Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Samples
2.2.1. Preparation of Nitrogen-Doped Carbon Nanosheets (N-CNS)
2.2.2. Preparation of Nitrogen-Doped Carbon Nanosheets-Supported Palladium Catalyst (Pd/N-CNS)
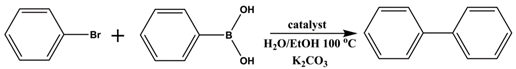
2.3. Catalytic Performance for Suzuki Cross-Coupling Reactions
2.4. Characterization
2.5. DFT Calculations
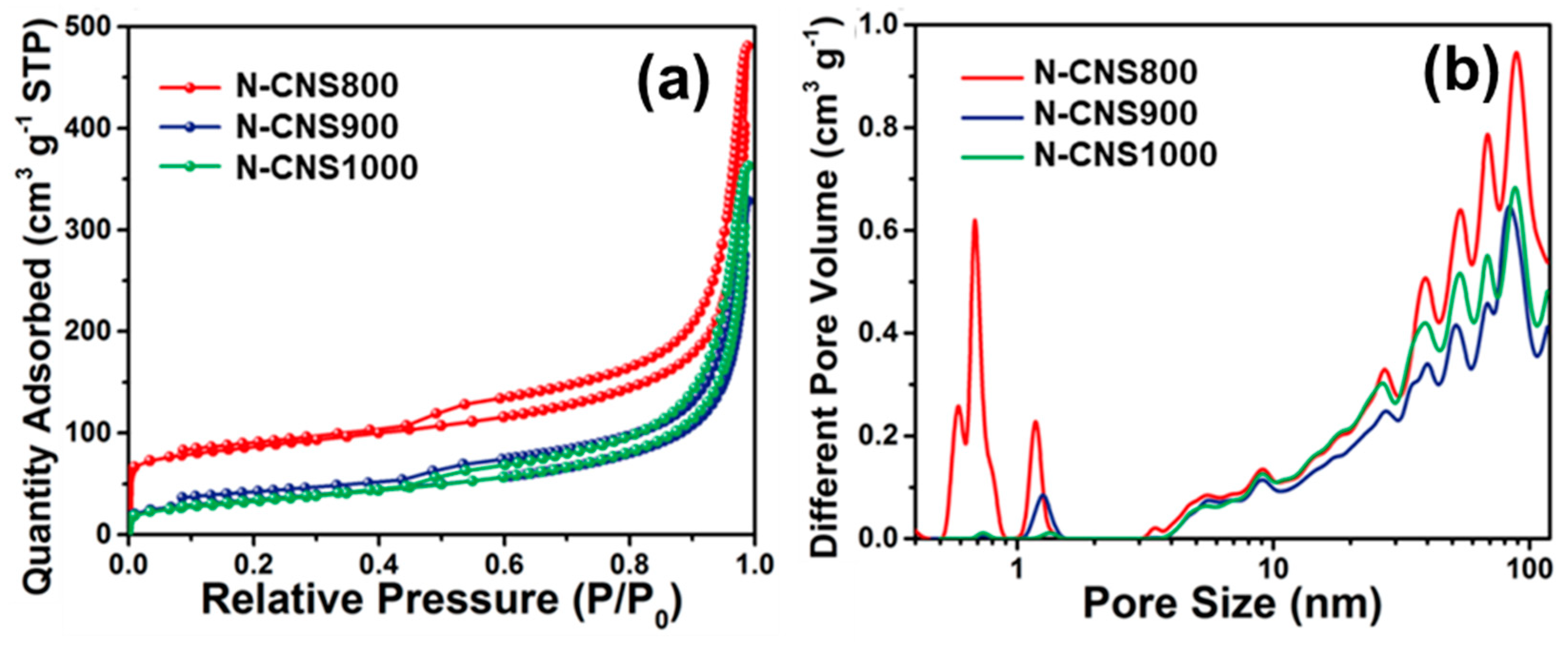
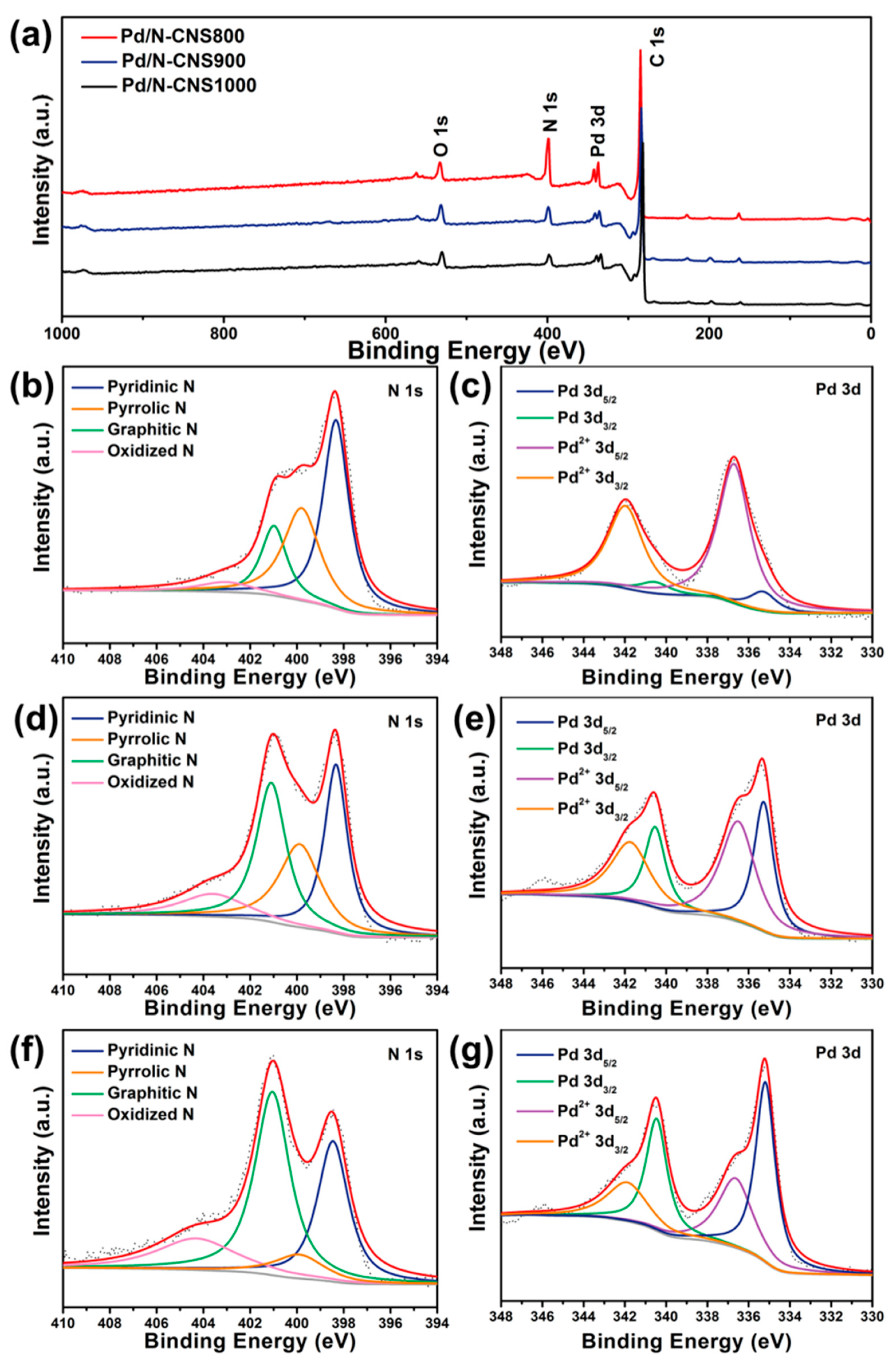
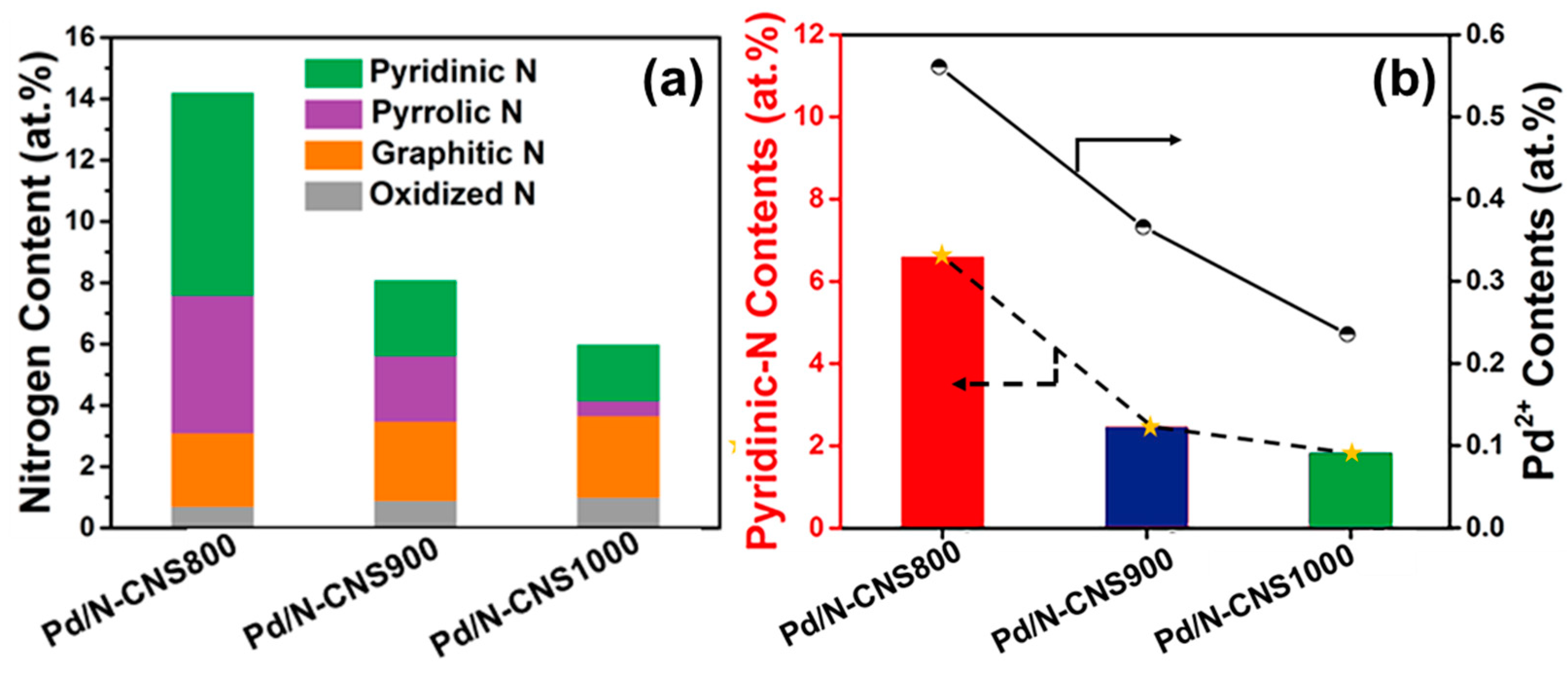
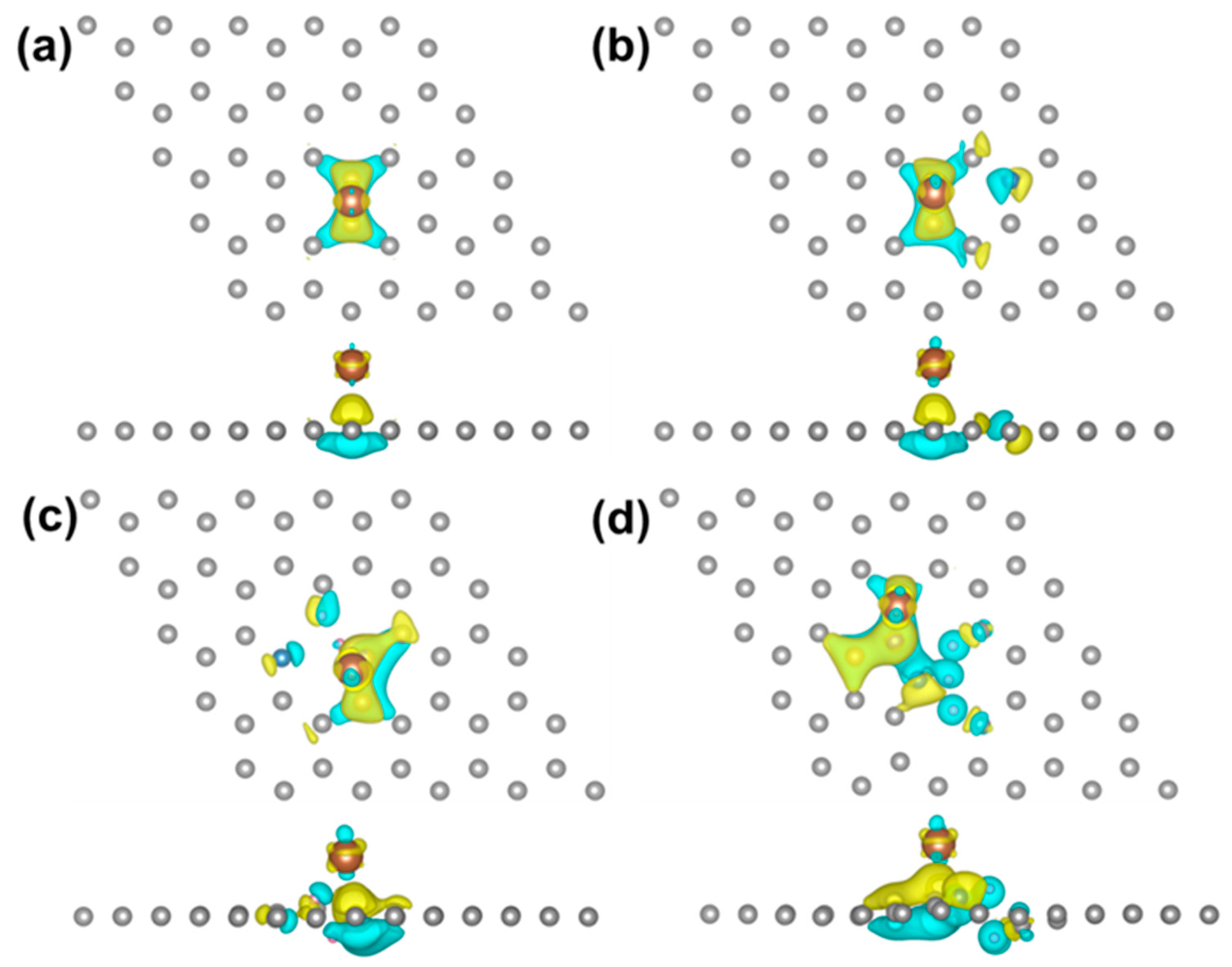
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noda, S.; Kondo, A. Recent advances in microbial production of aromatic chemicals and derivatives. Trends Biotechnol. 2017, 35, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Heeres, A.; Schenk, N.; Muizebelt, I.; Blees, R.; De Waele, B.; Zeeuw, A.J.; Meyer, N.; Carr, R.; Wilbers, E.; Heeres, H.J. Synthesis of bio-aromatics from black liquors using catalytic pyrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Kuchkina, N.; Sorokina, S.; Bykov, A.; Sulman, M.; Bronstein, L.; Shifrina, Z. Magnetically recoverable nanoparticulate catalysts for cross-coupling reactions: The dendritic support influences the catalytic performance. Nanomaterials 2021, 11, 3345. [Google Scholar] [CrossRef]
- Atisme, T.; Yu, C.; Tseng, E.; Chen, Y.C.; Hsu, P.; Chen, S. Interface Interactions in Conjugated Polymer Composite with Metal Oxide Nanoparticles. Nanomaterials 2019, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Kanchana, U.S.; Diana, E.J.; Mathew, T.V.; Anilkumar, G. Cyclodextrin based palladium catalysts for Suzuki reaction: An overview. Carbohydr. Res. 2020, 489, 107954. [Google Scholar] [CrossRef] [PubMed]
