Vanadium-Based MXenes: Types, Synthesis, and Recent Advances in Supercapacitor Applications
Abstract
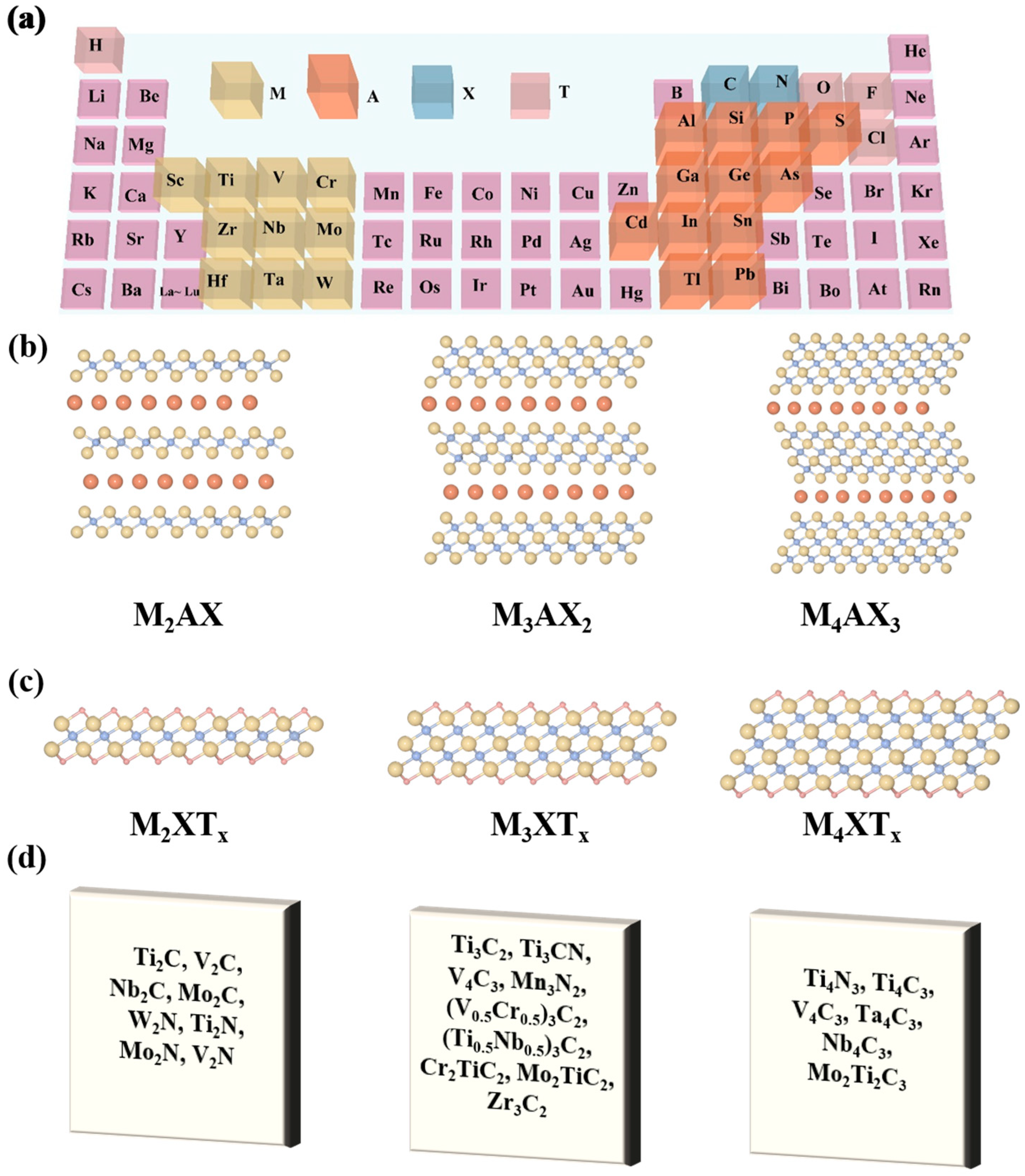
1. Introduction
2. Basic Properties of V-MXene
2.1. Multi-Valence Characteristics
2.2. High Surface Area and Conductivity
