Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Nanotubes
2.3. Characterization Systems
2.4. Measurement of Photocatalytic Bacteria Inactivation
3. Results and Discussion
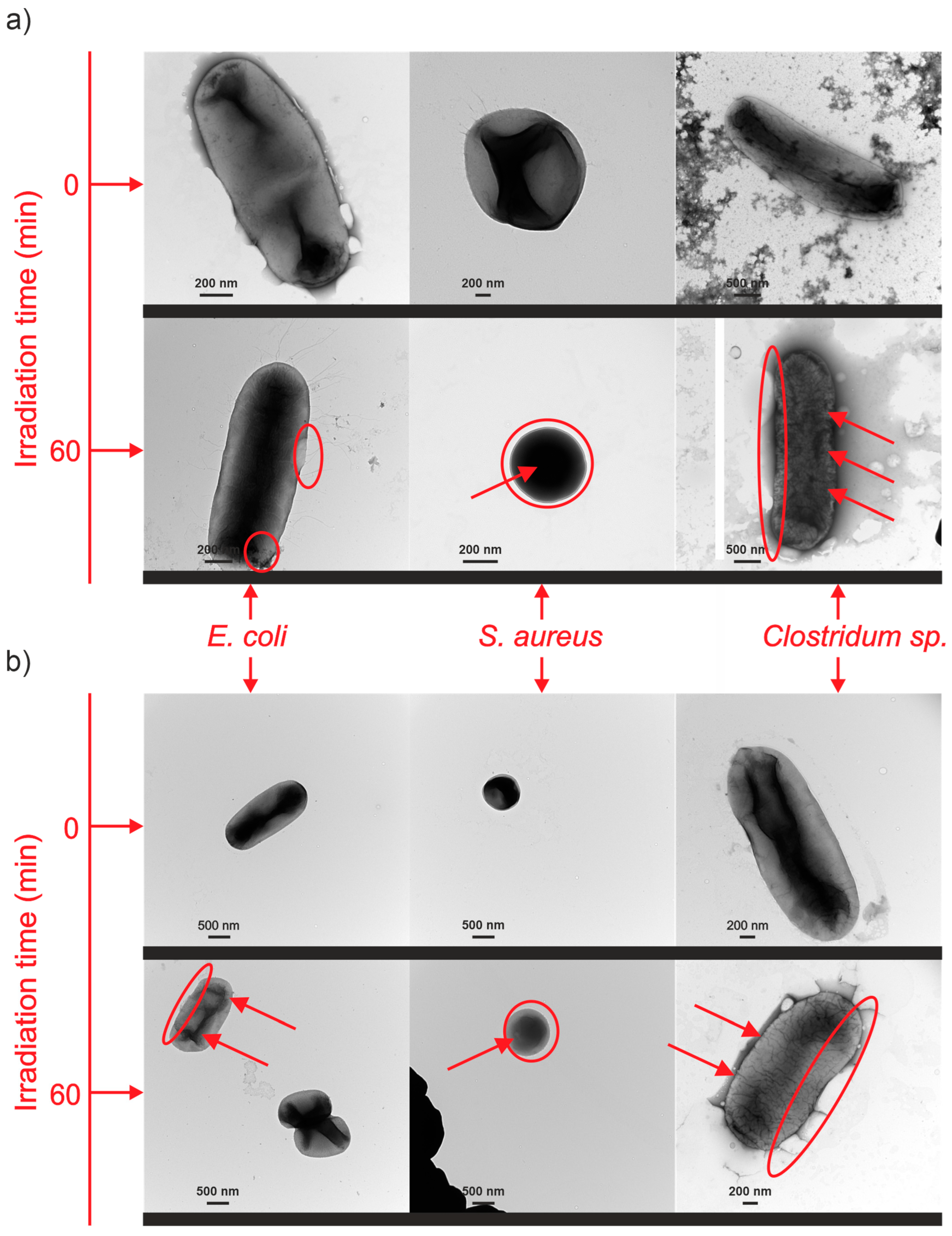
3.1. SEM and TEM Analysis
3.2. X-ray Diffraction
3.3. Raman Spectroscopy
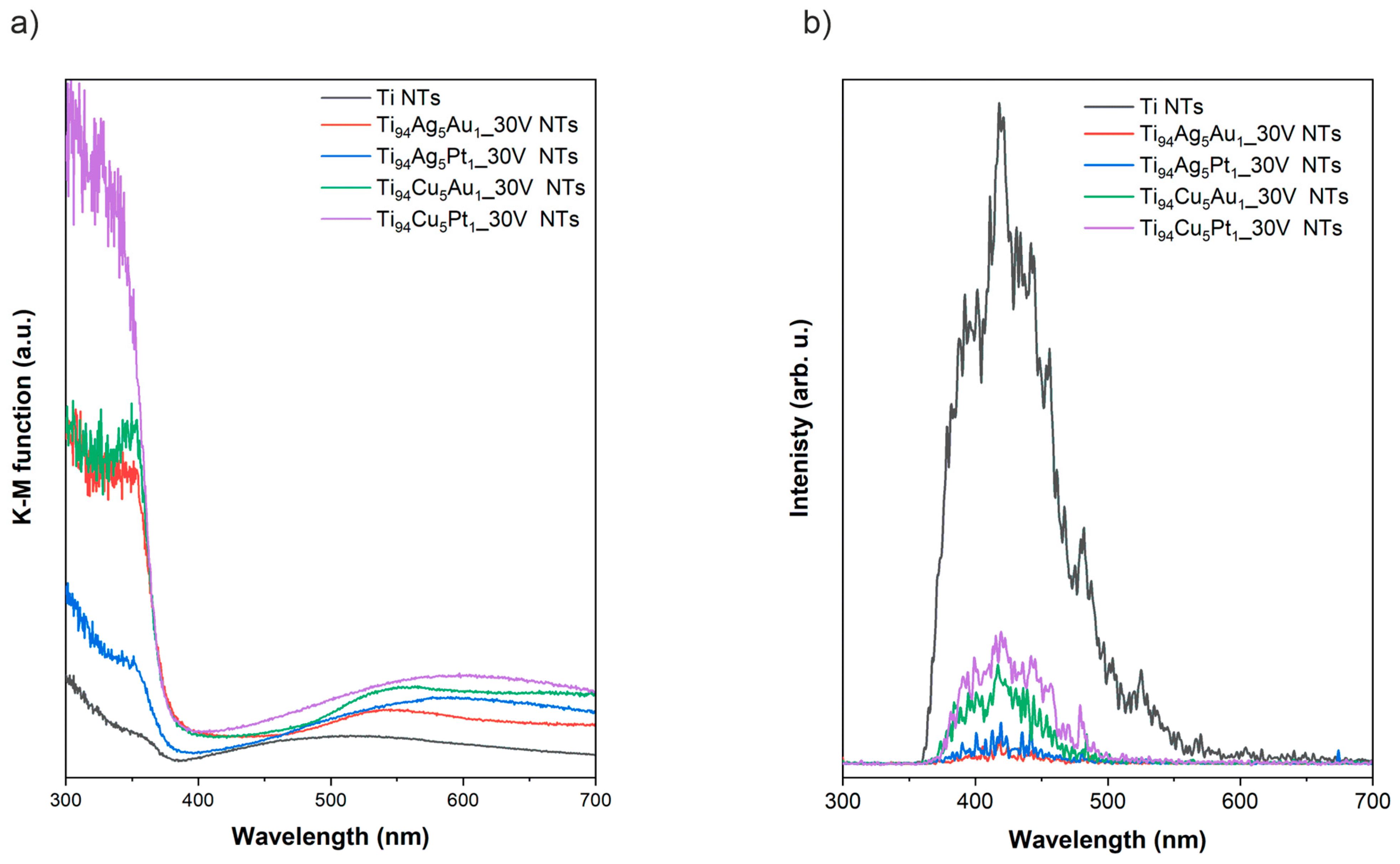
3.4. DRS and Photoluminescence
3.5. X-ray Photoelectron Spectroscopy (XPS)
3.6. Photocatalytic Antibacterial Activity
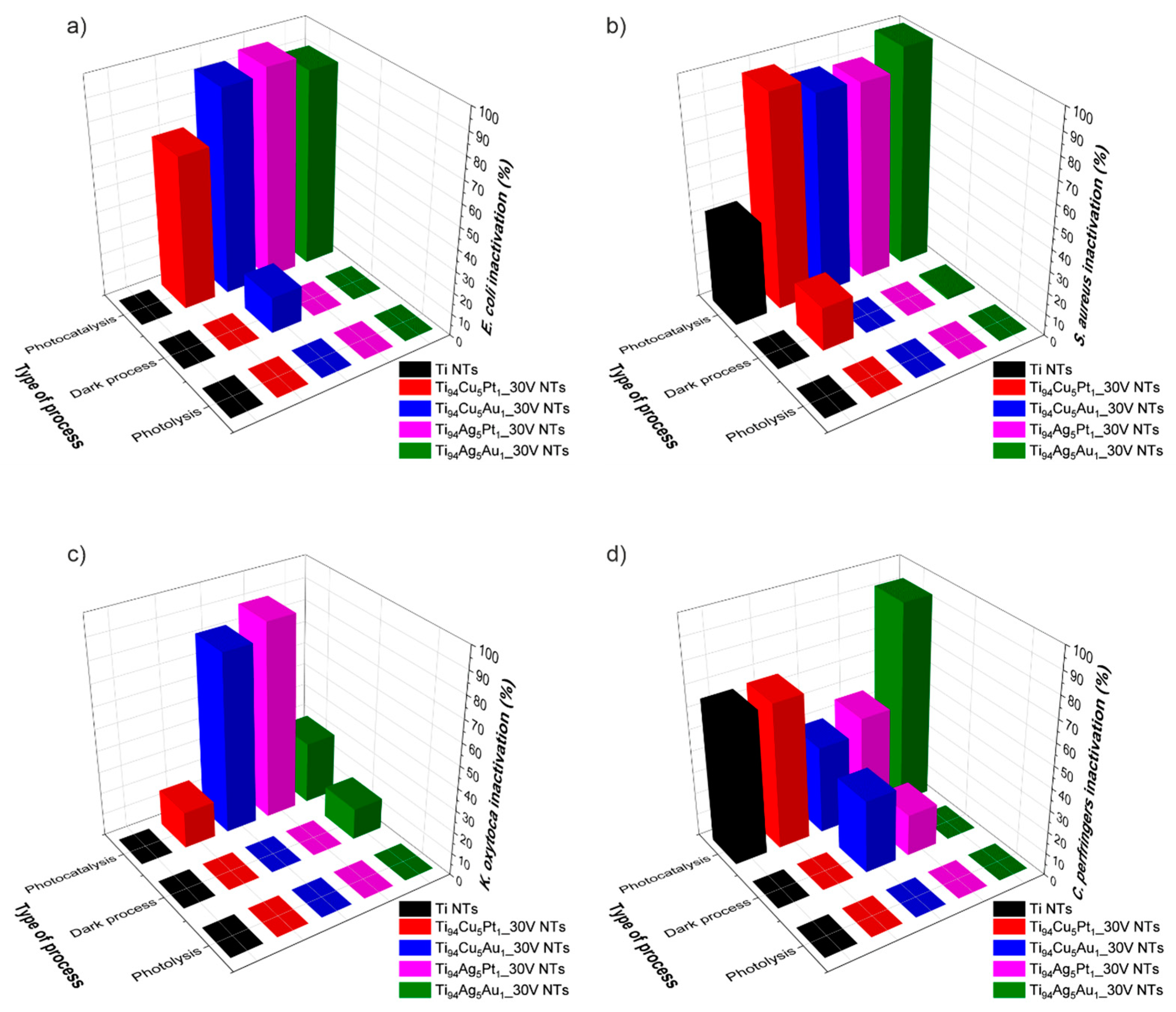
3.6.1. Effectiveness of Bacterial Inactivation
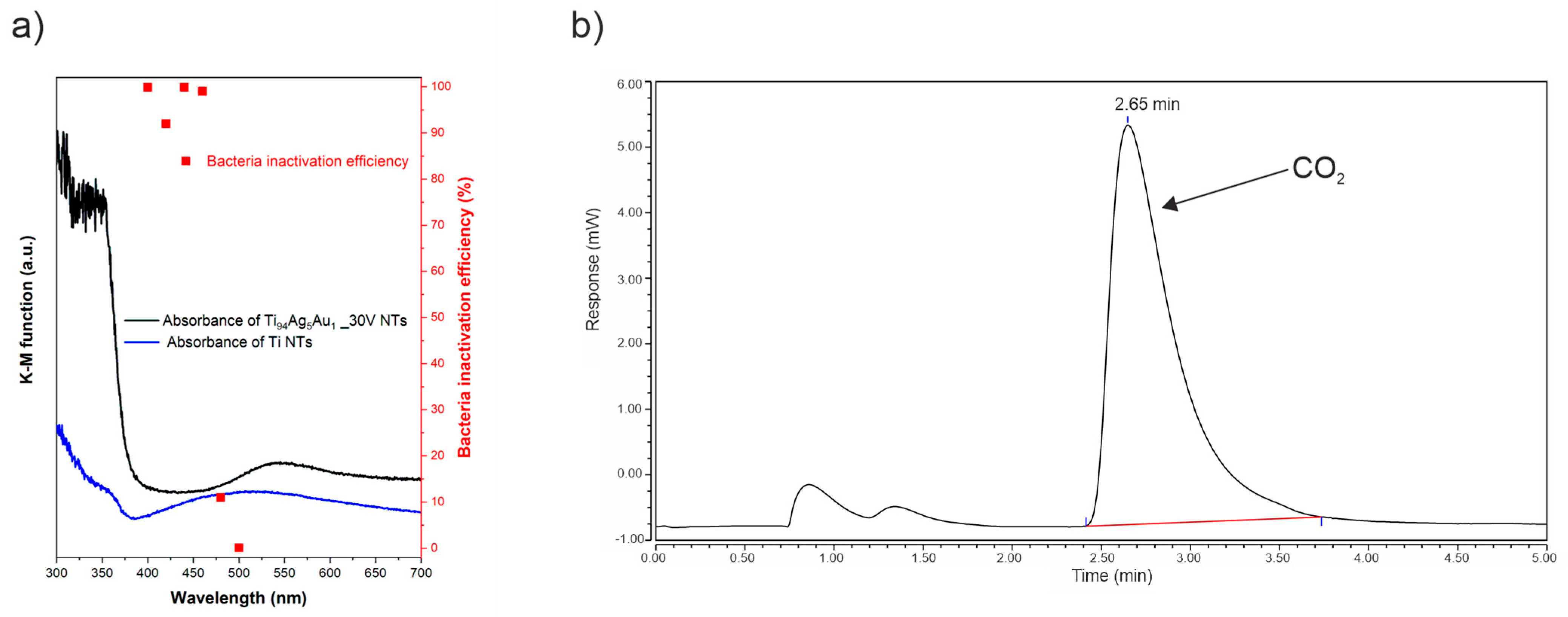
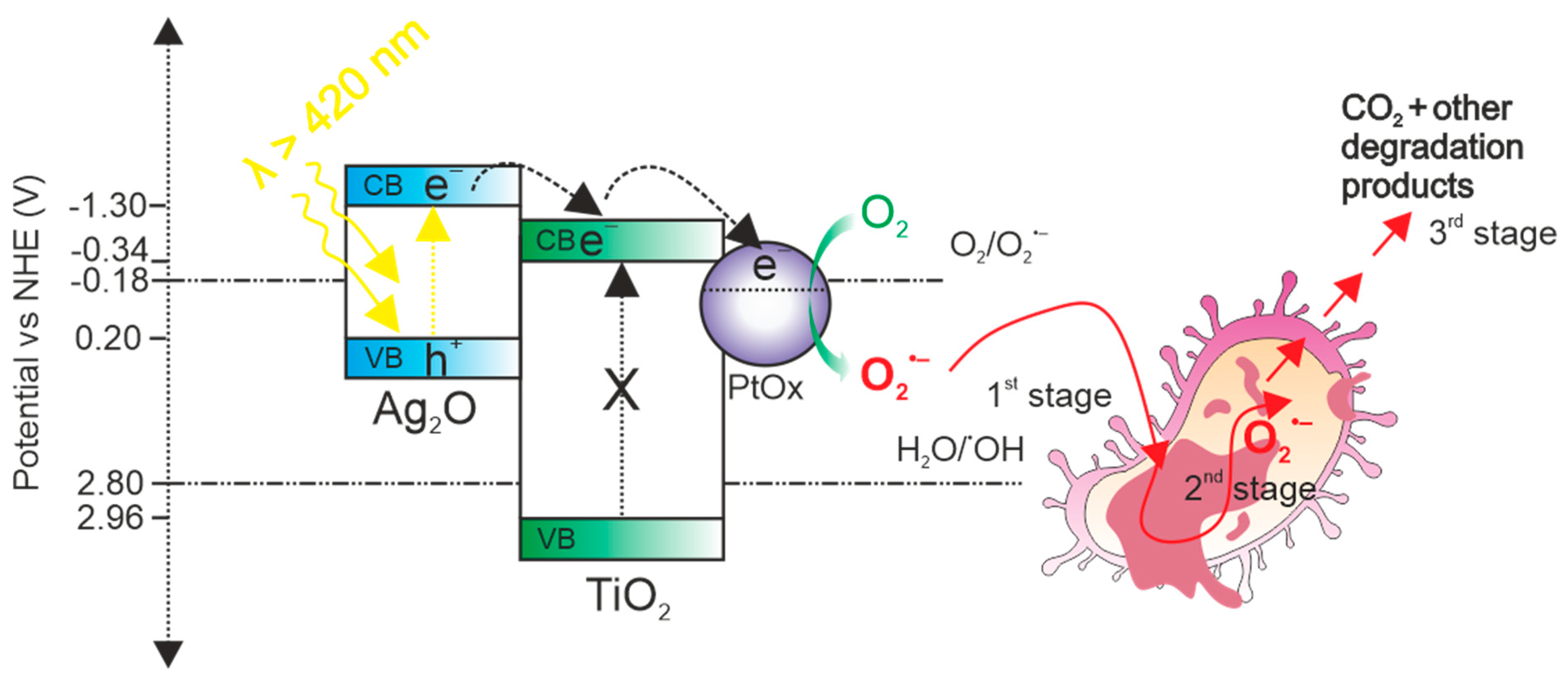
3.6.2. Mechanism of Bacterial Inactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.B.; Sohaib, M.; Sagir, M.; Rafique, M. Role of Nanotechnology in Photocatalysis. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 578–589. [Google Scholar] [CrossRef]
- Lofrano, G.; Ubaldi, F.; Albarano, L.; Carotenuto, M.; Vaiano, V.; Valeriani, F.; Libralato, G.; Gianfranceschi, G.; Fratoddi, I.; Meric, S.; et al. Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials 2022, 12, 2831. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Nah, Y.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. Chemphyschem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Paramasivam, I.; Macak, J.; Schmuki, P. Photocatalytic activity of TiO2 nanotube layers loaded with Ag and Au nanoparticles. Electrochem. Commun. 2008, 10, 71–75. [Google Scholar] [CrossRef]
- Mazierski, P.; Nadolna, J.; Nowaczyk, G.; Lisowski, W.; Winiarski, M.J.; Klimczuk, T.; Kobylański, M.P.; Jurga, S.; Zaleska-Medynska, A. Highly Visible-Light-Photoactive Heterojunction Based on TiO2 Nanotubes Decorated by Pt Nanoparticles and Bi2S3 Quantum Dots. J. Phys. Chem. C 2017, 121, 17215–17225. [Google Scholar] [CrossRef]
- Sewnet, A.; Abebe, M.; Asaithambi, P.; Alemayehu, E. Visible-Light-Driven g-C3N4/TiO2 Based Heterojunction Nanocomposites for Photocatalytic Degradation of Organic Dyes in Wastewater: A Review. Air Soil Water Res. 2022, 15, 11786221221117266. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and Antimicrobial Properties of Ag2O/TiO2 Heterojunction. Chemengineering 2019, 3, 3. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Kozak, M.; Mazierski, P.; Żebrowska, J.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237. [Google Scholar] [CrossRef]
- Mazierski, P.; Malankowska, A.; Kobylański, M.; Diak, M.; Kozak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. Photocatalytically Active TiO2/Ag2O Nanotube Arrays Interlaced with Silver Nanoparticles Obtained from the One-Step Anodic Oxidation of Ti-Ag Alloys. ACS Catal. 2017, 7, 2753–2764. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Kobylański, M.P.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Self-Organized TiO2-MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22, 564. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Mazierski, P.; Kobylański, M.P.; Szczepańska, G.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Growth, Structure, and Photocatalytic Properties of Hierarchical V2O5-TiO2 Nanotube Arrays Obtained from the One-step Anodic Oxidation of Ti-V Alloys. Molecules 2017, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Kobylański, M.P.; Mazierski, P.; Malankowska, A.; Kozak, M.; Diak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. TiO2CoxOy composite nanotube arrays via one step electrochemical anodization for visible light–induced photocatalytic reaction. Surf. Interfaces 2018, 12, 179–189. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, J.; Xu, X.; Liu, G.; Wang, X. Preparation and magnetic properties of iron titanium oxide nanotube arrays. Ceram. Int. 2014, 40, 5825–5830. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Wang, R.; Zhu, W.; Ren, X.; Luo, L.; Zhang, X.; Luo, S.; Ai, X.; Wang, J. Binary composite MoS2/TiO2 nanotube arrays as a recyclable and efficient photocatalyst for solar water disinfection. Chem. Eng. J. 2020, 401, 126052. [Google Scholar] [CrossRef]
