Interface Engineering of NCMA Cathodes with LATP Coatings for High-Performance Solid-State Lithium Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
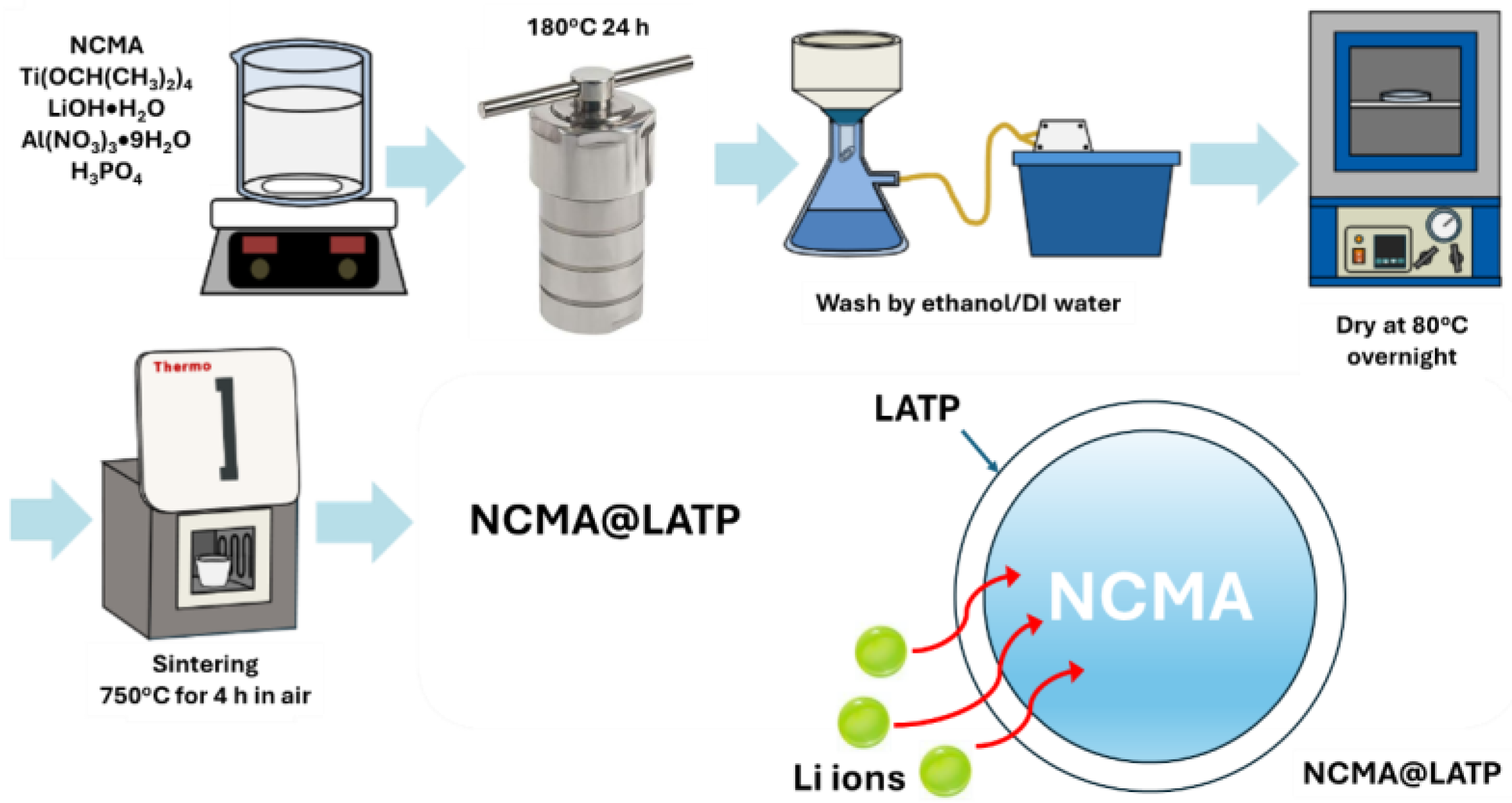
2.2. Synthesis of NCMA83@LATP-Coated Cathodes
2.3. Material Characterizations
2.4. Electrochemical Characterization
2.5. Cell Assembly for Lithium-Ion Battery Testing
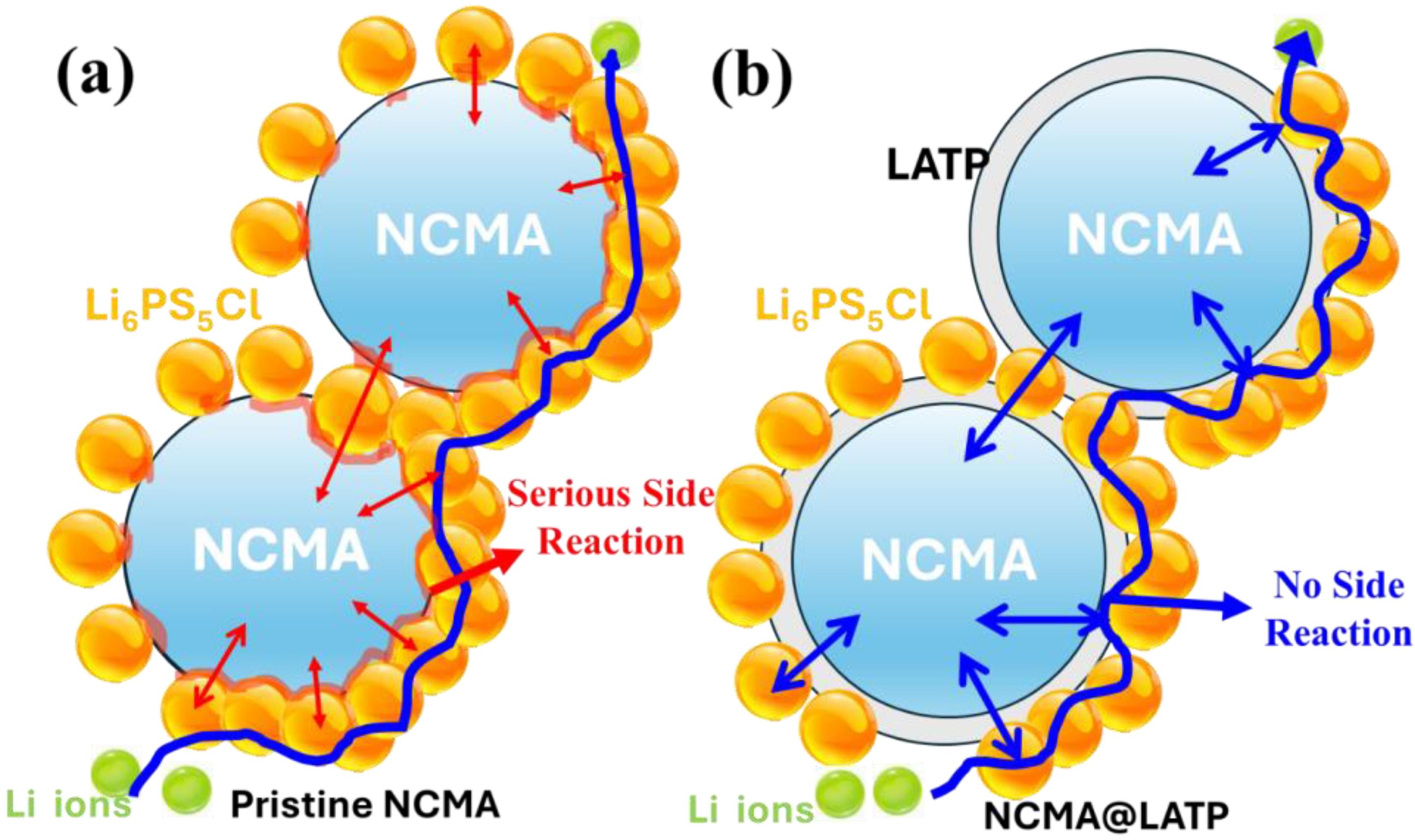
3. Results and Discussion
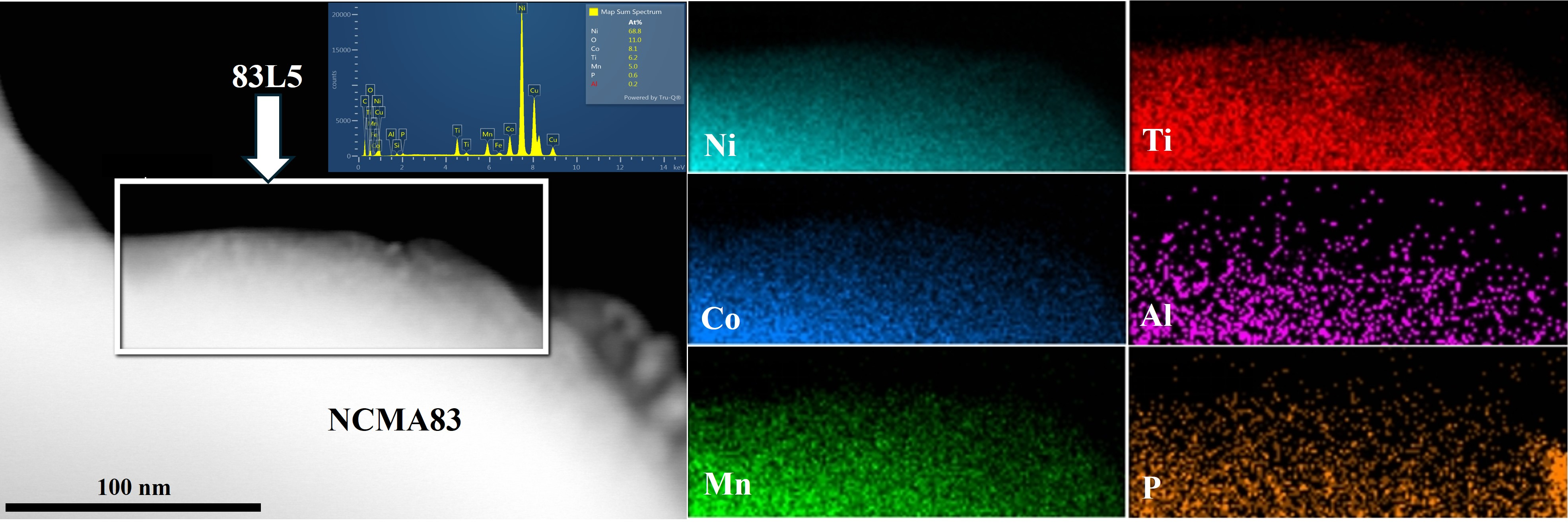
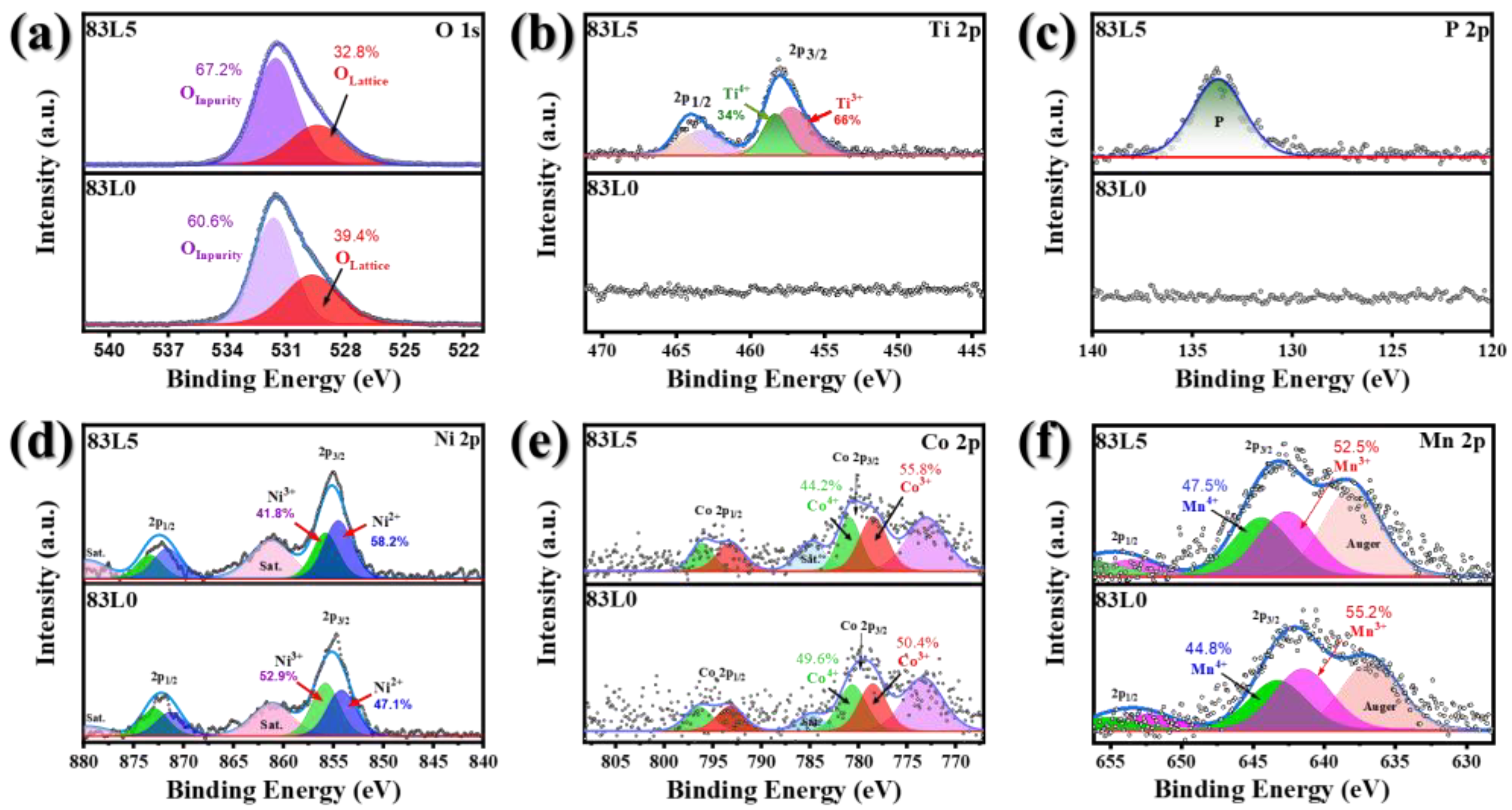
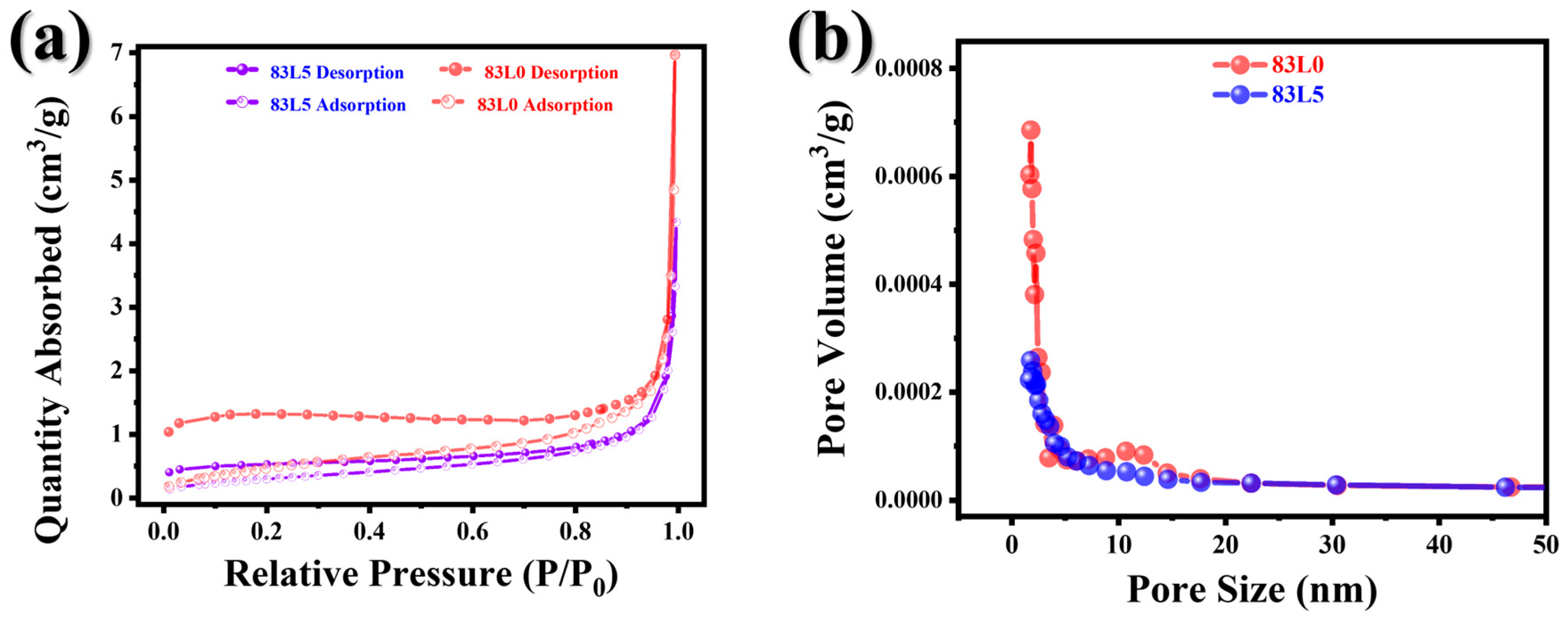
3.1. Structure and Morphology
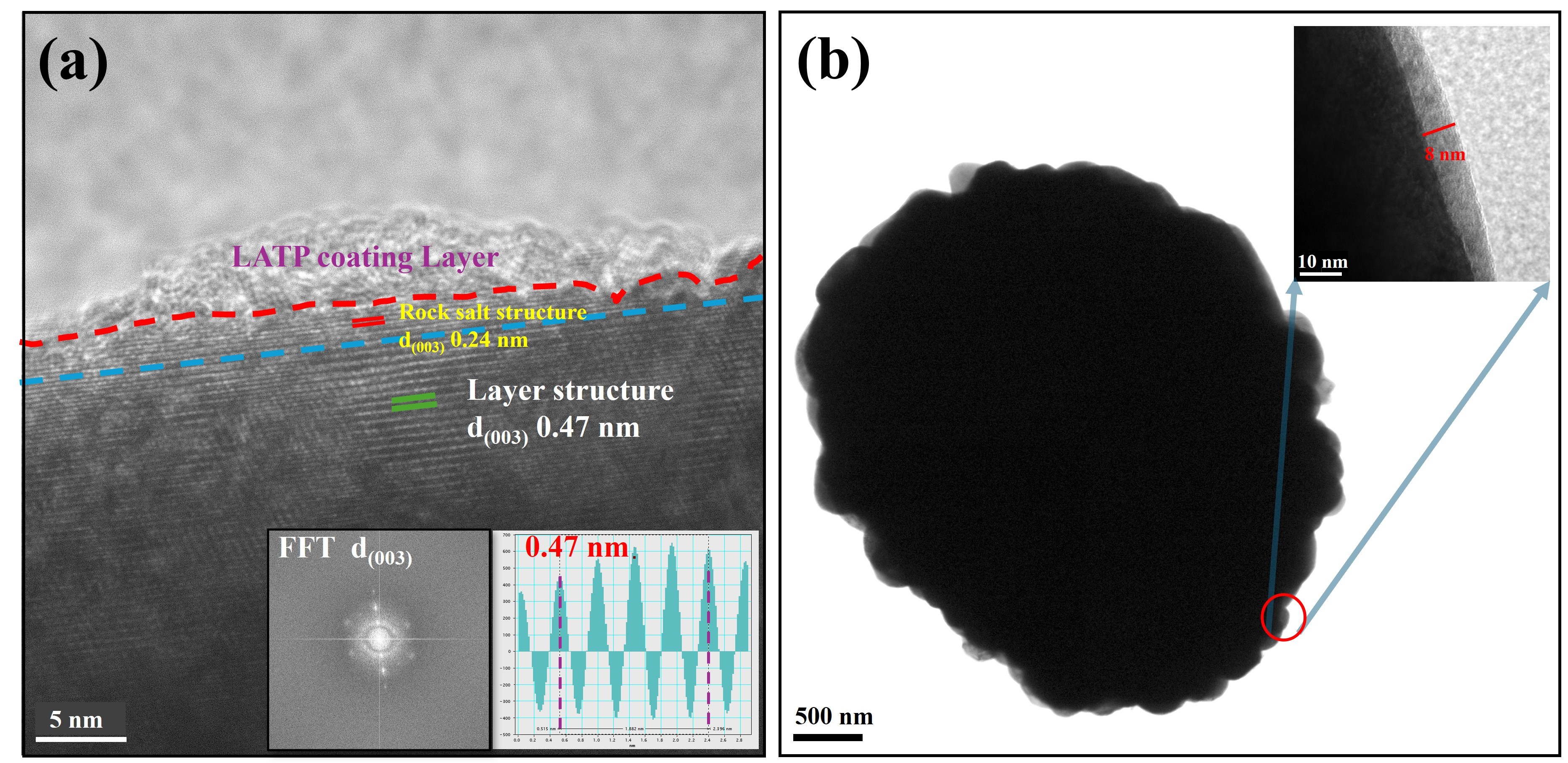
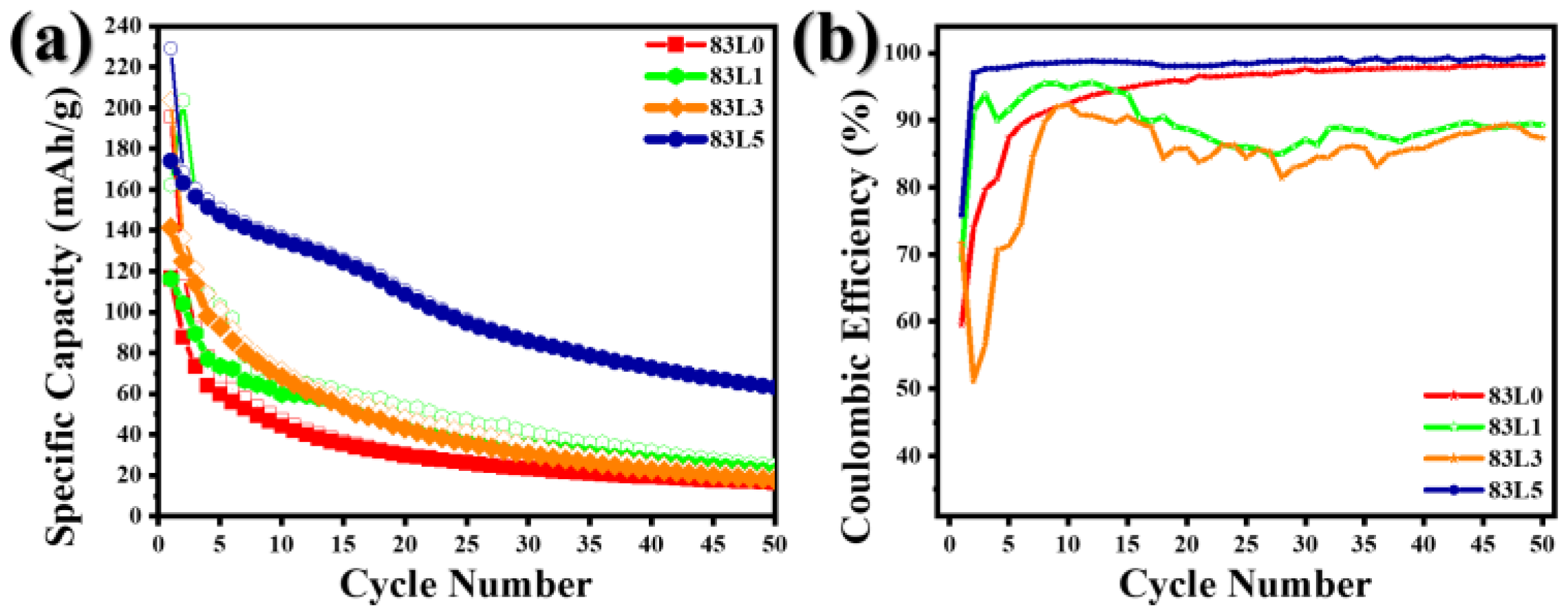
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.J.; Cai, X.Y.; Cai, S.M.; Shao, Y.X.; Hu, C.; Lu, S.R.; Ding, S.J. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Wu, D.X.; Wu, F. Toward better batteries: Solid-state battery roadmap 2035+. Etransportation 2023, 16, 100224–100228. [Google Scholar] [CrossRef]
- Schmaltz, T.; Hartmann, F.; Wicke, T.; Weymann, L.; Neef, C.; Janek, J. A Roadmap for Solid-State Batteries. Adv. Energy Mater. 2023, 13, 2301886. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Liu, Y.; Luo, D.; Xu, R. A Critical Review of Single-Crystal LiNixMnyCo1-x-yO2 Cathode Materials. Renewables 2024, 2, 25–51. [Google Scholar] [CrossRef]