2.3. Surface Functionalization and Heterostructures
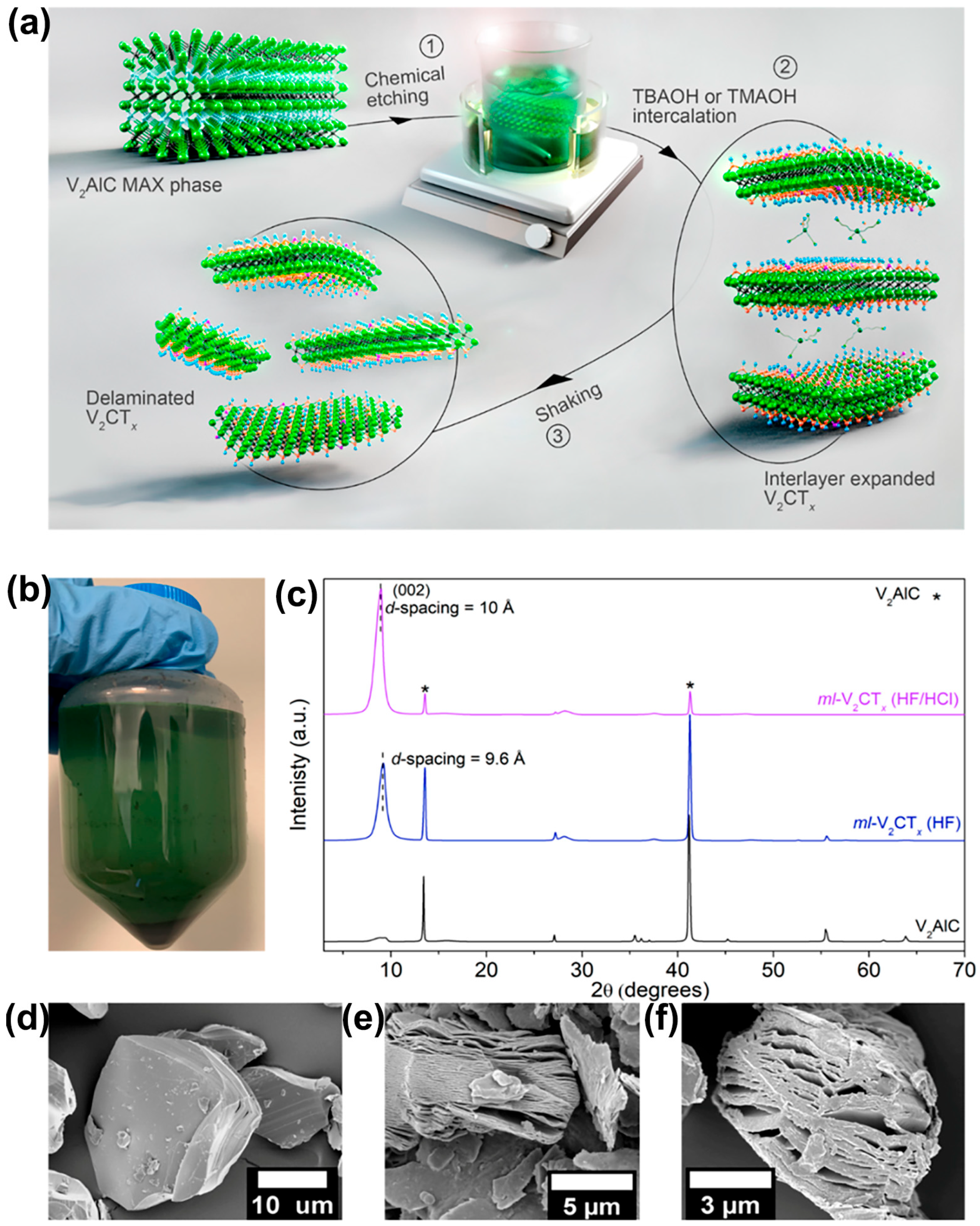
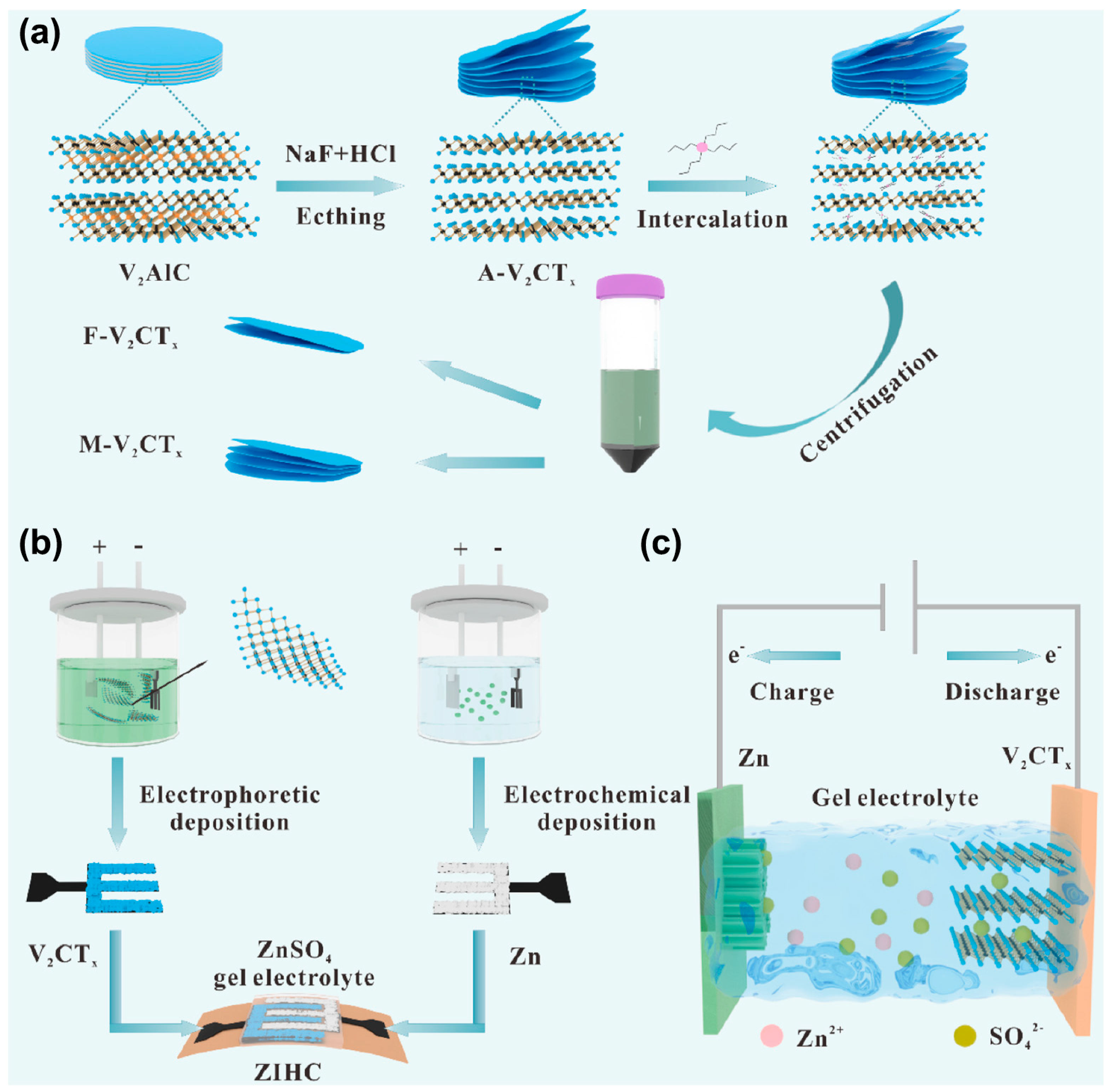
3. Synthesis Methods of V-MXene
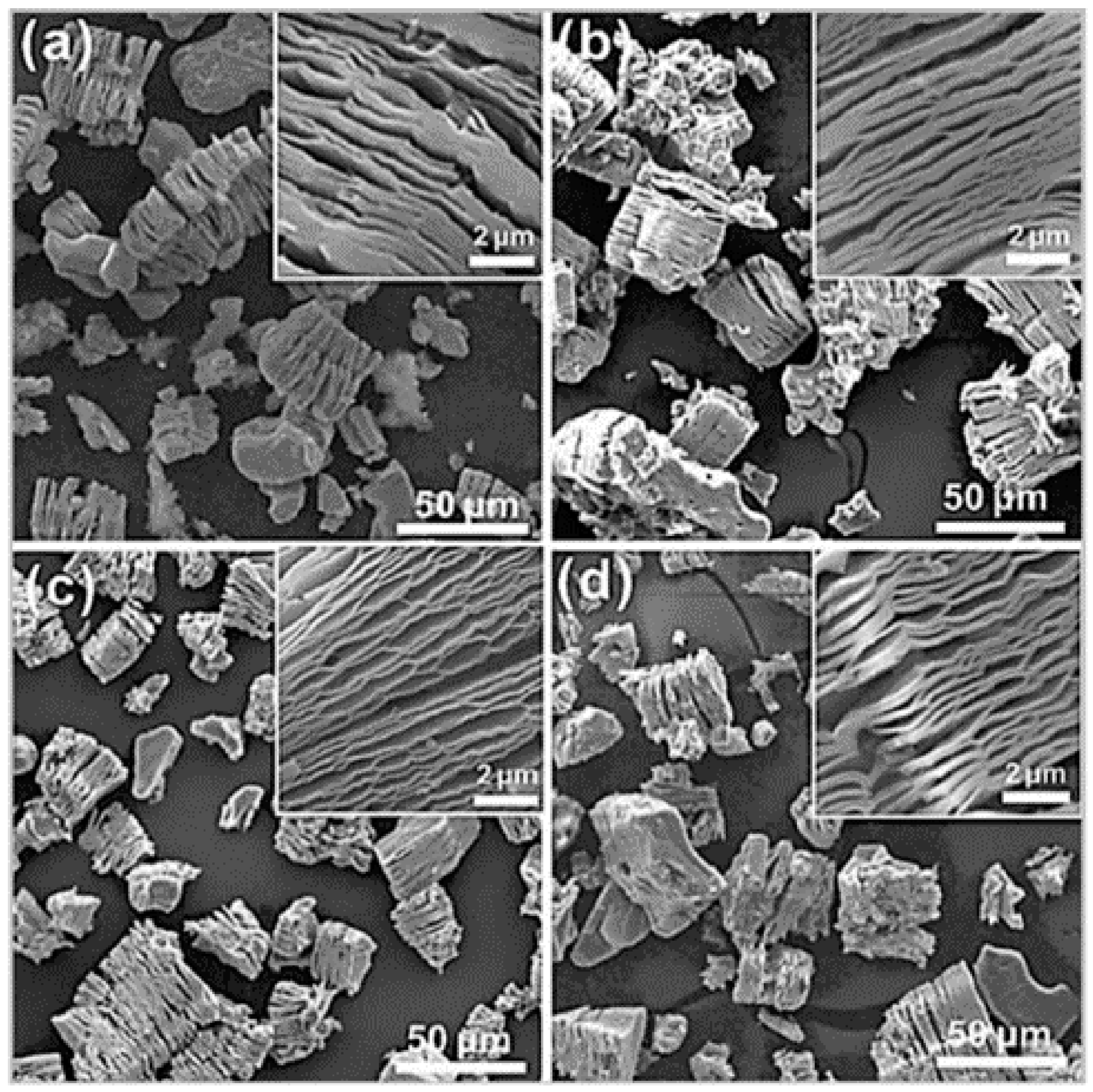
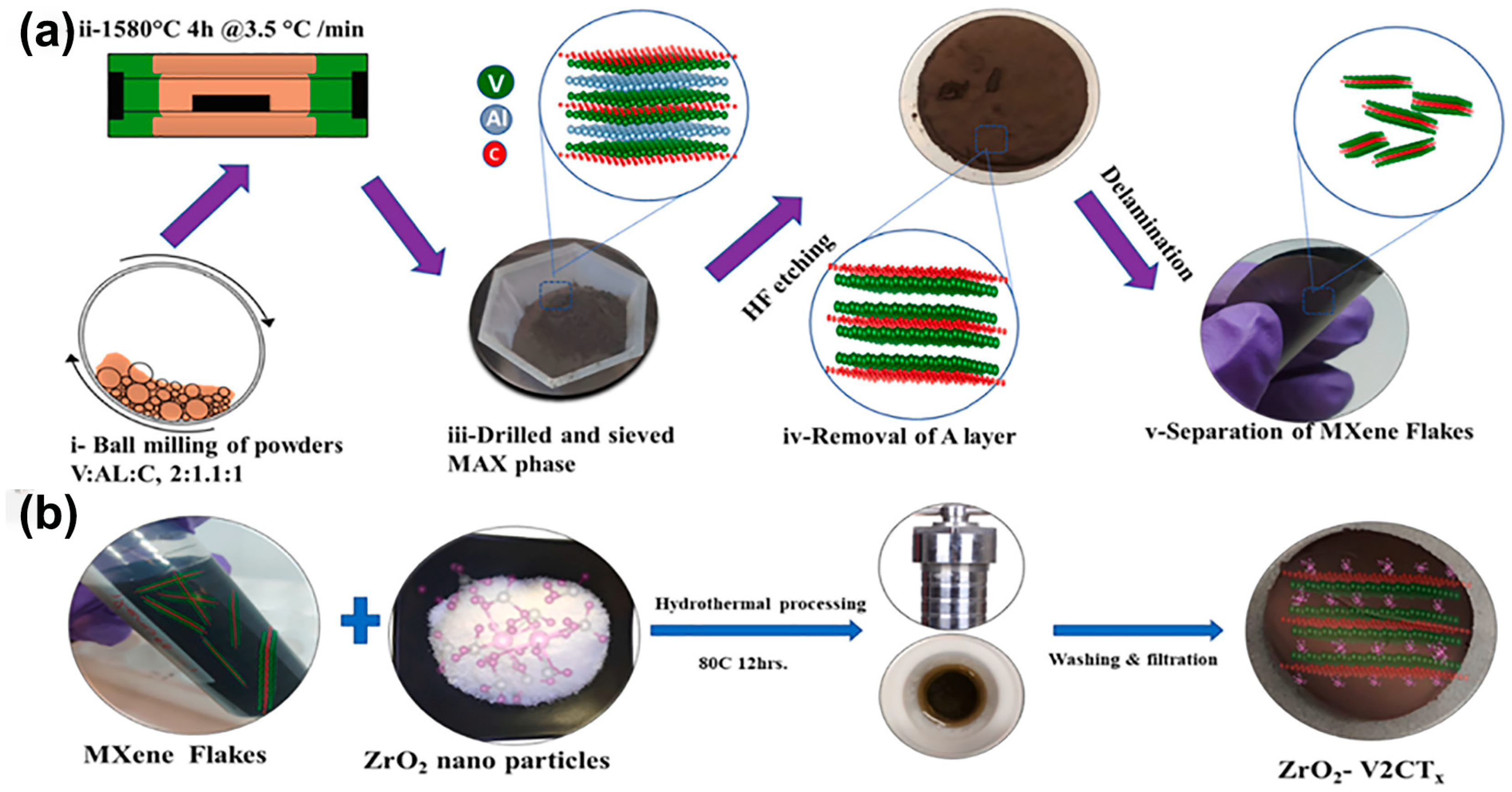
