Redox-Modified Nanostructured Electrochemical Surfaces for Continuous Glucose Monitoring in Complex Biological Fluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanocomposite Deposition
2.2. Fabrication of the Redox-Functionalized Glucose Sensor
2.3. Electrochemical Measurements
2.4. Sample Collection
3. Results
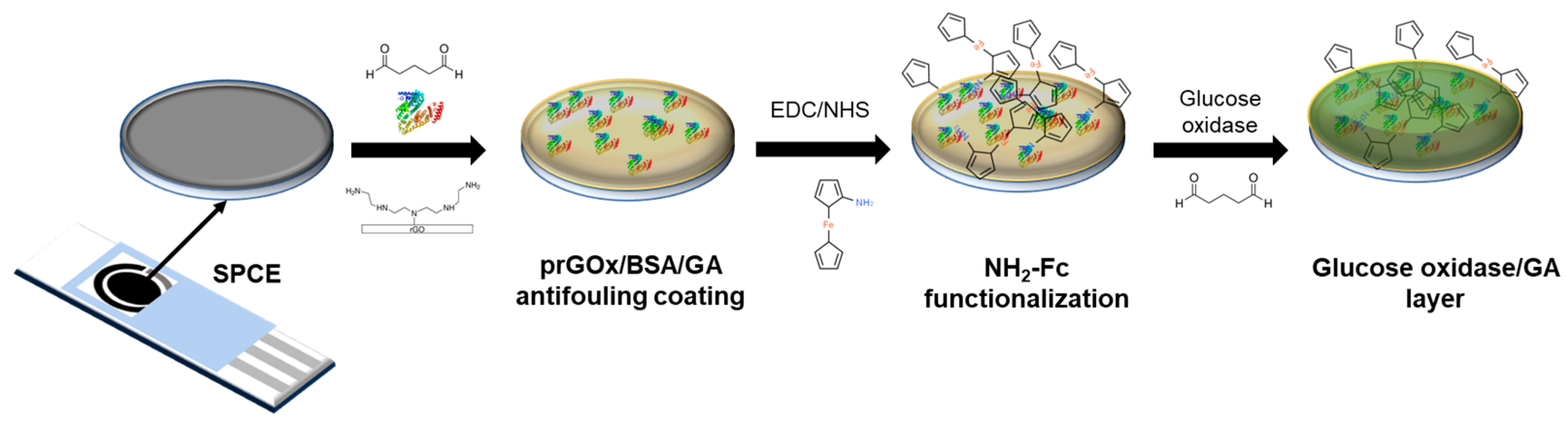
3.1. Fabrication of the Nanocomposite Interface with Confined Redox Probes
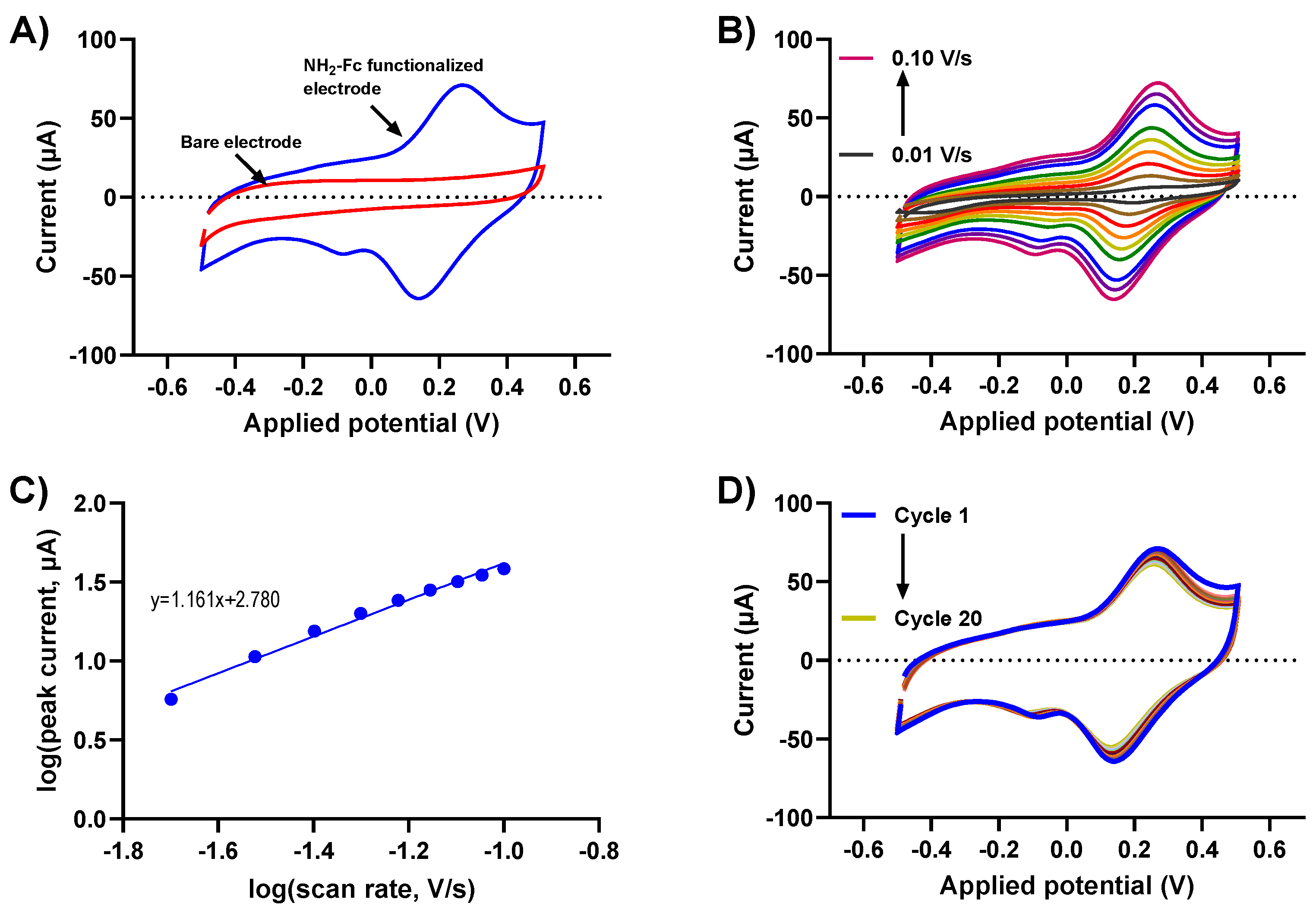
3.2. Electrochemical Characterization of the Redox Immobilized Glucose Sensor
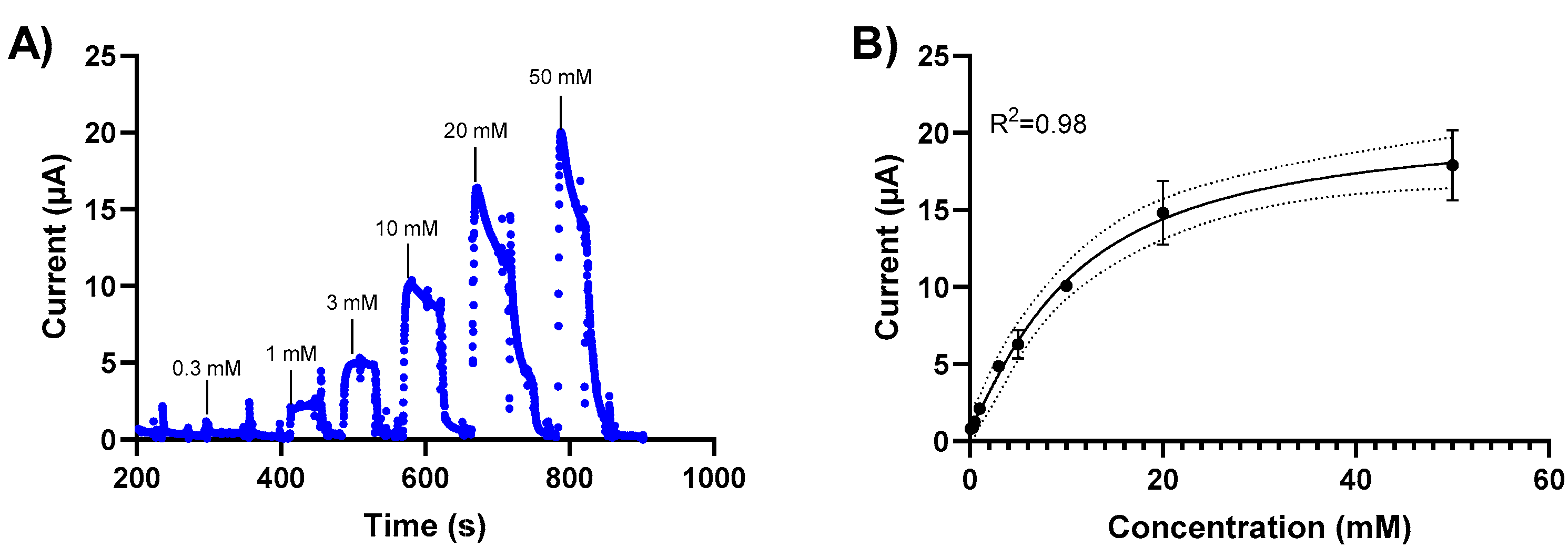
3.3. Glucose Detection in a Standard Solution
