A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60
Abstract
1. Introduction
2. Materials and Methods
3. Results
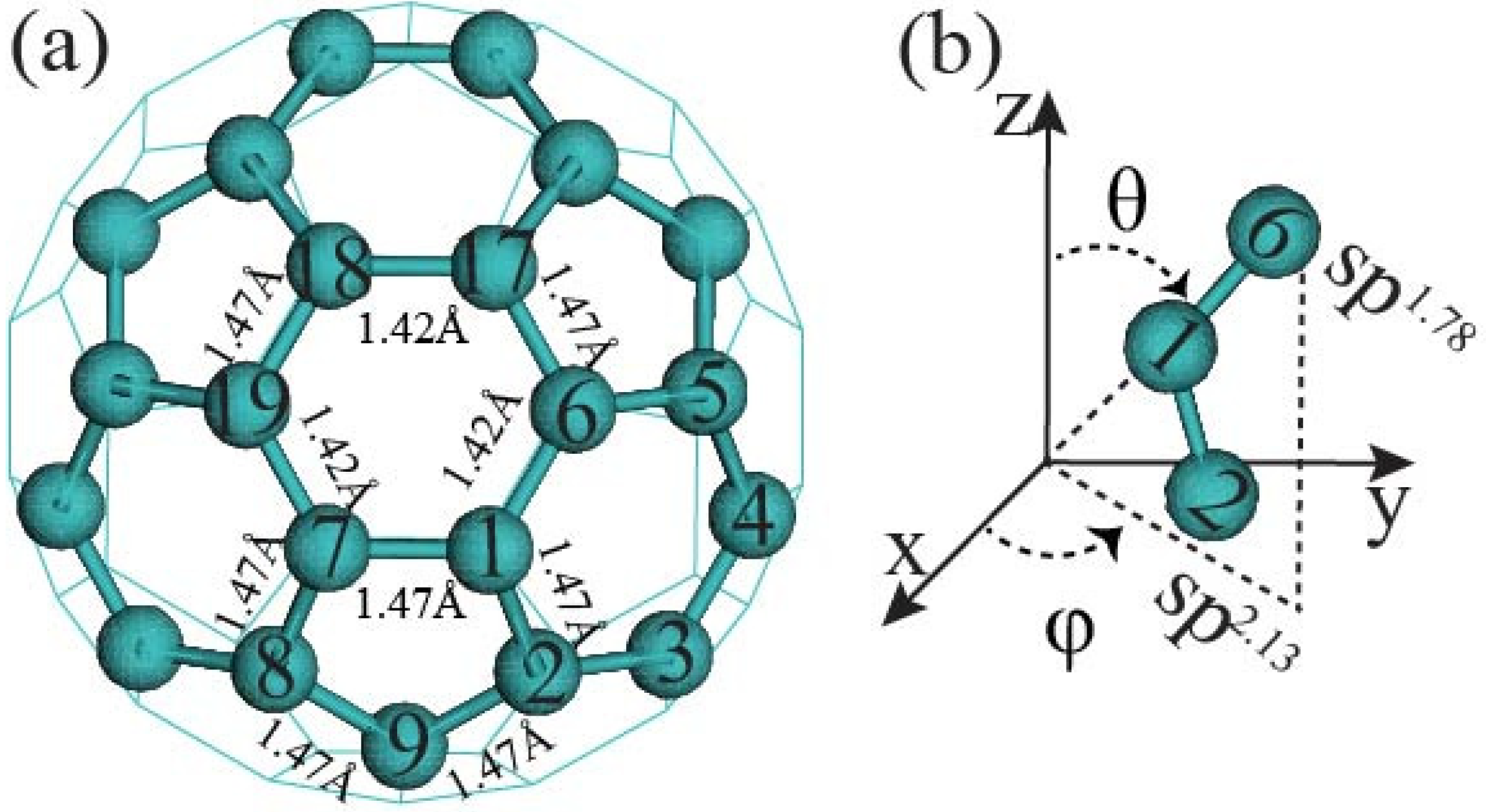
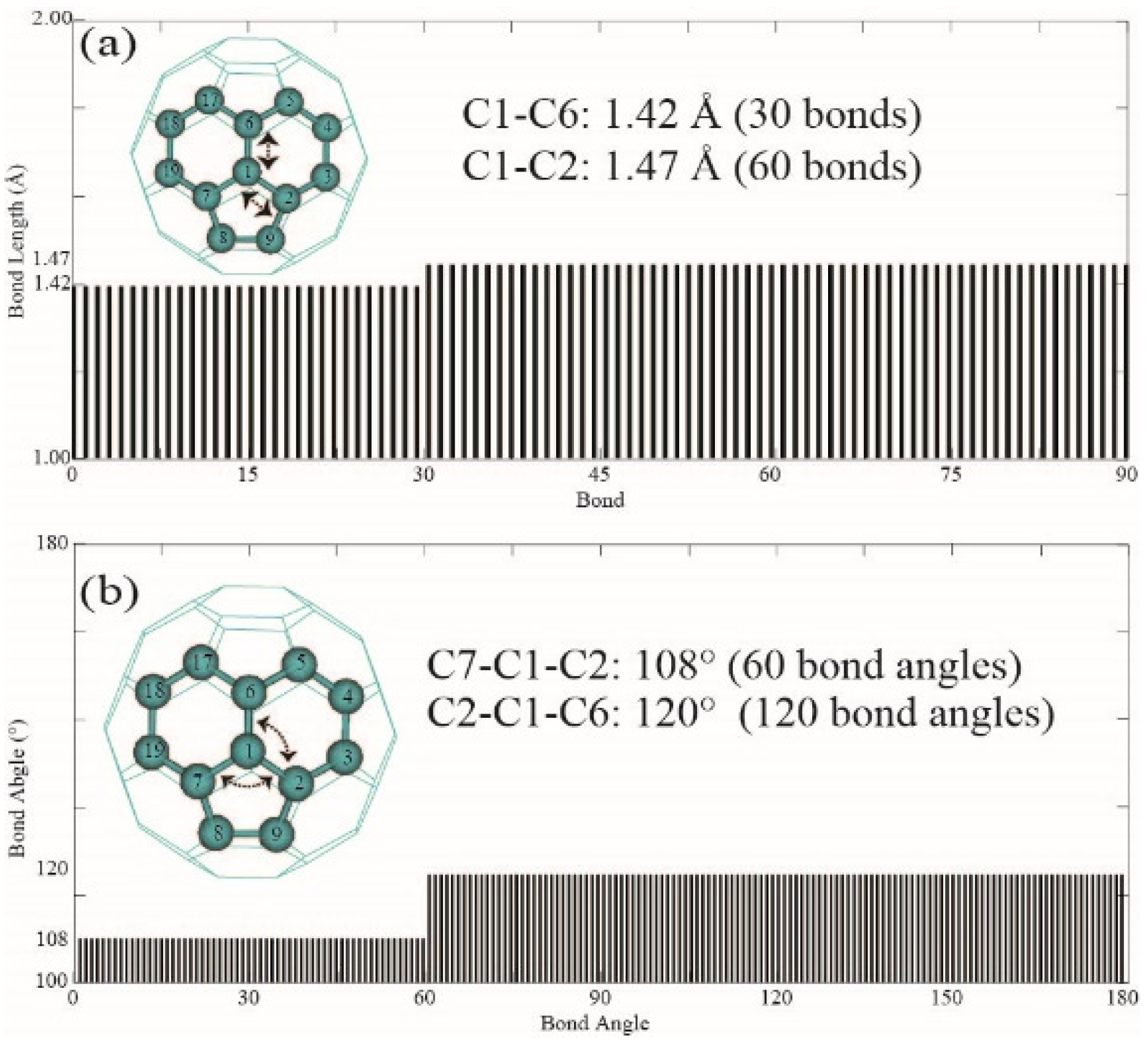
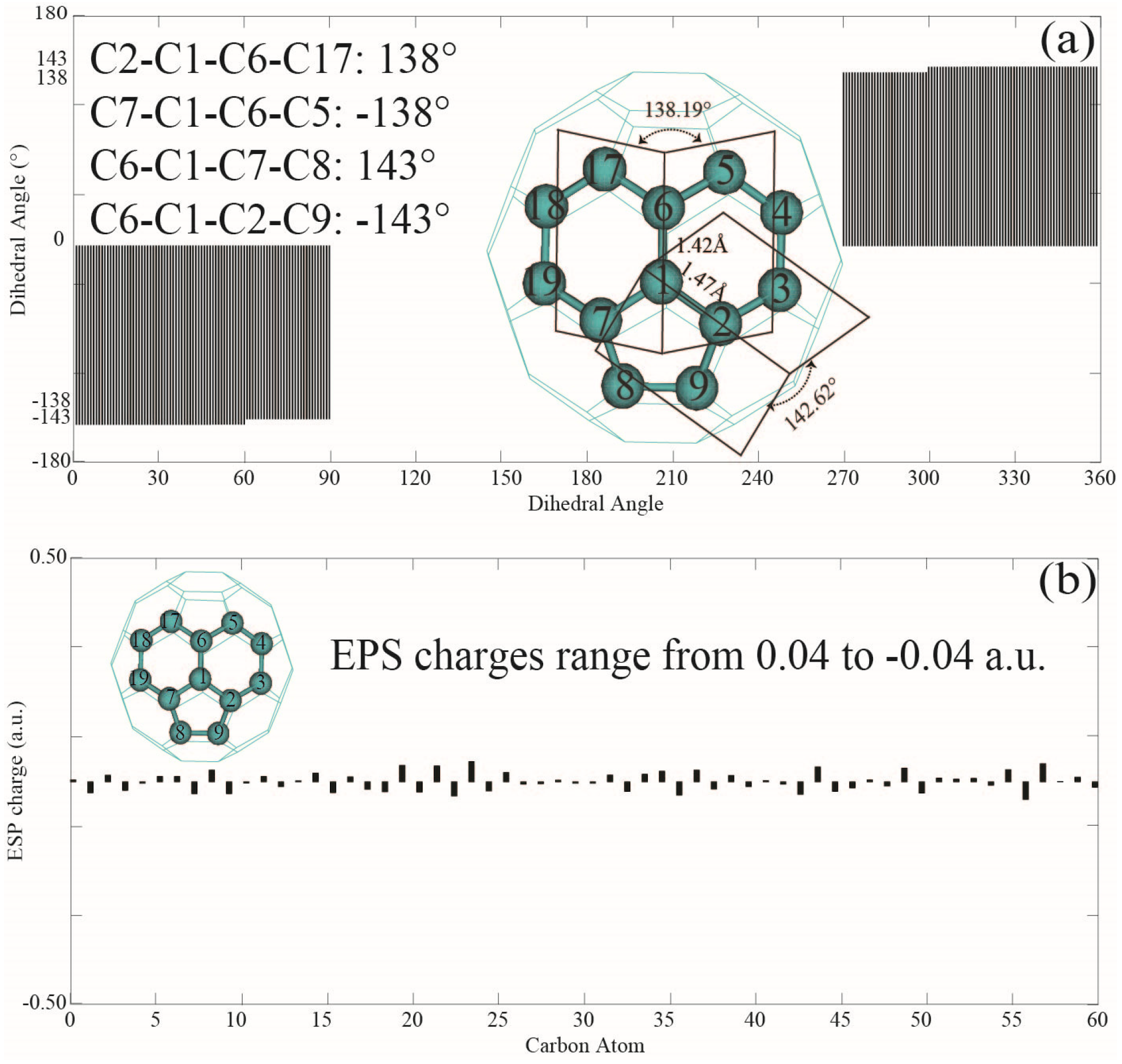
3.1. Bond Length, Bond Angle, and Dihedral Angles of Fullerene
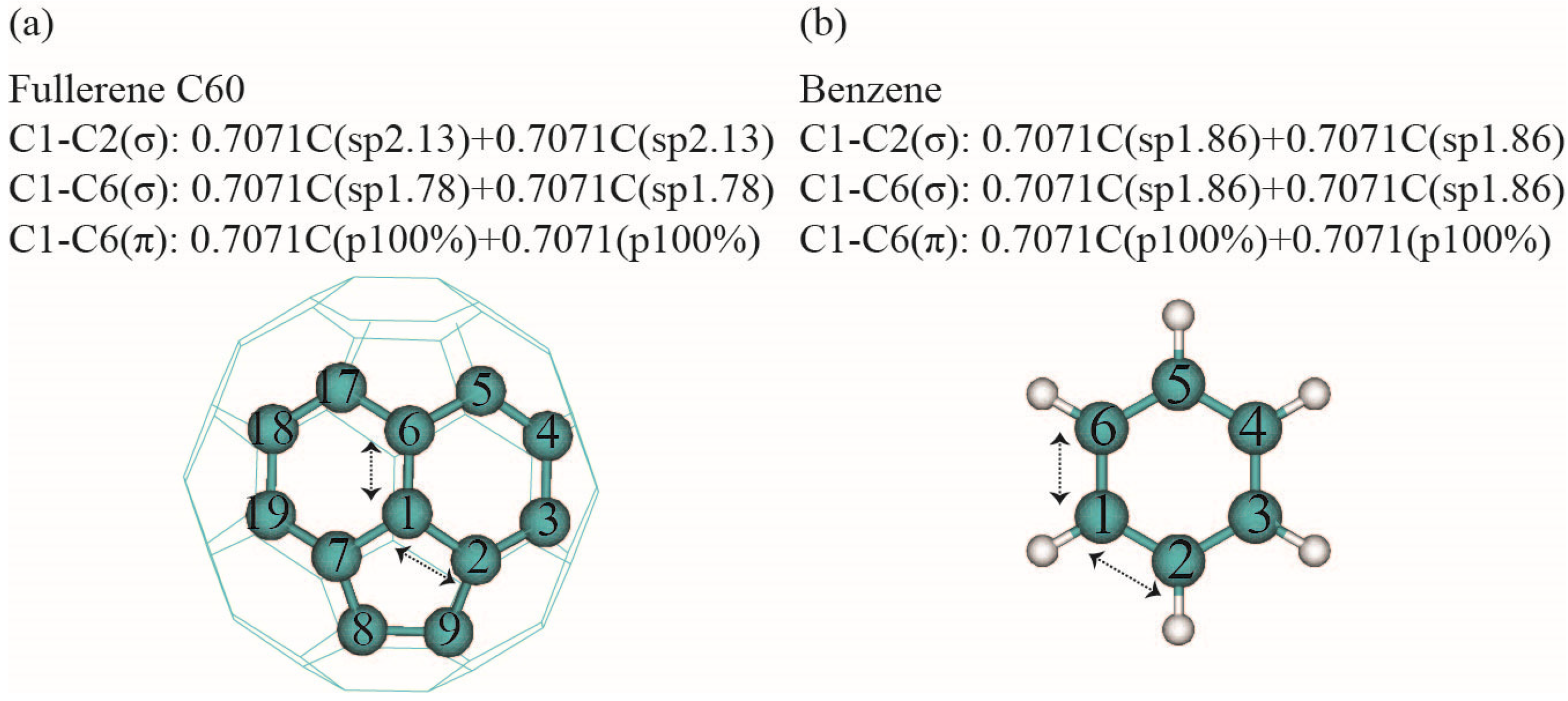
3.2. Natural Bond Analysis
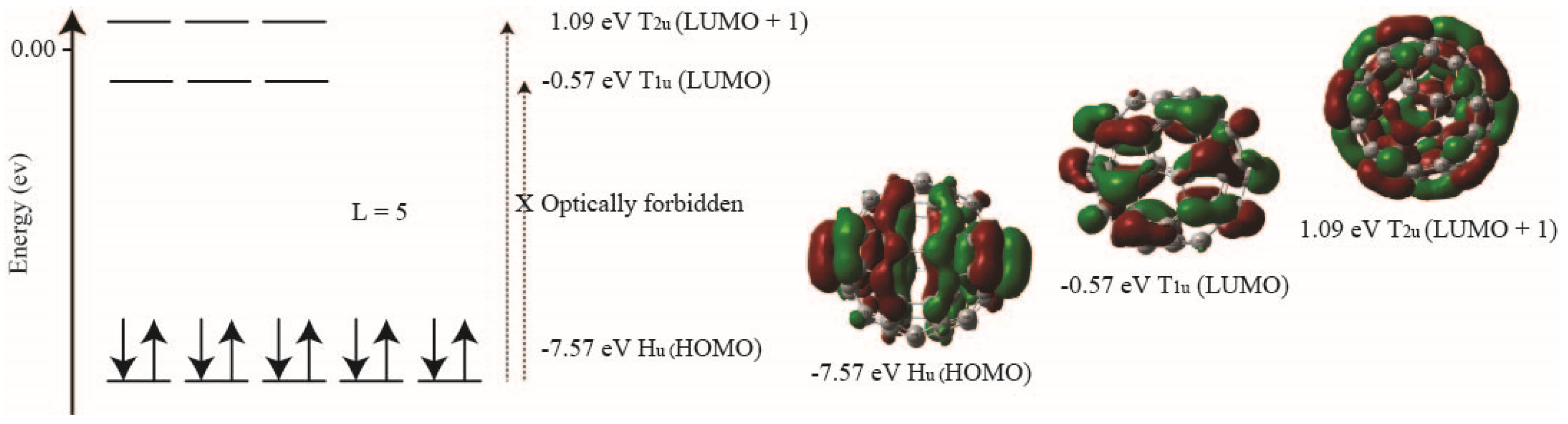
3.3. Correlation Energy and HOMO-LUMO Gap of α Electrons of Fullerene C60
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.H.D.R.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Paukov, M.; Kramberger, C.; Begichev, I.; Kharlamova, M.; Burdanova, M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Sitharaman, B. Multifunctional fullerene-and metallofullerene-based nanobiomaterials. Nano Life 2013, 3, 1342003. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomedicine 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Fjodorova, N.; Novič, M.; Venko, K.; Rasulev, B.; Türker Saçan, M.; Tugcu, G.; Sağ Erdem, S.; Toropova, A.P.; Toropov, A.A. Cheminformatics and Machine Learning Approaches to Assess Aquatic Toxicity Profiles of Fullerene Derivatives. Int. J. Mol. Sci. 2023, 24, 14160. [Google Scholar] [CrossRef]
- Sohlberg, K.; Foster, M.E. What’s the gap? A possible strategy for advancing theory, and an appeal for experimental structure data to drive that advance. RSC Adv. 2020, 10, 36887–36896. [Google Scholar] [CrossRef]
- Blase, X.; Attaccalite, C.; Olevano, V. First-principles GW calculations for fullerenes, porphyrins, phtalocyanine, and other molecules of interest for organic photovoltaic applications. Phys. Rev. B 2011, 83, 115103. [Google Scholar] [CrossRef]
- Haddon, R.C. Electronic structure, conductivity and superconductivity of alkali metal doped (C60). Acc. Chem. Res. 1992, 25, 127–133. [Google Scholar] [CrossRef]
- Yannoni, C.S.; Bernier, P.P.; Bethune, D.S.; Meijer, G.; Salem, J.R. NMR determination of the bond lengths in C60. J. Am. Chem. Soc. 1991, 113, 3190–3192. [Google Scholar] [CrossRef]
- Meirzadeh, E.; Evans, A.M.; Rezaee, M.; Milich, M.; Dionne, C.J.; Darlington, T.P.; Bao, S.T.; Bartholomew, A.K.; Handa, T.; Rizzo, D.J.; et al. A few-layer covalent network of fullerenes. Nature 2023, 613, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.; Wu, J.I.; Corminboeuf, C.; Bohmann, J.; Lu, X.; Hirsch, A.; von Rague Schleyer, P. Is C60 buckminsterfullerene aromatic? Phys. Chem. Chem. Phys. 2012, 14, 14886–14891. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tahara, T. Observation of an optically forbidden state of C60 by nondegenerate two-photon absorption spectroscopy. Chem. Phys. Lett. 2004, 390, 136–139. [Google Scholar] [CrossRef]
- Hoke, S.H., II; Molstad, J.; Dilettato, D.; Jay, M.J.; Carlson, D.; Kahr, B.; Cooks, R.G. Reaction of fullerenes and benzyne. J. Org. Chem. 1992, 57, 5069–5071. [Google Scholar] [CrossRef]
