Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia integrifolia Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Antioxidant Activities Determination of Natural Oils
2.2.1. 2,2′-Azinobis (3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Assay
2.2.2. Lipid Peroxidation Inhibition by the Ferric Thiocyanate (FTC) Assay
2.3. Hyaluronidase Inhibitory Activity Determination of Natural Oils
2.4. Determination of Required Hydrophilic Lipophilic Balance (RHLB) of Natural Oil
2.5. Conventional O/W Emulsion Development
2.6. Characterizations and Stability Tests of Conventional O/W Emulsion
2.7. O/W Nanoemulsion Development
2.8. Characterizations and Stability Tests of Nanoemulsions
2.9. Irritation Test
2.9.1. In Vitro Hen’s Egg–Chorioallantoic Membrane (HET-CAM) Test
2.9.2. In Vivo Human Patch Test
2.10. Evaluations of Skin Hydration Enhancement and Skin Wrinkle Reduction in Human Volunteers
2.11. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant and Hyaluronidase Inhibitory Activities of Natural Oils
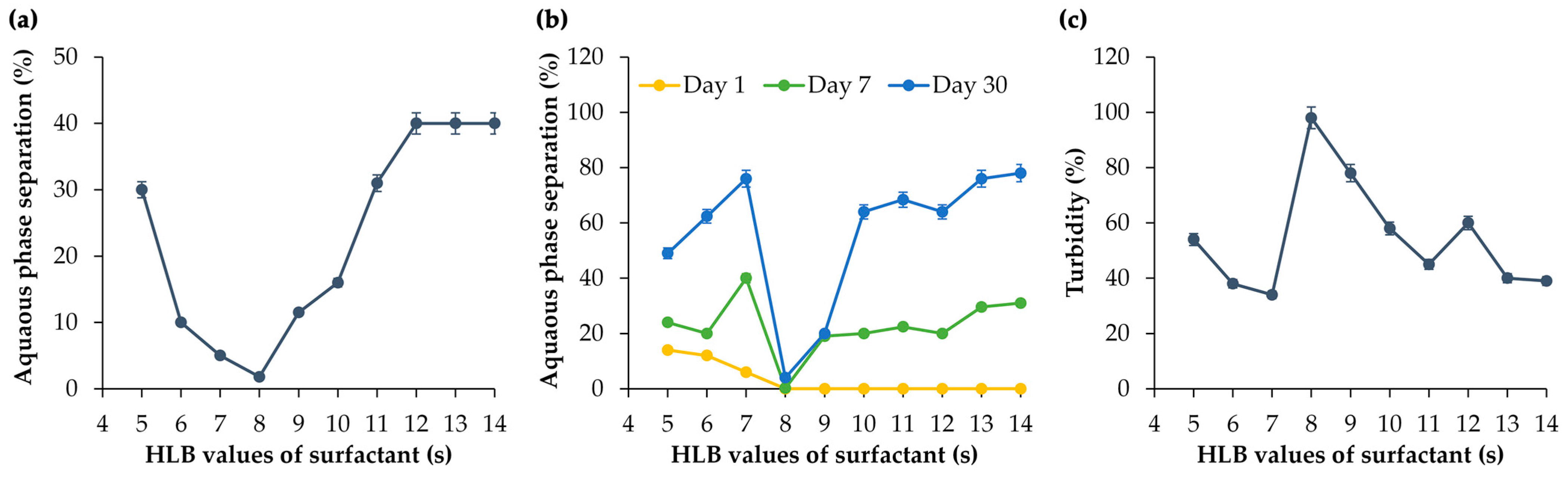
3.2. RHLB of M. integrifolia Oil
3.3. Conventional O/W Emulsions of M. integrifolia Oil
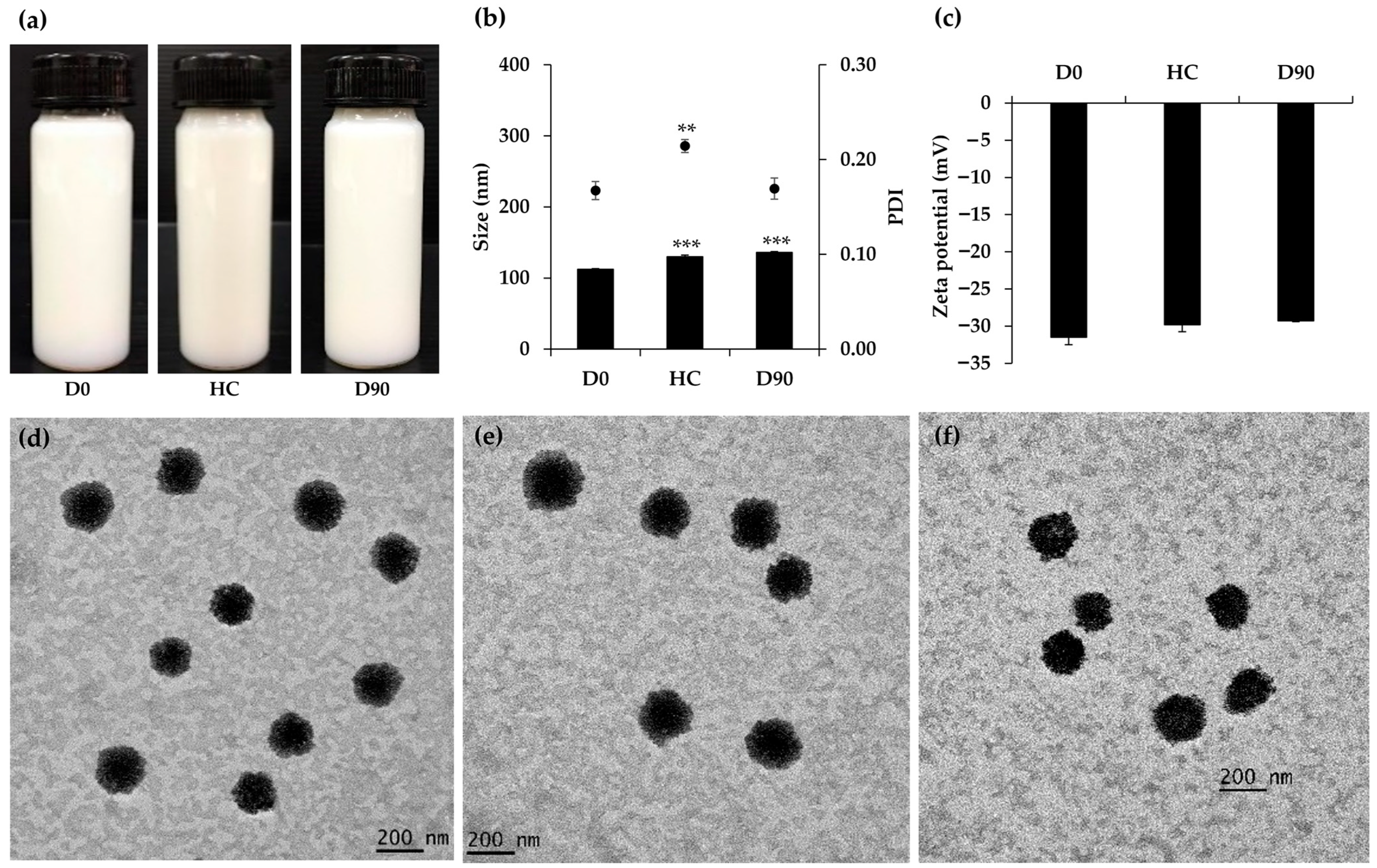
3.4. O/W Nanoemulsions of M. integrifolia Oil
3.5. Irritation Properties of Emulsion and Nanoemulsions of M. integrifolia Oil
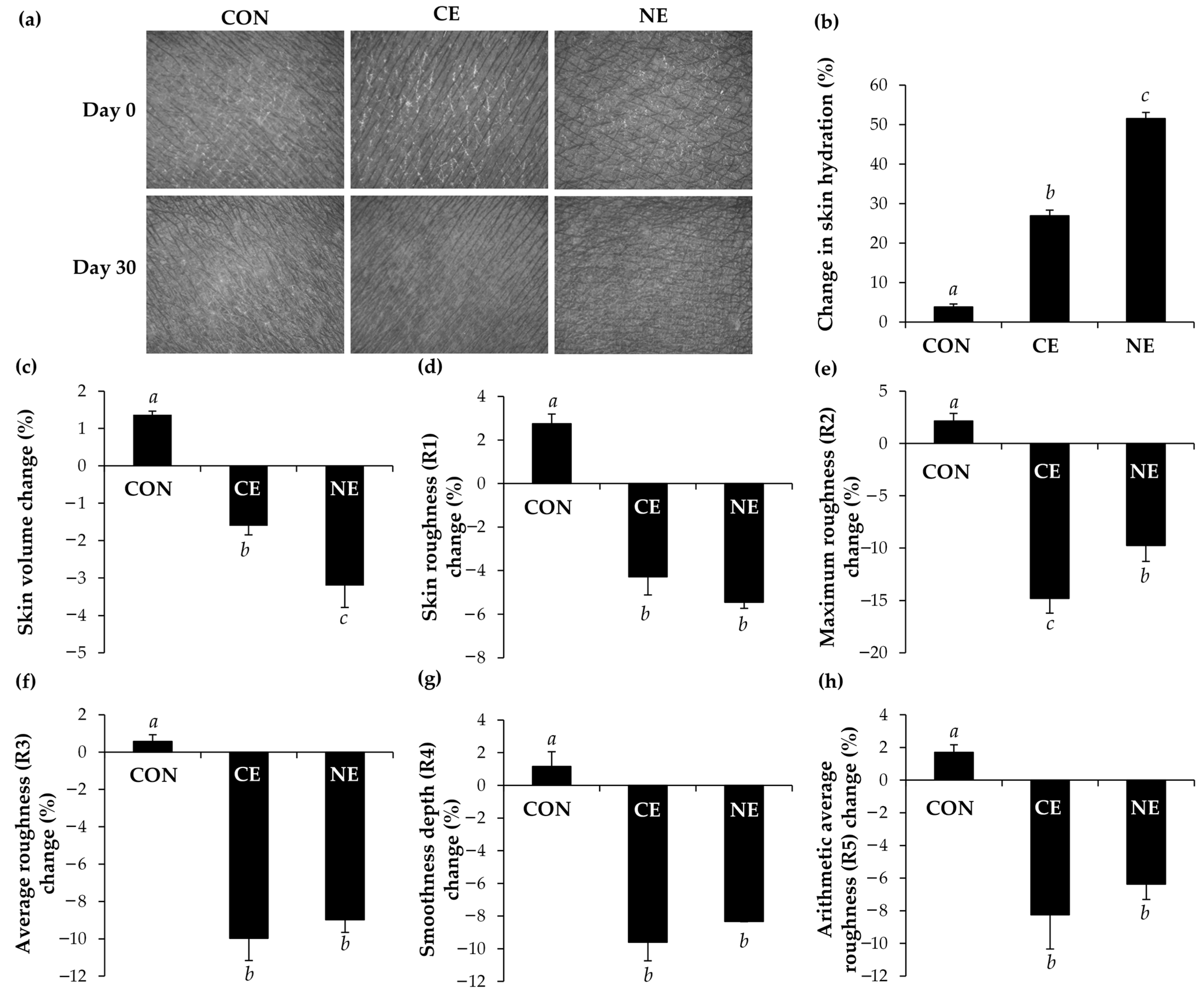
3.6. Skin Hydration Enhancement and Skin Wrinkle Reduction Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef] [PubMed]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Sheth, T.; Seshadri, S.; Prileszky, T.; Helgeson, M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020, 5, 214–228. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.; Parkinson, C.; Sherman, P. Factors affecting emulsion stability, and the HLB concept. J. Colloid Interface Sci. 1972, 41, 359–370. [Google Scholar] [CrossRef]
