Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
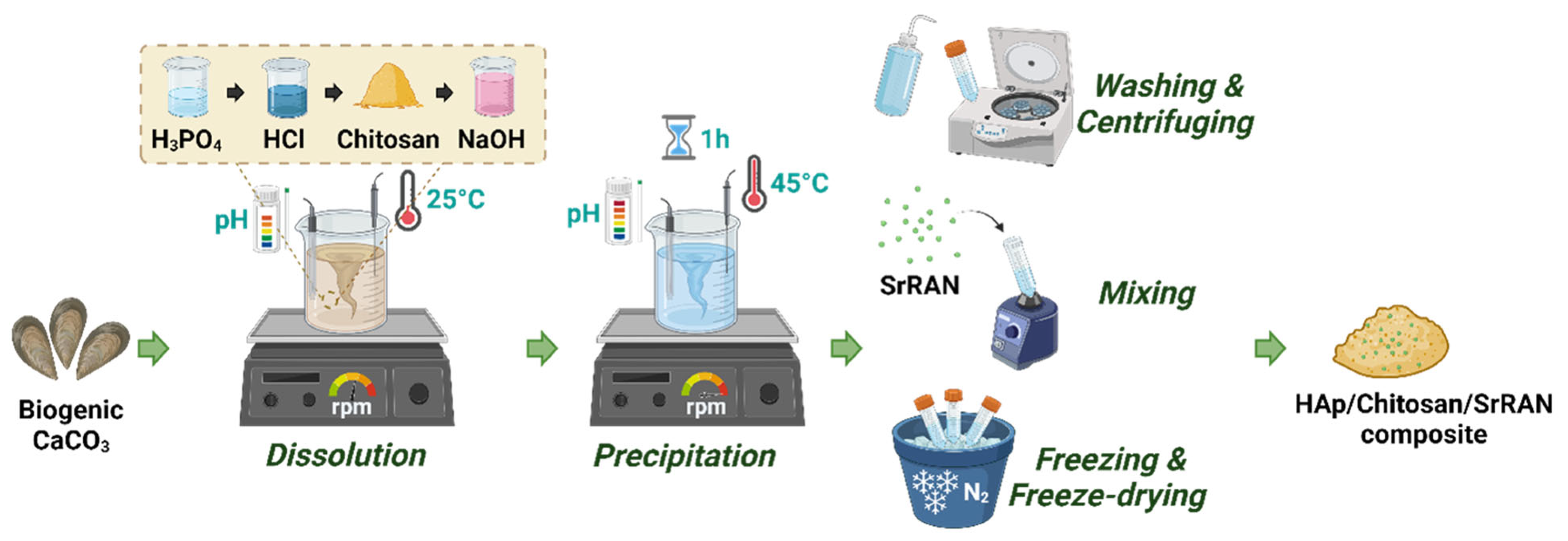
2.1. Synthesis and Cold Sintering of HAp and HAp/Chitosan Composites
2.2. Physicochemical Characterisation
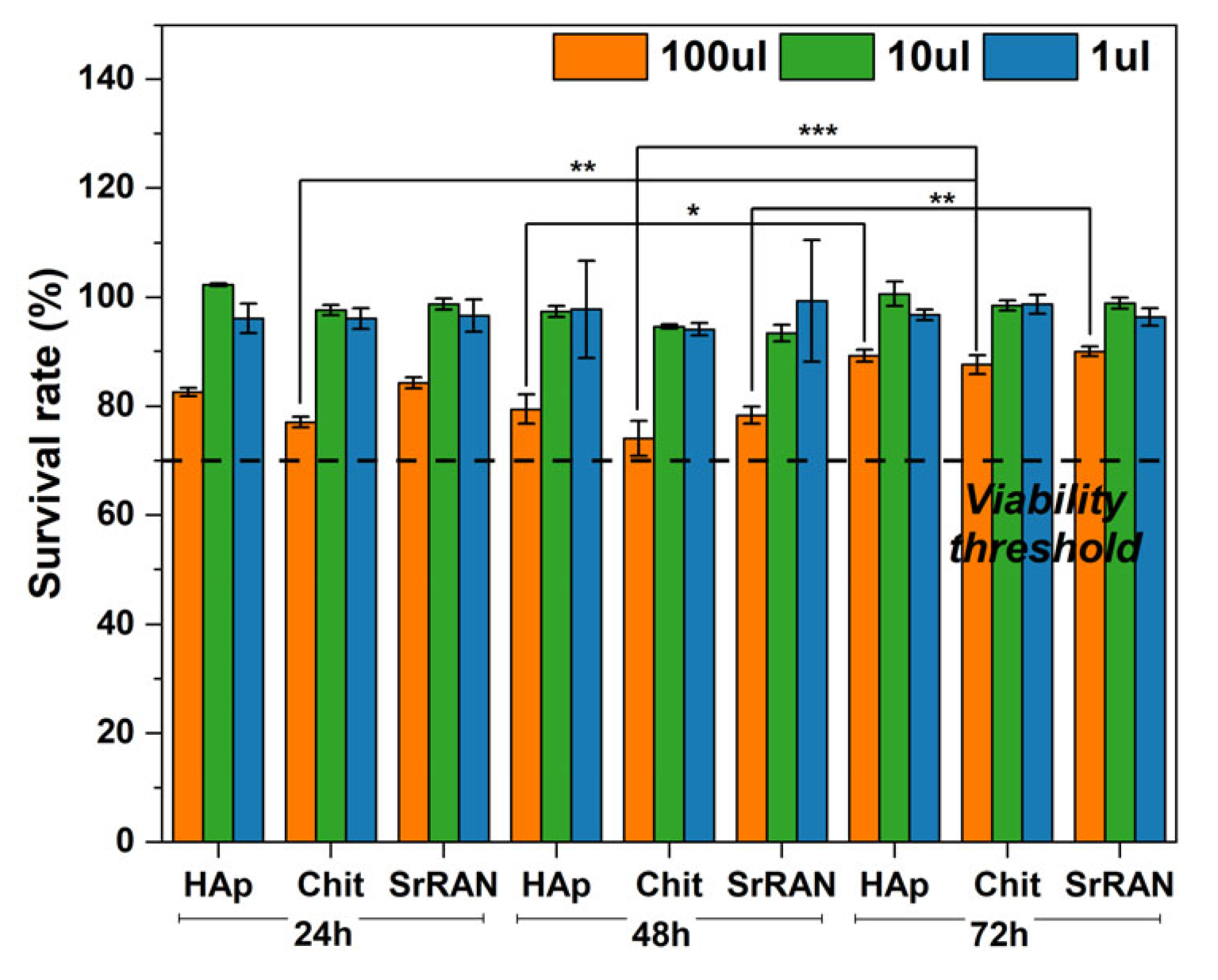
2.3. In Vitro Preliminary Assessment: Drug Release and Cytotoxicity
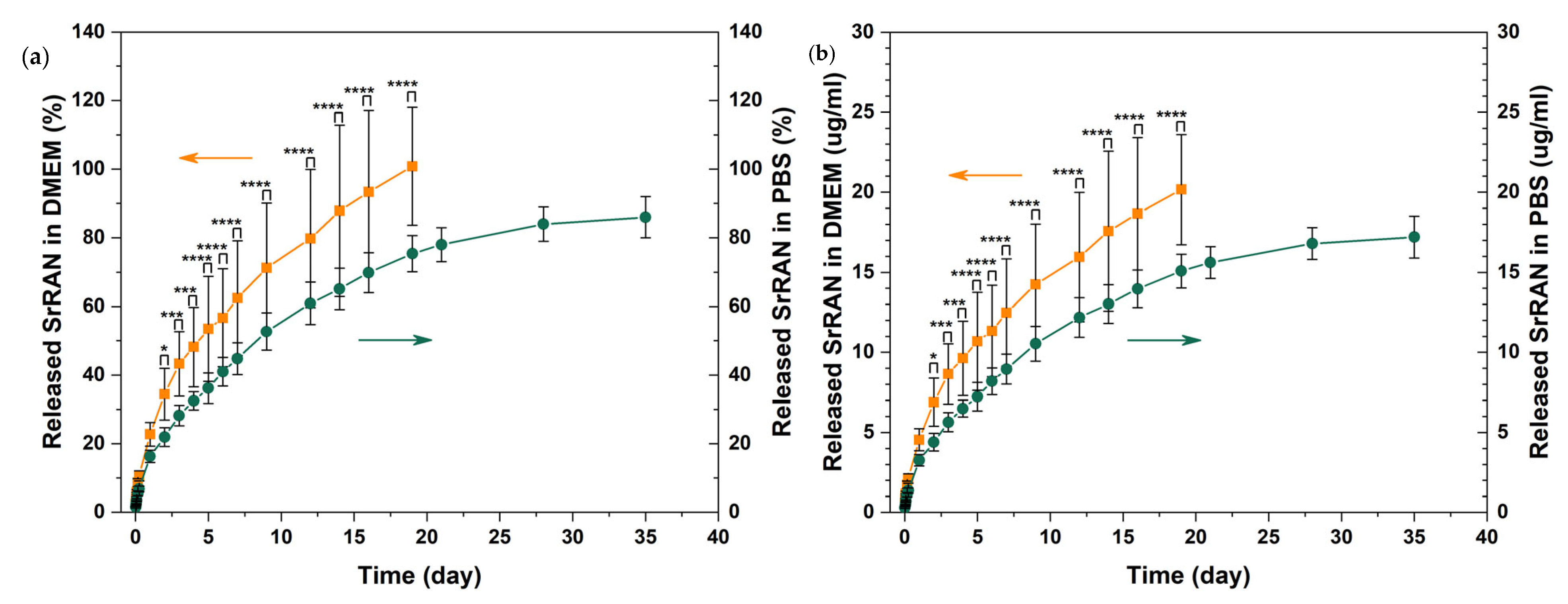
3. Results and Discussion
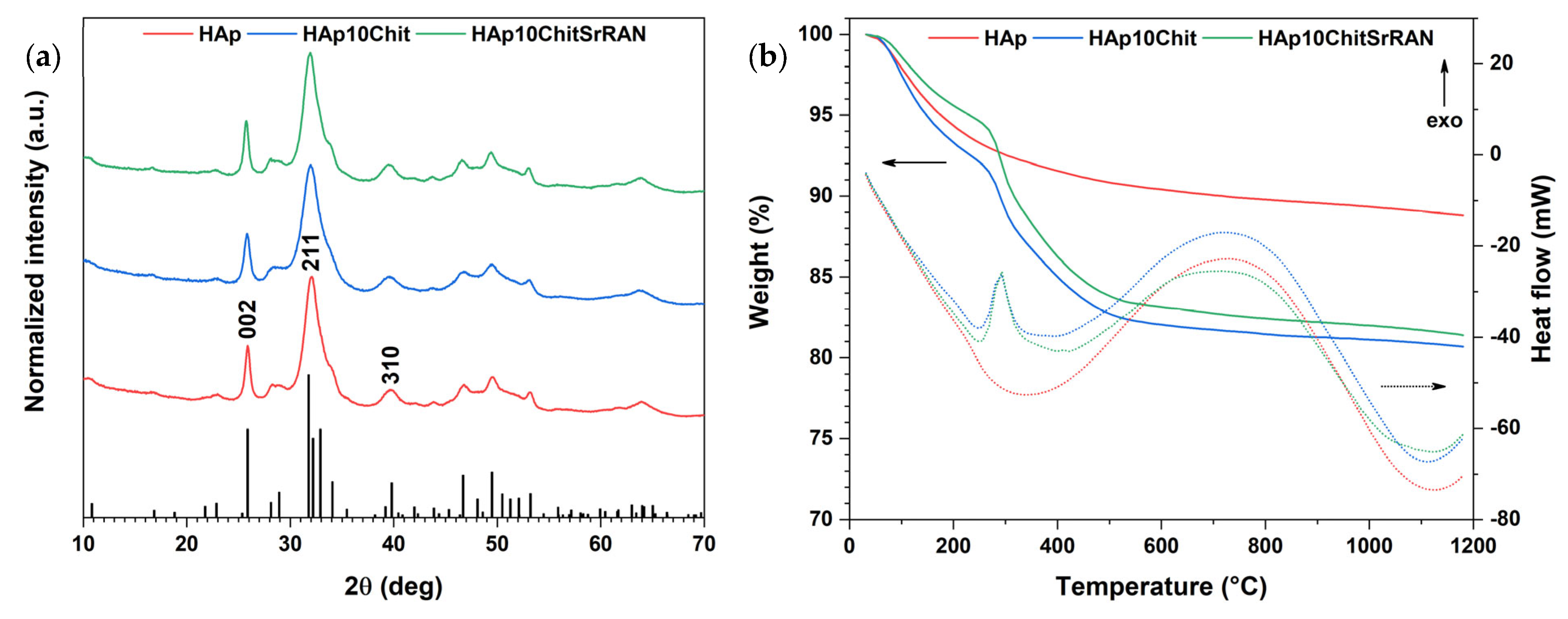
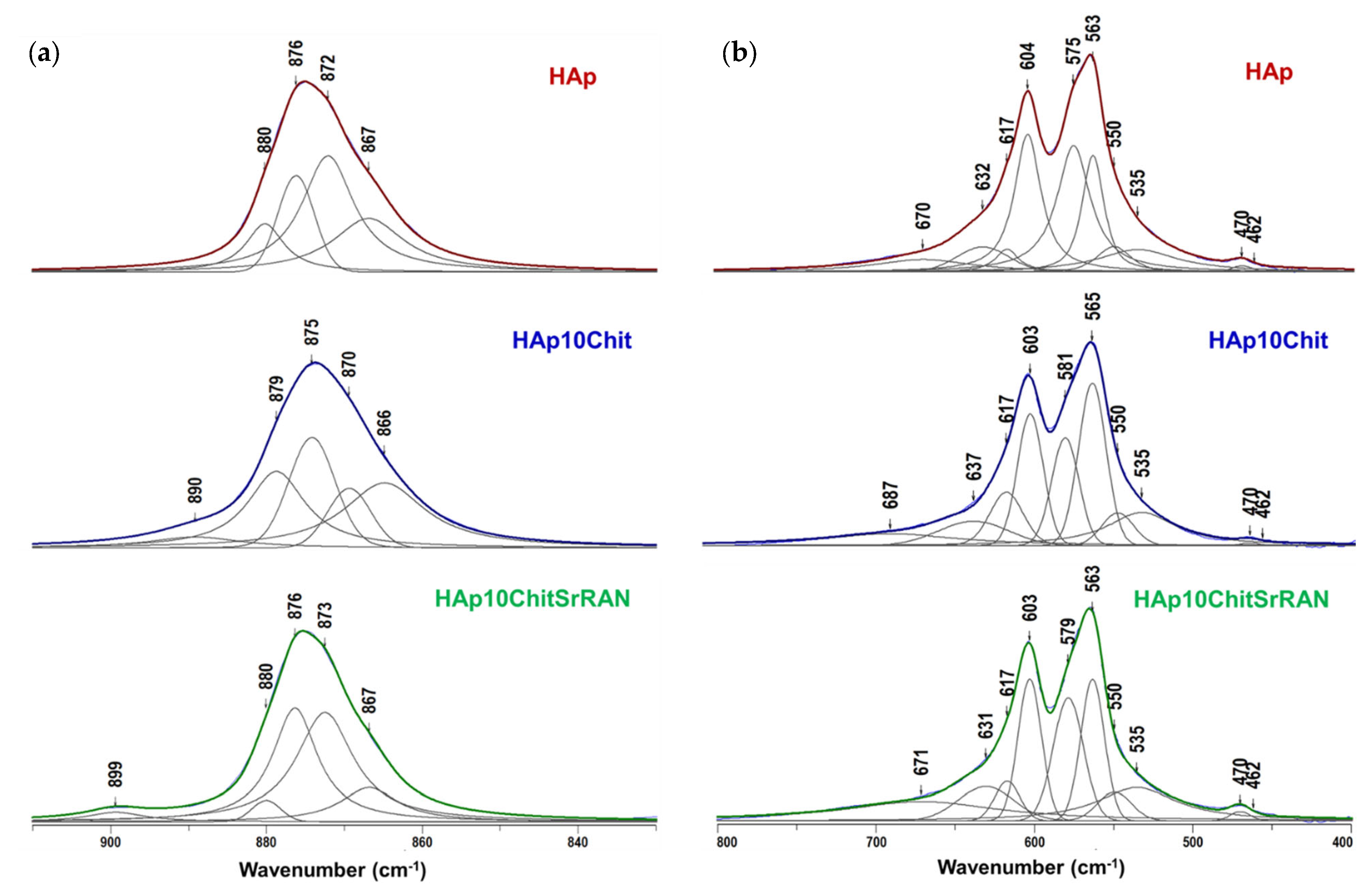
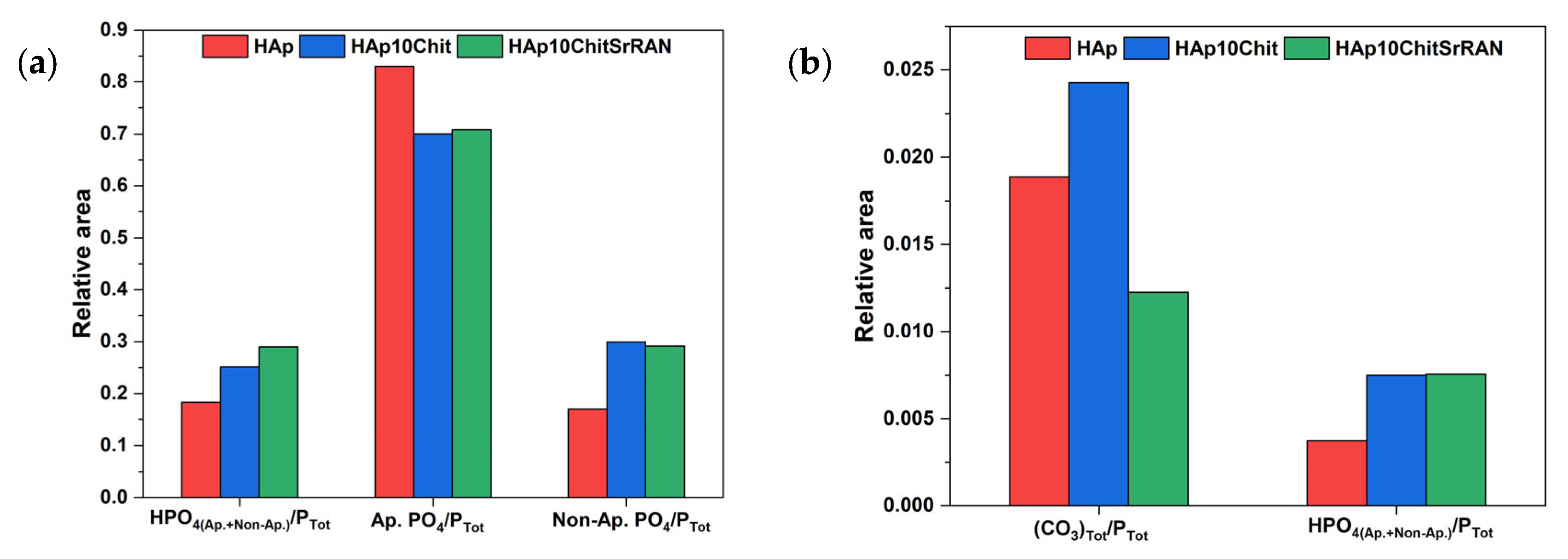
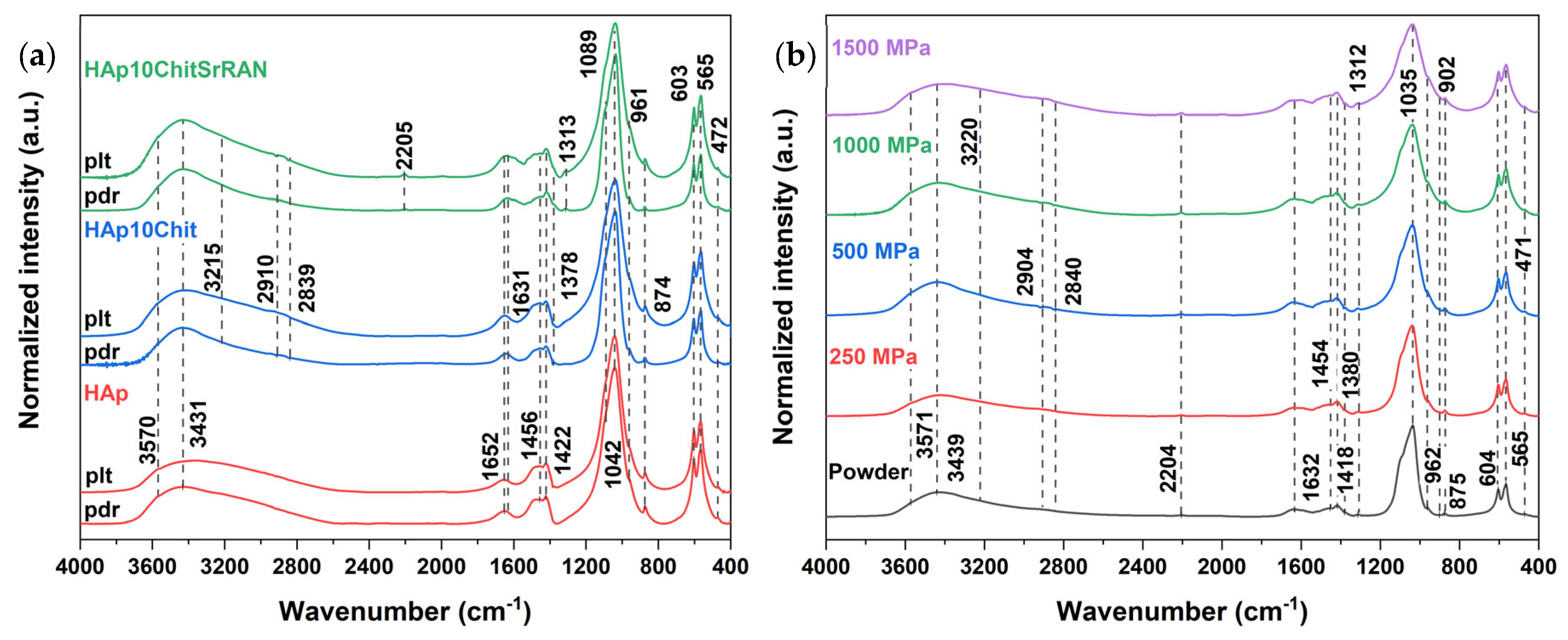
3.1. Dissolution–Precipitation Synthesis of HAp and HAp Composites
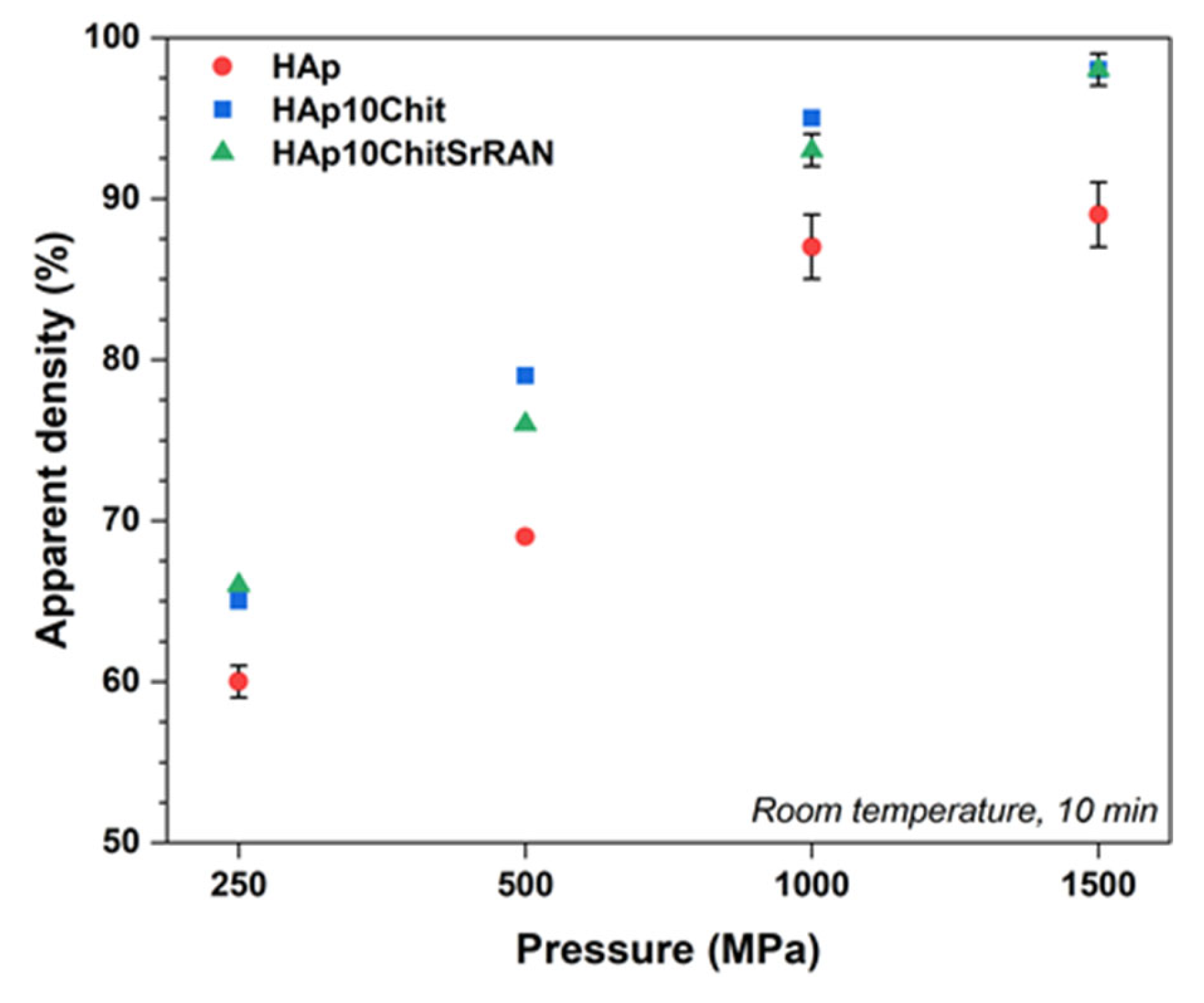
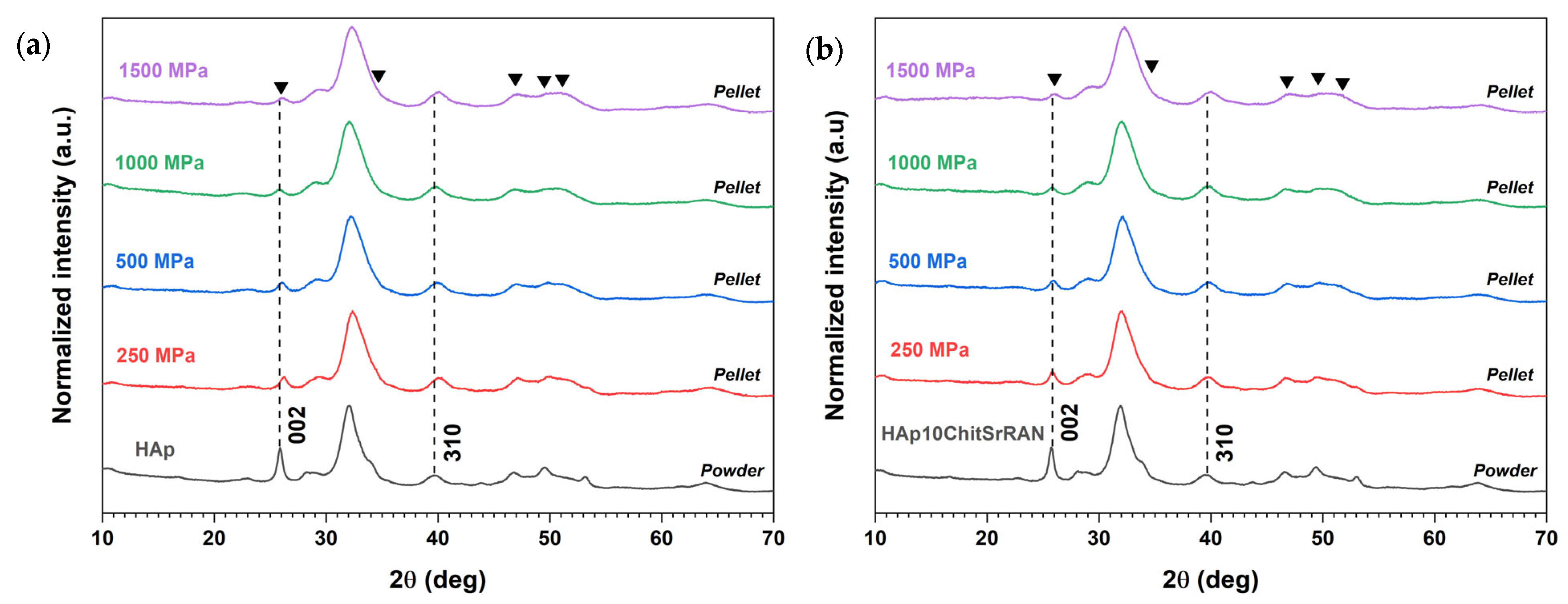
3.2. Cold Sintering of HAp and HAp-Based Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.C.; Lu, Z.F.; Zreiqat, H. Bioceramics for skeletal bone regeneration. In Bone Substitute Biomaterials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 180–216. [Google Scholar]
- Dorozhkin, S.V. Current State of Bioceramics. J. Ceram. Sci. Technol. 2018, 9, 353–370. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Muñoz, E.M. Hydroxyapatite-Based Materials: Synthesis and Characterization. In Biomedical Engineering—Frontiers and Challenges; Fazel-Rezai, R., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Jiang, Y.; Yuan, Z.; Huang, J. Substituted hydroxyapatite: A recent development. Mater. Technol. 2019, 35, 785–796. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 145–211. [Google Scholar]
- Ressler, A.; Antunović, M.; Teruel-Biosca, L.; Ferrer, G.G.; Babić, S.; Urlić, I.; Ivanković, M.; Ivanković, H. Osteogenic differentiation of human mesenchymal stem cells on substituted calcium phosphate/chitosan composite scaffold. Carbohydr. Polym. 2022, 277, 118883. [Google Scholar] [CrossRef]
- Cazalbou, S.; Eichert, D.; Rey, C.; Combes, C. Adaptative physico-chemistry of bio-related calcium phosphates. J. Mater. Chem. 2004, 14, 2148–2153. [Google Scholar] [CrossRef]
- Cazalbou, S.; Eichert, D.; Rey, C.; Glimcher, M.J.; Combes, C. Poorly crystalline apatites: Evolution and maturation in vitro and in vivo. J. Bone Miner. Metab. 2004, 22, 310–317. [Google Scholar] [CrossRef]
- Eichert, D.; Sfihi, H.; Combes, C.; Rey, C. Specific Characteristics of Wet Nanocrystalline Apatites. Consequences on Biomaterials and Bone Tissue. Key Eng. Mater. 2003, 254–256, 927–930. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Lafon, J.; Champion, E.; Bernache-Assollant, D. Processing of AB-type carbonated hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)y ceramics with controlled composition. J. Eur. Ceram. Soc. 2008, 28, 139–147. [Google Scholar] [CrossRef]
- Rupani, A.; Hidalgo-Bastida, L.A.; Rutten, F.; Dent, A.; Turner, I.; Cartmell, S. Osteoblast activity on carbonated hydroxyapatite. J. Biomed. Mater. Res. Part A 2012, 100A, 1089–1096. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Eichert, D.; Combes, C.; Drouet, C.; Rey, C. Formation and Evolution of Hydrated Surface Layers of Apatites. Key Eng. Mater. 2005, 284–286, 3–6. [Google Scholar] [CrossRef]
