Preparation of Low-Temperature Solution-Processed High-κ Gate Dielectrics Using Organic–Inorganic TiO2 Hybrid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AUPs
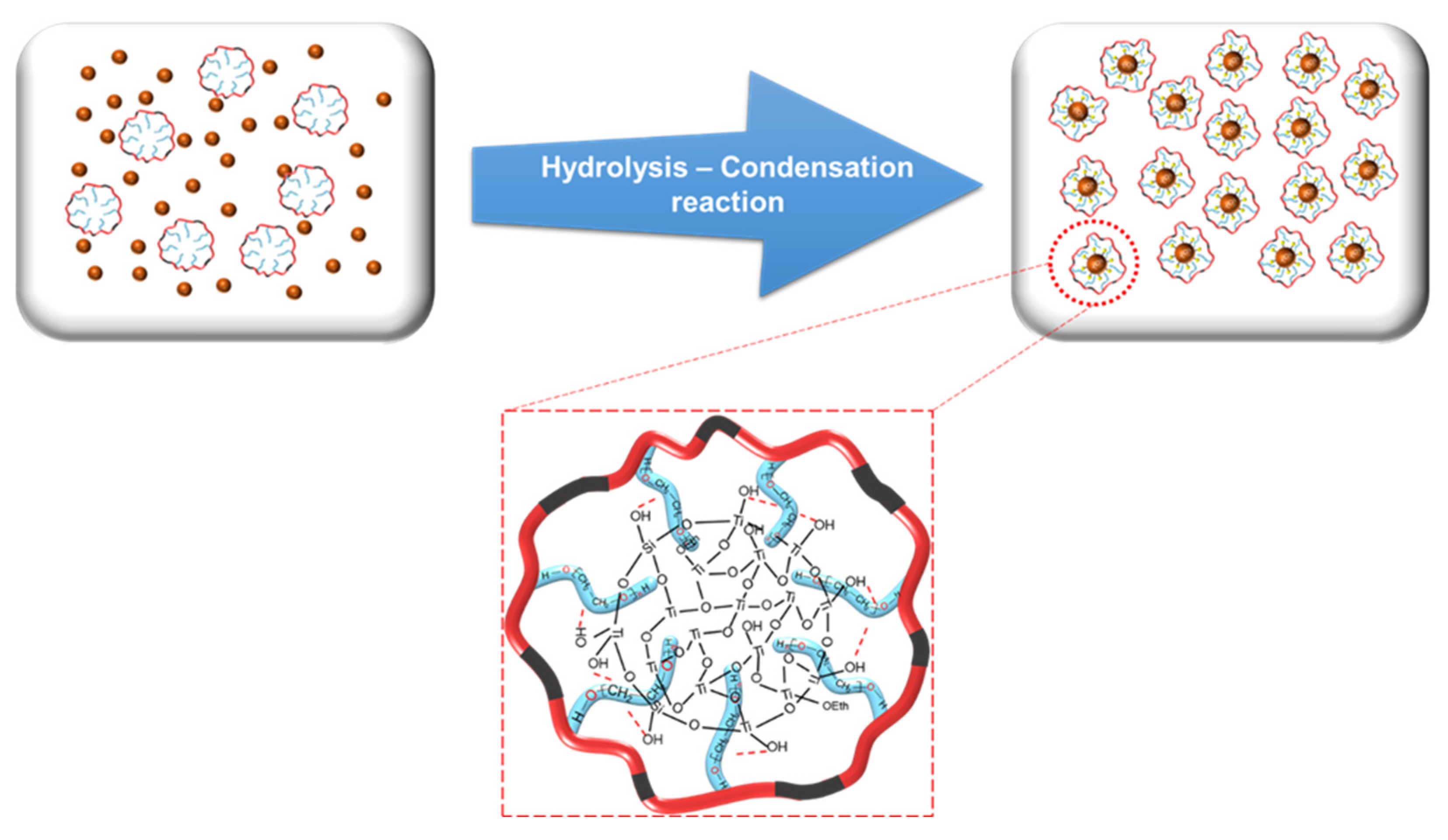
2.3. Preparation of O-I TiO2 Hybrid Nanoparticles
2.4. Preparation of TiO2/poly(AUP) Nanocomposites
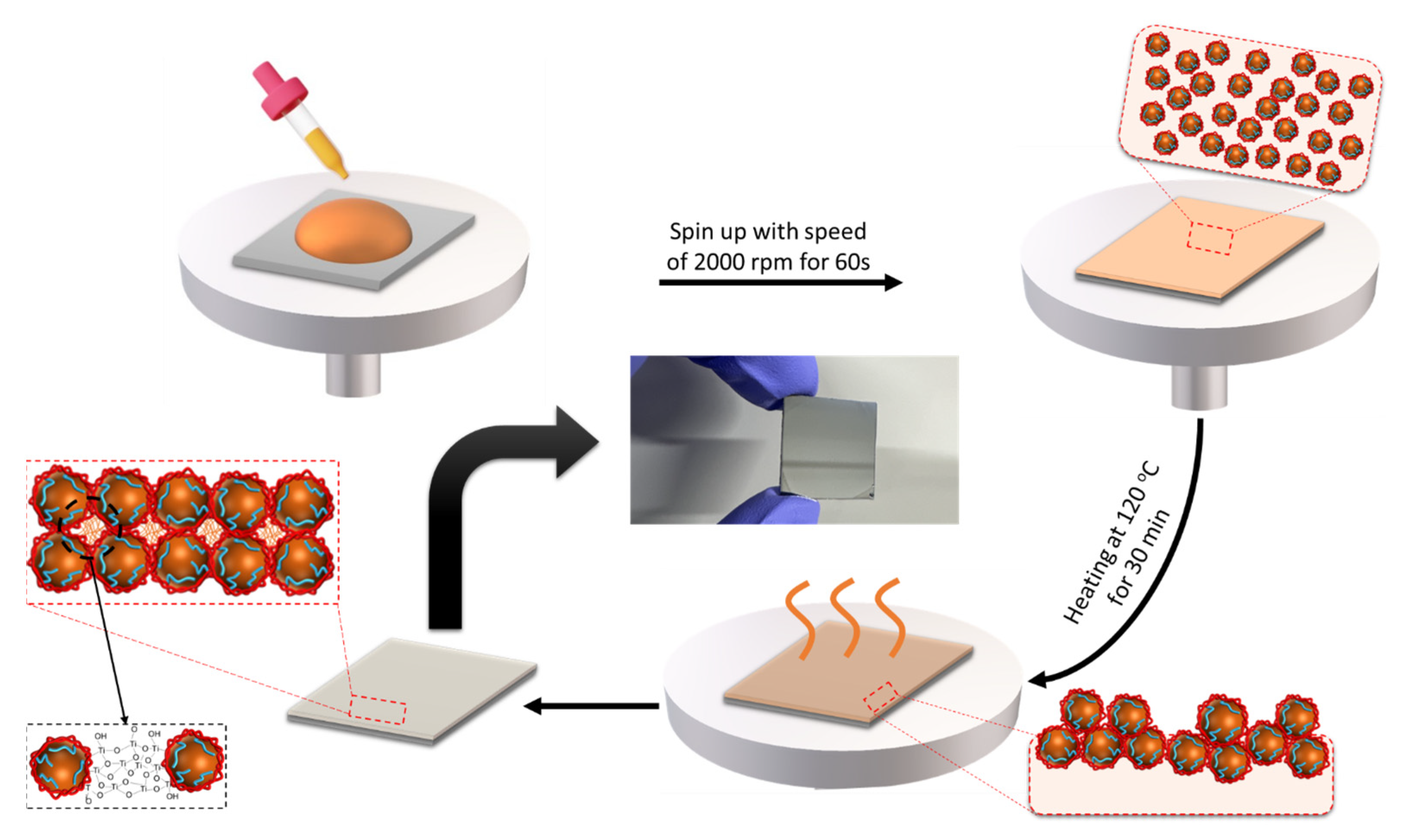
2.5. Sample Preparation (Dielectric Deposition and Device Fabrication)
2.5.1. Fabrication of Metal–Insulator–Metal (MIM) Device
2.5.2. Fabrication of OTFT Device
2.6. Characterization
3. Results and Discussion
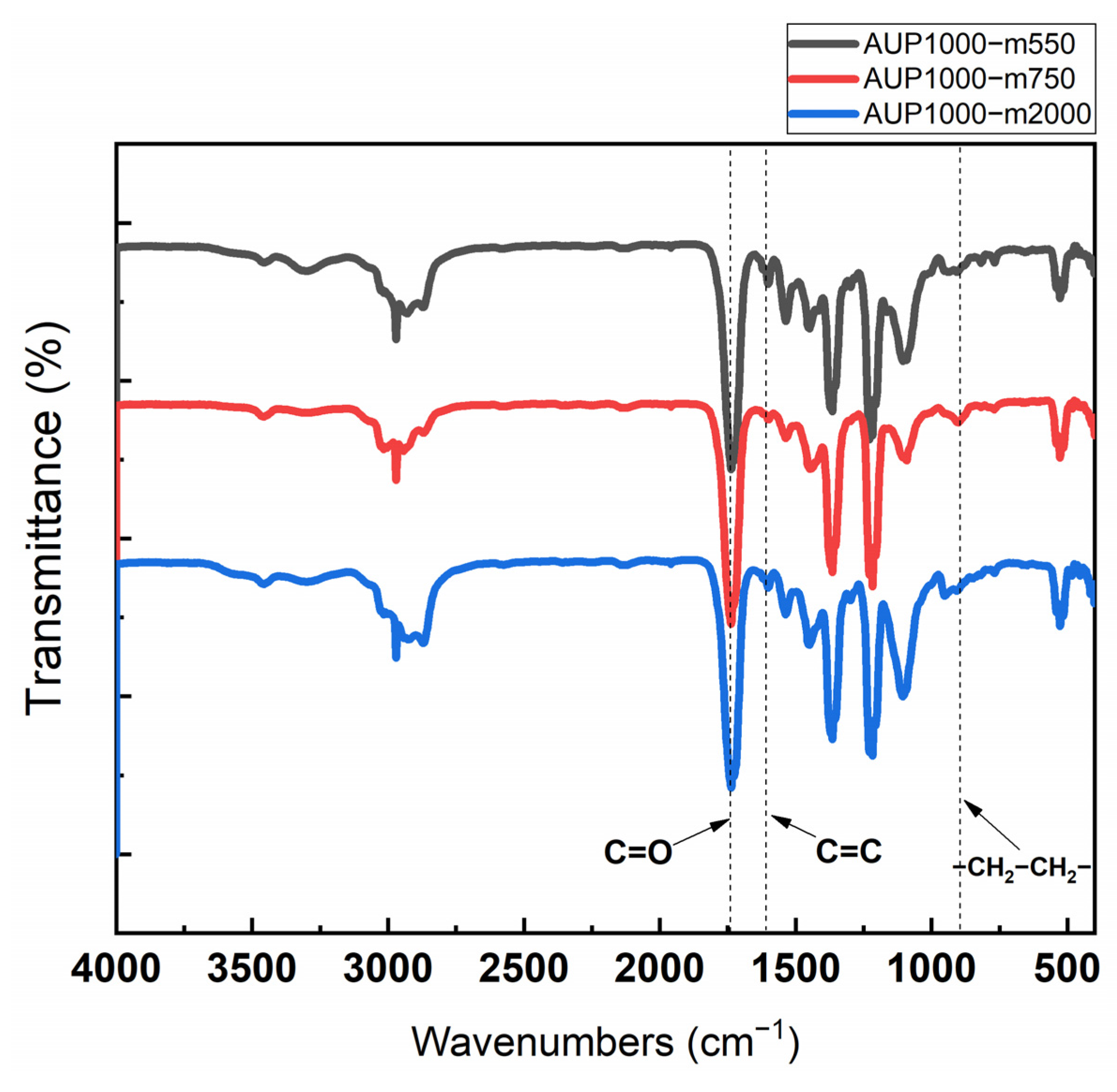
3.1. Characterization of AUPs
3.2. Preparation of Colloidally Stable O-I TiO2 Hybrid Sols
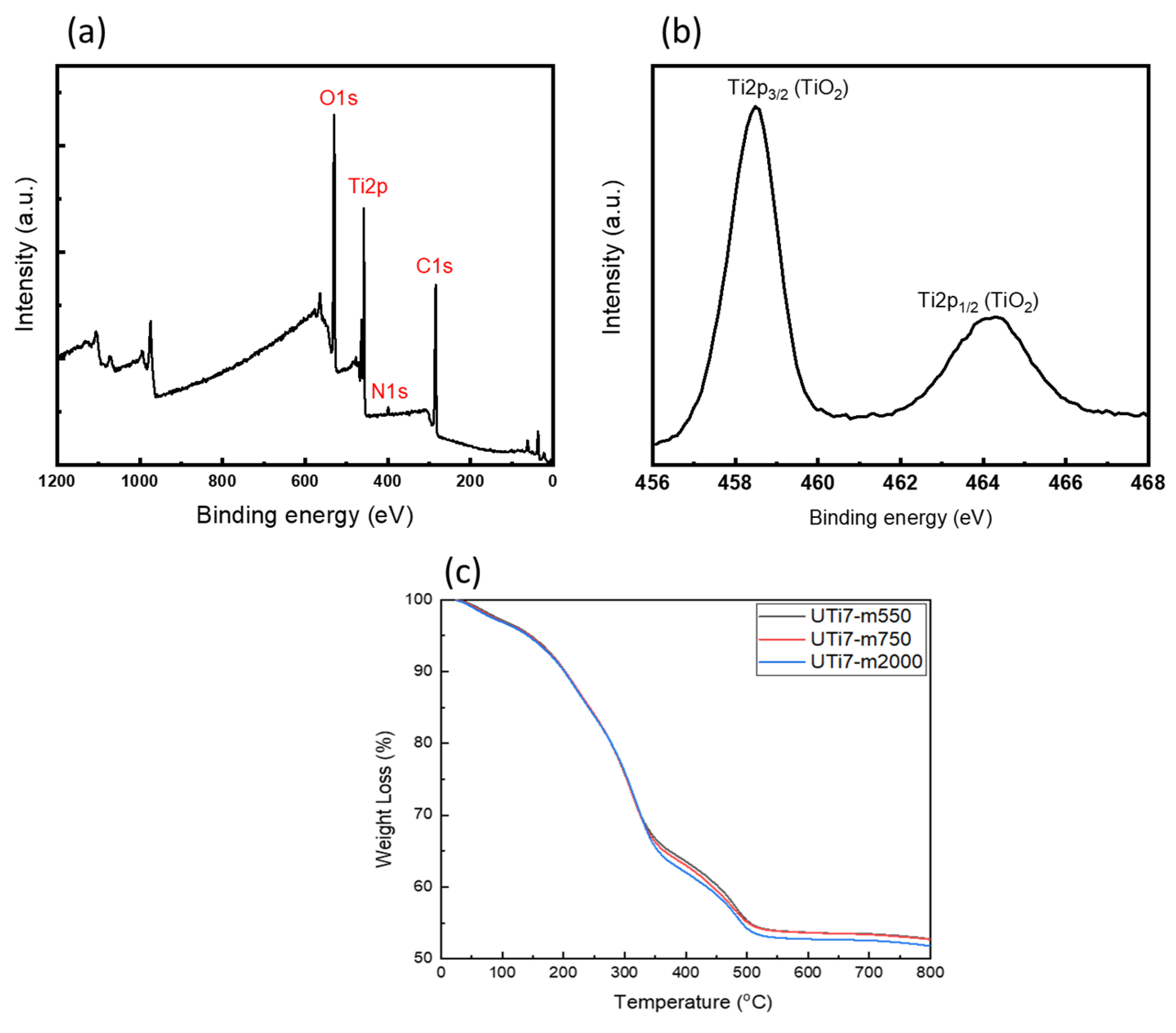
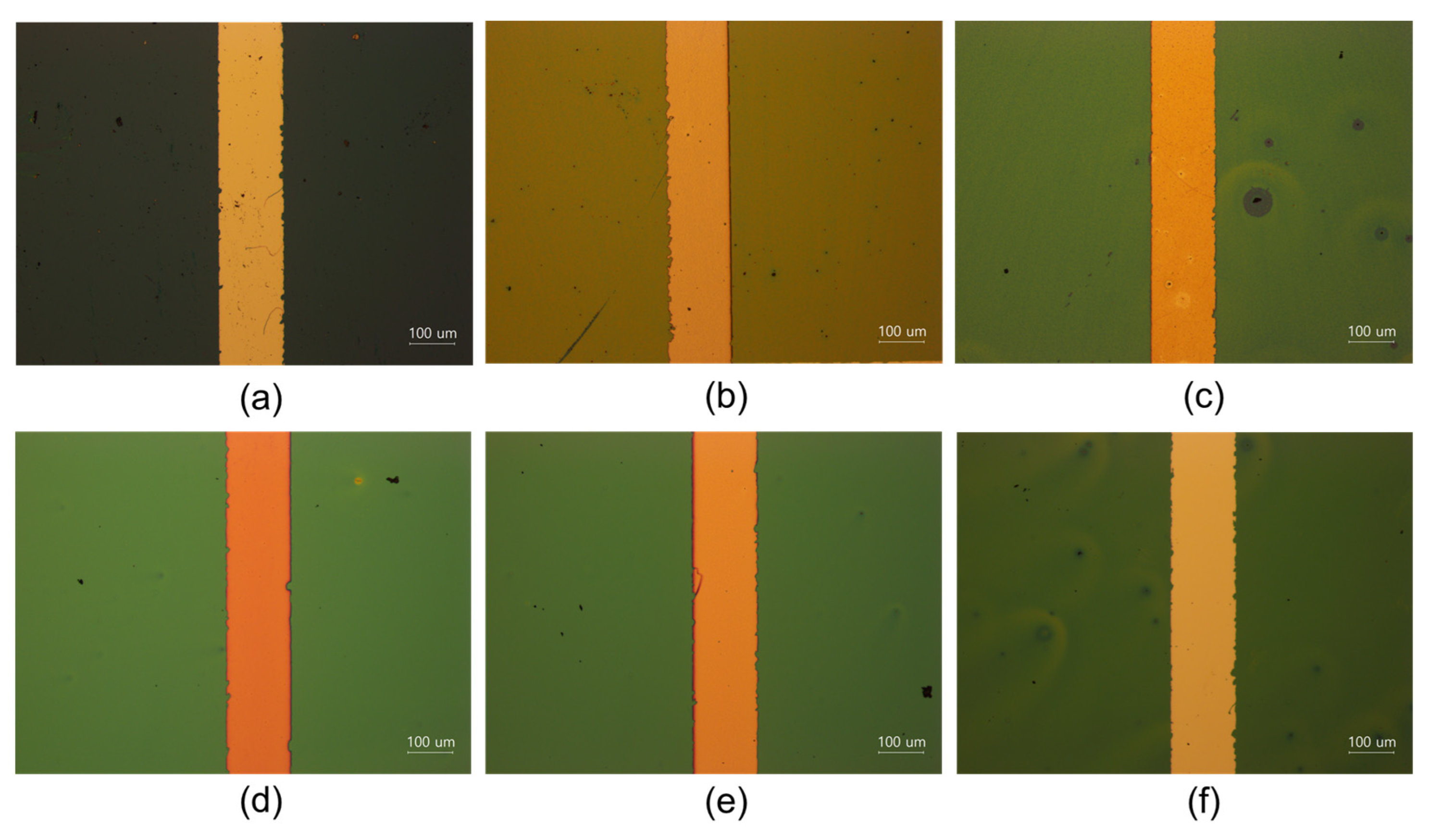
3.3. Characterization of O-I Hybrid TiO2 Coating Films
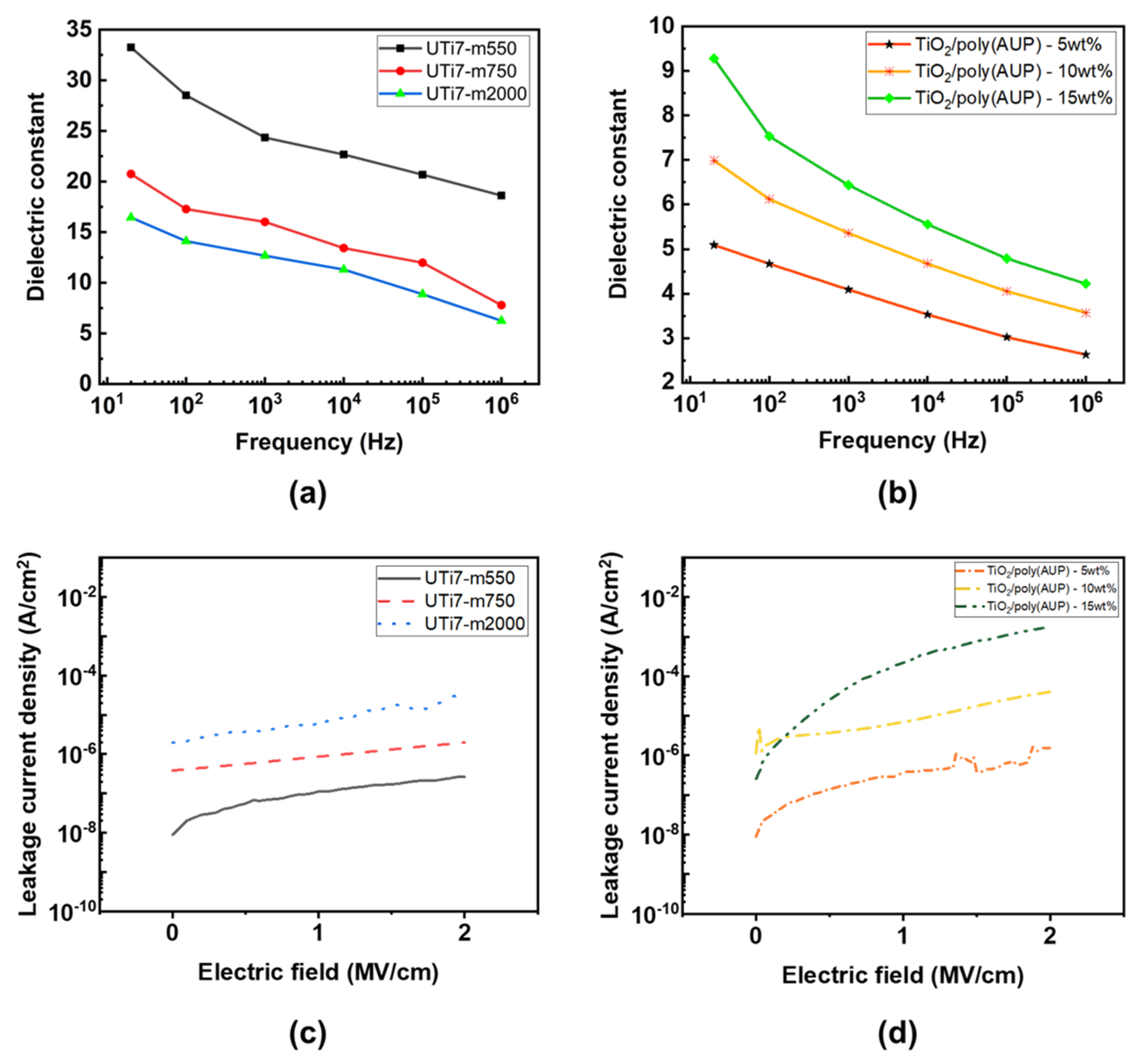
3.4. Electrical Characterization of O-I Hybrid Titania Coatings and Nanocomposite Coatings
3.5. Effect of AUPs on the Dielectric Properties of O-I Hybrid Coatings
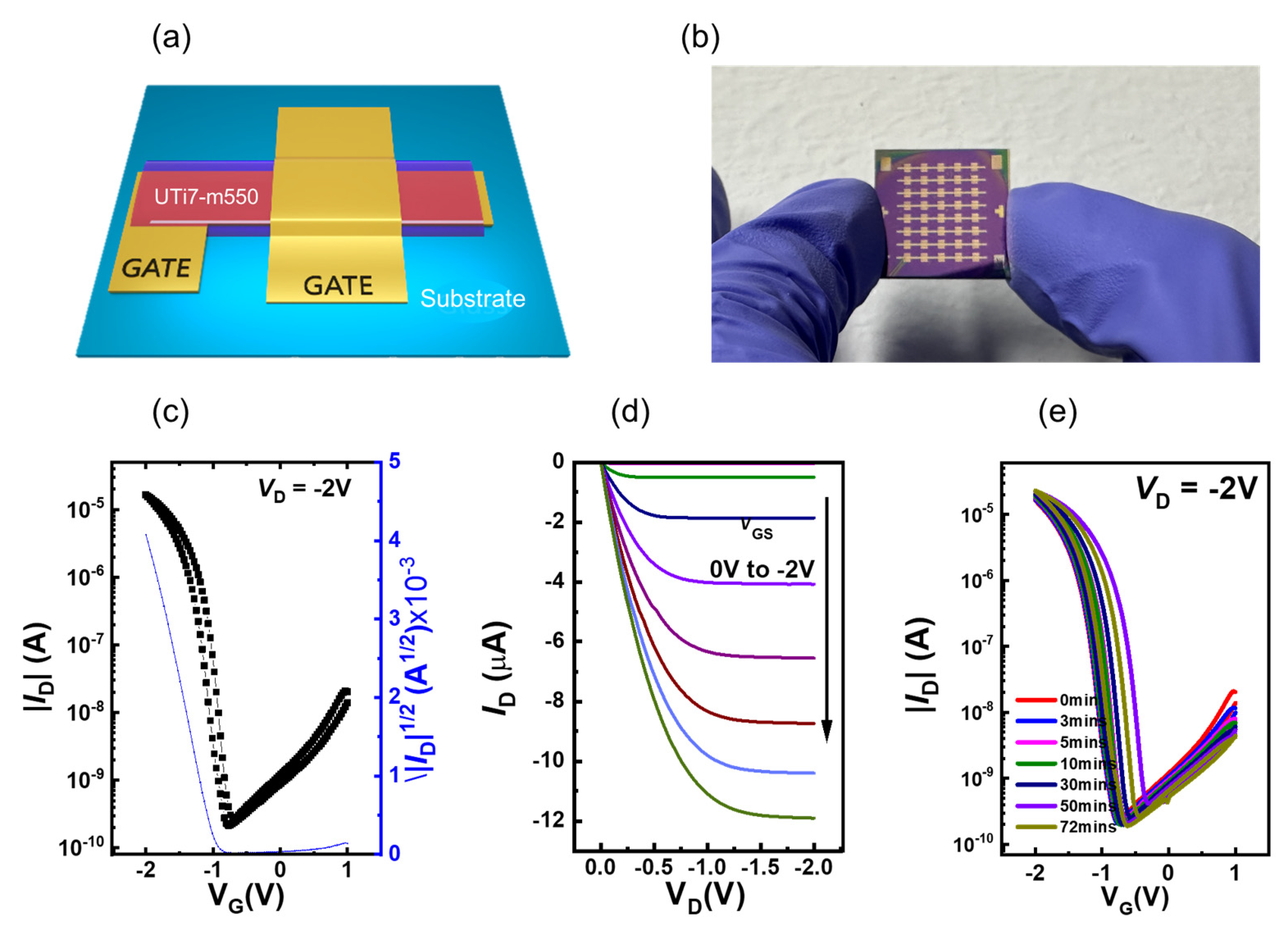
3.6. OTFT Device Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukuda, K.; Someya, T. Recent progress in the development of printed thin-film transistors and circuits with high-resolution printing technology. Adv. Mater. 2017, 29, 1602736. [Google Scholar] [CrossRef]
- Li, J.; Tang, W.; Wang, Q.; Sun, W.; Zhang, Q.; Guo, X.; Wang, X.; Yan, F. Solution-processable organic and hybrid gate dielectrics for printed electronics. Mater. Sci. Eng. R Rep. 2018, 127, 1–36. [Google Scholar] [CrossRef]
