Tellurium and Nano-Tellurium: Medicine or Poison?
Abstract
1. Introduction
2. Discovery of Tellurium
3. Tellurium’s Occurrence, Forms in Nature, and Characterization
3.1. Tellurium’s Occurrence
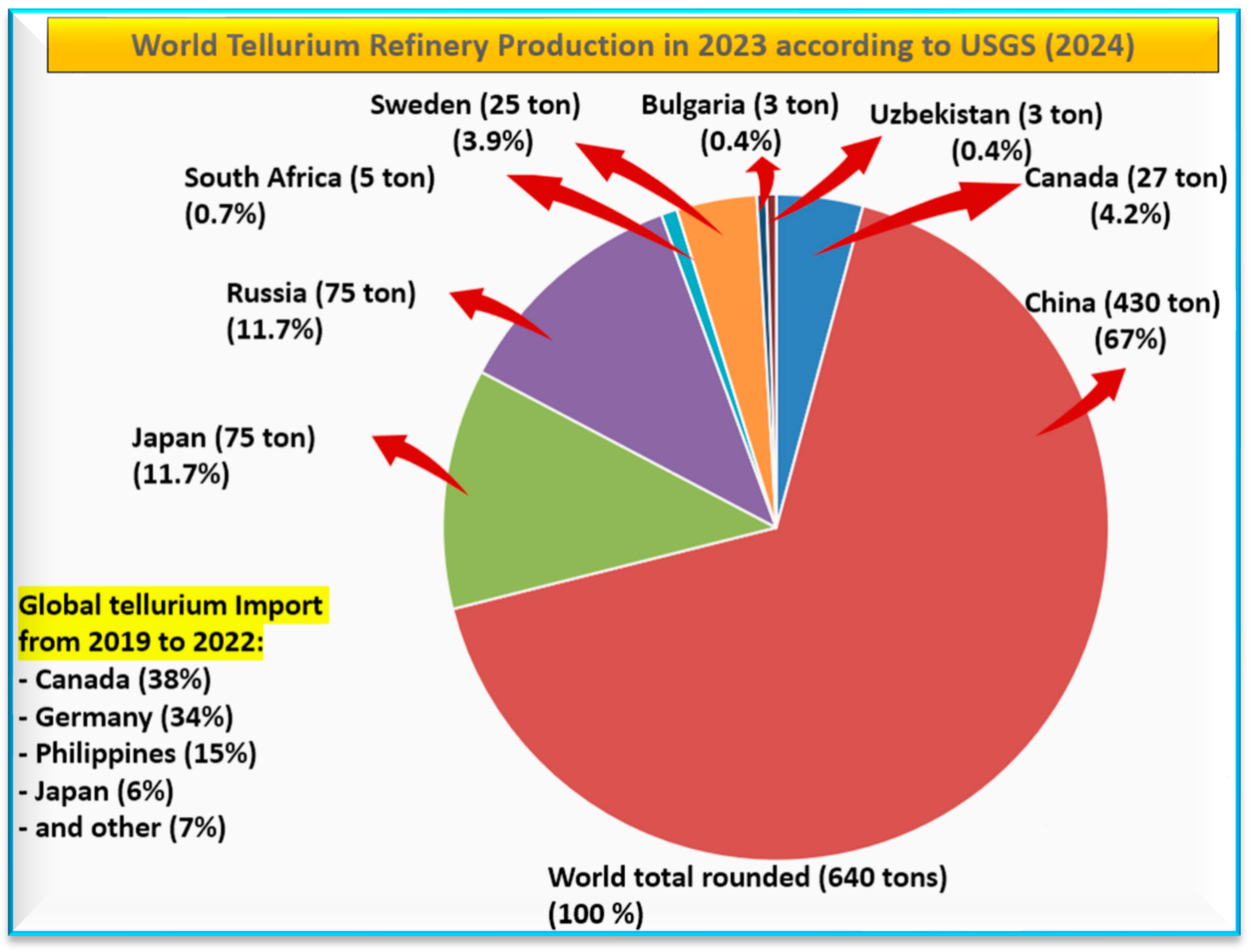
3.2. Global Tellurium Production
3.3. Tellurium Forms in Nature
3.4. Tellurium Characterization
4. Nano-Tellurium and Its Production
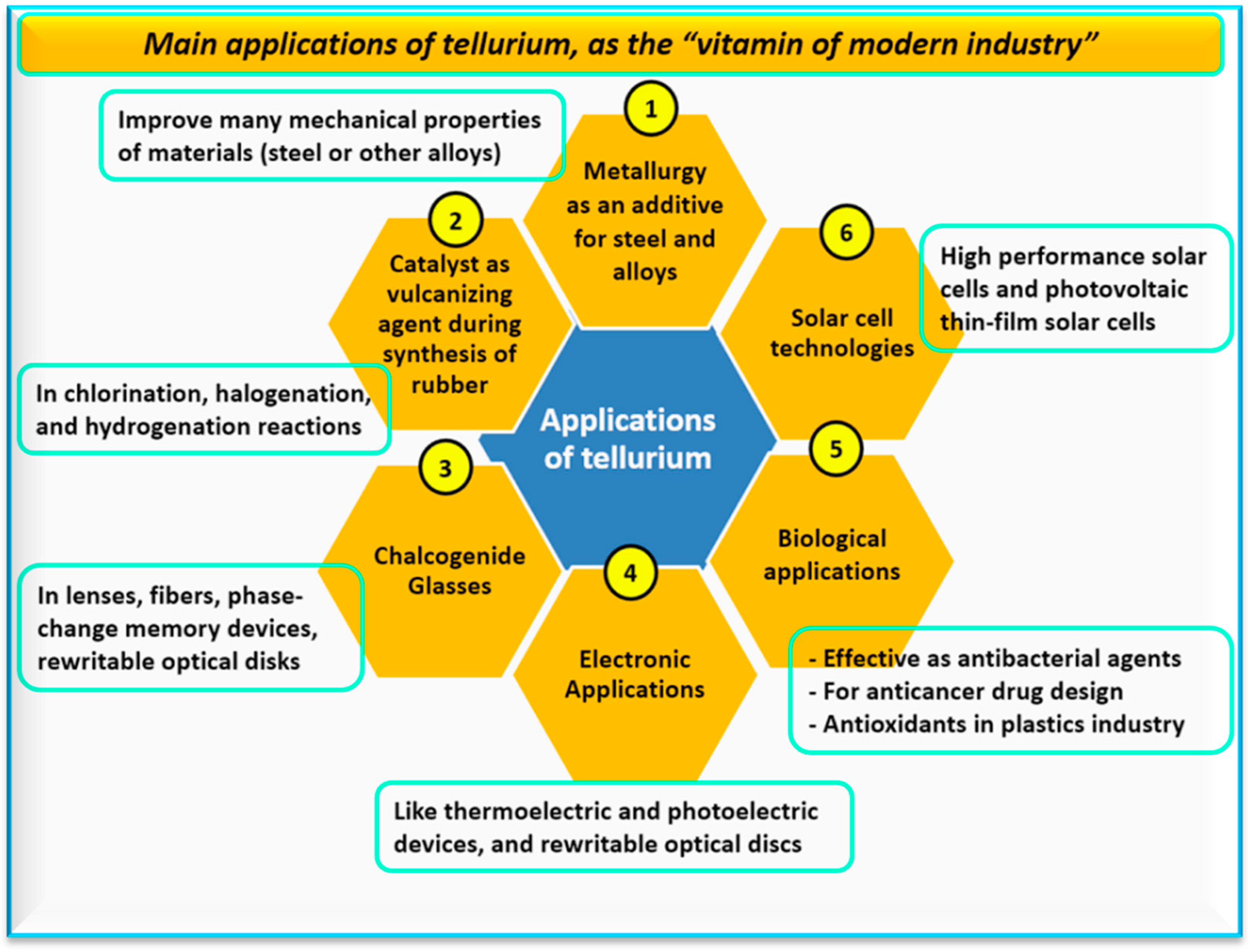
5. Applications of Tellurium and Nano-Tellurium
5.1. Pharmaceutical Applications
5.2. Biomedical Applications
6. Toxicity of Tellurium and Nano-Tellurium
6.1. Clinical Signs
6.2. Biogeochemistry
6.3. Environmental Effects
6.4. Toxicity of Nano-Tellurium
6.5. Tellurium Toxicology and Safety
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Li, X.; Abbas, M.; Fu, C.; Su, Z.; Tang, R.; Chen, S.; Fan, P.; Liang, G. Tellurium Doping Inducing Defect Passivation for Highly Effective Antimony Selenide Thin Film Solar Cell. Nanomaterials 2023, 13, 1240. [Google Scholar] [CrossRef]
- Yang, M.; Yang, M.; Li, Y.; Chen, Y.; Song, Y.; Jia, J.; Su, T. Optimizing Thermoelectric Performance of Tellurium via Doping with Antimony and Selenium. Molecules 2023, 28, 7287. [Google Scholar] [CrossRef] [PubMed]
- Rezaul Karim, S.; Khan, S.; Ansari, G.F.; Mishra, D.; Kumar, S.; Ashiq, M. X-ray Attenuation Performance of a Newly Synthesized Tellurium Based Lead-Free Radiation Shielding Glass System. Radiat. Phys. Chem. 2024, 216, 111477. [Google Scholar] [CrossRef]
- Pale, P.; Mamane, V. Chalcogen Bonding Catalysis: Tellurium, the Last Frontier? Chem. Eur. J. 2023, 29, e202302755. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, X.; Li, D.; Tian, Q.; Zhu, L. Selective Recovery of Sb and Te from the Sodium Sulfide Leach Solution of Te-Bearing Alkaline Skimming Slag by Drop-Wise H2O2 Addition Followed by Na2S–Na2SO3 Precipitation. Hydrometallurgy 2020, 191, 105219. [Google Scholar] [CrossRef]
- Lian, Q.; Liu, W.; Ma, D.; Liang, Z.; Tang, Z.; Cao, J.; He, C.; Xia, D. Precisely Orientating Atomic Array in One-Dimension Tellurium Microneedles Enhances Intrinsic Piezoelectricity for an Efficient Piezo-Catalytic Sterilization. ACS Nano 2023, 17, 8755–8766. [Google Scholar] [CrossRef] [PubMed]
- PubChem Tellurium. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6327182 (accessed on 17 March 2024).