- Bai, X.T.; Duan, Y.; Zhuang, W.D.; Yang, R.; Wang, J.T. Research progress in Li-argyrodite-based solid-state electrolytes. J. Mater. Chem. A 2020, 8, 25663–25686. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, F.P.; Luo, J.; Zhang, L.; Sun, X.L. Recent development of lithium argyrodite solid-state electrolytes for solid-state batteries: Synthesis, structure, stability and dynamics. Nano Energy 2021, 83, 105858. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; He, X.F.; Mo, Y.F. First principles study on electrochemical and chemical stability of solid electrolyte-electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 2016, 4, 3253–3266. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Kim, M.; An, H.; Min, K.; Park, K. A review of problems and solutions in Ni-rich cathode-based Li-ion batteries from two research aspects: Experimental studies and computational insights. J. Power Sources 2024, 597, 234132. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.B.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface Stability of Argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in Bulk All-Solid-State Batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Zhou, D.L.; Gao, X.; Hu, T.F.; Zhang, Z.Y.; Zhen, Z.; Chen, X.D.; Cui, L.F.; Wang, G.X. Elucidating and Minimizing the Space-Charge Layer Effect between NCM Cathode and Li6PS5Cl for Sulfide-Based Solid-State Lithium Batteries. Adv. Energy Mater. 2024, 14, 230443. [Google Scholar] [CrossRef]
