Mixed Systems of Quaternary Ammonium Foam Drainage Agent with Carbon Quantum Dots and Silica Nanoparticles for Improved Gas Field Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
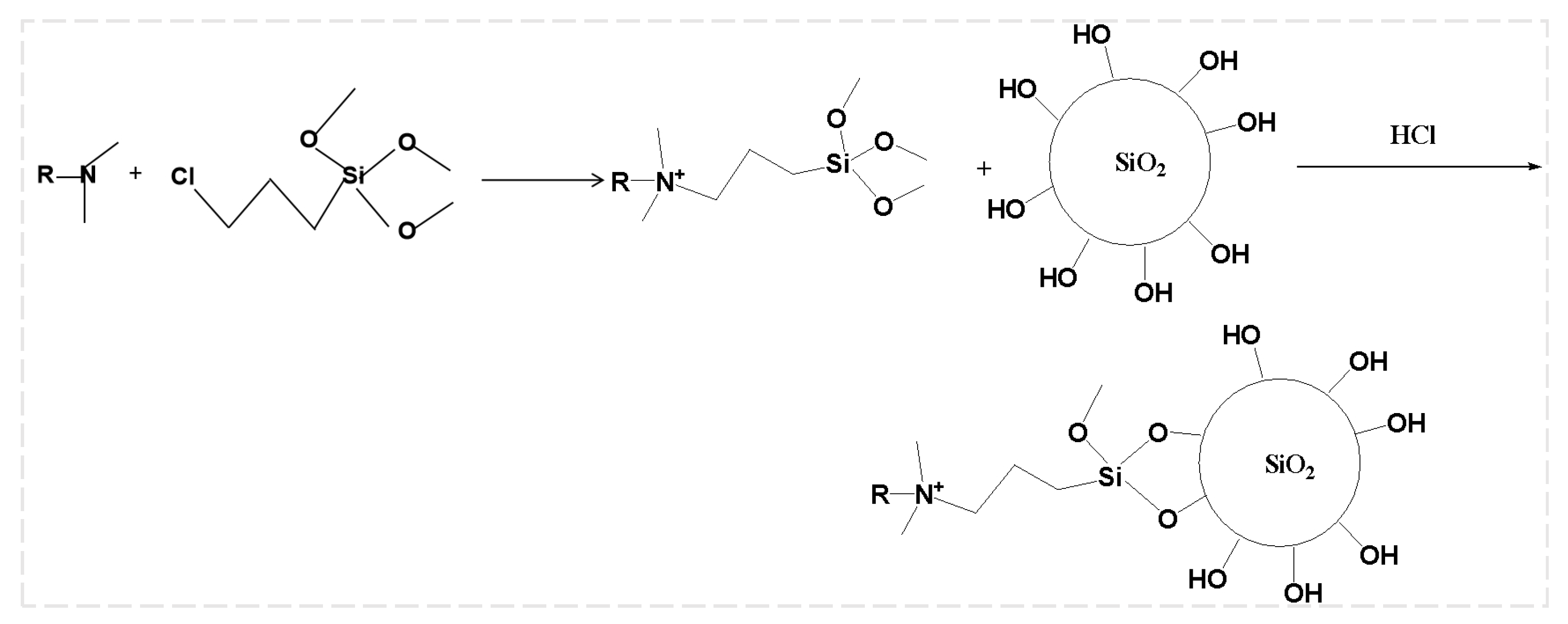
2.2.1. Synthesis of Foam Drainage Agent
Selection of Molecular Structure for Foam Drainage Agent
Synthesis Process of Foam Drainage Agent
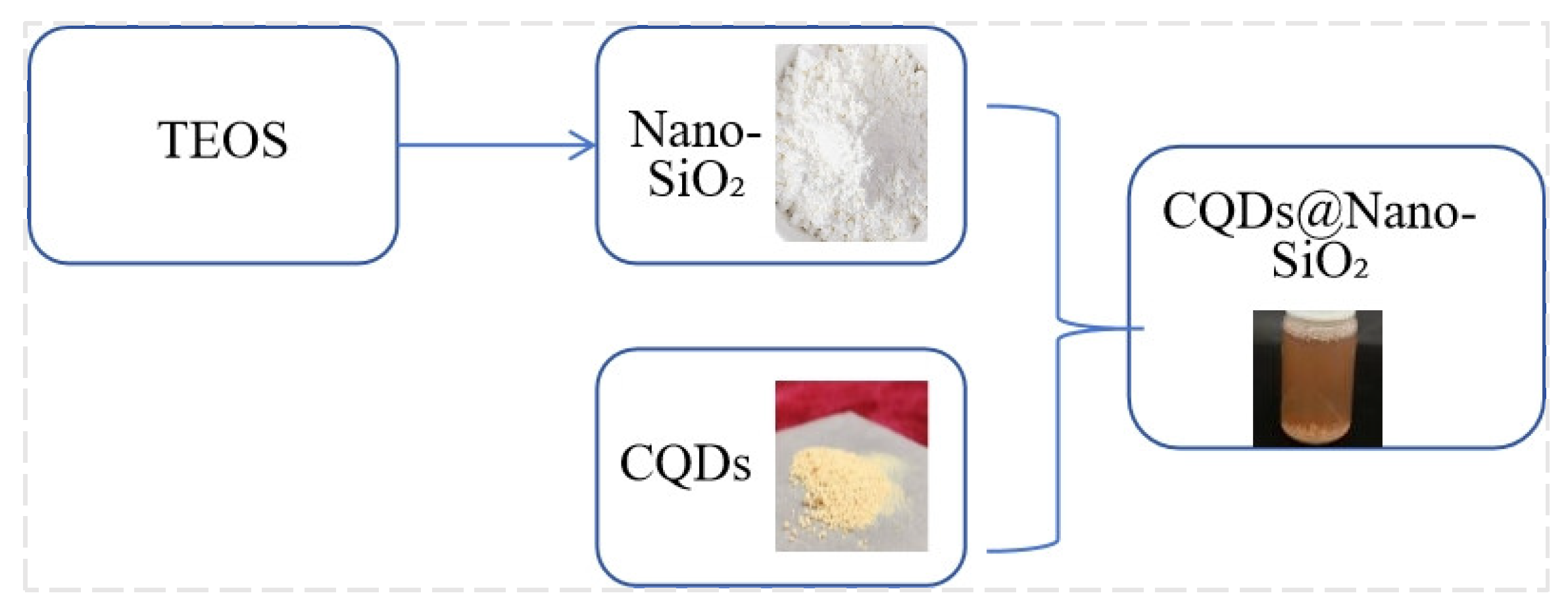
2.2.2. Synthesis of Foam Stabilizers
Synthesis of Silica Nanoparticles via the Stöber Method
Synthesis of Modified Nano-SiO2 Particles
Preparation of Carbon Quantum Dots
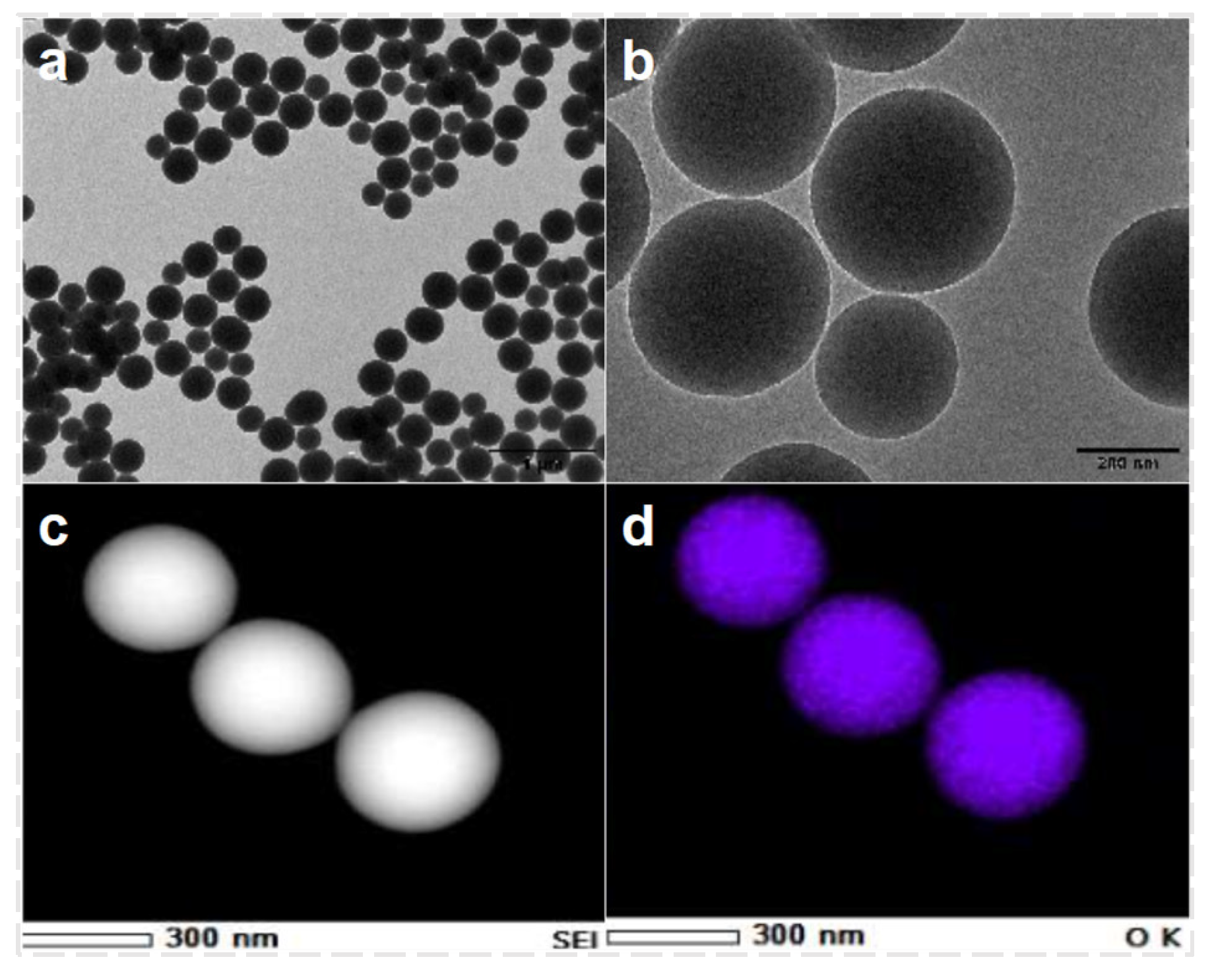
Assembly of CQDs/Nano-SiO2 Nanoparticles
3. Compound Experiment
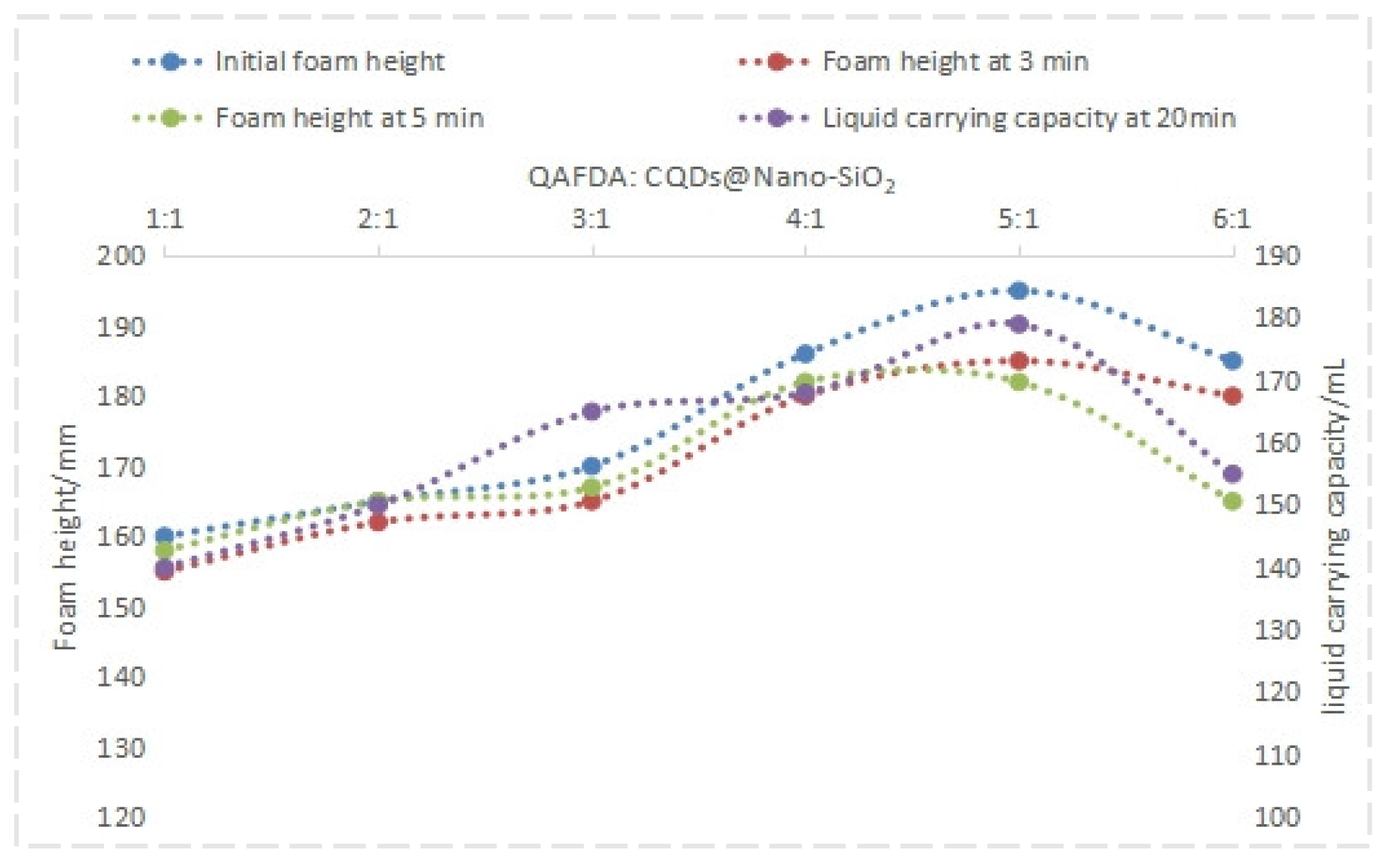
3.1. Determination of Foam Drainage Agent Dosage
3.2. Compound Experiment Condition
4. Results and Discussion
4.1. Performance Evaluation of Foam Drainage System
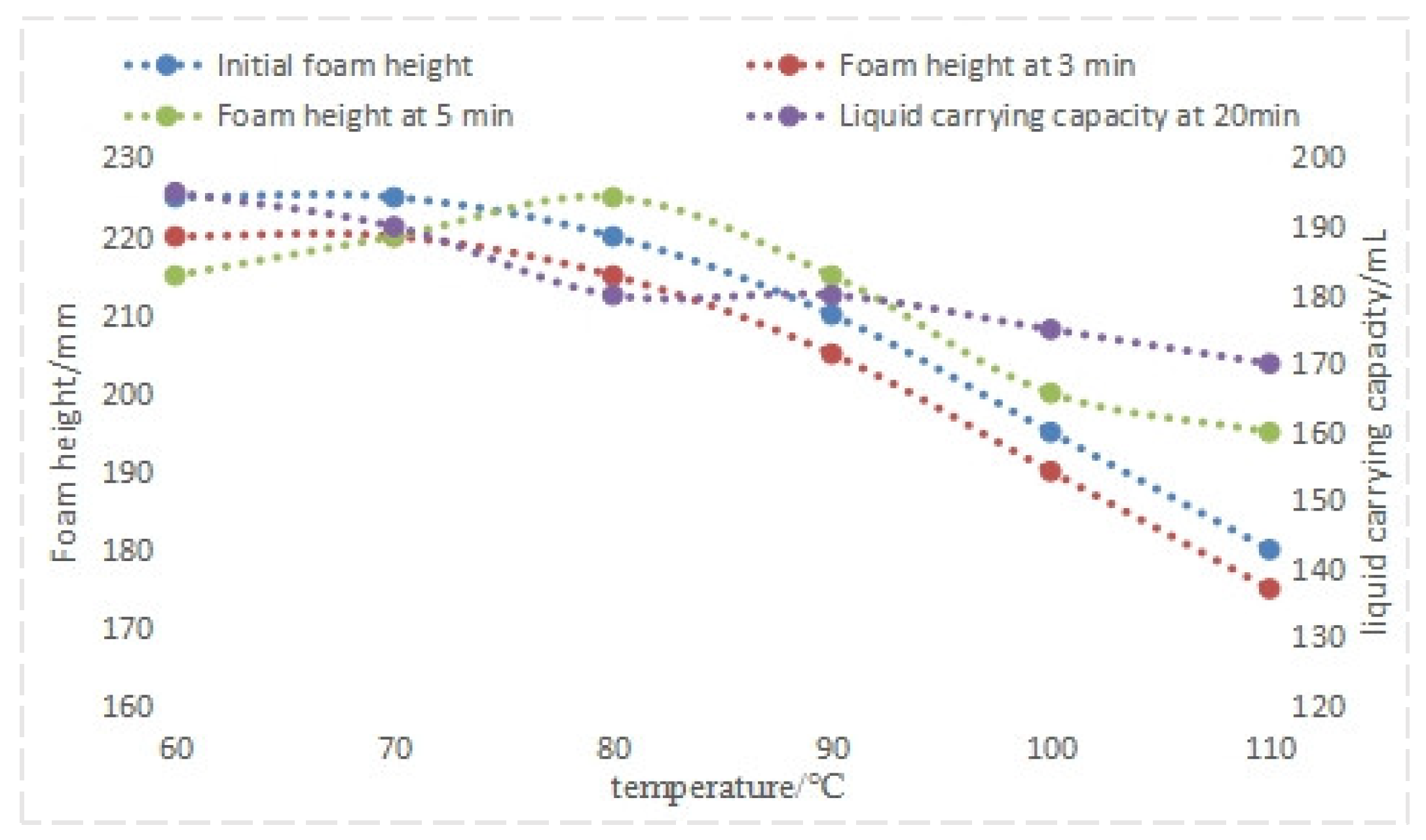
4.1.1. Evaluation of Temperature Resistance
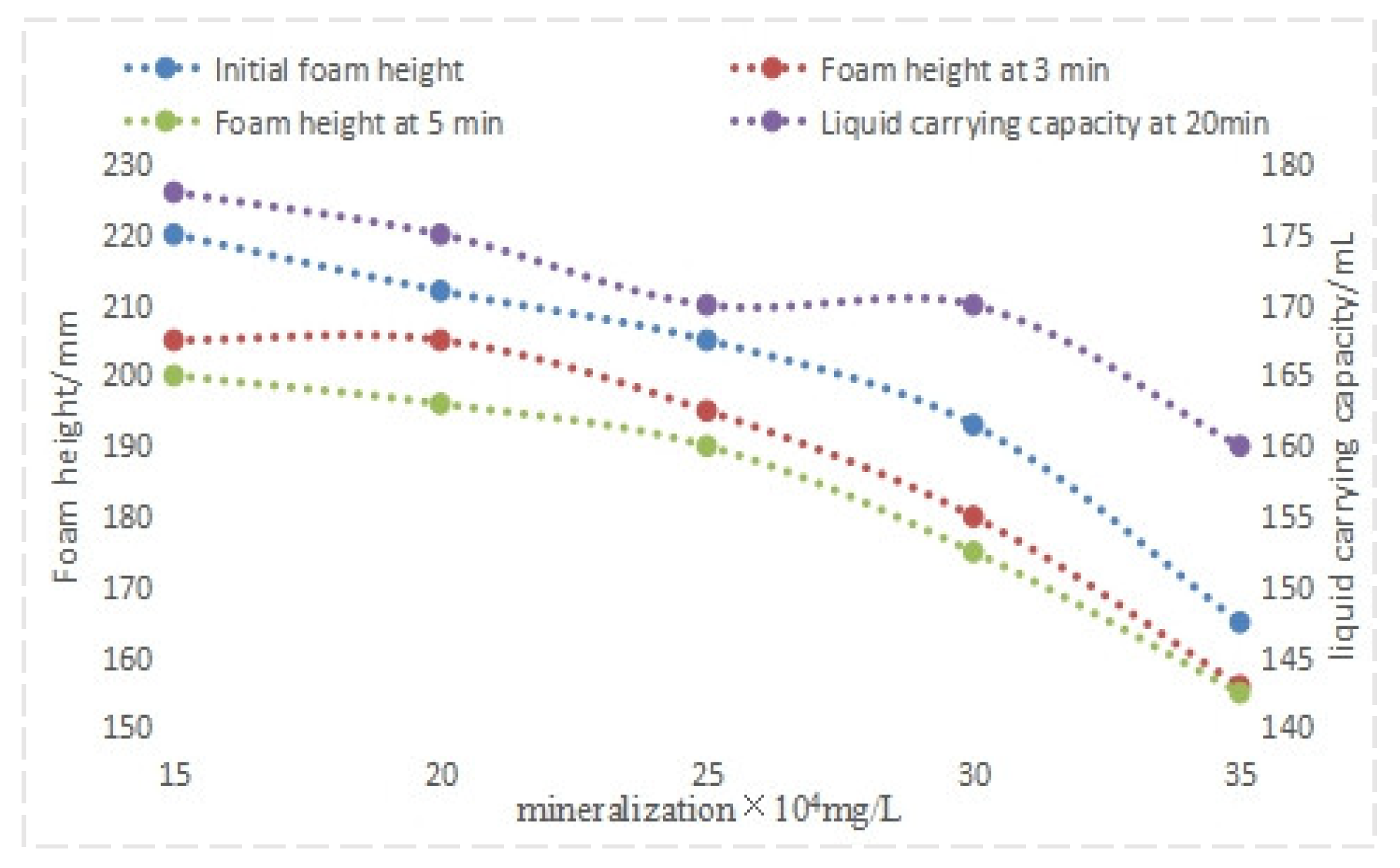
4.1.2. Evaluation of Mineralization Resistance
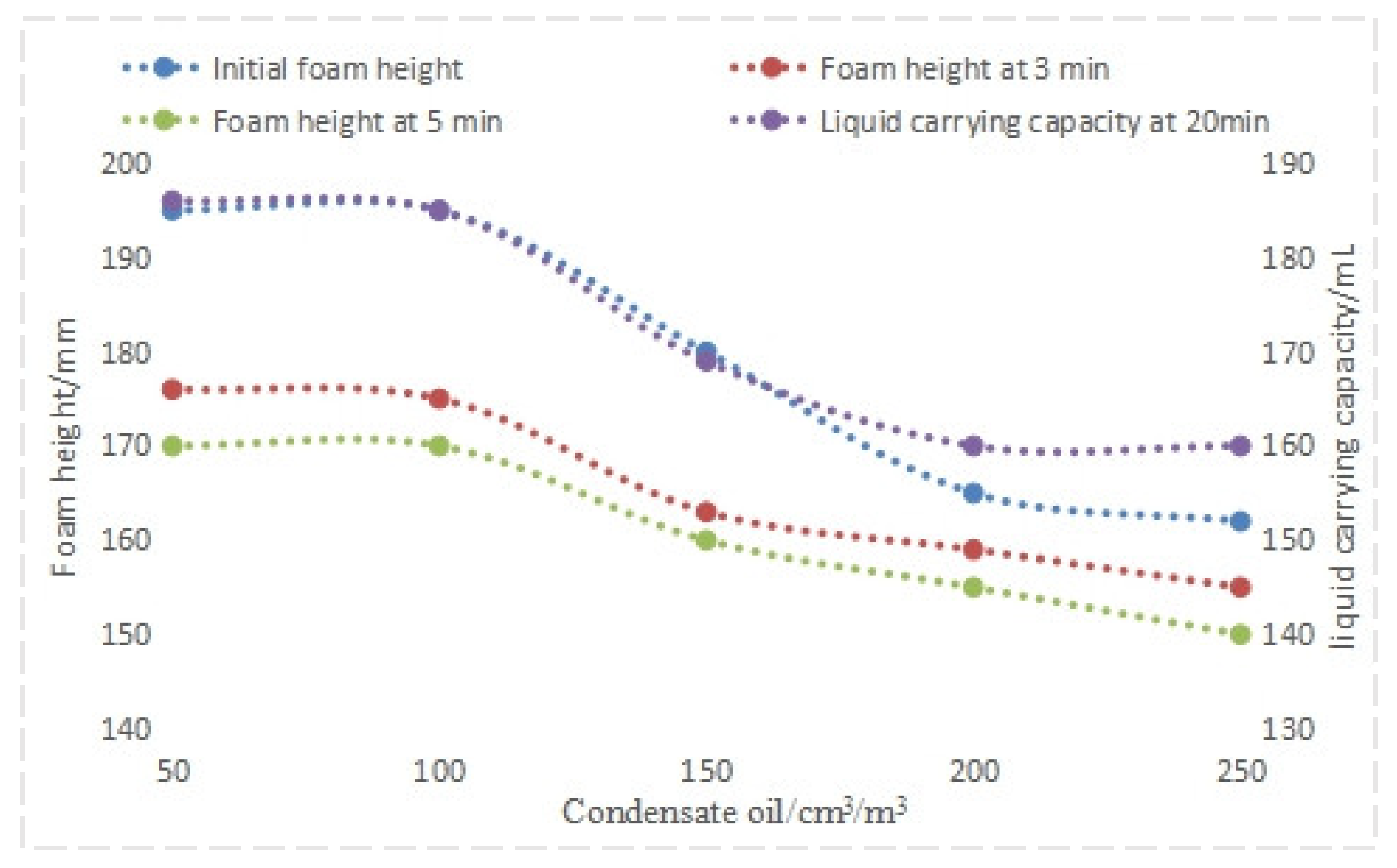
4.1.3. Evaluation of Anti-Condensate Oil Performance
4.1.4. Effect of Different Aging Times on the Performance of the Foam Drainage Agent System
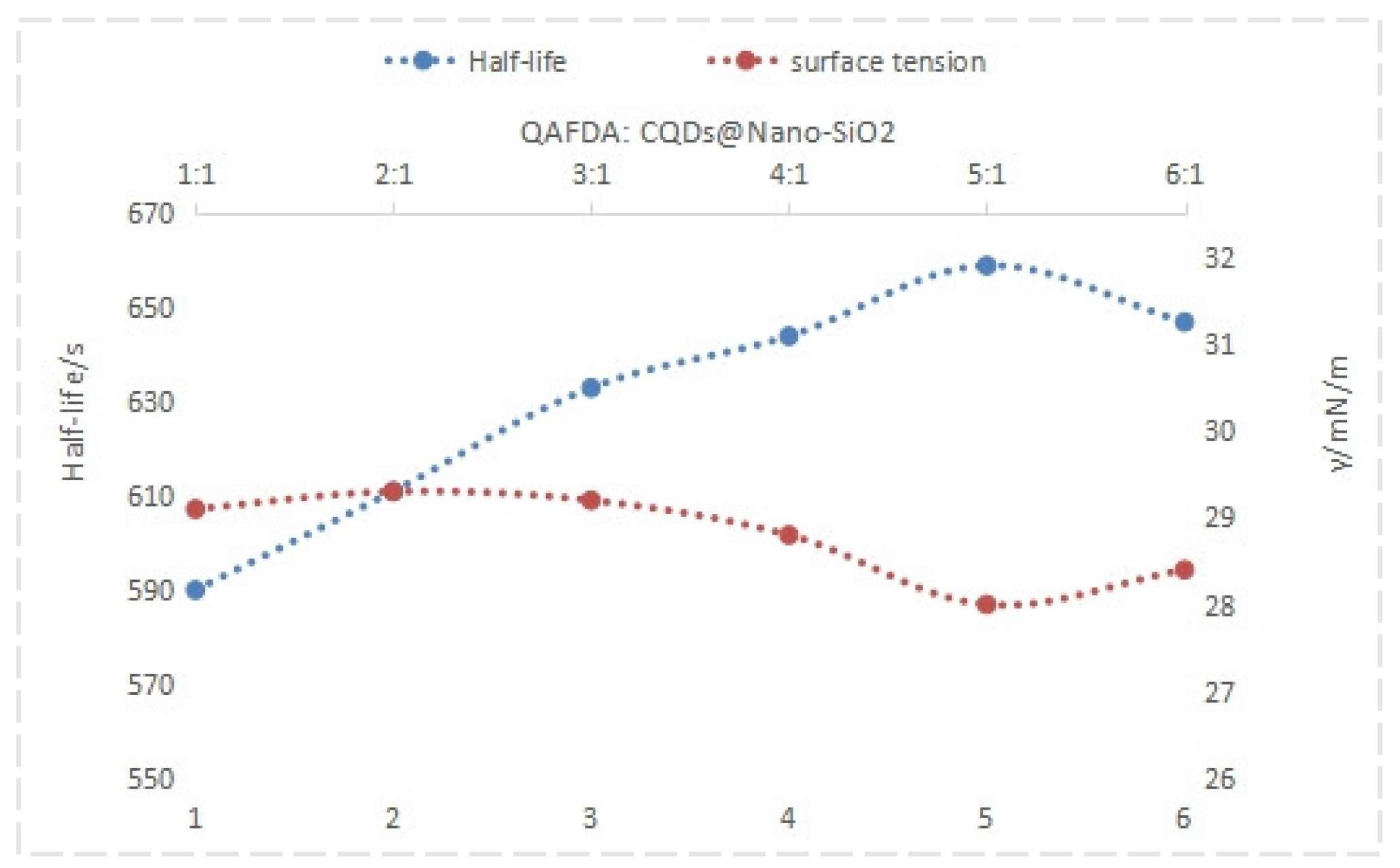
4.2. Effect of Foam Drainage Agent Concentration on Surface Tension and Half-Life
4.2.1. Effect of Foam Drainage Agent Concentration on Solution Surface Tension
4.2.2. Effect of Concentration on the Half-Life of Foam Drainage Agent
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Serial number | Abbreviated form | Full English name |
| 1 | CQDs | Carbon Quantum Dots |
| 2 | CQDs/Nano-SiO2 | Carbon Quantum Dots/Silica Nanoparticles |
| 3 | Nano-SiO2 | Silica Nanoparticles |
| 4 | QAFDA | Quaternary Ammonium Foam Drainage Agent |
| 5 | TEOS | Tetraethyl Orthosilicate |
References