- Drouet, C.; Aufray, M.; Rollin-Martinet, S.; Vandecandelaère, N.; Grossin, D.; Rossignol, F.; Champion, E.; Navrotsky, A.; Rey, C. Nanocrystalline apatites: The fundamental role of water. Am. Miner. 2018, 103, 550–564. [Google Scholar] [CrossRef]
- Grossin, D.; Rollin-Martinet, S.; Estournès, C.; Rossignol, F.; Champion, E.; Combes, C.; Rey, C.; Geoffroy, C.; Drouet, C. Biomimetic apatite sintered at very low temperature by spark plasma sintering: Physico-chemistry and microstructure aspects. Acta Biomater. 2010, 6, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. Preparation and evaluation of collagen-silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2014, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-F.; Qing, X.; Peng, Y.; Wang, H.; Shao, Z.; Zhang, K.-Q. Enhancement of mechanical and biological performance on hydroxyapatite/silk fibroin scaffolds facilitated by microwave-assisted mineralization strategy. Colloids Surf. B Biointerfaces 2020, 197, 111401. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2017, 36, 68–91. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2020, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hao, J.; Wang, C. Preparation and mechanical properties of nanocomposites of poly. Synthesis 2001, 22, 2867–2873. [Google Scholar]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2021, 110, 266–272. [Google Scholar] [CrossRef]
- Brahimi, S.; Ressler, A.; Boumchedda, K.; Hamidouche, M.; Kenzour, A.; Djafar, R.; Antunović, M.; Bauer, L.; Hvizdoš, P.; Ivanković, H. Preparation and characterization of biocomposites based on chitosan and biomimetic hydroxyapatite derived from natural phosphate rocks. Mater. Chem. Phys. 2021, 276, 125421. [Google Scholar] [CrossRef]
- Zima, A. Hydroxyapatite-chitosan based bioactive hybrid biomaterials with improved mechanical strength. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 175–184. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, D.; Shen, H.; Nan, J.; Ma, N.; Guo, Z.; Wang, X.; Jin, Q. Cold sintering constructed in situ drug-loaded high strength HA-PLA composites: Potential bone substitution material. Ceram. Int. 2023, 49, 11655–11663. [Google Scholar] [CrossRef]
- Nasiri-Tabrizi, B.; Fahami, A.; Ebrahimi-Kahrizsangi, R. Effect of milling parameters on the formation of nanocrystalline hydroxyapatite using different raw materials. Ceram. Int. 2013, 39, 5751–5763. [Google Scholar] [CrossRef]
- Ferro, A.C.; Guedes, M. Mechanochemical synthesis of hydroxyapatite using cuttlefish bone and chicken eggshell as calcium precursors. Mater. Sci. Eng. C 2019, 97, 124–140. [Google Scholar] [CrossRef]
