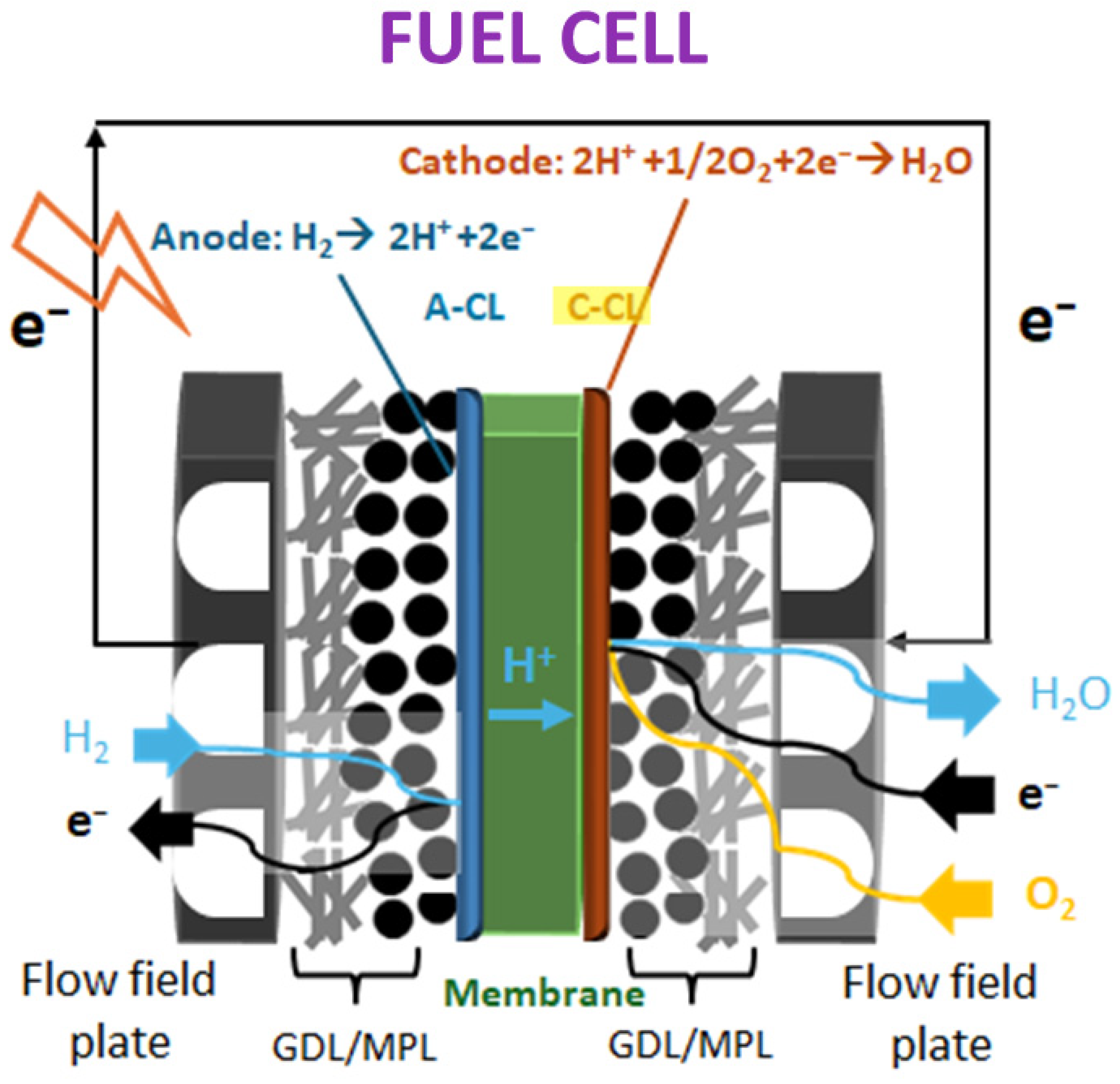
3.3. LIG for Proton Exchange Membrane Fuel Cells (PEMFCs)
3.4. LIG for Microbial Fuel Cells and Enzymatic Biofuel Cells
3.5. LIG Materials as Positive Cathode for Energy Storage Systems
Metal–Air Batteries
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Plane. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yin, J.; Meng, J.; Liu, Y.; Liao, M.; Wu, T.; Dresselhaus, M.; Xie, Y.; Wu, J.; Lu, C.; et al. Supermolecule Cucurbituril Subnanoporous Carbon Supercapacitor (SCSCS). Nano Lett. 2021, 21, 2156–2164. [Google Scholar] [CrossRef]
- Jin, X.; Song, L.; Yang, H.; Dai, C.; Xiao, Y.; Zhang, X.; Han, Y.; Bai, C.; Lu, B.; Liu, Q.; et al. Stretchable supercapacitor at −30 °C. Energy Environ. Sci. 2021, 14, 3075–3085. [Google Scholar] [CrossRef]
- Xu, T.; Li, Z.; Wang, D.; Zhang, M.; Ai, L.; Chen, Z.; Zhang, J.; Zhang, X.; Shen, L. A Fast Proton-Induced Pseudocapacitive Supercapacitor with High Energy and Power Density. Adv. Funct. Mater. 2022, 32, 2107720. [Google Scholar] [CrossRef]
- Lim, T.S.; Ock, I.W.; Lee, J.; Jo, S.G.; Jung, Y.W.; Kwon, S.-H.; Song, T.; Park, W.I.; Lee, J.W. Freestanding vanadium nitride nanowire/nitrogen-doped graphene paper with hierarchical pore structure for asymmetric supercapacitor anode. J. Alloys Compd. 2023, 934, 167858. [Google Scholar] [CrossRef]
- Diao, F.; Huang, W.; Ctistis, G.; Wackerbarth, H.; Yang, Y.; Si, P.; Zhang, J.; Xiao, X.; Engelbrekt, C. Bifunctional and Self-Supported NiFeP-Layer-Coated NiP Rods for Electrochemical Water Splitting in Alkaline Solution. ACS Appl. Mater. Interfaces 2021, 13, 23702–23713. [Google Scholar] [CrossRef]
- Jo, S.G.; Kim, C.S.; Kim, S.J.; Lee, J.W. Phase-Controlled NiO Nanoparticles on Reduced Graphene Oxide as Electrocatalysts for Overall Water Splitting. Nanomaterials 2021, 11, 3379. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.-Y.; Chen, X.-N.; Huang, M.-J.; Cai, S.-H.; Han, J.-Y.; Li, J.-S. Self-supported bimetallic phosphides with artificial heterointerfaces for enhanced electrochemical water splitting. Appl. Catal. B 2022, 304, 120914. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T.; et al. Electron Redistributed S-Doped Nickel Iron Phosphides Derived from One-Step Phosphatization of MOFs for Significantly Boosting Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Winter, M. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 2021, 371, 46. [Google Scholar]
- Pei, Z.; Ding, L.; Wang, C.; Meng, Q.; Yuan, Z.; Zhou, Z.; Zhao, S.; Chen, Y. Make it stereoscopic: Interfacial design for full-temperature adaptive flexible zinc–air batteries. Energy Environ. Sci. 2021, 14, 4926–4935. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, X.; Liu, S.; Han, Y.; Meng, H.; Jiang, Q.; Zhao, S.; Wei, F.; Sun, J.; Tan, T.; et al. Superdurable Bifunctional Oxygen Electrocatalyst for High-Performance Zinc–Air Batteries. J. Am. Chem. Soc. 2022, 144, 2694–2704. [Google Scholar] [CrossRef]
- Bello, I.T.; Jalaoso, L.A.; Ahmed, R.A.; Bello, A. Electrochemical energy conversion and Storage Systems: A perspective on the challenges and opportunities for sustainable energy in Africa. Energy Rev. 2025, 4, 10019. [Google Scholar] [CrossRef]
- Available online: https://joint-research-centre.ec.europa.eu/scientific-activities-z/hydrogen-electrolysers-and-fuel-cells-decarbonised-and-sustainable-europe-0_en (accessed on 19 May 2025).
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Balandin, A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Ge, B.; Zheng, F.; Zhang, N.; Zuo, M.; Yang, Y.; Chen, Q. Constructing Graphitic-Nitrogen-Bonded Pentagons in Interlayer-Expanded Graphene Matrix toward Carbon-Based Electrocatalysts for Acidic Oxygen Reduction Reaction. Adv. Mater. 2021, 33, 2103133. [Google Scholar] [CrossRef]