- Zhou, L.; Minafra, N.; Zeier, W.G.; Nazar, L.F. Innovative Approaches to Li-Argyrodite Solid Electrolytes for All-Solid-State Lithium Batteries. Acc. Chem. Res. 2021, 54, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Li, N.A.; Luo, J.Y.; Zhu, J.H.; Zhuang, X.D. Cathodic interface in sulfide-based all-solid-state lithium batteries. Energy Storage Mater. 2023, 63, 103034. [Google Scholar] [CrossRef]
- Meng, J.K.; Qu, G.; Huang, Y.H. Stabilization strategies for high-capacity NCM materials targeting for safety and durability improvements. Etransportation 2023, 16, 100233. [Google Scholar] [CrossRef]
- Sun, H.H.; Kim, U.H.; Park, J.H.; Park, S.W.; Seo, D.H.; Heller, A.; Mullins, C.B.; Yoon, C.S.; Sun, Y.K. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat. Commun. 2021, 12, 6552. [Google Scholar] [CrossRef]
- Morchhale, A.; Tang, Z.H.; Yu, C.Y.; Farahati, R.; Kim, J.H. Coating materials and processes for cathodes in sulfide-based all solid-state batteries. Curr. Opin. Electrochem. 2023, 39, 101251. [Google Scholar] [CrossRef]
- Okada, K.; Machida, N.; Naito, M.; Shigematsu, T.; Ito, S.; Fujiki, S.; Nakano, M.; Aihara, Y. Preparation and electrochemical properties of LiAlO2-coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries. Solid State Ion. 2014, 255, 120–127. [Google Scholar] [CrossRef]
- Negi, R.S.; Minnmann, P.; Pan, R.J.; Ahmed, S.; Herzog, M.J.; Volz, K.; Takata, R.; Schmidt, F.; Janek, J.; Elm, M.T. Stabilizing the Cathode/Electrolyte Interface Using a Dry-Processed Lithium Titanate Coating for All-Solid-State Batteries. Chem. Mater. 2021, 33, 6713–6723. [Google Scholar] [CrossRef]
