Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians
Abstract
1. Introduction
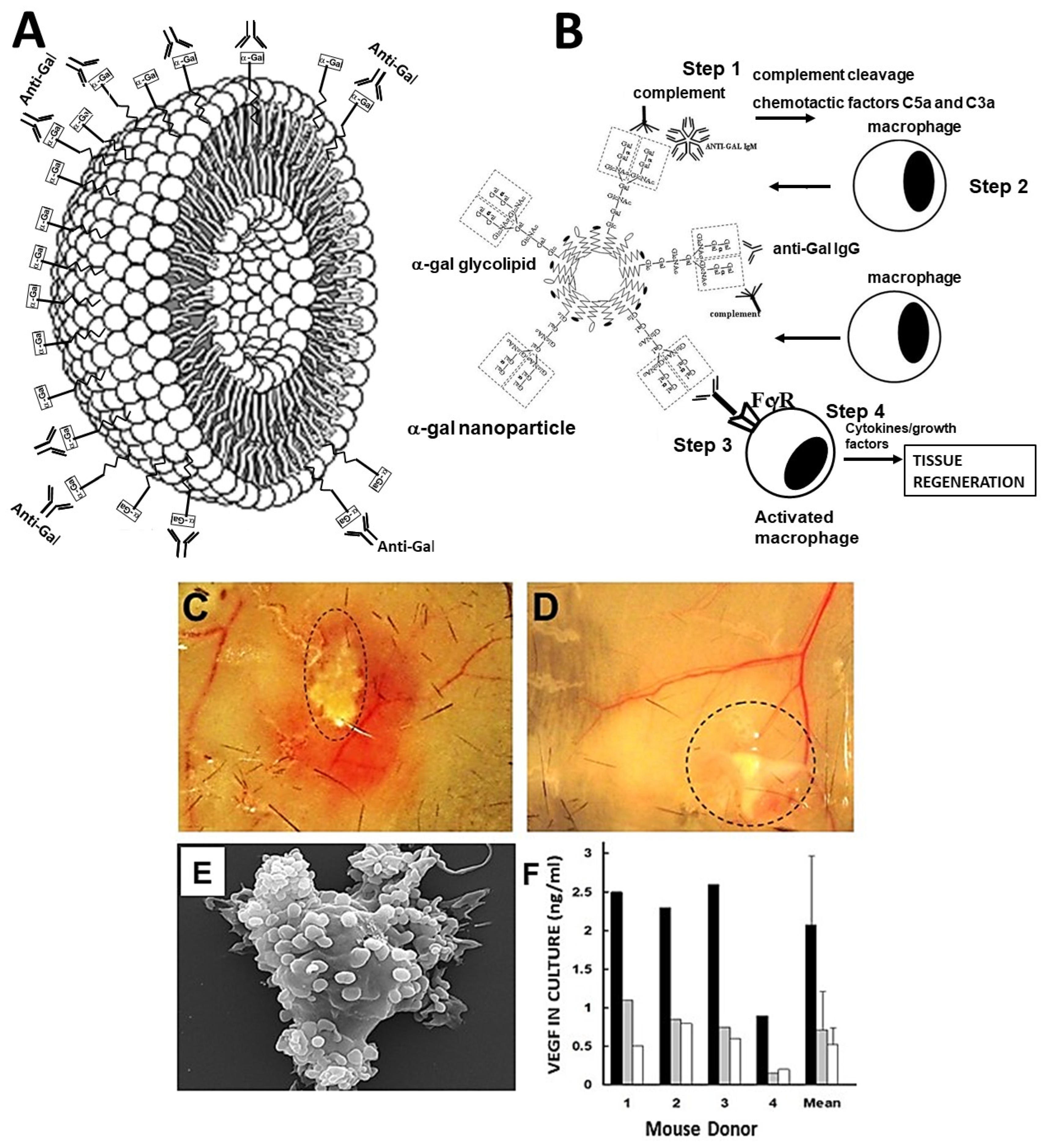
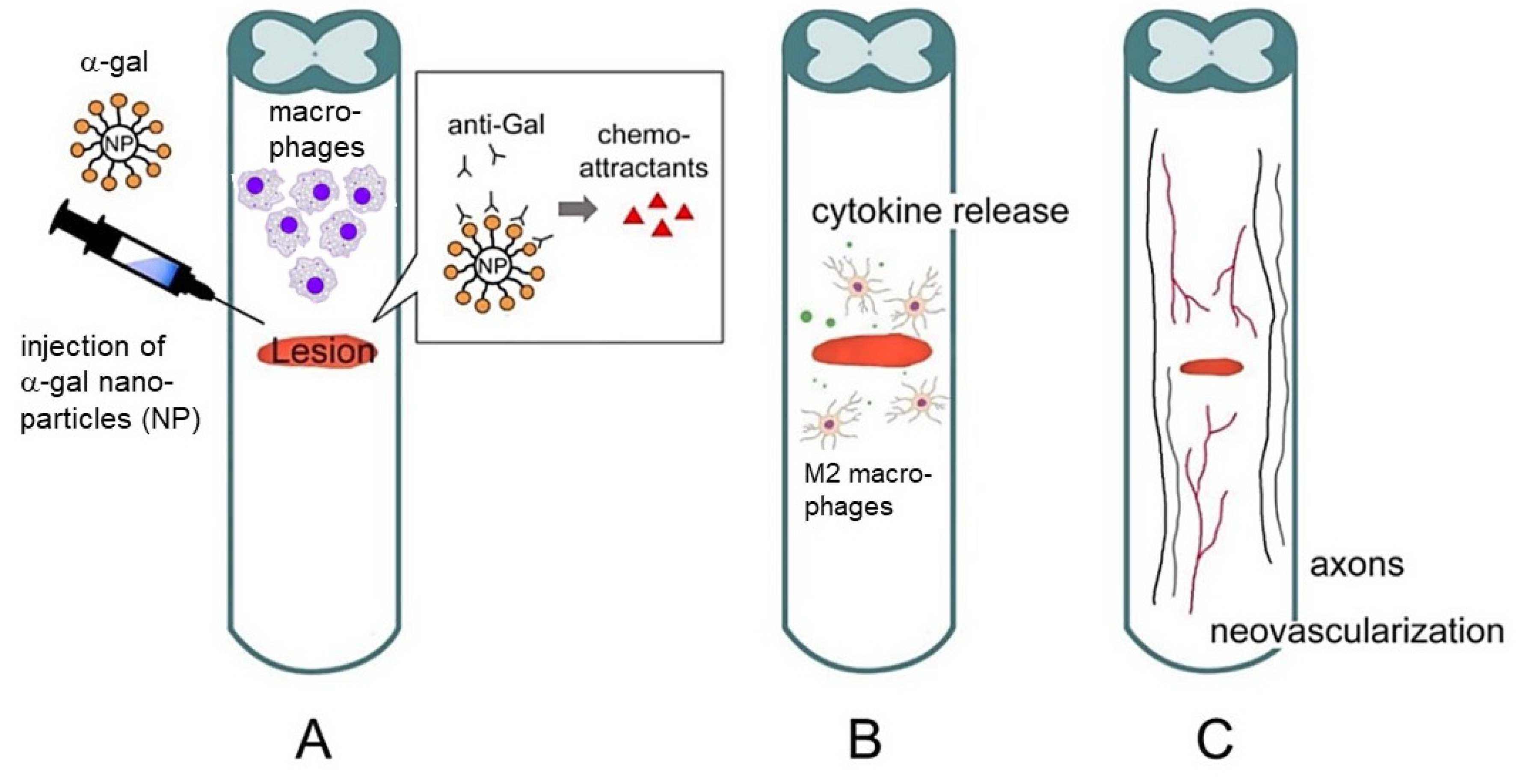
2. Hypothesis on the Regeneration of Injured Tissues in Adult Mice by α-Gal Nanoparticles
- Activation of the complement system occurs by anti-Gal binding to α-gal nanoparticles administered to the injury site.
- Macrophages are recruited to the injury site by the chemotactic complement cleavage peptides C5a and C3a.
- Binding of anti-Gal-coated α-gal nanoparticles to the recruited macrophages activates them to polarize into pro-regenerative macrophages.
- The pro-regenerative macrophages produce cytokines/growth factors that orchestrate regeneration of the treated injured tissue.
3. Experimental Demonstration of the Various Steps in the Hypothesis
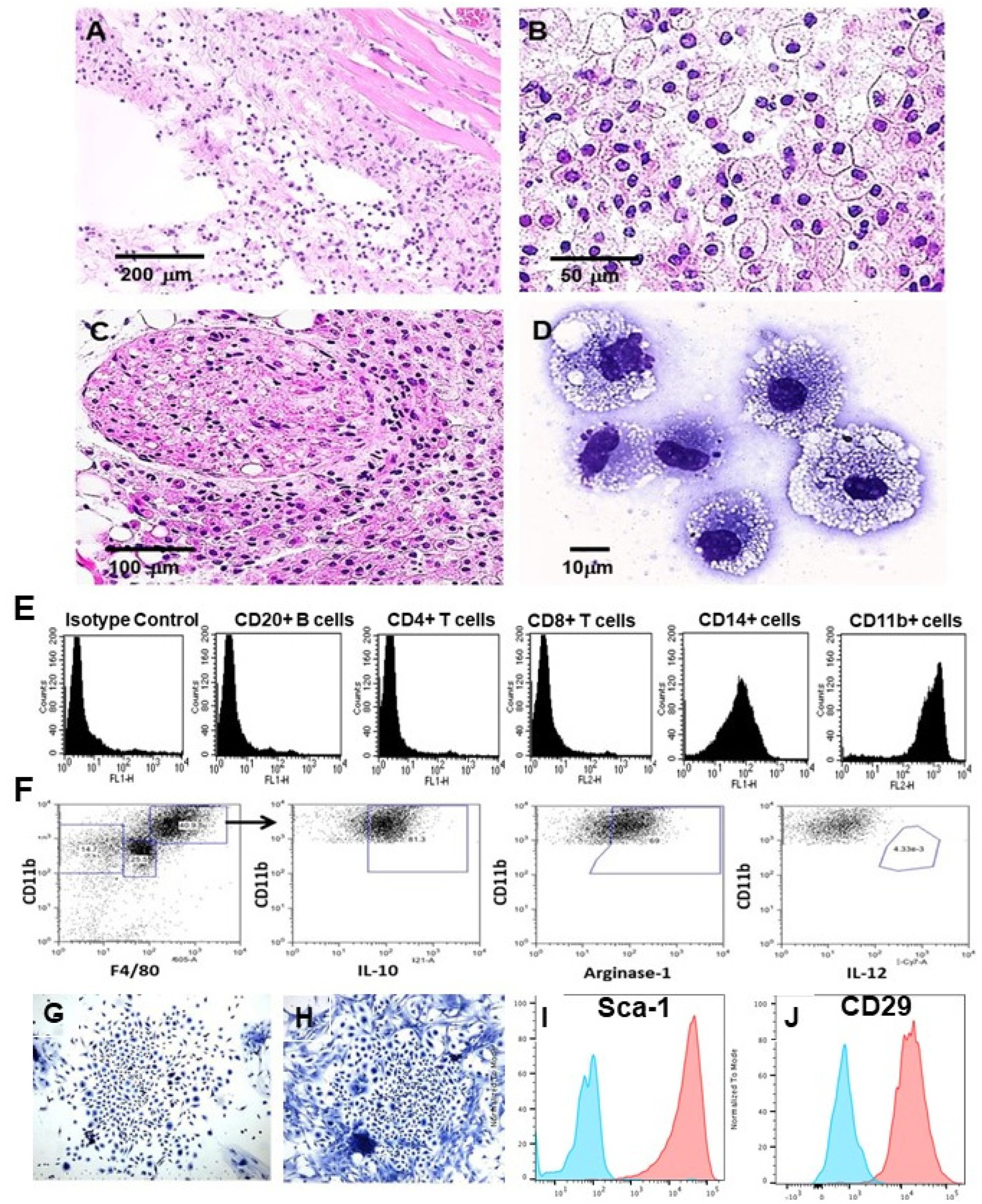
4. Accelerated Scar-Free Regeneration of α-Gal Nanoparticle-Treated Wounds
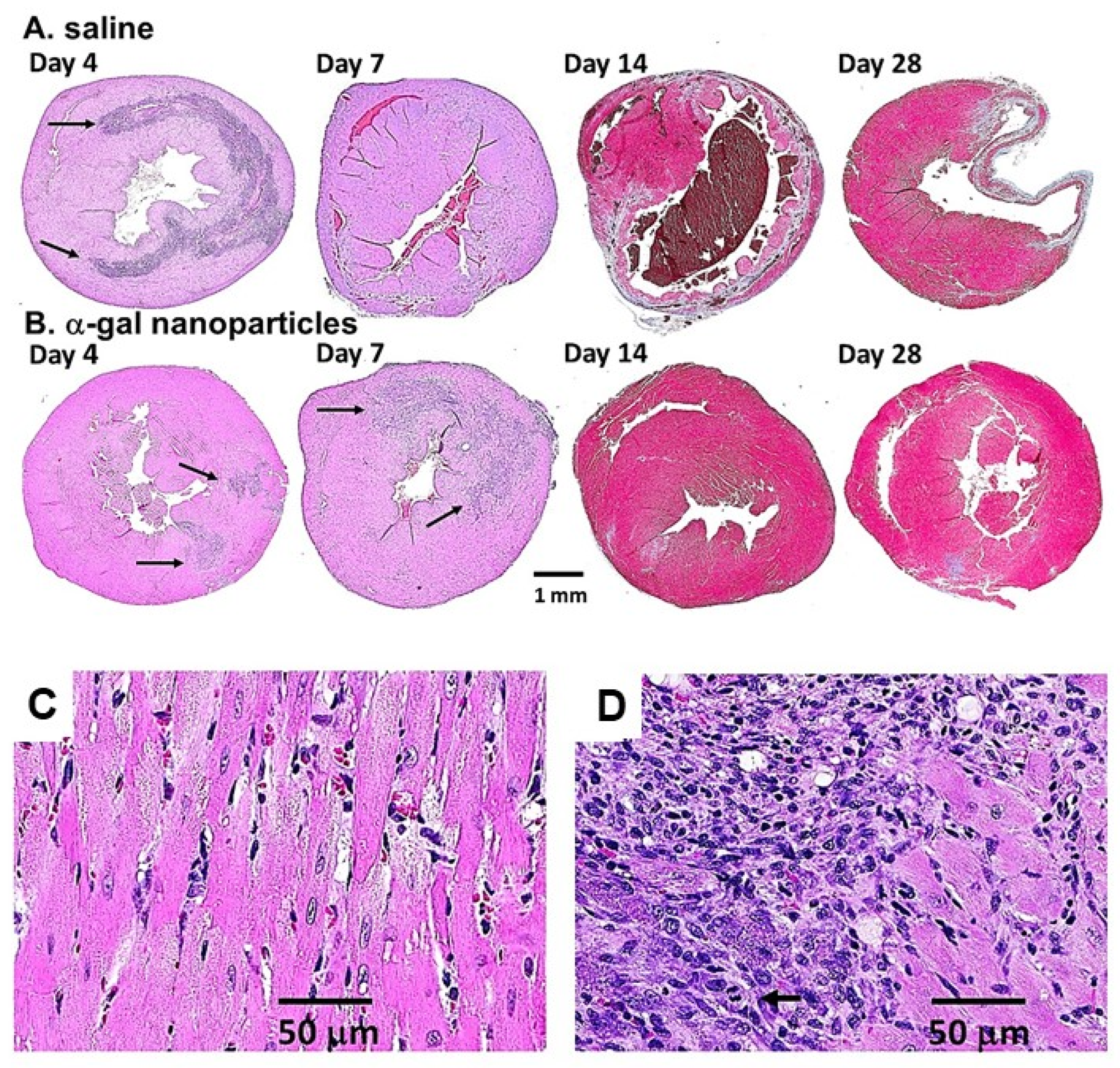
5. α-Gal Nanoparticles Induce the Regeneration of Post-MI Myocardium in Adult Mice
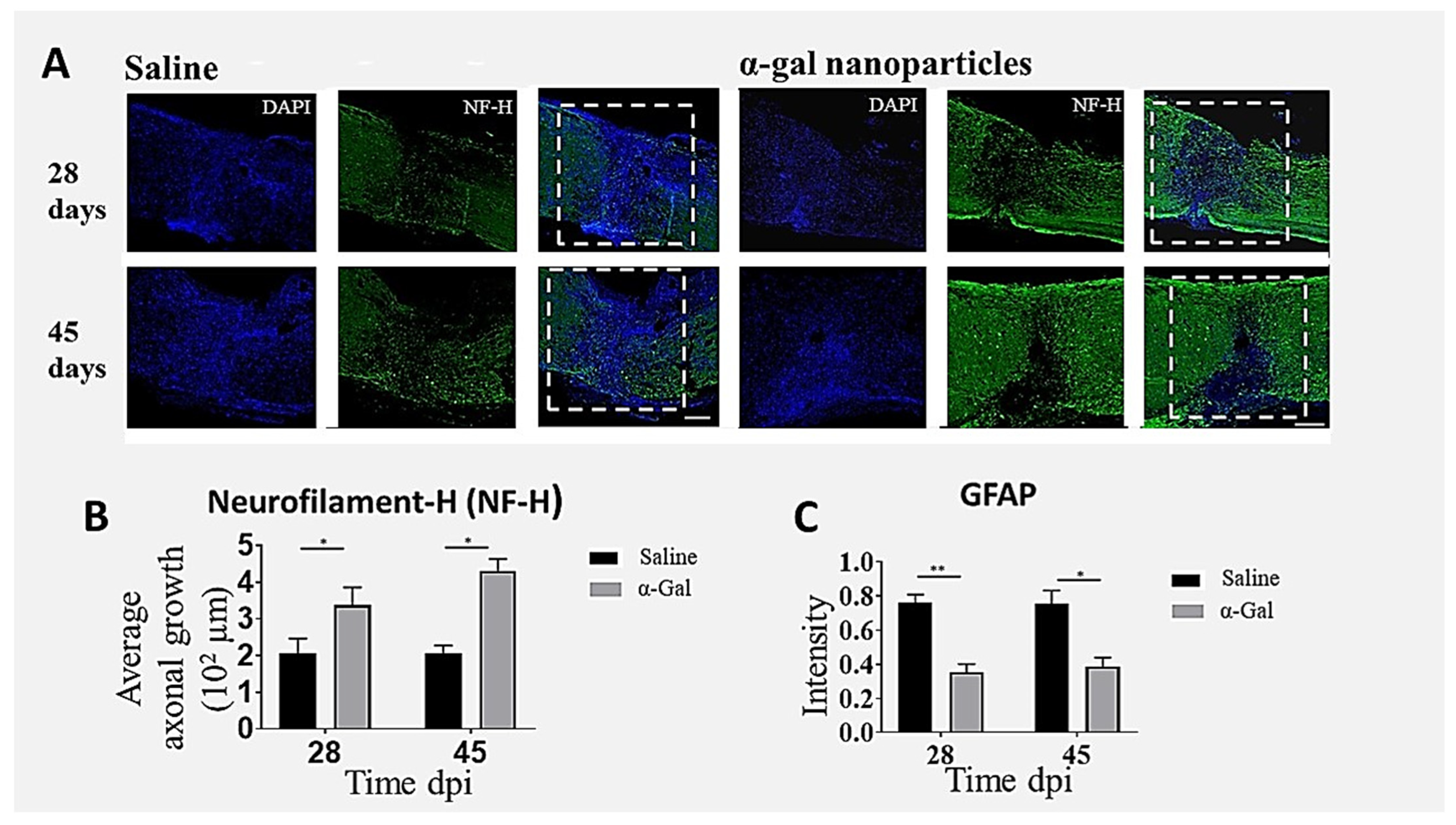
6. Nerve Regeneration in Injured Spinal Cord by α-Gal Nanoparticles
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Brockes, J.P. Amphibian limb regeneration: Rebuilding a complex structure. Science 1997, 276, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.; Bryant, S.V.; Gardiner, D.M. The axolotl limb blastema: Cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration 2015, 2, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Leigh, N.D.; Dunlap, G.S.; Johnson, K.; Mariano, R.; Oshiro, R.; Wong, A.Y.; Bryant, D.M.; Miller, B.M.; Ratner, A.; Chen, A.; et al. Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nat. Commun. 2018, 9, 5153. [Google Scholar] [CrossRef] [PubMed]
- Joven, A.; Elewa, A.; Simon, A. Model systems for regeneration: Salamanders. Development 2019, 146, dev167700. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.K.; Smith, J.J.; Voss, S.R. Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing. Exp. Cell Res. 2020, 394, 112149. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Castro, L.A.; Walters, H.E.; García Vázquez, R.O.; Yun, M.H. Immunity in salamander regeneration: Where are we standing and where are we headed? Dev. Dyn. 2021, 250, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.W.; Debuque, R.; Salimova, E.; Rosenthal, N.A. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen. Med. 2017, 2, 22. [Google Scholar] [CrossRef]
- Lévesque, M.; Villiard, É.; Roy, S. Skin wound healing in axolotls: A scarless process. J. Exp. Zool. 2010, 314B, 684–697. [Google Scholar] [CrossRef]