- Su, Y.-W.; Lan, S.-C.; Wei, K.-H. Organic photovoltaics. Mater. Today 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Jarvis, S.P.; Sang, H.; Junqueira, F.; Gordon, O.; Hodgkinson, J.E.; Saywell, A.; Rahe, P.; Mamone, S.; Taylor, S.; Sweetman, A.; et al. Chemical shielding of H2O and HF encapsulated inside a C60 cage. Commun. Chem. 2021, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, E.; Barron, A.R. Activation Effect of Fullerene C60 on the Carbon Dioxide Absorption Performance of Amine-Rich Polypropylenimine Dendrimers. ChemSusChem 2015, 8, 2635–2644. [Google Scholar] [CrossRef]
- Sternfeld, T.; Thilgen, C.; Hoffman, R.E.; del Rosario Colorado Heras, M.; Diederich, F.; Wudl, F.; Scott, L.T.; Mack, J.; Rabinovitz, M. An Insight into the Aromaticity of Fullerene Anions: Experimental Evidence for Diamagnetic Ring Currents in the Five-Membered Rings of C606− and C706−. J. Am. Chem. Soc. 2002, 124, 5734–5738. [Google Scholar] [CrossRef]
- Vyas, V.K.; Bacanu, G.R.; Soundararajan, M.; Marsden, E.S.; Jafari, T.; Shugai, A.; Light, M.E.; Nagel, U.; Rõõm, T.; Levitt, M.H.; et al. Squeezing formaldehyde into C60 fullerene. Nat. Commun. 2024, 15, 2515. [Google Scholar] [CrossRef]
- Froimowitz, M. Molecular geometries and heats of formation of C60 and C70 as computed by MM2-87. J. Comput. Chem. 1991, 12, 1129–1133. [Google Scholar] [CrossRef]
- Friedrich, M.; Piovano, P.; Stefanelli, U. The Geometry of C60: A Rigorous Approach via Molecular Mechanics. SIAM J. Appl. Math. 2016, 76, 2009–2029. [Google Scholar] [CrossRef]
- Liu, T.; Dennis, T.J.S. Conformational Analysis of [60]PCBM from DFT Simulations of Electronic Energies, Bond Strain and the 13C NMR Spectrum: Input Geometry Determination and Ester Bond Rotation Dynamics. C 2021, 7, 66. [Google Scholar] [CrossRef]
- Kitjanon, J.; Khuntawee, W.; Phongphanphanee, S.; Sutthibutpong, T.; Chattham, N.; Karttunen, M.; Wong-Ekkabut, J. Nanocomposite of Fullerenes and Natural Rubbers: MARTINI Force Field Molecular Dynamics Simulations. Polymers 2021, 13, 4044. [Google Scholar] [CrossRef]
- Wang, C.I.; Hua, C.C.; Chen, S.A. Dynamic solvation shell and solubility of C60 in organic solvents. J. Phys. Chem. B 2014, 118, 9964–9973. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, K.; Hedberg, L.; Bethune, D.S.; Brown, C.A.; Dorn, H.C.; Johnson, R.D.; De Vries, M. Bond Lengths in Free Molecules of Buckminsterfullerene, C60 from Gas-Phase Electron Diffraction. Science 1991, 254, 410–412. [Google Scholar] [CrossRef] [PubMed]
- David, W.I.F.; Ibberson, R.M.; Matthewman, J.C.; Prassides, K.; Dennis, T.J.S.; Hare, J.P.; Kroto, H.W.; Taylor, R.; Walton, D.R. Crystal structure and bonding of ordered C60. Nature 1991, 353, 147–149. [Google Scholar] [CrossRef]
- Yang, Y.; Arias, F.; Echegoyen, L.; Chibante, L.F.; Flanagan, S.; Robertson, A.; Wilson, L.J. Reversible Fullerene Electrochemistry: Correlation with the HOMO-LUMO Energy Difference for C60, C70, C76, C78, and C84. J. Am. Chem. Soc. 1995, 117, 7801–7804. [Google Scholar] [CrossRef]
