Novel Green Synthesis Route of ZnO Nanoparticles for Dielectric Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
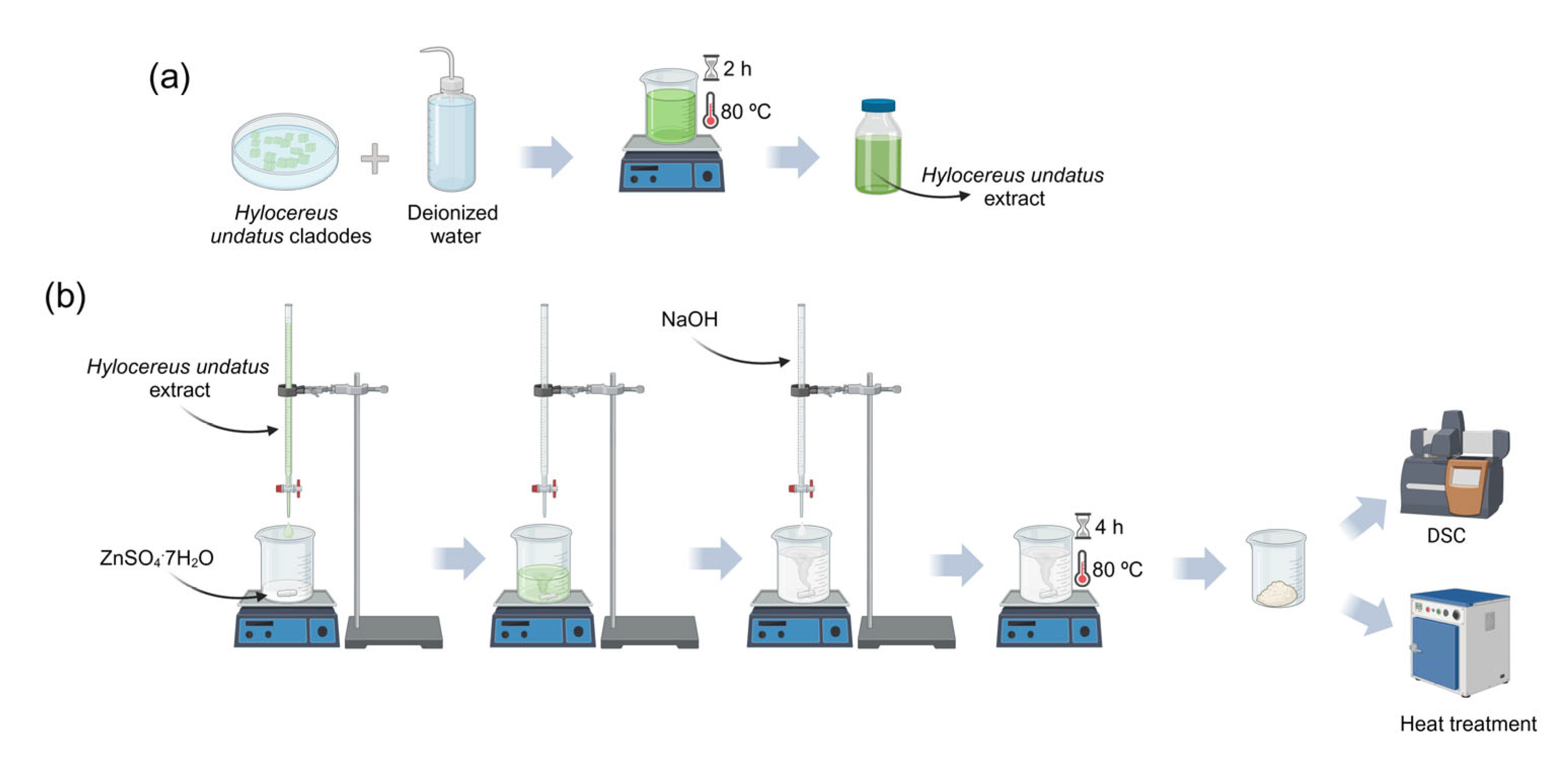
2.2. Preparation of the Hylocereus undatus Cladodes Extract
2.3. Synthesis of the ZnO Nanoparticles
2.4. Characterization Techniques
3. Results
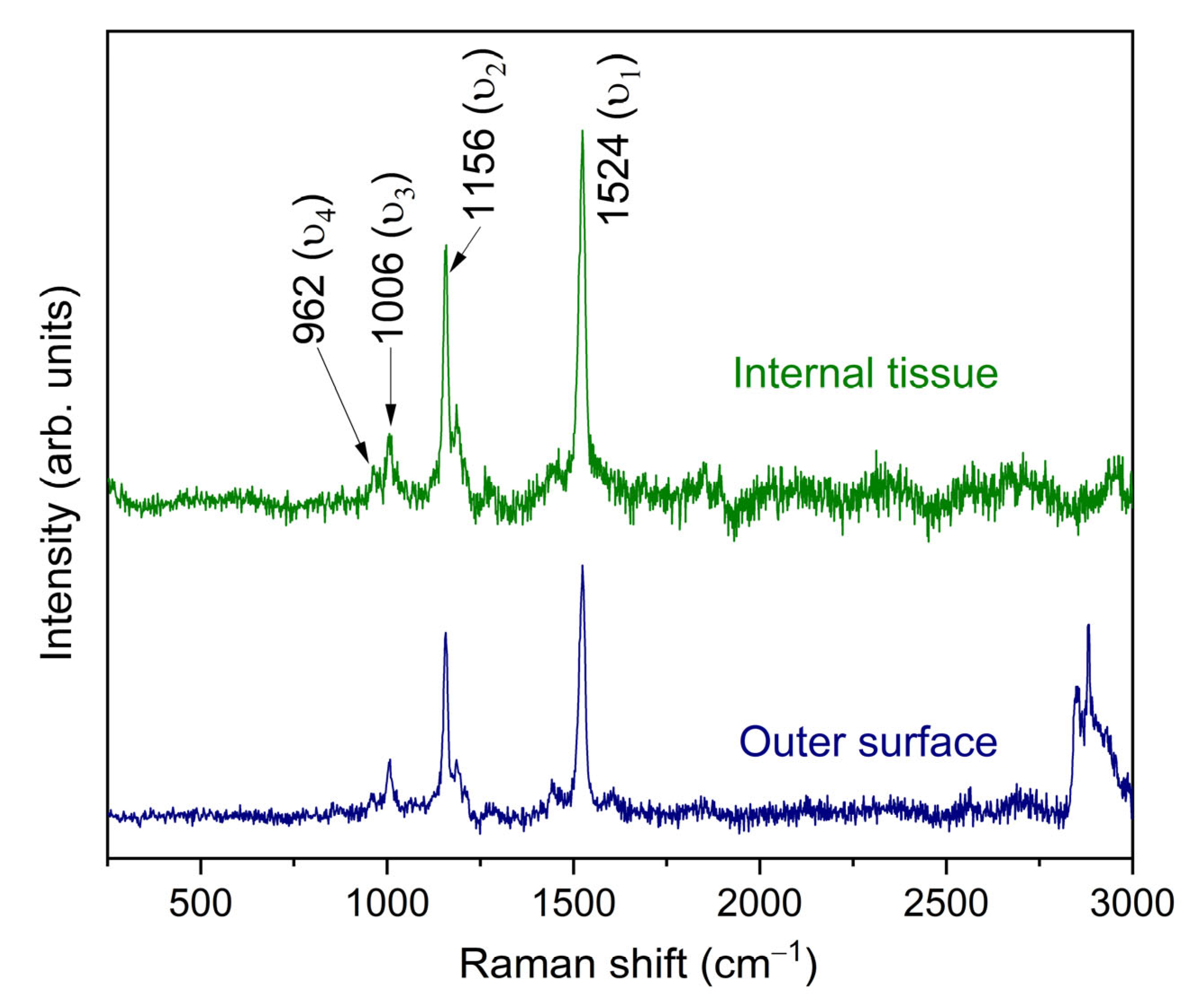
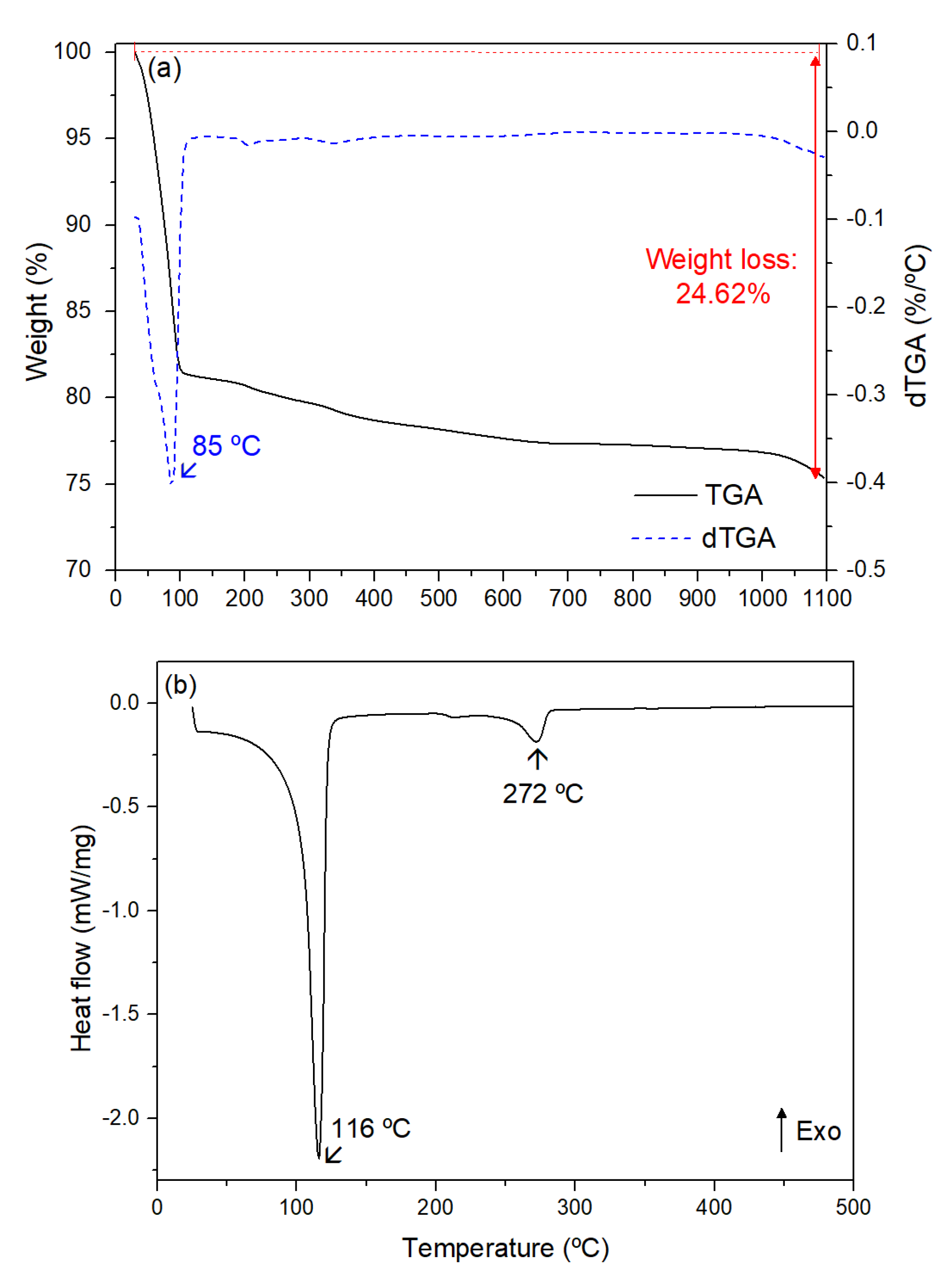
3.1. Characterization of Hylocereus undatus Cladodes
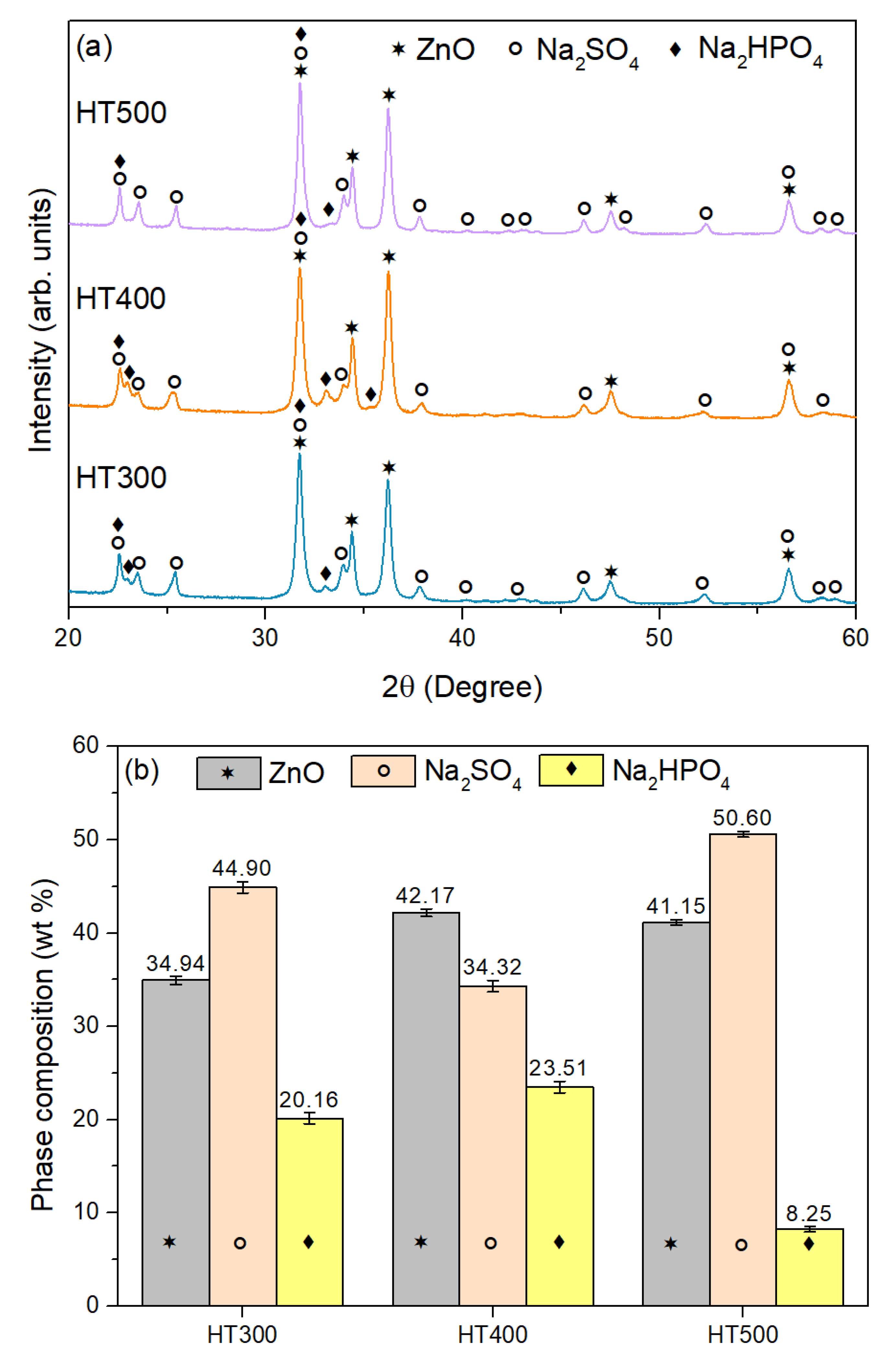
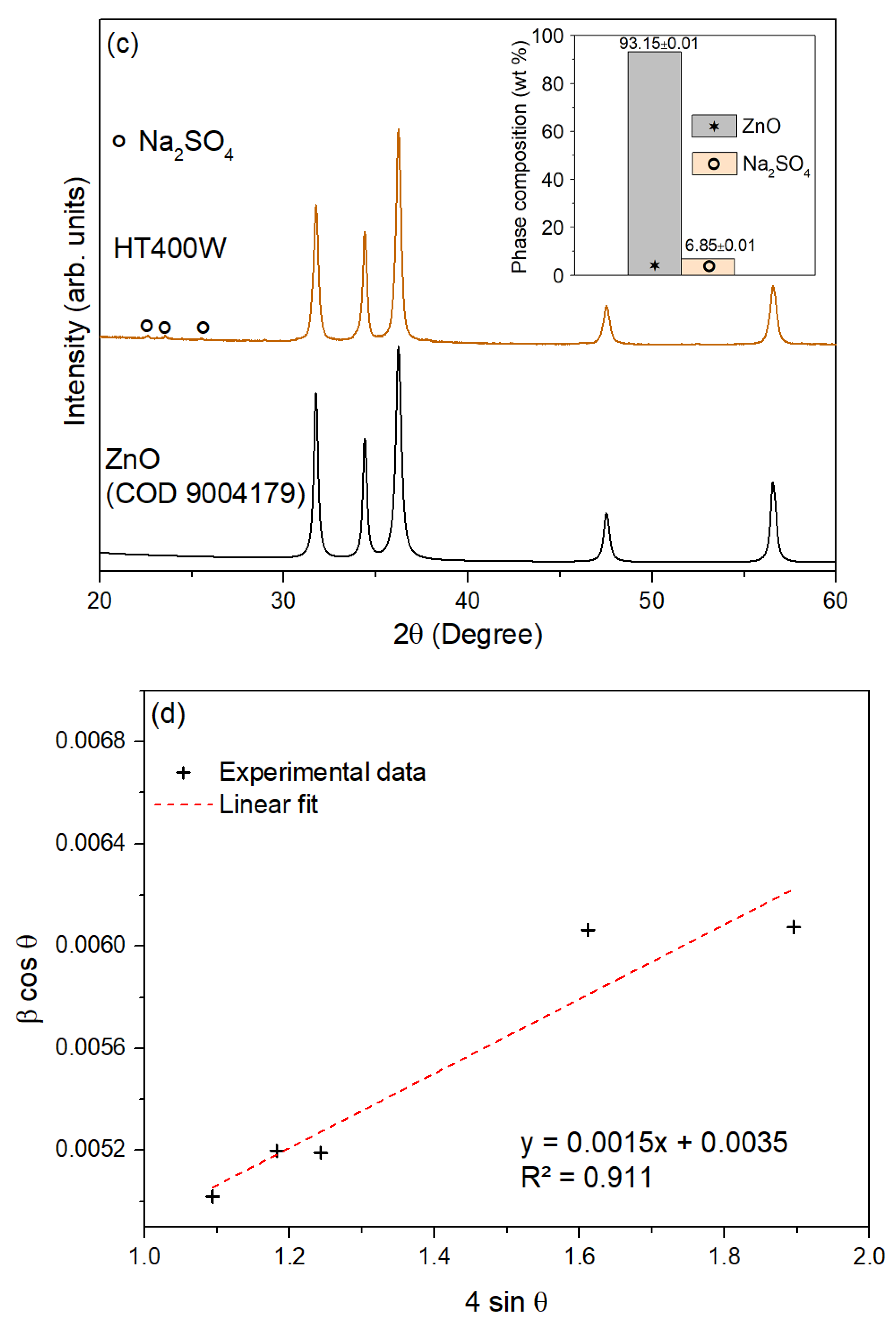
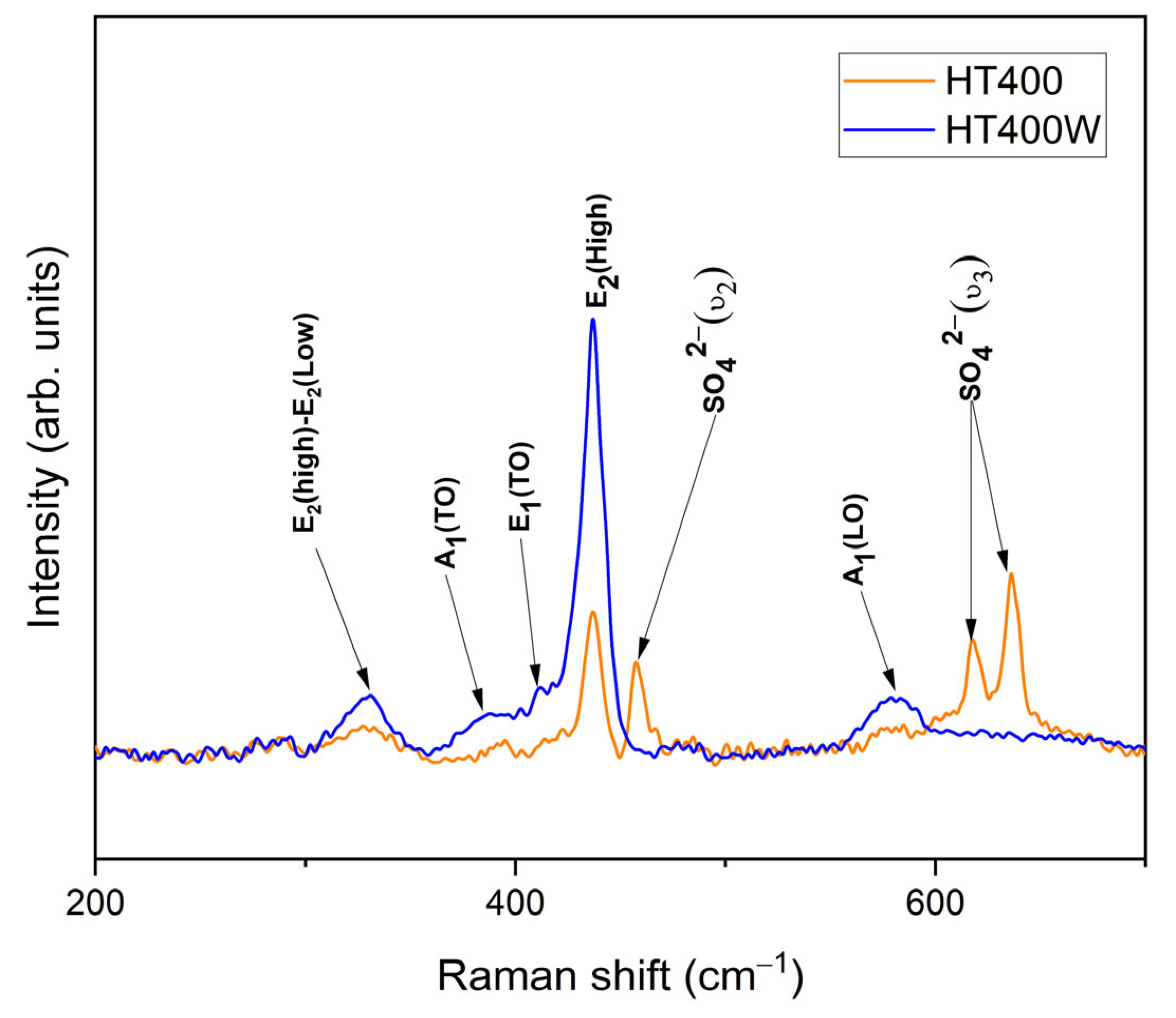
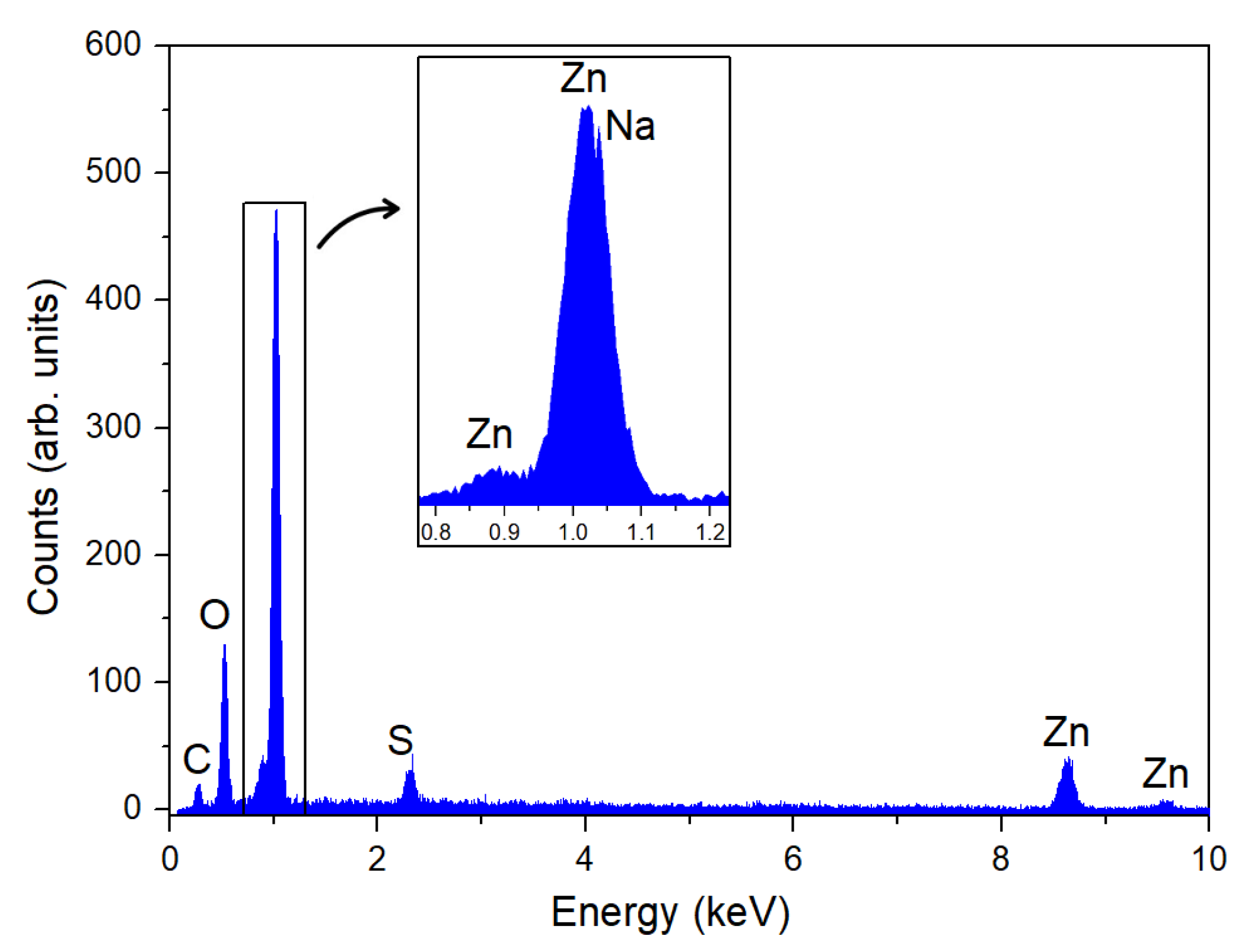
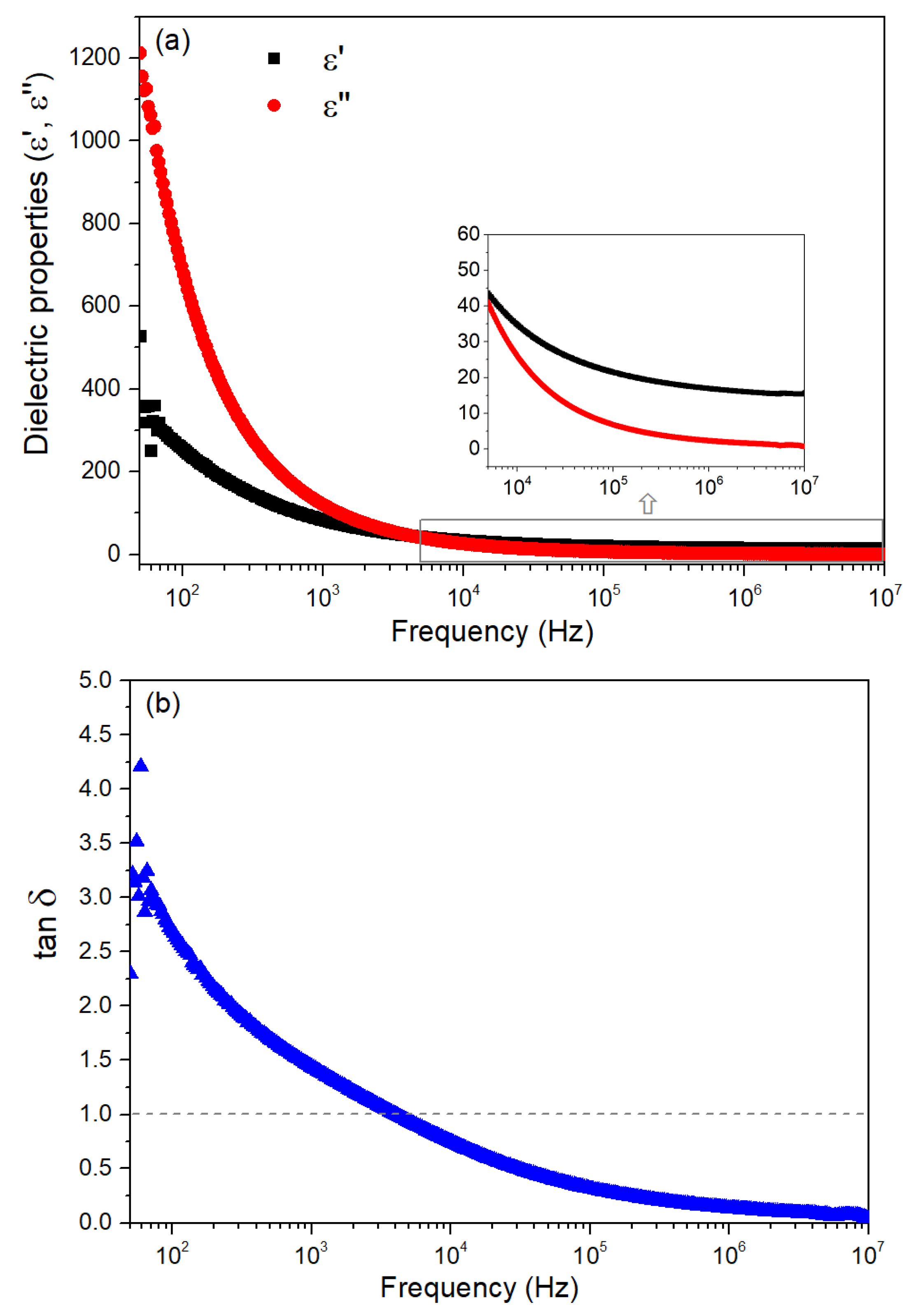
3.2. Characterization of ZnO Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basílio, J.A.J.; Mahendra, D.S. Colloidal Stability and Dielectric Behavior of Eco-Friendly Synthesized Zinc Oxide Nanostructures from Moringa Seeds. Sci. Rep. 2024, 14, 2310. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.; Poinern, G. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef] [PubMed]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Selvarajan, E.; Mohanasrinivasan, V. Biosynthesis and Characterization of ZnO Nanoparticles Using Lactobacillus plantarum VITES07. Mater. Lett. 2013, 112, 180–182. [Google Scholar] [CrossRef]
- Stalin Dhas, T.; Ganesh Kumar, V.; Karthick, V.; Jini Angel, K.; Govindaraju, K. Facile Synthesis of Silver Chloride Nanoparticles Using Marine Alga and Its Antibacterial Efficacy. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 416–420. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Balusamy, S.R.; Madhusudanan, M.; Singh, H.; Amsath Haseef, H.M.; Mijakovic, I. Advanced nanomaterials for cancer therapy: Gold, silver, and iron oxide nanoparticles in oncological applications. Adv. Healthc. Mater. 2025, 14, 2403059. [Google Scholar] [CrossRef]
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Green Synthesis and Characterization of Silver Nanoparticles Using Boerhaavia diffusa Plant Extract and Their Anti Bacterial Activity. Ind. Crops. Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Anguraj, A.; Michael, H.S.R.; Sugumaran, S.; Madhusudhanan, G.R.; Sivaraman, R.K. A Comparative Study on Biosynthesized Silver Nanoparticles from H. undatus Fruit Peel and Their Therapeutic Applications. Discov. Nano 2024, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Vindhya, P.S.; Jeyasingh, T.; Kavitha, V.T. Dielectric Properties of Zinc Oxide Nanoparticles Using Annona Muricata Leaf. