Biodegradation of Heterogeneous Industrial Multi-Walled Carbon Nanotubes by Pro-Inflammatory Macrophages
Abstract
1. Introduction
2. Materials and Methods
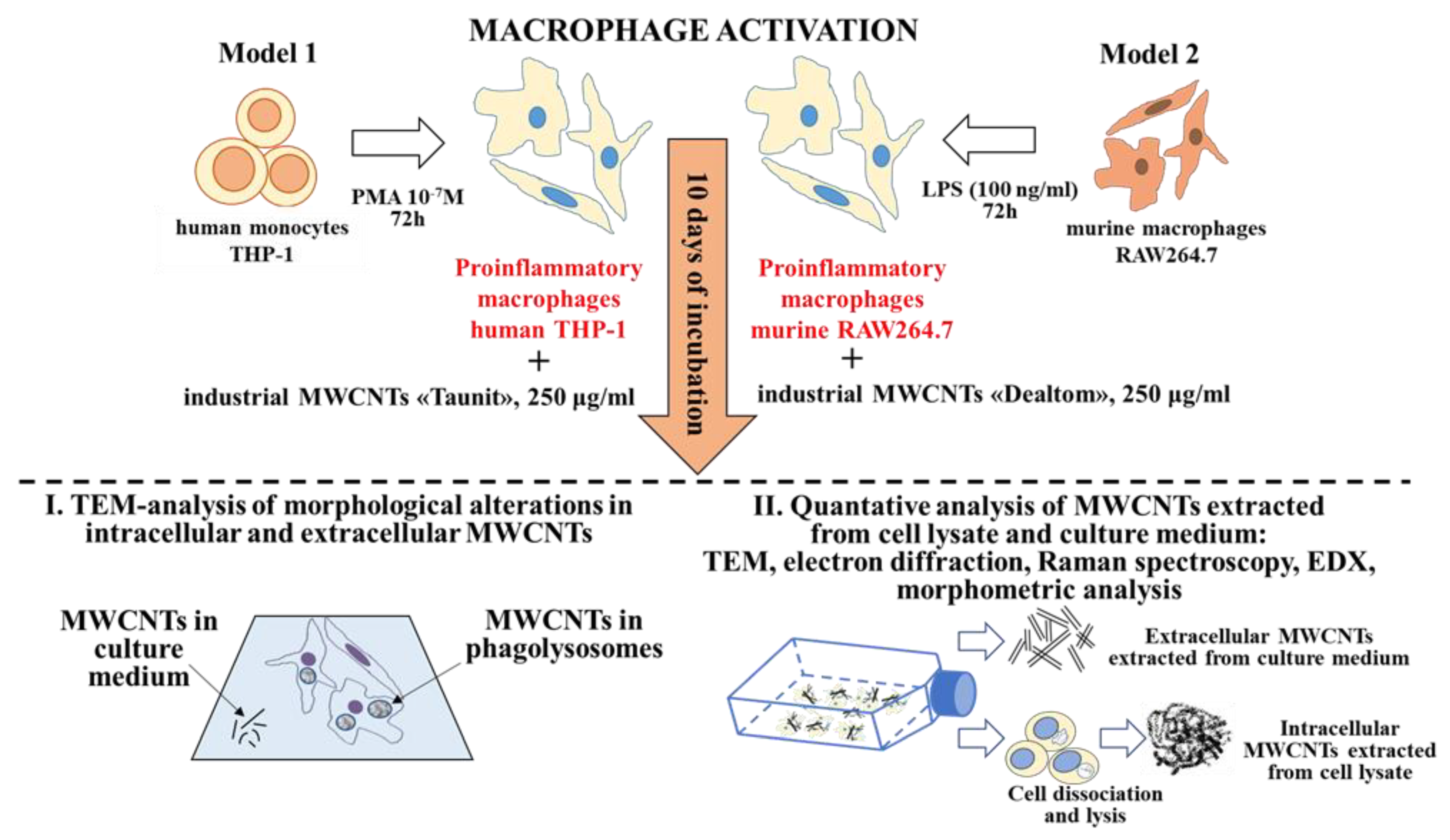
2.1. Experimental Design
2.2. Multi-Walled Carbon Nanotubes
2.3. Cell Lines
2.4. Macrophage Differentiation
2.5. Isolation of MWCNTs from the Cell Culture Medium and Cell Lysate