- Tans, S.J.; Devoret, M.H.; Groeneveld, R.J.; Dekker, C. Electron–electron correlations in carbon nanotubes. Nature 1998, 394, 761–764. [Google Scholar] [CrossRef]
- Ugwumadu, C.; Nepal, K.; Thapa, R.A.J.E.N.D.R.A.; Lee, Y.G.; Al Majali, Y.; Trembly, J.; Drabold, D.A. Simulation of multi-shell fullerenes using Machine-Learning Gaussian Approximation Potential. Carbon Trends 2023, 10, 100239. [Google Scholar] [CrossRef]
- Zhao, Y.; Yakobson, B.I.; Smalley, R.E. Dynamic Topology of Fullerene Coalescence. Phys. Rev. Lett. 2002, 88, 185501. [Google Scholar] [CrossRef]
- Zhao, Y.; Smalley, R.E.; Yakobson, B.I. Coalescence of fullerene cages: Topology, energetics, and molecular dynamics simulation. Phys. Rev. B 2002, 66, 195409. [Google Scholar] [CrossRef]
- Scuseria, G.E. Ab Initio Calculations of Fullerenes. Science 1996, 271, 942–945. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.L. (C=C=C=C)@C(60): A Bonding C(60)-Endohedral Molecular Allotrope of Carbon. ACS Omega 2020, 5, 26933–26937. [Google Scholar] [CrossRef] [PubMed]
- Sattarova, A.F.; Biglova, Y.N.; Mustafin, A.G. Quantum-chemical approaches in the study of fullerene and its derivatives by the example of the most typical cycloaddition reactions: A review. Int. J. Quantum Chem. 2022, 122, e26863. [Google Scholar] [CrossRef]
- Kłos, J.; Tiesinga, E.; Kotochigova, S. Quantum scattering of icosahedron fullerene C60 with noble-gas atoms. Sci. Rep. 2024, 14, 9267. [Google Scholar] [CrossRef]
- Argaman, U.; Makov, G. Structure and properties of graphullerene: A semiconducting two-dimensional C60 crystal. NPJ Comput. Mater. 2023, 9, 211. [Google Scholar] [CrossRef]
- Orozco-Ic, M.; Charistos, N.D.; Muñoz-Castro, A.; Islas, R.; Sundholm, D.; Merino, G. Core-electron contributions to the molecular magnetic response. Phys. Chem. Chem. Phys. 2022, 24, 12158–12166. [Google Scholar] [CrossRef]
- Trzaskowski, B.; Adamowicz, L.; Beck, W.; Muralidharan, K.; Deymier, P.A. Impact of Local Curvature and Structural Defects on Graphene–C60 Fullerene Fusion Reaction Barriers. J. Phys. Chem. C 2013, 117, 19664–19671. [Google Scholar] [CrossRef]
- Karton, A. Fullerenes Pose a Strain on Hybrid Density Functional Theory. J. Phys. Chem. A 2022, 126, 4709–4720. [Google Scholar] [CrossRef]
- An, W.; Shao, N.; Bulusu, S.; Zeng, X.C. Ab initio calculation of carbon clusters. II. Relative stabilities of fullerene and nonfullerene C24. J. Chem. Phys. 2008, 128, 084301. [Google Scholar] [CrossRef]
- Vance, S.J.; Desai, V.; Smith, B.O.; Kennedy, M.W.; Cooper, A. Aqueous solubilization of C60 fullerene by natural protein surfactants, latherin and ranaspumin-2. Biophys. Chem. 2016, 214-215, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Aihara, J.-i. Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys. Chem. Chem. Phys. 2000, 2, 3121–3125. [Google Scholar] [CrossRef]
- Fukuda, R.; Ehara, M. Electronic excitations of C60 fullerene calculated using the ab initio cluster expansion method. J. Chem. Phys. 2012, 137, 134304. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.L.; Troullier, N.; Weaver, J.H. Analysis of occupied and empty electronic states of C60. Chem. Phys. Lett. 1991, 180, 457–460. [Google Scholar] [CrossRef]