- Godwin, J.W.; Rosenthal, N. Scar-free wound healing and regeneration in amphibians: Immunological influences on regenerative success. Differentiation 2014, 87, 66–75. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Egar, M.W.; Chernoff, E.A. Reorganization of the ependyma during axolotl spinal cord regeneration: Changes in intermediate filament and fibronectin expression. Dev. Dyn. 1992, 193, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.L.; Wurdinger, T.; Figueiredo, J.S.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Pinto, A.R.; Epelman, S.; Kopecky, B.J.; Clemente-Casares, X.; Godwin, J.; Jason, N.; Kovacic, C. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). J. Am. Coll. Cardiol. 2018, 72, 2213–2230. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Cell biological mechanisms in regulation of the post-infarction inflammatory response. Curr. Opin. Physiol. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S.; Lorenz, H.P. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann. Surg. 1994, 220, 10–18. [Google Scholar] [CrossRef]
- Harty, M.; Neff, A.W.; King, M.W.; Mescher, A.L. Regeneration or scarring: An immunologic perspective. Dev. Dyn. 2003, 226, 268–279. [Google Scholar] [CrossRef]
- Ferguson, M.W.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Adamowicz-Brice, M.; Khadayate, S.; Tiefenthaler, V.; Metzler, B.; Aitman, T.; Penninger, J.M. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging 2012, 4, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; D’Agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early Regenerative Capacity in the Porcine Heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative potential of neonatal porcine hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.I.; Porrello, E.R. Turning back the cardiac regenerative clock: Lessons from the neonate. Trends Cardiovasc. Med. 2012, 22, 128–133. [Google Scholar] [CrossRef]
- Galili, U. Anti-Gal: An abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 2013, 140, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Madhavan, M.; Call, M.K.; Santiago, W.; Tsonis, P.A.; Lambris, J.D.; Del Rio-Tsonis, K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J. Immunol. 2003, 170, 2331–2339. [Google Scholar] [CrossRef]
- Mastellos, D.C.; Deangelis, R.A.; Lambris, J.D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013, 25, 29–38. [Google Scholar] [CrossRef]