2.6. Hydrogen Peroxide-Mediated MWCNT Degradation
2.7. Transmission Electron Microscopy
2.8. Analytical TEM
2.8.1. Electron Diffraction
2.8.2. EDS
2.9. Raman Spectroscopy
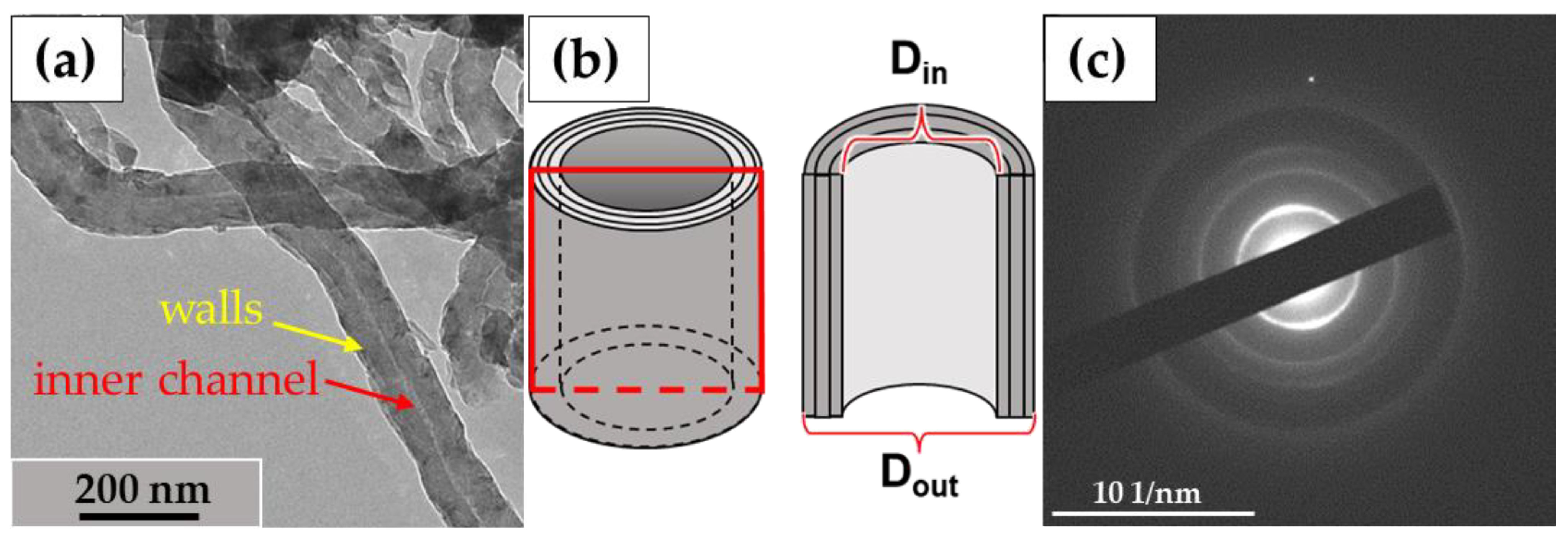
2.10. Morphometric Analysis
3. Results
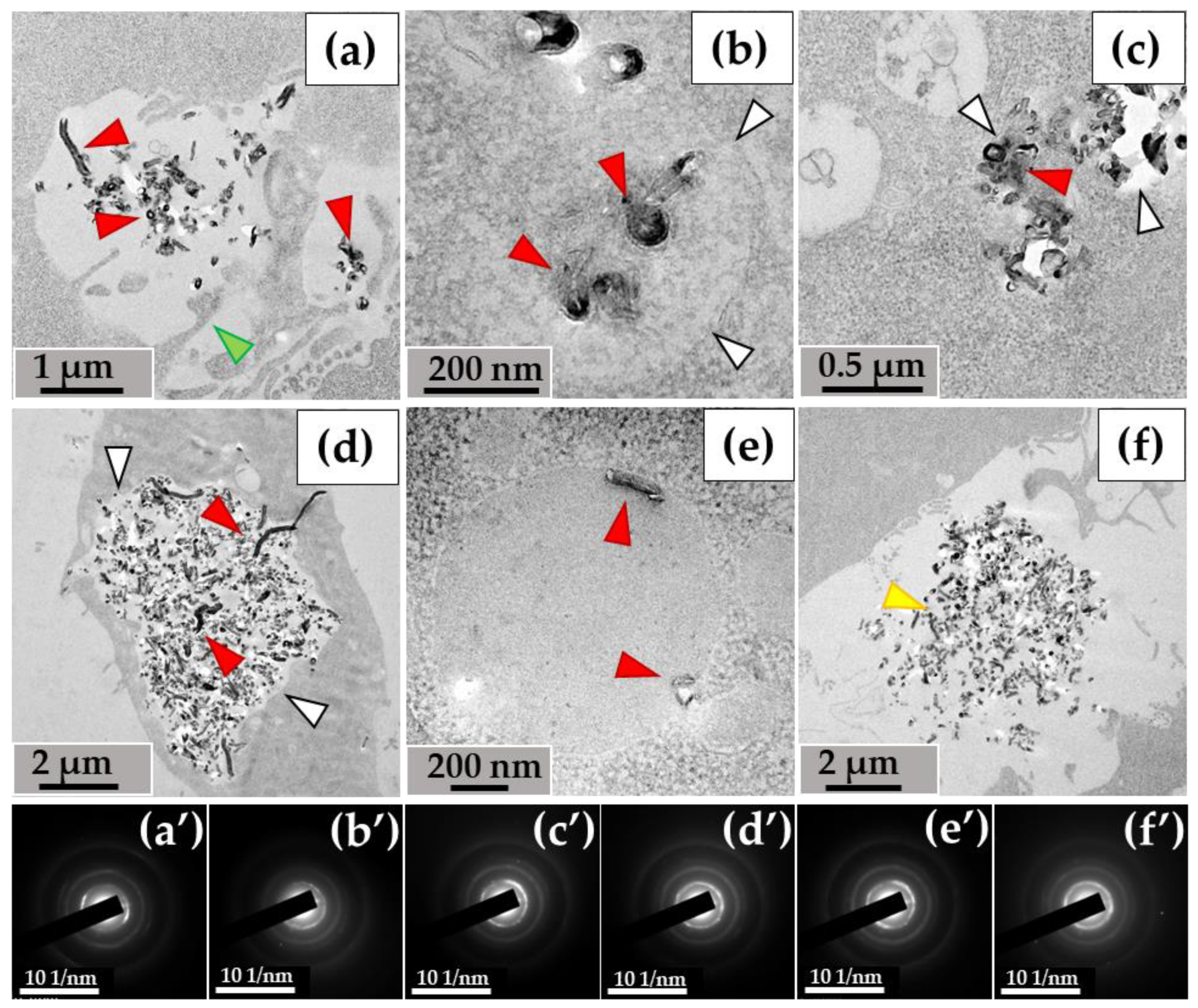