- Qingchun, Y. A Study of Management System Based on Energy Crisis. IOP Conf. Ser. Earth Environ. Sci. 2018, 153, 032001. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, S.; Wang, L.; Zhang, X.; Ma, J.; Zeng, K.; Tian, J.; Wang, M.; Li, R.; Jing, Z.; et al. Feasibility Study of Swash Plate Plunger Pump System in Drainage Gas Recovery Process. J. Phys. Conf. Ser. 2021, 1983, 012036. [Google Scholar] [CrossRef]
- Yang, S.; Xu, D.; Liu, L.; Duan, C.; Xiu, L. Research of Drainage Gas Recovery Technology in Gas Wells. OJFD 2014, 4, 154–162. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Xue, Y.; Yao, E.; Zhang, L.; Fan, F.; Wang, R. The Adsorption Properties of a Novel Ether Nanofluid for Gas Wetting of Tight Sandstone Reservoir. Pet. Sci. Technol. 2019, 37, 1436–1454. [Google Scholar] [CrossRef]
- Chen, M.; Sun, J.; Gao, E.; Tian, H. A Summary of Wellbore Fluid Accumulation and Drainage Gas Production Technology in Gas Wells. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012113. [Google Scholar] [CrossRef]
- Xiong, C.; Cao, G.; Zhang, J.; Li, N.; Xu, W.; Wu, J.; Li, J.; Zhang, N. Nanoparticle Foaming Agents for Major Gas Fields in China. Pet. Explor. Dev. 2019, 46, 1022–1030. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, Q.; Wang, Y.; Li, S.; Sun, S.; Hu, S. Study on the Influence of the External Conditions and Internal Components on Foam Performance in Gas Recovery. Chem. Eng. Sci. 2021, 231, 116279. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Panuganti, S.R.; Khemka, Y.; Valdes, H.; Vargas, F.M. Foam-Assisted Gas Lift: A Novel Experimental Setup to Investigate the Feasibility of Using a Commercial Surfactant for Increasing Oil Well Productivity. J. Pet. Sci. Eng. 2021, 201, 108496. [Google Scholar] [CrossRef]
- Bowman, C.W.; Collins, J.A. Increasing the Production from Marginal Gas Wells. In Proceedings of the SPE International Oilfield Corrosion Symposium, Aberdeen, UK, 30 May 2006; p. SPE-100514-MS. [Google Scholar]
