Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus globulus Leaf Extract on Pinus radiata Sawdust for Cationic Dye Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Phenolic Compounds
2.2. Synthesis of Iron Nanoparticles on Sawdust
2.3. Characterization Techniques
2.4. Design of Experiments
2.4.1. The Plackett–Burman Design
2.4.2. Steepest Ascent Test
2.4.3. Box–Behnken Design
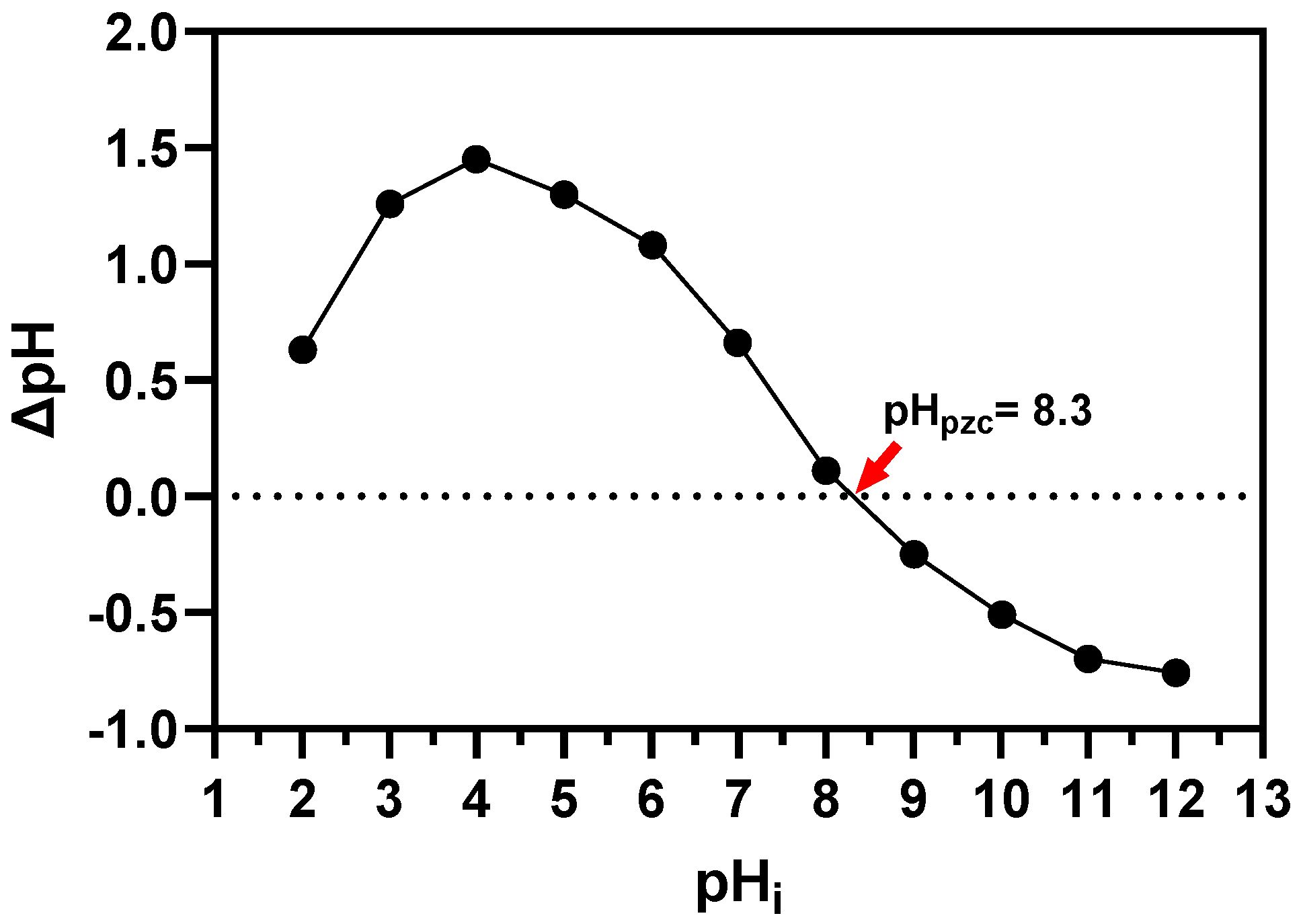
2.5. pHPZC Determination
2.6. Profile of RhB Adsorption by Fe2O3@PS
2.7. Reusability of Fe2O3@PS
3. Results
3.1. Synthesis and Characterization of Fe2O3 Nanoparticles
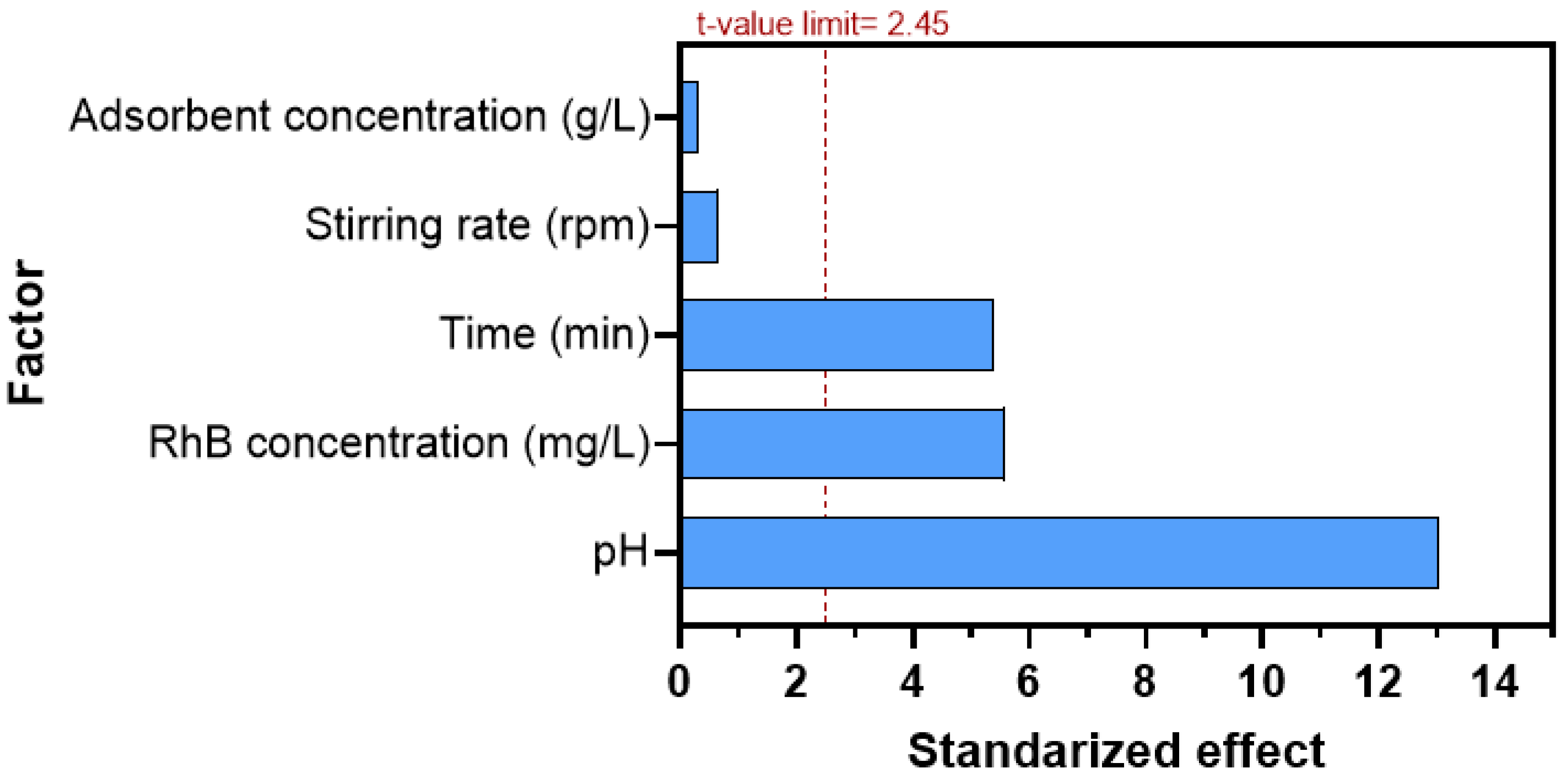
3.2. Selection of Significant Variables for RhB Adsorption Using the Plackett–Burman Design (PBD)
3.3. Determination of the Central Point Levels by the Steepest Ascent Experiments
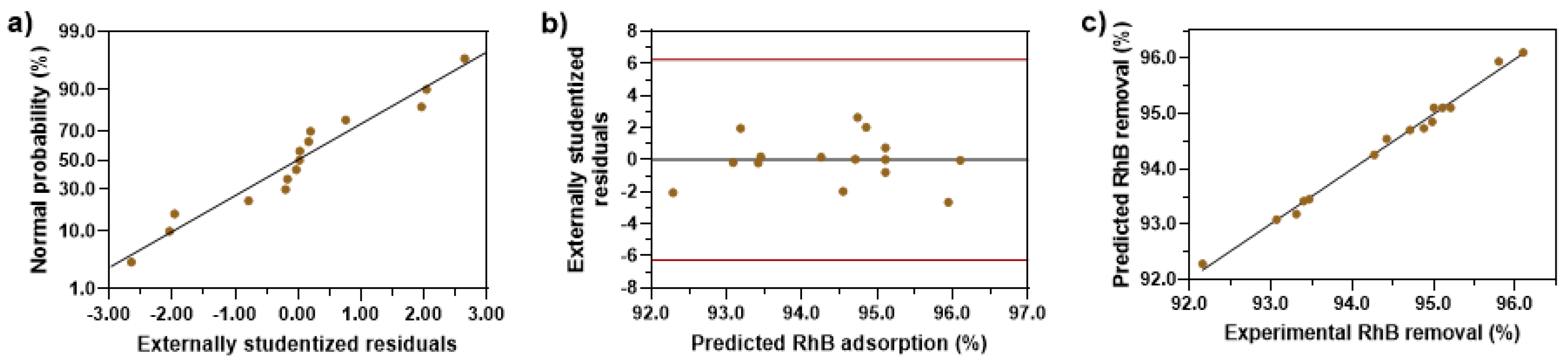
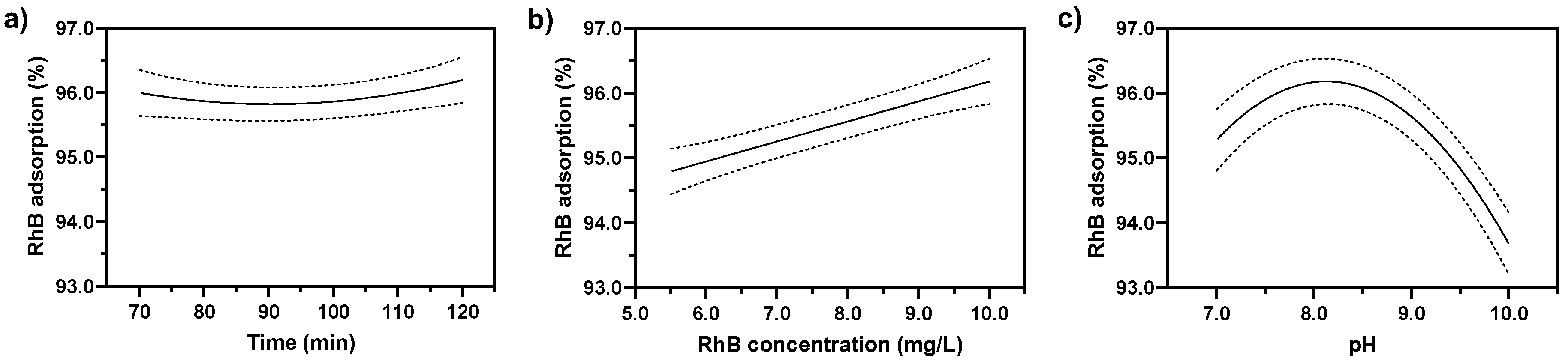
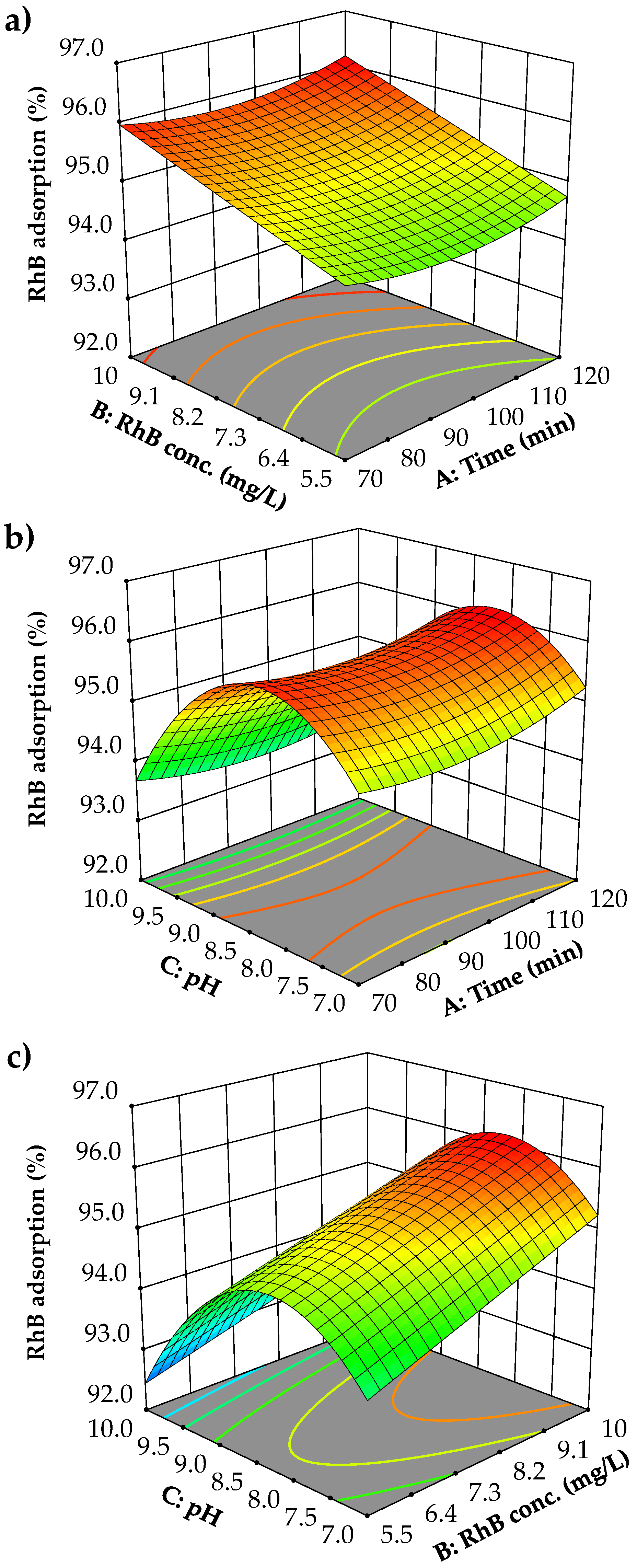
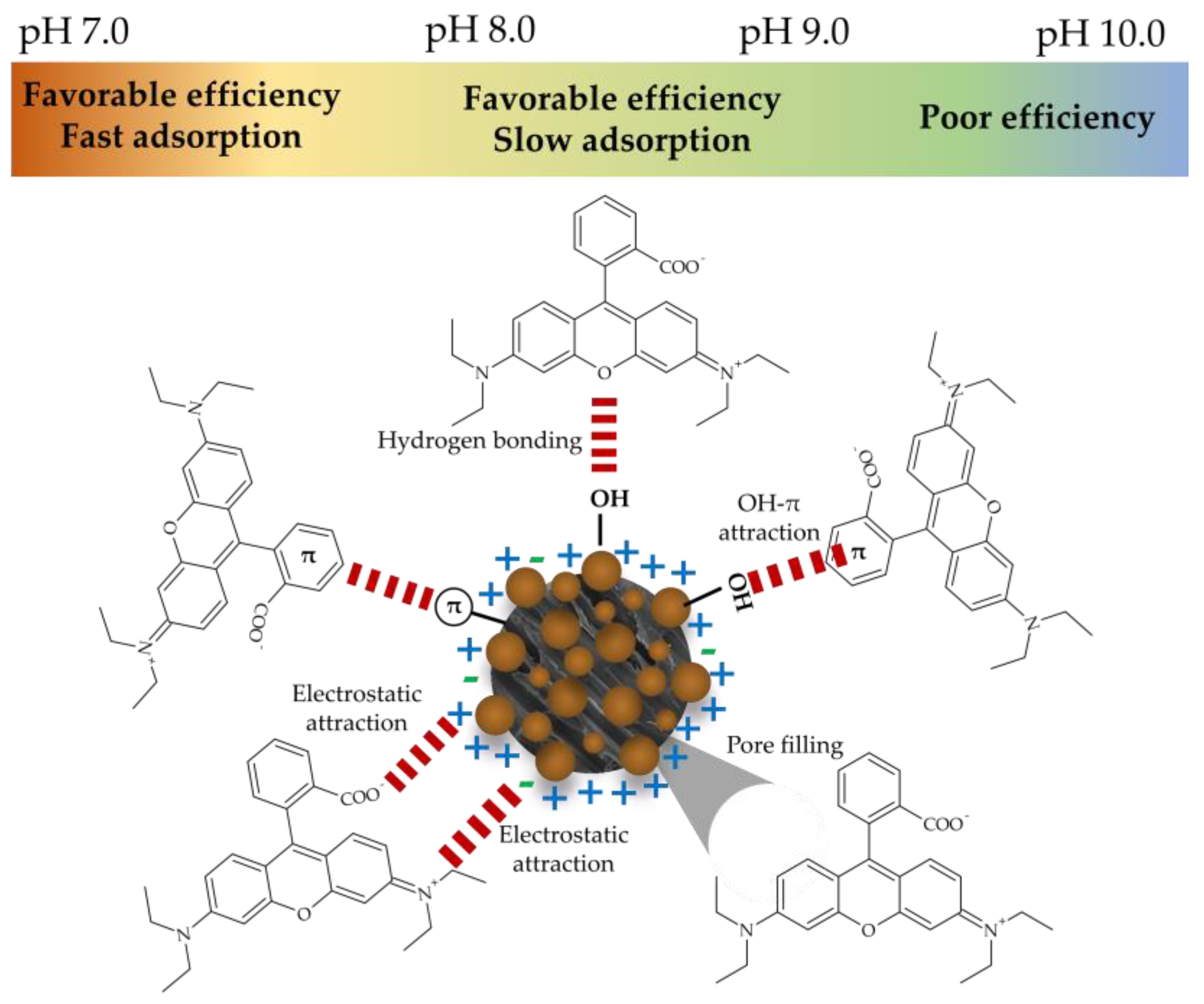
3.4. Response Surface Analysis by the BBD to Determine Optimal Conditions
3.5. Optimization of Parameter Combinations and Verification
3.6. Reusability
3.7. Profile of RhB Adsorption Efficiency by Fe2O3@PS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Gayathri, R.; Rathi, B.S. A review on adsorptive separation of toxic metals from aquatic system using biochar produced from agro-waste. Chemosphere 2021, 285, 131438. [Google Scholar] [CrossRef] [PubMed]
- Alderete, B.L.; da Silva, J.; Godoi, R.; da Silva, F.R.; Taffarel, S.R.; da Silva, L.P.; Garcia, A.L.H.; Júnior, H.M.; de Amorim, H.L.N.; Picada, J.N. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after Advanced Oxidation Process treatment. Chemosphere 2021, 263, 128291. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.P.; Thiruvengadaravi, K.V.; Senthil Kumar, P.; Nandagopal, J.; Sivanesan, S. Eco-Friendly Treatment Strategies for Wastewater Containing Dyes and Heavy Metals. In Environmental Contaminants: Measurement, Modelling and Control; Gupta, T., Agarwal, A.K., Agarwal, R.A., Labhsetwar, N.K., Eds.; Springer: Singapore, 2018; pp. 317–360. [Google Scholar]
- Shakir, K.; Elkafrawy, A.F.; Ghoneimy, H.F.; Elrab Beheir, S.G.; Refaat, M. Removal of rhodamine B (a basic dye) and thoron (an acidic dye) from dilute aqueous solutions and wastewater simulants by ion flotation. Water Res. 2010, 44, 1449–1461. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Jabbar, K.Q.; Barzinjy, A.A.; Hamad, S.M. Iron oxide nanoparticles: Preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100661. [Google Scholar] [CrossRef]
