Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
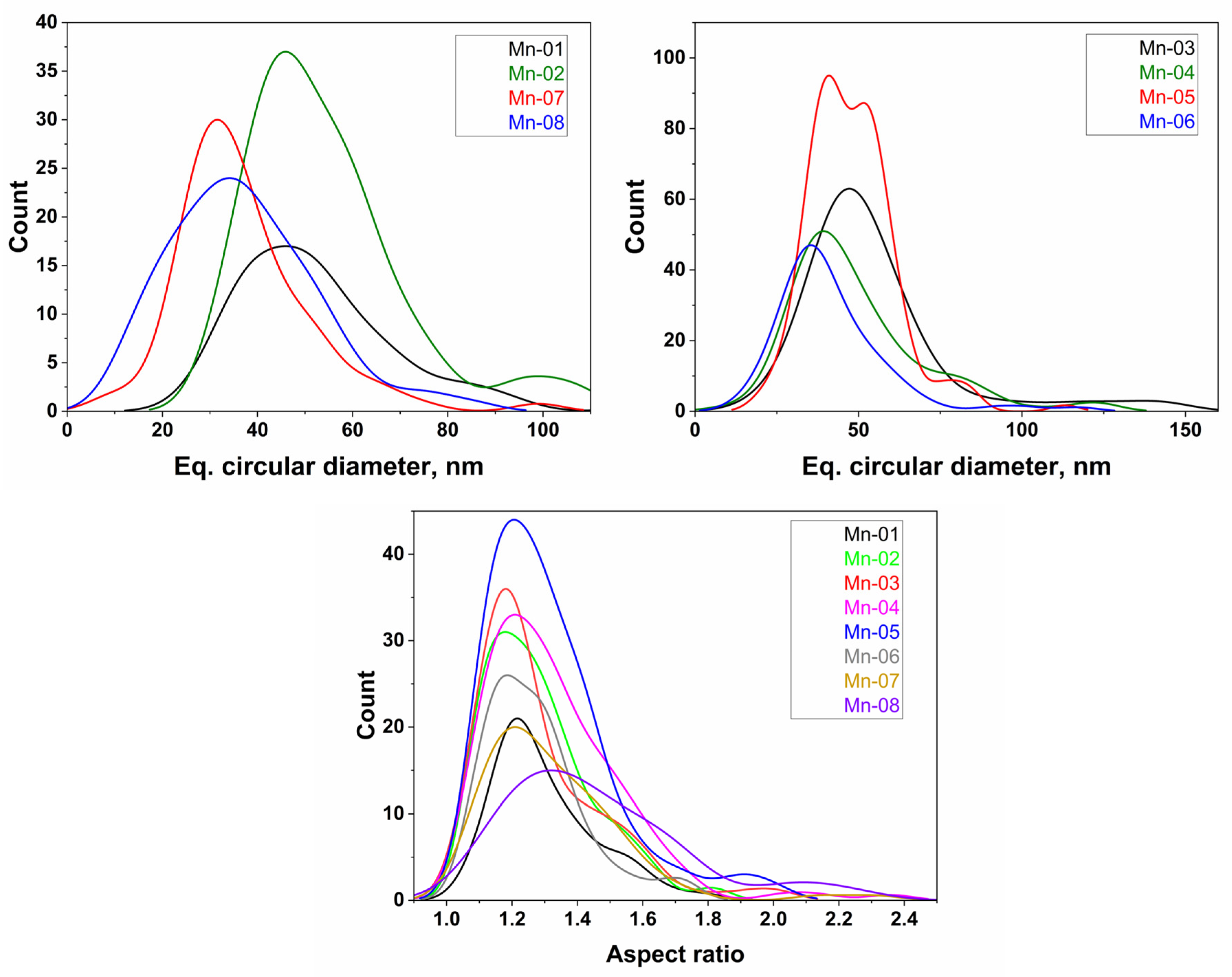
3.1. Nanomaterial Characterization
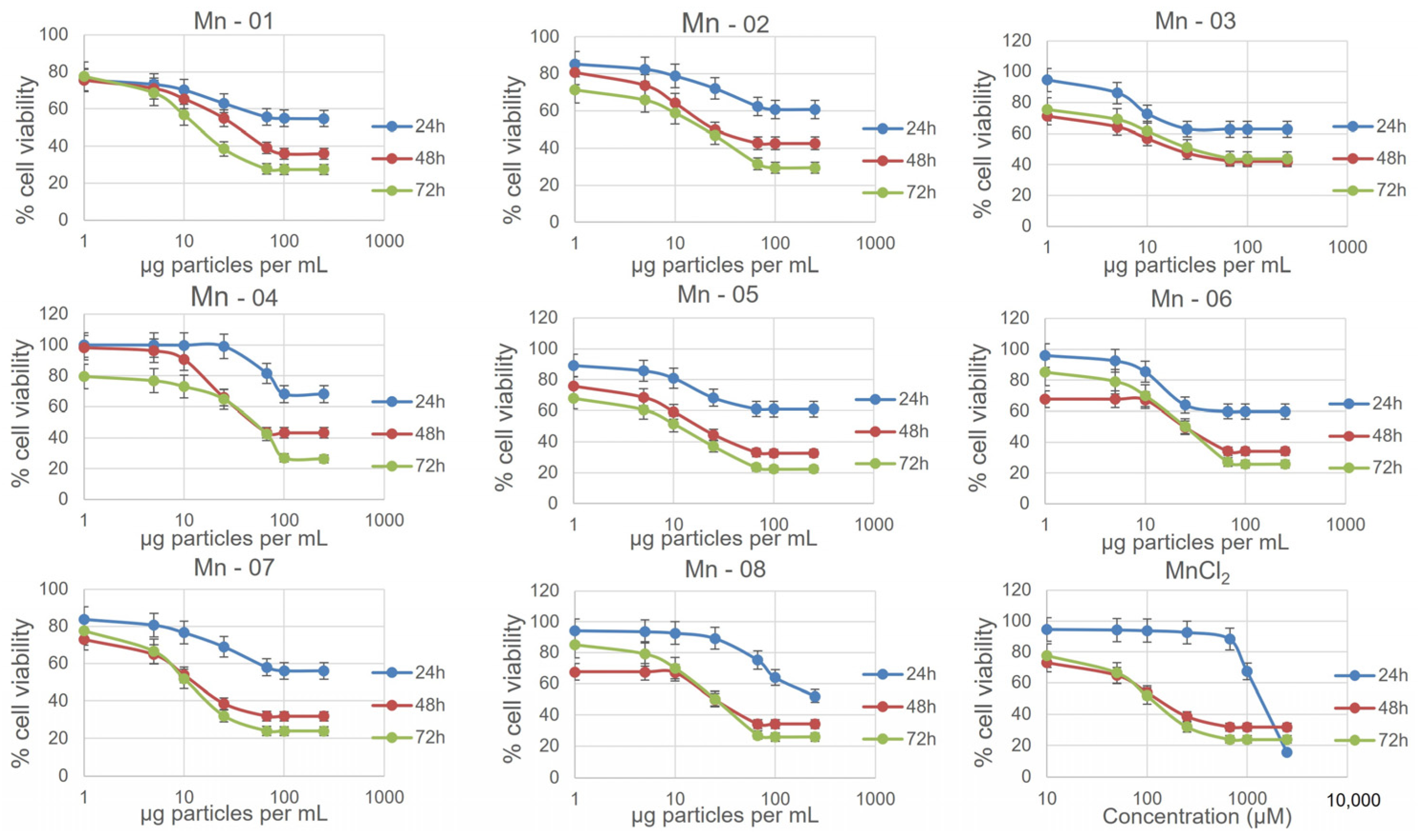
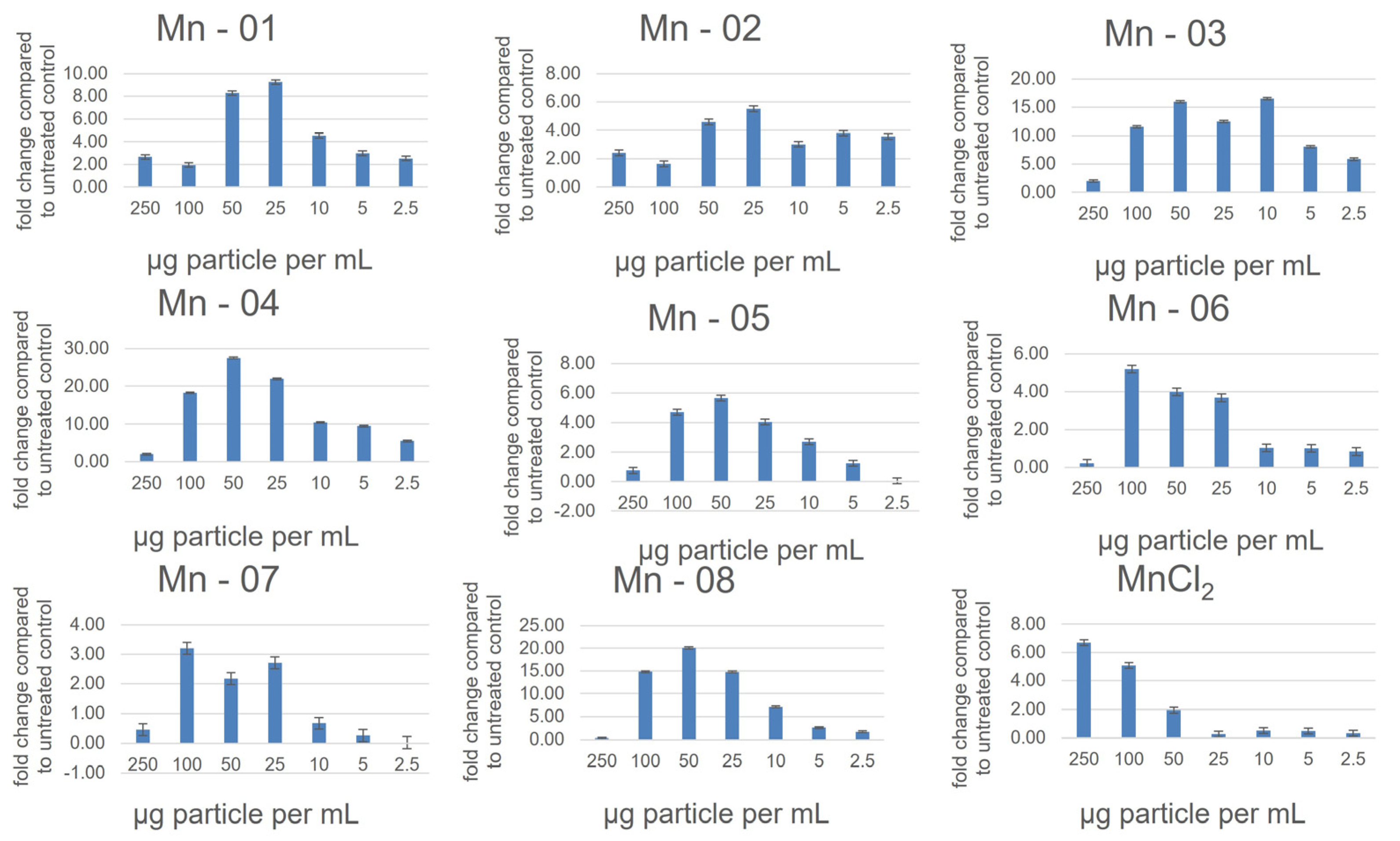
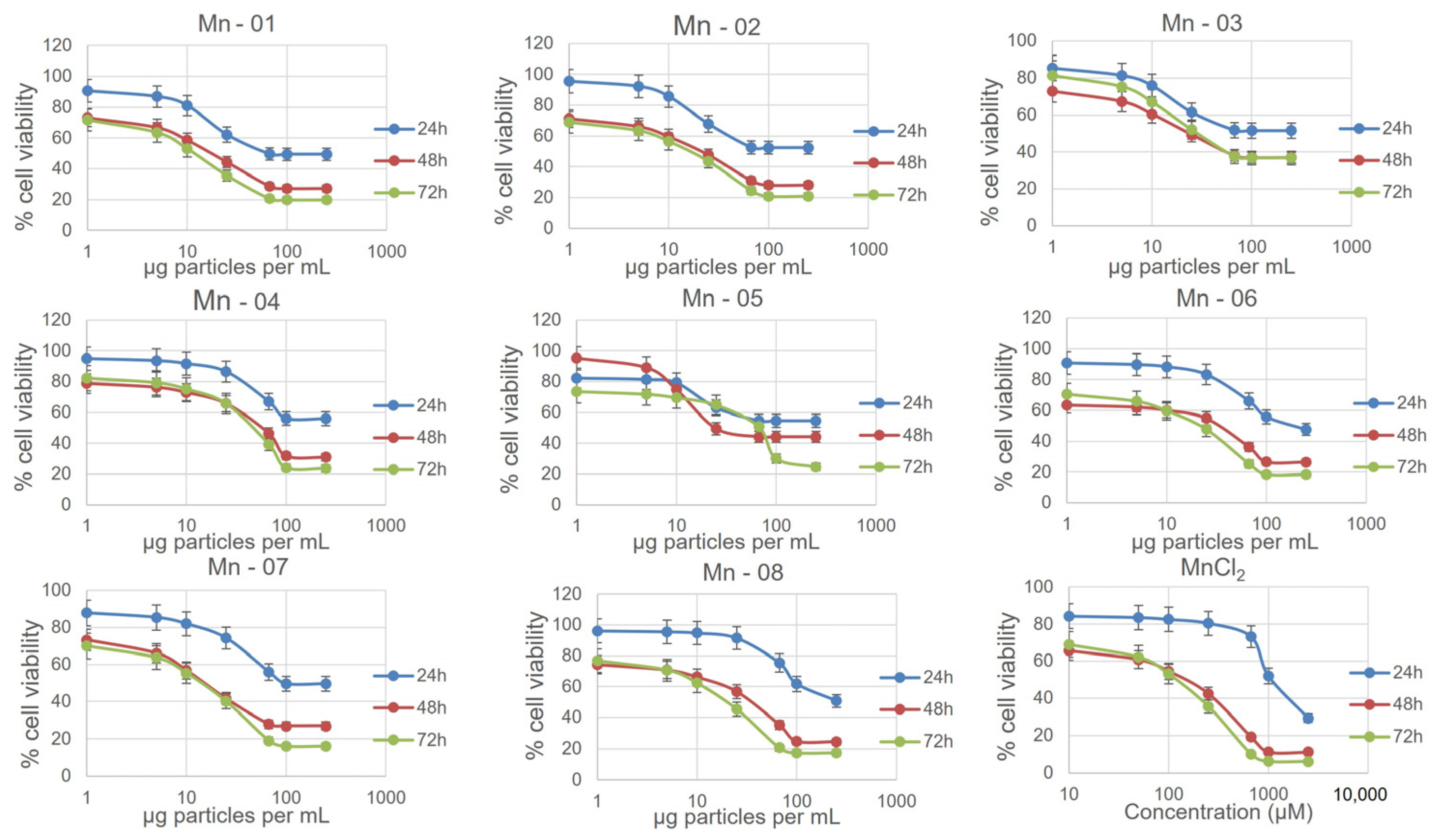
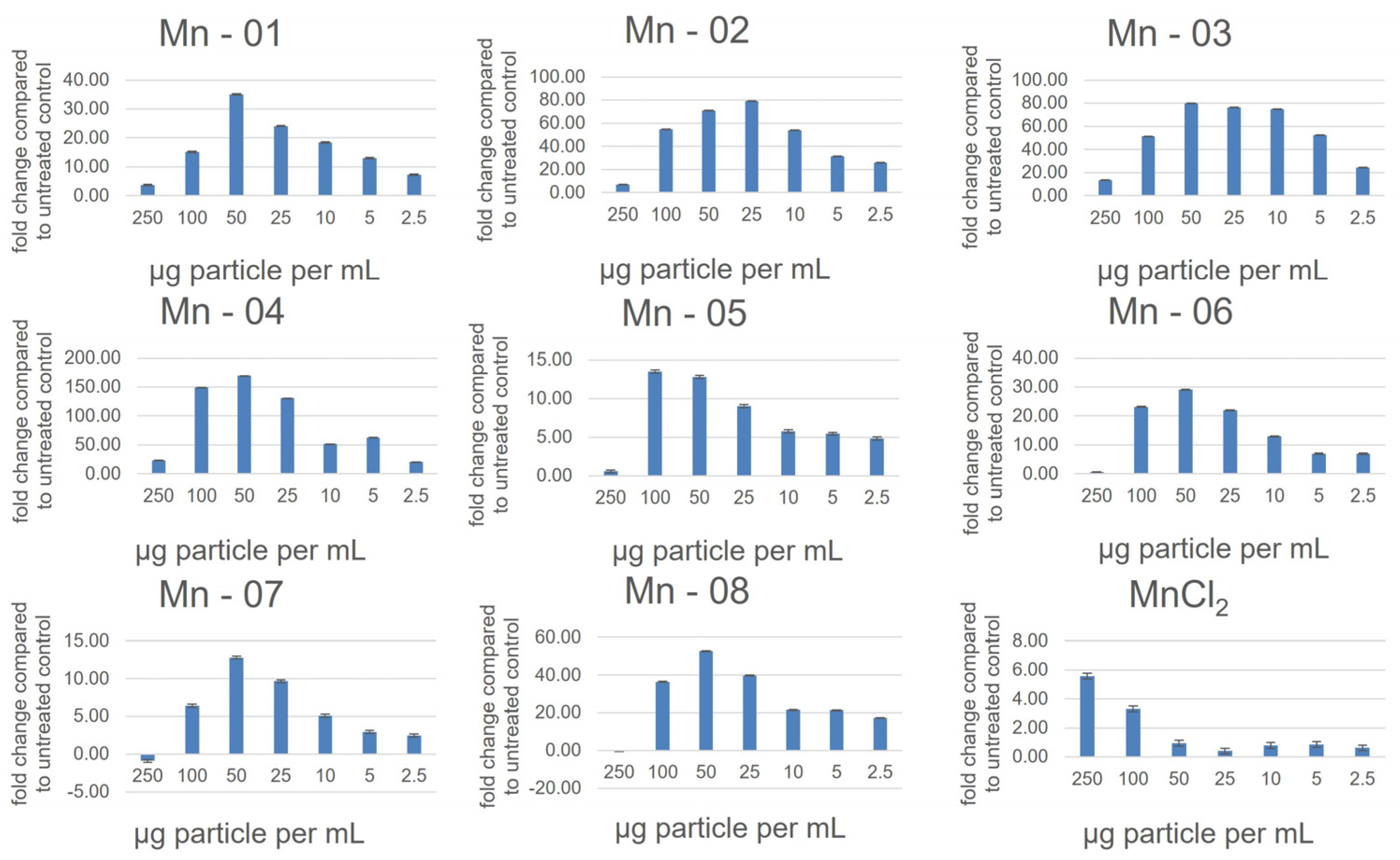
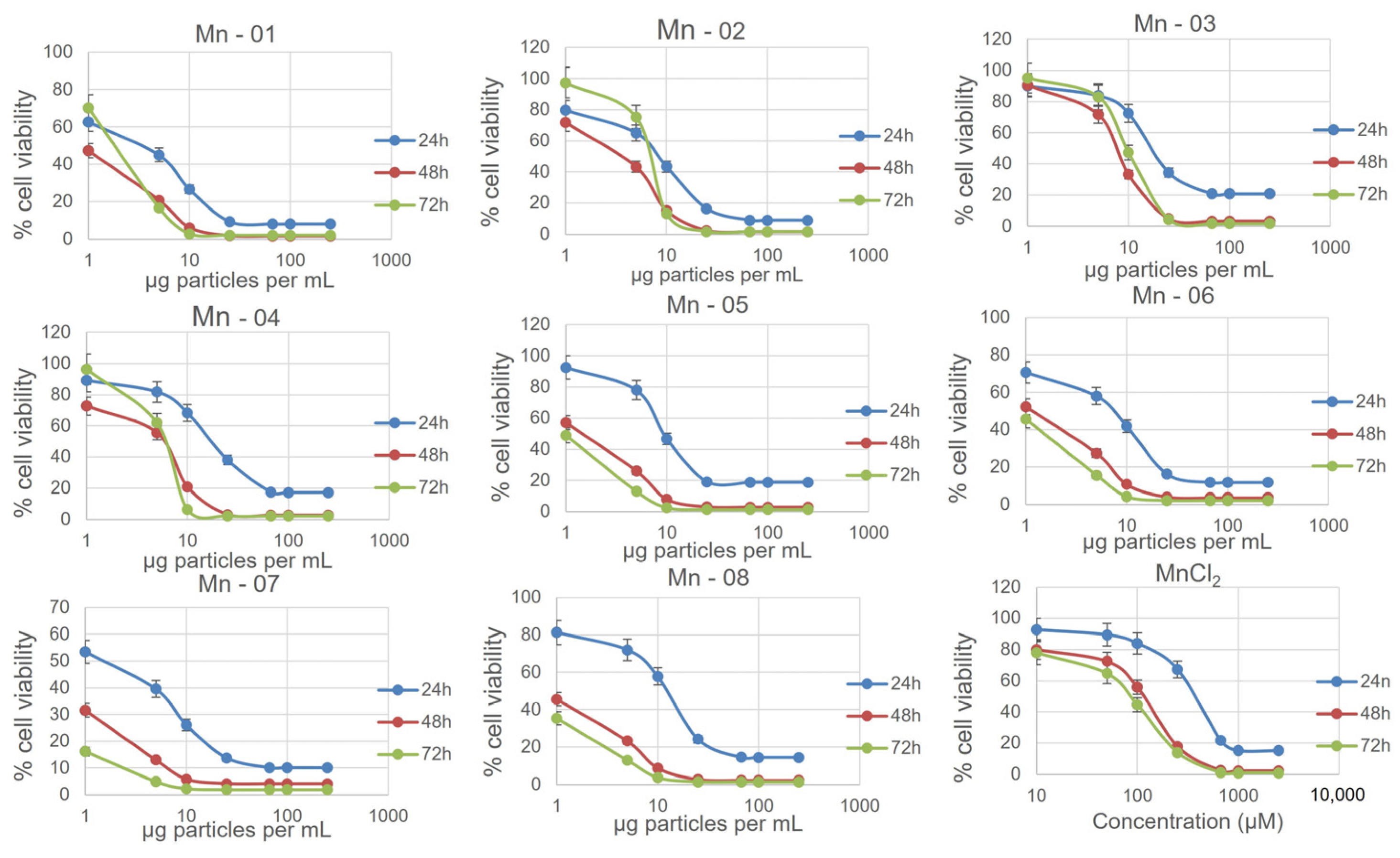
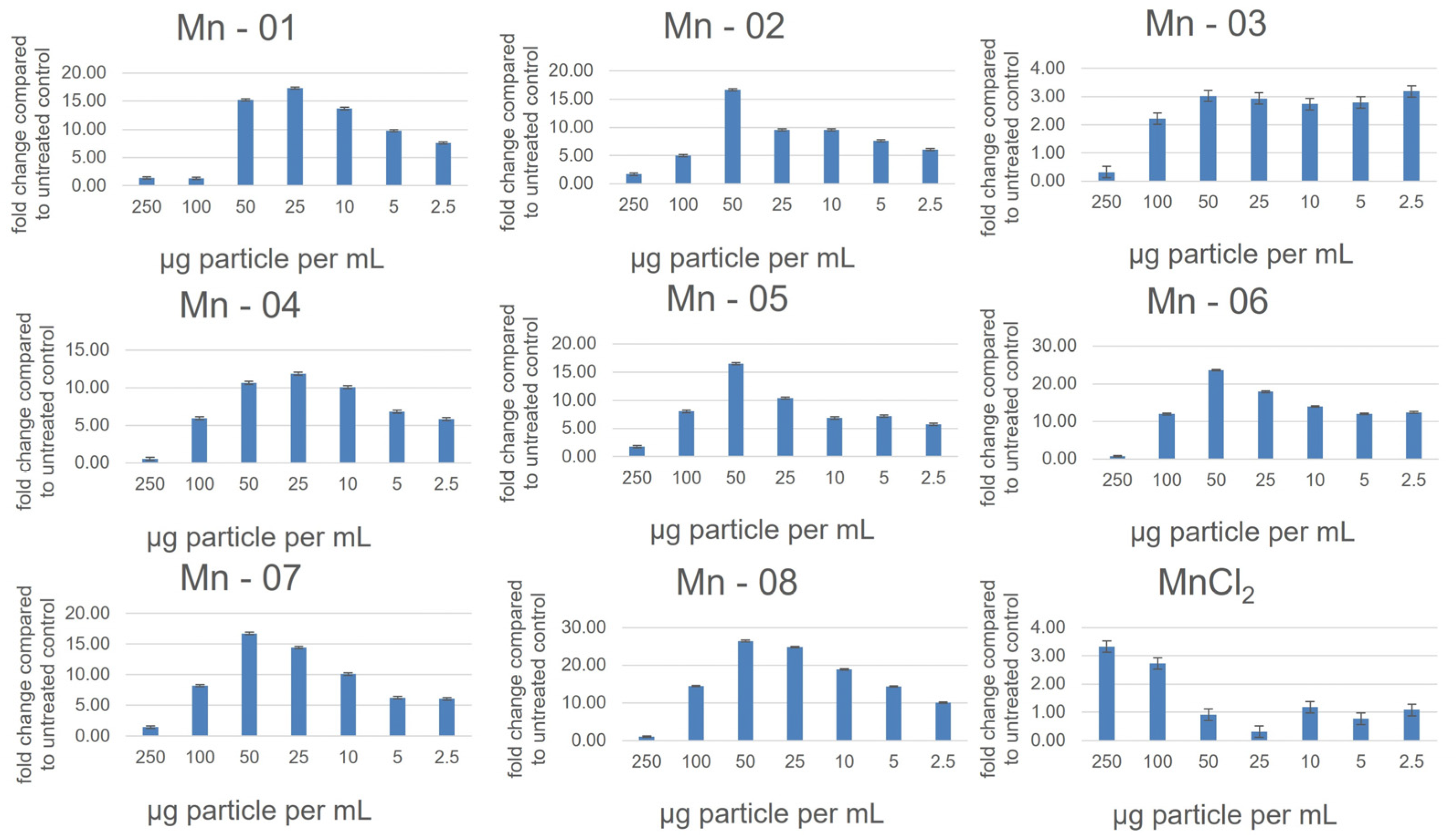
3.2. Cell Assays
3.3. A549 Lung Cells
3.4. HepG2 Liver Cells
3.5. J774A.1 Macrophage Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobańska, Z.; Roszak, J.; Kowalczyk, K.; Stępnik, M. Applications and biological activity of nanoparticles of manganese and manganese oxides in in vitro and in vivo models. Nanomaterials 2021, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: A sustainable approach for agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese oxide nanomaterials: Synthesis, properties, and theranostic applications. Adv. Mater. 2020, 32, 1905823. [Google Scholar] [CrossRef]
- Hsu, B.Y.W.; Kirby, G.; Tan, A.; Seifalian, A.M.; Li, X.; Wang, J. Relaxivity and toxicological properties of manganese oxide nanoparticles for MRI applications. RSC Adv. 2016, 6, 45462–45474. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, P.; Ghaffarifar, F.; Delavari, H.; Shahpari, N. Efficacy of manganese oxide (Mn2O3) nanoparticles against Leishmania major in vitro and in vivo. J. Trace Elem. Med. Biol. 2019, 56, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Razumov, I.A.; Zav’yalov, E.L.; Troitskii, S.Y.; Romashchenko, A.V.; Petrovskii, D.V.; Kuper, K.E.; Moshkin, M.P. Selective cytotoxicity of manganese nanoparticles against human glioblastoma cells. Bull. Exp. Biol. Med. 2017, 163, 561–565. [Google Scholar] [CrossRef]
- Xu, W.; Qing, X.; Liu, S.; Chen, Z.; Zhang, Y. Manganese oxide nanoparticles for bacterial infection detection and therapy. J. Mater. Chem. B 2022, 10, 1343–1358. [Google Scholar] [CrossRef]
- Jiang, X.; Gray, P.; Patel, M.; Zheng, J.; Yin, J.-J. Crossover between anti- and pro-oxidant activities of different manganese oxide nanoparticles and their biological implications. J. Mater. Chem. B 2020, 8, 1191–1201. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative stress-induced DNA damage by manganese dioxide nanoparticles in human neuronal cells. BioMed Res. Int. 2017, 5478790. [Google Scholar] [CrossRef]