- Farina, L.; Passucci, C.; Di Lullo, A.; Negri, E.; Anderson, S.; Page, S. Artificial Lift Optimization with Foamer Technology in the Alliance Shale Gas Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012; p. SPE-160282-MS. [Google Scholar]
- Guan, J.; Liang, L.; Zhao, Y.; Sun, N.; Lu, W.; Zhen, Y. Study on Screening and Evaluation of Foam Drainage Agents for Gas Wells with High Temperature and High Pressure. ACS Omega 2023, 8, 7940–7949. [Google Scholar] [CrossRef]
- Zhao, L.; Li, A.; Chen, K.; Tang, J.; Fu, S. Development and Evaluation of Foaming Agents for High Salinity Tolerance. J. Pet. Sci. Eng. 2012, 81, 18–23. [Google Scholar] [CrossRef]
- Qi, H.; Bai, Z.; Zhang, Q.; Lai, X. Synthesis of a Gemini Betaine Surfactant and Its Properties as Foam Drainage Agent. Tenside Surfactants Deterg. 2018, 55, 142–147. [Google Scholar] [CrossRef]
- Retamal, F.; Solar, C.; Saavedra, J.H.; Quezada, G.R.; Orvalho, S.; Toledo, P.G. Effect of Aliphatic Alcohol-Based and Polyglycol Polymer-Based Foaming Agents on the Water-Liquid-Vapor Interface by Means of Molecular Dynamics. J. Mol. Liq. 2024, 407, 125279. [Google Scholar] [CrossRef]
- Xu, X.; Saeedi, A.; Liu, K. Laboratory Studies on CO2 Foam Flooding Enhanced by a Novel Amphiphilic Ter-Polymer. J. Pet. Sci. Eng. 2016, 138, 153–159. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, Q.; Yuan, C.; Wang, H.; Li, C.; Sun, S.; Hu, S. Molecular Dynamics Simulation of the Influence of Polyacrylamide on the Stability of Sodium Dodecyl Sulfate Foam. Chem. Eng. Sci. 2017, 166, 313–319. [Google Scholar] [CrossRef]
- Truong, T.T.; Mondal, S.; Doan, V.H.M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.D.; Misra, M.; Lee, B.; Oh, J. Precision-Engineered Metal and Metal-Oxide Nanoparticles for Biomedical Imaging and Healthcare Applications. Adv. Colloid Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef]