- Strauss, F.; Teo, J.H.; Maibach, J.; Kim, A.Y.; Mazilkin, A.; Janek, J.; Brezesinski, T. Li2ZrO3-Coated NCM622 for Application in Inorganic Solid-State Batteries: Role of Surface Carbonates in the Cycling Performance. ACS Appl. Mater. Interfaces 2020, 12, 57146–57154. [Google Scholar] [CrossRef]
- James Abraham, J.; Nisar, U.; Monawwar, H.; Abdul Quddus, A.; Shakoor, R.A.; Saleh, M.I.; Kahraman, R.; Al-Qaradawi, S.; Aljaber, A.S. Improved electrochemical performance of SiO2-coated Li-rich layered oxides-Li1.2Ni0.13Mn0.54Co0.13O2. J. Mater. Sci. Mater. Electron. 2020, 31, 19475–19486. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
- You, M.J.; Jung, J.; Byeon, Y.S.; Jung, J.Y.; Hong, Y.; Park, M.S. Controlled crystallinity of LiTaO3 surface layer for single-crystalline Ni-rich cathodes for lithium-ion batteries and all-solid-state batteries. Chem. Eng. J. 2024, 483, 149199. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Shi, Q.; Lv, Z.; Lu, Y.; Bao, L.; Li, N.; Chen, S.; Wu, F. Roles of Fast-Ion Conductor LiTaO3 Modifying Ni-rich Cathode Material for Li-Ion Batteries. ChemSusChem 2021, 14, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Fan, Z.; Zhang, D.; Su, M.; Wang, X.; Tu, J. Surface Construction of a High-Ionic-Conductivity Buffering Layer on a LiNi0.6Co0.2Mn0.2O2 Cathode for Stable All-Solid-State Sulfide-Based Batteries. J. Electron. Mater. 