3.1. Ultrastructural Analysis of the Localization of ig-MWCNTs in Human and Murine Macrophages
3.1.1. THP-1 Macrophages/ig-MWCNT-T
3.1.2. RAW264.7 Macrophages/ig-MWCNT-D
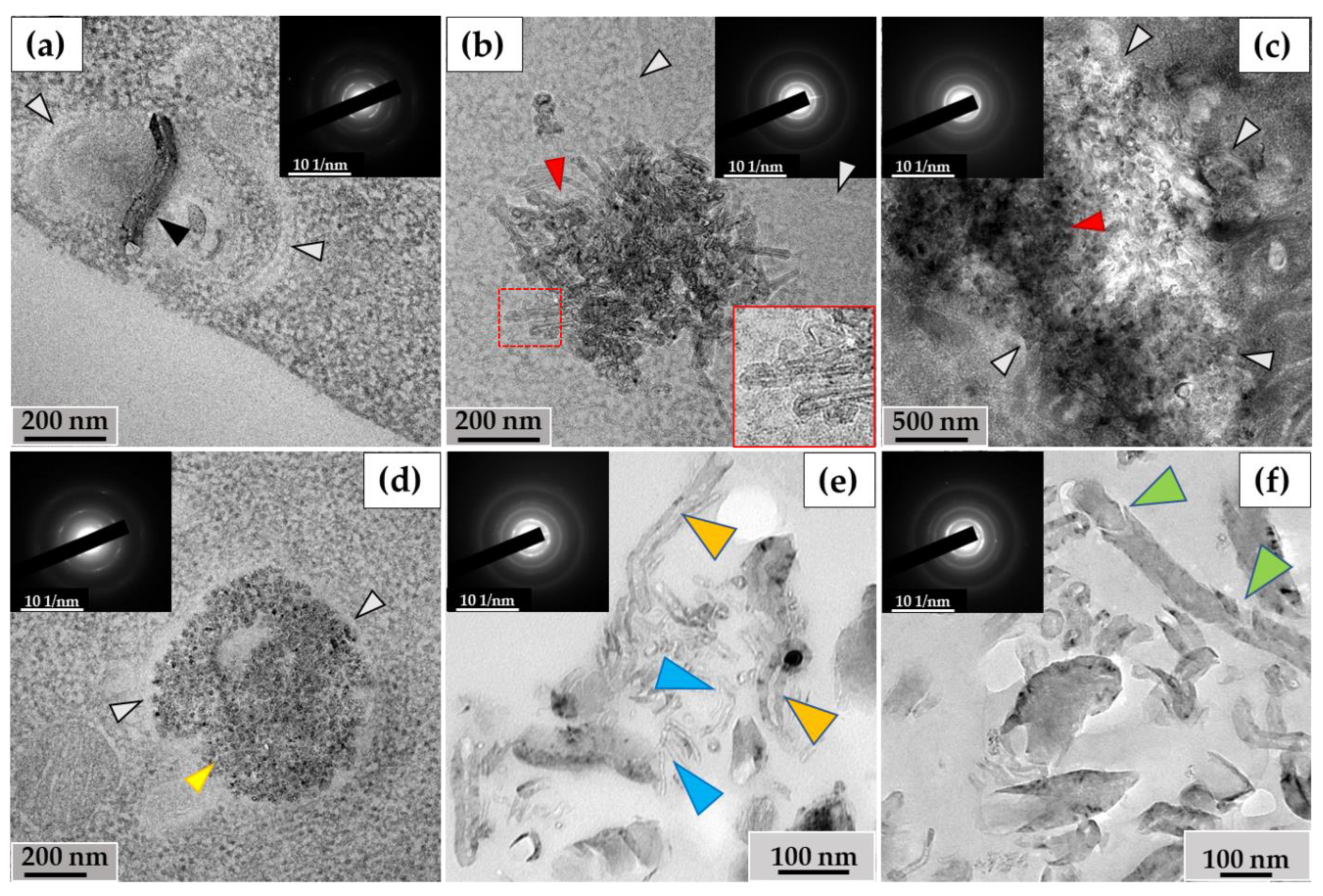
3.2. Morphological Analysis of Intra- and Extracellular ig-MWCNTs in Ultrathin Sections
3.2.1. THP-1 Macrophages/ig-MWCNT-T