3.1. Mixed-Acid Etching
3.2. Hydrothermal Etching Method
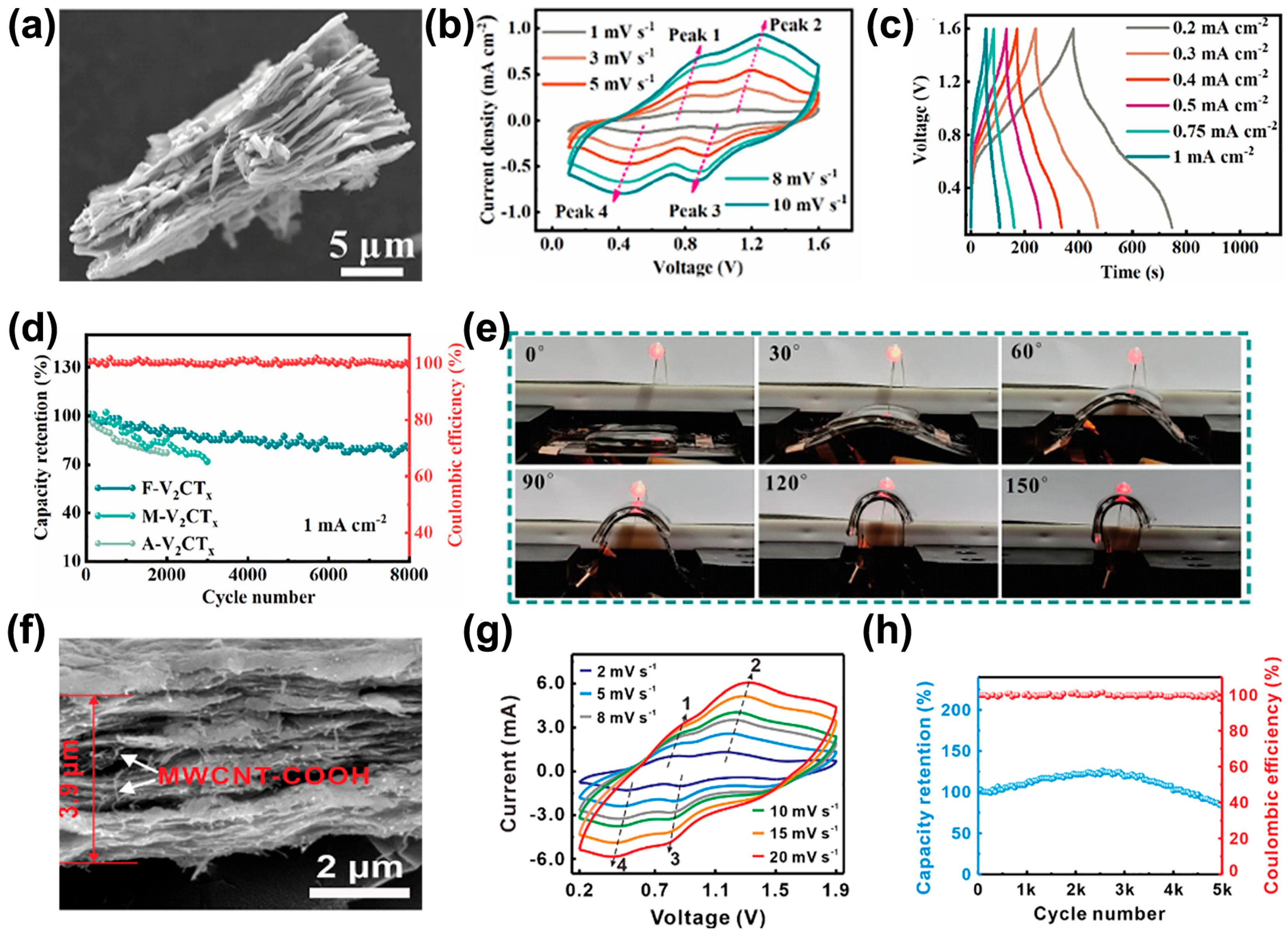
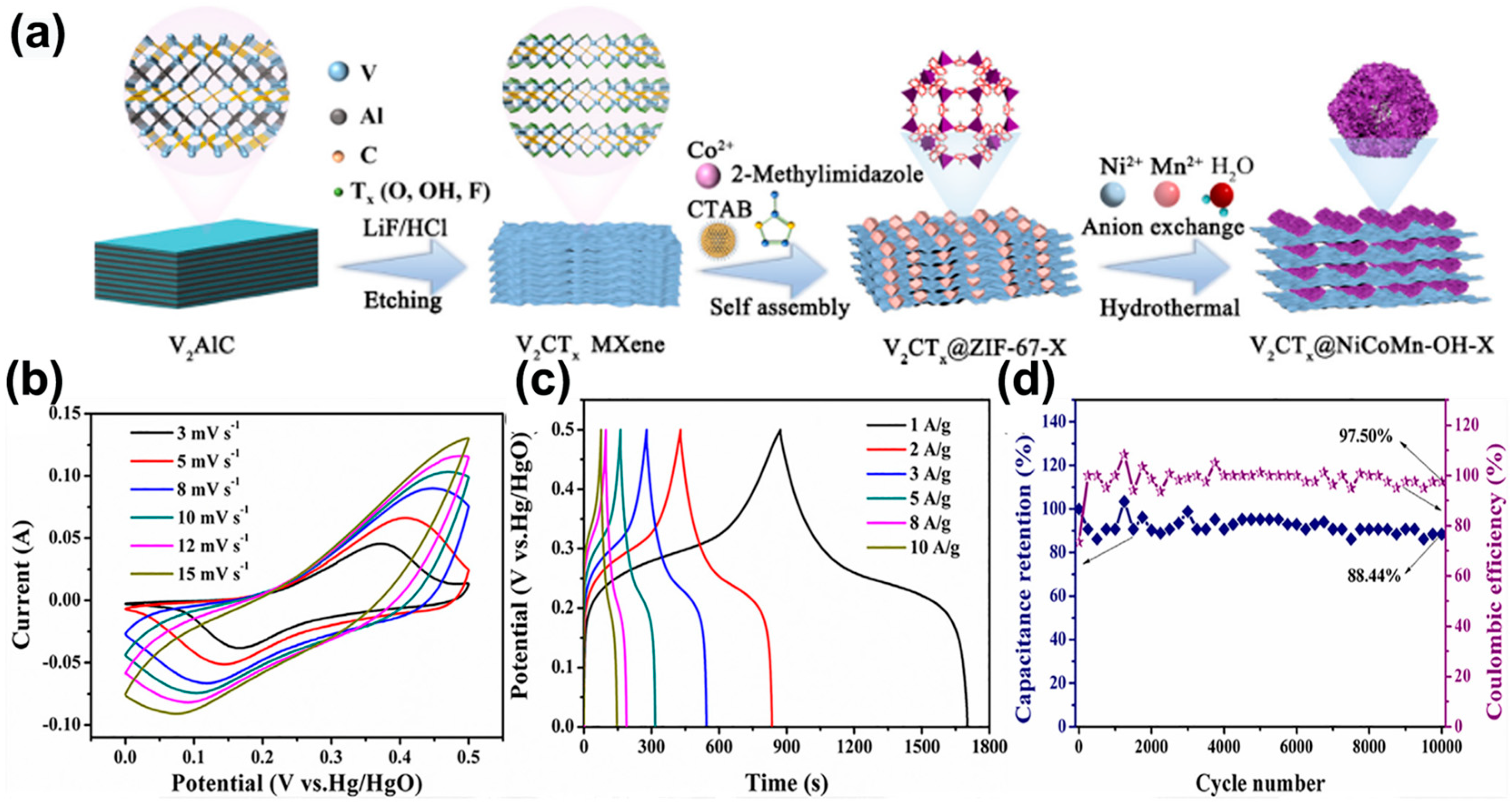
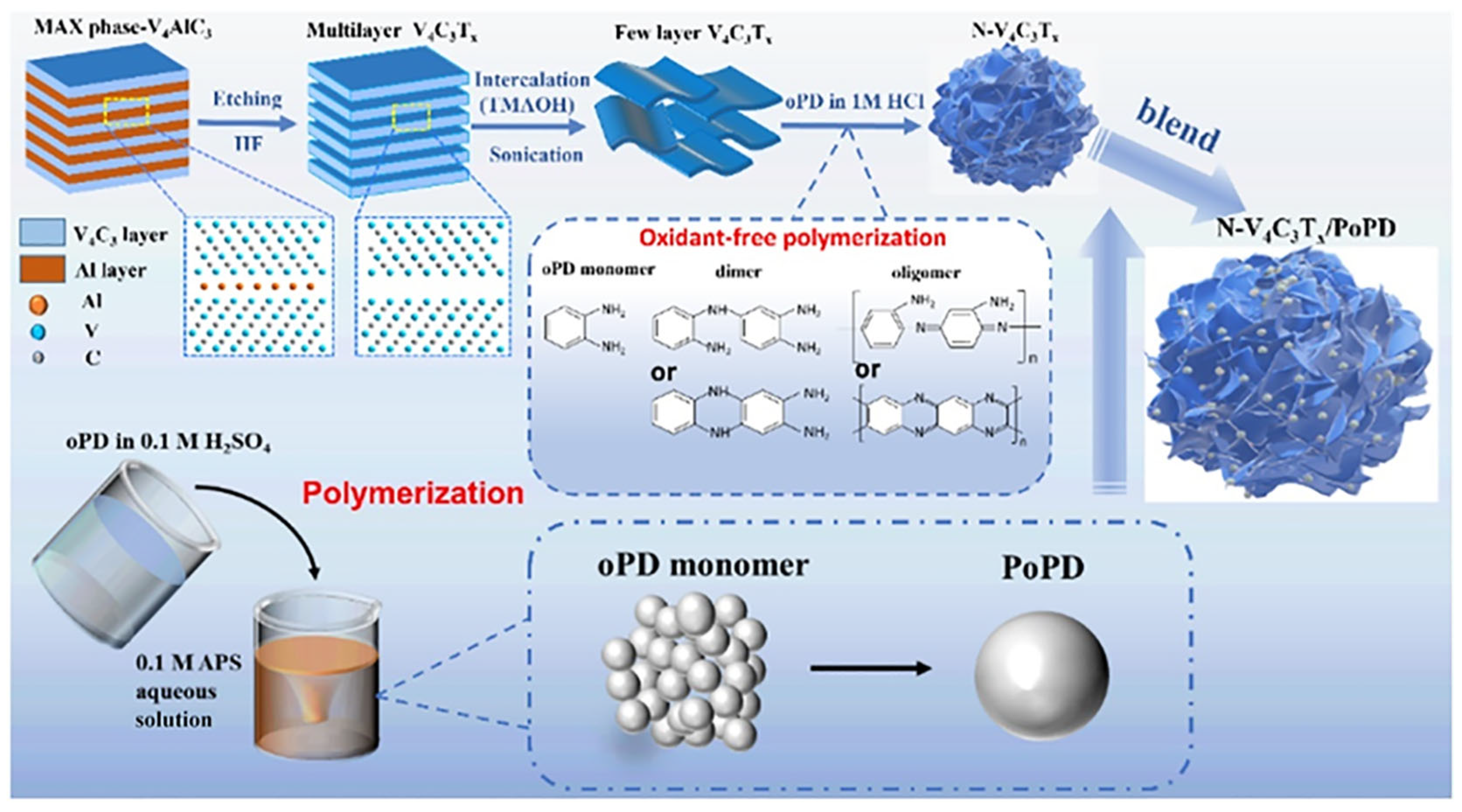
4. Application of V-MXene in Supercapacitors
5. Conclusions and Prospects
5.1. Conclusions
5.2. Prospects
- Enhancing synthesis efficiency and purity: Developing more efficient and green synthesis processes, reducing by-product generation, and optimizing reaction conditions to obtain high-purity V-based MXene.