- Wu, S.; Deng, D.; Zhang, E.; Li, H.; Xu, L. CoN nanoparticles anchored on ultra-thin N-doped graphene as the oxygen reduction electrocatalyst for highly stable zinc-air batteries. Carbon 2022, 196, 347–353. [Google Scholar] [CrossRef]
- Wang, N.; Ning, S.; Yu, X.; Chen, D.; Li, Z.; Xu, J.; Meng, H.; Zhao, D.; Li, L.; Liu, Q.; et al. Graphene composites with Ru-RuO2 heterostructures: Highly efficient Mott–Schottky-type electrocatalysts for pH-universal water splitting and flexible zinc–air batteries. Appl. Catal. B 2022, 302, 120838. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Kang, H.W.; Nahm, S.; Kim, B.H.; Kim, H.; Han, S.H. Flexible electrochromic and thermochromic hybrid smart window based on a highly durable ITO/graphene transparent electrode. Chem. Eng. J. 2021, 416, 129028. [Google Scholar] [CrossRef]
- Kang, J.H.; Choi, S.; Park, Y.J.; Park, J.S.; Cho, N.S.; Cho, S.; Walker, B.; Choi, D.S.; Shin, J.-W.; Seo, J.H. Cu/graphene hybrid transparent conducting electrodes for organic photovoltaic devices. Carbon 2021, 171, 341–349. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, R.; Zheng, J.; Khan, A.I.; Neilson, K.M.; Geiger, S.J.; Callahan, D.M.; Moebius, M.G.; Saxena, A.; Chen, M.E.; et al. Ultra-low-energy programmable non-volatile silicon photonics based on phase-change materials with graphene heaters. Nat. Nanotechnol. 2022, 17, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortadella, R.; Schwesig, G.; Jeschke, C.; Illa, X.; Gray, A.L.; Savage, S.; Stamatidou, E.; Schiessl, I.; Masvidal-Codina, E.; Kostarelos, K.; et al. Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat. Commun. 2021, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, D.; Zhou, M.; Zheng, Y.; Wang, T.; Lu, C.; Zhang, L.; Liu, H.; Liu, C. Wearable Strain Sensors Based on a Porous Polydimethylsiloxane Hybrid with Carbon Nanotubes and Graphene. ACS Appl. Mater. Interfaces 2021, 13, 15572–15583. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Chen, Y.; Nie, Z. Research Progress on the Preparation and Applications of Laser-Induced Graphene Technology. Nanomaterials 2022, 12, 2336. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Hasheminia, F.; Sadeghzadeh, S. Laser-induced graphene in energy storage-batteries. Nano-Struct. Nano-Objects 2024, 40, 101347. [Google Scholar] [CrossRef]
- Bressi, A.C.; Dallinger, A.; Steksova, Y.; Greco, F. Bioderived Laser-Induced Graphene for Sensors and Supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 35788–35814. [Google Scholar] [CrossRef]
- Asgar, M.A.; Van Tran, C.; Kim, J.; Jin, C.; Lee, S.; Kim, Y.K.; Lu, X.; In, J.B.; Kim, S.-M. Fabrication of Laser-Induced Graphene on Carbon Electrodes for Efficient Hydrogen Evolution Reaction. Int. J. Energy Res. 2024, 2024, 5541306. [Google Scholar] [CrossRef]
- Tiliakos, A.; Trefilov, A.M.I.; Tanasă, E.; Balan, A.; Stamatin, I. Laser-induced graphene as the microporous layer in proton exchange membrane fuel cells. Appl. Surf. Sci. 2020, 504, 144096. [Google Scholar] [CrossRef]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W.; et al. Recent Advances in Laser-Induced Graphene: Mechanism, Fabrication, Properties, and Applications in Flexible Electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Park, M.; Gu, Y.; Mao, X.; Zorba, V. Mechanisms of ultrafast GHz burst fs laser ablation. Sci. Adv. 2023, 9, eadf6397. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.A.; Eikabbash, M.; Zhang, J.; Garcell, E.M.; Singh, S.; Guo, C. Spectral absorption control of femtosecond laser-treated metals and application in solar-thermal devices. Light Sci. Appl. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, J.; Kim, J.C.; Kim, Y.G.; Hyeon, D.Y.; Hong, K.S.; Suh, J.; Shinm, D.; Jeong, H.Y.; Park, K.I. Piezoelectricity of picosecond laser-synthesized perovskite BaTiO3 nanoparticles. Appl. Surf. Sci. 2020, 11, 145614. [Google Scholar] [CrossRef]