- Natarajan, N.; Abbas, Y.; Bryant, D.M.; Gonzalez-Rosa, J.M.; Sharpe, M.; Uygur, A.; Cocco-Delgado, L.H.; Ho, N.N.; Gerard, N.P.; Gerard, C.J.; et al. Complement Receptor C5aR1 Plays an Evolutionarily Conserved Role in Successful Cardiac Regeneration. Circulation 2018, 137, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.R.; Epp, L.G.; Putta, S.; Page, R.B.; Walker, J.A.; Beachy, C.K.; Zhu, W.; Pao, G.M.; Verma, I.M.; Hunter, T.; et al. Microarray and cDNA sequence analysis of transcription during nerve-dependent limb regeneration. BMC Biol. 2009, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Wigglesworth, K.; Abdel-Motal, U.M. Accelerated healing of skin burns by anti-Gal/alpha-gal liposomes interaction. Burns 2010, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, K.M.; Raski, W.J.; Mishra, R.; Szomolanyi-Tsuda, E.; Greiner, D.L.; Galili, U. Rapid recruitment and activation of macrophages by anti-Gal/α-Gal liposome interaction accelerates wound healing. J. Immunol. 2011, 186, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Zhu, Z.; Chen, J.; Goldufsky, J.W.; Schaer, G.L. Near Complete Repair after Myocardial Infarction in Adult Mice by Altering the Inflammatory Response with Intramyocardial Injection of α-Gal Nanoparticles. Front. Cardiovasc. Med. 2021, 8, 719160. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.L.; Rojas, M.; Galili, U. Immunogenic Gal α1----3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J. Immunol. 1989, 142, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, I.M.; Comrack, C.A.; Sachs, D.H.; DerSimonian, H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal(α1,3)Galactose epitope. Transplantation 1997, 64, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Lin, S.S.; Yu, P.B.; Sood, A.; Nakamura, Y.C.; Song, A.; Platt, J.L. Naturally occurring anti-α-galactosyl antibodies: Relationship to xenoreactive anti-α-galactosyl antibodies. Glycobiology 1999, 9, 865–873. [Google Scholar] [CrossRef]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef]
- Mañez, R.; Blanco, F.J.; Díaz, I.; Centeno, A.; Lopez-Pelaez, E.; Hermida, M.; Davies, H.F.; Katopodis, A. Removal of bowel aerobic gram-negative bacteria is more effective than immunosuppression with cyclophosphamide and steroids to decrease natural alpha-galactosyl IgG antibodies. Xenotransplantation 2001, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bernth Jensen, J.M.; Petersen, M.S.; Ellerman-Eriksen, S.; Møller, B.K.; Jensenius, J.C.; Skov Sørensen, U.B.; Thiel, S. Abundant human anti-Galα3Gal antibodies display broad pathogen reactivity. Sci. Rep. 2020, 10, 4611. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Macher, B.A.; Buehler, J.; Shohet, S.B. Human natural anti-α-galactosyl IgG. II. The specific recognition of α[1,3]-linked galactose residues. J. Exp. Med. 1985, 162, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Rosenfelder, G.; Wieslander, J.; Avila, J.L.; Rojas, M.; Szarfman, A.; Esser, K.; Nowack, H.; Timpl, R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl [α1-3]-galactose epitopes. J. Exp. Med. 1987, 166, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Teneberg, S.; Lönnroth, I.; Torres Lopez, J.F.; Galili, U.; Olwegard Halvarsson, M.; Angstrom, J.; Angstrom, J.; Karlsson, K.A. Molecular mimicry in the recognition of glycosphingolipids by Galα3Galβ4GlcNAcβ-binding Clostridium difficile toxin A, human natural anti-α-galactosyl IgG and the monoclonal antibody Gal-13: Characterization of a binding-active human glycosphingolipid, non-identical with the animal receptor. Glycobiology 1996, 6, 599–609. [Google Scholar] [PubMed]
- Galili, U.; Clark, M.R.; Shohet, S.B.; Buehler, J.; Macher, B.A. Evolutionary relationship between the anti-Gal antibody and the Galα1-3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 1987, 84, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Shohet, S.B.; Kobrin, E.; Stults, C.L.M.; Macher, B.A. Man, apes, and Old-World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 1988, 263, 17755–17762. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Paleo-immunology of human anti-carbohydrate antibodies preventing primate extinctions. Immunology 2023, 168, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. α-Gal Nanoparticles in Wound and Burn Healing Acceleration. Adv. Wound Care. 2017, 6, 81–92. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Galili, U.; Dunbar, A.; Solorio, L.; Shi, R.; Li, J. alpha-Gal Nanoparticles in CNS Trauma: I. In Vitro Activation 3 of Microglia Towards a Pro-Healing State. Tissue Eng. Regen. Med. 2024, 21, 409–419. [Google Scholar] [CrossRef]
- Thall, A.D.; Maly, P.; Lowe, J.B. Oocyte Galα,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 1995, 270, 21437–21440. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, M.; Yin, D.; Chong, A.S.; Galili, U. Differential immune responses to α-gal epitopes on xenografts and allografts: Implications for accommodation in xenotransplantation. J. Clin. Investig. 2000, 105, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Benatuil, L.; Kaye, J.; Rich, R.F.; Fishman, J.A.; Green, W.R.; Iacomini, J. The influence of natural antibody specificity on antigen immunogenicity. Eur. J. Immunol. 2005, 35, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.M.; Middleton, J.; Wigglesworth, K.; Charlemagne, A.; Schulz, O.; Glossop, M.S.; Whalen, G.F.; Old, R.; Westby, M.; Pickford, C.; et al. AGI-134: A fully synthetic α-Gal glycolipid that converts tumors into in situ autologous vaccines, induces anti-tumor immunity and is synergistic with an anti-PD-1 antibody in mouse melanoma models. Cancer Cell Int. 2019, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Tearle, R.G.; Tange, M.J.; Zanettino, Z.L.; Katerelos, M.; Shinkel, T.A.; Van Denderen, B.J.; Lonie, A.J.; Lyonsm, I.; Nottle, M.B.; Cox, T.; et al. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation 1996, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- LaTemple, D.C.; Galili, U. Adult and neonatal anti-Gal response in knock-out mice for alpha1,3galactosyltransferase. Xenotransplantation 1997, 5, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. The Natural Anti-Gal Antibody as Foe Turned Friend in Medicine; Academic Press/Elsevier Publishers: London, UK, 2018; pp. 207–228. [Google Scholar]
- DiPietro, L.A.; Wilgus, T.A.; Koh, T.J. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int. J. Mol. Sci. 2021, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A.; Burdick, M.; Low, Q.E.; Kunkel, S.L.; Strieter, R.M. MIP-1a as a critical macrophage chemoattractant in murine wound repair. J. Clin. Investig. 1998, 101, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.T.; Wang, Y.; Sannomiya, P.; Piccolo, N.S.; Piccolo, M.S.; Hugli, T.E.; Ward, P.A.; Till, G.O. Chemotactic mediator requirements in lung injury following skin burns in rats. Exp. Mol. Pathol. 1999, 66, 220–226. [Google Scholar] [CrossRef]
- Heinrich, S.A.; Messingham, K.A.; Gregory, M.S.; Colantoni, A.; Ferreira, A.M.; Dipietro, L.A.; Kovacs, E.J. Elevated monocyte chemoattractant protein-1 levels following thermal injury precede monocyte recruitment to the wound site and are controlled, in part, by tumor necrosis factor-b. Wound Repair Regen. 2003, 11, 110–119. [Google Scholar] [CrossRef]
- Wood, G.W.; Hausmann, E.; Choudhuri, R. Relative role of CSF-1, MCP-1/JE, and RANTES in macrophage recruitment during successful pregnancy. Mol. Reprod. Dev. 1997, 46, 62–69. [Google Scholar] [CrossRef]
- Shukaliak, J.; Dorovini-Zis, K. Expression of the [β]-chemokines RANTES and MIP-1β by human brain microvessel endothelial cells in primary culture. J. Neuropathol. Exp. Neurol. 2000, 59, 339–352. [Google Scholar] [CrossRef]
- Shallo, H.; Plackett, T.P.; Heinrich, S.A.; Kovacs, E.J. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns 2003, 29, 641–647. [Google Scholar] [CrossRef]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- Gopalakrishnan, B.; Galili, U.; Seanger, M.; Burket, N.J.; Koss, W.; Lokender, M.S.; Wolfe, K.M.; Husak, S.J.; Stark, C.J.; Solorioet, C.; et al. alpha-Gal Nanoparticles in CNS Trauma: II. Improved Functional II. Outcomes After Spinal Cord Injury (SCI) via Immunomodulation. Tissue Eng. Regen. Med. 2024, 21, 437–453. [Google Scholar] [CrossRef]