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater. 2020, 32, 1905279. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, T.; Duan, L. Emerging self-emissive technologies for flexible displays. Adv. Mater. 2020, 32, 1902391. [Google Scholar] [CrossRef] [PubMed]
- Baeg, K.J.; Lee, J. Flexible electronic systems on plastic substrates and textiles for smart wearable technologies. Adv. Mater. Technol. 2020, 5, 2000071. [Google Scholar] [CrossRef]
- Joshi, K.; Velasco, V.; Esfandyarpour, R. A low-cost, disposable and portable inkjet-printed biochip for the developing world. Sensors 2020, 20, 3593. [Google Scholar] [CrossRef]
- Acharya, V.; Agarwal, K.; Mondal, S. Electronic materials for solution-processed TFTs. Mater. Res. Express 2023, 10, 082002. [Google Scholar] [CrossRef]
- Tixier-Mita, A.; Ihida, S.; Ségard, B.-D.; Cathcart, G.A.; Takahashi, T.; Fujita, H.; Toshiyoshi, H. Review on thin-film transistor technology, its applications, and possible new applications to biological cells. Jpn. J. Appl. Phys. 2016, 55, 04EA08. [Google Scholar] [CrossRef]
- Yusof, N.S.; Mohamed, M.F.P.; Ghazali, N.A.; Khan, M.F.A.J.; Shaari, S.; Mohtar, M.N. Evolution of solution-based organic thin-film transistor for healthcare monitoring—From device to circuit integration: A review. Alex. Eng. J. 2022, 61, 11405–11431. [Google Scholar] [CrossRef]