- Cummings, F.; Tshaka, A. Opto-electronic properties of anodized TiO2 nanotube arrays investigated using electron energy loss spectroscopy. Surf. Interfaces 2019, 17, 100347. [Google Scholar] [CrossRef]
- Ning, X.; Wang, X.; Yu, X.; Li, J.; Zhao, J. Preparation and capacitance properties of Mn-doped TiO2 nanotube arrays by anodisation of Ti-Mn alloy. J. Alloys Compd. 2016, 658, 177–182. [Google Scholar] [CrossRef]
- Jha, H.; Hahn, R.; Schmuki, P. Ultrafast oxide nanotube formation on TiNb, TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Mishra, T.; Wang, L.; Hahn, R.; Schmuki, P. In-situ Cr doped anodized TiO2 nanotubes with increased photocurrent response. Electrochim. Acta 2014, 132, 410–415. [Google Scholar] [CrossRef]
- Oliveira, N.T.; Verdério, J.F.; Bolfarini, C. Obtaining self-organized nanotubes on biomedical Ti-Mo alloys. Electrochem. Commun. 2013, 35, 139–141. [Google Scholar] [CrossRef]
- Hejazi, S.; Altomare, M.; Nguyen, N.T.; Mohajernia, S.; Licklederer, M.; Schmuki, P. Intrinsic Au-decoration on anodic TiO2 nanotubes grown from metastable Ti-Au sputtered alloys—High density co-catalyst decoration enhances the photocatalytic H2 evolution. Appl. Mater. Today 2019, 14, 118–125. [Google Scholar] [CrossRef]
- Lv, S.; Du, Y.; Wu, F.; Cai, Y.; Zhou, T. Review on LSPR assisted photocatalysis: Effects of physical fields and opportunities in multifield decoupling. Nanoscale Adv. 2022, 4, 2608–2631. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Mazierski, P.; Baluk, M.; Żebrowska, J.; Lisowski, W.; Trykowski, G.; Skowron, P.; Zaleska-Medynska, A. Anodized multi—Component titanium alloys carrying antibacterial features. Appl. Surf. Sci. 2023, 613, 156009. [Google Scholar] [CrossRef]
- Hang, R.; Liu, Y.; Zhao, L.; Gao, A.; Bai, L.; Huang, X.; Zhang, X.; Tang, B.; Chu, P.K. Fabrication of Ni-Ti-O nanotube arrays by anodization of NiTi alloy and their potential applications. Sci. Rep. 2014, 4, 7547. [Google Scholar] [CrossRef] [PubMed]
- Hang, R.; Zhao, F.; Yao, X.; Tang, B.; Chu, P.K. Self-assembled anodization of NiTi alloys for biomedical applications. Appl. Surf. Sci. 2020, 517, 146118. [Google Scholar] [CrossRef]
- Nah, Y.-C.; Ghicov, A.; Kim, D.; Berger, S.; Schmuki, P. TiO2-WO3 Composite Nanotubes by Alloy Anodization: Growth and Enhanced Electrochromic Properties. J. Am. Chem. Soc. 2008, 130, 16154–16155. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Misiolek, W.Z. Review of Fabrication Methods, Physical Properties, and Applications of Nanostructured Copper Oxides Formed via Electrochemical Oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef]
- Elgohary, E.A.; Mohamed, Y.M.A.; El Nazer, H.A.; Baaloudj, O.; Alyami, M.S.S.; El Jery, A.; Assadi, A.A.; Amrane, A. A Review of the Use of Semiconductors as Catalysts in the Photocatalytic Inactivation of Microorganisms. Catalysts 2021, 11, 1498. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs:Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-SusceptibleStaphylococcus aureusBloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Ng, S.; Kuberský, P.; Krbal, M.; Prikryl, J.; Gärtnerová, V.; Moravcová, D.; Sopha, H.; Zazpe, R.; Yam, F.K.; Jäger, A.; et al. ZnO Coated Anodic 1D TiO2 Nanotube Layers: Efficient Photo-Electrochemical and Gas Sensing Heterojunction. Adv. Eng. Mater. 2018, 20, 1700589. [Google Scholar] [CrossRef]