- Mannepalli, L.K.; Gadipelly, C.; Deshmukh, G.; Likhar, P.; Pottabathula, S. Advances in C-C coupling reactions catalyzed by homogeneous phosphine free palladium catalysts. Bull. Chem. Soc. Jpn. 2020, 93, 355–372. [Google Scholar] [CrossRef]
- Billingsley, K.L.; Anderson, K.W.; Buchwald, S.L. A highly active catalyst for Suzuki-Miyaura cross-coupling reactions of heteroaryl compounds. Angew. Chem. Int. Ed. Engl. 2006, 45, 3484–3488. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lyu, S.; Zhang, P.; Tian, X.; Wang, D.; Huang, W.; Liu, Z. Nitrogen-bonded ultrasmall palladium clusters over the nitrogen-doped carbon for promoting Suzuki cross-coupling reactions. Adv. Compos. Hybrid Mater. 2022, 5, 1396–1403. [Google Scholar]
- Li, X.-X.; Zhao, Q.-S.; Feng, X.; Pan, L.; Wu, Z.-Z.; Wu, X.-C.; Ma, T.-W.; Liu, J.-L.; Pan, Y.-Y.; Song, Y.; et al. Pyridinic nitrogen-doped graphene nanoshells boost the catalytic efficiency of palladium nanoparticles for the N-allylation reaction. ChemSusChem 2019, 12, 858–865. [Google Scholar] [CrossRef]
- Liu, H.; Huang, M.-Y.; Tao, W.-L.; Han, L.-L.; Zhang, J.-Q.; Zhao, Q.-S. A palladium catalyst supported on boron-doped porous carbon for efficient dehydrogenation of formic acid. Nanomaterials 2024, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Li, M.B.; Yang, J.; Yang, Y.; Xu, G.Y.; Luo, G.; Yang, J.; Backvall, J.E. Amino-supported palladium catalyst for chemo- and stereoselective domino reactions. Angew. Chem. Int. Ed. Engl. 2021, 60, 670–674. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Flanagan, K.A.; Hain, H. Suzuki coupling activity of an aqueous phase Pd nanoparticle dispersion and a carbon nanotube/Pd nanoparticle composite. Catal. Today 2009, 145, 108–113. [Google Scholar] [CrossRef]