- Abu-Huwaij, R.; Al-Assaf, S.F.; Hamed, R. Recent exploration of nanoemulsions for drugs and cosmeceuticals delivery. J. Cosmet. Dermatol. 2022, 21, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crop. Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Steiling, W.; Bracher, M.; Courtellemont, P.; De Silva, O. The HET–CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. Vitr. 1999, 13, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.A.; Chamberlain, M.; Griffiths, H.A.; Rowson, M.; Whittle, E.; York, M. The classification of skin irritants by human patch test. Food Chem. Toxicol. 1997, 35, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shan, Y.; Gong, R.; Lin, D.; Zhang, M.; Wang, C.; Wang, L. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Front. Bioeng. Biotechnol. 2023, 10, 1082403. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F. Harmful free radicals in aging: A narrative review of their detrimental effects on health. Indian. J. Clin. Biochem. 2023, 39, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liu, D.; Liu, T.C.; Li, K.; Wang, S.; Liu, J.; Regenstein, J.M.; Wu, Y.; Zhou, P. The combined effect of commercial tilapia collagen peptides and antioxidants against UV-induced skin photoaging in mice. Food Funct. 2023, 14, 5936–5948. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Moon, J.Y.; Lee, Y.C. Antioxidants for improved skin appearance: Intracellular mechanism, challenges and future strategies. Int. J. Cosmet. Sci. 2023, 45, 299–314. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A novel hyaluronic acid matrix ingredient with regenerative, anti-aging and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 4774. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K. The biological significance of hyaluronic acid and hyaluronidase. Physiol. Rev. 1947, 27, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Tzellos, T.G.; Klagas, I.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Karakiulakis, G.; Zouboulis, C.C.; Papakonstantinou, E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. Int. Sch. Res. Not. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed]
- Miele, N.A.; Volpe, S.; Torrieri, E.; Cavella, S. Improving physical properties of sodium caseinate based coating with the optimal formulation: Effect on strawberries’ respiration and transpiration rates. J. Food Eng. 2022, 331, 111123. [Google Scholar] [CrossRef]