- Sopha, H.; Kashimbetova, A.; Hromadko, L.; Saldan, I.; Celko, L.; Montufar, E.B.; Macak, J.M. Anodic TiO2 Nanotubes on 3D-Printed Titanium Meshes for Photocatalytic Applications. Nano Lett. 2021, 21, 8701–8706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z. Freestanding TiO2 Nanotube Arrays with Ultrahigh Aspect Ratio via Electrochemical Anodization. Chem. Mater. 2008, 20, 1257–1261. [Google Scholar] [CrossRef]
- Smith, K.A.; Savva, A.I.; Deng, C.; Wharry, J.P.; Hwang, S.; Su, D.; Wang, Y.; Gong, J.; Xu, T.; Butt, D.P.; et al. Effects of proton irradiation on structural and electrochemical charge storage properties of TiO2 nanotube electrodes for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 11815–11824. [Google Scholar] [CrossRef]
- Ren, Y.; Shi, X.; Xia, P.; Li, S.; Lv, M.; Wang, Y.; Mao, Z. In Situ Raman Investigation of TiO2 Nanotube Array-Based Ultraviolet Photodetectors: Effects of Nanotube Length. Molecules 2020, 25, 1854. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Lai, Y.-K.; Huang, J.-Y.; Zhang, H.-F.; Subramaniam, V.-P.; Tang, Y.-X.; Gong, D.-G.; Sundar, L.; Sun, L.; Chen, Z.; Lin, C.-J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard. Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Yaremchuk, I.; Meškinis, Š.; Bulavinets, T.; Vasiliauskas, A.; Andrulevičius, M.; Fitio, V.; Bobitski, Y.; Tamulevičius, S. Effect of oxidation of copper nanoparticles on absorption spectra of DLC:Cu nanocomposites. Diam. Relat. Mater. 2019, 99, 107538. [Google Scholar] [CrossRef]
- Fernández-Ponce, C.; Muñoz-Miranda, J.P.; Santos, D.M.d.L.; Aguado, E.; García-Cozar, F.; Litrán, R. Influence of size and surface capping on photoluminescence and cytotoxicity of gold nanoparticles. J. Nanopart. Res. 2018, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, H.; Ao, C.; Lee, S.; Yu, J.C.; Ho, W. Preparation, characterization and photocatalytic activity of in situ Fe-doped TiO2 thin films. Thin Solid Films 2006, 496, 273–280. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, C.; Ma, G.; Du, Z. Electronic State Characterization of TiO2 Ultrafine Particles by Luminescence Spectroscopy. J. Solid State Chem. 1998, 139, 124–127. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Chansaenpak, K.; Channei, D. Evaluating the photocatalytic efficiency of the BiVO4/rGO photocatalyst. Sci. Rep. 2019, 9, 16091. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. In NIST Standard Reference Database 20, Version 4.1; NIST Standard Reference Materials: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Schierbaum, K.; Fischer, S.; Torquemada, M.; de Segovia, J.; Román, E.; Martín-Gago, J. The interaction of Pt with TiO2(110) surfaces: A comparative XPS, UPS, ISS, and ESD study. Surf. Sci. 1996, 345, 261–273. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Moma, J.A.; Scurrell, M.S.; Jordaan, W.A. Effects of incorporation of ions into Au/TiO2 catalysts for carbon monoxide oxidation. Top. Catal. 2007, 44, 167–172. [Google Scholar] [CrossRef]
- Meister, T.L.; Fortmann, J.; Breisch, M.; Sengstock, C.; Steinmann, E.; Köller, M.; Pfaender, S.; Ludwig, A. Nanoscale copper and silver thin film systems display differences in antiviral and antibacterial properties. Sci. Rep. 2022, 12, 7193. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. UV and solar photocatalytic disinfection of municipal wastewater: Inactivation, reactivation and regrowth of bacterial pathogens. Int. J. Environ. Sci. Technol. 2019, 16, 3687–3696. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Z.; Tang, R.; Ouyang, K.; Liao, C.; Fang, Y.; Ding, C.; Yang, L.; Su, L.; Gong, D. What will happen when microorganisms “meet” photocatalysts and photocatalysis? Environ. Sci. Nano 2020, 7, 702–723. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible Light-Enhanced Antibacterial and Osteogenic Functionality of Au and Pt Nanoparticles Deposited on TiO2 Nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, H.; Li, H.; Yang, Y.; Zhang, S.; Rauf, A.; Zhao, Q.; Ning, G. Highly synergistic antimicrobial activity of spherical and flower-like hierarchical titanium dioxide/silver composites. J. Colloid Interface Sci. 2017, 504, 448–456. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Myles, A.; Quilty, B.; McCormack, D.E.; Fagan, R.; Hinder, S.J.; Dionysiou, D.D.; Pillai, S.C. Antibacterial properties of F-doped ZnO visible light photocatalyst. J. Hazard. Mater. 2017, 324, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Deng, J.; Li, M.; Xu, T.; Tong, M. Bactericidal activity and mechanism of Ti-doped BiOI microspheres under visible light irradiation. Colloids Surf. B Biointerfaces 2016, 147, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Z.; Yu, M.; Xu, Z.; Liu, Y.; Li, F.; Wang, L. Preparation of enhanced AgI@MnO2 heterojunction photocatalysts for rapid sterilization under visible light. J. Alloys Compd. 2021, 887, 161431. [Google Scholar] [CrossRef]
- Shanmugam, V.; Sanjeevamuthu, S.; Jeyaperumal, K.S.; Vairamuthu, R. Fabrication of heterostructured vanadium modified g-C3N4/TiO2 hybrid photocatalyst for improved photocatalytic performance under visible light exposure and antibacterial activities. J. Ind. Eng. Chem. 2019, 76, 318–332. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhang, W.; Zhang, S.; Su, H.; Tan, T. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan—TiO2 organic–inorganic composites for water disinfection. Appl. Catal. B Environ. 2015, 170–171, 255–262. [Google Scholar] [CrossRef]
- Luthfiah, A.; Permana, M.D.; Deawati, Y.; Firdaus, M.L.; Rahayu, I.; Eddy, D.R. Photocatalysis of nanocomposite titania—Natural silica as antibacterial against Staphylococcus aureus and Pseudomonas aeruginosa. RSC Adv. 2021, 11, 38528–38536. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-D.; Lee, B.-K. Disinfection of Staphylococcus aureus in indoor aerosols using Cu-TiO2 deposited on glass fiber under visible light irradiation. J. Photochem. Photobiol. A Chem. 2015, 307–308, 16–22. [Google Scholar] [CrossRef]
- Afzal, M.I.; Shahid, S.; Mansoor, S.; Javed, M.; Iqbal, S.; Hakami, O.; Yousef, E.S.; Al-Fawzan, F.F.; Elkaeed, E.B.; Pashameah, R.A.; et al. Fabrication of a Ternary Nanocomposite g-C3N4/Cu@CdS with Superior Charge Separation for Removal of Organic Pollutants and Bacterial Disinfection from Wastewater under Sunlight Illumination. Toxics 2022, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Achouri, F.; BenSaid, M.; Bousselmi, L.; Corbel, S.; Schneider, R.; Ghrabi, A. Comparative study of Gram-negative bacteria response to solar photocatalytic inactivation. Environ. Sci. Pollut. Res. 2019, 26, 18961–18970. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Keulemans, M.; Goris, B.; Blommaerts, N.; Bals, S.; Martens, J.A.; Lenaerts, S. Plasmonic ‘rainbow’ photocatalyst with broadband solar light response for environmental applications. Appl. Catal. B Environ. 2016, 188, 147–153. [Google Scholar] [CrossRef]
- Tian, Y.; Notsu, H.; Tatsuma, T. Visible-light-induced patterning of Au- and Ag-TiO2 nanocomposite film surfaces on the basis of plasmon photoelectrochemistry. Photochem. Photobiol. Sci. 2005, 4, 598–601. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Karthik, M.; Neppolian, B.; Madhavan, J.; Shankar, M. Solar light sensitized p-Ag2O/n-TiO2 nanotubes heterojunction photocatalysts for enhanced hydrogen production in aqueous-glycerol solution. Sol. Energy Mater. Sol. Cells 2016, 154, 78–87. [Google Scholar] [CrossRef]