2023, 52, 2904–2912. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, L.H.; Zhang, K.; Zhang, Z.Y.; Zhang, L.L.; Li, Z.; Kong, T.Y.; Xie, Y.H.; Wang, Y.G. High-Performance Sulfide All-Solid-State Batteries Enabled by High-Voltage Ni-Rich Cathode with a Conformal and Conductive Protective Layer. ACS Appl. Energy Mater. 2024, 7, 2524–2532. [Google Scholar] [CrossRef]
- Wu, E.A.; Jo, C.; Tan, D.H.S.; Zhang, M.H.; Doux, J.M.; Chen, Y.T.; Deysher, G.; Meng, Y.S. A Facile, Dry-Processed Lithium Borate-Based Cathode Coating for Improved All-Solid-State Battery Performance. J. Electrochem. Soc. 2020, 167, 130516. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Z.; Wu, D.; Yu, Y.; Wang, Z.; Fang, Y.; Chen, D.; Shang, S.; Qu, X.; Li, P. Low-cost BPO4 In Situ Synthetic Li3PO4 Coating and B/P-Doping to Boost 4.8 V Cyclability for Sulfide-Based All-Solid-State Lithium Batteries. Small 2024, 20, e2307030. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, J.; Yu, R.; Zhuo, H.; Wang, C.; Ren, Z.; Wang, J. Practical Application of Li-Rich Materials in Halide All-Solid-State Batteries and Interfacial Reactions between Cathodes and Electrolytes. ACS Appl. Mater. Interfaces 2023, 15, 8190–8199. [Google Scholar] [CrossRef]
- Cha, H.; Yun, J.; Kim, S.; Kang, J.; Cho, M.; Cho, W.; Lee, J.-W. Stabilizing the interface between high-Ni oxide cathode and Li6PS5Cl for all-solid-state batteries via dual-compatible halides. J. Power Sources 2024, 617, 235157. [Google Scholar] [CrossRef]