- Spechler, J.A.; Nagamatsu, K.A.; Sturm, J.C.; Arnold, C.B. Improved efficiency of hybrid organic photovoltaics by pulsed laser sintering of silver nanowire network transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 10556–10562. [Google Scholar] [CrossRef]
- Paeng, D.; Yoo, J.-H.; Yeo, J.; Lee, D.; Kim, E.; Ko, S.H.; Grigoropoulos, C.P. Low-Cost Facile Fabrication of Flexible Transparent Copper Electrodes by Nanosecond Laser Ablation. Adv. Mater. 2015, 27, 2762–2767. [Google Scholar] [CrossRef]
- Ko, S.H.; Chung, J.; Pan, H.; Grigoropoulos, C.P.; Poulikakos, D. Fabrication of multilayer passive and active electric components on polymer using inkjet printing and low temperature laser processing. Sens. Actuators A Phys. 2007, 134, 161–168. [Google Scholar] [CrossRef]
- Long, J.; Eliceiri, M.H.; Wang, L.; Vangelatos, Z.; Ouyang, Y.; Xie, X.; Zhang, Y.; Grigoropoulos, C.P. Capturing the final stage of the collapse of cavitation bubbles generated during nanosecond laser ablation of submerged targets. Opt. Laser Technol. 2021, 134, 106647. [Google Scholar] [CrossRef]
- Grigoropoulos, C.; Rogers, M.; Ko, S.H.; Golovin, A.A.; Matkowsky, B.J. Explosive crystallization in the presence of melting. Phys. Rev. B 2006, 73, 184125. [Google Scholar] [CrossRef]
- Park, K.-I.; Son, J.H.; Hwang, G.-T.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef]
- He, D.; Jin, J.; Yuan, Z. Effect of ArF excimer laser annealing on morphology and surface plasmon resonance properties of Ag nanoparticles. Appl. Phys. A 2019, 125, 423. [Google Scholar] [CrossRef]
- Angmo, D.; Larsen-Olsen, T.T.; Jørgensen, M.; Søndergaard, R.R.; Krebs, F.C. Roll-to-Roll Inkjet Printing and Photonic Sintering of Electrodes for ITO Free Polymer Solar Cell Modules and Facile Product Integration. Adv. Energy Mater. 2012, 3, 172–175. [Google Scholar] [CrossRef]
- Seok, J.Y.; Kim, S.; Yang, I.; Park, J.H.; Lee, J.; Kwon, S.; Woo, K. Strategically Controlled Flash Irradiation on Silicon Anode for Enhancing Cycling Stability and Rate Capability toward High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 15205–15215. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Park, J.H.; Jang, D.; Shin, J.H.; Im, T.H.; Lee, J.H.; Hong, S.K.; Wang, H.S.; Kwak, M.S.; Peddigari, M.; et al. Optogenetic brain neuromodulation by stray magnetic field via flash-enhanced magneto-mechano-triboelectric nanogenerator. NanoEnergy 2020, 75, 104951. [Google Scholar] [CrossRef]
- Okoroanyanwu, U.; Watkins, A.B.J. Large Area Millisecond Preparation of High-Quality, Few-Layer Graphene Films on Arbitrary Substrates via Xenon Flash Lamp Photothermal Pyrolysis and Their Application for High-Performance Micro-supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 13495–13507. [Google Scholar] [CrossRef]
- Park, J.H.; Pattipaka, S.; Hwang, G.-T.; Park, M.; Woo, Y.M.; Kim, Y.B.; Lee, H.E.; Jeong, C.K.; Zhang, T.; Min, Y.; et al. Light–Material Interactions Using Laser and Flash Sources for Energy Conversion and Storage Application. Nano-Micro Lett. 2024, 16, 276. [Google Scholar] [CrossRef]
- Gilje, S.; Dubin, S.; Badakhshan, A.; Farrar, J.; Danczyk, S.A.; Kaner, R.B. Photothermal Deoxygenation of Graphene Oxide for Patterning and Distributed Ignition Applications. Adv. Mater. 2010, 22, 419–423. [Google Scholar] [CrossRef]
- Jeon, T.; Jin, H.M.; Lee, S.H.; Lee, J.M.; Park, H.I.; Kim, M.K.; Lee, K.J.; Shin, B.; Kim, S.O. Laser Crystallization of Organic–Inorganic Hybrid Perovskite Solar Cells. ACS Nano 2016, 10, 7907–7914. [Google Scholar] [CrossRef]