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2082, p. 080005. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green Synthesis of Zinc Oxide Nanoparticles Using Sea Lavender (Limonium pruinosum L. Chaz.) Extract: Characterization, Evaluation of Anti-Skin Cancer, Antimicrobial and Antioxidant Potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef] [PubMed]
- Meshkatalsadat, M.H.; Momeni, A.; Abdollahzadeh, M.R. Biosynthesis of Zinc Oxide Nanoparticles Using Punica granatum L. Waste Peel Extract, and Assessment of Antioxidant and Catalytic Activity. Nano Biomed. Eng. 2023, 15, 378–388. [Google Scholar] [CrossRef]
- Asan-Ozusaglam, M.; Celik, I. Formulating edible films with red pitahaya extract and probiotic. Foods Raw Mater. 2025, 13, 144–154. [Google Scholar] [CrossRef]
- Hernández-Ramos, L.; García-Mateos, M.D.R.; Castillo-González, A.M.; Ybarra-Moncada, C.; Nieto-Ángel, R. Fruits of the pitahaya Hylocereus undatus and H. ocamponis: Nutritional components and antioxidants. J. Appl. Bot. Food Qual. 2020, 93, 197–203. [Google Scholar]
- Canute Kamikawachi, R.; Carrara, V.; Vilegas, W. Dragon Fruit Farming By-Products as an Important Source of Several Glycosylated Flavonoids. Food Res. Int. 2023, 173, 113400. [Google Scholar] [CrossRef]
- Sunitha, N.; Yamini, B.; Madhuri, M.; Rakesh, R.; Bhavani, R.; Nagajyothi, Y.; Thangabalan, B. Complete Novel Review on Selenicereus Undatus. World J. Adv. Res. Rev. 2024, 21, 1596–1608. [Google Scholar] [CrossRef]
- Balendres, M.A.; Bengoa, J.C. Diseases of Dragon Fruit (Hylocereus Species): Etiology and Current Management Options. Crop Prot. 2019, 126, 104920. [Google Scholar] [CrossRef]
- Abirami, K.; Swain, S.; Baskaran, V.; Venkatesan, K.; Sakthivel, K.; Bommayasamy, N. Distinguishing Three Dragon Fruit (Hylocereus spp.) Species Grown in Andaman and Nicobar Islands of India Using Morphological, Biochemical and Molecular Traits. Sci. Rep. 2021, 11, 2894. [Google Scholar] [CrossRef]
- Vishnupriya, B.; Nandhini, G.R.E.; Anbarasi, G. Biosynthesis of Zinc Oxide Nanoparticles Using Hylocereus undatus Fruit Peel Extract against Clinical Pathogens. Mater. Today Proc. 2022, 48, 164–168. [Google Scholar] [CrossRef]