- Kassem, M.G.; Ahmed, A.M.M.; Abdel-Rahman, H.H.; Moustafa, A.H. Use of Span 80 and Tween 80 for blending gasoline and alcohol in spark ignition engines. Energy Rep. 2019, 5, 221–230. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef]
- Jiao, J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1663–1673. [Google Scholar] [CrossRef]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Rastgo, M.; Zadeh, M.M.; Vereecken, H. Particle size distribution models, their characteristics and fitting capability. J. Hydrol. 2015, 529, 872–889. [Google Scholar] [CrossRef]
- Danaei, M.R.M.M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, D.; Manjula, N.; Pise, A.; Pise, S. Ban of cosmetic testing on animals: A brief overview. Int. J. Curr. Res. Rev. 2020, 12, 113. [Google Scholar] [CrossRef]
- Fischer, K. Animal testing and marketing bans of the EU cosmetics legislation. Eur. J. Risk Regul. 2015, 6, 613–621. [Google Scholar] [CrossRef]
- Wilson, T.D.; Steck, W.F. A modified HET–CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Yenilmez, E.; Şenel, B.; Kıyan, H.T.; Güven, U.M. Effect of different molecular weight PLGA on flurbiprofen nanoparticles: Formulation, characterization, cytotoxicity, and in vivo anti-inflammatory effect by using HET-CAM assay. Drug Dev. Ind. Pharm. 2020, 46, 682–695. [Google Scholar] [CrossRef]
- Pereira, G.; Fernandes, C.; Dhawan, V.; Dixit, V. Preparation and development of nanoemulsion for skin moisturizing. In Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–47. [Google Scholar]
- Barreto, S.M.A.G.; Maia, M.S.; Benica, A.M.; de Assis, H.R.B.S.; Leite-Silva, V.R.; da Rocha-Filho, P.A.; de Negreiros, M.M.F.; de Oliveira-Rocha, H.A.; Ostrosky, E.A.; Lopes, P.S.; et al. Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: Development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind. Crop. Prod. 2017, 108, 470–479. [Google Scholar] [CrossRef]