- Suresh, C.H.; Lincy, T.L.; Mohan, N.; Rakhi, R. Aromatization Energy and Strain Energy of Buckminsterfullerene from Homodesmotic Reactions. J. Phys. Chem. A 2015, 119, 6683–6688. [Google Scholar] [CrossRef]
- Panahian Jand, S.; Nourbakhsh, Z.; Site, L.D. Nuclear quantum effects in fullerene-fullerene aggregation in water. Front. Chem. 2022, 10, 1072665. [Google Scholar] [CrossRef]
- Moztarzadeh, O.; Jamshidi, M.; Taherpour, A.A.; Babuska, V. Molecular modelling of fullerene C60 functionalized by nitric oxide for use in biological environment. Sci. Rep. 2024, 14, 2565. [Google Scholar] [CrossRef]
- Aouane, M.; Armstrong, J.; Walkey, M.; Hoffman, G.; Bacanu, G.R.; Whitby, R.J.; Levitt, M.H.; Rols, S. A combined inelastic neutron scattering and simulation study of the 3He@C60 endofullerene. Phys. Chem. Chem. Phys. 2023, 25, 20295–20301. [Google Scholar] [CrossRef]
- Karton, A.; Chan, B.; Raghavachari, K.; Radom, L. Evaluation of the Heats of Formation of Corannulene and C60 by Means of High-Level Theoretical Procedures. J. Phys. Chem. A 2013, 117, 1834–1842. [Google Scholar] [CrossRef]
- Chan, B. Fullerene Thermochemical Stability: Accurate Heats of Formation for Small Fullerenes, the Importance of Structural Deformation on Reactivity, and the Special Stability of C60. J. Phys. Chem. A 2020, 124, 6688–6698. [Google Scholar] [CrossRef]
- Choi, C.H.; Kertesz, M.; Mihaly, L. Vibrational Assignment of All 46 Fundamentals of C60 and C606−: Scaled Quantum Mechanical Results Performed in Redundant Internal Coordinates and Compared to Experiments. J. Phys. Chem. A 2000, 104, 102–112. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Osawa, E. Information Entropy of Fullerenes. J. Chem. Inf. Model. 2015, 55, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, Y.; Yakobson, B.I. Fullerene shape transformations via Stone-Wales bond rotations. Phys. Rev. B 2003, 68, 233403. [Google Scholar] [CrossRef]
- Häser, M.; Almlöf, J.; Scuseria, G.E. The equilibrium geometry of C60 as predicted by second-order (MP2) perturbation theory. Chem. Phys. Lett. 1991, 181, 497–500. [Google Scholar] [CrossRef]
- Bühl, M.; Patchkovskii, S.; Thiel, W. Interaction energies and NMR chemical shifts of noble gases in C60. Chem. Phys. Lett. 1997, 275, 14–18. [Google Scholar] [CrossRef]
- Shameema, O.; Ramachandran, C.N.; Sathyamurthy, N. Blue Shift in X−H Stretching Frequency of Molecules Due to Confinement. J. Phys. Chem. A 2006, 110, 2–4. [Google Scholar] [CrossRef]
- Jaworski, A.; Hedin, N. Local energy decomposition analysis and molecular properties of encapsulated methane in fullerene (CH4@C60). Phys. Chem. Chem. Phys. 2021, 23, 21554–21567. [Google Scholar] [CrossRef]
- Meloni, G.; Giustini, A.; Park, H. CO2 Activation within a Superalkali-Doped Fullerene. Front. Chem. 2021, 9, 712960. [Google Scholar] [CrossRef]