- Basu, A.; Schilling, K.; Halliday, A.N.; Wasserman, N.; Johnson, T.M. Te(IV) Immobilization by Siderite: Reaction Kinetics, Mechanism, and Te Isotopic Fractionation. Chem. Geol. 2022, 612, 121123. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A Maverick among the Chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Lausberg, E.R.; Brugger, J.; Etschmann, B.; Mills, S.J.; Momma, K.; Ram, R.; Maruyama, M.; Fang, X.-Y.; Melchiorre, E.; et al. Natural Nanoparticles of the Critical Element Tellurium. J. Hazard. Mater. Lett. 2022, 3, 100053. [Google Scholar] [CrossRef]
- Abed, N.N.; Abou El-Enain, I.M.M.; El-Husseiny Helal, E.; Yosri, M. Novel Biosynthesis of Tellurium Nanoparticles and Investigation of Their Activity against Common Pathogenic Bacteria. J. Taibah Univ. Med. Sci. 2023, 18, 400–412. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, S.; Guo, Q.; Missen, O.P.; Xia, X. Microbial Mechanisms to Transform the Super-Trace Element Tellurium: A Systematic Review and Discussion of Nanoparticulate Phases. World J. Microbiol. Biotechnol. 2023, 39, 262. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, L.; Wang, K.; Liu, H.; Zhang, J.; Yan, S. Progress in the Synthesis and Application of Tellurium Nanomaterials. Nanomaterials 2023, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Mithun, K.P.; Tripathi, S.; Roy, A.; Ravishankar, N.; Sood, A.K. Ultrafast Time-Resolved Carrier Dynamics in Tellurium Nanowires Using Optical Pump Terahertz Probe Spectroscopy. Nanoscale 2023, 15, 12670–12678. [Google Scholar] [CrossRef] [PubMed]
- Bhol, P.; Jagdale, P.B.; Jadhav, A.H.; Saxena, M.; Samal, A.K. All-Solid-State Supercapacitors Based on Cobalt Magnesium Telluride Microtubes Decorated with Tellurium Nanotubes. ChemSusChem 2024, e202301009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Chu, J.; Yang, L.; Zhao, X.; Zhang, Y.; Liu, T.; Lu, Y.; Chen, C.; Hou, X.; et al. Tailoring the Epitaxial Growth of Oriented Te Nanoribbon Arrays. iScience 2023, 26, 106177. [Google Scholar] [CrossRef] [PubMed]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghany, M.N.; Hamdi, S.A.; Korany, S.M.; Elbaz, R.M.; Farahat, M.G. Biosynthesis of Novel Tellurium Nanorods by Gayadomonas Sp. TNPM15 Isolated from Mangrove Sediments and Assessment of Their Impact on Spore Germination and Ultrastructure of Phytopathogenic Fungi. Microorganisms 2023, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Sári, D.; Ferroudj, A.; Muthu, A.; Prokisch, J.; Fawzy, Z.F.; Brevik, E.C.; Solberg, S.Ø. Nanofarming: Promising Solutions for the Future of the Global Agricultural Industry. Agronomy 2023, 13, 1600. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Longa, I. A three times discovered tellurium or geologists in the magic flute. Ipjogvéd. És Szerzői Jogi Szle. 2006, 11, 276. [Google Scholar]
- 12 October 1825—Death of Franz-Joseph Müller, Discoverer of Tellurium. Rincón Educ. Available online: https://rinconeducativo.org/en/anniversaries/12-october-1825-death-of-franz-joseph-muller-discoverer-of-tellurium/#:~:text=Cooperative%20learning-,12%20October%201825%20%2D%20Death%20of%20Franz%2DJoseph%20M%C3%BCller%2C%20discoverer,specialized%20in%20mineralogy%20and%20chemistry (accessed on 5 February 2024).
- Klaproth, Martin Heinrich. Available online: https://www.vilaglex.hu/Lexikon/Html/Klaproth_htm (accessed on 17 March 2024).
- Kitaibel Pál. Available online: https://mek.oszk.hu/02000/02060/html/kitaibel.htm (accessed on 17 March 2024).
- Christy, A.G.; Mills, S.J.; Kampf, A.R. A Review of the Structural Architecture of Tellurium Oxycompounds. Mineral. Mag. 2016, 80, 415–545. [Google Scholar] [CrossRef]
- Cohen, B.L. Anomalous Behavior of Tellurium Abundances. Geochim. Cosmochim. Acta 1984, 48, 203–205. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Berger, B.R.; George, M.W.; Seal II, R.R. Tellurium; Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; US Geological Survey: Reston, VA, USA, 2017; pp. R1–R27.
- Wiklund, J.A.; Kirk, J.L.; Muir, D.C.G.; Carrier, J.; Gleason, A.; Yang, F.; Evans, M.; Keating, J. Widespread Atmospheric Tellurium Contamination in Industrial and Remote Regions of Canada. Environ. Sci. Technol. 2018, 52, 6137–6145. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xiong, Y.; Song, Y.; Zhang, G.; Zhang, F.; Yang, Y.; Hua, Z.; Tian, Y.; You, J.; Zhao, Z. Recycling of Copper Telluride from Copper Anode Slime Processing: Toward Efficient Recovery of Tellurium and Copper. Hydrometallurgy 2020, 196, 105436. [Google Scholar] [CrossRef]
- US Geological Survey Mineral Commodity Summaries 2024. 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-tellurium.pdf (accessed on 5 February 2024).