- Tsai, Y.; Lin, C.-G.; Chen, W.-L.; Huang, Y.-C.; Chen, C.-Y.; Huang, K.-F.; Yang, C.-H. Evaluation of the Antioxidant and Wound-Healing Properties of Extracts from Different Parts of Hylocereus polyrhizus. Agronomy 2019, 9, 27. [Google Scholar] [CrossRef]
- Kaur, D.; Bharti, A.; Sharma, T.; Madhu, C. Dielectric Properties of ZnO-Based Nanocomposites and Their Potential Applications. Int. J. Opt. 2021, 2021, 9950202. [Google Scholar] [CrossRef]
- Abdel–Baset, T.A.; Belhaj, M. Structural Characterization, Dielectric Properties and Electrical Conductivity of ZnO Nanoparticles Synthesized by Co-Precipitation Route. Phys. B Condens. Matter 2021, 616, 413130. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO Nanoparticles Using Jacaranda mimosifolia Flowers Extract: Synergistic Antibacterial Activity and Molecular Simulated Facet Specific Adsorption Studies. J. Photochem. Photobiol. B 2016, 162, 199–207. [Google Scholar] [CrossRef]
- Saadi, H.; Benzarti, Z.; Sanguino, P.; Pina, J.; Abdelmoula, N.; de Melo, J.S.S. Enhancing the Electrical Conductivity and the Dielectric Features of ZnO Nanoparticles through Co Doping Effect for Energy Storage Applications. J. Mater. Sci. Mater. Electron. 2023, 34, 116. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Mohamed, A.M.H.A.; Sharaky, M.; Mohamed, N.A.; Diab, Y.M. Green Approach for the Synthesis of ZnO Nanoparticles Using Cymbopogon citratus Aqueous Leaf Extract: Characterization and Evaluation of Their Biological Activities. Chem. Biol. Technol. Agric. 2023, 10, 63. [Google Scholar] [CrossRef]
- Saadi, H.; Khaldi, O.; Pina, J.; Costa, T.; Seixas de Melo, J.S.; Vilarinho, P.; Benzarti, Z. Effect of Co Doping on the Physical Properties and Organic Pollutant Photodegradation Efficiency of ZnO Nanoparticles for Environmental Applications. Nanomaterials 2024, 14, 122. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Hoang Bui, V.K.; Kumar, M.K.; Alinaghibeigi, M.; Moolayadukkam, S.; Eskandarinejad, S.; Mahmoudi, S.; Mirzamohammadi, S.; Rezaei-khamseh, M. A Review on Zinc Oxide Composites for Energy Storage Applications: Solar Cells, Batteries, and Supercapacitors. J. Compos. Compd. 2021, 3, 182–193. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, N.; Ji, Y.; Zhang, J.; Zhu, Y.; Yi, T. Nanosized Zinc Oxides-Based Materials for Electrochemical Energy Storage and Conversion: Batteries and Supercapacitors. Chin. Chem. Lett. 2022, 33, 714–729. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Kurapova, O.; Faia, P.; Pazheltsev, V.; Zaripov, A.; Konakov, V. Polyantimonic Acid-Based Materials Evaluated as Moisture Sensors at Ambient Temperature. J. Solid State Electrochem. 2023, 27, 611–625. [Google Scholar] [CrossRef]
- Neiva, J.; Benzarti, Z.; Carvalho, S.; Devesa, S. Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization. Materials 2024, 17, 5709. [Google Scholar] [CrossRef]
- Udensi, J.; Loskutova, E.; Loughman, J.; Byrne, H.J. Quantitative Raman Analysis of Carotenoid Protein Complexes in Aqueous Solution. Molecules 2022, 27, 4724. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Naffa, R.; Zhang, W.; Schreurs, N.M.; Waterland, M.; Cooper, S.; Holmes, G. Validity and Reliability of Raman Spectroscopy for Carotenoid Assessment in Cattle Skin. Biochem. Biophys. Rep. 2021, 27, 101036. [Google Scholar] [CrossRef]
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; Perea-Flores, M.d.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and Structural Properties, Biological Activity, and Polyphenols Profile. Food Sci. Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Eucalyptus globulus Labill. Leaf Extract and Zinc Nitrate Hexahydrate Salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Meena, J.; Pavithra, G.; Anusha, D.; Kumar, A.S.; Santhakumar, K. The Green Approach of ZnO NPs and Its Antioxidant, Hemolytic, and Photocatalytic Activity and Functionalized r-GO-ZnO for Energy Storage Application. J. Mater. Sci. Mater. Electron. 2023, 34, 1131. [Google Scholar] [CrossRef]
- Jamil, H.; Faizan, M. Facile Synthesis of ZnO Nanoparticles Using Nigella sativa Extract and Its Role as Catalyst in Production of Bio-Oil and Degradation of Methylene Blue Dye. Heliyon 2024, 10, e35828. [Google Scholar] [CrossRef]
- Heer, A.S.K. Spectral Characterization and Anti-Fungal Activity of Zinc Oxide (ZnO) Nanoparticles Synthesized Using Cynodon dactylon Leaf Extract. Arvind. World J. Pharm. Res. 2017, 6, 1588–1596. [Google Scholar] [CrossRef]
- Kihara, K.; Donnay, G. Anharmonic Thermal Vibrations in ZnO. Can. Mineral. 1985, 23, 647–654. [Google Scholar]