- Ridassepri, A.F.; Umejima, Y.; Nakamura, J. B-Doped Fullerene as a Potential Metal-Free Catalyst Material for CO Reduction Reaction. J. Phys. Chem. C 2024, 128, 9513–9519. [Google Scholar] [CrossRef]
- Vícha, J.; Vaara, J.; Straka, M. The essential role of symmetry in understanding 3He chemical shifts in endohedral helium fullerenes. Phys. Chem. Chem. Phys. 2023, 25, 10620–10627. [Google Scholar] [CrossRef]
- Cioslowski, J. Endohedral Magnetic Shielding in the C60 Cluster. J. Am. Chem. Soc. 1994, 116, 3619–3620. [Google Scholar] [CrossRef]
- Bühl, M.; Hirsch, A. Spherical Aromaticity of Fullerenes. Chem. Rev. 2001, 101, 1153–1184. [Google Scholar] [CrossRef] [PubMed]
- Cleland, D.M.; Fletcher, E.K.; Kuperman, A.; Per, M.C. Electron correlation effects in isomers of C20. J. Phys. Mater. 2020, 3, 025006. [Google Scholar] [CrossRef]
- Karton, A.; Waite, S.L.; Page, A.J. Performance of DFT for C60 Isomerization Energies: A Noticeable Exception to Jacob’s Ladder. J. Phys. Chem. A 2019, 123, 257–266. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Fan, H.; Johnson, B.R.; Briggs, J.M. Pharmacophore Oriented MP2 Characterization of Charge Distribution for Anti-SARS-CoV-2 Inhibitor Nirmatrelvir. J. Mol. Struct. 2023, 1290, 135871. [Google Scholar] [CrossRef]
- Liu, Y.; Gallo, A.A.; Liu, Y.; Hall, M.B.; Johnson, B.R. QM evaluation of the intramolecular aromatic π-π interactions of Ir(I) complex transition states. J. Mol. Struct. 2023, 1291, 135907. [Google Scholar] [CrossRef]
- Liu, Y.; Sulaiman, H.F.; Johnson, B.R.; Ma, R.; Gao, Y.; Fernando, H.; Amarasekara, A.; Ashley-Oyewole, A.; Fan, H.; Ingram, H.N.; et al. QM/MM study of N501 involved intermolecular interaction between SARS-CoV-2 receptor binding domain and antibody of human origin. Comput. Biol. Chem. 2023, 102, 107810. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D.; Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density functional calculations of molecular bond energies. J. Chem. Phys. 1986, 84, 4524–4529. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. A density-functional model of the dispersion interaction. J. Chem. Phys. 2005, 123, 154101. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Schein, S.; Sands-Kidner, M. A geometric principle may guide self-assembly of fullerene cages from clathrin triskelia and from carbon atoms. Biophys. J. 2008, 94, 958–976. [Google Scholar] [CrossRef]
- Singh, U.C.; Kollman, P.A. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 1984, 5, 129–145. [Google Scholar] [CrossRef]
- Johari, G.P. Entropy of buckminsterfullerene at 0 K. J. Chem. Phys. 1994, 100, 2220–2222. [Google Scholar] [CrossRef]