- Sanabria-Arenas, B.E.; Mazare, A.; Yoo, J.; Nguyen, N.T.; Hejazi, S.; Bian, H.; Diamanti, M.V.; Pedeferri, M.P.; Schmuki, P. Intrinsic AuPt-alloy particles decorated on TiO2 nanotubes provide enhanced photocatalytic degradation. Electrochim. Acta 2018, 292, 865–870. [Google Scholar] [CrossRef]
- Martínez, M.C.N.; Mazierski, P.; Nowaczyk, G.; Lisowski, W.; Trykowski, G.; Zaleska-Medynska, A. Insights into the Intrinsic Creation of Heterojunction-Based Ordered TiO2 Nanotubes Obtained from the One-Step Anodic Oxidation of Titanium Alloys. J. Phys. Chem. C 2021, 125, 7097–7108. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyła, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process. Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Sambhy, V.; MacBride, M.M.; Peterson, B.R.; Sen, A. Silver Bromide Nanoparticle/Polymer Composites: Dual Action Tunable Antimicrobial Materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- He, W.; Huang, H.; Yan, J.; Zhu, J. Photocatalytic and antibacterial properties of Au-TiO2 nanocomposite on monolayer graphene: From experiment to theory. J. Appl. Phys. 2013, 114, 204701. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Qian, S.; Liu, Z.; Feng, J.; Jin, P.; Liu, X. Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl. Phys. Lett. 2014, 104, 261110. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Smith, J.A. Incorporation of copper nanoparticles into paper for point-of-use water purification. Water Res. 2014, 63, 245–251. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Deng, C.-H.; Gong, J.-L.; Zeng, G.-M.; Zhang, P.; Song, B.; Zhang, X.-G.; Liu, H.-Y.; Huan, S.-Y. Graphene sponge decorated with copper nanoparticles as a novel bactericidal filter for inactivation of Escherichia coli. Chemosphere 2017, 184, 347–357. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Kusiak-Nejman, E.; Morawski, A.W. Photocatalytic water disinfection under the artificial solar light by fructose-modified TiO2. Chem. Eng. J. 2019, 372, 203–215. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.-L.; Khiew, P.S.; Lim, S.S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.-Y.; Leong, C.-O. Enhanced marine antifouling performance of silver-titania nanotube composites from hydrothermal processing. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 701–711. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, J.; Hovde, C.J. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef]
- Cooper, I.R. Introduction to biomaterials and medical device-associated infections. In Biomaterials and Medical Device—Associated Infections; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–17. [Google Scholar] [CrossRef]
- Available online: https://www.healthline.com/health/mssa (accessed on 21 April 2023).
- Camacho-Cruz, J.; Gutiérrez, I.F.; Brand-López, K.; Sosa-Rodríguez, Y.A.; Vásquez-Hoyos, P.; Gómez-Cortés, L.C.; Romero-Higuera, L.N.; Rojas-Rojas, D.P.; Ortiz-Mendez, C.A.; Camacho-Moreno, G.; et al. Differences Between Methicillin-susceptible Versus Methicillin-resistant Staphylococcus aureus Infections in Pediatrics. Pediatr. Infect. Dis. J. 2022, 41, 12–19. [Google Scholar] [CrossRef]
- Singh, L.; Cariappa, M.P.; Kaur, M. Klebsiella oxytoca: An emerging pathogen? Med. J. Armed. Forces India 2016, 72, S59–S61. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.L.; Wilkins, T.D. Medical Microbiology. 4th Edition. 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8219/ (accessed on 25 April 2023).