- Kaymakcalan, O.E.; Abadeer, A.; Goldufsky, J.W.; Galili, U.; Karinja, S.J.; Dong, X.; Jin, J.L.; Samadi, A.; Spector, J.A. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp. Dermatol. 2020, 29, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Galili, U. Accelerated Burn Healing in a Mouse Experimental Model Using α-Gal Nanoparticles. Bioengineering 2023, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; De la Garza, M.; Fang, R.C.; Hong, S.J.; Galiano, R.D. Excisional wound healing is delayed in a murine model of chronic kidney disease. PLoS ONE 2013, 8, e59979. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, O.E.; Karinja, S.; Abadeer, A.; Dong, X.; Jin, J.L.; Galili, U.; Spector, J.A. Antigen-Mediated, Macrophage-Stimulated, Accelerated Wound Healing Using α-Gal Nanoparticles. Ann. Plast. Surg. 2018, 80 (Suppl. 4), S196–S203. [Google Scholar] [CrossRef]
- Hurwitz, Z.; Ignotz, R.; Lalikos, J.; Galili, U. Accelerated porcine wound healing with α-gal nanoparticles. Plast. Reconstr. Surg. 2012, 129, 242–251. [Google Scholar] [CrossRef]
- Samadi, A.; Buro, J.; Dong, X.; Weinstein, A.; Lara, D.O.; Celie, K.B.; Wright, M.A.; Gadijko, M.A.; Galili, U.; Spector, J.A. Topical α-Gal Nanoparticles Enhance Wound Healing in Radiated Skin. Ski. Pharmacol. Physiol. 2022, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020, 142, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Clarke, J.D.; Golding, J.P.; Goodbrand, I.A.; Tonge, D.A. Macrophage response during axonal regeneration in the axolotl central and peripheral nervous system. Neuroscience 1993, 54, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Enos, N.; Takenaka, H.; Scott, S.; Salfity, H.V.N.; Kirk, M.; Egar, M.W.; Sarria, D.A.; Slayback-Barry, D.; Belecky-Adams, T.; Chernoff, E.A.G. Meningeal Foam Cells and Ependymal Cells in Axolotl Spinal Cord Regeneration. Front. Immunol. 2019, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.P.; Cafferty, W.B.; Budel, S.O.; Strittmatter, S.M. Extracellular regulators of axonal growth in the adult central nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1593–1610. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.A.; Liebscher, T.; Niedeggen, A.; Laufer, S.; Brommer, B.; Jungehulsing, G.J.; Strittmatter, S.M.; Dirnagl, U.; Schwab, J.M. Smal l-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res. 2012, 349, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, Y.; Li, S. Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res. 2015, 1619, 22–35. [Google Scholar] [CrossRef]
- Zochodne, D.W.; Nguyen, C. Angiogenesis at the site of neuroma formation in transected peripheral nerve. J. Anat. 1997, 191, 23–30. [Google Scholar] [CrossRef]
- Facchiano, F.; Fernandez, E.; Mancarella, S.; Maira, G.; Miscusi, M.; D’Arcangelo, D.; Cimino-Reale, G.; Falchetti, M.L.; Capogrossi, M.C.; Pallini, R. Promotion of regeneration of corticospinal tract axons in rats with recombinant vascular endothelial growth factor alone and combined with adenovirus coding for this factor. J. Neurosurg. 2002, 97, 161–168. [Google Scholar] [CrossRef]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Hobson, M.I.; Green, C.J.; Terenghi, G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000, 197, 591–605. [Google Scholar] [CrossRef]