- Taylor, A. Biochemistry of Tellurium. Biol. Trace Elem. Res. 1996, 55, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Takahashi, Y. Origin of the Difference in the Distribution Behavior of Tellurium and Selenium in a Soil–Water System. Geochim. Cosmochim. Acta 2008, 72, 1281–1294. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halliday, A.N. Global Occurrence of Tellurium-Rich Ferromanganese Crusts and a Model for the Enrichment of Tellurium. Geochim. Cosmochim. Acta 2003, 67, 1117–1127. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W. Tellurium in the Environment: A Critical Review Focused on Natural Waters, Soils, Sediments and Airborne Particles. Appl. Geochem. 2015, 63, 83–92. [Google Scholar] [CrossRef]
- Vávrová, S.; Struhárňanská, E.; Turňa, J.; Stuchlík, S. Tellurium: A Rare Element with Influence on Prokaryotic and Eukaryotic Biological Systems. Int. J. Mol. Sci. 2021, 22, 5924. [Google Scholar] [CrossRef] [PubMed]
- Anan, Y.; Yoshida, M.; Hasegawa, S.; Katai, R.; Tokumoto, M.; Ouerdane, L.; Łobiński, R.; Ogra, Y. Speciation and Identification of Tellurium-Containing Metabolites in Garlic, Allium Sativum. Metallomics 2013, 5, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Haider, S.I.; Solangi, A.R.; Memon, A.F. Toxicity of Tellurium and Its Compounds. Phys. Sci. Rev. 2023, 8, 4375–4390. [Google Scholar] [CrossRef]
- Medina-Cruz, D.; Tien-Street, W.; Vernet-Crua, A.; Zhang, B.; Huang, X.; Murali, A.; Chen, J.; Liu, Y.; Garcia-Martin, J.M.; Cholula-Díaz, J.L.; et al. Tellurium, the Forgotten Element: A Review of the Properties, Processes, and Biomedical Applications of the Bulk and Nanoscale Metalloid. In Racing for the Surface; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 723–783. ISBN 978-3-030-34470-2. [Google Scholar]
- Vernet Crua, A.; Medina, D.; Zhang, B.; González, M.U.; Huttel, Y.; García-Martín, J.M.; Cholula Díaz, J.L.; Webster, T.J. Comparison of Cytocompatibility and Anticancer Properties of Traditional and Green Chemistry-Synthesized Tellurium Nanowires. Int. J. Nanomed. 2019, 14, 3155–3176. [Google Scholar] [CrossRef] [PubMed]
- Mahalakhsmi, A.; Baskar, G. Greener Synthesis and Characterization of Cadmium-Tellurium Quantum Dots Using Aqueous Extract of Waste Orange Peel. Indian J. Biochem. Biophys. 2021, 58, 56–61. [Google Scholar] [CrossRef]
- Gómez-Gómez, B.; Sanz-Landaluce, J.; Pérez-Corona, M.T.; Madrid, Y. Fate and Effect of In-House Synthesized Tellurium Based Nanoparticles on Bacterial Biofilm Biomass and Architecture. Challenges for Nanoparticles Characterization in Living Systems. Sci. Total Environ. 2020, 719, 137501. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium Chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. IJPR 2018, 17, 87–97. [Google Scholar] [PubMed]
- Beleneva, I.A.; Kharchenko, U.V.; Kukhlevsky, A.D.; Boroda, A.V.; Izotov, N.V.; Gnedenkov, A.S.; Egorkin, V.S. Biogenic Synthesis of Selenium and Tellurium Nanoparticles by Marine Bacteria and Their Biological Activity. World J. Microbiol. Biotechnol. 2022, 38, 188. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Lashani, E.; Moghimi, H. Simultaneous Bioremediation of Phenol and Tellurite by Lysinibacillus Sp. EBL303 and Characterization of Biosynthesized Te Nanoparticles. Sci. Rep. 2023, 13, 1243. [Google Scholar] [CrossRef] [PubMed]
- Vaigankar, D.C.; Dubey, S.K.; Mujawar, S.Y.; D’Costa, A.; Shyama, S.K. Tellurite Biotransformation and Detoxification by Shewanella Baltica with Simultaneous Synthesis of Tellurium Nanorods Exhibiting Photo-Catalytic and Anti-Biofilm Activity. Ecotoxicol. Environ. Saf. 2018, 165, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Ao, B.; He, F.; Lv, J.; Tu, J.; Tan, Z.; Jiang, H.; Shi, X.; Li, J.; Hou, J.; Hu, Y.; et al. Green Synthesis of Biogenetic Te(0) Nanoparticles by High Tellurite Tolerance Fungus Mortierella Sp. AB1 with Antibacterial Activity. Front. Microbiol. 2022, 13, 1020179. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and Investigations on Tellurium Myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Biosynthesis of Gallic Acid Fabricated Tellurium Nanoparticles (GA-Te NPs) for Enhanced Antibacterial, Antioxidant, and Cytotoxicity Applications. Environ. Res. 2024, 240, 117461. [Google Scholar] [CrossRef] [PubMed]
- Rachitha, P.; Krupashree, K.; Kandikattu, H.K.; Nagaraj, G.; Alahmadi, T.A.; Alharbi, S.A.; Shanmuganathan, R.; Brindhadevi, K.; Raghavendra, V.B. Nanofabrication of Cobalt-Tellurium Using Allium Sativum Extract and Its Protective Efficacy against H2O2-Induced Oxidative Damage in HaCaT Cells. Environ. Res. 2023, 226, 115659. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Borghese, R.; Zannoni, D. Microbial Processing of Tellurium as a Tool in Biotechnology. Biotechnol. Adv. 2012, 30, 954–963. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, F.; Tian, Q.; Yue, X.; Zhang, T. Production and Recovery of Tellurium from Metallurgical Intermediates and Electronic Waste-A Comprehensive Review. J. Clean. Prod. 2022, 366, 132796. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, R.; Khan, K.; Tareen, A.K.; Liu, X.; Liang, W.; Zhang, Y.; Ma, C.; Guo, Z.; Luo, X.; et al. Two-Dimensional Tellurium: Progress, Challenges, and Prospects. Nano-Micro Lett. 2020, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.I.; Kar, Y.B.; Doroody, C.; Kiong, T.S.; Rahman, K.S.; Harif, M.N.; Amin, N. A Comprehensive Review of Flexible Cadmium Telluride Solar Cells with Back Surface Field Layer. Heliyon 2023, 9, e21622. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Ram, R.; Mills, S.J.; Etschmann, B.; Reith, F.; Shuster, J.; Smith, D.J.; Brugger, J. Love Is in the Earth: A Review of Tellurium (Bio)Geochemistry in Surface Environments. Earth-Sci. Rev. 2020, 204, 103150. [Google Scholar] [CrossRef]
- Tao, J.-J.; Jiang, J.; Zhao, S.-N.; Zhang, Y.; Li, X.-X.; Fang, X.; Wang, P.; Hu, W.; Lee, Y.H.; Lu, H.-L.; et al. Fabrication of 1D Te/2D ReS 2 Mixed-Dimensional van Der Waals p-n Heterojunction for High-Performance Phototransistor. ACS Nano 2021, 15, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Poborchii, V.V.; Fokin, A.V.; Shklyaev, A.A. Optical Properties of Extreme Tellurium Nanowires Formed in Subnanometer-Diameter Channels. Nanoscale Adv. 2023, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, R.; Zhang, Y. Tellurium Nanotubes and Chemical Analogues from Preparation to Applications: A Minor Review. Nanomaterials 2022, 12, 2151. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.; Lee, S.-H.; Yu, S.; Kim, W.; Bae, J.-S.; Ahn, C.-Y.; Shim, H.; Lee, J.E.; Yu, S.-H. Te Hexagonal Nanotubes with Fast 1-Dimensional Zn Ion Diffusion for High-Performance Zinc-Ion Battery Cathodes. Chem. Eng. J. 2024, 481, 148256. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Santa, R.; Crnac, Z.; Bošnjaković, T. Environmental Impact of PV Power Systems. Sustainability 2023, 15, 11888. [Google Scholar] [CrossRef]
- Scarpulla, M.A.; McCandless, B.; Phillips, A.B.; Yan, Y.; Heben, M.J.; Wolden, C.; Xiong, G.; Metzger, W.K.; Mao, D.; Krasikov, D.; et al. CdTe-Based Thin Film Photovoltaics: Recent Advances, Current Challenges and Future Prospects. Sol. Energy Mater. Sol. Cells 2023, 255, 112289. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Papež, N.; Holcman, V. Overview of the Current State of Flexible Solar Panels and Photovoltaic Materials. Materials 2023, 16, 5839. [Google Scholar] [CrossRef] [PubMed]
- National Food Institute—Technical University of Denmark; Doulgeridou, A.; Amlund, H.; Sloth, J.J.; Hansen, M. Review of Potentially Toxic Rare Earth Elements, Thallium and Tellurium in Plant-based Foods. EFSA J. 2020, 18, e181101. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.T.; Kim, H.; Frenzel, M.; Moats, M.S.; Hayes, S.M. Global Tellurium Supply Potential from Electrolytic Copper Refining. Resour. Conserv. Recycl. 2022, 184, 106434. [Google Scholar] [CrossRef]