- Li, Y.; Yin, H.; Cai, Y.; Luo, H.; Yan, C.; Dang, Z. Regulating the exposed crystal facets of α-Fe2O3 to promote Fe2O3-modified biochar performance in heavy metals adsorption. Chemosphere 2023, 311, 136976. [Google Scholar] [CrossRef]
- Salgado, P.; Mártire, D.O.; Vidal, G. Eucalyptus extracts-mediated synthesis of metallic and metal oxide nanoparticles: Current status and perspectives. Mater. Res. Express 2019, 6, 082006. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Baabu, P.R.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B. Iron oxide nanoparticles: A review on the province of its compounds, properties and biological applications. Materials 2023, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Márquez, K.; Rubilar, O.; Contreras, D.; Vidal, G. The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity. Appl. Nanosci. 2019, 9, 371–385. [Google Scholar] [CrossRef]
- Kumar Prajapati, A.; Kumar Mondal, M. Green synthesis of Fe3O4-onion peel biochar nanocomposites for adsorption of Cr(VI), methylene blue and congo red dye from aqueous solutions. J. Mol. Liq. 2022, 349, 118161. [Google Scholar] [CrossRef]
- Selvaraj, R.; Pai, S.; Vinayagam, R.; Varadavenkatesan, T.; Kumar, P.S.; Duc, P.A.; Rangasamy, G. A recent update on green synthesized iron and iron oxide nanoparticles for environmental applications. Chemosphere 2022, 308, 136331. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Biochar-supported nanomaterials for environmental applications. J. Ind. Eng. Chem. 2019, 78, 21–33. [Google Scholar] [CrossRef]
- Alipour Atmianlu, P.; Badpa, R.; Aghabalaei, V.; Baghdadi, M. A review on the various beds used for immobilization of nanoparticles: Overcoming the barrier to nanoparticle applications in water and wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106514. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Guerrouache, M.; Carbonnier, B.; Chehimi, M.M. Polymer-immobilized nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 43–68. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishra, G.K.; Rajagopal, C.; Nagar, P.N. Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 2008, 150, 604–611. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdellatif, F.H.H.; Hasanin, M.S.; Abdellatif, M.M. Fabrication, characterization, and potential application of modified sawdust sorbents for efficient removal of heavy metal ions and anionic dye from aqueous solutions. J. Clean. Prod. 2022, 332, 130021. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V.K. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 208, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the removal of heavy metals from water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, K.A.; Adesina, O.O.; Okon-Akan, O.A.; Adegoke, O.R.; Olabintan, A.B.; Ajala, O.A.; Olagoke, H.; Maxakato, N.W.; Bello, O.S. Sawdust-biomass based materials for sequestration of organic and inorganic pollutants and potential for engineering applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100274. [Google Scholar] [CrossRef]
- Labbé, R.; Niklitschek, M.; Contreras, M. Effect of climate change on the land rent of radiata pine plantations in Chile: Site productivity and forest fires. For. Policy Econ. 2023, 156, 103068. [Google Scholar] [CrossRef]
- Santos, J.; Escobar-Avello, D.; Fuentealba, C.; Cabrera-Barjas, G.; González-Álvarez, J.; Martins, J.M.; Carvalho, L.H. Forest by-product valorization: Pilot-scale Pinus radiata and Eucalyptus globulus bark mixture extraction. Forests 2023, 14, 895. [Google Scholar] [CrossRef]
- Salgado, P.; Márquez, K.; Vidal, G. Biogenic synthesis based on cuprous oxide nanoparticles using Eucalyptus globulus extracts and its effectiveness for removal of recalcitrant compounds. Catalysts 2024, 14, 525. [Google Scholar] [CrossRef]
- Venkataraghavan, R.; Thiruchelvi, R.; Sharmila, D. Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using Plackett-Burman design and response surface methodology. Heliyon 2020, 6, e05219. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Arce, E.; Cartelle, A.; Suárez-García, A. Use of Plackett–burman and box–behnken designs to optimize bioelectricity production from winery residues. Water 2023, 15, 3051. [Google Scholar] [CrossRef]
- Ben, Z.; Sun, X.; Bai, Y.; Yang, D.; Chen, K.; Dong, Y. Parameter calibration of discrete element model for gluten densification molding. J. Food Sci. 2024, 89, 3700–3712. [Google Scholar] [CrossRef]
- Salgado, P.; Frontela, J.L.; Vidal, G. Optimization of fenton technology for recalcitrant compounds and bacteria inactivation. Catalysts 2020, 10, 1483. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, M.; Karimzadeh, F.; Raeissi, K.; Giannakis, S.; Pulgarin, C. Improving visible light photocatalytic inactivation of E. coli by inducing highly efficient radical pathways through peroxymonosulfate activation using 3-D, surface-enhanced, reduced graphene oxide (rGO) aerogels. Chem. Eng. J. 2020, 396, 125189. [Google Scholar] [CrossRef]
- Chihi, S.; Bouafia, A.; Meneceur, S.; Laouini, S.E.; Ahmed, R.Z. Effect of precursor concentration on the bandgap energy and particles size for green synthesis of hematite α-Fe2O3 nanoparticles by the aqueous extract of Moltkia ciliata and evaluation of the antibacterial activity. Biomass Convers. Biorefinery 2023, 1–14. [Google Scholar] [CrossRef]