- Nitti, A.; Scagliotti, M.; Beverina, L.; Mariucci, L.; Rapisarda, M.; Pasini, D. Solution-processable thin-film transistors from anthradithiophene (ADT) and naphthothiopene (NT) small molecule-based p-type organic semiconductors. Mater. Adv. 2023, 4, 4590–4597. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Ogier, S.; Ng, T.N.; Caironi, M.; Perinot, A.; Li, L.; Zhao, J.; Tang, W.; Sporea, R.A.; et al. Current status and opportunities of organic thin-film transistor technologies. IEEE Trans. Electron Devices 2017, 64, 1906–1921. [Google Scholar] [CrossRef]
- Raveendran, R.; Nagaraj, M.; Namboothiry, M.A.G. High-Performance, Transparent Solution-Processed Organic Field-Effect Transistor with Low-k Elastomeric Gate Dielectric and Liquid Crystalline Semiconductor: Promises and Challenges. ACS Appl. Electron. Mater. 2020, 2, 3336–3345. [Google Scholar] [CrossRef]
- Bilgaiyan, A.; Cho, S.I.; Abiko, M.; Watanabe, K.; Mizukami, M. Solution Processed Organic Transistors on Polymeric Gate Dielectric with Mobility Exceeding 15 cm2V−1s−1. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2020, 14, 2000156. [Google Scholar] [CrossRef]
- Yao, R.; Zheng, Z.; Xiong, M.; Zhang, X.; Li, X.; Ning, H.; Fang, Z.; Xie, W.; Lu, X.; Peng, J. Low-temperature fabrication of sputtered high-k HfO2 gate dielectric for flexible a-IGZO thin film transistors. Appl. Phys. Lett. 2018, 112, 103503. [Google Scholar] [CrossRef]
- Auciello, O.; Fan, W.; Kabius, B.; Saha, S.; Carlisle, J.A.; Chang, R.P.H.; Lopez, C.; Irene, E.A.; Baragiola, R.A. Hybrid titanium–aluminum oxide layer as alternative high-k gate dielectric for the next generation of complementary metal–oxide–semiconductor devices. Appl. Phys. Lett. 2005, 86, 042904. [Google Scholar] [CrossRef]