- Frick, R.; Müller-Edenborn, B.; Schlicker, A.; Rothen-Rutishauser, B.; Raemy, D.O.; Günther, D.; Hattendorf, B.; Stark, W.; Beck-Schimmer, B. Comparison of manganese oxide nanoparticles and manganese sulfate with regard to oxidative stress, uptake and apoptosis in alveolar epithelial cells. Toxicol. Lett. 2011, 205, 163–172. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. 2017, 56, 14267–14271. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Chaturvedi, V.K.; Yadav, C.B.; Singh, V.; Singh, M.P. In vitro cytotoxicity and antioxidant efficiency of synthesized mixed phase manganese oxide nanomaterial. J. Exp. Zool. India 2021, 24, 95–100. [Google Scholar]
- Stebounova, L.V.; Gonzalez-Pech, N.I.; Peters, T.M.; Grassian, V.H. Physicochemical properties of air discharge-generated manganese oxide nanoparticles: Comparison to welding fumes. Environ. Sci. Nano 2018, 5, 696–707. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, B.H.; Singh, G.; Hak, S.; Bandyopadhyay, S.; Augestad, I.L.; Peddis, D.; Sandvig, I.; Sandvig, A.; Glomm, W.R. L-DOPA-coated manganese oxide nanoparticles as dual MRI contrast agents and drug-delivery vehicles. Small 2016, 12, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, H.Y.; Ji, Z.X.; Rallo, R.; Xia, T.; Chang, C.H.; Nel, A.; Cohen, Y. Development of structure-activity relationship for metal oxide nanoparticles. Nanoscale 2013, 5, 5644–5653. [Google Scholar] [CrossRef]
- Mirshafiee, V.; Sun, B.; Chang, C.H.; Liao, Y.-P.; Jiang, W.; Jiang, J.; Liu, X.; Wang, X.; Xia, T.; Nel, A.E. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in Kupffer cells and macrophages versus apoptosis in hepatocytes. ACS Nano 2018, 12, 3836–3851. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Chusuei, C.C.; Wu, C.-H.; Mallavarapu, S.; Hou, F.Y.S.; Hsu, C.M.; Winiarz, J.G.; Aronstam, R.S.; Huang, Y.-W. Cytotoxicity in the age of nano: The role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem.-Biol. Interact. 2013, 206, 319–326. [Google Scholar] [CrossRef]
- Tolliver, L.M.; Holl, N.J.; Hou, F.Y.S.; Lee, H.-J.; Cambre, M.H.; Huang, Y.-W. Differential cytotoxicity induced by transition metal oxide nanoparticles is a function of cell killing and suppression of cell proliferation. Int. J. Mol. Sci. 2020, 21, 1731. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of commercial metal oxide nanomaterials: Crystalline phase, crystallite and particle size and specific surface area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef] [PubMed]