- Ibrahim, J.E.F.M.; Tihtih, M.; Gömze, L.A. Environmentally-friendly ceramic bricks made from zeolite-poor rock and sawdust. Constr. Build. Mater. 2021, 297, 123715. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Yang, A.L.; Li, S.P.; Wang, Y.J.; Wang, L.L.; Bao, X.C.; Yang, R.Q. Fabrication of Cu2O@Cu2O core–shell nanoparticles and conversion to Cu2O@Cu core–shell nanoparticles in solution. Trans. Nonferrous Met. Soc. China 2015, 25, 3643–3650. [Google Scholar] [CrossRef]
- Benyoucef, S.; Harrache, D.; Djaroud, S.; Sail, K.; Gallart-Mateu, D.; de la Guardia, M. Preparation and characterization of novel microstructure cellulosic sawdust material: Application as potential adsorbent for wastewater treatment. Cellulose 2020, 27, 8169–8180. [Google Scholar] [CrossRef]
- Ahamad, Z.; Ahmed, M.; Mashkoor, F.; Nasar, A. Chemically modified Azadirachta indica sawdust for adsorption of methylene blue from aqueous solutions. Biomass Convers. Biorefinery 2024, 14, 19929–19946. [Google Scholar] [CrossRef]
- Rahman, N.u.; Ullah, I.; Alam, S.; Khan, M.S.; Shah, L.A.; Zekker, I.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Vincevica-Gaile, Z.; et al. Activated ailanthus altissima sawdust as adsorbent for removal of acid yellow 29 from wastewater: Kinetics approach. Water 2021, 13, 2136. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Quaranta, N.; Gass, S.; Procaccini, R.; Cristóbal, A. Ceramic bricks containing Ni ions from contaminated biomass used as an adsorbent. Sustain. Environ. Res. 2020, 30, 26. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef]
- Gopal, R.A.; Song, M.; Yang, D.; Lkhagvaa, T.; Chandrasekaran, S.; Choi, D. Synthesis of hierarchically structured ɤ-Fe2O3–PPy nanocomposite as effective adsorbent for cationic dye removal from wastewater. Environ. Pollut. 2020, 267, 115498. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Liu, J.; Zeng, M.-J.; Feng, X.; Jia, X.; Yu, Z.-Z. Coating of wood with Fe2O3-decorated carbon nanotubes by one-step combustion for efficient solar steam generation. ACS Appl. Mater. Interfaces 2021, 13, 22845–22854. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Zhou, J.; Wu, R. Structure and properties of cellulose/Fe2O3 nanocomposite fibers spun via an effective pathway. J. Phys. Chem. C 2008, 112, 4538–4544. [Google Scholar] [CrossRef]
- Phan, A.D.T.; D’Arcy, B.R.; Gidley, M.J. Polyphenol–cellulose interactions: Effects of pH, temperature and salt. Int. J. Food Sci. Technol. 2016, 51, 203–211. [Google Scholar] [CrossRef]