- Salas, J.; Benzo, Z.; Gonzalez, G.; Marcano, E.; Gómez, C. Effect of Ca/P ratio and milling material on the mechanochemical preparation of hydroxyapaptite. J. Mater. Sci. Mater. Med. 2009, 20, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ozyegin, L.S.; Chou, J.; Samur, R.; Oktar, F.N.; Ben-Nissan, B. An alternative synthesis method for di calcium phosphate (monetite) powders from mediterranean mussel (mytilus galloprovincialis) shells. J. Aust. Ceram. Soc. 2013, 49, 122–128. [Google Scholar]
- Lin, K.; Liu, P.; Wei, L.; Zou, Z.; Zhang, W.; Qian, Y.; Shen, Y.; Chang, J. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. 2013, 222, 49–59. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Hydrothermal synthesis of hydroxyapatite rods. J. Cryst. Growth 2007, 308, 133–140. [Google Scholar] [CrossRef]
- Benataya, K.; Lakrat, M.; Elansari, L.; Mejdoubi, E. Synthesis of B-type carbonated hydroxyapatite by a new dissolution-precipitation method. Mater. Today Proc. 2020, 31, S83–S88. [Google Scholar] [CrossRef]
- Lakrat, M.; Jodati, H.; Mejdoubi, E.M.; Evis, Z. Synthesis and characterization of pure and Mg, Cu, Ag, and Sr doped calcium-deficient hydroxyapatite from brushite as precursor using the dissolution-precipitation method. Powder Technol. 2023, 413, 118026. [Google Scholar] [CrossRef]
- Zalite, V.; Lungevics, J.; Vecstaudza, J.; Stipniece, L.; Locs, J. Nanosized calcium deficient hydroxyapatites for tooth enamel protection. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Gudelj, A.; Zadro, K.; Antunović, M.; Cvetnić, M.; Ivanković, M.; Ivanković, H. From Bio-waste to Bone Substitute: Synthesis of Biomimetic Hydroxyapatite and Its Use in Chitosan-based Composite Scaffold Preparation. Chem. Biochem. Eng. Q. 2020, 34, 59–71. [Google Scholar] [CrossRef]
- Laonapakul, T. Synthesis of hydroxyapatite from biogenic wastes. KKU Eng. J. 2015, 42, 269–275. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-Products. Appl. Sci. 2021, 11, 3387. [Google Scholar] [CrossRef]
- Gergely, G.; Wéber, F.; Lukács, I.; Illés, L.; Tóth, A.; Horváth, Z.; Mihály, J.; Balázsi, C. Nano-hydroxyapatite preparation from biogenic raw materials. Open Chem. 2010, 8, 375–381. [Google Scholar] [CrossRef]
- Mohd Pu’Ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Ma, Q.; Rubenis, K.; Sigurjónsson, E.; Hildebrand, T.; Standal, T.; Zemjane, S.; Locs, J.; Loca, D.; Haugen, H.J. Eggshell-derived amorphous calcium phosphate: Synthesis, characterization and bio-functions as bone graft materials in novel 3D osteoblastic spheroids model. Smart Mater. Med. 2023, 4, 522–537. [Google Scholar] [CrossRef]
- Lee, S.-W.; Balázsi, C.; Balázsi, K.; Seo, D.-H.; Kim, H.S.; Kim, C.-H.; Kim, S.-G. Comparative Study of hydroxyapatite prepared from seashells and eggshells as a bone graft material. Tissue Eng. Regen. Med. 2014, 11, 113–120. [Google Scholar] [CrossRef]
- Owuamanam, S.; Cree, D. Progress of Bio-Calcium Carbonate Waste Eggshell and Seashell Fillers in Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Galotta, A.; Agostinacchio, F.; Motta, A.; Dirè, S.; Sglavo, V.M. Mechanochemical synthesis and cold sintering of mussel shell-derived hydroxyapatite nano-powders for bone tissue regeneration. J. Eur. Ceram. Soc. 2023, 43, 639–647. [Google Scholar] [CrossRef]