- Han, D.; Kang, J.; Lin, C.; Han, R. Reliability characteristics of high-K gate dielectrics HfO2 in metal-oxide semiconductor capacitors. Microelectron. Eng. 2003, 66, 643–647. [Google Scholar] [CrossRef]
- Cai, H.; Tuokedaerhan, K.; Lu, Z.; Du, H.; Zhang, R. Effect of Heat Treatment on Zirconium Oxide High-k Gate Dielectric in Silicon-Based Metal Oxide Semiconductor Capacitors. Phys. Status Solidi (A) 2023, 220, 2200903. [Google Scholar] [CrossRef]
- El-Gamal, A.A. Enhancement of the Dielectric Properties of Polyvinyl Alcohol by Adding TiO2 Nanoparticles. Phys. Status Solidi (A) 2022, 219, 2200190. [Google Scholar] [CrossRef]
- Mullapudi, G.S.R.; Velazquez-Nevarez, G.A.; Avila-Avendano, C.; Torres-Ochoa, J.A.; Quevedo-López, M.A.; Ramírez-Bon, R. Low-temperature deposition of inorganic–organic HfO2–PMMA hybrid gate dielectric layers for high-mobility ZnO thin-film transistors. ACS Appl. Electron. Mater. 2019, 1, 1003–1011. [Google Scholar] [CrossRef]
- Rao, M.S.; Reddy, K.C.S.; Meza-Arroyo, J.; Murthy, L.N.; Daunis, T.B.; Pintor-Monroy, M.I.; Hsu, J.W.; Ramirez-Bon, R. ZrHfO2-PMMA hybrid dielectric layers for high-performance all solution-processed In2O3-based TFTs. Mater. Res. Bull. 2022, 150, 111768. [Google Scholar]