- Aydoğan, C.; Beltekin Çakan, B.; Alharthi, S.; Ali, A.; Göktürk, I.; Yılmaz, F.; Denizli, A.; Rassi, Z.E. Recent Advances and Applications in Drug Analysis by Nano-Scale Separation Techniques. Green Anal. Chem. 2024, 10, 100131. [Google Scholar] [CrossRef]
- Yadav, K.K.; Cabral-Pinto, M.M.S.; Gacem, A.; Fallatah, A.M.; Ravindran, B.; Rezania, S.; Algethami, J.S.; Bashier Eltayeb, L.; Abbas, M.; Hassan Al-shareef, T.; et al. Recent Advances in the Application of Nanoparticle-Based Strategies for Water Remediation as a Novel Clean Technology–A Comprehensive Review. Mater. Today Chem. 2024, 40, 102226. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, M.; He, J.; Ni, P. A Porous Cross-Linked Gel Polymer Electrolyte Separator for Lithium-Ion Batteries Prepared by Using Zinc Oxide Nanoparticle as a Foaming Agent and Filler. Electrochim. Acta 2018, 292, 769–778. [Google Scholar] [CrossRef]
- Chaudhry, A.U.; Muneer, R.; Lashari, Z.A.; Hashmet, M.R.; Osei-Bonsu, K.; Abdala, A.; Rabbani, H.S. Recent Advancements in Novel Nanoparticles as Foam Stabilizer: Prospects in EOR and CO2 Sequestration. J. Mol. Liq. 2024, 407, 125209. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Wang, Y.; Niu, J.; Qin, J. Preparation and Properties of Carbon Dioxide Foamed Concrete Using Nanoparticles as Foam Stabilizer and Pre–Carbonated Cement Slurry. Cem. Concr. Compos. 2024, 151, 105585. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bryant, S.L.; Huh, C.; Johnston, K.P. Carbon Dioxide-in-water Foams Stabilized with Nanoparticles and Surfactant Acting in Synergy. AIChE J. 2013, 59, 3490–3501. [Google Scholar] [CrossRef]