- Structure and function optimization: Explore different surface modification and functionalization strategies to enhance the stability, conductivity, and reactivity of the material, further improving its electrochemical performance.
- Composite materials and heterostructures: By compounding with other two-dimensional materials or nanomaterials, novel heterostructures are developed. Utilizing the built-in electric field within the heterojunction can accelerate electron transfer and expose more electrochemical sites at the grain boundaries, thereby synergistically enhancing the electrochemical performance.
- Large-scale production and practical applications: advancing the fabrication technologies of V-based MXene materials at industrial scales, paving the way for their energy storage and conversion applications.
Funding
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Varma, A.; Pol, V.G. Carbon anodes for nonaqueous alkali metal-ion batteries and their thermal safety aspects. Adv. Energy Mater. 2019, 9, 1900550. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.; Sofer, Z. Beyond graphene: Chemistry of group 14 graphene analogues: Silicene, germanene, and stanene. ACS Nano 2019, 13, 8566–8576. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, K.; Yan, D.; Chen, R.; Zou, Y.; Liu, H.; Wang, S. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater. 2018, 30, 1800295. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Liu, M.; Su, B.; Tang, Y.; Jiang, X.; Yu, A. Recent advances in nanostructured vanadium oxides and composites for energy conversion. Adv. Energy Mater. 2017, 7, 1700885. [Google Scholar] [CrossRef]
- Zavabeti, A.; Jannat, A.; Zhong, L.; Haidry, A.A.; Yao, Z.; Ou, J.Z. Two-dimensional materials in large-areas: Synthesis, properties and applications. Nano-Micro Lett. 2020, 12, 66. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-dimensional building blocks for future materials and devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Feng, J.; Qian, Y. MXenes for advanced separator in rechargeable batteries. Mater. Today 2022, 57, 146–179. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Hemanth, N.R.; Kandasubramanian, B. Recent advances in 2D MXenes for enhanced cation intercalation in energy harvesting applications: A review. Chem. Eng. J. 2020, 392, 123678. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage; Jenny Stanford Publishing: Singapore, 2023; pp. 677–722. [Google Scholar]
- Zhang, S.; Han, W.Q. Recent advances in MXenes and their composites in lithium/sodium batteries from the viewpoints of components and interlayer engineering. Phys. Chem. Chem. Phys. 2020, 22, 16482–16526. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, B.C.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable mechanical and tribological properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. MXenes: Trends, growth, and future directions. Graphene 2D Mater. 2022, 7, 75–79. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared structure design of MXene with ultra-large interlayer spacing for high-performance lithium-ion capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, E.A.; Anasori, B.; et al. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2019, 14, 204–217. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Karimiziarani, M.; Moradkhani, H.; Elliott, M.; Anasori, B. MXenes: The two-dimensional influencers. Mater. Today Adv. 2022, 13, 100202. [Google Scholar] [CrossRef]
- Ahmed, B.; Ghazaly, A.E.; Rosen, J. i-MXenes for energy storage and catalysis. Adv. Funct. Mater. 2020, 30, 2000894. [Google Scholar] [CrossRef]
- Hu, T.; Wang, J.; Zhang, H.; Li, Z.; Hu, M.; Wang, X. Vibrational properties of Ti3C2 and Ti3C2Tx (T = O, F, OH) monosheets by first-principles calculations: A comparative study. Phys. Chem. Chem. Phys. 2015, 17, 9997–10003. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Zhao, S.; Guo, M.; Wu, D.; Luo, S.; Zhang, C.; Huang, T.; He, W.; Li, M.; Zhou, X.; et al. Nitrogen doping engineering of V2CTx based zinc ion hybrid microcapacitors with quadruple high energy density. Chem. Eng. J. 2024, 499, 156668. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Jenny Stanf. Publ. 2023, 23, 15–29. [Google Scholar]