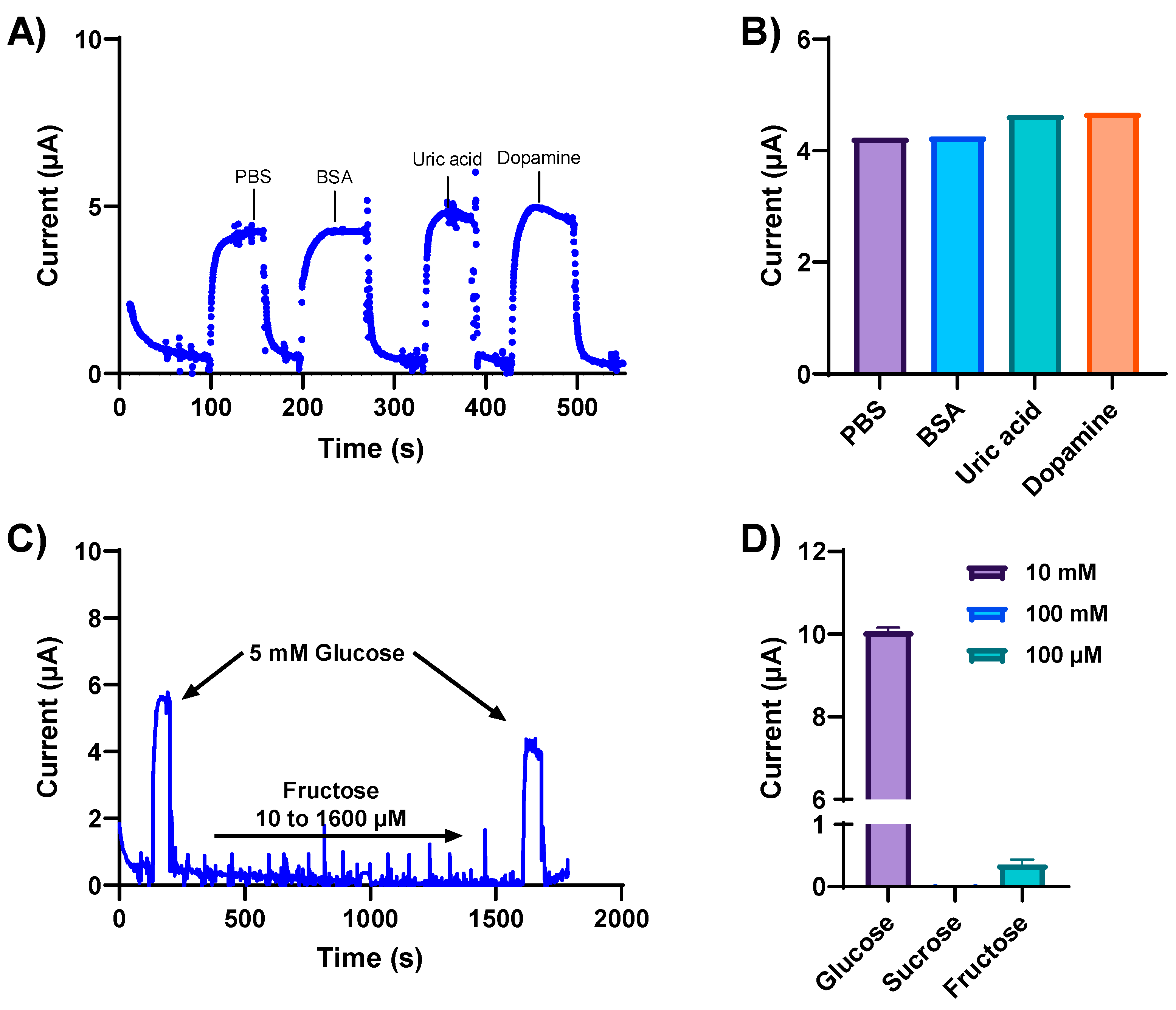
3.4. Specificity and Selectivity
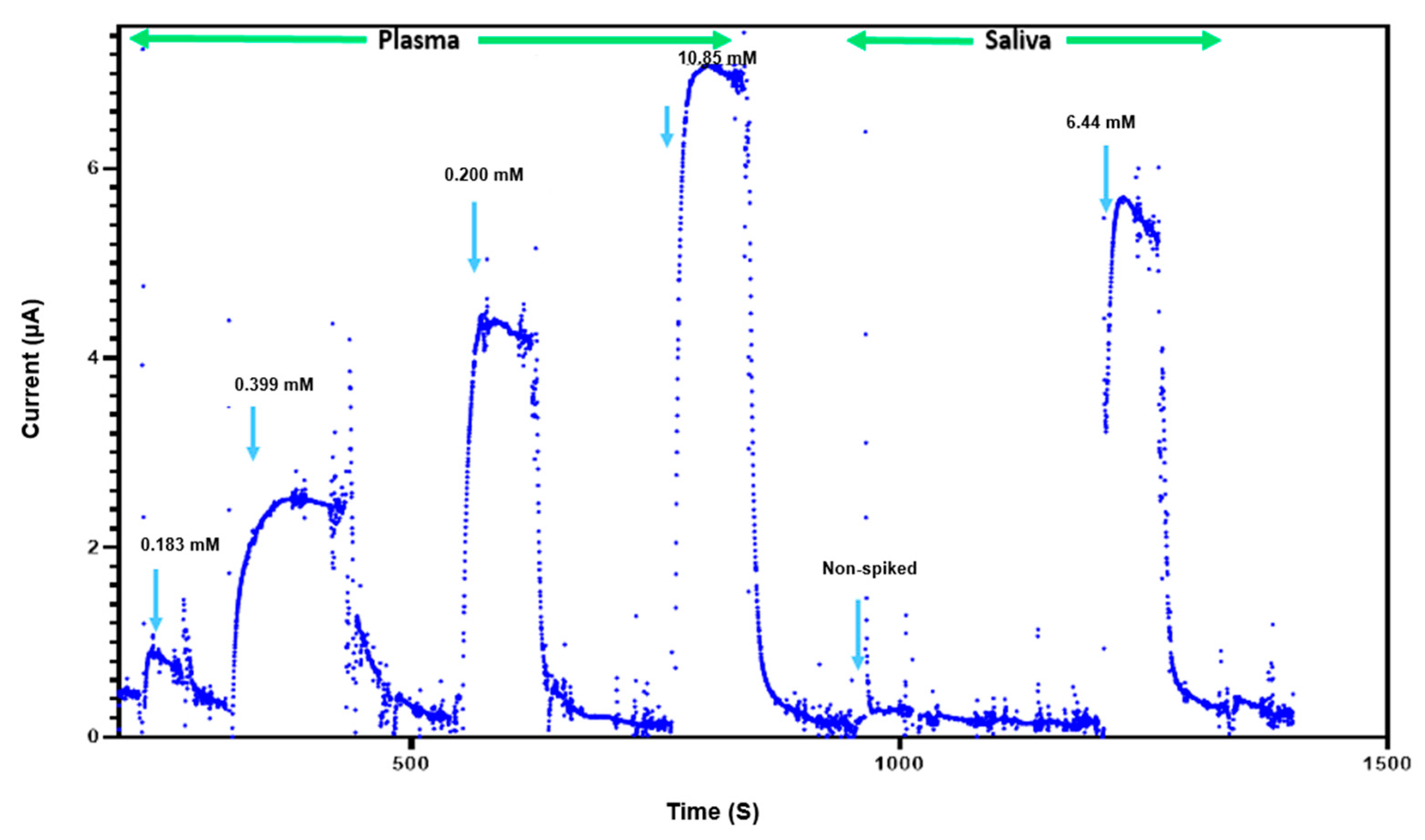
3.5. Glucose Detection in Complex Biological Fluids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xie, X.; Tan, Q.; Kang, H.; Cui, J.; Zhang, X.; Li, W.; Feng, G. Blood glucose sensors and recent advances: A review. J. Innov. Opt. Health Sci. 2022, 15, 2230003. [Google Scholar] [CrossRef]
- CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. 2023. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdiabetes%2Fdata%2Fstatistics-report%2Fdiagnosed-undiagnosed-diabetes.html# (accessed on 1 June 2023).