- El-Bassyouni, G.T.; Eldera, S.S.; Kenawy, S.H.; Hamzawy, E.M. Hydroxyapatite nanoparticles derived from mussel shells for in vitro cytotoxicity test and cell viability. Heliyon 2020, 6, e04085. [Google Scholar] [CrossRef] [PubMed]
- Kizalaite, A.; Grigoraviciute-Puroniene, I.; Asuigui, D.R.C.; Stoll, S.L.; Cho, S.H.; Sekino, T.; Kareiva, A.; Zarkov, A. Dissolution–Precipitation Synthesis and Characterization of Zinc Whitlockite with Variable Metal Content. ACS Biomater. Sci. Eng. 2021, 7, 3586–3593. [Google Scholar] [CrossRef]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Locs, J. Novel preparation route of stable amorphous calcium phosphate nanoparticles with high specific surface area. J. Alloys Compd. 2017, 700, 215–222. [Google Scholar] [CrossRef]
- Rubenis, K.; Zemjane, S.; Vecstaudza, J.; Bitenieks, J.; Locs, J. Densification of amorphous calcium phosphate using principles of the cold sintering process. J. Eur. Ceram. Soc. 2020, 41, 912–919. [Google Scholar] [CrossRef]
- Galotta, A.; Rubenis, K.; Locs, J.; Sglavo, V.M. Dissolution-precipitation synthesis and cold sintering of mussel shells-derived hydroxyapatite and hydroxyapatite/chitosan composites for bone tissue engineering. Open Ceram. 2023, 15, 100418. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan–hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.A.; Freire, A.d.O.; Carvalho, H.C.O.; Silva, G.E.B.; Vasconcelos, J.W.; Guerra, R.N.M.; Cartágenes, M.D.S.d.S.; Garcia, J.B.S. Prophylactic and Therapeutic Use of Strontium Ranelate Reduces the Progression of Experimental Osteoarthritis. Front. Pharmacol. 2018, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Loca, D.; Smirnova, A.; Locs, J.; Dubnika, A.; Vecstaudza, J.; Stipniece, L.; Makarova, E.; Dambrova, M. Development of local strontium ranelate delivery systems and long term in vitro drug release studies in osteogenic medium. Sci. Rep. 2018, 8, 16754. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Nani, E.P.; Teixeira, L.N.; Peruzzo, D.C.; Joly, J.C.; Napimoga, M.H.; Martinez, E.F. Strontium ranelate increases osteoblast activity. Tissue Cell 2016, 48, 183–188. [Google Scholar] [CrossRef]
- Horák, P.; Skácelová, M.; Kazi, A. Role of Strontium Ranelate in the Therapy of Osteoporosis. J. Rheum. Dis. Treat. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Chiang, C.-W.; Chen, C.-H.; Manga, Y.B.; Huang, S.-C.; Chao, K.-M.; Jheng, P.-R.; Wong, P.-C.; Nyambat, B.; Satapathy, M.K.; Chuang, E.-Y. Facilitated and Controlled Strontium Ranelate Delivery Using GCS-HA Nanocarriers Embedded into PEGDA Coupled with Decortication Driven Spinal Regeneration. Int. J. Nanomed. 2021, 16, 4209–4224. [Google Scholar] [CrossRef]