3.2.2. RAW264.7 Macrophages/ig-MWCNT-D
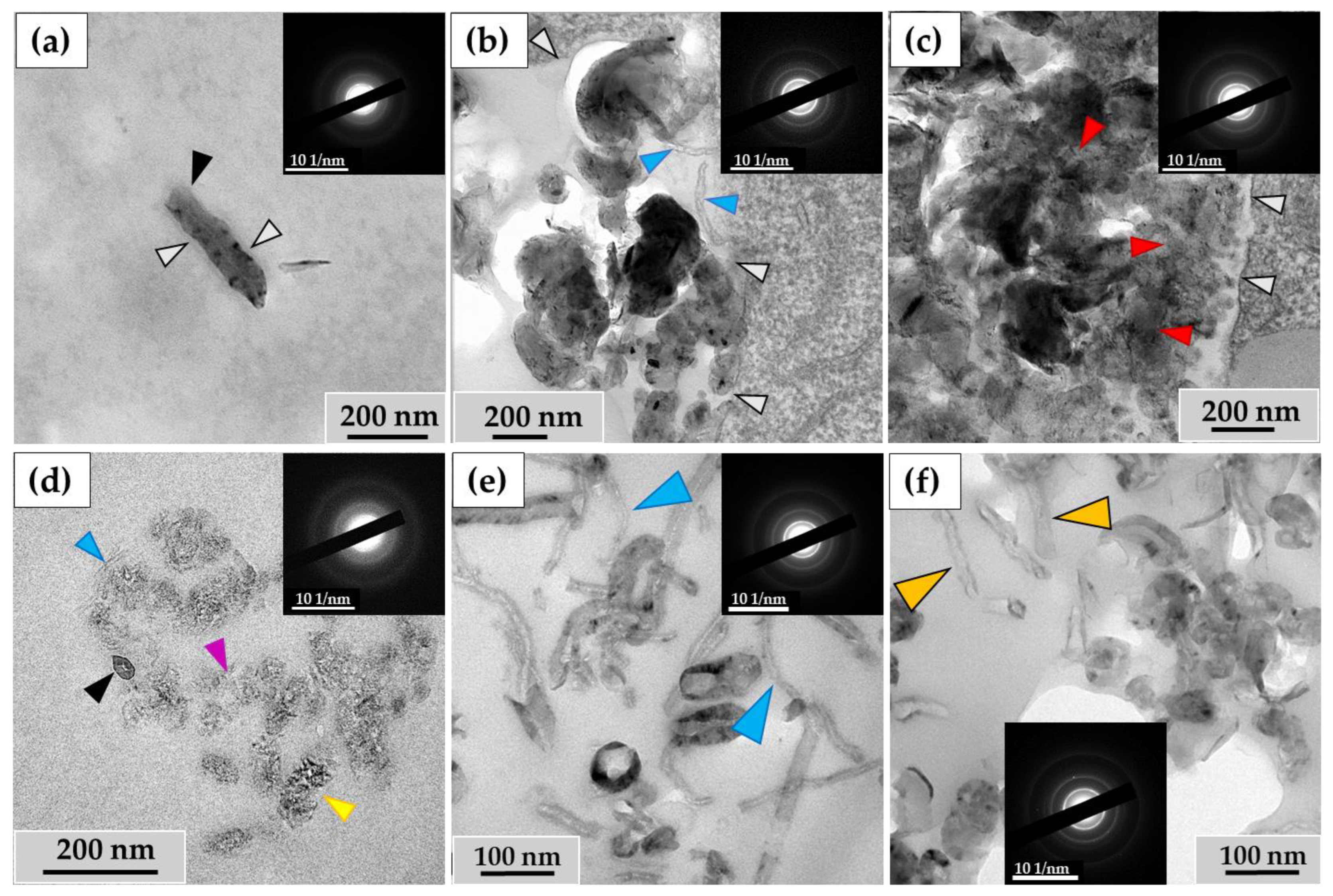
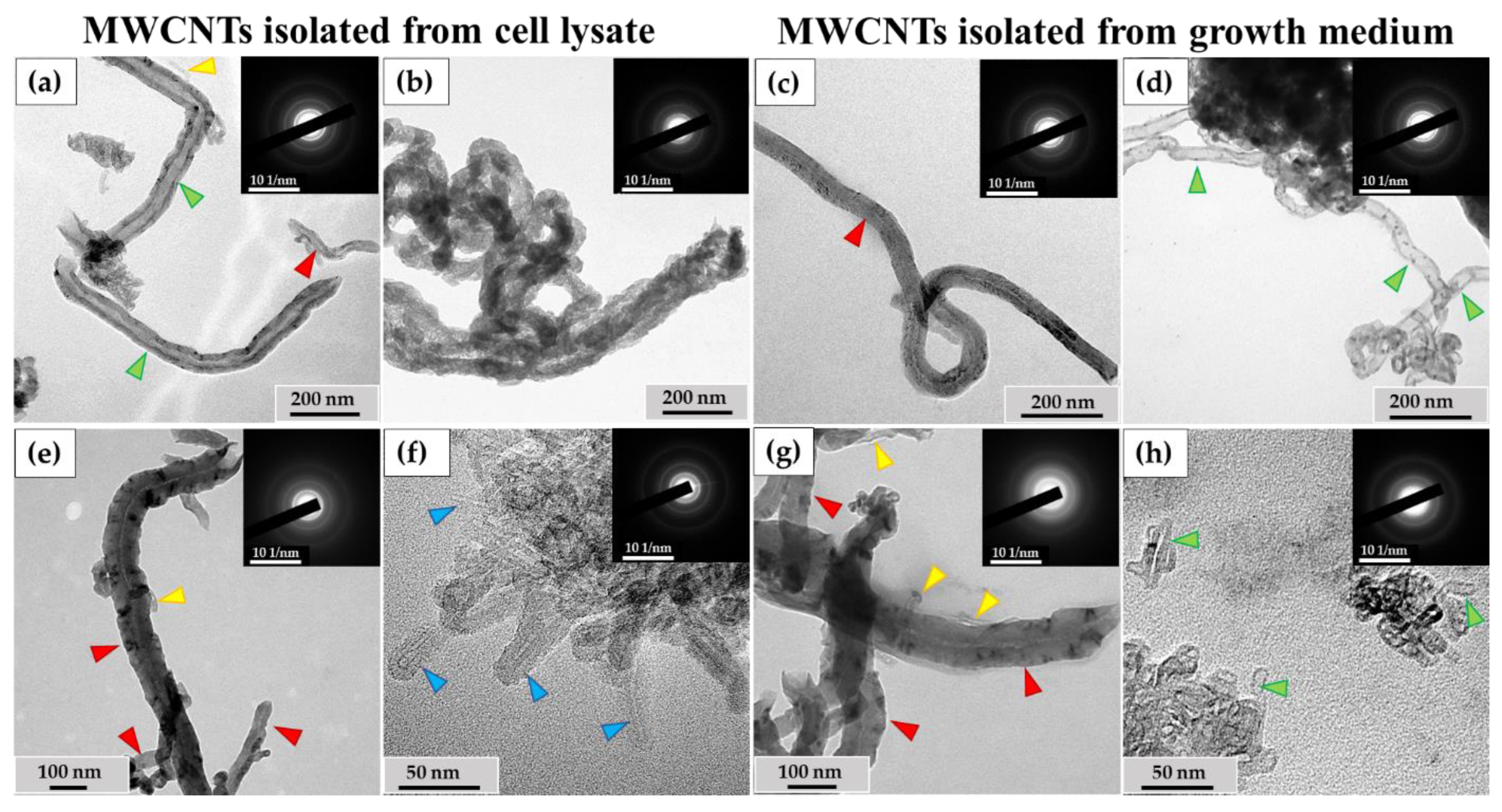
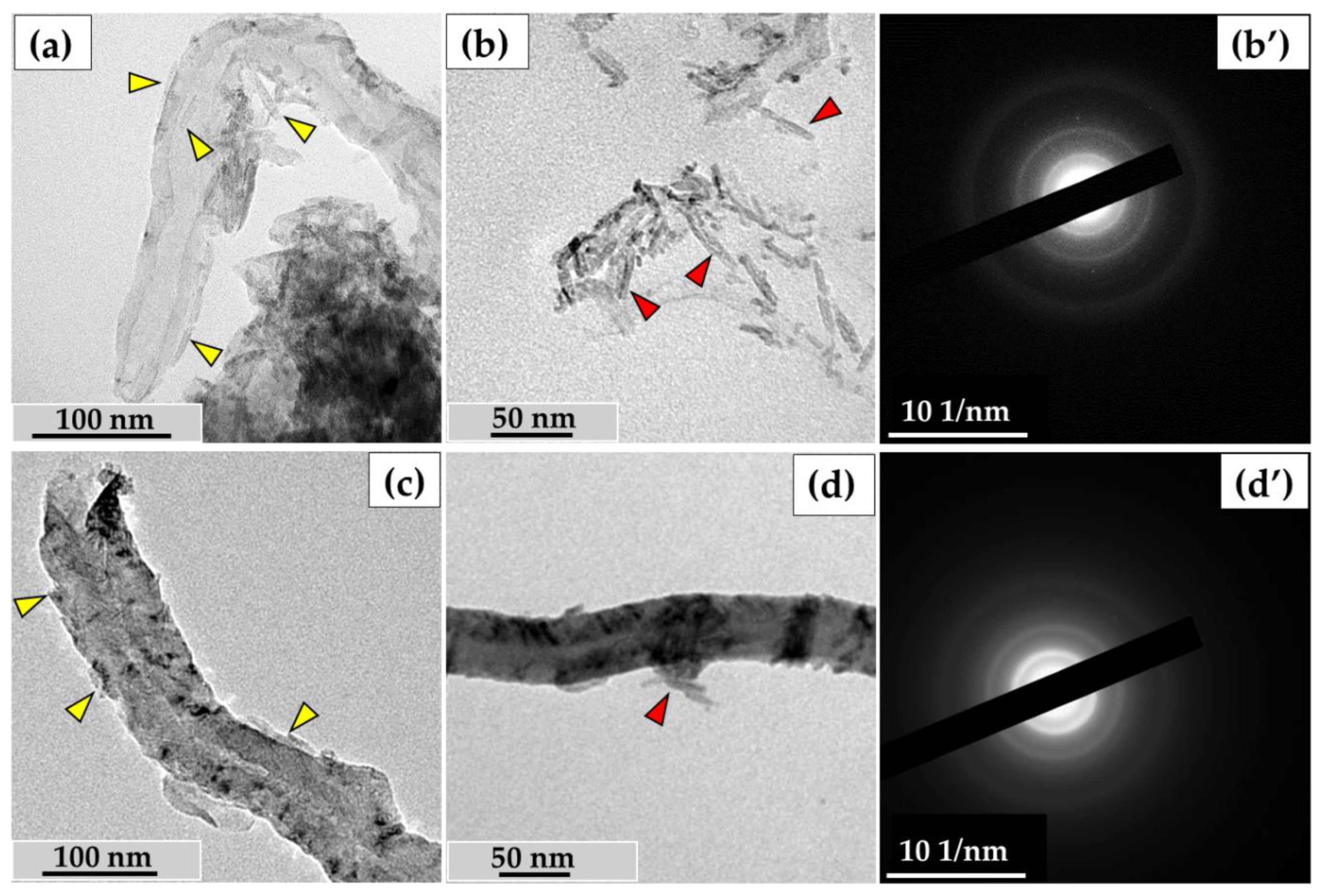
3.3. Analysis of the Ultrastructure of ig-MWCNTs Isolated from Cell Lysate and Extracellular Medium
3.4. Quantification of Changes in ig-MWCNTs after Incubation with Macrophages