- Back, S.; Park, J.H.; Kang, B. Microsupercapacitive Stone Module for Natural Energy Storage. ACS Nano 2022, 16, 11708–11719. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Grigoropoulos, C.P.; King, T.-J. High-performance thin-silicon-film transistors fabricated by double laser crystallization. J. Appl. Phys. 2006, 99, 034508. [Google Scholar] [CrossRef]
- Yuan, G.; Wan, T.; BaQais, Z.; Mu, Y.; Cui, D.; Amin, M.A.; Li, X.; Xu, B.B.; Zhu, X.; Algadi, H.; et al. Boron and fluorine Co-doped laser-induced graphene towards high-performance micro-supercapacitors. Carbon 2023, 212, 118101. [Google Scholar] [CrossRef]
- Gu, M.K.G.; Jeong, H.; Song, E.; Jeon, J.W.; Huh, K.-M.; Kang, P.; Kim, S.-K.; Kim, B.G. Laser Scribing of Fluorinated Polyimide Films to Generate Microporous Structures for High-Performance Micro-supercapacitor Electrodes. ACS Appl. Energy Mater. 2021, 4, 208–214. [Google Scholar]
- Le, T.D.; Park, S.; An, J.; Lee, P.S.; Kim, Y.-J. Ultrafast Laser Pulses Enable One-Step Graphene Patterning on Woods and Leaves for Green Electronics. Adv. Funct. Mater. 2019, 29, 1902771. [Google Scholar] [CrossRef]
- Nam, H.K.; Choi, J.; Jing, T.; Yang, D.; Lee, Y.; Kim, Y.R.; Le, T.S.D.; Kim, B.; Yu, L.; Kim, S.W.; et al. Laser-induced graphene formation on recycled woods forgreen smart furniture. EcoMat 2024, 6, 12447. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Bakhtiyari, A.N.; Zheng, H. A comparative study of laser induced graphene by CO2 infrared laser and 355 nm ultraviolet (UV) laser. Micromachines 2020, 11, 1094. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Zhou, S.; Shi, D.; Huang, Y.; Chen, X.; Gao, J.; Zhao, N.; Wong, C.P. UV laser-induced polyimide-to-graphene conversion: Modeling, fabrication, and application. Small Methods 2019, 3, 1900208. [Google Scholar] [CrossRef]
- Gaudin, J.; Peyrusse, O.; Chalupský, J.; Toufarová, M.; Vyšín, L.; Hájková, V.; Sobierajski, R.; Burian, T.; Dastjani-Farahani, S.; Graf, A.; et al. Amorphous to crystalline phase transition in carbon induced by intense femtosecond x-ray free-electron laser pulses. Phys. Rev. B 2012, 86, 024103. [Google Scholar] [CrossRef]
- Gosh, A.; Kaur, S.; Verma, G.; Dolle, C.; Azmi, R.; Heissler, S.; Eggeler, Y.M.; Mondal, K.; Mager, D.; Gupta, A.; et al. Enhanced Performance of Laser-Induced Graphene Supercapacitors via Integration with Candle-Soot Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 40313–40325. [Google Scholar] [CrossRef]
- Dong, Y.; Rismiller, S.C.; Lin, J. Molecular dynamic simulation of layered graphene clusters formation from polyimides under extreme conditions. Carbon 2016, 104, 47–55. [Google Scholar] [CrossRef]
- Avinash, K.; Patolsky, F. Laser-induced graphene structures: From synthesis and applications to future prospects. Mater. Today 2023, 70, 104–136. [Google Scholar] [CrossRef]
- Haider, R. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, T.; Rosa, A.; Ornelas, C.; Coelho, J.; Fortunato, E.; Marques, A.C.; Martin, R. Influence of CO2 laser beam modelling on electronic and electrochemical properties of paper-based laser-induced graphene for disposable pH electrochemical sensors. Carbon Trends 2023, 11, 100271. [Google Scholar] [CrossRef]
- Vashisth, A.; Kowalik, M.; Gerringer, J.C.; Ashraf, C.; van Duin, A.C.T.; Green, M.J. ReaxFF Simulations of Laser-Induced Graphene (LIG) Formation for Multifunctional Polymer Nanocomposites. ACS Appl. Nano Mater. 2020, 3, 1881. [Google Scholar] [CrossRef]