- Indurkar, A.; Choudhary, R.; Rubenis, K.; Locs, J. Advances in Sintering Techniques for Calcium Phosphates Ceramics. Materials 2021, 14, 6133. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N. Ceramic Processing and Sintering, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Al-Maawi, S.; Barbeck, M.; Vizcaíno, C.H.; Egli, R.; Sader, R.; Kirkpatrick, C.J.; Bohner, M.; Ghanaati, S. Thermal treatment at 500 °C significantly reduces the reaction to irregular tricalcium phosphate granules as foreign bodies: An in vivo study. Acta Biomater. 2022, 142, 414–429. [Google Scholar] [CrossRef]
- Egli, R.J.; Gruenenfelder, S.; Doebelin, N.; Hofstetter, W.; Luginbuehl, R.; Bohner, M. Thermal Treatments of Calcium Phosphate Biomaterials to Tune the Physico-Chemical Properties and Modify the In Vitro Osteoclast Response. Adv. Eng. Mater. 2010, 13, B102–B107. [Google Scholar] [CrossRef]
- Brouillet, F.; Laurencin, D.; Grossin, D.; Drouet, C.; Estournes, C.; Chevallier, G.; Rey, C. Biomimetic apatite-based composite materials obtained by spark plasma sintering (SPS): Physicochemical and mechanical characterizations. J. Mater. Sci. Mater. Med. 2015, 26, 1–11. [Google Scholar] [CrossRef]
- Biesuz, M.; Grasso, S.; Sglavo, V.M. What’s new in ceramics sintering? A short report on the latest trends and future prospects. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A review of cold sintering processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef]
- Shen, H.-Z.; Guo, N.; Liang, Y.-H.; Shen, P. Synthesis and densification of hydroxyapatite by mechanochemically-activated reactive cold sintering. Scr. Mater. 2021, 194, 113717. [Google Scholar] [CrossRef]
- Hassan, M.U.; Venkatesan, S.; Ryu, H.J. Non-volatile immobilization of iodine by the cold-sintering of iodosodalite. J. Hazard. Mater. 2020, 386, 121646. [Google Scholar] [CrossRef]
- Ortali, C.; Julien, I.; Vandenhende, M.; Drouet, C.; Champion, E. Consolidation of bone-like apatite bioceramics by spark plasma sintering of amorphous carbonated calcium phosphate at very low temperature. J. Eur. Ceram. Soc. 2018, 38, 2098–2109. [Google Scholar] [CrossRef]
- Drouet, C.; Largeot, C.; Raimbeaux, G.; Estournès, C.; Dechambre, G.; Combes, C.; Rey, C. Bioceramics: Spark Plasma Sintering (SPS) of Calcium Phosphates. Adv. Sci. Technol. 2006, 49, 45–50. [Google Scholar] [CrossRef]
- Shen, H.-Z.; Guo, N.; Zhao, L.; Shen, P. Role of ion substitution and lattice water in the densification of cold-sintered hydroxyapatite. Scr. Mater. 2019, 177, 141–145. [Google Scholar] [CrossRef]
- Guo, J.; Berbano, S.S.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Randall, C.A. Cold Sintering Process of Composites: Bridging the Processing Temperature Gap of Ceramic and Polymer Materials. Adv. Funct. Mater. 2016, 26, 7115–7121. [Google Scholar] [CrossRef]
- Yu, T.; Cheng, J.; Li, L.; Sun, B.; Bao, X.; Zhang, H. Current understanding and applications of the cold sintering process. Front. Chem. Sci. Eng. 2019, 13, 654–664. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, X.; De Beauvoir, T.H.; Seo, J.-H.; Berbano, S.S.; Baker, A.L.; Azina, C.; Randall, C.A. Recent Progress in Applications of the Cold Sintering Process for Ceramic–Polymer Composites. Adv. Funct. Mater. 2018, 28, 1801724. [Google Scholar] [CrossRef]