- Beyaz, K.; Abdi, Y.; Bagtache, R.; Trari, M.; Benaboura, A. Hematite (Fe2O3)-modified biopolymer for Rhodamine B degradation under visible light. Int. J. Polym. Anal. Charact. 2024, 29, 496–507. [Google Scholar] [CrossRef]
- Hosny, N.M.; Sherif, Y.E. Synthesis, optical band gap and anti-rheumatic activity of Fe2O3 nanocrystals via solid state decomposition of 4-aminophenol precursor. Chem. Data Collect. 2022, 37, 100813. [Google Scholar] [CrossRef]
- Sulaiman, S.; Azis, R.a.S.; Ismail, I.; Man, H.C.; Yusof, K.F.M.; Abba, M.U.; Katibi, K.K. Adsorptive removal of copper (ii) ions from aqueous solution using a magnetite nano-adsorbent from mill scale waste: Synthesis, characterization, adsorption and kinetic modelling studies. Nanoscale Res. Lett. 2021, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.A.; Abidin, Z.Z.; Pudza, M.Y.; Zentou, H. Efficient removal of Cu(II) from aqueous systems using enhanced quantum yield nitrogen-doped carbon nanodots. RSC Adv. 2020, 10, 14979–14990. [Google Scholar] [CrossRef]
- Ghani, U.; Hussain, S.; Imtiaz, M.; Khan, S.A. Laterite clay-based geopolymer as a potential adsorbent for the heavy metals removal from aqueous solutions. J. Saudi Chem. Soc. 2020, 24, 874–884. [Google Scholar] [CrossRef]
- Park, S.-K.; Shin, H. Effect of HCl and H2SO4 treatment of TiO2 powder on the photosensitized degradation of aqueous rhodamine b under visible light. J. Nanosci. Nanotechnol. 2014, 14, 8122–8128. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Huang, H.; Cao, W.; Li, T. Removal of rhodamine B and Cr(VI) from aqueous solutions by a polyoxometalate adsorbent. Chem. Eng. Res. Des. 2015, 100, 192–202. [Google Scholar] [CrossRef]
- Danchenko, Y.; Andronov, V.; Rybka, E.; Skliarov, S. Investigation into acid-basic equilibrium on the surface of oxides with various chemical nature. East. -Eur. J. Enterp. Technol. 2017, 4, 17–25. [Google Scholar] [CrossRef][Green Version]
- Danchenko, Y.; Andronov, V.; Barabash, E.; Rybka, E.; Khmyrova, A. Acid-basic surface properties of dispersed fillers based on metal oxides TiO2, Al2O3, CaO and Fe2O3. IOP Conf. Ser. Mater. Sci. Eng. 2019, 708, 012083. [Google Scholar] [CrossRef]
- Karpova, S.S.; Moshnikov, V.A.; Maksimov, A.I.; Mjakin, S.V.; Kazantseva, N.E. Study of the effect of the acid-base surface properties of ZnO, Fe2O3 and ZnFe2O4 oxides on their gas sensitivity to ethanol vapor. Semiconductors 2013, 47, 1026–1030. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Haouti, R.; El Guerdaoui, A.; Haounati, R.; Amaterz, E.; Addi, A.A.; Akbal, F.; Taha, M.L. Experimental and molecular dynamics simulation study on the adsorption of Rhodamine B dye on magnetic montmorillonite composite γ-Fe2O3@Mt. J. Mol. Liq. 2020, 309, 113142. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y. Removal of gentian violet and rhodamine B using banyan aerial roots after modification and mechanism studies of differential adsorption behaviors. Environ. Sci. Pollut. Res. 2020, 27, 9152–9166. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Purwiandono, G.; Hidayat, A.; Sagadevan, S.; Kamari, A. Mechanistic insight into the adsorption and photocatalytic activity of a magnetically separable γ-Fe2O3/Montmorillonite nanocomposite for rhodamine B removal. Chem. Phys. Lett. 2022, 792, 139410. [Google Scholar] [CrossRef]
- Cavali, M.; Soccol, C.R.; Tavares, D.; Zevallos Torres, L.A.; Oliveira de Andrade Tanobe, V.; Zandoná Filho, A.; Woiciechowski, A.L. Effect of sequential acid-alkaline treatment on physical and chemical characteristics of lignin and cellulose from pine (Pinus spp.) residual sawdust. Bioresour. Technol. 2020, 316, 123884. [Google Scholar] [CrossRef] [PubMed]
- Hacıosmanoğlu, G.G.; Doğruel, T.; Genç, S.; Oner, E.T.; Can, Z.S. Adsorptive removal of bisphenol A from aqueous solutions using phosphonated levan. J. Hazard. Mater. 2019, 374, 43–49. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, S.; Huang, T.; Cui, F.; Xing, B. pH-Dependent adsorption of aromatic compounds on graphene oxide: An experimental, molecular dynamics simulation and density functional theory investigation. J. Hazard. Mater. 2020, 395, 122680. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef]

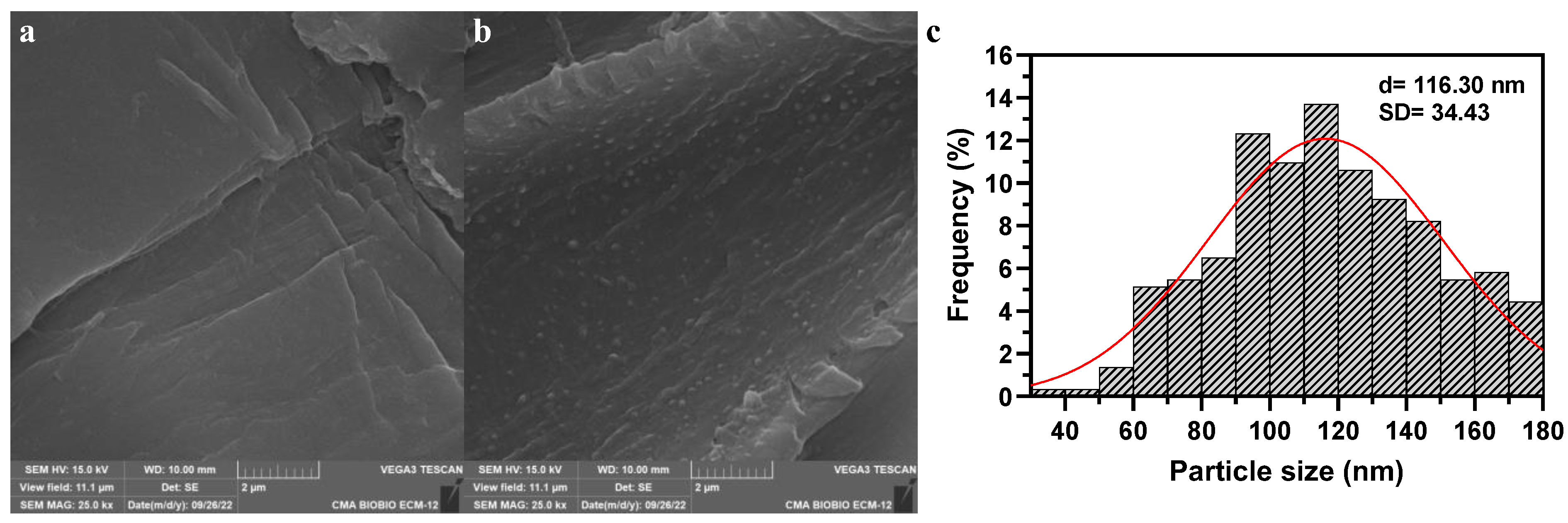
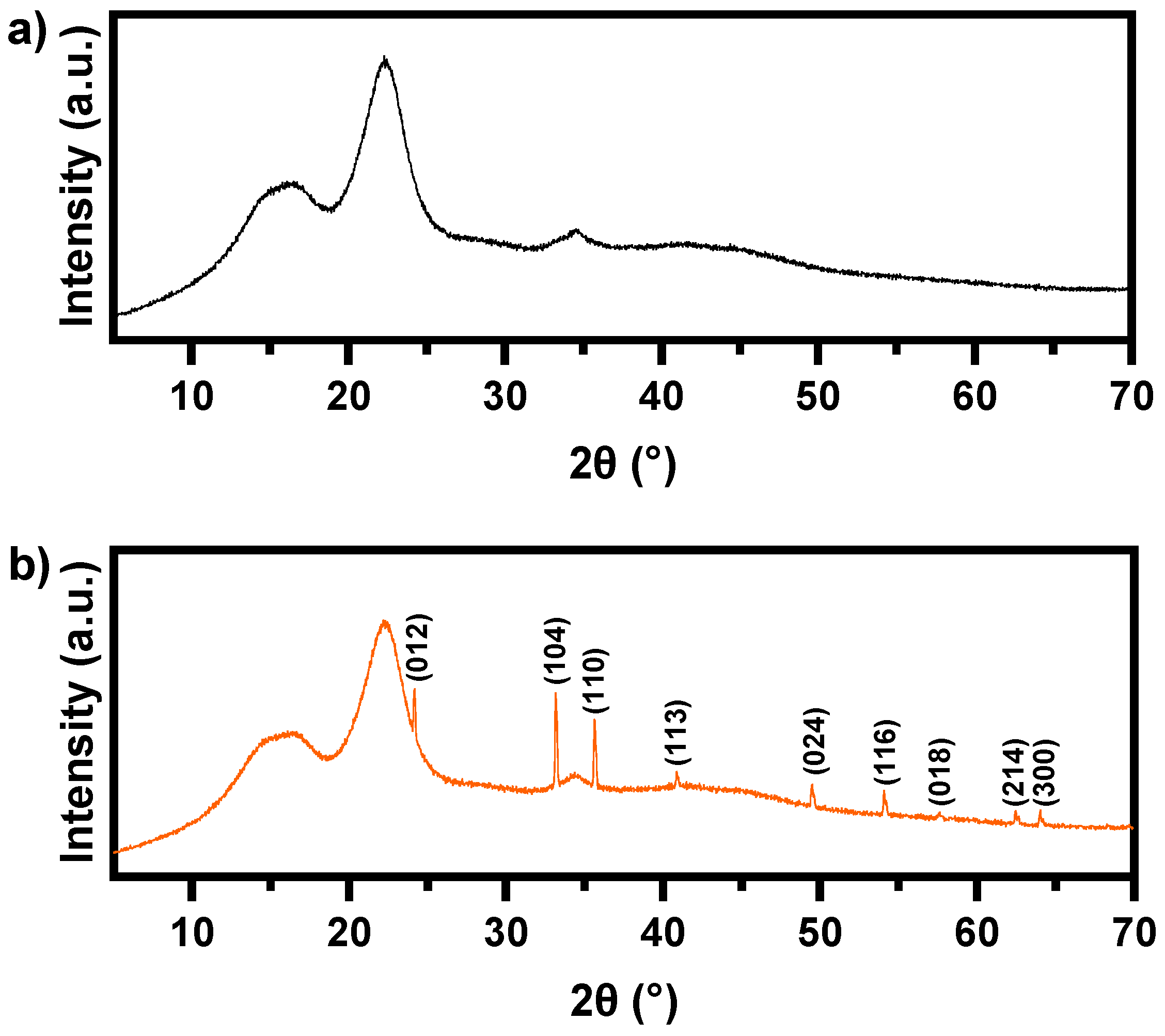
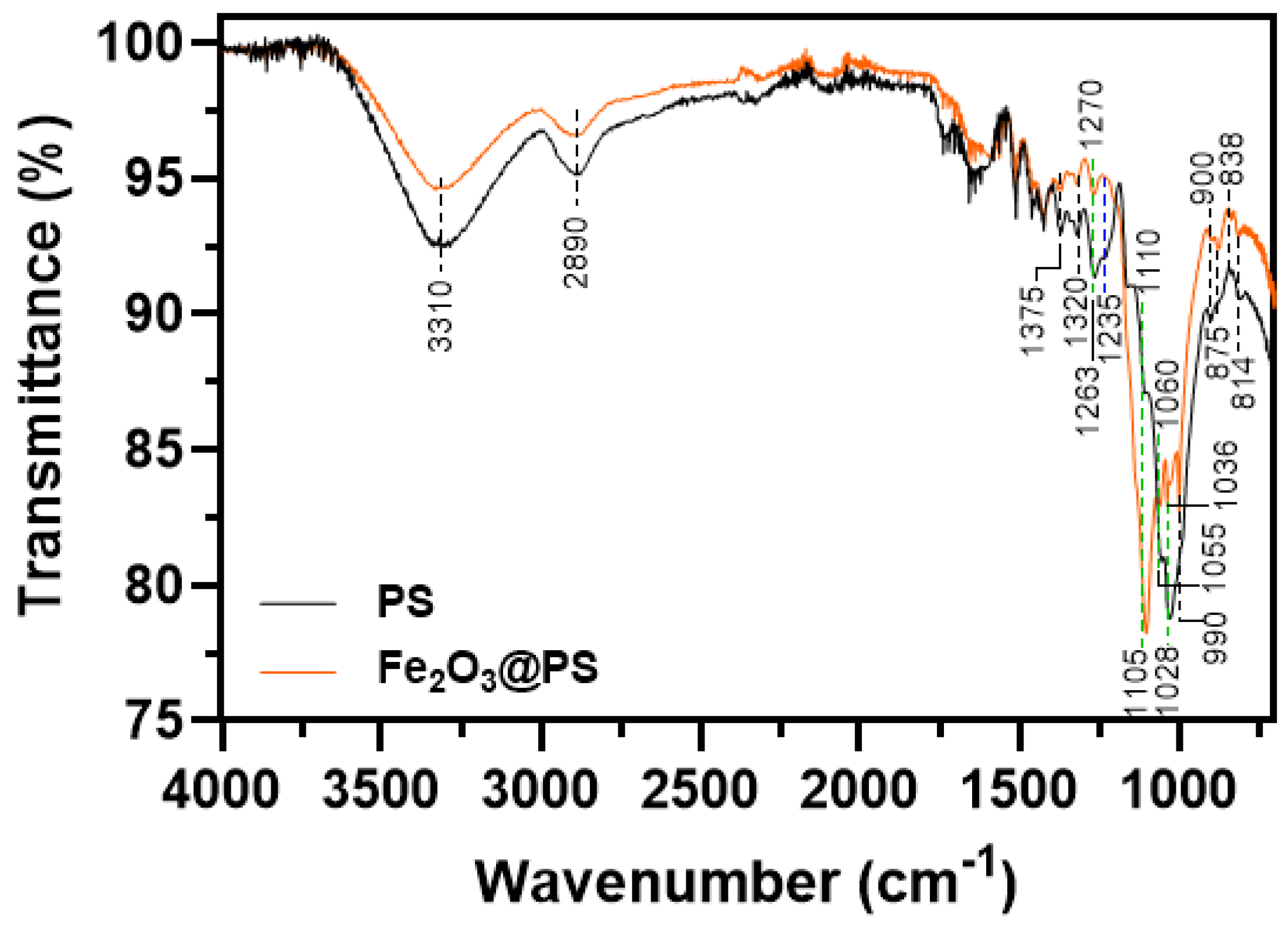
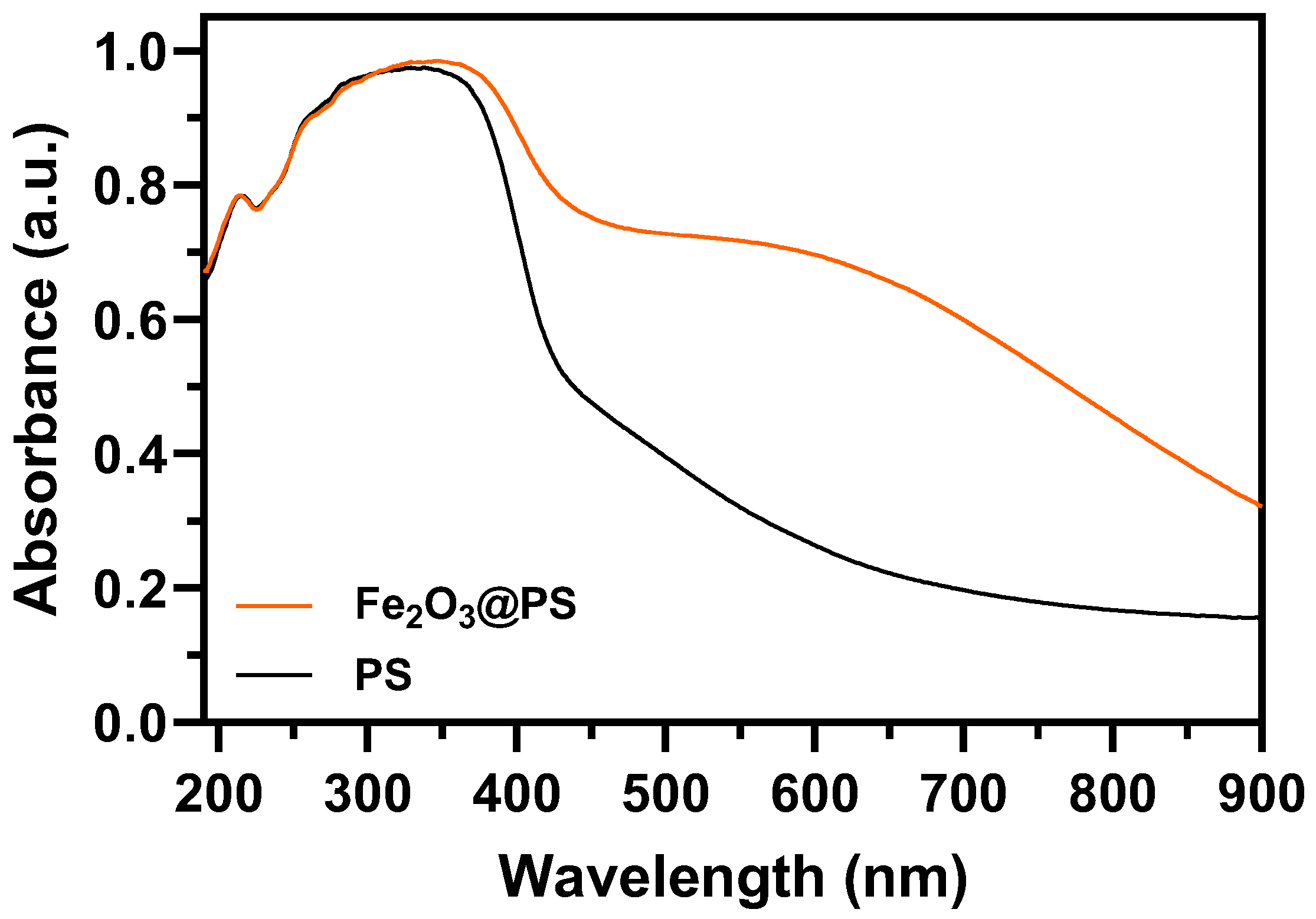


| Parameters | Units | Experimental Value | |
|---|---|---|---|
| Low (−) | High (+) | ||
| pH | - | 4 | 10 |
| RhB concentration | mg/L | 1 | 10 |
| Fe2O3@PS dose | g/L | 0.5 | 10 |
| Stirring rate | rpm | 200 | 1000 |
| Time | Minutes | 20 | 120 |
| Variables | Nomenclature | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Time (min) | A | 70.0 | 95.0 | 120.0 |
| RhB concentration (mg/L) | B | 5.50 | 7.75 | 10.00 |
| pH | C | 7.0 | 8.5 | 10.0 |
| Wavenumber (cm−1) | Vibration | Reference | |
|---|---|---|---|
| Pristine PS | Fe2O3@PS | ||
| 3310 | 3310 | Inter-molecular hydrogen bonding C(3)OH⋯C(6)O | [37] |
| 2890 | 2890 | Asymmetric stretching vibration of –CH2 | [38] |
| 1375 | 1375 | C–O vibration of lignin methoxy groups | [39] |
| 1320 | 1320 | C–O vibration of lignin methoxy groups | [39] |
| 1263 | 1270 | C–O stretching of the phenolic group | [40] |