- Šulka, M.; Šulková, K.; Jurečka, P.; Dubecký, M. Dynamic and Nondynamic Electron Correlation Energy Decomposition Based on the Node of the Hartree-Fock Slater Determinant. J. Chem. Theory Comput. 2023, 19, 8147–8155. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Quantum Theory of Many-Particle Systems. III. Extension of the Hartree-Fock Scheme to Include Degenerate Systems and Correlation Effects. Phys. Rev. 1955, 97, 1509–1520. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Kristensen, K.; Høyvik, I.M.; Jansik, B.; Jørgensen, P.; Kjærgaard, T.; Reine, S.; Jakowski, J. MP2 energy and density for large molecular systems with internal error control using the Divide-Expand-Consolidate scheme. Phys. Chem. Chem. Phys. 2012, 14, 15706–15714. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, S. Analysis of Bonding by Quantum Chemistry─Resolving Delocalization Stabilization in a Mechanistic Basis and New Hückel Model. J. Phys. Chem. A 2023, 127, 3449–3471. [Google Scholar] [CrossRef] [PubMed]
- Damour, Y.; Véril, M.; Kossoski, F.; Caffarel, M.; Jacquemin, D.; Scemama, A.; Loos, P.F. Accurate full configuration interaction correlation energy estimates for five- and six-membered rings. J. Chem. Phys. 2021, 155, 134104. [Google Scholar] [CrossRef] [PubMed]
- Grunenberg, J. Ill-defined chemical concepts: The problem of quantification. Int. J. Quantum Chem. 2017, 117, e25359. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.K. Structural Aspects of Aromaticity. Chem. Rev. 2001, 101, 1385–1420. [Google Scholar] [CrossRef]
- Cyrański, M.K. Energetic Aspects of Cyclic Pi-Electron Delocalization: Evaluation of the Methods of Estimating Aromatic Stabilization Energies. Chem. Rev. 2005, 105, 3773–3811. [Google Scholar] [CrossRef]
- Feixas, F.; Matito, E.; Poater, J.; Solà, M. Quantifying aromaticity with electron delocalisation measures. Chem. Soc. Rev. 2015, 44, 6434–6451. [Google Scholar] [CrossRef]
- Zanasi, R.; Lazzeretti, P.; Malagoli, M.; Piccinini, F. Molecular magnetic properties within continuous transformations of origin of the current density. J. Chem. Phys. 1995, 102, 7150–7157. [Google Scholar] [CrossRef]
- Matsumoto, F.; Iwai, T.; Moriwaki, K.; Takao, Y.; Ito, T.; Mizuno, T.; Ohno, T. Design of Fullerene Derivatives for Stabilizing LUMO Energy using Donor Groups Placed in Spatial Proximity to the C60 Cage. J. Org. Chem. 2012, 77, 9038–9043. [Google Scholar] [CrossRef]
- Saito, S.; Oshiyama, A. Cohesive mechanism and energy bands of solid C60. Phys. Rev. Lett. 1991, 66, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.H.; Martins, J.L.; Komeda, T.; Chen, Y.; Ohno, T.R.; Kroll, G.H.; Troullier, N.; Haufler, R.E.; Smalley, R.E. Electronic structure of solid C60: Experiment and theory. Phys. Rev. Lett. 1991, 66, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.S.; Knupfer, M.; Fink, J.; Armbruster, J.F.; Cummins, T.R.; Romberg, H.A.; Roth, M.; Sing, M.; Schmidt, M.; Sohmen, E. The electronic structure of fullerenes and fullerene compounds from high-energy spectroscopy. J. Phys. Condens. Matter 1995, 7, 8219. [Google Scholar] [CrossRef]
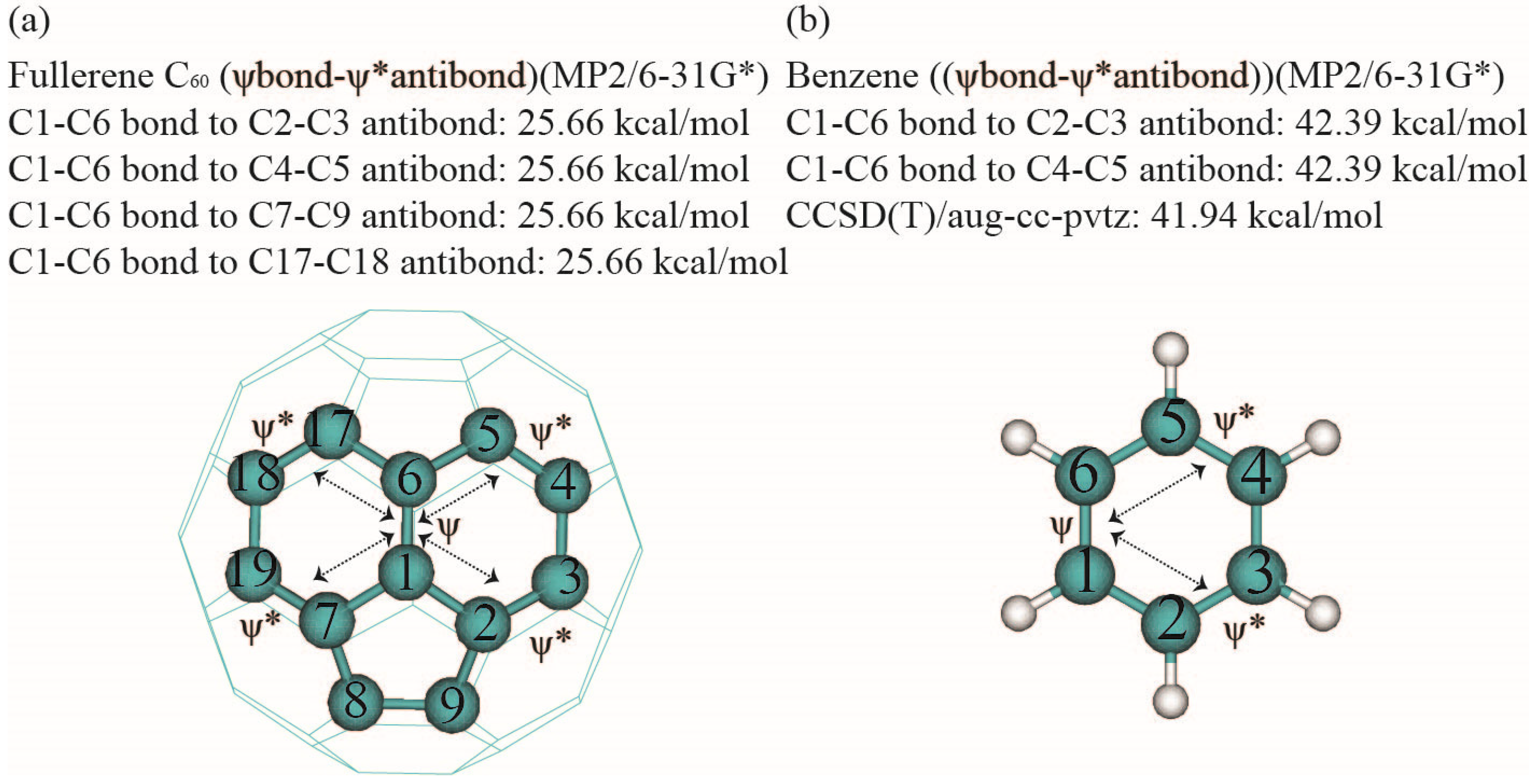

| Model | C1 | C6 | C2 | Minimum | Maximum | Median |
|---|---|---|---|---|---|---|
| ESP | −0.031000 | 0.009000 | −0.035000 | −0.040000 | 0.040000 | 0.0 |
| NBO | −0.000020 | 0.000010 | 0.000010 | −0.000030 | 0.000030 | 0.0 |