- Maria, J.-P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Current status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef]
- Guo, N.; Shen, H.-Z.; Shen, P. Cold sintering of chitosan/hydroxyapatite composites. Materialia 2021, 21, 101294. [Google Scholar] [CrossRef]
- Gee, A.; Deitz, V.R. Determination of Phosphate by Differential Spectrophotometry. Anal. Chem. 1953, 25, 1320–1324. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic apatite-based biomaterials: On the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef]
- Luo, D.; Sang, L.; Wang, X.; Xu, S.; Li, X. Low temperature, pH-triggered synthesis of collagen–chitosan–hydroxyapatite nanocomposites as potential bone grafting substitutes. Mater. Lett. 2011, 65, 2395–2397. [Google Scholar] [CrossRef]
- Somrani, S.; Rey, C.; Jemal, M. Thermal evolution of amorphous tricalcium phosphate. J. Mater. Chem. 2003, 13, 888–892. [Google Scholar] [CrossRef]
- Siddiqi, S.A.; Azhar, U. Carbonate Substituted Hydroxyapatite; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ansari, Z.; Kalantar, M.; Soriente, A.; Fasolino, I.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. In-Situ Synthesis and Characterization of Chitosan/Hydroxyapatite Nanocomposite Coatings to Improve the Bioactive Properties of Ti6Al4V Substrates. Materials 2020, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Ivanković, M.; Ivanković, H. Preparation and characterization of nano-hydroxyapatite within chitosan matrix. Mater. Sci. Eng. C 2013, 33, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, D.; Yin, J.; Liu, Y.; Yao, F.; Yao, K. Formation of nano-hydroxyapatite crystal in situ in chitosan–pectin polyelectrolyte complex network. Mater. Sci. Eng. C 2010, 30, 795–803. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Surface Chemical and Morphological Analysis of Chitosan/1,3-β-d-Glucan Polysaccharide Films Cross-Linked at 90 °C. Int. J. Mol. Sci. 2022, 23, 5953. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Hao, J.; Zhao, E.; Zhao, X.; Guo, J.; Wang, H.; Randall, C.A. Preparation of zinc oxide/poly-ether-ether-ketone (PEEK) composites via the cold sintering process. Acta Mater. 2021, 215, 117036. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, C.H.; Heo, J.H.; Lee, J.H. Progress and perspectives of metal-ion-substituted hydroxyapatite for bone tissue engineering: Comparison with hydroxyapatite. J. Korean Ceram. Soc. 2022, 59, 271–288. [Google Scholar] [CrossRef]