- Latif, W.M.S.M.; Sharbini, S.N.; Wan Sulaiman, W.R.; Idris, A.K. Utilization of Silicon Dioxide Nanoparticles in Foam Enhanced Oil Recovery—A Comprehensive Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 469, 012027. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, X.; Liu, J.; Sun, D.; Zhang, X.; Zhang, C.; Liu, J. Effects of Inorganic Salts and Polymers on the Foam Performance of 1-Tetradecyl-3-methylimidazolium Bromide Aqueous Solution. J. Surfact. Deterg. 2012, 15, 613–621. [Google Scholar] [CrossRef]
- Lai, L.; Zhang, T.; Zheng, C. Study of Foam Drainage Agent Based on G-C3N4 Nanosheets Reinforced Stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130607. [Google Scholar] [CrossRef]
- Lai, N.; He, Y.; Zhang, X.; Deng, J.; Wang, Z. Synthesis and Performance Evaluation of Temperature and Salt-Resistant Foam Drainage Agent XY-1. Arab. J. Sci. Eng. 2023, 48, 8911–8923. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Li, C.; Zhang, D.; Cao, X.; Song, X.; Wang, Q.; Li, Y. Molecular Array Behavior and Synergistic Effect of Sodium Alcohol Ether Sulphate and Carboxyl Betaine/Sulfobetaine in Foam Film under High Salt Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 138–148. [Google Scholar] [CrossRef]
- Osei-Bonsu, K.; Shokri, N.; Grassia, P. Foam Stability in the Presence and Absence of Hydrocarbons: From Bubble- to Bulk-Scale. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 514–526. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hou, Q.F.; Zhu, Y.Y.; Li, W.J.; Chang, Z.D. Foam Stability of Mixed System of Fluorocarbon and Hydrocarbon Surfactants: Effect of Polymer and Oil. AMR 2013, 803, 85–89. [Google Scholar] [CrossRef]
- Simjoo, M.; Rezaei, T.; Andrianov, A.; Zitha, P.L.J. Foam Stability in the Presence of Oil: Effect of Surfactant Concentration and Oil Type. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 148–158. [Google Scholar] [CrossRef]