- Park, H.W.; Jeon, H.J.; Chang, M.S. Vascular endothelial growth factor enhances axonal outgrowth in organotypic spinal cord slices via vascular endothelial growth factor receptor 1 and 2. Tissue Eng. Regen. Med. 2016, 13, 601–609. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Rao, Z.; Deng, R.; Xu, Y.; Qiu, S.; Long, H.; Zhu, Q.; Liu, X.; Bai, Y.; et al. Facilitate Angiogenesis and Neurogenesis by Growth Factors Integrated Decellularized Matrix Hydrogel. Tissue Eng. Part A 2021, 27, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.W.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef]
- An, N.; Yang, J.; Wang, H.; Sun, S.; Wu, H.; Li, L.; Li, M. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021, 11, 41. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; Passos Dos Santos, R.; Gaestel, M.; David, S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Finkel, Z.; Rodriguez, B.; Joseph, A.; Cai, L. Current Advancements in Spinal Cord Injury Research-Glial Scar Formation and Neural Regeneration. Cells 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Barouch, R.; Appel, E.; Kazimirsky, G.; Brodie, C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J. Neuroimmunol. 2001, 112, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.S.; Tsai, M.J.; Huang, M.C.; Chiu, C.W.; Tsai, C.Y.; Lee, M.J.; Huang, W.C.; Lin, Y.L.; Kuo, W.C.; Cheng, H. Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J. Neurosci. 2011, 31, 4137–4147. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Site-specific neural hyperactivity via the activation of MAPK and PKA/CREB pathways triggers neuronal degeneration in methylmercury-intoxicated mice. Toxicol. Lett. 2017, 271, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.; Zheng, Q.; Hu, X.; Wang, Z.; Liang, Z.; Li, K.; Song, J.; Ding, T.; Shen, X.; et al. Low-level laser therapy 810-nm up-regulates macrophage secretion of neurotrophic factors via PKA-CREB and promotes neuronal axon regeneration in vitro. J. Cell. Mol. Med. 2020, 24, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Whalen, G.F.; Sullivan, M.; Piperdi, B.; Wasseff, W.; Galili, U. Cancer immunotherapy by intratumoral injection of α-gal glycolipids. Anticancer Res. 2012, 32, 3861–3868. [Google Scholar]
- Albertini, M.R.; Ranheim, E.A.; Zuleger, C.L.; Sondel, P.M.; Hank, J.A.; Bridges, A.; Newton, M.A.; McFarland, T.; Collins, J.; Clements, E.; et al. Phase I study to evaluate toxicity and feasibility of intratumoral injection of α-gal glycolipids in patients with advanced melanoma. Cancer Immunol. Immunother. 2016, 65, 897–907. [Google Scholar] [CrossRef]