- Liu, J.; Gui, J.; Zhou, W.; Tian, X.; Liu, Z.; Wang, J.; Liu, J.; Yang, L.; Zhang, P.; Huang, W.; et al. Self-regulating and asymmetric evaporator for efficient solar water-electricity generation. Nano Energy 2021, 86, 106112. [Google Scholar] [CrossRef]
- Holz, J.; Pfeffer, C.; Zuo, H.; Beierlein, D.; Richter, G.; Klemm, E.; Peters, R. In situ generated gold nanoparticles on active carbon as reusable highly efficient catalysts for a C sp3 -C sp3 stille coupling. Angew. Chem. Int. Ed. Engl. 2019, 58, 10330–10334. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, Y.; Cao, J.P. Carbon-based material-supported palladium nanocatalysts in coupling reactions: Discussion on their stability and heterogeneity. Appl. Organomet. Chem. 2020, 34, e5539. [Google Scholar] [CrossRef]
- Afshari, R.; Emad Hooshmand, S.; Atharnezhad, M.; Shaabani, A. An insight into the novel covalent functionalization of multi-wall carbon nanotubes with pseudopeptide backbones for palladium nanoparticles immobilization: A versatile catalyst towards diverse cross-coupling reactions in bio-based solvents. Polyhedron 2020, 175, 114238. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Wang, K.; Phan, N.T.S.; Zhang, F. Microwave-assisted aqueous carbon–carbon cross-coupling reactions of aryl chlorides catalysed by reduced graphene oxide supported palladium nanoparticles. Green Chem. 2020, 22, 3239–3247. [Google Scholar] [CrossRef]
- Lin, T.B.; Chung, D.L.; Chang, J.R. Ethyl acetate production from water-containing ethanol catalyzed by supported Pd catalysts: Advantages and disadvantages of hydrophobic supports. Ind. Eng. Chem. Res. 1999, 38, 1271–1276. [Google Scholar] [CrossRef]