- Gieg, L.M.; Duncan, K.E.; Suflita, J.M. Bioenergy Production via Microbial Conversion of Residual Oil to Natural Gas. Appl. Environ. Microbiol. 2008, 74, 3022–3029. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Lee, J.; Nikolov, A.; Wasan, D. Surfactant Micelles Containing Solubilized Oil Decrease Foam Film Thickness Stability. J. Colloid Interface Sci. 2014, 415, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, G.; Yuan, S. The Effect of Betaine on the Foam Stability: Molecular Simulation. Appl. Surf. Sci. 2017, 407, 156–161. [Google Scholar] [CrossRef]
- Xu, L.; Rad, M.D.; Telmadarreie, A.; Qian, C.; Liu, C.; Bryant, S.L.; Dong, M. Synergy of Surface-Treated Nanoparticle and Anionic-Nonionic Surfactant on Stabilization of Natural Gas Foams. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 176–185. [Google Scholar] [CrossRef]
- Madhu, H.C.; Kailas, S.V. Fabrication of Localised Aluminium Foam by a Novel Polymeric Blowing Agent. Mater. Charact. 2018, 142, 340–351. [Google Scholar] [CrossRef]
- Yaagoob, I.Y.; Goni, L.K.M.O.; Ossoss, K.M.; Mazumder, M.A.J.; Ali, S.A.; Alfantazi, A.; Verma, C. Synthesis, Characterization, and Inhibition Potential of a Tosylate-Based Dicationic Surfactant for Mild Steel in Pickling Solution. Arab. J. Sci. Eng. 2024. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, L.; Cheng, H.; Xu, W.; Li, G.; Zhang, Y.; Gu, J.; Chen, L.; Xie, Z.; Li, Z.; et al. Negatively Charged Carbon Dots Employed Symplastic and Apoplastic Pathways to Enable Better Plant Delivery than Positively Charged Carbon Dots. ACS Nano 2024, 18, 34. [Google Scholar] [CrossRef]
- Pavanello, L.; Cortês, I.T.; De Carvalho, R.D.P.; Picolo, M.Z.D.; Cavalli, V.; Silva, L.T.S.; Boaro, L.C.C.; Prokopovich, P.; Cogo-Müller, K. Physicochemical and Biological Properties of Dental Materials and Formulations with Silica Nanoparticles: A Narrative Review. Dent. Mater. 2024, in press. [Google Scholar] [CrossRef]
- Farid Shayegan, F.; Babaei, R.; Mizban, A. Nano Coating Containing SiO2 Nanoparticles with Non-Stick Properties for Easy Peeling of Car Paint from Mild Steel Surfaces. Int. J. Adhes. Adhes. 2024, 134, 103800. [Google Scholar] [CrossRef]