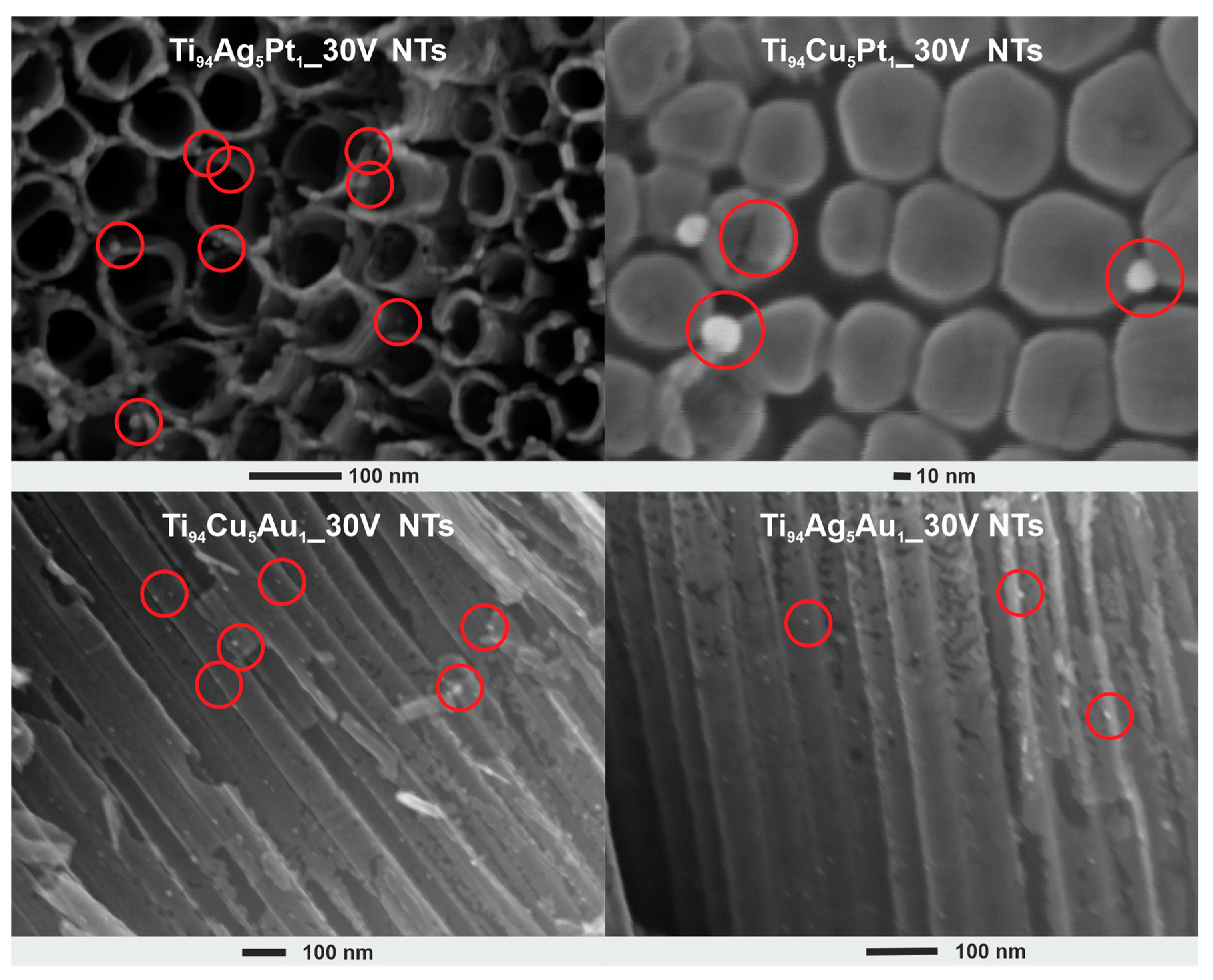
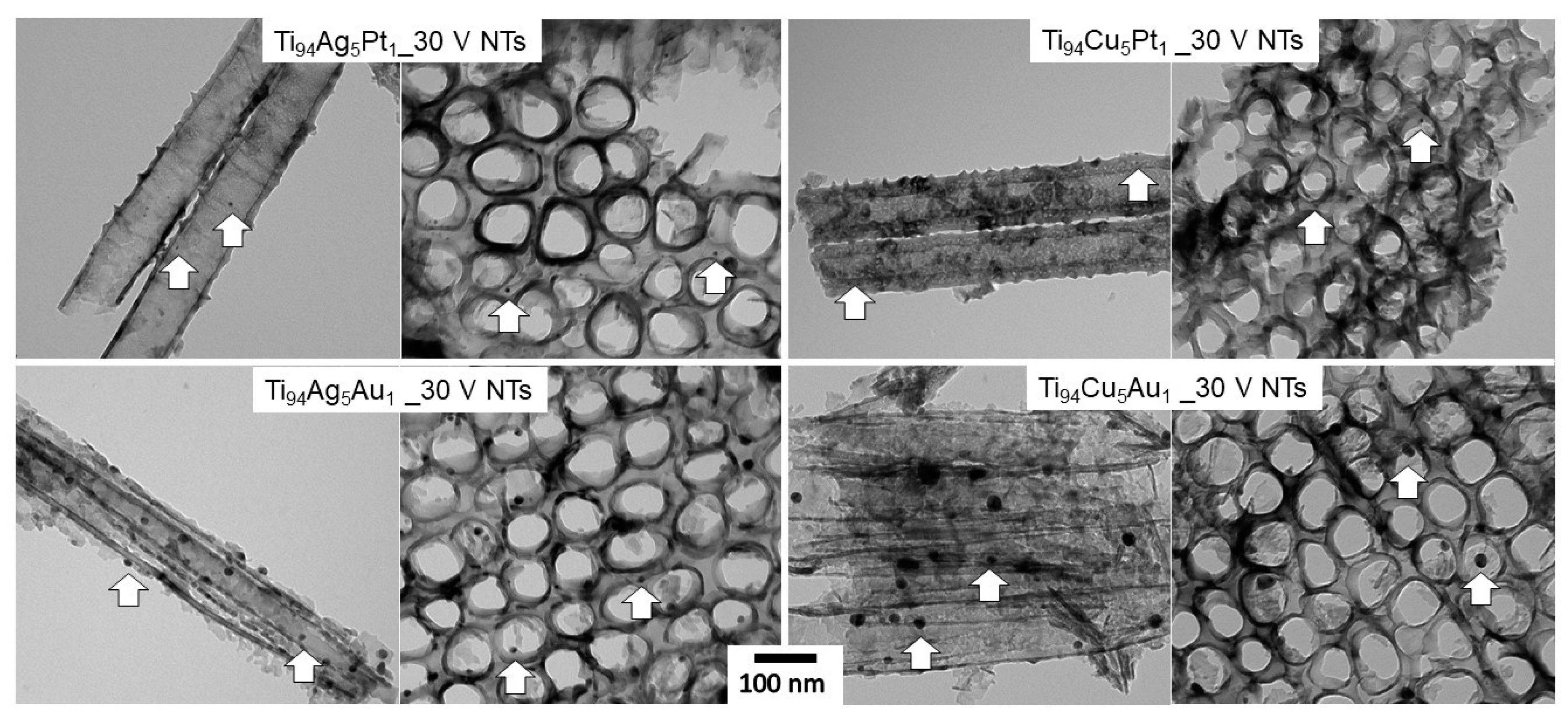
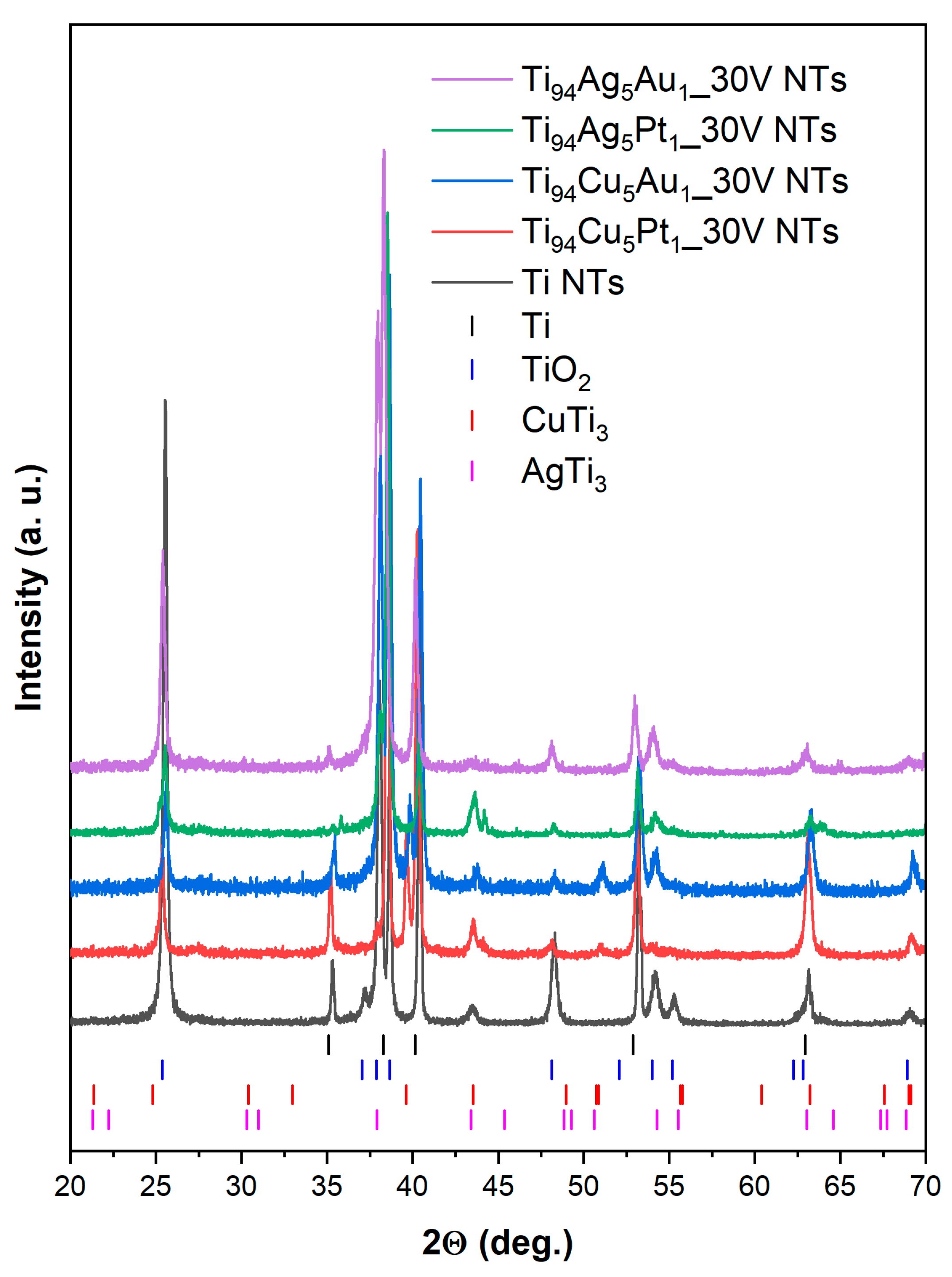
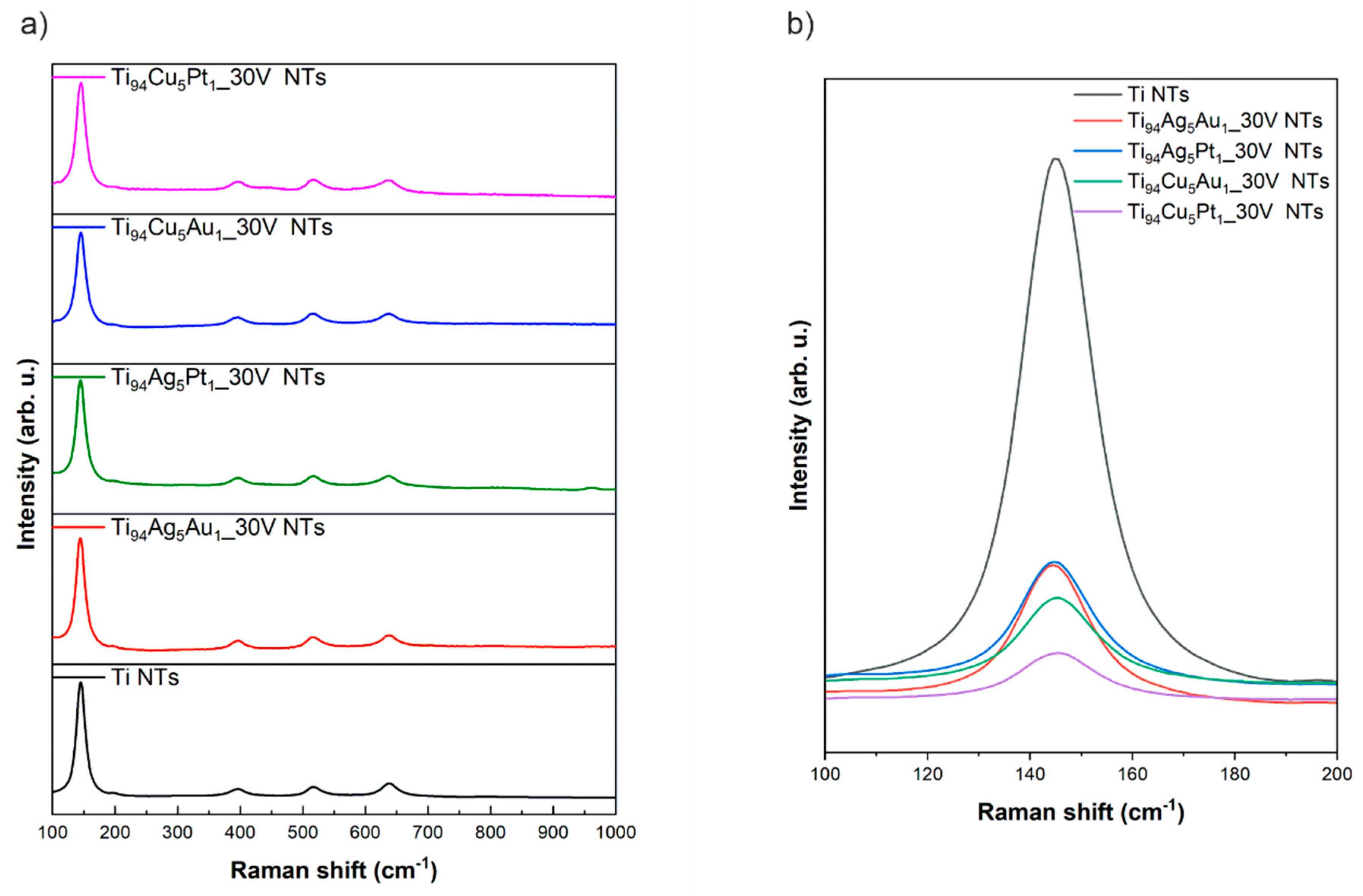

| Sample Label | Composition of Alloy (wt. %) | Synthesis Parameters | Morphology of Obtained Nanotubes Based on SEM Imaging | ||
|---|---|---|---|---|---|
| Voltage (V) | Time (min) | Inner diameter (nm) | Length (µm) | ||
| Ti NTs | 100% Ti | 30 V | 90 min | 64.3 ± 4.9 | 2.6 ± 0.1 |
| Ti94Ag5Au1_30V NTs | 94% Ti, 5% Ag, 1% Au | 30 V | 60 min | 58.8 ± 6.8 | 2.3 ± 0.1 |
| Ti94Ag5Pt1_30V NTs | 94% Ti, 5% Ag, 1% Pt | 30 V | 60 min | 54.3 ± 5.5 | 2.5 ± 0.2 |
| Ti94Cu5Au1_30V NTs | 94% Ti, 5% Cu, 1% Au | 30 V | 60 min | 55.2 ± 6.2 | 2.6 ± 0.1 |
| Ti94Cu5Pt1_30V NTs | 94% Ti, 5% Cu, 1% Pt | 30 V | 60 min | 57.5 ± 6.2 | 2.5 ± 0.1 |
| Sample Label | Elemental Composition (Atomic %.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | O | C | N | Cu | Ag | Pt | Au | O/Ti | |
| Ti NTs | 24.23 | 62.16 | 12.70 | 0.91 | - | - | - | - | 2.56 |
| Ti94Ag5Au1_30V NTs | 24.40 | 63.19 | 10.95 | 0.62 | - | 0.47 | - | 0.38 | 2.59 |
| Ti94Cu5Pt1_30V NTs | 22.12 | 62.22 | 13.65 | 1.41 | 0.11 | - | 0.49 | - | 2.81 |
| Ti94Cu5Au1_30V NTs | 24.56 | 62.56 | 11.45 | 0.59 | 0.16 | - | - | 0.68 | 2.55 |
| Ti94Ag5Pt1_30V NTs | 24.07 | 64.35 | 10.56 | 0.74 | - | 0.23 | 0.05 | - | 2.67 |
| Sample Label | Ti/Me Ratio Based on XPS Analysis (Nominal Ti/Me Ratio of Ti-Alloys) | Me5/Me1 Ratio Based on XPS Analysis (Nominal Me5/Me1 Ratio of Ti-Alloys) | Ti 2p3/2 Fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti/Ag | Ti/Cu | Ti/Pt | Ti/Au | Ag/Au | Cu/Pt | Cu/Au | Ag/Pt | Ti(4+) 458.8 ± 0.2 eV | Ti(3+) 457.3 ± 0.3 eV | |