- Lee, D.; Cui, Z.; Goodenough, J.B.; Manthiram, A. Interphase Stabilization of LiNi0.5Mn1.5O4 Cathode for 5 V-Class All-Solid-State Batteries. Small 2024, 20, e2306053. [Google Scholar] [CrossRef]
- Demuth, T.; Fuchs, T.; Walther, F.; Pokle, A.; Ahmed, S.; Malaki, M.; Beyer, A.; Janek, J.; Volz, K. Influence of the sintering temperature on LLZO-NCM cathode composites for solid-state batteries studied by transmission electron microscopy. Matter 2023, 6, 2324. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, J.; Jeong, J.; Lee, H.J.; Kim, S.C.; Hah, H.J.; Song, M.S.; Oh, K.; Lee, S.H. Effect of Polyacrylonitrile Surface Coating on Electrochemical Performance of LiNi0.8Mn0.1Co0.1O2 in All Solid-State Batteries. J. Electrochem. Soc. 2022, 169, 060541. [Google Scholar] [CrossRef]
- Wu, M.; Song, H.; Zhou, X.; Qin, L.; Fan, X.; Wang, H. Li1.4Al0.4Ti1.6(PO4)3 coating surface modification enables improved electrochemical performance of LiNi0.83Co0.11Mn0.06O2 cathode. Mater. Today Commun. 2024, 41, 110270. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Chen, Y.; Yu, T.; Dong, Z.; Yang, H.; Yu, Y.-G. Stabilizing Phase Transition in Ni Rich Layered Oxide Cathode Material by Nanoscale Nasicon Polyanion Groups. 2024. Available online: https://ssrn.com/abstract=4809101 (accessed on 1 January 2020).