- Ramesh, P.; Okazaki, T.; Taniguchi, R.; Kimura, J.; Sugai, T.; Sato, K.; Ozeki, Y.; Shinohara, H. Selective Chemical Vapor Deposition Synthesis of Double-Wall Carbon Nanotubes on Mesoporous Silica. J. Phys. Chem. B 2005, 109, 1141–1147. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Fang, C.; Zhao, T.; Zhao, W.; Du, Y.; Chen, J. Assembly of CQDs/Mesoporous SiO2/VO2 Composites with Wide Optical Response and Abnormal Phase Transition Temperature. Diam. Relat. Mater. 2023, 137, 110138. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, C.; Zhang, L.; Yang, W. Synthesis of Silica Particles with Controlled Microstructure via the Choline Hydroxide Cocatalyzed Stöber Method. Langmuir 2024, 40, 15933–15940. [Google Scholar] [CrossRef]
- Naat, J.N.; Suyanta, S.; Nuryono, N. Hydrophobic Modification of Naturally Magnetic Silica with Methyltrimethoxysilane for Enhanced Adsorption of Chloramphenicol and Ciprofloxacin. Case Stud. Chem. Environ. Eng. 2024, 10, 100878. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, Y.; Lai, X.; Li, H.; Chen, W.; Zeng, X. Synergistically Improving Tracking Resistance of Addition-Cure Liquid Silicone Rubber with Aminepropyltriethoxysilane-Immobilized Silica and Platinum Catalyst. Silicon 2024. [Google Scholar] [CrossRef]
- Slewa, L.H. Antifungal Films for Strawberry Packaging Using Carbon Quantum Dots Derived from Lemon and Onion Juice via Green Hydrothermal Method. Food Biosci. 2024, 61, 104653. [Google Scholar] [CrossRef]
- Han, X.; Han, Y.; Huang, H.; Zhang, H.; Zhang, X.; Liu, R.; Liu, Y.; Kang, Z. Synthesis of Carbon Quantum Dots/SiO2 Porous Nanocomposites and Their Catalytic Ability for Photo-Enhanced Hydrocarbon Selective Oxidation. Dalton Trans. 2013, 42, 10380. [Google Scholar] [CrossRef]