- Mohamad Nor, N.; Ridhuan, N.S.; Abdul Razak, K. Progress of Enzymatic and Non-Enzymatic Electrochemical Glucose Biosensor Based on Nanomaterial-Modified Electrode. Biosensors 2022, 12, 1136. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- Olejnik, A.; Karczewski, J.; Dołęga, A.; Siuzdak, K.; Grochowska, K. Novel approach to interference analysis of glucose sensing materials coated with Nafion. Bioelectrochemistry 2020, 135, 107575. [Google Scholar] [CrossRef] [PubMed]
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef]
- Erbach, M.; Freckmann, G.; Hinzmann, R.; Kulzer, B.; Ziegler, R.; Heinemann, L.; Schnell, O. Interferences and Limitations in Blood Glucose Self-Testing: An Overview of the Current Knowledge. J. Diabetes Sci. Technol. 2016, 10, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; Risler, Z.; Gupta, K. Erroneous Causes of Point-of-Care Glucose Readings. Cureus 2023, 15, e36356. [Google Scholar] [CrossRef]
- Klonoff, D.C. Continuous glucose monitoring: Roadmap for 21st century diabetes therapy. Diabetes Care 2005, 28, 1231–1239. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: Current problems and future promises. J. Diabetes Sci. Technol. 2010, 4, 1540–1562. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Janapala, R.N.; Jayaraj, J.S.; Fathima, N.; Kashif, T.; Usman, N.; Dasari, A.; Jahan, N.; Sachmechi, I. Continuous Glucose Monitoring Versus Self-monitoring of Blood Glucose in Type 2 Diabetes Mellitus: A Systematic Review with Meta-analysis. Cureus 2019, 11, e5634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kalita, D.; Naskar, U.; Mishra, B.K.; Kumar, P.; Mirza, K.B. Prediction of Glucose Sensor Sensitivity in the Presence of Biofouling Using Machine Learning and Electrochemical Impedance Spectroscopy. IEEE Sens. J. 2023, 23, 18785–18797. [Google Scholar] [CrossRef]
- Sabaté del Río, J.; Henry, O.Y.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, U.; Jolly, P.; Estrela, P.; Moschou, D.; Ingber, D.E. Graphene Enabled Low-Noise Surface Chemistry for Multiplexed Sepsis Biomarker Detection in Whole Blood. Adv. Funct. Mater. 2021, 31, 2010638. [Google Scholar] [CrossRef]
- Timilsina, S.S.; Ramasamy, M.; Durr, N.; Ahmad, R.; Jolly, P.; Ingber, D.E. Biofabrication of multiplexed electrochemical immunosensors for simultaneous detection of clinical biomarkers in complex fluids. Adv. Healthc. Mater. 2022, 11, 2200589. [Google Scholar] [CrossRef] [PubMed]
- Najjar, D.; Rainbow, J.; Sharma Timilsina, S.; Jolly, P.; de Puig, H.; Yafia, M.; Durr, N.; Sallum, H.; Alter, G.; Li, J.Z. A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 2022, 6, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Lim, Y.; Lee, Y.; Shin, H. Glucose sensor based on redox-cycling between selectively modified and unmodified combs of carbon interdigitated array nanoelectrodes. Anal. Chim. Acta 2015, 889, 194–202. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. Redox-Functionalized Graphene Oxide Architecture for the Development of Amperometric Biosensing Platform. ACS Appl. Mater. Interfaces 2013, 5, 4791–4798. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Bradford, V.Q.; Whitaker, R.G. Enzyme electrode studies of glucose oxidase modified with a redox mediator. Talanta 1991, 38, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Zuo, X.; Yang, R.; Xia, F.; Plaxco, K.W.; White, R.J. Comparing the Properties of Electrochemical-Based DNA Sensors Employing Different Redox Tags. Anal. Chem. 2009, 81, 9109–9113. [Google Scholar] [CrossRef] [PubMed]