| Mulliken | 0.000011 | −0.000022 | −0.000022 | −0.000049 | 0.000046 | 0.0 |
| Hirshfeld | −0.000020 | 0.000013 | 0.000010 | −0.000022 | 0.000023 | 0.0 |
| CM5 | −0.000020 | 0.000013 | 0.000010 | −0.000022 | 0.000023 | 0.0 |
| Bond | Line of Center between Two Nuclei | Hybrid 1 | Hybrid 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Hybrid 1–2 | θ (Theta) | φ (Phi) | θ | φ | Dev | θ | φ | Dev |
| Fullerene C60 | ||||||||
| C1–C2 (σ) | 129.4 | 264.9 | 140.6 | 255.2 | 13.1 | 62.4 | 91.8 | 13.1 |
| C1–C6 (σ) | 47.9 | 167.4 | 58.7 | 154.1 | 15.1 | 140.8 | 5.5 | 15.1 |
| C1–C6 (π) | 47.9 | 167.4 | 50.3 | 276.1 | 75.8 | 71.9 | 297.4 | 75.8 |
| Benzene | ||||||||
| C1–C2 (σ) | 90.0 | 22.7 | 90.0 | 17.9 | 4.1 | 90.0 | 207.6 | 4.1 |
| C1–C6 (σ) | 90.0 | 142.7 | 90.0 | 147.6 | 4.1 | 90.0 | 317.9 | 4.1 |
| C1–C6 (π) | 90.0 | 142.7 | 0.0 | 0.0 | 90.0 | 0.0 | 0.0 | 90.0 |
| Molecule | Bond | Energy (Hartree) | Geminal | Vicinal | Energy (kcal/mol) |
|---|---|---|---|---|---|
| Fullerene C60 | C1–C2 (σ) | −0.88405 | 4 | 12 | 34.98 |
| Total | C1–C2 | 4 | 12 | 34.98 (100% + 38%) | |
| C1–C6 (σ) | −0.93273 | 4 | 8 | 26.33 | |
| C1–C6 (π) | −0.34383 | 4 | 8 | 110.14 | |
| Total | C1–C6 | 8 | 16 | 143.70 (100% + 22%) | |
| Benzene | C1–C2 (σ) | −0.90988 | 4 | 6 | 21.74 |
| Total | C1–C2 | 4 | 6 | 21.76 (100%) | |
| C1–C6 (σ) | −0.91019 | 4 | 6 | 21.76 | |
| C1–C6 (π) | −0.29735 | 0 | 4 | 87.66 | |
| Total | C1–C6 | 4 | 10 | 111.70 (100%) |
| Fullerene C60 | Benzene(MP2/6–31G*/CCSD(T)/aug-cc-pvtz) | ||||||
|---|---|---|---|---|---|---|---|
| Donor | Type | Acceptor | E (kcal/mol) | Donor | Type | Acceptor | E (kcal/mol) |
| C1–C2 (σ) | Rydberg | C3 | 0.8 | C1–C2 (σ) | Rydberg | C3 | 1.36/0.92 |
| Rydberg | C3 | 1.64 | Rydberg | C3 | 2.02/1.93 | ||
| Rydberg | C6 | 0.8 | Rydberg | C6 | 1.36/0.92 | ||
| Rydberg | C6 | 1.64 | Rydberg | C6 | 2.02/1.93 | ||
| Rydberg | C7 | 1.31 | Antibond | C1–C6 | 3.24/2.25 | ||
| Rydberg | C9 | 1.31 | Antibond | C1–H7 | 1.49/0.91 | ||
| Antibond | C1–C6 | 3.91 | Antibond | C2–C3 | 3.24/2.25 | ||
| Antibond | C1–C7 | 2.05 | Antibond | C2–H8 | 1.49/0.91 | ||
| Antibond | C2–C3 | 3.91 | Antibond | C3–H9 | 2.77/3.15 | ||
| Antibond | C2–C9 | 2.05 | Antibond | C6-H12 | 2.77/3.15 | ||
| Antibond | C3–C11 | 3.48 | Total | C1–C2 | 10 | 21.76/18.32 | |
| Antibond | C6–C17 | 3.48 | C1–C6 (σ) | Rydberg | C2 | 1.36/0.92 | |
| Antibond | C7–C19 | 3.8 | Rydberg | C2 | 2.02/1.93 | ||
| Antibond | C7–C19 | 0.5 | Rydberg | C5 | 1.36/0.92 | ||
| Antibond | C9–C10 | 3.8 | Rydberg | C5 | 2.02/1.93 | ||
| Antibond | C9–C10 | 0.5 | Antibond | C1–C2 | 3.24/2.25 | ||
| Total | C1–C2 | 16 | 34.98 | Antibond | C1–H7 | 1.50/0.91 | |
| C1–C6 (σ) | Rydberg | C2 | 1.61 | Antibond | C2–H8 | 2.77/3.15 | |
| Rydberg | C5 | 1.61 | Antibond | C5–C6 | 3.24/2.25 | ||
| Rydberg | C7 | 1.61 | Antibond | C5–H11 | 2.77/3.15 | ||
| Rydberg | C7 | 1.61 | Antibond | C6–H12 | 1.50/0.91 | ||
| Antibond | C1–C2 | 3.68 | Total | 10 | 21.78/18.32 | ||
| Antibond | C1–C7 | 3.68 | C1–C6 (π) | Rydberg | C2 | 2.57/1.88 | |