- Jiang, J.; Li, P.; Huang, J.; Deng, K.; Xiong, J.; Dao, F.; Xie, J. Preparation of recyclable magnetic palladium nanocatalysts by dispersion strategy based on sodium alginate for reduction of p-nitrophenol and Suzuki-Miyaura coupling. Int. J. Biol. Macromol. 2024, 258, 129100. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Greiner, M.T.; Liu, P.; Hansen, T.W.; Dreyer, K.S.; Hibbitts, D.D.; Tessonnier, J.-P. Oxygen-doped carbonsupports modulate the hydrogenation activity of palladium nanoparticles through electronic metal–support interactions. ACS Catal. 2022, 12, 7344–7356. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.-F.; Liu, Y.-N.; Xu, A.-W. Erbium oxide as a novel support for palladium nanocatalysts with strong metal–support interactions: Remarkable catalytic performance in hydrogenation reactions. New J. Chem. 2018, 42, 19901–19907. [Google Scholar] [CrossRef]
- Ji, S.; Lu, X.; Zhang, M.; Leng, L.; Liu, H.; Yin, K.; Xu, C.; He, C.; Horton, J.H.; Zhang, J.; et al. Construction of a single-atom palladium catalyst by electronic metal-support interaction and interface confinement effect with remarkable performance in Suzuki coupling reaction. Chem. Eng. J. 2023, 452, 139205. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, S.; Wang, G.; Chen, Z.; Li, P.; Hu, J. Novel highly utilized PdNi bimetallic-loaded N-doped carbon catalysts prepared from MOF for Suzuki coupling reaction and hydro reduction of nitroaromatics. J. Environ. Chem. Eng. 2024, 12, 113324. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, P.; Wang, K.; Lan, K.; Huang, X.; Yang, M.; Gong, L.; Jia, Q.; Mu, X.; Xiong, Y.; et al. Phosphorus and nitrogen-doped palladium nanomaterials support on coral-like carbon materials as the catalyst for semi-hydrogenation of phenylacetylene and mechanism study. J. Alloys Compd. 2021, 868, 159047. [Google Scholar] [CrossRef]