- Commins, S.P.; Platts-Mills, T.A.E. Tick Bites and Red Meat Allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 354–359. [Google Scholar] [CrossRef] [PubMed]
- van Nunen, S. Tick-induced Allergies: Mammalian Meat Allergy, Tick Anaphylaxis and Their Significance. Asia Pac. Allergy 2015, 5, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Hamsten, C.; Starkhammar, M.; Tran, T.A.; Johansson, M.; Bengtsson, U.; Ahlen, G.; Sällberg, M.; Grönlund, H.; van Hage, M. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy 2013, 68, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, A.; Mateos-Hernández, L.; de la Fuente, J.; Cabezas-Cruz, A. Delayed hypersensitivity reaction to mammalian galactose-α-1,3-galactose (α-Gal) after repeated tick bites in a patient from France. Ticks Tick Borne Dis. 2019, 10, 1057–1059. [Google Scholar] [CrossRef]
- González-Orozco, J.C.; Escobedo-Avila, I.; Velasco, I. Transcriptome Profiling after Early Spinal Cord Injury in the Axolotl and Its Comparison with Rodent Animal Models through RNA-Seq Data Analysis. Genes 2023, 14, 2189. [Google Scholar] [CrossRef]


| Tissue Injury | Experimental Animal | Treatment Results | References |
|---|---|---|---|
| 1. Skin Wound | GT-KO mouse a | Accelerated healing by scar-free regeneration | [34,70] |
| 2. Chronic Skin Wound (diabetes) | GT-KO mouse | Chronic wound healing | [49,67] |
| 3. Skin Burn and Radiation | GT-KO mouse | Accelerated healing | [33,68,72] |
| 4. Skin Wound | GT-KO pig b | Accelerated healing by scar-free regeneration | [71] |
| 5. Myocardial Ischemia (post-MI) | GT-KO mouse | Near-complete myocardial regeneration | [35] |
| 6. Spinal Cord Crush | GT-KO mouse | Accelerated axonal growth and scar-free regeneration | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galili, U.; Li, J.; Schaer, G.L. Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians. Nanomaterials 2024, 14, 730. https://doi.org/10.3390/nano14080730
Galili U, Li J, Schaer GL. Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians. Nanomaterials. 2024; 14(8):730. https://doi.org/10.3390/nano14080730
Chicago/Turabian StyleGalili, Uri, Jianming Li, and Gary L. Schaer. 2024. "Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians" Nanomaterials 14, no. 8: 730. https://doi.org/10.3390/nano14080730
APA StyleGalili, U., Li, J., & Schaer, G. L. (2024). Regeneration in Mice of Injured Skin, Heart, and Spinal Cord by α-Gal Nanoparticles Recapitulates Regeneration in Amphibians. Nanomaterials, 14(8), 730. https://doi.org/10.3390/nano14080730