| Ti94Ag5Au1_30V NTs | 52.1 (18.8) | - | - | 64.2 (94) | 1.2 (5) | - | - | - | 97.30 | 2.70 |
| Ti94Cu5Pt1_30V NTs | - | 201.1 (18.8) | 45.1 (94) | - | - | 0.2 (5) | - | - | 97.36 | 2.64 |
| Ti94Cu5Au1_30V NTs | - | 153.5 (18.8) | - | 36.1 (94) | - | - | 0.2 (5) | - | 97.70 | 2.30 |
| Ti94Ag5Pt1_30V NTs | 104.7 (18.8) | - | 481.4 (94) | - | - | - | - | 4.6 (5) | 97.76 | 2.24 |
| Ti NTs | N/A | N/A | 96.57 | 3.43 | ||||||
| Photocatalyst Type | Form | Preparation Method | Time of the Irradiation (min) | The Initial Concentration of Bacteria (CFC/mL or UFC/mL) | Inactivation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Ag/TiO2 | nanoparticles | Template induction | - | 106 | 100 | [60] |
| Cu/TiO2 | particles | Sol–gel | - | - | 99.9 | [59] |
| Au/TiO2 | nanotubes | Magnetron sputtering | - | 105 | <50 | [58] |
| Pt–TiO2 | nanotubes | Photodeposition | - | 105 | <40 | [58] |
| F-ZnO | particles | Sol–gel | 360 | - | 99.9 | [61] |
| Ti- BiOI | particles | Solvothermal method | 45 | 3 × 106 | 100 | [62] |
| AgI@MnO2 | particles | Deposition | 25 | 99.4 | [63] | |
| MoS2/TiO2 | nanotubes | Two-step anodization and hydrothermal method | 150 | >108 | 100 | [18] |
| g-C3N4-V-TiO2 | particles | Hydrothermal calcination | 60 | - | 99.5 | [64] |
| ZnCl2/TiO2 | nanoparticles | Sol–gel calcination | 120 | 105–106 | >90 | [65] |
| Zn(Ac)2/TiO2 | nanoparticles | Sol–gel calcination | 120 | 105–106 | >80 | [65] |
| Zn(NO3)2/TiO2 | nanoparticles | Sol–gel calcination | 120 | 105–106 | >95 | [65] |