- Azzouz, L.; Halit, M.; Charifi, Z.; Matta, C.F. Tellurium Doping and the Structural, Electronic, and Optical Properties of NaYS 2(1–x) Te 2 x Alloys. ACS Omega 2019, 4, 11320–11331. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, T.; Li, D. Unexpected D−p Orbital Covalent Interaction Between the Non-d-Block Main-Group Metal Tellurium and Fluorine at High Pressure. Fundam. Res. 2023, S2667325823001279. [Google Scholar] [CrossRef]
- Jiang, B.; Xue, H.; Wang, P.; Du, H.; Kang, Y.; Zhao, J.; Wang, S.; Zhou, W.; Bian, Z.; Li, H.; et al. Noble-Metal–Metalloid Alloy Architectures: Mesoporous Amorphous Iridium–Tellurium Alloy for Electrochemical N2 Reduction. J. Am. Chem. Soc. 2023, 145, 6079–6086. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shi, T.; Li, Y.; Yang, B.; Tian, Y.; Xu, B.; Yang, H.; Chen, X.; Chen, C. Selective Sulfidation-Vacuum Volatilization Processes for Tellurium and Bismuth Recovery from Bismuth Telluride Waste Thermoelectric Material. J. Environ. Manag. 2023, 327, 116845. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zha, W.; Yuan, R.; Lou, B.; Sa, R. Indirect-to-Direct Band Gap Transition and Optical Properties of Metal Alloys of Cs2 Te1−x Tix I6: A Theoretical Study. RSC Adv. 2020, 10, 36734–36740. [Google Scholar] [CrossRef]
- Faizan, M.; Xie, J.; Murtaza, G.; Echeverría-Arrondo, C.; Alshahrani, T.; Bhamu, K.C.; Laref, A.; Mora-Seró, I.; Haidar Khan, S. A First-Principles Study of the Stability, Electronic Structure, and Optical Properties of Halide Double Perovskite Rb2Sn1−xTexI6 for Solar Cell Applications. Phys. Chem. Chem. Phys. 2021, 23, 4646–4657. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Q.; Zhang, W.; Huang, S. Amorphous Tellurium-Embedded Hierarchical Porous Carbon Nanofibers as High-Rate and Long-Life Electrodes for Potassium-Ion Batteries. Small 2022, 18, 2202750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Freschi, D.J.; Liu, J. High-Performance Potassium-Tellurium Batteries Stabilized by Interface Engineering. Small 2022, 18, 2200085. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Mu, Z.; Qian, K.; Guo, C.; Li, M.; Li, J. Biochar-Derived Hierarchical Porous Carbon as Tellurium Host for High-Performance Potassium-Tellurium Batteries. Chem. Eur. J. 2023, 29, e202302121. [Google Scholar] [CrossRef] [PubMed]
- Hellier, K.; Stewart, D.A.; Read, J.; Sfadia, R.; Abbaszadeh, S. Tuning Amorphous Selenium Composition with Tellurium to Improve Quantum Efficiency at Long Wavelengths and High Applied Fields. ACS Appl. Electron. Mater. 2023, 5, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, H.; Kobarfard, F.; Alizadeh, A.; Saravanan, M.; Barabadi, H. Green Nanotechnology-Based Tellurium Nanoparticles: Exploration of Their Antioxidant, Antibacterial, Antifungal and Cytotoxic Potentials against Cancerous and Normal Cells Compared to Potassium Tellurite. Inorg. Chem. Commun. 2021, 124, 108385. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium–Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Rahimi-Nasrabadi, M.; Pourmasud, S.; Eghbali-Arani, M.; Banafshe, H.R.; Ahmadi, F.; Ganjali, M.R.; Sobhani Nasab, A. CdTe Quantum Dots Prepared Using Herbal Species and Microorganisms and Their Anti-Cancer, Drug Delivery and Antibacterial Applications; a Review. Ceram. Int. 2020, 46, 9979–9989. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, K.; Sharma, M.; Modi, S.K.; Nenavathu, B.P. Novel Tellurium Doped CeO2 Nano Wools as a next Generation Antibacterial Therapeutic Agent. Mater. Chem. Phys. 2023, 307, 128172. [Google Scholar] [CrossRef]
- Gautam, M.; Kim, J.O.; Yong, C.S. Fabrication of Aerosol-Based Nanoparticles and Their Applications in Biomedical Fields. J. Pharm. Investig. 2021, 51, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Dutton, W.A.; Cooper, W.C. The Oxides and Oxyacids of Tellurium. Chem. Rev. 1966, 66, 657–675. [Google Scholar] [CrossRef]
- Avila, D.S.; Soares, A.; Salgueiro, W.G. Toxicology and Pharmacology of Organotellurium Compounds. In Tellurium: Properties, Uses and Research; Grey, D., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 137–169. [Google Scholar]
- Sahoo, B.M.; Banik, B.K.; Tiwari, A.; Tiwari, V.; Jain, A.; Borah, P. Synthesis and Application of Organotellurium Compounds. Phys. Sci. Rev. 2023, 8, 4435–4460. [Google Scholar] [CrossRef]
- Tripathi, A.; Khan, A.; Kiran, P.; Shetty, H.; Srivastava, R. Screening of AS101 Analog, Organotellurolate (IV) Compound 2 for Its in Vitro Biocompatibility, Anticancer, and Antibacterial Activities. Amino Acids 2023, 55, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and Tellurium in the Development of Novel Small Molecules and Nanoparticles as Cancer Multidrug Resistance Reversal Agents. Drug Resist. Updat. 2022, 63, 100844. [Google Scholar] [CrossRef] [PubMed]
- Grey, D. (Ed.) Tellurium: Properties, Uses, and Research; Chemistry Research and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2017; ISBN 978-1-5361-0587-2. [Google Scholar]
- Zigman-Hoffman, E.; Sredni, B.; Meilik, B.; Naparstek, E.; Tartakovsky, B. Tellurium Compound Provides Pro-Apoptotic Signaling in Drug Resistant Multiple Myeloma. Leuk. Lymphoma 2021, 62, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Liu, T.; Chen, T. Near-Infrared Laser-Triggered Drug Release in a Tellurium Nanosystem for Simultaneous Chemo-Photothermal Cancer Therapy. Biomater. Sci. 2021, 9, 1767–1778. [Google Scholar] [CrossRef]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial Activity of Tellurium-loaded Polymeric Fiber Meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Zare, B.; Nami, M.; Shahverdi, A.-R. Tracing Tellurium and Its Nanostructures in Biology. Biol. Trace Elem. Res. 2017, 180, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, C.; Li, Y.; Yang, Y.; Li, W.; Feng, C.; Li, L. Ultrathin Tellurium Nanosheets for Simultaneous Cancer Thermo-Chemotherapy. Bioact. Mater. 2022, 13, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Zare, H.; Taghavinia, N.; Irajizad, A.; Aghaei, M.; Panjehpour, M. Cadmium Telluride Quantum Dots Induce Apoptosis in Human Breast Cancer Cell Lines. Toxicol. Ind. Health 2018, 34, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ke, H.; Wang, Q.; Tang, Y.; Deng, Y.; Yang, H.; Yang, X.; Yang, P.; Ling, D.; Chen, C.; et al. Bifunctional Tellurium Nanodots for Photo-Induced Synergistic Cancer Therapy. ACS Nano 2017, 11, 10012–10024. [Google Scholar] [CrossRef] [PubMed]
- Najimi, S.; Shakibaie, M.; Jafari, E.; Ameri, A.; Rahimi, N.; Forootanfar, H.; Yazdanpanah, M.; Rahimi, H.R. Acute and Subacute Toxicities of Biogenic Tellurium Nanorods in Mice. Regul. Toxicol. Pharmacol. 2017, 90, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Missen, O.P.; Etschmann, B.; Mills, S.J.; Sanyal, S.K.; Ram, R.; Shuster, J.; Rea, M.A.D.; Raudsepp, M.J.; Fang, X.-Y.; Lausberg, E.R.; et al. Tellurium Biogeochemical Transformation and Cycling in a Metalliferous Semi-Arid Environment. Geochim. Cosmochim. Acta 2022, 321, 265–292. [Google Scholar] [CrossRef]
- Muthu, A.; Sári, D.; Ferroudj, A.; El-Ramady, H.; Béni, Á.; Badgar, K.; Prokisch, J. Microbial-Based Biotechnology: Production and Evaluation of Selenium-Tellurium Nanoalloys. Appl. Sci. 2023, 13, 11733. [Google Scholar] [CrossRef]
- Blackadder, E.S.; Manderson, W.G. Occupational Absorption of Tellurium: A Report of Two Cases. Occup. Environ. Med. 1975, 32, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Toxocology—International Occupational Safety & Health Information Centre. Available online: https://www.ilo.org/static/english/protection/safework/cis/products/safetytm/toxic.htm (accessed on 17 March 2024).