3.4.1. Morphometric Analysis of ig-MWCNTs Isolated from Cell Lysate and Extracellular Medium
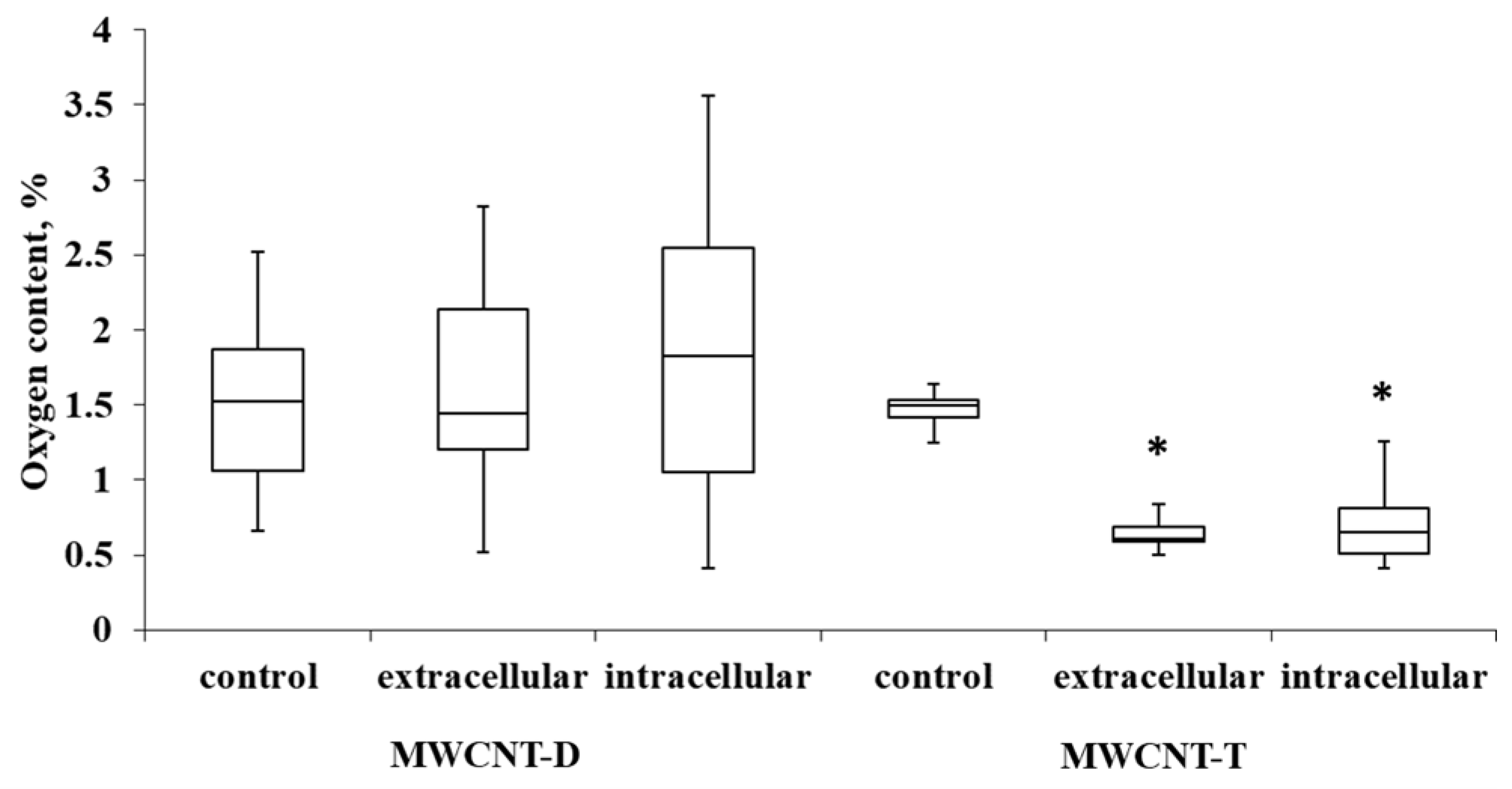
3.4.2. Evaluation of Oxygen Content of ig-MWCNTs after Incubation with Macrophages
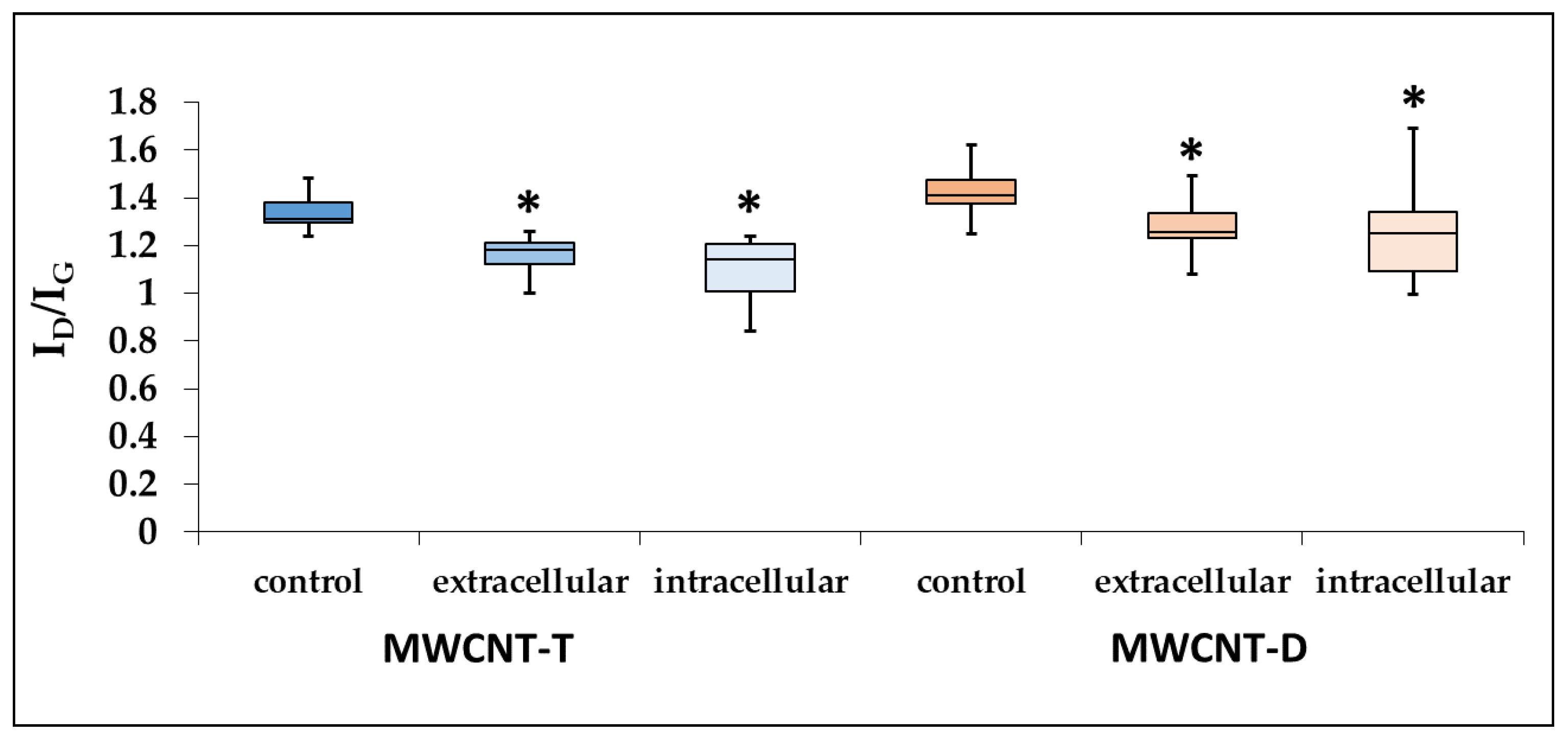
3.4.3. Evaluation of Defect Density of ig-MWCNTs after Incubation with Macrophages
3.5. Evaluation of Morphological Changes in ig-MWCNT Hydrogen Peroxide Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanotubes Database. Available online: https://www.nanowerk.com/carbon-nanotubes-suppliers.php (accessed on 15 May 2024).
- MWCNTs Applications. Available online: https://www.us-nano.com/mwcnts_applications (accessed on 15 May 2024).