- Frevel, L.K. The Crystal Structure of Sodium Sulfate III. J. Chem. Phys. 1940, 8, 290. [Google Scholar] [CrossRef]
- Rhimi, T.; Leroy, G.; Duponchel, B.; Khirouni, K.; Guermazi, S.; Toumi, M. Electrical Conductivity and Dielectric Analysis of NaH2PO4 Compound. Ionics 2018, 24, 3507–3514. [Google Scholar] [CrossRef]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Asmi, D.; Low, I.M. Manufacture of Graded Ceramic Matrix Composites Using Infiltration Techniques. In Advances in Ceramic Matrix Composites; Zhang, J., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 109–140. [Google Scholar] [CrossRef]
- Pieczara, G.; Manecki, M.; Rzepa, G.; Borkiewicz, O.; Gaweł, A. Thermal Stability and Decomposition Products of P–Doped Ferrihydrite. Materials 2020, 13, 4113. [Google Scholar] [CrossRef]
- Devesa, S.; Rooney, A.P.; Graça, M.P.; Cooper, D.; Costa, L.C. Williamson-hall analysis in estimation of crystallite size and lattice strain in Bi1.34Fe0.66NO6.35 prepared by the sol-gel method. Mater. Sci. Eng. B 2021, 263, 114830. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Hajer, S.; Khaldi, O.; Dahri, A.; Abdelmoula, N.; Hammami, I.; Pedro Fernandes Graça, M.; Benzarti, Z. Influence of (Co+Al) Co−doping on Structural, Micro-Structural, Optical and Electrical Properties of Nanostructured Zinc Oxide. Ceram. Int. 2024, 50, 44151–44164. [Google Scholar] [CrossRef]
- Thyr, J.; Österlund, L.; Edvinsson, T. Polarized and Non-polarized Raman Spectroscopy of ZnO Crystals: Method for Determination of Crystal Growth and Crystal Plane Orientation for Nanomaterials. J. Raman Spectrosc. 2021, 52, 1395–1405. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Fdez-Ortiz de Vallejuelo, S.; Veneranda, M.; Lama, E.; Castro, K.; Arana, G.; Larrañaga, A.; Madariaga, J.M. The Raman Spectra of the Na2SO4-K2SO4 System: Applicability to Soluble Salts Studies in Built Heritage. J. Raman Spectrosc. 2019, 50, 175–183. [Google Scholar] [CrossRef]
- Dey, S.; Mohanty, D.L.; Divya, N.; Bakshi, V.; Mohanty, A.; Rath, D.; Das, S.; Mondal, A.; Roy, S.; Sabui, R. A Critical Review on Zinc Oxide Nanoparticles: Synthesis, Properties and Biomedical Applications. Intell. Pharm. 2025, 3, 53–70. [Google Scholar] [CrossRef]