- Oh, S.; Jeong, J.; Kim, M.; Jin, X.; Zheng, S.; Kim, Y.M.; Yi, T.H. A study of anti-wrinkle functions and improvement of cream with Phaseolus angularis. Int. J. Cosmet. Sci. 2023, 46, 318–332. [Google Scholar] [CrossRef]
- Ryu, J.H.; Seo, Y.K.; Boo, Y.C.; Chang, M.Y.; Kwak, T.J.; Koh, J.S. A quantitative evaluation method of skin texture affected by skin ageing using replica images of the cheek. Int. J. Cosmet. Sci. 2014, 36, 247–252. [Google Scholar] [CrossRef] [PubMed]



| Stability Test | Homogeneity | pH | Viscosity (mPas) |
|---|---|---|---|
| Before stability test | √ | 6.28 ± 0.01 | 1.75 ± 0.11 |
| After 6 cycles of heating–cooling | √ | 6.13 ± 0.03 *** | 1.85 ± 0.10 |
| After 3 months in room temperature | √ | 6.21 ± 0.00 ** | 2.09 ± 0.09 * |
| Stability Test | Homogeneity | pH | Viscosity (mPas) |
|---|---|---|---|
| Before stability test | √ | 6.37 ± 0.02 | 0.21 ± 0.02 |
| After 6 cycles of heating–cooling | √ | 6.21 ± 0.01 *** | 0.16 ± 0.00 ** |
| After 3 months in room temperature | √ | 6.32 ± 0.02 * | 0.36 ± 0.00 *** |
| Tested Compounds | Irritation Score (IS) | Severity |
|---|---|---|
| Positive control | 18.5 ± 0.1 | Severe irritation |
| Negative control | 0.0 ± 0.0 | No irritation |
| Conventional O/W emulsion | 0.0 ± 0.0 | No irritation |
| O/W Nanoemulsion | 0.0 ± 0.0 | No irritation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somwongin, S.; Chaiyana, W. Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia integrifolia Seed Oil. Nanomaterials 2024, 14, 724. https://doi.org/10.3390/nano14080724
Somwongin S, Chaiyana W. Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia integrifolia Seed Oil. Nanomaterials. 2024; 14(8):724. https://doi.org/10.3390/nano14080724
Chicago/Turabian StyleSomwongin, Suvimol, and Wantida Chaiyana. 2024. "Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia integrifolia Seed Oil" Nanomaterials 14, no. 8: 724. https://doi.org/10.3390/nano14080724
APA StyleSomwongin, S., & Chaiyana, W. (2024). Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia integrifolia Seed Oil. Nanomaterials, 14(8), 724. https://doi.org/10.3390/nano14080724