- Zhang, M.; Zhang, P.; Wen, W.D.; Wang, H.W.; He, B.B.; Gong, Y.S.; Jin, J.; Wang, R. Enhanced Electrochemical Performance of LiNi1/3Co1/3Mn1/3O2 at a High Cut-Off Voltage of 4.6 V by Li1.3Al0.3Ti1.7(PO4)3 Coating. Coatings 2022, 12, 1964. [Google Scholar] [CrossRef]
- Yen, P.Y.; Lee, M.L.; Gregory, D.H. and Liu, W.R. Optimization of sintering process on Li1+xAlxTi2-x(PO4)3 solid electrolytes for all-solid-state Lithium-ion batteries. Ceram. Int. 2020, 46, 20529–20536. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Miara, L.J.; Wang, Y.; Ceder, G. Computational Screening of Cathode Coatings for Solid-State Batteries. Joule 2019, 3, 1252–1275. [Google Scholar] [CrossRef]
- Benabed, Y.; Rioux, M.; Rousselot, S.; Hautier, G.; Dollé, M. Assessing the Electrochemical Stability Window of NASICON-Type Solid Electrolytes. Front. Energy Res. 2021, 9, 682008. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, X.Q.; Dai, W.L.; Yao, C.C.; Qian, J.C.; Chen, Z.G.; Liu, C.B. Optimized Ni-rich LiNi0.83Co0.06Mn0.06Al0.05O2 cathode material with a Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor coating for Lithium-ion batteries. J. Alloys Compd. 2022, 923, 166277. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Subashini, C.; Sivasubramanian, R.; Sundaram, M.M.; Priyadharsini, N. The evolution of allotropic forms of Na2CoP2O7 electrode and its role in future hybrid energy storage. J. Energy Storage 2025, 130, 117390. [Google Scholar] [CrossRef]
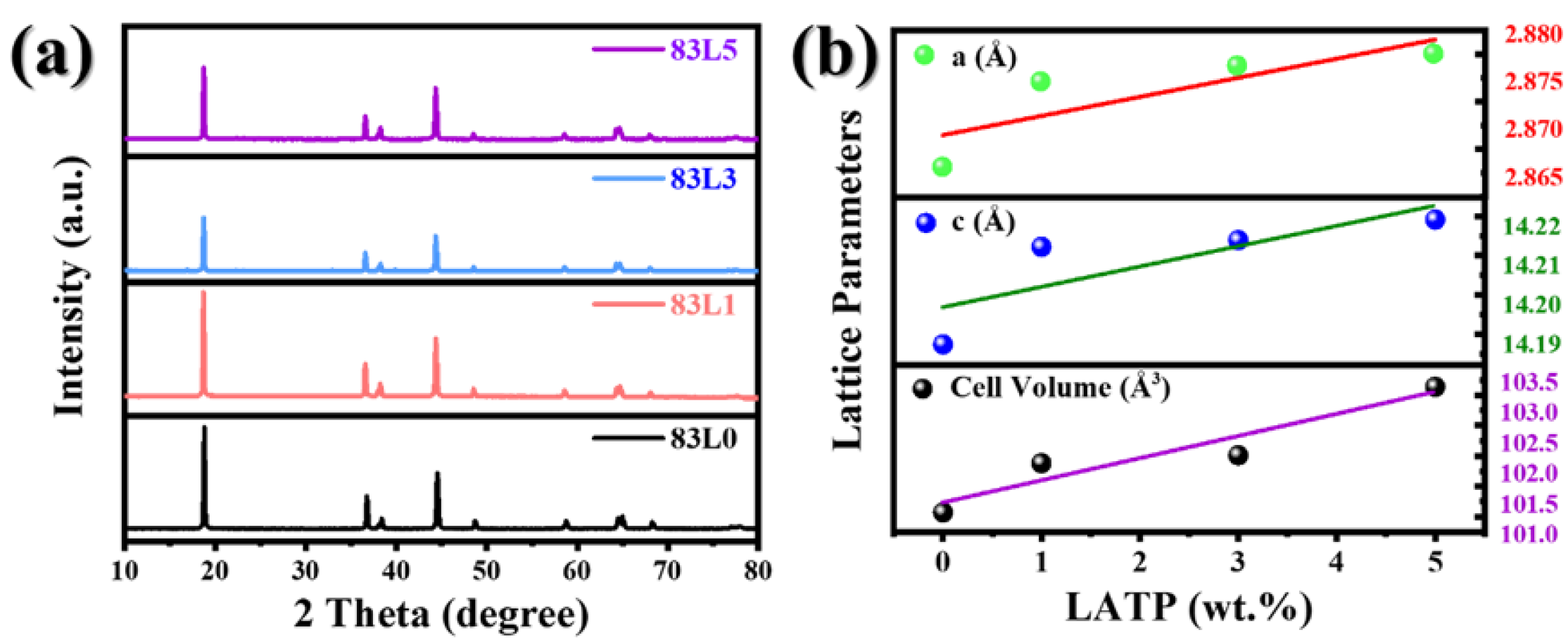
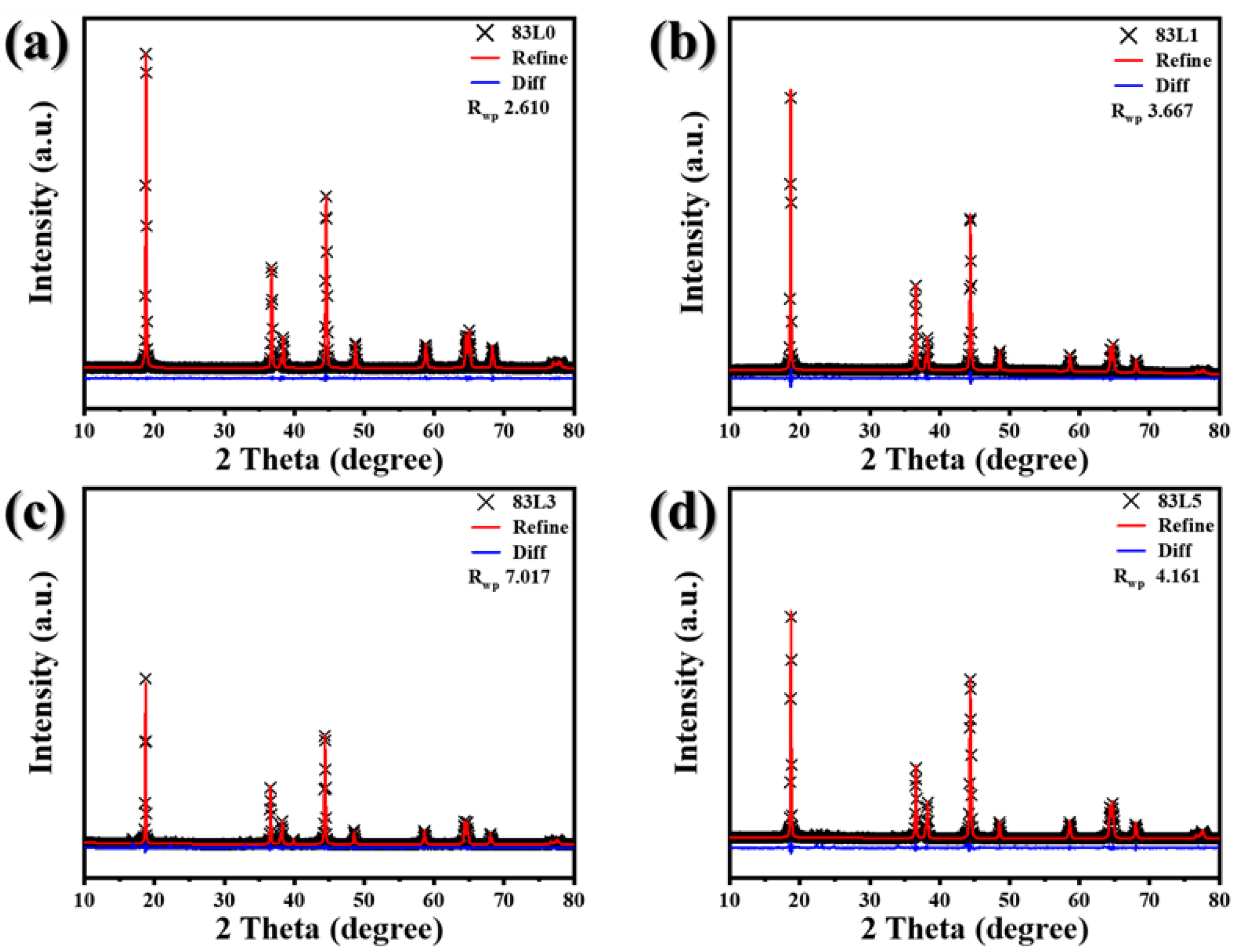