- Bushell, M.; Kunc, F.; Du, X.; Zborowski, A.; Johnston, L.J.; Kennedy, D.C. Characterization of engineered cerium oxide nanoparticles and their effects on lung and macrophage cells. Int. J. Transl. Med. 2022, 2, 522–536. [Google Scholar] [CrossRef]
- Kunc, F.; Bushell, M.; Du, X.; Zborowski, A.; Johnston, L.J.; Kennedy, D.C. Physical Characterization and Cellular Toxicity Studies of Commercial NiO Nanoparticles. Nanomaterials 2022, 12, 1822. [Google Scholar] [CrossRef] [PubMed]
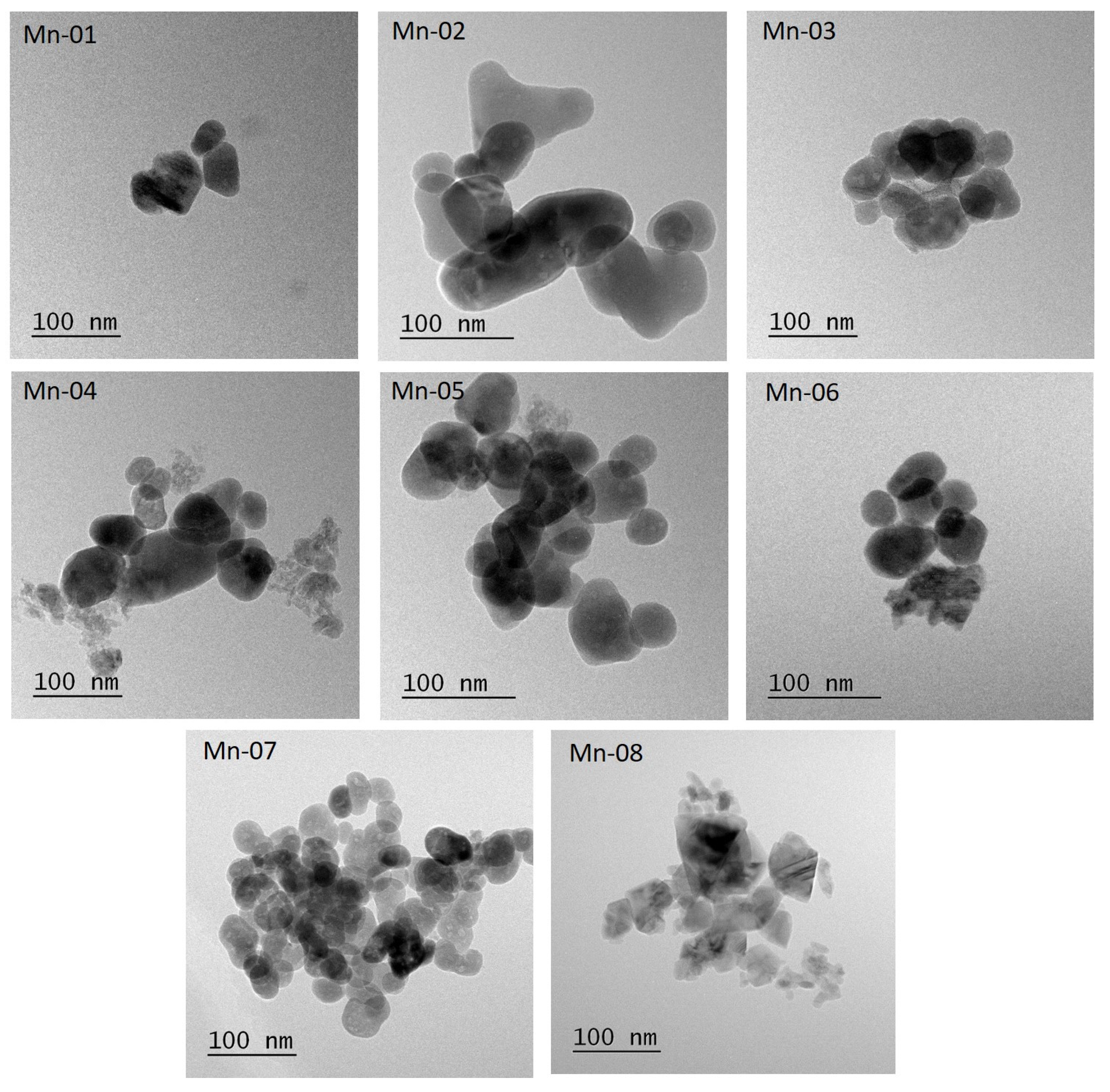
| Sample | Supplier | Surface Coating | Size, nm | Specific Surface Area, m2/g | |
|---|---|---|---|---|---|
| BET | Supplier 1 | ||||
| Mn-01 | US Research Nanomaterials | uncoated | 30 | 30.6 | 150 |
| Mn-02 | US Research Nanomaterials | PVP | 30 | 19.6 | - 2 |
| Mn-03 | US Research Nanomaterials | Stearic acid | 30 | 9.3 | - 2 |
| Mn-04 | US Research Nanomaterials | silane | 30 | 36 | - 2 |
| Mn-05 | US Research Nanomaterials | uncoated | <100 | 40.0 | 68 |
| Mn-06 | mKNano | uncoated | 50 | 28.9 | |