- Yu, Y.-Y.; Yang, C.-H. Preparation and application of organic-inorganic nanocomposite materials in stretched organic thin film transistors. Polymers 2020, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, R.; Crasmareanu, E.C.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New Hybrid Materials Synthesized with Different Dyes by Sol-Gel Method. Adv. Mater. Sci. Eng. 2017, 2017, 4537039. [Google Scholar] [CrossRef]
- Choi, J.; Lee, C.; Kang, J.; Lee, C.; Lee, S.M.; Oh, J.; Choi, S.; Im, S.G. A Sub-20 nm Organic/Inorganic Hybrid Dielectric for Ultralow-Power Organic Thin-Film Transistor (OTFT) With Enhanced Operational Stability. Small 2022, 18, 2203165. [Google Scholar] [CrossRef] [PubMed]
- Di Gianni, A.; Trabelsi, S.; Rizza, G.; Sangermano, M.; Althues, H.; Kaskel, S.; Voit, B. Hyperbranched Polymer/TiO2 Hybrid Nanoparticles Synthesized via an In Situ Sol-Gel Process. Macromol. Chem. Phys. 2007, 208, 76–86. [Google Scholar] [CrossRef]
- Rahman, R.A.; Islam, S.; Othaman, Z.; Riaz, S.; Saeed, M.A.; Naseem, S. Preparation and Characterization of Crack-Free Sol-Gel Based SiO2-TiO2Hybrid Nanoparticle Film. J. Sol-Gel Sci. Technol. 2013, 68, 162. [Google Scholar]
- Kwon, H.J.; Ye, H.; Baek, Y.; Hong, J.; Wang, R.; Choi, Y.; Kim, S.H. Printable Ultra-Flexible Fluorinated Organic–Inorganic Nanohybrid Sol–Gel Derived Gate Dielectrics for Highly Stable Organic Thin-Film Transistors and Other Practical Applications. Adv. Funct. Mater. 2021, 31, 2009539. [Google Scholar] [CrossRef]
- de Urquijo-Ventura, M.S.; Rao, M.S.; Meraz-Davila, S.; Torres-Ochoa, J.A.; Quevedo-Lopez, M.A.; Ramirez-Bon, R. PVP-SiO2 and PVP-TiO2 hybrid films for dielectric gate applications in CdS-based thin film transistors. Polymer 2020, 191, 122261. [Google Scholar] [CrossRef]