- Shen, H.; He, J.; He, F.; Xue, Y.; Li, Y.; Li, Y. Nitrogen-doped graphdiyne for effective metal deposition and heterogeneous Suzuki-Miyaura coupling catalysis. Appl. Catal. A 2021, 623, 118244. [Google Scholar] [CrossRef]
- Chen, Z.; Vorobyeva, E.; Mitchell, S.; Fako, E.; Ortuno, M.A.; Lopez, N.; Collins, S.M.; Midgley, P.A.; Richard, S.; Vile, G.; et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 2018, 13, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Song, S.; Wang, J.; Xie, Y.; Chu, W.; Li, H.; Xu, H.; Tian, C.; Fu, H. Nitrogen-doped graphene supported Pd@PdO core-shell clusters for C-C coupling reactions. Nano Res. 2014, 7, 1280–1290. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Preview 2020, 120, 1250–1349. [Google Scholar]
- Wang, L.; Yin, P.; Zhang, L.-L.; Shen, S.-C.; Xu, S.-L.; Chen, P.; Liang, H.-W. Nitrogen-fixing of ultrasmall Pd-based bimetallic nanoclusters on carbon supports. J. Catal. 2020, 389, 297–304. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.X.; Yan, Q.Q.; Xu, S.L.; Chu, S.Q.; Chen, P.; Lin, Y. Hai-Wei Liang. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 2019, 5, eaax6322. [Google Scholar] [CrossRef]
- Pylypenko, S.; Queen, A.; Olson, T.S.; Dameron, A.; O’Neill, K.; Neyerlin, K.C.; Pivovar, B.; Dinh, H.N.; Ginley, D.S.; Gennett, T.; et al. Tuning Carbon-Based Fuel Cell Catalyst Support Structures via Nitrogen Functionalization. II. Investigation of Durability of Pt–Ru Nanoparticles Supported on Highly Oriented Pyrolytic Graphite Model Catalyst Supports As a Function of Nitrogen Implantation Dose. J. Phys. Chem. C 2011, 115, 13676–13684. [Google Scholar]