- Swanson, J.J.; Febo, R.; Boies, A.M.; Kittelson, D.B. Fuel Sulfur and Iron Additives Contribute to the Formation of Carbon Nanotube-like Structures in an Internal Combustion Engine. Environ. Sci. Technol. Lett. 2016, 3, 364–368. [Google Scholar] [CrossRef]
- Lara-Romero, J.; Campos-García, J.; Dasgupta-Schubert, N.; Borjas-García, S.; Tiwari, D.K.; Paraguay-Delgado, F.; Jiménez-Sandoval, S.; Alonso-Nuñez, G.; Gómez-Romero, M.; Lindig-Cisneros, R.; et al. Biological effects of carbon nanotubes generated in forest wildfire ecosystems rich in resinous trees on native plants. PeerJ 2017, 5, e3658. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef] [PubMed]
- Masyutin, A.G.; Erokhina, M.V.; Sychevskaya, K.A.; Gusev, A.A.; Vasyukova, I.A.; Tkachev, A.G.; Smirnova, E.A.; Onishchenko, G.E. Multiwalled Carbon Nanotubules Induce Pathological Changes in the Digestive Organs of Mice. Bull. Exp. Biol. Med. 2016, 161, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Just, J.; Hartman, K.B.; Laoudi, Y.; Boudjemaa, S.; Alloyeau, D.; Szwarc, H.; Wilson, L.J.; Moussa, F. Anthropogenic Carbon Nanotubes Found in the Airways of Parisian Children. EBioMedicine 2015, 2, 1697–1704. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Moussa, F. Anthropogenic Carbon Nanotubes and Air Pollution. Emiss. Control Sci. Technol. 2017, 3, 230–232. [Google Scholar] [CrossRef]
- Elgrabli, D.; Dachraoui, W.; Marmier, H.D.; Ménard-Moyon, C.; Bégin, D.; Bégin-Colin, S.; Bianco, A.; Alloyeau, D.; Gazeau, F. Intracellular degradation of functionalized carbon nanotube/iron oxide hybrids is modulated by iron via Nrf2 pathway. Sci. Rep. 2017, 7, 40997. [Google Scholar] [CrossRef]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Dupre-Crochet, S.; Erard, M.; Nusse, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Mahalingam, P.; Parasuram, B.; Maiyalagan, T.; Sundaram, S. Chemical Methods For Purification Of Carbon Nanotubes-A Review. J. Environ. Nanotechnol. 2012, 1, 53–61. [Google Scholar]
- Masyutin, A.G.; Bagrov, D.V.; Vlasova, I.I.; Nikishin, I.I.; Klinov, D.V.; Sychevskaya, K.A.; Onishchenko, G.E.; Erokhina, M.V. Wall thickness of industrial multi-walled carbon nanotubes is not a crucial factor for their degradation by sodium hypochlorite. Nanomaterials 2018, 8, 715. [Google Scholar] [CrossRef] [PubMed]
- Elgrabli, D.; Dachraoui, W.; Ménard-Moyon, C.; Liu, X.J.; Bégin, D.; Bégin-Colin, S.; Bianco, A.; Gazeau, F.; Alloyeau, D. Carbon Nanotube Degradation in Macrophages: Live Nanoscale Monitoring and Understanding of Biological Pathway. ACS Nano 2015, 9, 10113–10124. [Google Scholar] [CrossRef]
- Zhao, Y.; Allen, B.L.; Star, A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A 2011, 115, 9536–9544. [Google Scholar] [CrossRef] [PubMed]
- González, V.J.; Vega-Díaz, S.M.; Morelos-Gómez, A.; Fujisawa, K.; Endo, M.; Cadiz, O.M.; Llido, J.B.; Terrones, M. H2O2/UV layer-by-layer oxidation of multiwall carbon nanotubes: The “onion effect” and the control of the degree of surface crystallinity and diameter. Carbon 2018, 139, 1027–1034. [Google Scholar] [CrossRef]
- Kurynina, A.V.; Erokhina, M.V.; Makarevich, O.A.; Sysoeva, V.Y.; Lepekha, L.N.; Kuznetsov, S.A.; Onishchenko, G.E. Plasticity of Human THP–1 Cell Phagocytic Activity during Macrophagic Differentiation. Biochemistry 2018, 83, 200–214. [Google Scholar] [CrossRef]
- Pi, J.; Li, T.; Liu, J.; Su, X.; Wang, R.; Yang, F.; Bai, H.; Jin, H.; Cai, J. Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron 2014, 65, 1–9. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Nayfeh, M.H. (Ed.) Chapter 7—Characterization and Simulation Technologies of Nanomaterial. In Fundamentals and Applications of Nano Silicon in Plasmonics and Fullerines; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2008; pp. 153–167. ISBN 978-0-323-48057-4. [Google Scholar]
- Ayer, R.; Scanlon, J.C.; Ebert, L.B. Electron diffraction of carbon nanotubes. J. Phys. Chem. 1995, 99, 9639–9641. [Google Scholar] [CrossRef]
- Qin, L.C. Electron diffraction from carbon nanotubes. Rep. Prog. Phys. 2006, 69, 2761–2821. [Google Scholar] [CrossRef]
- Masyutin, A.G.; Tarasova, E.K.; Onishchenko, G.E.; Erokhina, M. V Identifying Carbon Nanoparticles in Biological Samples by Means of Transmission Electron Microscopy. Bull. Russ. Acad. Sci. Phys. 2023, 87, 1443–1448. [Google Scholar] [CrossRef]
- Masyutin, A.G.; Erokhina, M.V.; Sychevskaya, K.A.; Gusev, A.A.; Vasyukova, I.A.; Smirnova, E.A.; Onishchenko, G.E. Multi-walled carbon nanotubes: Biodegradation by gastric agents in vitro and effect on murine intestinal system. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012008. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Gou, P.; Vlasova, I.I.; Kapralov, A.A.; Konduru, N.; Kagan, V.E.; Star, A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008, 8, 2903–3899. [Google Scholar] [CrossRef]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Russier, J.; Ménard-Moyon, C.; Venturelli, E.; Gravel, E.; Marcolongo, G.; Meneghetti, M.; Doris, E.; Bianco, A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale 2011, 3, 893–896. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Sacchetti, C.; El-Sayed, R.; Fornara, A.; Kotchey, G.P.; Gaugler, J.A.; Star, A.; Bottini, M.; Fadeel, B. Enzymatic “stripping” and degradation of PEGylated carbon nanotubes. Nanoscale 2014, 6, 14686–14690. [Google Scholar] [CrossRef]