- Cho, J.S.; Um, S.; Yoo, D.S.; Chung, Y.; Chung, S.H.; Lee, J.; Rhee, S. Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 1046–1062. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and Mg,CO3-substituted hydroxyapatites: Synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Stipniece, L.; Wilson, S.; Curran, J.; Chen, R.; Salma-Ancane, K.; Sharma, P.; Meenan, B.; Boyd, A. Strontium substituted hydroxyapatite promotes direct primary human osteoblast maturation. Ceram. Int. 2020, 47, 3368–3379. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Ortali, C.; Julien, I.; Drouet, C.; Champion, E. Influence of carbonation on the low-temperature consolidation by Spark Plasma Sintering of carbonated calcium phosphate bioceramics. Ceram. Int. 2019, 46, 5799–5810. [Google Scholar] [CrossRef]
- CRey, C.; Combes, C.; Drouet, C.; Lebugle, A.; Sfihi, H.; Barroug, A. Nanocrystalline apatites in biological systems: Characterisation, structure and properties. Mater. Und Werkst. 2007, 38, 996–1002. [Google Scholar] [CrossRef]
- Reed, J.S. Principles of Ceramics and Processing, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Nayir, S.; Waryoba, D.R.; Rajagopalan, R.; Arslan, C.; Randall, C.A. Cold Sintering of a Covalently Bonded MoS2/Graphite Composite as a High Capacity Li-Ion Electrode. Chemnanomat 2018, 4, 1088–1094. [Google Scholar] [CrossRef]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates Using Vibrational Spectroscopies. In Advances in Calcium Phosphate Biomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–266. [Google Scholar]
- Williams, Q.; Knittle, E. Infrared and raman spectra of Ca5(PO4)3F2-fluorapatite at high pressures: Compression-induced changes in phosphate site and Davydov splittings. J. Phys. Chem. Solids 1996, 57, 417–422. [Google Scholar] [CrossRef]
- Le Grill, S.; Drouet, C.; Marsan, O.; Coppel, Y.; Mazel, V.; Barthelemy, M.-C.; Brouillet, F. Consolidation of Spray-Dried Amorphous Calcium Phosphate by Ultrafast Compression: Chemical and Structural Overview. Nanomaterials 2024, 14, 152. [Google Scholar] [CrossRef]
- De Carmejane, O.; Morris, M.D.; Davis, M.K.; Stixrude, L.; Tecklenburg, M.; Rajachar, R.M.; Kohan, D.H. Bone Chemical Structure Response to Mechanical Stress Studied by High Pressure Raman Spectroscopy. Calcif. Tissue Int. 2005, 76, 207–213. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Bettini, R.; Santi, P.; De Ascentiis, A.; Peppas, N. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J. Control. Release 1996, 39, 231–237. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Development of a liposomal nanodelivery system for nevirapine. J. Biomed. Sci. 2010, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, S.; Lu, M.; Shao, Z.; Lu, J.; Xia, L.; Lin, K.; Zou, D. Dose-dependent Effects of Strontium Ranelate on Ovariectomy Rat Bone Marrow Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells. Int. J. Biol. Sci. 2016, 12, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; D’asta, F.; Brandi, M.L. A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 2013, 5, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Luginina, M.; Orru, R.; Cao, G.; Grossin, D.; Brouillet, F.; Chevallier, G.; Thouron, C.; Drouet, C. First successful stabilization of consolidated amorphous calcium phosphate (ACP) by cold sintering: Toward highly-resorbable reactive bioceramics. J. Mater. Chem. B 2019, 8, 629–635. [Google Scholar] [CrossRef]