- Dudkaitė, V.; Bagdžiūnas, G. Functionalization of Glucose Oxidase in Organic Solvent: Towards Direct Electrical Communication across Enzyme-Electrode Interface. Biosensors 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Şenel, M.; Abasıyanık, M.F. Construction of a Novel Glucose Biosensor Based on Covalent Immobilization of Glucose Oxidase on Poly(glycidyl methacrylate-co-vinylferrocene). Electroanalysis 2010, 22, 1765–1771. [Google Scholar] [CrossRef]
- Jayakumar, K.; Bennett, R.; Leech, D. Electrochemical glucose biosensor based on an osmium redox polymer and glucose oxidase grafted to carbon nanotubes: A design-of-experiments optimisation of current density and stability. Electrochim. Acta 2021, 371, 137845. [Google Scholar] [CrossRef]
- Chung, R.J.; Wang, A.N.; Liao, Q.L.; Chuang, K.Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef]
| Platform | Redox | Detection Method | Linear Range | Detection Limit | Comments |
|---|---|---|---|---|---|
| Pyrolytic carbon [20] | Potassium ferro/ferricyanide | Amperometry | 0.001–1 | 0.4 µM | Redox species in solution |
| Gold [24] | GOx modified with ferrocencecarboxaldehyde | Cyclic voltammetry | 1.0–5.0 mM | 5.2–210 µM | Variable response due to high oxygen levels |
| Glassy carbon electrode [25] | Poly(glycidyl methacrylateco-vinylferrocene) redox copolymer | Amperometry | 0.5 to 6 mM | 3 µM | Requires FAD as a cofactor |
| Multiwalled carbon nanotubes [26] | Osmium redox polymer | Voltammetry /amperometry | - | - | Moderate stability |
| ZnO Nanorods [27] | - | Cyclic voltammetry | 1–13.8 mM | 1 mM | Non-enzymatic sensing may have selectivity issues |
| prGOx/GA/BSA (This work) | NH2-Fc | Amperometry | 0.0–10 mM | 1 mM | Scalable and cost effective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janfaza, S.; Radha Shanmugam, N.; Jolly, P.; Kovur, P.; Singh, U.; Mackay, S.; Wishart, D.; Ingber, D.E. Redox-Modified Nanostructured Electrochemical Surfaces for Continuous Glucose Monitoring in Complex Biological Fluids. Nanomaterials 2024, 14, 796. https://doi.org/10.3390/nano14090796
Janfaza S, Radha Shanmugam N, Jolly P, Kovur P, Singh U, Mackay S, Wishart D, Ingber DE. Redox-Modified Nanostructured Electrochemical Surfaces for Continuous Glucose Monitoring in Complex Biological Fluids. Nanomaterials. 2024; 14(9):796. https://doi.org/10.3390/nano14090796
Chicago/Turabian StyleJanfaza, Sajjad, Nandhinee Radha Shanmugam, Pawan Jolly, Prashanthi Kovur, Upasana Singh, Scott Mackay, David Wishart, and Donald E. Ingber. 2024. "Redox-Modified Nanostructured Electrochemical Surfaces for Continuous Glucose Monitoring in Complex Biological Fluids" Nanomaterials 14, no. 9: 796. https://doi.org/10.3390/nano14090796
APA StyleJanfaza, S., Radha Shanmugam, N., Jolly, P., Kovur, P., Singh, U., Mackay, S., Wishart, D., & Ingber, D. E. (2024). Redox-Modified Nanostructured Electrochemical Surfaces for Continuous Glucose Monitoring in Complex Biological Fluids. Nanomaterials, 14(9), 796. https://doi.org/10.3390/nano14090796