- Tellurium—ESPI Metals. Available online: https://www.espimetals.com/index.php/msds/285-Tellurium (accessed on 17 March 2024).
- Tellurium Dioxide CAS 7446-07-3|112356. Available online: https://www.emdmillipore.com/US/en/product/Tellurium-dioxide,MDA_CHEM-112356?bd=1 (accessed on 17 March 2024).
- Gerhardsson, L. Tellurium. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2007; pp. 815–825. ISBN 978-0-12-369413-3. [Google Scholar]
- Hayes, S.M.; Ramos, N.A. Surficial Geochemistry and Bioaccessibility of Tellurium in Semiarid Mine Tailings. Environ. Chem. 2019, 16, 251. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Givelet, L.; Amlund, H.; Sloth, J.J.; Hansen, M. Risk Assessment of Rare Earth Elements, Antimony, Barium, Boron, Lithium, Tellurium, Thallium and Vanadium in Teas. EFSA J. 2022, 20, e200410. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.C.; Srivastava, R.; Srivastava, T.N.; Jain, S.P. Effect of Organo-Tellurium Compounds on the Enzymatic Alterations in Rats. Toxicol. Lett. 1983, 16, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F. Toxicity of Glutathione-Binding Metals: A Review of Targets and Mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chai, L.; Xie, Z.; Zhang, H. Recent Advance of Tellurium for Biomedical Applications. Chem. Res. Chin. Univ. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Jia, Y.; Nitz, M. Bio-Incorporation of TePhe, a Tellurium-Containing Phenylalanine Analogue, Preserves Protein Structure & Stability. Available online: https://sciforum.net/manuscripts/10192/slides.pdf (accessed on 5 February 2024).
- Chen, C.; Huang, Z. Tellurium-Modified Nucleosides, Nucleotides, and Nucleic Acids with Potential Applications. Molecules 2022, 27, 8379. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, E.; Engman, L.; Svensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.-M.; Brattsand, R. Antioxidative Properties of Organotellurium Compounds in Cell Systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Trindade, C.; Juchem, A.L.M.; Guecheva, T.N.; De Oliveira, I.M.; Dos Santos Silveira, P.; Vargas, J.E.; Puga, R.; Pessoa, C.Ó.; Henriques, J.A.P. Diphenyl Ditelluride: Redox-Modulating and Antiproliferative Properties. Oxid. Med. Cell. Longev. 2019, 2019, 2510936. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Kumar, M.; Bendi, A.; Garg, S. Theoretical and Experimental In-vitro Studies of Novel Thiophene Based Organotellurium(IV) Complexes. Chem. Biodivers. 2024, 21, e202301544. [Google Scholar] [CrossRef] [PubMed]
- Juchem, A.L.M.; Trindade, C.; Da Silva, J.B.; Machado, M.D.S.; Guecheva, T.N.; Rocha, J.C.; Saffi, J.; De Oliveira, I.M.; Henriques, J.A.P.; Escargueil, A. Diphenyl Ditelluride Anticancer Activity and DNA Topoisomerase I Poisoning in Human Colon Cancer HCT116 Cells. Oncotarget 2023, 14, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Takada, S.; Kumagai, K.; Kobayashi, K.; Hokura, A.; Ogra, Y. Elucidation of Tellurium Biogenic Nanoparticles in Garlic, Allium Sativum, by Inductively Coupled Plasma-Mass Spectrometry. J. Trace Elem. Med. Biol. 2020, 62, 126628. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Zschiesche, W.; Steffen, H.M.; Schaller, K.H. Tellurium-Intoxication. Klin. Wochenschr. 1989, 67, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.A.; Buckman, J.; Balassa, J.J. Abnormal Trace Elements in Man: Tellurium. J. Chronic Dis. 1967, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Czapla, M.; Grygoyć, K. Speciation and Fractionation of Less-StudiedTechnology-Critical Elements (Nb, Ta, Ga, In, Ge, Tl, Te): A Review. Pol. J. Environ. Stud. 2021, 30, 1477–1486. [Google Scholar] [CrossRef]
- Filella, M.; Reimann, C.; Biver, M.; Rodushkin, I.; Rodushkina, K. Tellurium in the Environment: Current Knowledge and Identification of Gaps. Environ. Chem. 2019, 16, 215. [Google Scholar] [CrossRef]
- Dickson, R.S.; Glowa, G.A. Tellurium Behaviour in the Fukushima Dai-Ichi Nuclear Power Plant Accident. J. Environ. Radioact. 2019, 204, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Bajpai, S. Accessing the Environmental Impact of Tellurium Metal. Phys. Sci. Rev. 2023, 8, 4903–4913. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.-M.; Wu, D.-D.; Yao, Y.; Liang, Z.-L.; Tan, H.W.; Lau, A.T.Y. Acute and Chronic Cadmium Telluride Quantum Dots-Exposed Human Bronchial Epithelial Cells: The Effects of Particle Sizes on Their Cytotoxicity and Carcinogenicity. Biochem. Biophys. Res. Commun. 2018, 495, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Fortier, M.; Gagne, F.; Gagnon, C.; Turcotte, P.; Tayabali, A.; Davis, T.L.; Auffret, M.; Fournier, M. Size Distribution Effects of Cadmium Tellurium Quantum Dots (CdS/CdTe) Immunotoxicity on Aquatic Organisms. Environ. Sci. Process. Impacts 2013, 15, 596. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Maysinger, D.; Jain, M.; Röder, B.; Hackbarth, S.; Winnik, F.M. Long-Term Exposure to CdTe Quantum Dots Causes Functional Impairments in Live Cells. Langmuir 2007, 23, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Seligy, V.L.; Tayabali, A.F. Cadmium Telluride Quantum Dot Nanoparticle Cytotoxicity and Effects on Model Immune Responses to Pseudomonas aeruginosa. Nanotoxicology 2013, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, M.; Feng, Z.; Xu, X.; Chen, L.; Ma, Z.; Li, L. Biocompatible Tellurium Nanoneedles with Long-Term Stable Antibacterial Activity for Accelerated Wound Healing. Mater. Today Bio 2022, 15, 100271. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liang, Y.; Wei, T.; Huang, X.; Zhang, T.; Tang, M. Protein Corona Exacerbated Inflammatory Response in Macrophages Elicited by CdTe Quantum Dots. NanoImpact 2024, 33, 100494. [Google Scholar] [CrossRef] [PubMed]
- Pedram Jarf, M.; Kamali, A.; Khara, H.; Pourang, N.; Shekarabi, S.P.H. Microplastic Pollution and Heavy Metal Risk Assessment in Perca Fluviatilis from Anzali Wetland: Implications for Environmental Health and Human Consumption. Sci. Total Environ. 2024, 907, 167978. [Google Scholar] [CrossRef] [PubMed]