- Rizzoli, R.; Reginster, J.-Y.; Boonen, S.; Bréart, G.; Diez-Perez, A.; Felsenberg, D.; Kaufman, J.-M.; Kanis, J.A.; Cooper, C. Adverse Reactions and Drug–Drug Interactions in the Management of Women with Postmenopausal Osteoporosis. Calcif. Tissue Int. 2011, 89, 91–104. [Google Scholar] [CrossRef]
- Kaufman, J.-M.; Audran, M.; Bianchi, G.; Braga, V.; Diaz-Curiel, M.; Francis, R.M.; Goemaere, S.; Josse, R.; Palacios, S.; Ringe, J.D.; et al. Efficacy and Safety of Strontium Ranelate in the Treatment of Osteoporosis in Men. J. Clin. Endocrinol. Metab. 2013, 98, 592–601. [Google Scholar] [CrossRef][Green Version]
- Vaz, L.M.; Branco, R.; Morais, P.V.; Guiomar, A.J. Sterilized Polyhexanide-Releasing Chitosan Membranes with Potential for Use in Antimicrobial Wound Dressings. Membranes 2023, 13, 877. [Google Scholar] [CrossRef]


| Powders | SSA (m2/g) | Measured Density (g/cm3) | Average Particle Size (nm) * | Average Crystallite Length (nm) ** | Average Crystallite Width/Thickness (nm) ** |
|---|---|---|---|---|---|
| HAp | 138 ± 2 | 2.68 ± 0.02 | 16 ± 0 | 17 | 6 |
| HAp10Chit | 143 ± 4 | 2.43 ± 0.01 | 17 ± 1 | 14 | 5 |
| HAp10ChitSrRAN | 142 ± 2 | 2.45 ± 0.01 | 17 ± 0 | 18 | 6 |
| HAp | HAp10Chit | HAp10ChitSrRAN | |
|---|---|---|---|
| Ca2+ (wt%) | 32.45 ± 0.02 | 29.65 ± 0.02 | 29.04 ± 0.02 |
| P− (wt%) | 18.13 ± 0.02 | 18.25 ± 0.02 | 17.70 ± 0.02 |
| HPO42− (wt%) | 0.15 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.02 |
| Na+ (wt%) | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.29 ± 0.02 |
| Mg2+ (wt%) | 0.05 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 |
| Sr2+ (wt%) | 0.09 ± 0.02 | 0.08 ± 0.02 | 1.36 ± 0.02 |
| CO32− (wt%) | 3.3 ± 0.2 | 3.1 ± 0.2 | 2.6 ± 0.2 |
| Ca/P | 1.38 | 1.26 | 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galotta, A.; Demir, Ö.; Marsan, O.; Sglavo, V.M.; Loca, D.; Combes, C.; Locs, J. Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications. Nanomaterials 2024, 14, 441. https://doi.org/10.3390/nano14050441
Galotta A, Demir Ö, Marsan O, Sglavo VM, Loca D, Combes C, Locs J. Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications. Nanomaterials. 2024; 14(5):441. https://doi.org/10.3390/nano14050441
Chicago/Turabian StyleGalotta, Anna, Öznur Demir, Olivier Marsan, Vincenzo M. Sglavo, Dagnija Loca, Christèle Combes, and Janis Locs. 2024. "Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications" Nanomaterials 14, no. 5: 441. https://doi.org/10.3390/nano14050441
APA StyleGalotta, A., Demir, Ö., Marsan, O., Sglavo, V. M., Loca, D., Combes, C., & Locs, J. (2024). Apatite/Chitosan Composites Formed by Cold Sintering for Drug Delivery and Bone Tissue Engineering Applications. Nanomaterials, 14(5), 441. https://doi.org/10.3390/nano14050441