- Ye, R.; Chyan, Y.; Zhang, J.; Han, X.; Kittrel, C.; Tour, J.M.l. Laser-induced graphene formation on wood. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Fu, Z.; Gao, H.; Shao, Y.; Li, X.; Yan, X. Laser-Induced Porous Graphene-Based Materials and Its Potential Application in Lithium–Ion Batterie. ACS Omega 2025, 10, 11241–11249. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible light laser-induced graphene from phenolic resin: A new approach for directly writing graphene-based electrochemical devices on various substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Lei, Y.; Alshareef, A.H.; Zhao, W.; Inal, S. Laser-Scribed Graphene Electrodes Derived from Lignin for Biochemical Sensing. ACS Appl. Nano Mater. 2020, 3, 1166–1174. [Google Scholar] [CrossRef]
- Massaglia, G.; Verpoorten, E.; Pirri, C.F.; Quaglio, M. Laser Writing in Ambient Conditions for Carbonization of Synthetic Polymers, Including Nanostructured Polymers Treated in Alkaline Media. WO 2023/242701, 21 December 2023. [Google Scholar]
- Wang, H.; Wang, H.; Wang, Y.; Su, X.; Wang, C.; Zhang, M.; Jian, M.; Xia, K.; Liang, X.; Lu, H.; et al. Laser Writing of Janus Graphene/Kevlar Textile for Intelligent Protective Clothing. ACS Nano 2020, 14, 3219–3226. [Google Scholar] [CrossRef]
- Lamberti, A.; Serrapede, M.; Ferraro, G.; Fontana, M.; Perrucci, F.; Bianco, S.; Chiolerio, A.; Bocchini, S. All-SPEEK flexible supercapacitor exploiting laser-induced graphenization. 2D Mater. 2017, 4, 035012. [Google Scholar] [CrossRef]
- Samouco, A.; Marques, A.C.; Pimentel, A.; Martins, R.; Fortunato, E. Laser-induced electrodes towards low-cost flexible UV ZnO sensors. Flex. Print. Electron. 2018, 3, 044002. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-Doped Laser-Induced Porous Graphene Derived from Polysulfone-Class Polymers and Membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hong, Q.; Li, H.; Liu, C.; Feng, L. Direct-Laser-Writing of Metal Sulfide-Graphene Nanocomposite Photoelectrode toward Sensitive Photoelectrochemical Sensing. Adv. Funct. Mater. 2019, 29, 190400. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.G.; Li, J.; Tour, J.M. In situ formation of Metal Oxide Nanocrystals Embedded n Laser Induced Graphene. ACS Nano 2015, 9, 9244–9251. [Google Scholar] [CrossRef]
- Gutru, R.; Turtayev, Z.; Xu, F.; Maranzana, G.; Thimmappa, R.; Mamlouk, M.; Desforges, A.; Vigolo, B. Recent progress in heteroatom doped carbon based electrocatalysts for oxygen reduction reaction in anion exchange membrane fuel cells. Int. J. Hydrogen Enrergy 2023, 48, 3593–3631. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Wang, L.; Li, Y.; Yakobson, B.I.; Tou, J.M. Oxidized Laser-Induced Graphene for Efficient Oxygen Electrocatalysis. Adv. Adv. Mater. 2018, 30, 1707319. [Google Scholar] [CrossRef]
- Song, W.; Zhu, J.; Gan, B.; Zhao, S.; Wang, H.; Li, C.; Wang, J. Flexible, Stretchable, and Transparent Planar Microsupercapacitors Based on 3D Porous Laser-Induced Graphene. Small 2017, 14, 1702249. [Google Scholar] [CrossRef]
- Khandelwal, M.; Van Tran, C.; In, J.B. Nitrogen and phosphorous Co-Doped Laser-Induced Graphene: A High-Performance electrode material for supercapacitor applications. Appl. Surf. Sci. 2022, 576, 151714. [Google Scholar] [CrossRef]
- Kim, K.Y.; Choi, H.; Van Tran, C.; In, J.B. Simultaneous densification and nitrogen doping of laser-induced graphene by duplicated pyrolysis for supercapacitor applications. J. Power Sources 2019, 441, 227199. [Google Scholar] [CrossRef]
- Wan, Z.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.-T.; Trinchi, A.; Shiddiky, M.J.A.; Gao, Y.; Li, Q. Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhou, C.; Zhou, K.; Yu, J.; Su, Y.; Hu, N.; Zhang, Y. Laser-Induced MoOx/Sulfur-Doped Graphene Hybrid Frameworks as Efficient Antibacterial Agents. Langmuir 2021, 37, 1596–1604. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible Boron-Doped Laser-Induced Graphene Microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G.L. Flow Batteries: Current Status and Trends. Chem. Rev. 2015, 115, 11533. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Uchaker, E.; Candelaria, S.L.; Cao, G.Z. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127. [Google Scholar] [CrossRef]