- Kramer, S.; Mielby, J.; Buss, K.; Kasama, T.; Kegnæs, S. Nitrogen-doped carbon-encapsulated nickel/cobalt nanoparticle catalysts for olefin migration in allylarenes. ChemCatChem 2017, 9, 2930–2934. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Mostafa, Y.S.; Alamri, S.A.; El-Mehasseb, I.M. A nanoelectrode of hybrid nanomaterials of palladium oxide with cadmium sulfide based on 2D-carbon nanosheets for developing electron transfer efficiency for supercapacitor applications. New J. Chem. 2024, 48, 11932–11948. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; Ding, X.; Wang, H.; Qiu, W.; Song, J.; Pang, S. Pd single atoms on N-Doped hollow carbon nanosheet assemblies for Suzuki cross-coupling reactions of aryl chlorides. ACS Appl. Nano Mater. 2024, 7, 8063–8073. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Shen, B.; Zhang, Y.; He, H.; Jiang, Q.; Yang, L.; Nanjundan, A.K.; Na, J.; Xu, X.; et al. Synthesis of multiple-twinned Pd nanoparticles anchored on graphitic carbon nanosheets for use as highly-active multifunctional electrocatalyst in formic acid and methanol oxidation reactions. Adv. Mater. Interfaces 2020, 7, 2000142. [Google Scholar] [CrossRef]
- Fan, M.; Long, Y.; Zhu, Y.; Hu, X.; Dong, Z. Two-dimensional covalent-organic-framework-derived nitrogen-rich carbon nanosheets modified with small Pd nanoparticles for the hydrodechlorination of chlorophenols and hydrogenation of phenol. Appl. Catal. A 2018, 568, 130–138. [Google Scholar] [CrossRef]