- Xu, G.; Gong, X.; Yu, Y.; Chen, X. A Rapid Method for the Determination of SBS Content Based on the Principle of Orthogonal Testing. Appl. Sci. 2021, 11, 10911. [Google Scholar] [CrossRef]
- Ablat, M.A.; Qattawi, A.; Jaman, M.S.; Alafaghani, A.; Yau, C.; Soshi, M.; Sun, J.-Q. An Experimental and Analytical Model for Force Prediction in Sheet Metal Forming Process Using Perforated Sheet and Origami Principles. Procedia Manuf. 2020, 48, 407–418. [Google Scholar] [CrossRef]




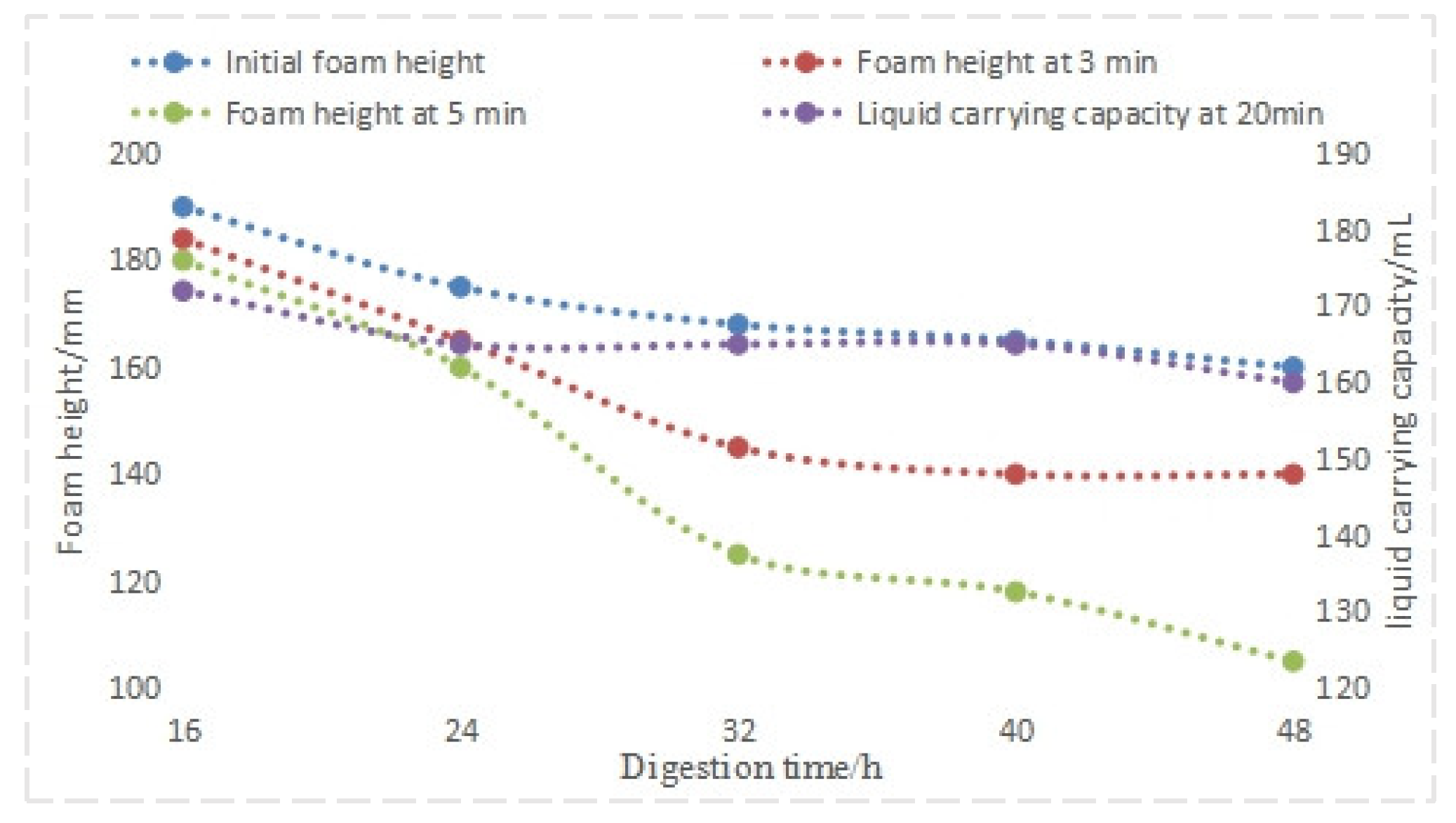
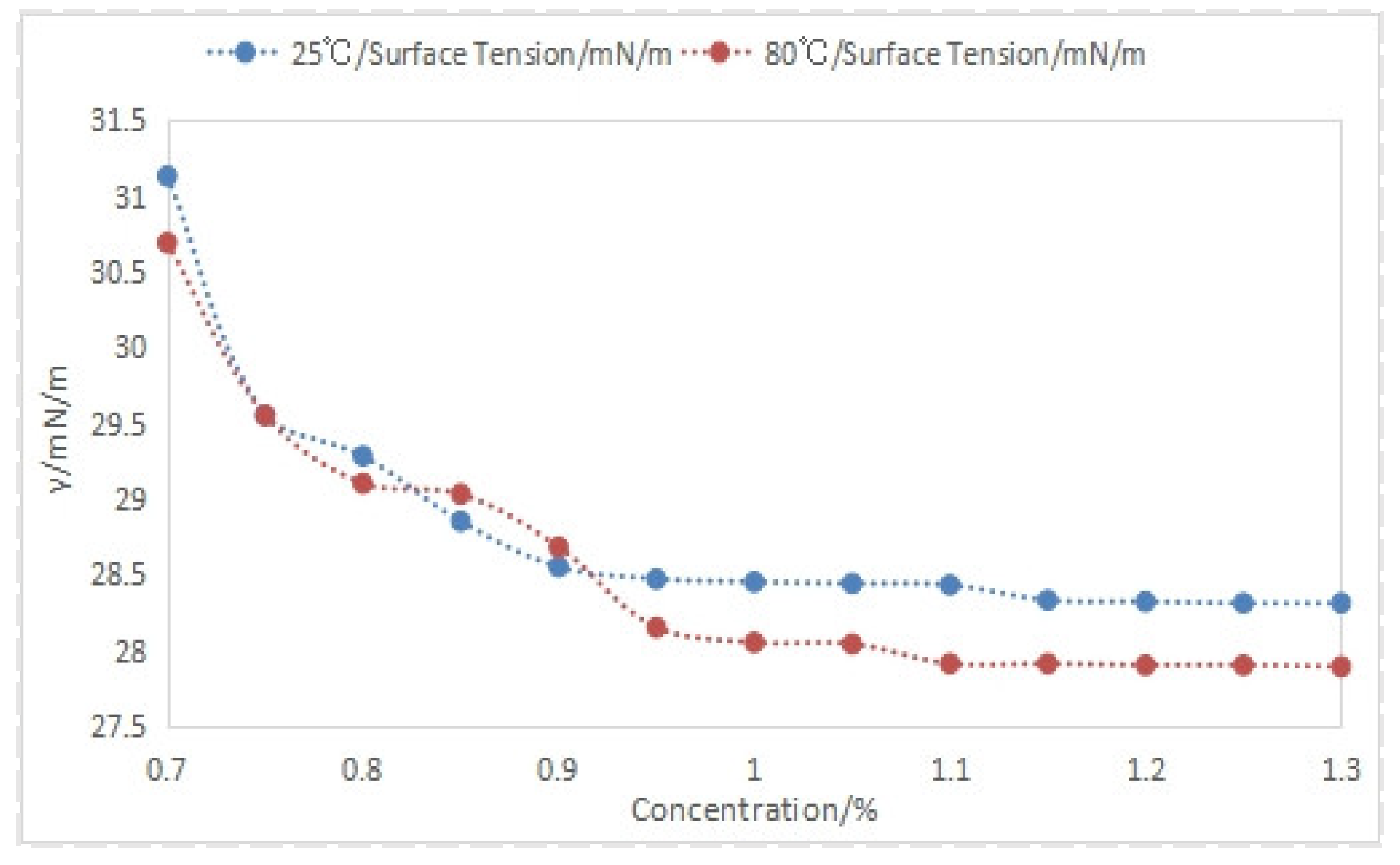


| Concentration/% | Formation Water (29.84 × 104 mg/L) | Simulated Water (35 × 104 mg/L) |
|---|---|---|
| 1.0 | 725 | 632 |
| 1.1 | 789 | 659 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Y.; Wei, A.; Shan, X.; Liu, Q.; Fan, Z.; Sun, A.; Zhu, L.; Kong, L. Mixed Systems of Quaternary Ammonium Foam Drainage Agent with Carbon Quantum Dots and Silica Nanoparticles for Improved Gas Field Performance. Nanomaterials 2024, 14, 1590. https://doi.org/10.3390/nano14191590
Sun Y, Zhang Y, Wei A, Shan X, Liu Q, Fan Z, Sun A, Zhu L, Kong L. Mixed Systems of Quaternary Ammonium Foam Drainage Agent with Carbon Quantum Dots and Silica Nanoparticles for Improved Gas Field Performance. Nanomaterials. 2024; 14(19):1590. https://doi.org/10.3390/nano14191590
Chicago/Turabian StyleSun, Yongqiang, Yongping Zhang, Anqi Wei, Xin Shan, Qingwang Liu, Zhenzhong Fan, Ao Sun, Lin Zhu, and Lingjin Kong. 2024. "Mixed Systems of Quaternary Ammonium Foam Drainage Agent with Carbon Quantum Dots and Silica Nanoparticles for Improved Gas Field Performance" Nanomaterials 14, no. 19: 1590. https://doi.org/10.3390/nano14191590
APA StyleSun, Y., Zhang, Y., Wei, A., Shan, X., Liu, Q., Fan, Z., Sun, A., Zhu, L., & Kong, L. (2024). Mixed Systems of Quaternary Ammonium Foam Drainage Agent with Carbon Quantum Dots and Silica Nanoparticles for Improved Gas Field Performance. Nanomaterials, 14(19), 1590. https://doi.org/10.3390/nano14191590