- Luo, B.; Ye, D.; Wang, L. Recent Progress on Integrated Energy Conversion and Storage Systems. Adv. Sci. 2017, 4, 1700104. [Google Scholar] [CrossRef] [PubMed]
- Niakolas, D.K.; Daletou, M.; Neophytides, S.G. Fuel cells are a commercially viable alternative for the production of “clean” energy. Ambio 2016, 45, 32–37. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallon, E.; Cazorla-Amoros, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuelcells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Macchi, S.; Denmark, I.; Le, T.; Forson, M.; Bashiru, M.; Jalihal, A.; Siraj, N. Recent Advancements in the Synthesis and Application of Carbon-Based Catalysts in the ORR. Electrochem 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Tang, J.; Chen, D.; Yao, Q.; Xie, J.; Yang, J. Recent advances in noble metal-based nanocomposites for electrochemical reactions. Mater. Today Energy 2017, 6, 115–127. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of metal catalysts for oxygen reduction reaction: From nanoscale engineering to atomic design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Zhong, H.; Campos-Rold, C.A.; Zhao, Y.; Zhang, S.; Feng, Y.; Alonso-Vante, N. Recent advances of cobalt-based electrocatalysts for oxygen electrode reactions and hydrogen evolution reaction. Catalysts 2018, 8, 559. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Liang, Z.; Yin, Y.; Mei, B.; Song, F.; Sun, F.; Gu, S.; Jiang, Z.; Wu, Y.; et al. Role of local coordination in bimetallic sites for oxygen reduction: A theoretical analysis. J. Energy Chem. 2020, 44, 131–137. [Google Scholar] [CrossRef]
- Li, T.; Deng, H.; Liu, J.; Jin, C.; Song, Y.; Wang, F. First-row transition metals and nitrogen co-doped carbon nanotubes: The exact origin of the enhanced activity for oxygen reduction reaction. Carbon 2019, 143, 859–868. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, G.; He, C.; Wang, T.; Yang, L.; Yang, Z.; Ma, D. Novel catalytic activity for oxygen reduction reaction on MnN4 embedded graphene: A dispersion corrected density functional theory study. Carbon 2014, 84, 500–508. [Google Scholar] [CrossRef]
- Passaponti, M.; Rosi, L.; Savastano, M.; Giurlani, W.; Miller, H.A.; Lavacchi, A.; Filippi, J.; Zangari, G.; Vizza, F.; Innocenti, M. Recycling of waste automobile tires: Transforming char into oxygen reduction reaction catalysts for alkaline fuel cells. J. Power Sources 2019, 427, 85–90. [Google Scholar] [CrossRef]
- Veksha, A.; Latiff, N.M.; Chen, W.; Ng, J.E.; Lisak, G. Heteroatom doped carbon nanosheets from waste tires as electrode materials for electrocatalytic oxygen reduction reaction: Effect of synthesis techniques on properties and activity. Carbon 2020, 167, 104–113. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Wu, Q.M.; Deng, D.K.; He, Y.L.; Zhou, Z.C.; Sang, S.B.; Zhou, Z.H. Fe/N-doped mesoporous carbons derived from soybeans: A highly efficient and low-cost non-precious metal catalyst for ORR. J. Cent. South Univ. 2020, 27, 344–355. [Google Scholar] [CrossRef]
- Liu, Y.; Su, M.; Li, D.; Li, S.; Li, X.; Zhao, J.; Liu, F. Soybean straw biomass-derived Fe-N co-doped porous carbon as an efficient electrocatalyst for oxygen reduction in both alkaline and acidic media. RSC Adv. 2020, 10, 6763–6771. [Google Scholar] [CrossRef]