- Ding, X.; Chen, Y.; Nan, J.; Dai, H.; Wang, Y.; Bai, G.; Qiu, W. Ultrasmall palladium nanoparticles anchored on N-doped nestlike carbon nanosheets for selective hydrogenation of quinolines. ACS Sustain. Chem. Eng. 2022, 10, 14011–14023. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]



 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Reaction Time (min) | Yield (%) | Final TOF (h−1) |
| 1 | Blank | 50 | 0 | — |
| 2 | N-CNS800 | 50 | 0.6 | — |
| 3 | N-CNS900 | 50 | 0.5 | — |
| 4 | N-CNS1000 | 50 | 0.8 | — |
| 5 | Pd/N-CNS800 | 50 | 99.6 | 2390 |
| 6 | Pd/N-CNS900 | 50 | 86.8 | 2083 |
| 7 | Pd/N-CNS1000 | 50 | 90.8 | 2179 |
| 8 | Pd(II)/N-CNS800 | 50 | 85.7 | 2057 |
| 9 | Pd/AC | 50 | 36.7 | 881 |
| 10 | Pd/rGO | 50 | 48.9 | 1173 |
| 11 | Commercial Pd/C | 50 | 11.5 | 276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Xu, D.; Wang, Z.; Wang, L.; Zhao, Y.; Deng, W.; Zhao, Q.; Wu, M. Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions. Nanomaterials 2024, 14, 1690. https://doi.org/10.3390/nano14211690
Cui S, Xu D, Wang Z, Wang L, Zhao Y, Deng W, Zhao Q, Wu M. Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions. Nanomaterials. 2024; 14(21):1690. https://doi.org/10.3390/nano14211690
Chicago/Turabian StyleCui, Shihao, Dejian Xu, Zhiyuan Wang, Libo Wang, Yikun Zhao, Wei Deng, Qingshan Zhao, and Mingbo Wu. 2024. "Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions" Nanomaterials 14, no. 21: 1690. https://doi.org/10.3390/nano14211690
APA StyleCui, S., Xu, D., Wang, Z., Wang, L., Zhao, Y., Deng, W., Zhao, Q., & Wu, M. (2024). Pd Nanoparticles Immobilized on Pyridinic N-Rich Carbon Nanosheets for Promoting Suzuki Cross-Coupling Reactions. Nanomaterials, 14(21), 1690. https://doi.org/10.3390/nano14211690