- Andón, F.T.; Kapralov, A.A.; Yanamala, N.; Feng, W.; Baygan, A.; Chambers, B.J.; Hultenby, K.; Ye, F.; Toprak, M.S.; Brandner, B.D.; et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small 2013, 9, 2720–2729. [Google Scholar] [CrossRef]
- Bhattacharya, K.; El-Sayed, R.; Andón, F.T.; Mukherjee, S.P.; Gregory, J.; Li, H.; Zhao, Y.; Seo, W.; Fornara, A.; Brandner, B.; et al. Lactoperoxidase-mediated degradation of single-walled carbon nanotubes in the presence of pulmonary surfactant. Carbon 2015, 91, 506–517. [Google Scholar] [CrossRef]
- Hou, J.; Wan, B.; Yang, Y.; Ren, X.M.; Guo, L.H.; Liu, J.F. Biodegradation of single-walled carbon nanotubes in macrophages through respiratory burst modulation. Int. J. Mol. Sci. 2016, 17, 409. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tian, R.; Yang, Z.; Chen, J.; Lu, N. NADPH oxidase-dependent degradation of single-walled carbon nanotubes in macrophages. J. Mater. Sci. Mater. Med. 2017, 28, 7. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Kapralov, A.A.; St Croix, C.M.; Watkins, S.C.; Kisin, E.R.; Kotchey, G.P.; Balasubramanian, K.; Vlasova, I.I.; Yu, J.; Kang Kim, X.; et al. Lung Macrophages “Digest” Carbon Nanotubes Using a Superoxide/Peroxynitrite Oxidative Pathway. ACS nano 2014, 8, 5610–5621. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Vakhrusheva, T.V.; Sokolov, A.V.; Kostevich, V.A.; Gusev, A.A.; Gusev, S.A.; Melnikova, V.I.; Lobach, A.S. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012, 264, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Pinault, M.; Tchankouo, S.; Charon, Ï.; Ridoux, A.; Boczkowski, J.; Mayne-L’Hermite, M.; Lanone, S. Early signs of multi-walled carbon nanotbues degradation in macrophages, via an intracellular pH-dependent biological mechanism importance of length and functionalization. Part. Fibre Toxicol. 2016, 13, 61. [Google Scholar] [CrossRef]
- Lam, T.I.; Brennan-Minnella, A.M.; Won, S.J.; Shen, Y.; Hefner, C.; Shi, Y.; Sun, D.; Swanson, R.A. Intracellular pH reduction prevents excitotoxic and ischemic neuronal death by inhibiting NADPH oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, E4362–E4368. [Google Scholar] [CrossRef]
- Levine, A.P.; Duchen, M.R.; De Villiers, S.; Rich, P.R.; Segal, A.W. Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS ONE 2015, 10, e0125906. [Google Scholar] [CrossRef]
- Morgan, D.; Cherny, V.V.; Murphy, R.; Katz, B.Z.; DeCoursey, T.E. The pH dependence of NADPH oxidase in human eosinophils. J. Physiol. 2005, 569, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Hadad, C.; Prato, M.; Bianco, A.; Kostarelos, K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale 2016, 8, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G. Defects and Disorder in Carbon Nanotubes. In Oxford Handbook of Nanoscience and Technology; Oxford University Press: Oxford, UK, 2009; pp. 1–73. [Google Scholar]
- Nunes, A.; Bussy, C.; Gherardini, L.; Meneghetti, M.; Herrero, M.A.; Bianco, A.; Prato, M.; Pizzorusso, T.; Al-Jamal, K.T.; Kostarelos, K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine 2012, 7, 1485–1494. [Google Scholar] [CrossRef]
- Weiss, S.J.; Epstein, F.H.; Weiss, S.J. Tissue Destruction by Neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef]


| RAW264.7/MWCNT-D | THP-1/MWCNT-T | |||||
|---|---|---|---|---|---|---|
| Diameter (D), nm | Control | Extracellular MWCNTs | Intracellular MWCNTs # | Control | Extracellular MWCNTs | Intracellular MWCNTs # |
| Dout | 69 ± 26 | 50 ± 28 * | 47 ± 27 * | 46 ± 15 | 35 ± 14 * | 41 ± 17 |
| Din | 10 ± 3 | 8 ± 3 | 8 ± 3 | 9 ± 4 | 17 ± 7 * | 9 ± 4 |
| Average wall thickness | 29 ± 12 (100%) | 22 ± 14 (≈75%) | 20 ± 11 (≈68%) | 18 ± 7 (100%) | 9 ± 5 * (≈50%) | 16 ± 7 (≈89%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masyutin, A.G.; Tarasova, E.K.; Samsonov, D.A.; Onishchenko, G.E.; Erokhina, M.V. Biodegradation of Heterogeneous Industrial Multi-Walled Carbon Nanotubes by Pro-Inflammatory Macrophages. Nanomaterials 2024, 14, 1616. https://doi.org/10.3390/nano14201616
Masyutin AG, Tarasova EK, Samsonov DA, Onishchenko GE, Erokhina MV. Biodegradation of Heterogeneous Industrial Multi-Walled Carbon Nanotubes by Pro-Inflammatory Macrophages. Nanomaterials. 2024; 14(20):1616. https://doi.org/10.3390/nano14201616
Chicago/Turabian StyleMasyutin, Alexander G., Ekaterina K. Tarasova, Daniil A. Samsonov, Galina E. Onishchenko, and Maria V. Erokhina. 2024. "Biodegradation of Heterogeneous Industrial Multi-Walled Carbon Nanotubes by Pro-Inflammatory Macrophages" Nanomaterials 14, no. 20: 1616. https://doi.org/10.3390/nano14201616
APA StyleMasyutin, A. G., Tarasova, E. K., Samsonov, D. A., Onishchenko, G. E., & Erokhina, M. V. (2024). Biodegradation of Heterogeneous Industrial Multi-Walled Carbon Nanotubes by Pro-Inflammatory Macrophages. Nanomaterials, 14(20), 1616. https://doi.org/10.3390/nano14201616