- Gao, J.; Chu, X.; He, C.; Yin, Z.; Lu, H.; Li, X.; Feng, J.; Tan, X. Biomass-derived carbon for ORR: Pine needles as a single source for efficient carbon electrocatalyst. J. Appl. Electrochem. 2020, 50, 1257–1267. [Google Scholar] [CrossRef]
- Gong, T.; Qi, R.; Liu, X.; Li, H.; Zhang, Y. N, F-codoped microporous carbon nanofibers as efficient metal-free electrocatalysts for ORR. Nano-Micro Lett. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sibul, R.; KibenaPõldsepp, E.; Mäeorg, U.; Merisalu, M.; Kikas, A.; Kisand, V.; Treshchalov, A.; Sammelselg, V.; Tammeveski, K. Sulphur and nitrogen co-doped graphene-based electrocatalysts for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2019, 109, 106603. [Google Scholar] [CrossRef]
- Men, B.; Sun, Y.; Li, M.; Hu, C.; Zhang, M.; Wang, L.; Tang, Y.; Chen, Y.; Wan, P.; Pan, J. Hierarchical metal-free nitrogen-doped porous graphene/carbon composites as an efficient oxygen reduction reaction catalyst. ACS Appl. Mater. Interfaces 2016, 8, 1415–1423. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B. Catalyst-Free Synthesis of Nitrogen Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Guo, M.Q.; Huang, J.Q.; Kong, X.Y.; Peng, H.J.; Shui, H.; Qian, F.Y.; Zhu, L.; Zhu, W.C.; Zhang, Q. Hydrothermal synthesis of porous phosphorus-doped carbon nanotubes and their use in the oxygen reduction reaction and lithium-sulfur batteries. New Carbon Mater. 2016, 31, 352–362. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.Z. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. Int. Ed. 2013, 52, 3110–3116. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Hsiao, Y.-J.; Jiang, J.-C. Dopamine sensing by boron and nitrogen co-doped single-walled carbon nanotubes: A first-principles study. Appl. Surf. Sci. 2019, 473, 59–64. [Google Scholar] [CrossRef]
- Magerusan, L.; Pogacean, F.; Pruneanu, S. Eco-friendly synthesis of sulphur-doped graphenes with applicability in caffeic acid electrochemical assay. Bioelectrochemistry 2022, 148, 108228. [Google Scholar] [CrossRef]
- Rao, L.T.; Dubey, S.K.; Javed, A.; Goel, S. Laser induced graphene electrodes enhanced with carbon nanotubes for membraneless microfluidic fuel cell. Sustain. Energy Technol. Assess. 2021, 45, 101176. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Wanjari, V.P.; Kanwar, B.; Singh, S.P. Laser-Induced Graphene as a Cathode Catalyst for Microbial Fuel Cell. ACS Appl. Nano Mater. 2024, 7, 2639–2649. [Google Scholar] [CrossRef]
- Rewatkar, P.; Kothuru, A.; Goel, S. PDMS-Based Microfluidic Glucose Biofuel Cell Integrated with Optimized Laser-Induced Flexible Graphene Bioelectrodes. IEEE Trans. Electron Devices 2020, 67, 1832–1838. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Rewatkar, P.; Goel, S. Miniaturized polymeric enzymatic biofuel cell with integrated microfluidic device and enhanced laser ablated bioelectrodes. Int. J. Hydrogen Energy 2021, 46, 3183–3192. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Tour, J.M. Laser-Induced Graphene Hybrid Catalysts for Rechargeable Zn-Air Batteries. ACS Appl. Energy Mater. 2019, 2, 1460–1468. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Tour, J.M. Laser-induced graphene synthesis of Co3O4 in graphene for oxygen electrocatalysis and metal-air batteries. Carbon 2018, 139, 880–887. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Fan, M.; Ajayan, P.M.; Tour, J.M. Li-Breathing Air Batteries Catalyzed by MnNiFe/Laser-Induced Graphene Catalysts. Adv. Mater. Interfaces 2019, 6, 1901035. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Zhang, C.; Stanford, M.G.; Chyan, Y.; Yao, Y.; Tour, J.M. Quasi-Solid-State Li–O2 Batteries with Laser-Induced Graphene Cathode Catalysts. ACS Appl. Energy Mater. 2020, 3, 1702–1709. [Google Scholar] [CrossRef]