- Abidin, A.U.; Maziya, F.B.; Susetyo, S.H.; Yoneda, M.; Matsui, Y. Heavy Metal Air Pollution in an Indonesian Landfill Site: Characterization, Sources, and Health Risk Assessment for Informal Workers. Environ. Adv. 2024, 15, 100512. [Google Scholar] [CrossRef]
- Mwelwa, S.; Chungu, D.; Tailoka, F.; Beesigamukama, D.; Tanga, C.M. Data to Understand the Biotransfer of Heavy Metals along the Soil-Plant-Edible Insect-Human Food Chain in Africa. Data Brief 2023, 49, 109434. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Nandimandalam, J.R. Environmental Health Risk Assessment and Source Apportion of Heavy Metals Using Chemometrics and Pollution Indices in the Upper Yamuna River Basin, India. Chemosphere 2024, 346, 140570. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C. Soil, Food Security, and Human Health. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publishers: Abu Dhabi, United Arab Emirates, 2010; Volume III, pp. 161–195. ISBN 978-1-84826-879-7. [Google Scholar]
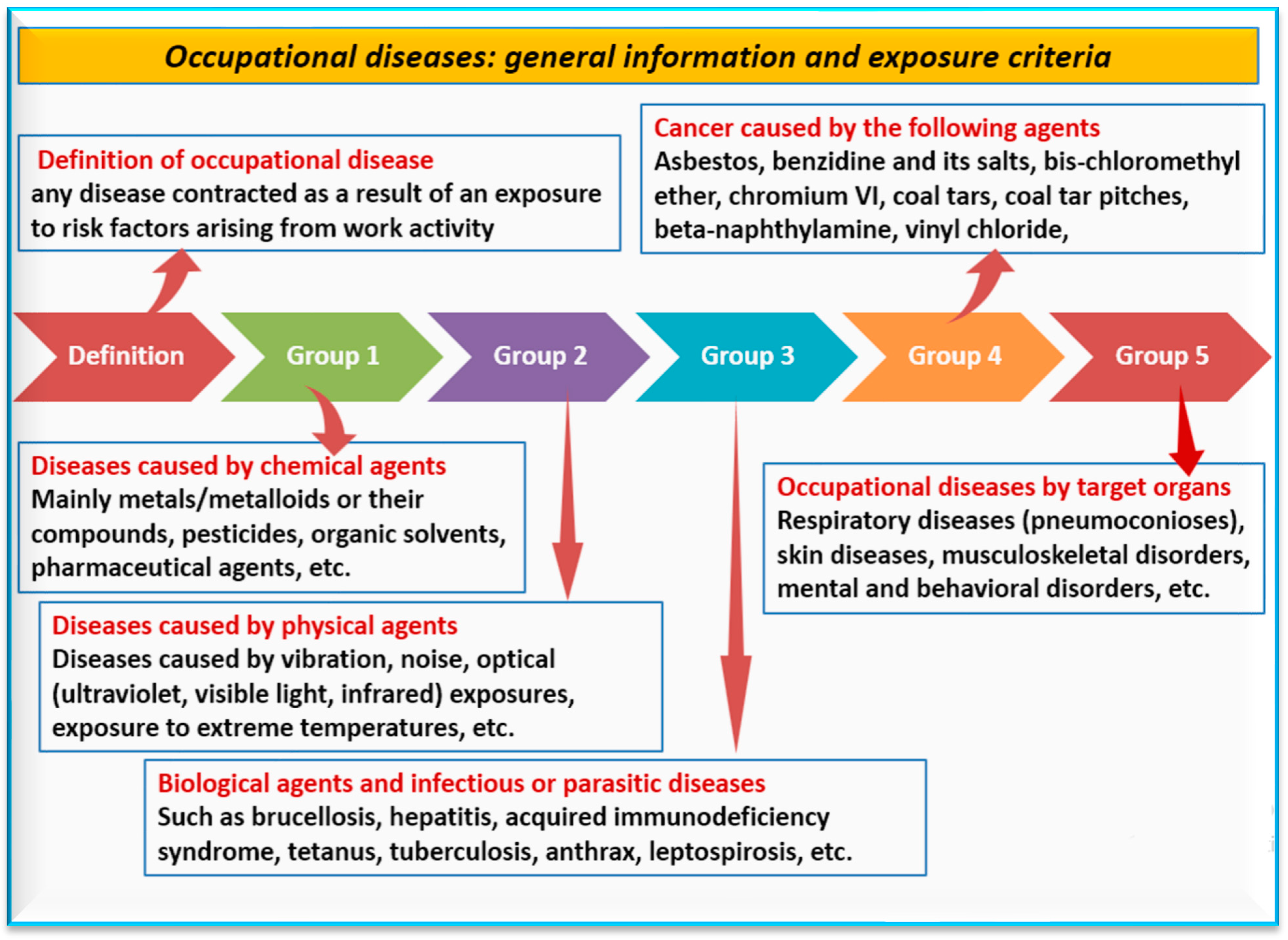
- Niu, S.; Colosio, C.; Carugno, M.; Adisesh, A. Diagnostic and Exposure Criteria for Occupational Diseases Guidance Notes for Diagnosis and Prevention of the Diseases in the ILO List of Occupational Diseases (Revised 2010); International Labour Organization (ILO): Geneva, Switzerland, 2022. [Google Scholar]
- Bazaluk, O.; Tsopa, V.; Cheberiachko, S.; Deryugin, O.; Radchuk, D.; Borovytskyi, O.; Lozynskyi, V. Ergonomic Risk Management Process for Safety and Health at Work. Front. Public Health 2023, 11, 1253141. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fresneda, M.A.; Morales-Hidalgo, M.; Povedano-Priego, C.; Jroundi, F.; Hidalgo-Iruela, J.; Cano-Cano, M.; Pérez-Muelas, E.; Merroun, M.L.; Martín-Sanchez, I. Unlocking the Key Role of Bentonite Fungal Isolates in Tellurium and Selenium Bioremediation and Biorecovery: Implications in the Safety of Radioactive Waste Disposal. Sci. Total Environ. 2024, 912, 169242. [Google Scholar] [CrossRef] [PubMed]


| Term(s) | Definition or More Details |
|---|---|
| Nano-materials (NMs) | A material in which at least one dimension of measurement is between 1 and 100 nm. |
| Nano-tellurium | Nano-tellurium or elemental Te-nano-particles (Te-NPs) is the nano form (diameter ranges from 1 to 100 nm) of the Te metalloid that can be found naturally in regolith samples produced by chemical or biological approaches. |
| Te-NP synonyms | Tellurium nano-powder, nano-tellurium, nano-Te |
| Natural nano-materials | A nano-material made naturally through (bio)geochemical or mechanical processes, without a direct or indirect connection to a human activity or anthropogenic process |
| Engineered nano-materials | Chemical substances/materials that are purposely created by humans with particle sizes between 1 and 100 nm in at least one dimension |
| Nano-alloy of tellurium | Produced by combining Te with other elements to create desirable properties. Common examples include combining Te with Se using microbes (e.g., Lactobacillus casei NCAIM B 1147), Pd–Te nano-clusters, CdTe QDs, CdTe/CdS QD nano-sensors, etc. |
| Common tellurium nano-materials | Nano-Te structures, nano-Te wires, nano-Te tubes, nano-Te rods, and nano-Te ribbons |
| Quantum dots (QDs) | Quantum dots (QDs) are semi-conducting nano-crystals with unique optical properties. |
| Cadmium telluride quantum dots (CdTe QDs) | CdTe-based quantum dots (QDs) are colloidal structures that have unique luminescence and electronic properties and are used in diagnostic and biomedical research. CdTe QDs have a low excitation energy and small band gap compared to those of CdS and CdSe materials. |
| Nano-needles | Needles in the nano-size range, used to deliver therapeutics intracellularly. |
| Nano-Te structures | Nano-forms of Te compounds that have a unique van der Waals structure and intriguing chemical and physical properties, such as nano-wires, nano-tubes, nano-cables, belt-shaped structures, etc. |
| Nano-particles (NPs) | Individual particles that range from 1 to 100 nm in diameter. Also sometimes used for nano-wires and nano-tubes. |
| Nano-spheres | Spherical particles with a diameter between 1 and 100 nm. |
| Nano-wires (NWs) | Nano-wires are structures with a diameter of ∼10 nm and a much greater length. |
| Nano-rods (NRs) | Nano-rods have a typical nano-size between 1 and 100 nm with standard aspect ratios (length divided by width) of 3–5. |
| Nano-tubes (NTs) | Nano-tubes are NMs with a cylindrical shape around a hollow center; their diameters typically range from 200 to 600 nm and their lengths from 4 to 15 nm. |
| Nano-ribbons (NRs) | Nano-ribbons are rectangular in shape, very thin with an appreciably greater width, and a length that can be hundreds of nm. Two dimensions are less than 100 nm. |
| Nano-plates | Nano-plates are rectangular in shape with only one dimension in the nano-meter range. |
| Dimensional Te nano-structures | NMs come in a variety of dimensions. Nano-particles are considered zero-dimensional (0D); nano-rods, nano-tubes, and nano-wires are one-dimensional (1D); nano-ribbons are two-dimensional (2D); and flower-like three-dimensional (3D) Te NMs have been created. |
| Physical Parameter(s) | Value |
|---|---|
| Density in solid form at room T | 6.0 (amorphous) and 6.25 (crystalline) g cm−3 |
| Density in liquid form | 5.70 g cm−3 |
| Thermal conductivity | 1.0–3.4 W/(m·K) in a single crystal |
| Electrical resistivity | 1–50 mΩ·m |
| Electron affinity | 1.971 eV or 1.96 eV |
| Electronegativity | 2.1 (Pauling scale), 2.158 (Allen scale) |
| Van der Waals radius | 206 pm |
| Ionization energy | 9.010 eV |
| Band gap energy | 0.35 eV at room temperature |
| Molar volume | 2.05 × 10−5 m3 |
| Crystal structure | Trigonal, orthorhombic, or hexagonal form |
| Atomic radius | 123 pm |
| Covalent radius | 138 pm |
| Atomic number | 52 |
| Melting point | 449.5 °C (amorphous) and 452 °C (crystal) |
| Boiling point | 988 °C (amorphous) and 1390 °C (crystalline) |