| Samples | a (Å) | c (Å) | Cell Volume (Å3) | Rwp (%) | I(003)/I(104) |
|---|---|---|---|---|---|
| 83L0 | 2.86810(3) | 14.1874(7) | 101.069(9) | 2.610 | 1.78513 |
| 83L1 | 2.87706(5) | 14.2122(4) | 101.881(5) | 3.666 | 1.76049 |
| 83L3 | 2.87873(4) | 14.2139(7) | 102.011(4) | 7.017 | 1.51346 |
| 83L5 | 2.87999(1) | 14.2191(7) | 103.137(9) | 4.161 | 1.37251 |
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| 83L0 | 1.6499 | 0.007480 | 16.52651 |
| 83L5 | 1.0773 | 0.005147 | 18.10328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-P.; Hameed, M.U.; Hsieh, C.-T.; Liu, W.-R. Interface Engineering of NCMA Cathodes with LATP Coatings for High-Performance Solid-State Lithium Batteries. Nanomaterials 2025, 15, 1057. https://doi.org/10.3390/nano15141057
Cho S-P, Hameed MU, Hsieh C-T, Liu W-R. Interface Engineering of NCMA Cathodes with LATP Coatings for High-Performance Solid-State Lithium Batteries. Nanomaterials. 2025; 15(14):1057. https://doi.org/10.3390/nano15141057
Chicago/Turabian StyleCho, Shih-Ping, Muhammad Usman Hameed, Chien-Te Hsieh, and Wei-Ren Liu. 2025. "Interface Engineering of NCMA Cathodes with LATP Coatings for High-Performance Solid-State Lithium Batteries" Nanomaterials 15, no. 14: 1057. https://doi.org/10.3390/nano15141057
APA StyleCho, S.-P., Hameed, M. U., Hsieh, C.-T., & Liu, W.-R. (2025). Interface Engineering of NCMA Cathodes with LATP Coatings for High-Performance Solid-State Lithium Batteries. Nanomaterials, 15(14), 1057. https://doi.org/10.3390/nano15141057