- Aldhafeeri, T.R.; Uceda, M.; Singh, A.; Valappil, M.O.; Fowler, M.W.; Pope, M.A. Embedded Platinum–Cobalt Nanoalloys in Biomass-Derived Laser-Induced Graphene as Stable, Air-Breathing Cathodes for Zinc–Air Batteries. ACS Appl. Nano Mater. 2023, 6, 8302–8314. [Google Scholar] [CrossRef]



| Carbon-Based Materials as Electrochemical Active Catalyst Layers for ORR | |||||
|---|---|---|---|---|---|
| Typer of Catalyst Layers | Onset Potential | Number of Electrons | Electrolyte Solution | Reference | |
| Doped carbon electrocatalysts derived from organic waste | Efficient ORR catalysts obtained by pyrolysis implemented on waste-tire | −90 mV | 4 e− | Alkaline solution for fuel cells and metal air batteries | [105] |
| Tire waste as source for the synthesis of heteroatoms doped carbon nanosheets (CNS) | −0.217 V to −0.220 V | – | – | [106] | |
| Sewage sludge rich in N and P | −0.04 V | 4 e− | Alkaline solution | [107] | |
| Fe-modified mesoporous N-doped carbons | 0.890 V | – | – | [108] | |
| N-doped carbon based catalysts derived from soybeans | Honeycomb-like Fe–N co-doped porous carbon material | 0.886 V in acidic conditions; 0.989 V in alkaline solutions | – | Both alkaline and acidic solutions | [109] |
| Doped and/or Co-doped carbon based electrocatalysts | P-doped porous carbon | 0.96 V | – | – | [110] |
| N and F co-doped carbon nanofibers | 0.94 V | 4 e− | – | [111] | |
| Triazine polymers as self-doping N, F, and P carbon based catalysts | 0.93 V | – | Both alkaline and acidic solutions | [112] | |
| Sulphur and nitrogen co-doped graphene | from −0.11 V to −0.13 V | – | Alkaline solution | [113] | |
| N-doped porous graphene with a certain amount of carbon, obtained starting from pyrolysis implemented on glucose | 0.91 V vs. RHE | Close to 4 e− | [114] | ||
| N-doped graphene obtained by implementing a thermal annealing treatment obtained from graphene oxide mixed with melamine | −0.1 V vs. RHE | Equal to 3.4 e−–3.6 e− | Alkaline solution: 0.1 M KOH | [115] | |
| Phosphorous-doped graphite | 0.1 V vs. RHE | Close to 3 e− | Alkaline solution: 0.1 M KOH | [116] | |
| Co-doping of P and N carbon materials | 0.94 V vs. RHE | Close to 4 e− | Alkaline solution: 0.1 M KOH | [117] | |
| Co-doping of Born and Nitrogen graphene N-co-doped carbon nanotubes | The onset potential achieved is similar to the one obtained by Pt/C catalyst layer | Equal to 3.97 e− | Alkaline solution: 0.1 M KOH | [118] | |
| Sulfur (S)-doped graphene | close to −0.15 V vs. RHE | equal to 3.81 e− | Alkaline solution: 0.1 M KOH | [119] | |
| Co-doped P-N sites in carbon nanotube and graphene foam | 0.91 V close to 20 wt% of Pt | close to 4 e− | Acidic media | [120] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaglia, G.; Quaglio, M. A Review on Laser-Induced Graphene-Based Electrocatalysts for the Oxygen Reduction Reaction in Electrochemical Energy Storage and Conversion. Nanomaterials 2025, 15, 1070. https://doi.org/10.3390/nano15141070
Massaglia G, Quaglio M. A Review on Laser-Induced Graphene-Based Electrocatalysts for the Oxygen Reduction Reaction in Electrochemical Energy Storage and Conversion. Nanomaterials. 2025; 15(14):1070. https://doi.org/10.3390/nano15141070
Chicago/Turabian StyleMassaglia, Giulia, and Marzia Quaglio. 2025. "A Review on Laser-Induced Graphene-Based Electrocatalysts for the Oxygen Reduction Reaction in Electrochemical Energy Storage and Conversion" Nanomaterials 15, no. 14: 1070. https://doi.org/10.3390/nano15141070
APA StyleMassaglia, G., & Quaglio, M. (2025). A Review on Laser-Induced Graphene-Based Electrocatalysts for the Oxygen Reduction Reaction in Electrochemical Energy Storage and Conversion. Nanomaterials, 15(14), 1070. https://doi.org/10.3390/nano15141070