| Heat of fusion | 17.5 kJ mol−1 |
| Heat of vaporization | 48 kJ mol−1 |
| Specific heat | 199–219 J/(kg.K) |
| Refractive index | 1.002495 at λ = 589 nm (vapor) 1.26 at 260 nm and 4.5 at 720 nm (thin film) |
| Chemical Parameter(s) | Details |
|---|---|
| Oxidation states | Telluride (Te2−) (−2), elemental (Te0) (0), tellurite (TeO32−) (+4), and tellurate (TeO42−) (+6). Tellurite (TeO32−) (+4) is the most common. |
| Common Te alloys | Bismuth telluride (Bi2Te3) with Se or Sb, mercury cadmium telluride (HgCdTe), cadmium zinc telluride (CdZnTe), etc. |
| Common Te minerals | Calaverite (AuTe2), sylvanite (AgAuTe4), krennerite (AuTe2), nagyagite [AuPb(Sb, Bi)Te2–3S6], and tellurobismuthite (Bi2Te3) |
| Main Te-oxides in crystalline, amorphous, and colloidal forms | Telluride (Te2−), tellurite (TeO32−), tellurate (TeO4−), tellurium dioxide (TeO2), and tellurium trioxide (TeO3) |
| Tellurium hydrides | Hydrogen telluride (H2Te), hydrogen-rich varieties such as H4Te, and H5Te2 |
| Tellurium acids | Tellurous acid (H2TeO3) and telluric acid [Te(OH)6] |
| Tellurium electron conifiguration | [Kr] 5s2 4d10 5p4 |
| Organo-tellurium compounds | Functional group –TeH is called tellurols and they are found in compounds such as dimethyl telluride (Te(CH3)2) and diphenyl ditelluride (C12H10Te2) |
| Isotopes of tellurium | Stable forms are 120Te, 124Te, 125Te, and 126Te |
| Common Te crystal morphologies | n-whiskers, f-whiskers, s-whiskers, etc. |
| Te Compound (s) | Molecular Formula | Molecular Weight | Synonyms |
|---|---|---|---|
| Tellurous acid | H2O3Te | 177.6 g/mol | Dihydrogen trioxotellurate |
| Tellurinic acid | H2O2Te | 161.6 g/mol | Hydridohydroxidooxidotellurium |
| Tellane | H2Te | 129.6 g/mol | Hydrogen telluride |
| Tellurium, mol. | Te2 | 255.2 g/mol | Di tellurium |
| Copper telluride | CuTe | 191.1 g/mol | Copper monotelluride |
| Copper tellurate | CuO4Te | 255.1 g/mol | Copper(2+) tellurium tetraoxide |
| Cuprous telluride | Cu2Te | 254.7 g/mol | Copper (I) telluride |
| Tellurium trioxide | TeO3 | 175.6 g/mol | Tellurium(VI) oxide |
| Tellurium dioxide | TeO2 | 159.6 g/mol | Tellurium oxide |
| Cadmium telluride | CdTe | 240.0 g/mol | Cadmium monotelluride |
| Cadmium tellurate | CdTeO4 | 304.0 g/mol | Cadmium tellurium tetraoxide |
| Bismuth telluride | Bi2Te3 | 800.8 g/mol | Bismuth selenide telluride |
| Mercury telluride | HgTe | 328.2 g/mol | Tellanylidenemercury |
| Dimethyl telluride | C2H6Te | 157.7 g/mol | Dimethyltellurium |
| Diphenyl ditelluride | C12H10Te2 | 409.4 g/mol | Ditelluride, diphenyl |
| Potassium tellurite | K2TeO3 | 253.8 g/mol | Dipotassium trioxotellurate |
| Potassium tellurate | K2TeO4 | 269.8 g/mol | Potassium tellurate(VI) |
| Sodium tellurite | Na2TeO3 | 221.6 g/mol | Disodium trioxotellurate |
| Sodium tellurate | Na2TeO4 | 237.6 g/mol | Disodium tetraoxotellurate |
| Tellurium hexafluoride | F6Te | 241.6 g/mol | Tellurium(VI) fluoride |
| Phenol, 4,4′-tellurobis- | C12H10O2Te | 313.8 g/mol | bis(4-hydroxyphenyl)telluride |
| Diphenyl ditelluride | C12H10Te2 | 409.4 g/mol | Phenyl ditelluride |
| Synthesis Type | Produced by | Nanparticle Features | Main Purpose of the Study | Refs. |
|---|---|---|---|---|
| Biosynthesis | Streptomyces graminisoli. | Crystal shape (12–25 nm) | Antibacterial activity; minimum inhibitory concentration was 50 μg mL−1 | [11] |
| Biogenic method | Penicillium chrysogenum PTCC 5031 | 50.16 nm | Exploited biomolecules and enzymes secreted from P. chrysogenum at room temperature | [42] |
| Biosynthesis of nano-Te rods | Gayadomonas sp. TNPM15 | 15–23 nm | Acted against phytopathogenic fungi by disruption of integrity and membrane permeability of fungal spores | [18] |
| Biogenic Te-NPs | Bacterial marine isolates | Smaller than 100 nm | Antimicrobial activity | [43] |
| Biosynthesized Te-NPs | Lysinibacillus sp. EBL303. | Rod-shaped (22–148 nm) | Bioremediation of tellurite and phenol at polluted sites | [44] |
| Tellurium nano-rods | Shewanella baltica | From 8–75 nm | Reduced methylene blue through photo-catalytic and anti-biofilm activity | [45] |
| Biogenetic nano-Te particles | Mortierella sp. AB1 | From 100–500 nm | Antibacterial against Escherichia coli, Shigella dysenteriae, Salmonella typhimurium, and Enterobacter sakazakii | [46] |
| Biogenic Te-NPs. | Aspergillus welwitschiae | Spherical shape (60.80 nm) | Antibacterial activity against Staphylococcus aureus and E. coli | [47] |
| Biosynthesis of Te-NPs | Biomolecules of gallic acid | 19.74 nm | Multifunctional agents and biomedical applications | [48] |
| Green synthesis of Te-NPs | Allium sativum extract | 350 nm | Evaluation of the cytoprotective and antioxidant activities of Co-Te-NPs | [49] |
| Nano-Te Structures | Common Form | Method of Synthesis | Additional Details |
|---|---|---|---|
| Zero-dimensional Te nano-structures | NPs in a spherical morphology | Green and chemical synthesis or laser ablation in liquids | Sizes of produced Te-NPs depend on solvents used |
| One-dimensional (1D) Te nano-structures | Nano-wires (Te-NWs) | Microwave-assisted synthesis, hydrothermal methods, and vapor–solid method | Te-NWs are controlled by the temperature of reaction, substrate, and growth time |
| Nano-tubes (Te-NTs) | Physical vapor deposition | Te-NTs are controlled by substrate and deposition temperature | |
| Nano-ribbons (Te-NRs) | Hydrothermal and vapor deposition methods | Te-NRs are controlled by pH, temperature, and reaction time | |
| Tellurium nano-rods | Hydrothermal methods | Surfactants can control diameters and lengths of nano-Te rods | |
| Tellurium belt-shaped structures (Te-NPs) | Thermal evaporation and deposition methods | Temperature and ambient atmosphere control NPs | |
| Two-dimensional (2D) nano-Te structure | 2D tellurene lesser layers | Physical vapor deposition and liquid-phase evolution | Temperature and thermodynamics control NPs |
| Three-dimensional (3D) nano-Te structure | Flower-like 3D Te nano-structures | Solvothermal method, dissolution, and recrystallization | Type of solvents (e.g., water, amide, or alcohol) and temperatures control NPs |