| Mn-07 | American Elements | uncoated | <100 | 29.4 | 20–50 |
| Mn-08 | Nanografi | uncoated | 28 | 30.7 | >155 |
| Sample | TEM | Supplier 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Shape 2 | n 3 | deq (Std Error), nm | SD, nm | Aspect Ratio | SD | Shape | Size, nm | |
| Mn-01 | SP, EL, SQ | 70 3 | 53 (2) | 17 | 1.3 | 0.2 | rods, flakes | 30 |
| Mn-02 | SP, EL, IRR | 111 | 54 (2) | 17 | 1.3 | 0.2 | 30 | |
| Mn-03 | SP, EL, IRR | 113 | 56 (3) | 27 | 1.3 | 0.2 | 30 | |
| Mn-04 | SP, EL, IRR, rods, plate | 130 | 49 (2) | 21 | 1.3 | 0.2 | 30 | |
| Mn-05 | SP, EL, rods | 163 | 47 (2) | 13 | 1.3 | 0.2 | spherical, rods | <100, aver 35 |
| Mn-06 | SP, EL, rods, plates | 83 | 41 (2) | 17 | 1.3 | 0.2 | 50 | |
| Mn-07 | SP, EL, rods, plates | 81 | 37 (2) | 14 | 1.3 | 0.2 | <100 | |
| Mn-08 | SP, EL, IRR | 84 | 37 (2) | 15 | 1.5 | 0.3 | spherical | 28 |
| Sample | Z-Average, nm | PDI | Zeta Potential, mV | Mn Content (%) (ICP-MS) |
|---|---|---|---|---|
| Mn-01 | 212 (5) | 0.34 (0.05) | 28.5 (0.2) | 63 (1) |
| Mn-02 | 266 (9) | 0.36 (0.03) | −11.2 (0.3) | 62 (2) |
| Mn-03 | >1000 | >0.4 | 2.1 (0.2) | 61 (1) |
| Mn-04 | 202 (5) | 0.27 (0.03) | 32.5 (0.6) | 63 (2) |