| ZnSO4/TiO2 | nanoparticles | Sol–gel calcination | 120 | 105–106 | 100 | [65] |
| TiO2–SiO2 | nanocomposite | Sonochemistry method | 120 | - | 98.6 | [66] |
| Cu–TiO2/GF | particles | Immersion–drying process | - | - | 67.49 | [67] |
| g-C3N4/Cu@CdS | nanocomposite | Co-precipitation method | - | - | 40 | [68] |
| TiO2/Ag2O/Au | nanotubes | One-step anodization | 60 | 103–104 | 99.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Mazierski, P.; Żebrowska, J.; Klimczuk, T.; Lisowski, W.; Żak, A.M.; Skowron, P.M.; Zaleska-Medynska, A. Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts. Nanomaterials 2024, 14, 409. https://doi.org/10.3390/nano14050409
Kozak M, Mazierski P, Żebrowska J, Klimczuk T, Lisowski W, Żak AM, Skowron PM, Zaleska-Medynska A. Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts. Nanomaterials. 2024; 14(5):409. https://doi.org/10.3390/nano14050409
Chicago/Turabian StyleKozak, Magda, Paweł Mazierski, Joanna Żebrowska, Tomasz Klimczuk, Wojciech Lisowski, Andrzej M. Żak, Piotr M. Skowron, and Adriana Zaleska-Medynska. 2024. "Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts" Nanomaterials 14, no. 5: 409. https://doi.org/10.3390/nano14050409
APA StyleKozak, M., Mazierski, P., Żebrowska, J., Klimczuk, T., Lisowski, W., Żak, A. M., Skowron, P. M., & Zaleska-Medynska, A. (2024). Detailed Insight into Photocatalytic Inactivation of Pathogenic Bacteria in the Presence of Visible-Light-Active Multicomponent Photocatalysts. Nanomaterials, 14(5), 409. https://doi.org/10.3390/nano14050409