| 1235 | - | C–O stretching vibration of hemicellulose | [41] |
| 1110 | 1105 | C–OH bending of lignin | [42] |
| 1055 | 1060 | C–O stretching vibration of cellulose | [37] |
| 1028 | 1036 | C–OH groups of cellulose | [37] |
| 990 | 990 | C–O and ring stretching modes of cellulose | [37] |
| 900 | 900 | Deformation of –C1–O–C4 in β-glycosidic bond of cellulose | [43] |
| 875 | 875 | Aromatic C–H bond of lignin | [25] |
| 838 | 838 | Glycosidic bond | [44] |
| 814 | 814 | Plane bending of =C–H | [45] |
| # | Variables | |||||
|---|---|---|---|---|---|---|
| pH | RhB Conc. (mg/L) | Fe2O3@PS Dose (g/L) | Stirring Rate (rpm) | Time (min) | RhB Adsorption (%) | |
| 1 | 4.0 | 1.0 | 10.0 | 1000 | 120 | 61.91 |
| 2 | 4.0 | 10.0 | 0.5 | 200 | 20 | 62.99 |
| 3 | 10.0 | 1.0 | 0.5 | 200 | 120 | 89.73 |
| 4 | 4.0 | 10.0 | 10.0 | 200 | 120 | 67.42 |
| 5 | 10.0 | 10.0 | 10.0 | 200 | 120 | 99.53 |
| 6 | 10.0 | 10.0 | 0.5 | 1000 | 20 | 87.25 |
| 7 | 4.0 | 1.0 | 0.5 | 200 | 20 | 41.44 |
| 8 | 4.0 | 1.0 | 0.5 | 1000 | 120 | 59.88 |
| 9 | 4.0 | 10.0 | 10.0 | 1000 | 20 | 62.36 |
| 10 | 10.0 | 10.0 | 10.0 | 1000 | 20 | 76.43 |
| 11 | 10.0 | 1.0 | 0.5 | 1000 | 120 | 96.96 |
| 12 | 10.0 | 10.0 | 10.0 | 200 | 20 | 74.96 |
| Term | Sum of Squares | df | F-Value | t-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3226.32 | 5 | 46.14 | 68.00 | <0.0001 |
| pH | 2376.14 | 1 | 169.93 | 13.04 | <0.0001 |
| RhB concentration (mg/L) | 433.92 | 1 | 31.03 | 5.57 | <0.001 |
| Adsorbent concentration (g/L) | 1.58 | 1 | 0.11 | 0.34 | 0.748 |
| Stirring rate (rpm) | 6.34 | 1 | 0.45 | 0.67 | 0.526 |
| Time (min) | 408.33 | 1 | 29.20 | 5.40 | 0.002 |
| Error | 83.90 | 6 | - | - | - |
| Variables | 0 (1) | 0 + 1∆ (2) | 0 + 2∆ (3) | 0 + 3∆ (4) | 0 + 4∆ (5) |
|---|---|---|---|---|---|
| Time (min) | 20 | 45 | 70 | 95 | 120 |
| RhB concentration (mg/L) | 1.00 | 3.25 | 5.50 | 7.75 | 10.00 |
| pH | 4.00 | 5.50 | 7.00 | 8.50 | 10.00 |
| RhB adsorption (%) | 51.73 | 55.13 | 77.94 | 89.09 | 87.3 |
| # | Variables | Experimental Response | Predicted Response | ||
|---|---|---|---|---|---|
| Time (min) | RhB Concentration (mg/L) | pH | RhB Adsorption (%) | RhB Adsorption (%) | |
| 1 | 70 (−1) | 5.50 (−1) | 8.5 (0) | 94.71 | 94.71 |
| 2 | 120 (+1) | 5.50 (−1) | 8.5 (0) | 94.88 | 94.74 |
| 3 | 70 (−1) | 10.00 (+1) | 8.5 (0) | 95.80 | 95.94 |
| 4 | 120 (+1) | 10.00 (+1) | 8.5 (0) | 96.10 | 96.10 |
| 5 | 70 (−1) | 7.75 (0) | 7.0 (−1) | 94.27 | 94.26 |
| 6 | 120 (+1) | 7.75 (0) | 7.0 (−1) | 94.42 | 94.55 |
| 7 | 70 (−1) | 7.75 (0) | 10.0 (+1) | 93.31 | 93.19 |
| 8 | 120 (+1) | 7.75 (0) | 10.0 (+1) | 93.07 | 93.08 |