| Mn-05 | 229 (6) | 0.24 (0.02) | 33.2 (0.8) | 65 (1) |
| Mn-06 | 233 (7) | 0.33 (0.04) | 29.1 (0.4) | 63 (1) |
| Mn-07 | 244 (8) | 0.36 (0.06) | 28.3 (0.9) | 63 (2) |
| Mn-08 | 211 (5) | 0.29 (0.05) | 24.7 (0.3) | 64 (2) |
| Sample | 0 h | 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|---|---|
| Z-Average | PDI | Z-Average | PDI | Z-Average | PDI | Z-Average | PDI | |
| Mn-01 | 225 (6) | 0.19 (0.03) | 215 (7) | 0.16 (0.04) | 223 (3) | 0.16 (0.03) | 234 (4) | 0.22 (0.03) |
| Mn-02 | 247 (9) | 0.23 (0.01) | 244 (8) | 0.26 (0.03) | 253 (5) | 0.26 (0.04) | 255 (9) | 0.28 (0.04) |
| Mn-03 | >1000 | >0.5 | >1000 | >0.5 | >1000 | >0.5 | >1000 | >0.5 |
| Mn-04 | 258 (8) | 0.25 (0.05) | 252 (9) | 0.24 (0.04) | 247 (7) | 0.21 (0.03) | 253 (9) | 0.23 (0.03) |
| Mn-05 | 236 (6) | 0.23 (0.03) | 235 (3) | 0.19 (0.02) | 235 (3) | 0.21 (0.02) | 247 (7) | 0.26 (0.03) |
| Mn-06 | 244 (9) | 0.21 (0.01) | 237 (6) | 0.22 (0.01) | 247 (8) | 0.24 (0.03) | 247 (5) | 0.22 (0.03) |
| Mn-07 | 237 (5) | 0.23 (0.01) | 243 (2) | 0.22 (0.01) | 238 (5) | 0.21 (0.01) | 239 (6) | 0.22 (0.03) |
| Mn-08 | 218 (5) | 0.24 (0.04) | 234 (4) | 0.30 (0.05) | 234 (3) | 0.26 (0.04) | 228 (6) | 0.24 (0.03) |