- Kim, H.; Pramanik, N.C.; Ahn, B.Y.; Seok, S.I. Preparation of inorganic–organic hybrid titania sol–gel nanocomposite films, and their dielectric properties. Phys. Status Solidi (A) 2006, 203, 1962–1970. [Google Scholar] [CrossRef]
- Yu, S.; Yao, K.; Shannigrahi, S.; Hock, F.T.E. Effects of poly(ethylene glycol) additive molecular weight on the microstructure and properties of sol-gel-derived lead zirconate titanate thin films. J. Mater. Res. 2003, 18, 737–741. [Google Scholar] [CrossRef]
- Sing Liow, K.; Sipaut, C.S.; Mansa, R.F.; Ung, M.C.; Ebrahimi, S. Effect of PEG Molecular Weight on the Polyurethane-Based Quasi-Solid-State Electrolyte for Dye-Sensitized Solar Cells. Polymers 2022, 14, 3603. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, D.-H.; Ihn, K.-J. Synthesis of Poly(urethane acrylate-co-styrene) Films Containing Silver Nanoparticles by a Simultaneous Copolymerization/in situ Electron Transfer Reaction. Macromol. Chem. Phys. 2005, 206, 794–801. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chon, Y.-S.; Yoo, D.-J.; Suh, K.-D. Microphase-separated structure of telechelic urethane acrylate anionomers and their network in various solvents. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2081–2095. [Google Scholar] [CrossRef]
- Le, H.N.; Lee, C.; Jung, W.; Kim, J. Effect of the Solvent Type on the Colloidal Stability and the Degree of Condensation of Silica Sols Stabilized by Amphiphilic Urethane Acrylate and the Properties of Their Coating Films. Coatings 2023, 13, 1997. [Google Scholar] [CrossRef]