| 9 | 95 (0) | 5.50 (−1) | 7.0 (−1) | 93.40 | 93.42 |
| 10 | 95 (0) | 10.00 (+1) | 7.0 (−1) | 94.98 | 94.85 |
| 11 | 95 (0) | 5.50 (−1) | 10.0 (+1) | 92.16 | 92.29 |
| 12 | 95 (0) | 10.00 (+1) | 10.0 (+1) | 93.47 | 93.45 |
| 13 | 95 (0) | 7.75 (0) | 8.5 (0) | 95.00 | 95.11 |
| 14 | 95 (0) | 7.75 (0) | 8.5 (0) | 95.21 | 95.11 |
| 15 | 95 (0) | 7.75 (0) | 8.5 (0) | 95.11 | 95.11 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Observations |
|---|---|---|---|---|---|---|
| Model | 16.76 | 9 | 1.86 | 73.03 | <0.0001 | significant |
| A | 0.0180 | 1 | 0.0180 | 0.7078 | 0.4386 | not significant |
| B | 3.38 | 1 | 3.38 | 132.53 | <0.0001 | significant |
| C | 3.20 | 1 | 3.20 | 125.49 | <0.0001 | significant |
| AB | 0.0042 | 1 | 0.0042 | 0.1657 | 0.7008 | not significant |
| AC | 0.0380 | 1 | 0.0380 | 1.49 | 0.2765 | not significant |
| BC | 0.0182 | 1 | 0.0182 | 0.7146 | 0.4365 | not significant |
| A2 | 0.2601 | 1 | 0.2601 | 10.20 | 0.0242 | significant |
| B2 | 6.41 × 10−7 | 1 | 6.41 × 10−7 | 0.0000 | 0.9962 | not significant |
| C2 | 9.51 | 1 | 9.51 | 372.76 | <0.0001 | significant |
| Residual | 0.1275 | 5 | 0.0255 | - | ||
| Lack of fit | 0.1055 | 3 | 0.0352 | 3.19 | 0.2480 | not significant |
| Pure error | 0.0221 | 2 | 0.0110 | - | ||
| Cor total | 16.89 | 14 | - | - |
| Time (min) | RhB Concentration (mg/L) | pH | Predicted Response (%) | Experimental Response (%) | Error | |
|---|---|---|---|---|---|---|
| Conditions 1 | 119.3 | 9.99 | 8.20 | 96.18 | 96.25 | 0.07 |
| Conditions 2 | 70.0 | 10.00 | 7.00 | 94.94 | 95.05 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, P.; Aedo, E.; Vidal, G. Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus globulus Leaf Extract on Pinus radiata Sawdust for Cationic Dye Adsorption. Nanomaterials 2024, 14, 1832. https://doi.org/10.3390/nano14221832
Salgado P, Aedo E, Vidal G. Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus globulus Leaf Extract on Pinus radiata Sawdust for Cationic Dye Adsorption. Nanomaterials. 2024; 14(22):1832. https://doi.org/10.3390/nano14221832
Chicago/Turabian StyleSalgado, Pablo, Eduardo Aedo, and Gladys Vidal. 2024. "Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus globulus Leaf Extract on Pinus radiata Sawdust for Cationic Dye Adsorption" Nanomaterials 14, no. 22: 1832. https://doi.org/10.3390/nano14221832
APA StyleSalgado, P., Aedo, E., & Vidal, G. (2024). Green Synthesis of Fe2O3 Nanoparticles Using Eucalyptus globulus Leaf Extract on Pinus radiata Sawdust for Cationic Dye Adsorption. Nanomaterials, 14(22), 1832. https://doi.org/10.3390/nano14221832