| Antibond | C2–C9 | 1.75 | Rydberg | C5 | 2.57/1.88 | ||
| Antibond | C5–C6 | 3.68 | Antibond | C2–C3 | 42.39/41.94 | ||
| Antibond | C5–C15 | 1.75 | Antibond | C4–C5 | 42.39/41.94 | ||
| Antibond | C6–C17 | 3.68 | Total | 4 | 89.92/87.64 | ||
| Antibond | C7–C8 | 1.75 | |||||
| Antibond | C6–C17 | 1.75 | |||||
| Total | 12 | 28.16 | |||||
| C1–C6 (π) | Rydberg | C1 | 0.53 | ||||
| Rydberg | C2 | 1.75 | |||||
| Rydberg | C5 | 1.75 | |||||
| Rydberg | C6 | 0.53 | |||||
| Rydberg | C7 | 1.75 | |||||
| Rydberg | C17 | 1.75 | |||||
| Antibond | C1–C2 | 1.2 | |||||
| Antibond | C1–C7 | 1.2 | |||||
| Antibond | C2–C3 | 25.66 | |||||
| Antibond | C4–C5 | 25.66 | |||||
| Antibond | C5–C6 | 1.2 | |||||
| Antibond | C6–C17 | 1.2 | |||||
| Antibond | C7–C19 | 25.66 | |||||
| Antibond | C17–C18 | 25.66 | |||||
| Total | 14 | 115.54 | |||||
| Total | C1–C6 | 26 | 143.70 (122%) | Total | C1–C6 | 14 | 111.70 (100%) |
| Total | C1–C2 | 16 | 34.98 (138%) | Total | C1–C2 | 10 | 21.76 (100%) |
| Method | MP2 (Hartree) | HF (Hartree) | MP2—HF (kcal/mol) | (MP2—HF)/ (kcal/mol e) |
|---|---|---|---|---|
| Fullerene C60 | ||||
| MP2/3–21G* | −2264.4378913 | −2258.9901941 | −3418.48 | −9.50 |
| MP2/6–31G* | −2279.6317630 | −2271.8025400 | −4912.92 | −13.65 |
| MP2/def2svp | −2277.8316660 | −2270.0928633 | −4865.21 | −13.49 |
| Benzene (The CCSD(T)/aug-cc-pvtz recovered correlation is counted by CCSD(T)-HF) | ||||
| MP2/3–21G* | −229.9376703 | −229.4155101 | −327.657 | −7.80 |
| MP2/6–31G* | −231.4577321 | −230.7018849 | −474.302 | −11.29 |
| MP2/def2svp | −231.3162080 | −230.5345726 | −490.477 | −11.68 |
| CCSD(T)/aug-cc-pvtz | −231.8204089 | −230.7804015 | −652.615 | −15.54 |
| Method (α Electron) | HOMO H1u (eV) | LUMO T1u (eV) | LUMO + 1 T2u (eV) | H1u to T1u (eV) | H1u to T2u (eV) |
|---|---|---|---|---|---|
| MP2/3-21G* | −8.19 | −0.92 | 0.75 | −7.27 | −8.94 |
| MP2/6–31G* | −7.57 | −0.57 | 1.09 | −7.00 | −8.66 |
| Experimental [7] | −4.90 | ||||
| B3LYP/Def2svp | −7.63 | −5.95 | −5.01 | −1.68 | −2.62 |
| BP86/Def2svp | −5.94 | −4.13 | −3.07 | −1.81 | −2.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, Y.; Altalhi, T.; Liu, D.-J.; Yakobson, B.I. A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60. Nanomaterials 2024, 14, 1576. https://doi.org/10.3390/nano14191576
Liu Y, Gao Y, Altalhi T, Liu D-J, Yakobson BI. A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60. Nanomaterials. 2024; 14(19):1576. https://doi.org/10.3390/nano14191576
Chicago/Turabian StyleLiu, Yuemin, Yunxiang Gao, Tariq Altalhi, Di-Jia Liu, and Boris I. Yakobson. 2024. "A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60" Nanomaterials 14, no. 19: 1576. https://doi.org/10.3390/nano14191576
APA StyleLiu, Y., Gao, Y., Altalhi, T., Liu, D.-J., & Yakobson, B. I. (2024). A Quantum Mechanical MP2 Study of the Electronic Effect of Nonplanarity on the Carbon Pyramidalization of Fullerene C60. Nanomaterials, 14(19), 1576. https://doi.org/10.3390/nano14191576