- Sharma, B.; Agarwal, R.; Jassal, M.; Agrawal, A.K. Stabilizer-free low-acid rapid synthesis of highly stable transparent aqueous titania nano sol and its photocatalytic activity. J. Mol. Liq. 2020, 305, 112842. [Google Scholar] [CrossRef]
- Kim, N.; Li, X.; Kim, S.H.; Kim, J. Colloidally stable organic–inorganic hybrid nanoparticles prepared using alkoxysilane-functionalized amphiphilic polymer precursors and mechanical properties of their cured coating film. J. Ind. Eng. Chem. 2018, 68, 209–219. [Google Scholar] [CrossRef]
- Pang, Z.; Sun, H.; Guo, Y.; Du, J.; Li, L.; Li, Q.; Yang, J.; Zhang, J.; Wu, W.; Yang, S. Research Advances of Porous Polyimide—Based Composites with Low Dielectric Constant. Polymers 2023, 15, 3341. [Google Scholar] [CrossRef]
- Rayssi, C.; Kossi, S.E.; Dhahri, J.; Khirouni, K. Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1−xCo4x/3O3 (0 ≤ x ≤ 0.1). Rsc. Adv. 2018, 8, 17139–17150. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.R.; Hwang, D.Y.; Lee, D.H.; Shin, D.H. Effect of surface roughness on leakage current and corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]


| AUPs | Mw (g/mol) | PDI | Particle Size of AUPs Dispersed in Ethanol (nm) |
|---|---|---|---|
| AUP 1000-m2000 | 38,765 | 2.1 | 33.60 ± 0.2 |
| AUP 1000-m750 | 9060 | 1.33 | 9.21 ± 0.07 |
| AUP 1000-m550 | 8996 | 1.32 | 4.74 ± 0.01 |