| Sample ID | Caspase 1 Activity versus Control (±3%) | Caspase 3 Activity versus Control (±3%) |
|---|---|---|
| Mn-01 | 76% | 170% |
| Mn-02 | 100% | 170% |
| Mn-03 | 82% | 142% |
| Mn-04 | 82% | 200% |
| Mn-05 | 100% | 149% |
| Mn-06 | 84% | 110% |
| Mn-07 | 100% | 158% |
| Mn-08 | 100% | 152% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, L.J.; Du, X.; Zborowski, A.; Kennedy, D.C. Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles. Nanomaterials 2024, 14, 198. https://doi.org/10.3390/nano14020198
Johnston LJ, Du X, Zborowski A, Kennedy DC. Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles. Nanomaterials. 2024; 14(2):198. https://doi.org/10.3390/nano14020198
Chicago/Turabian StyleJohnston, Linda J., Xiaomei Du, Andre Zborowski, and David C. Kennedy. 2024. "Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles" Nanomaterials 14, no. 2: 198. https://doi.org/10.3390/nano14020198
APA StyleJohnston, L. J., Du, X., Zborowski, A., & Kennedy, D. C. (2024). Characterization and Cellular Toxicity Studies of Commercial Manganese Oxide Nanoparticles. Nanomaterials, 14(2), 198. https://doi.org/10.3390/nano14020198