- Haemers, J.; Gusmão, R.; Sofer, Z. Synthesis protocols of the most common layered carbide and nitride MAX phases. Small Methods 2020, 4, 1900780. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Li, H.; Xu, H. Phase transition induced unusual electrochemical performance of V2CTx MXene for aqueous zinc hybrid-ion battery. ACS Nano 2020, 14, 541–551. [Google Scholar] [CrossRef]
- Song, J.; Cao, X.; Huang, Z. Diatomite-chitosan composite with abundant functional groups as efficient adsorbent for vanadium removal: Key influencing factors and influence of surface functional groups. J. Mol. Liq. 2022, 367, 120428. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Zhao, S.; Zhang, H.; Li, X.; Luo, X.; Cao, M.; Zhang, C.; Luo, S.; Yue, Y.; et al. All-MXenes zinc ion hybrid micro-supercapacitor with wide voltage window based on V2CTx cathode and Ti3C2Tx anode. Nano Energy 2023, 111, 108383. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Bhattarai, R.M.; Lohani, P.C.; Pathak, I.; Ji, S.; Ko, T.H.; et al. Hollow carbon nanofibers with inside-outside decoration of bi-metallic MOF derived Ni-Fe phosphides as electrode materials for asymmetric supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Xu, W.; Liu, C.; Wu, Q.; Kim, W.Y.; Lee, S.Y.; Gwon, J. A stretchable solid-state zinc ion battery based on a cellulose nanofiber–polyacrylamide hydrogel electrolyte and a Mg0.23V2O5·1.0H2O cathode. J. Mater. Chem. A 2020, 8, 18327–18337. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, H.; Kong, F.; Tian, H.; Meng, G.; Wang, S.; Mao, X.; Cui, X.; Hou, X.; Shi, J. V2C MXene synergistically coupling FeNi LDH nanosheets for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2021, 297, 120474. [Google Scholar] [CrossRef]
- Stone, K.H.; Schelhas, L.T.; Garten, L.M.; Shyam, B.; Mehta, A. Influence of amorphous structure on polymorphism in vanadia. APL Mater. 2016, 4, 076103. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Zhang, B.; Yan, J.; Qiu, J. Ultrahigh Pyridinic/Pyrrolic N Enabling N/S Co-Doped Holey Graphene with Accelerated Kinetics for Alkali-Ion Batteries. Adv. Mater. 2024, 36, 2407570. [Google Scholar] [CrossRef]
- Huang, P.; Han, W.Q. Recent advances and perspectives of lewis acidic etching route: An emerging preparation strategy for MXenes. Nano-Micro Lett. 2023, 15, 68. [Google Scholar] [CrossRef]
- Liu, X.; Li, G.; Wu, J.; Zhang, D.; Li, L. Fabrication of VO nanorings on a porous carbon architecture for high-performance Li-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 9454–9463. [Google Scholar] [CrossRef]
- Ji, Q.; Li, C.; Wang, J.; Niu, J.; Gong, Y.; Zhang, Z.; Fang, Q.; Zhang, Y.; Shi, J.; Liao, L.; et al. Metallic vanadium disulfide nanosheets as a platform material for multifunctional electrode applications. Nano Lett. 2017, 17, 4908–4916. [Google Scholar] [CrossRef]
- Dong, Y.; Huo, J.; Xu, C.; Ji, D.; Zhao, H.; Li, L.; Lei, Y. Research Progress on Vanadium Sulfide Anode Materials for Sodium and Potassium-Ion Batteries. Adv. Mater. Technol. 2024, 9, 2301840. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, X.; Cheng, Y.; Shi, Z.; Huang, T. A flexible zinc ion hybrid capacitor integrated system with layers-dependent V2CTx MXene. Chem. Eng. J. 2023, 454, 140360. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, S.; Huang, T.; He, W.; Zhou, X.; Wang, G.; Guo, M.; Luo, X.; Cao, M.; Yue, Y.; et al. Self-charging V2CTx/CNT-based zinc ion micro-supercapacitor for wearable electronics. Chem. Eng. J. 2024, 490, 151589. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, K.; Liu, X.; Yao, S.; Li, X.; Si, Y.; Li, X. 3D porous PEDOT/MXene scaffold toward high-performance supercapacitors. Chem. Eng. J. 2024, 496, 154348. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Z.; Wang, W.; Li, H.; Dong, J.; Gao, S.; Xu, D.; Li, L.; Shen, J.; Ye, M. Superior-performance aqueous zinc-ion batteries based on the in situ growth of MnO2 nanosheets on V2CTx MXene. ACS Nano 2021, 15, 2971–2983. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; An, Y.; Wei, H.; Wei, C.; Tao, Y.; Li, Y.; Feng, J.; Qian, Y. Micron-sized nanoporous vanadium pentoxide arrays for high-performance gel zinc-ion batteries and potassium batteries. Chem. Mater. 2020, 32, 4054–4064. [Google Scholar] [CrossRef]