| O-I TiO2 Hybrid Sols | Size of TiO2 Nanoparticles after Preparation (nm) | Size of TiO2 Nanoparticles 6 Months after Preparation (nm) |
|---|---|---|
| UTi7-m2000 | 9.70 ± 0.11 | 10.3 ± 0.44 |
| UTi7-m750 | 4.36 ± 0.04 | 4.44 ± 0.10 |
| UTi7-m550 | 4.35 ± 0.02 | 4.37 ± 0.01 |
| Semiconductor | Circumstance | Ci (nF·cm−2) | μFET (cm2·V−1·s−1) | Vth (V) | Ion/Ioff | SS (V/dec) | |
|---|---|---|---|---|---|---|---|
| C10-DNTT | 1-2 | SiO2—100 nm | 5.01 × 10−7 | 6.39 | −0.95 | 1.76 × 104 | 0.272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.N.; Wang, R.; Hou, B.; Kim, S.; Kim, J. Preparation of Low-Temperature Solution-Processed High-κ Gate Dielectrics Using Organic–Inorganic TiO2 Hybrid Nanoparticles. Nanomaterials 2024, 14, 488. https://doi.org/10.3390/nano14060488
Le HN, Wang R, Hou B, Kim S, Kim J. Preparation of Low-Temperature Solution-Processed High-κ Gate Dielectrics Using Organic–Inorganic TiO2 Hybrid Nanoparticles. Nanomaterials. 2024; 14(6):488. https://doi.org/10.3390/nano14060488
Chicago/Turabian StyleLe, Hong Nhung, Rixuan Wang, Benliang Hou, Sehyun Kim, and Juyoung Kim. 2024. "Preparation of Low-Temperature Solution-Processed High-κ Gate Dielectrics Using Organic–Inorganic TiO2 Hybrid Nanoparticles" Nanomaterials 14, no. 6: 488. https://doi.org/10.3390/nano14060488
APA StyleLe, H. N., Wang, R., Hou, B., Kim, S., & Kim, J. (2024). Preparation of Low-Temperature Solution-Processed High-κ Gate Dielectrics Using Organic–Inorganic TiO2 Hybrid Nanoparticles. Nanomaterials, 14(6), 488. https://doi.org/10.3390/nano14060488