| Chiral-shaped Te nano-structures | Chiral nano-materials (NMs) | Using chiral biomolecules as initiators | Chiral NMs are controlled by different synthetic conditions |
| Te-Alloys | Molecular Formula | Main Findings | Refs. |
|---|---|---|---|
| Alloys from sodium, yttrium, sulfur, and tellurium | NaYS2(1−x)Te2x alloys (with x = 0, 0.33, 0.67, and 1) | These alloys are potential light energy converters and considered attractive for photovoltaic applications | [64] |
| Alloys of tellurium fluorides | Te-F binary system | Stable Te-fluorides (TeF4, TeF6, and TeF8,) support strong d–p covalent interactions in the Te-F system at high pressure | [65] |
| Iridium–tellurium alloy | IrTe | Can promote adsorption of N2 and lower the Gibbs free energy for electrocatalytic N2 reduction reactions | [66] |
| Bismuth–telluride alloy | Bi2Te3 | This alloy has good performance in thermoelectric materials near room temperature | [67] |
| Cesium–tellurium–titanium alloy | Cs2Te1−xTixI6 | This alloy possesses large absorption coefficients in the visible light region as stable, eco-friendly and high-efficiency light absorbers used in optoelectronic applications | [68] |
| Rubidium–tin–tellurium alloy | Rb2Sn1−xTexI6 | Promising alloy using the Sn–Te mixture as a potential substitute for lead in photovoltaic materials | [69] |
| Tellurium-embedded carbon nano-fibers | Te@C-NF electrode | Poly-tellurides and K2Te-embedded carbon nano-fibers are high-rate and long-life electrodes for high-energy-storage materials | [70] |
| Potassium–tellurium battery system | K-Te | Converting Te to K2Te3 and ultimately to K5Te3 in a carbonate electrolyte-based K-Te battery system to promote and develop high-energy-density K-S/Se/Te batteries | [71] |
| Potassium–tellurium battery system | K-Te | Utilizes biochar from mangosteen shell in a hierarchical porous host to Te during K+ storage in K-Te battery | [72] |
| Amorphous selenium (a-Se)–tellurium alloy | Se-Te alloys | Improving quantum efficiency and conversion efficiencies for a-Se1−xTex (x = 0, 0.03, 0.05, 0.08) devices as a function of applied field, along with different band gaps in Se-Te alloys | [73] |
| Comparison Item | Bulk-Tellurium | Nano-Tellurium |
|---|---|---|
| Main common forms | Soluble oxyanionic forms | Natural and engineered nano-particles (NPs) |
| Abundance | 1–5 ppb in Earth’s crust 0.008–0.03 ppm in soil 15 ppb in seawater Around 0.27 ppm in plants | >4 ppm in the regolith depending on weathering of Te-ores >100 ppm in hotspots |
| Essentiality | Non-essential | Not confirmed yet |
| Exposure pathway(s) | Food (ingestion) followed by inhalation and then dermal. | Bioavailability of Te-NPs through dermal absorption, ingestion, or inhalation. |
| Foodstuff exposure and human daily intake | Dairy products, meat, and cereals; in general, there is <1 mg Te kg−1 in food and humans should not exceed an intake of >0.1 mg of Te day−1 | Depends on natural or engineered NPs and their properties |
| Main sources of exposure | Mainly Cd-Te in solar panels and from copper mining refineries | Cd-Te-quantum dots (QDs) and other nano-alloys of tellurium |
| Main applications | Te can be used as an alloy for Peltier devices, phase change optical magnetic disks, and solar panels | Alloys of Te with selenium, cadmium, zinc, and other metals can be used to produce NMs such as QDs |
| Mobility in the environment | Tellurium is a mobile element in the environment (mainly mining) | Te-NPs may transport similarly to other natural nano-materials like Au-NPs |
| Suggestied mechanisms to enhance human health | Boosts antioxidant defenses, acts as pro-oxidants, generates ROS, and induces apoptosis | Exerts antioxidant, lipid-lowering, and free radical scavenging activities; can be used as antitumor and chemopreventive agents |
| Toxicity (established) | Low concentrations of Te species are toxic | Elemental (Te0) is non-toxic to organisms |
| Toxicity (exposure dose) | TeIVO32-(aq) toxic to microbes at about ~1 mg L−1 (4 μM) | Bio-Te-NPs caused toxicity to Pseudomonas pseudoalcaligenes in mice at 6 mg kg−1 |
| Occupational exposure limits | Threshold limit value (TLV): 0.1 mg m−3 as 8 h total weighted average (TWA) | Not yet known |
| Toxicity (sources) | Highly toxic forms: tellurite, IV (TeO32−) and tellurate, VI (TeO66−) | Chemical Te-NPs are generally more toxic than biological or green forms |
| Toxicity (forms) | Organo-Te compounds are generally less toxic compared to mineral forms | Generally, the common toxic nano-form is Cd-Te QDs depending on size of NPs |
| Median lethal dose (LD50)—oral | K2TeO3 caused complete toxicity at 12.5 mg kg−1 in mice | Biogenic nano-Te rods had acute toxicity at 60 mg kg−1 in mice |
| Metabolic pathway | Reduces TeO32− and TeO42− in the liver, methylates to (CH3)2Te and (CH3)3Te)+, binds to hemoglobin, accumulates in the blood cells in humans | In general, bio-Te-NPs are insoluble in plants, depending on type of nano-tellurium (e.g., TeO2-NPs, TeO2-NP–acetic acid, and TeO2-NP–gallic acid) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sári, D.; Ferroudj, A.; Semsey, D.; El-Ramady, H.; Brevik, E.C.; Prokisch, J. Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials 2024, 14, 670. https://doi.org/10.3390/nano14080670
Sári D, Ferroudj A, Semsey D, El-Ramady H, Brevik EC, Prokisch J. Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials. 2024; 14(8):670. https://doi.org/10.3390/nano14080670
Chicago/Turabian StyleSári, Daniella, Aya Ferroudj, Dávid Semsey, Hassan El-Ramady, Eric C. Brevik, and József Prokisch. 2024. "Tellurium and Nano-Tellurium: Medicine or Poison?" Nanomaterials 14, no. 8: 670. https://doi.org/10.3390/nano14080670
APA StyleSári, D., Ferroudj, A., Semsey, D., El-Ramady, H., Brevik, E. C., & Prokisch, J. (2024). Tellurium and Nano-Tellurium: Medicine or Poison? Nanomaterials, 14(8), 670. https://doi.org/10.3390/nano14080670