- Eswari, K.M.; Asaithambi, S.; Karuppaiah, M.; Sakthivel, P.; Balaji, V.; Ponelakkia, D.K.; Yuvakkumar, R.; Kumar, P.; Vijayaprabhu, N. Green Synthesis of ZnO Nanoparticles Using Abutilon indicum and Tectona grandis Leaf Extracts for Evaluation of Anti-Diabetic, Anti-Inflammatory and in-Vitro Cytotoxicity Activities. Ceram. Int. 2022, 48, 33624–33634. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green synthesis of copper oxide (CuO) nanoparticles by Punica granatum peel extract. Mater. Today Proc. 2021, 36, 751–755. [Google Scholar] [CrossRef]
- You, W.; Ahn, J.C.; Boopathi, V.; Arunkumar, L.; Rupa, E.J.; Akter, R.; Kong, B.M.; Lee, G.S.; Yang, D.C.; Kang, S.C.; et al. Enhanced Antiobesity Efficacy of Tryptophan Using the Nanoformulation of Dendropanax morbifera Extract Mediated with ZnO Nanoparticle. Materials 2021, 14, 824. [Google Scholar] [CrossRef]
- Venu Gopal, V.R.; Kamila, S. Effect of Temperature on the Morphology of ZnO Nanoparticles: A Comparative Study. Appl. Nanosci. 2017, 7, 75–82. [Google Scholar] [CrossRef]
- Chan, Y.; Selvanathan, V.; Tey, L.-H.; Akhtaruzzaman, M.; Anur, F.; Djearamane, S.; Watanabe, A.; Aminuzzaman, M. Effect of Calcination Temperature on Structural, Morphological and Optical Properties of Copper Oxide Nanostructures Derived from Garcinia mangostana L. Leaf Extract. Nanomaterials 2022, 12, 3589. [Google Scholar] [CrossRef]
- Farooqi, M.A.; Bae, S.; Kim, S.; Bae, S.; Kausar, F.; Farooqi, H.M.U.; Kim, J.; Park, H.; Kang, C.U. Eco-Friendly Synthesis of Bioactive Silver Nanoparticles from Black Roasted Gram (Cicer arietinum) for Biomedical Applications. Sci. Rep. 2024, 14, 22922. [Google Scholar] [CrossRef]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesis of Copper Oxide/Carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon 2020, 6, e03323. [Google Scholar] [CrossRef]
- Patel, S.; Desai, R.; Patel, B.; Ali, D.; Dawane, V.; Gadhvi, K.; Sharma, P.; Kumar, A.; Patel, A. Phytonanofabrication of Iron Oxide Particles from the Acacia jacquemontii Plant and Their Potential Application for the Removal of Brilliant Green and Congo Red Dye from Wastewater. Front. Bioeng. Biotechnol. 2023, 11, 1319927. [Google Scholar] [CrossRef]
- Devesa, S.; Ferreira, N.M.; Peixoto, M.V.; Costa, F.M.; Graça, M.P.F. Growth Rate Effect on the Dielectric Properties of FeNbO4 Fibres Processed by the Laser Floating Zone Technique. Ceram. Int. 2024, 50, 34143–34152. [Google Scholar] [CrossRef]