- Matthews, K.; Zhang, T.; Shuck, C.E.; VahidMohammadi, A. Guidelines for synthesis and processing of chemically stable two-dimensional V2CTx MXene. Chem. Mater. 2021, 34, 499–509. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; He, P.; Hu, Q.; Fan, L.Z.; Zhou, A. Synthesis of two-dimensional carbide Mo2CTx MXene by hydrothermal etching with fluorides and its thermal stability. Ceram. Int. 2020, 46, 19550–19556. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Wang, S.; Wang, B.; Shen, C.; Wang, L.; Hu, Q.; Huang, Q.; Zhou, A. Preparation of high-purity V2C MXene and electrochemical properties as Li-ion batteries. J. Electrochem. Soc. 2017, 164, A709. [Google Scholar] [CrossRef]
- Guan, Y.; Jiang, S.; Cong, Y.; Wang, J.; Dong, Z.; Zhang, Q.; Yuan, G.; Li, Y.; Li, X. A hydrofluoric acid-free synthesis of 2D vanadium carbide (V2C) MXene for supercapacitor electrodes. 2D Mater. 2020, 7, 025010. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Liang, W.; Mojtabavi, M.; Wanunu, M.; Beidaghi, M. 2D titanium and vanadium carbide MXene heterostructures for electrochemical energy storage. Energy Storage Mater. 2021, 41, 554–562. [Google Scholar] [CrossRef]
- Zahra, S.A.; Ceesay, E.; Rizwan, S. Zirconia-decorated V2CTx MXene electrodes for supercapacitors. J. Energy Storage 2022, 55, 105721. [Google Scholar] [CrossRef]
- Yadav, A.; Singal, S.; Soni, P.; Singh, G.; Sharma, R.K. Structural engineering and carbon enrichment in V2CTx MXene: An approach for enhanced supercapacitive charge storage. J. Alloys Compd. 2023, 934, 167859. [Google Scholar] [CrossRef]
- Yu, T.; Li, S.; Zhang, L.; Li, F.; Wang, J.; Pan, H.; Zhang, D. In situ growth of ZIF-67-derived nickel-cobalt-manganese hydroxides on 2D V2CTx MXene for dual-functional orientation as high-performance asymmetric supercapacitor and electrochemical hydroquinone sensor. J. Colloid Interface Sci. 2023, 629, 546–558. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Li, J.; Yuan, Z.; Li, D.; Wang, L.; Han, W. Self-assembled Cobalt-doped NiMn-layered double hydroxide (LDH)/V2CTx MXene hybrids for advanced aqueous electrochemical energy storage properties. Chem. Eng. J. 2022, 430, 132992. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Synthesis, properties and performance evaluation of vanadium carbide MXene as supercapacitor electrodes. Ceram. Int. 2020, 46, 5323–5330. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Tong, H.; Huang, Y.; Tong, P.; Zhao, B.; Dai, J.; Liang, C.; Wang, H.; Zhu, X.; et al. Two-dimensional V4C3 MXene as high performance electrode materials for supercapacitors. Electrochim. Acta 2019, 307, 414–421. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Yang, L.; Yin, S. Poly (o-phenylenediamine)-Decorated V4C3Tx MXene/Poly (o-phenylenediamine) Blends as Electrode Materials to Boost Storage Capacity for Supercapacitors and Lithium-Ion Batteries. ACS Appl. Nano Mater. 2023, 6, 9186–9196. [Google Scholar] [CrossRef]
- Geng, Z.; Chen, W.; Qiu, Z.; Xu, H.; Pan, D.; Chen, S. Hierarchical V4C3TX@NiO-reduced graphene oxide heterostructure hydrogels and defective reduced graphene oxide hydrogels as free-standing anodes and cathodes for high-performance asymmetric supercapacitors. Phys. Chem. Chem. Phys. 2023, 25, 9140–9151. [Google Scholar] [CrossRef]
- Chen, K.; Guan, Y.; Cong, Y.; Zhu, H.; Li, K.; Wu, J.; Dong, Z.; Yuan, G.; Zhang, Q.; Li, X. Vertically pillared V2CTx/Ti3C2Tx flexible films for high-performance supercapacitors. J. Alloys Compd. 2022, 906, 164302. [Google Scholar] [CrossRef]
- Chen, C.; Pang, D.; Wang, X.; Chen, G.; Du, F. Electrochemical behavior of vanadium carbide in neutral aqueous electrolytes. Chin. Phys. Lett. 2021, 38, 058201. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Lamiel, C.; Ahmad, M.; Ashraf, M.A.; Chen, Y.; Gu, S.; Javed, M.S.; Zhang, K. Phosphorus containing layered quadruple hydroxide electrode materials on lab waste recycled flexible current collector. J. Colloid Interface Sci. 2022, 609, 566–574. [Google Scholar] [CrossRef]
- Mateen, A.; Ahmad, Z.; Ali, S.; Hassan, N.U.; Ahmed, F.; Alshgari Peng, K.Q. Silicon intercalation on MXene nanosheets towards new insights into a superior electrode material for high-performance Zn-ion supercapacitor. J. Energy Storage 2023, 71, 108151. [Google Scholar] [CrossRef]