- Toloman, D.; Gungor, A.; Popa, A.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Varadi, A.; Yildirim, I.D.; Suciu, R.; Nesterovschi, I.; et al. Morphological Impact on the Supercapacitive Performance of Nanostructured ZnO Electrodes. Ceram. Int. 2025, 51, 353–365. [Google Scholar] [CrossRef]
- da Silva, G.M.G.; Faia, P.M.; Mendes, S.R.; Araújo, E.S. A Review of Impedance Spectroscopy Technique: Applications, Modelling, and Case Study of Relative Humidity Sensors Development. Appl. Sci. 2024, 14, 5754. [Google Scholar] [CrossRef]
- Devesa, S.; Amorim, C.O.; Belo, J.H.; Araújo, J.P.; Teixeira, S.S.; Graça, M.P.F.; Costa, L.C. Comprehensive Characterization of Bi1.34Fe0.66Nb1.34O6.35 Ceramics: Structural, Morphological, Electrical, and Magnetic Properties. Magnetochemistry 2024, 10, 79. [Google Scholar] [CrossRef]
- Devesa, S.; Graça, M.P.F.; Pereira, W.O.; Santos, G.L.; da Silva Neto, J.F.; Amaral, F.M.B.; Hammami, I.; Mendes, F.; Macêdo, A.A.M. Dielectric Characterization of Solutions of Galactomannan Extracted from Adenanthera pavonina L.: Effects of Purification and Ethanol Concentration. Polymers 2024, 16, 1476. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Kakimoto, K.I. Impedance Spectroscopy and Mechanical Response of Porous Nanophase Hydroxyapatite–Barium Titanate Composite. Mater. Sci. Eng. C 2016, 63, 211–221. [Google Scholar] [CrossRef]
- Devesa, S.; Graça, M.P.; Costa, L.C. Dielectric Behaviour and Electrical Conductivity of α-BiNbO4 and β-BiNbO4 Ceramics. Micro 2022, 2, 549–563. [Google Scholar] [CrossRef]
- Saadi, H.; Benzarti, Z.; Mourad, S.; Sanguino, P.; Hadouch, Y.; Mezzane, D.; Abdelmoula, N.; Khemakhem, H. Electrical Conductivity Improvement of (Fe + Al) Co-Doped ZnO Nanoparticles for Optoelectronic Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 8065–8085. [Google Scholar] [CrossRef]
- Bhakta, N.; Chakrabarti, P.K. XRD Analysis, Raman, AC Conductivity and Dielectric Properties of Co and Mn Co-Doped SnO2 Nanoparticles. Appl. Phys. A 2019, 125, 73. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.-S.; Pode, R.B. Low Temperature Dielectric Studies of Zinc Oxide (ZnO) Nanoparticles Prepared by Precipitation Method. Adv. Powder Technol. 2013, 24, 331–335. [Google Scholar] [CrossRef]
- Taha, T.A.; Alshammari, M.; Alshammari, A.H.; Eessaa, A.K.; Azab, A.A. Surface Morphology, Structure, and Dielectric Relaxation Investigations of ZnO/Iron Nanostructures. J. Mater. Sci. Mater. Electron. 2023, 34, 739. [Google Scholar] [CrossRef]
- Zulfiqar; Zubair, M.; Khan, A.; Hua, T.; Ilyas, N.; Fashu, S.; Afzal, A.M.; Safeen, M.A.; Khan, R. Oxygen Vacancies Induced Room Temperature Ferromagnetism and Enhanced Dielectric Properties in Co and Mn Co-Doped ZnO Nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 9463–9474. [Google Scholar] [CrossRef]
- Dinesha, M.L.; Prasanna, G.D.; Naveen, C.S.; Jayanna, H.S. Structural and Dielectric Properties of Fe Doped ZnO Nanoparticles. Indian J. Phys. 2013, 87, 147–153. [Google Scholar] [CrossRef]

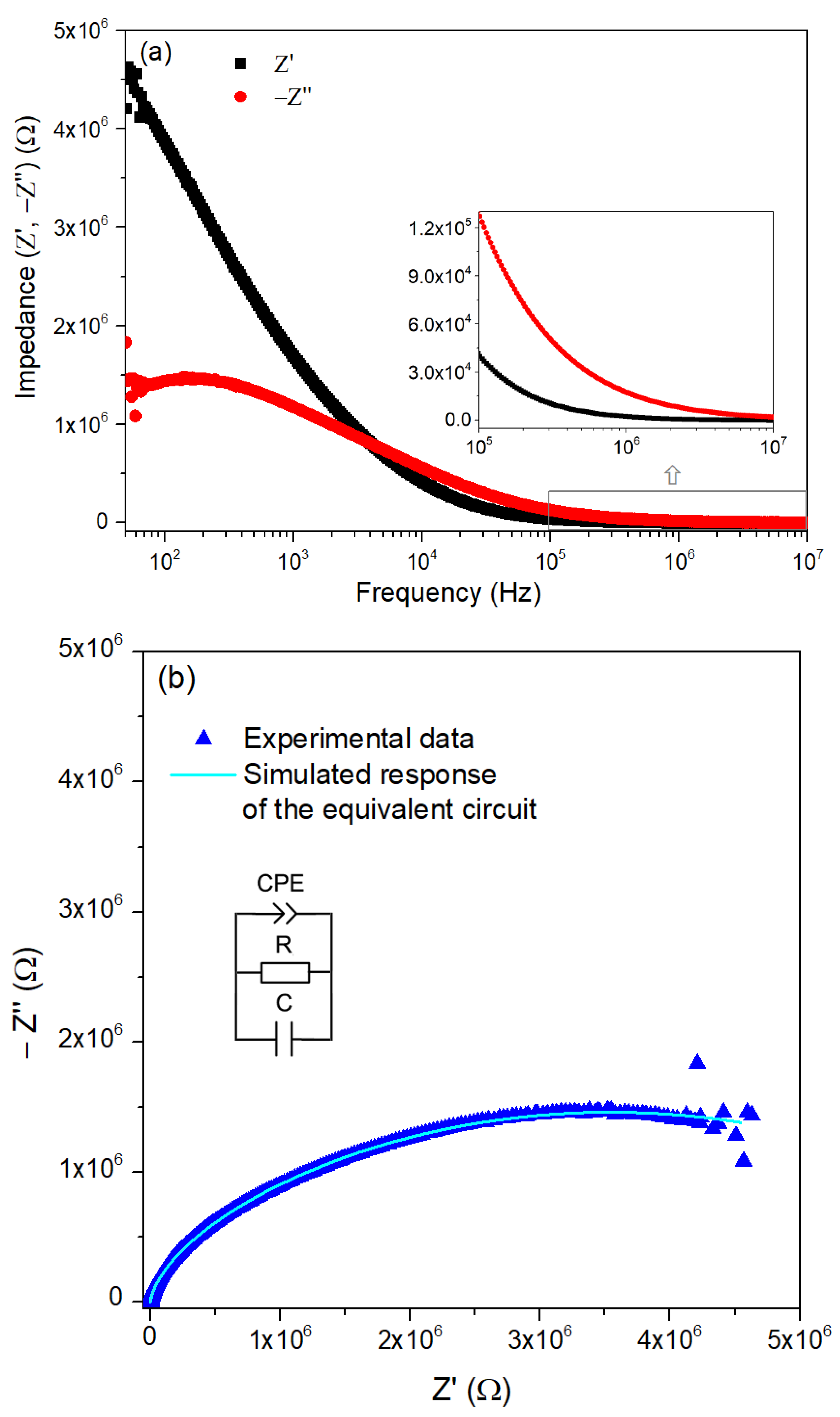
| R (MΩ) | C (pF) | Q (ns) | n (a.u.) |
|---|---|---|---|
| 7.57 | 8.34 | 6.97 | 0.447 |
| Reference | Synthesis Method | Calcination Temperature | ε′ | ε″ | Tan δ |
|---|---|---|---|---|---|
| This work | Green sol-gel | 400 °C | 16.96 | 2.32 | 0.14 |
| Lanje et al. [71] | Precipitation | 100 °C | 10.26 | 2.15 | 0.21 |
| Dinesha et al. [74] | Combustion | 400 °C | ≈15 | ≈12 | ≈0.8 |
| Saadi et al. [26] | Coprecipitation | 500 °C | ≈400 | ≈120 | 0.3 |
| Zulfiqar et al. [73] | Chemical precipitation | 600 °C | ≈2.5 | ≈1 | ≈0.4 |
| Taha et al. [72] | Sol-gel | 550 °C | ≈10 | ≈6 | ≈0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzarti, Z.; Neiva, J.; Faia, P.; Silva, E.; Carvalho, S.; Devesa, S. Novel Green Synthesis Route of ZnO Nanoparticles for Dielectric Applications. Nanomaterials 2025, 15, 991. https://doi.org/10.3390/nano15130991
Benzarti Z, Neiva J, Faia P, Silva E, Carvalho S, Devesa S. Novel Green Synthesis Route of ZnO Nanoparticles for Dielectric Applications. Nanomaterials. 2025; 15(13):991. https://doi.org/10.3390/nano15130991
Chicago/Turabian StyleBenzarti, Zohra, Joana Neiva, Pedro Faia, Eduardo Silva, Sandra Carvalho, and Susana Devesa. 2025. "Novel Green Synthesis Route of ZnO Nanoparticles for Dielectric Applications" Nanomaterials 15, no. 13: 991. https://doi.org/10.3390/nano15130991
APA StyleBenzarti, Z., Neiva, J., Faia, P., Silva, E., Carvalho, S., & Devesa, S. (2025). Novel Green Synthesis Route of ZnO Nanoparticles for Dielectric Applications. Nanomaterials, 15(13), 991. https://doi.org/10.3390/nano15130991