- Zhang, T.; Matthews, K.; VahidMohammadi, A.; Han, M.; Gogotsi, Y. Pseudocapacitance of vanadium carbide MXenes in basic and acidic aqueous electrolytes. ACS Energy Lett. 2022, 7, 3864–3870. [Google Scholar] [CrossRef]
- Pan, J.; Li, S.; Zhang, L.; Li, F.; Zhang, Z.; Yu, T.; Zhang, D. Designed formation of 2D/2D hierarchical V2CTx MXene/NiV layered double hydroxide heterostructure with boosted electrochemical performance for asymmetric supercapacitors. J. Energy Storage 2022, 55, 105415. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, H.; Lin, S.; Zhao, B.; Dai, J.; Sun, Y. Capacitance improvements of V4C3Tx by NH3 annealing. J. Alloys Compd. 2019, 784, 923–930. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Huang, Y.; Tong, P.; Zhao, B.; Zhu, X.; Sun, Y. Synthesis and lithium-ion storage performance of two-dimensional V4C3 MXene. Chem. Eng. J. 2019, 373, 203–212. [Google Scholar] [CrossRef]
| Material | Etching Method | Synthesis Time | Synthesis Temperature | Capacity | Reference |
|---|---|---|---|---|---|
| V2CTx | NaF + HCL (6 M) | 7 day | 90 °C | 0.1 mA cm−2 54.12 mF cm−2 | [50] |
| V2CTx | LiF + HCL | 120 h | 90 °C | 2 mV s−1 164 F g−1 | [59] |
| V2CTx | LiF + HCL (6 M) | 24 h | 35 °C | 2 mV s−1 1473 F cm−3 | [60] |
| V2CTx | 50% HF | 92 h | 25 °C | 5 mV s−1 1200 F g−1 | [61] |
| V2CTx | 48% HF | 120 h | 70 °C | 2 mV s−1 1842 F g−1 | [62] |
| V2CTx | LiF + HCL (12 M) | 72h | 90 °C | 1 A g−1 827.45 C g−1 | [63] |
| V2CTx | LiF + HCL (12 M) | 72 h | 90 °C | 1 A g−1 1658.2 F g−1 | [64] |
| V4C3Tx | 40% HF | 3 day | 25 °C | 5 mV s−1 330 F g−1 | [65] |
| V4C3Tx | 40% HF | 96 h | 55 °C | 2 mV s−1 209 F g−1 | [66] |
| Electrode Material | Electrolyte | Potential Window | Capacitance | Scan Rate/Current Density | Reference |
|---|---|---|---|---|---|
| V2CTx | 2 M Zn2SO4 | 0.1~1.6 | 54.12 mF cm−2 | 0.1 mA cm−2 | [50] |
| V2CTx/CNT | 2 M Zn2SO4 | 0.2~1.9 | 246.88 mF cm−2 | 0.5 mA cm−2 | [51] |
| V2C | 1 M Na2SO4 | −0.3~−0.9 | 164 F g−1 | 2 mV s−1 | [59] |
| V2CTx | 3 M H2SO4 | −0.4~0.2 | 1473 F cm−3 | 2 mV s−1 | [60] |
| V2CTx/ZrO2 | 3 M H2SO4 | −0.5~0.3 | 1200 F g−1 | 5 mV s−1 | [61] |
| V2CTx@C | 1 M H2SO4 | 0.0~1.2 | 551 F g−1 | 2 A g−1 | [62] |
| V2CTx@NiCoMn-OH | 3 M KOH | 0.0~0.5 | 827.45 C g−1 | 1 A g−1 | [63] |
| V2CTx/Co-LDH | 6 M KOH | −0.1~0.4 | 1005 F g−1 | 1 A g−1 | [64] |
| V4C3Tx | 1 M H2SO4 | −0.4~0.4 | 330 F g−1 | 5 mV s−1 | [65] |
| V4C3 | 1 M H2SO4 | −0.3~0.1 | 209 F g−1 | 2 mV s−1 | [66] |
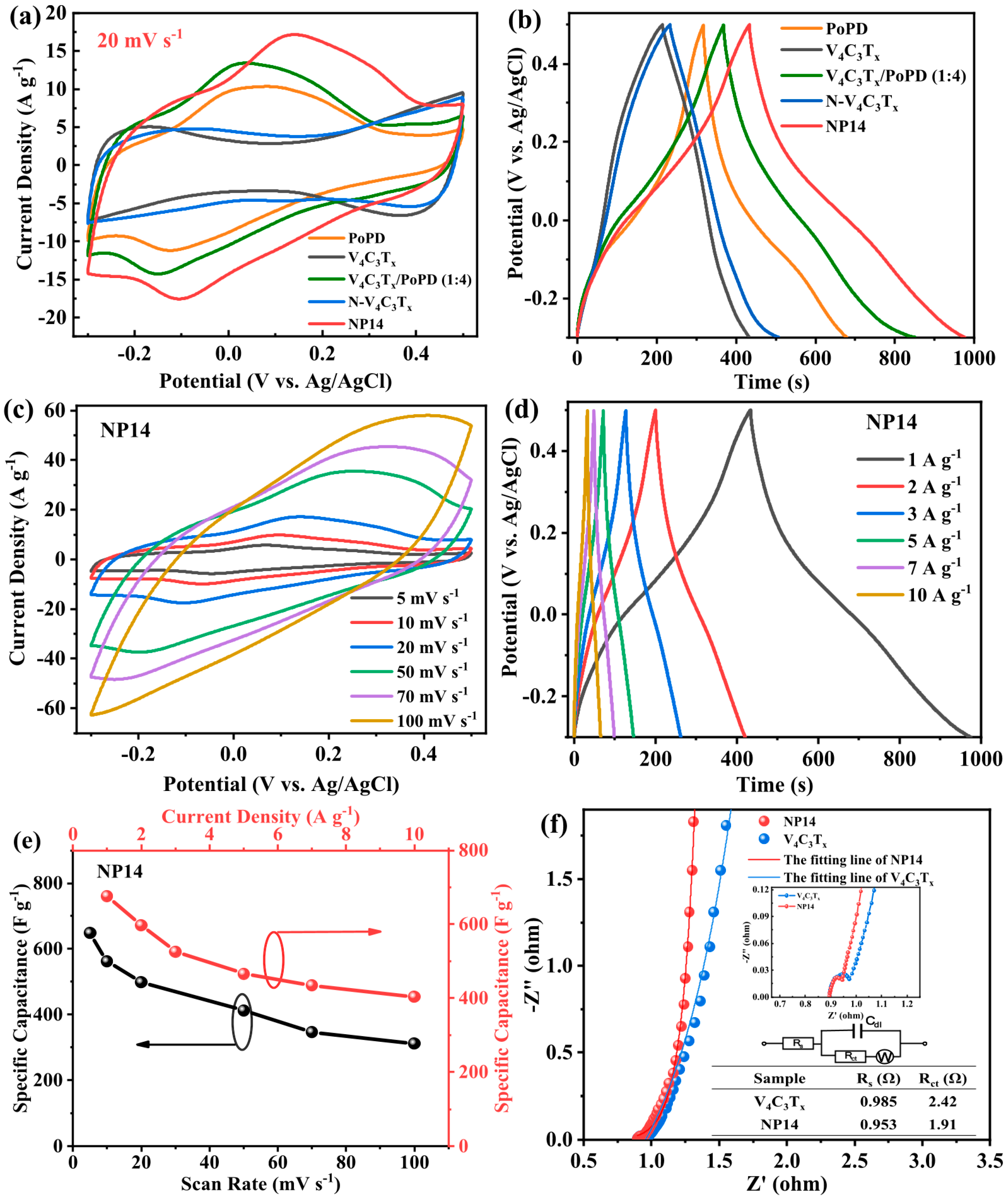
| V4C3Tx/POPD | 1 M H2SO4 | −0.2~0.4 | 676.5 F g−1 | 1 A g−1 | [67] |
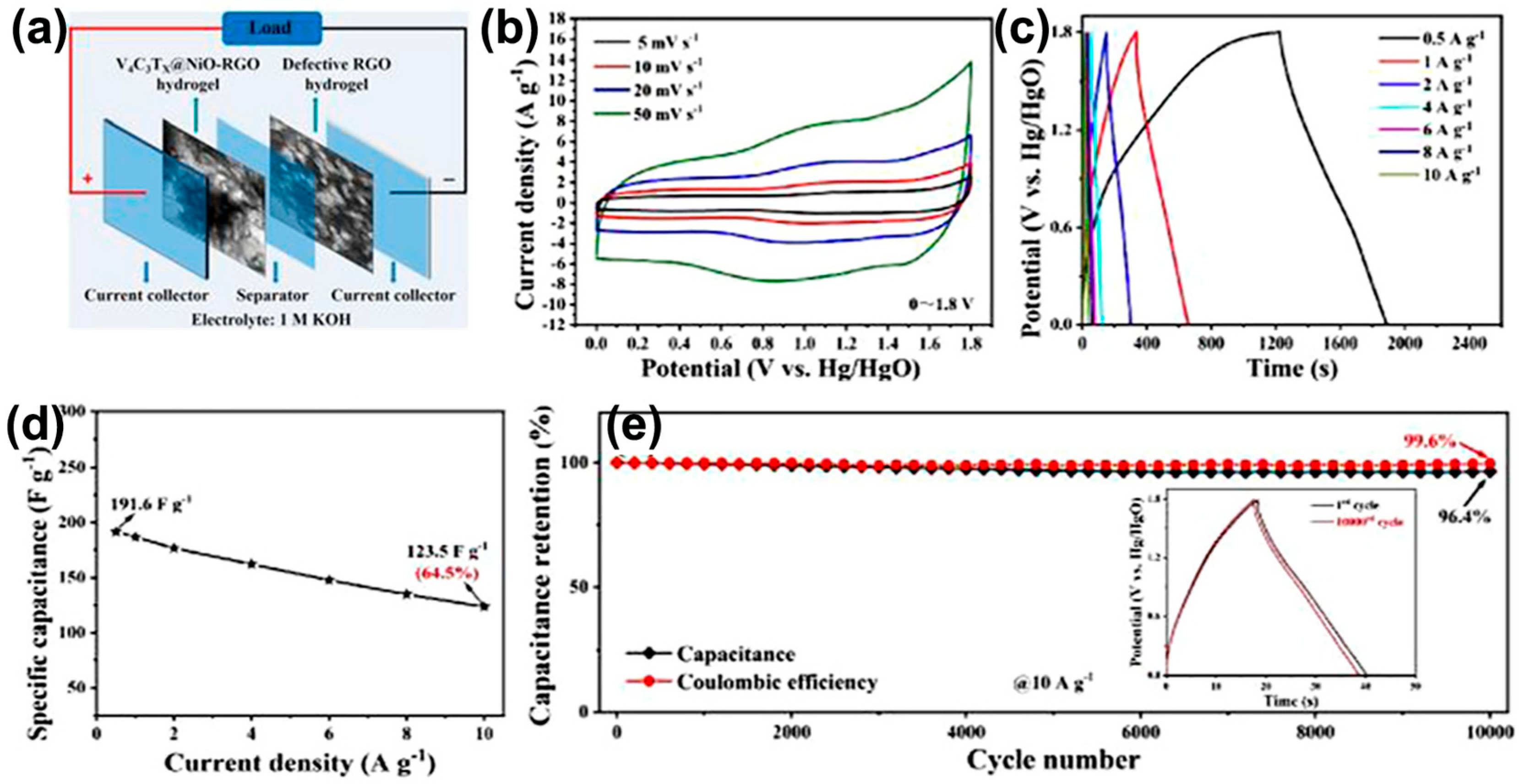
| V4C3Tx@NiO-RGO | 1 M KOH | −1.0~0.8 | 1021 F g−1 | 0.5 A g−1 | [68] |
| V2CTx/Ti3C2Tx | 1 M H2SO4 | −0.6~0.2 | 365 F g−1 | 1 A g−1 | [69] |
| V2C | 1 M Na2SO4 | −1.0~−0.2 | 120 F g−1 | 2 mV s−1 | [70] |
| V2CTx | 2 M Zn2SO4 | −0.9~0.3 | 481 F g−1 | 1 A g−1 | [71] |
| V2CTx@Si | 1 M Zn2SO4 | 0~1.0 | 557 F g−1 | 1 A g−1 | [72] |
| V2CTx | 1 M LiOH | −1.4~0.8 | 386 F g−1 | 2 mV s−1 | [73] |
| V2CTx/NiV-LDH | 6 M KOH | 0.0~0.5 | 1658.2 F g−1 | 1 A g−1 | [74] |
| V4C3Tx | 1 M H2SO4 | −0.3~0.3 | 210 F g−1 | 10 mV s−1 | [75] |
| NiCoAl-LDH/V4C3Tx | 1 M KOH | −0.1~0.5 | 627 F g−1 | 1 A g−1 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Shi, D.; Xu, J.; Hai, T.; Zhao, Y.; Qin, M.; Li, J. Vanadium-Based MXenes: Types, Synthesis, and Recent Advances in Supercapacitor Applications. Nanomaterials 2025, 15, 1038. https://doi.org/10.3390/nano15131038
Gao Z, Shi D, Xu J, Hai T, Zhao Y, Qin M, Li J. Vanadium-Based MXenes: Types, Synthesis, and Recent Advances in Supercapacitor Applications. Nanomaterials. 2025; 15(13):1038. https://doi.org/10.3390/nano15131038
Chicago/Turabian StyleGao, Zhiwei, Donghu Shi, Jiawei Xu, Te Hai, Yao Zhao, Meng Qin, and Jian Li. 2025. "Vanadium-Based MXenes: Types, Synthesis, and Recent Advances in Supercapacitor Applications" Nanomaterials 15, no. 13: 1038. https://doi.org/10.3390/nano15131038
APA StyleGao, Z., Shi, D., Xu, J., Hai, T., Zhao, Y., Qin, M., & Li, J. (2025). Vanadium-Based MXenes: Types, Synthesis, and Recent Advances in Supercapacitor Applications. Nanomaterials, 15(13), 1038. https://doi.org/10.3390/nano15131038





