Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Conversion of Bioethanol
3. Results and Discussion
3.1. Characterization of the Catalysts
3.2. Catalytic Performance
3.3. Characterization of Deactivated Catalysts
4. Comparison of NH4F-HF Modification and HF Modification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morad, M.H. Current status and future perspectives of efficient catalytic conversion of bioethanol to value-added chemicals and fuels. Arab. J. Chem. 2024, 17, 105560. [Google Scholar] [CrossRef]
- Matheus, C.R.; Sousa-Aguiar, E.F. Main catalytic challenges in ethanol chemistry: A review. Catal. Rev. 2024, 66, 174–213. [Google Scholar] [CrossRef]
- Xia, W.; Huang, Y.X.; Ma, C.; Wang, X.; Li, S.S.; Chen, K.; Liu, D. The role of crystalline phase of zirconia in catalytic conversion of ethanol to propylene. Ceram. Int. 2023, 49, 12258–12266. [Google Scholar] [CrossRef]
- Xia, W.; Huang, Y.X.; Ma, C.; Li, S.S.; Wang, X.; Chen, K.; Liu, D. Multiple important roles of phosphorus modification on the ZSM-5 in ethanol to olefin reaction: Acidity adjustment, hydrothermal stability and anti-coking. Fuel 2023, 341, 127675. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Vu, K.B.; Busca, G. (Bio)Propylene production processes: A critical review. J. Environ. Chem. Eng. 2021, 9, 105673. [Google Scholar] [CrossRef]
- Matheus, C.R.V.; Chagas, L.H.; Gonzalez, G.G.; Aguiar, E.F.S.; Appel, L.G. Synthesis of propene from ethanol: A mechanistic study. ACS Catal. 2018, 8, 7667–7678. [Google Scholar] [CrossRef]
- Iwamoto, M. Selective catalytic conversion of bio-ethanol to propene: A review of catalysts and reaction pathways. Catal. Today 2015, 242, 243–248. [Google Scholar] [CrossRef]
- Iwamoto, M.; Tanaka, M.; Hirakawa, S.; Mizuno, S.; Kurosawa, M. Pulse and IR study on the reaction pathways for the conversion of ethanol to propene over scandium-loaded indium oxide catalysts. ACS Catal. 2014, 4, 3463–3469. [Google Scholar] [CrossRef]
- Bai, T.; Li, X.H.; Ding, L.; Wang, J.; Xiao, Y.S.; Cao, B. Direct conversion of ethanol to propylene over Zn-modified HBeta zeolite: Influence of zinc precursors. Catalysts 2024, 14, 276. [Google Scholar] [CrossRef]
- Takahashi, A.; Xia, W.; Wu, Q.; Furukawa, T.; Nakamura, I.; Shimada, H.; Fujitani, T. Difference between the mechanisms of propylene production from methanol and ethanol over ZSM-5 catalysts. Appl. Catal. A 2013, 467, 380–385. [Google Scholar] [CrossRef]
- Takamitsu, Y.; Yamamoto, K.; Yoshida, S.; Ogawa, H.; Sano, T. Effect of crystal size and surface modification of ZSM-5 zeolites on conversion of ethanol to propylene. J. Porous Mater. 2014, 21, 433–440. [Google Scholar] [CrossRef]
- Inaba, M.; Murata, K.; Takahara, I.; Inoue, K.i. Production of olefins from ethanol by Fe and/or P-modified H-ZSM-5 zeolite catalysts. J. Chem. Technol. Biotechnol. 2011, 86, 95–104. [Google Scholar] [CrossRef]
- Liu, X.R.; Sun, Y.Q. Effect of ethanol on the morphology and textual properties of ZSM-5 zeolite. Catalysts 2020, 10, 198. [Google Scholar] [CrossRef]
- De Reviere, A.; Gilson, J.P.; Valtchev, V.; Thybaut, J.W.; Sabbe, M.K.; Verberckmoes, A. Enhancing the catalytic performance of H-ZSM-5 in bio n-butanol dehydration by zeolite crystal engineering. Appl. Catal. B 2024, 357, 124351. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Chetty, M.; Khotseng, L.; Kiambi, S.L.; Maharaj, L.; Oboirien, B.; Isa, Y.M. Stability, deactivation and regeneration study of a newly developed HZSM-5 and Ni-doped HZSM-5 zeolite catalysts for ethanol-to-hydrocarbon conversion. Catal. Commun. 2024, 186, 106802. [Google Scholar] [CrossRef]
- Quiroga, E.; García, N.; Cifuentes, B.; Cogua, R.; Becerra, J.; Berenguer, J.M.; Cobo, M. Industrial crude bioethanol dehydration to ethylene: Doping ZSM-5 to enhance selectivity and stability. J. Environ. Chem. Eng. 2024, 12, 111803. [Google Scholar] [CrossRef]
- Badghaiya, D.; Parikh, J.K.; Parikh, P.A. Valorization of bio-renewably available ethanol over alkali-exchanged ZSM-5: Improved aromatic selectivity and catalyst life. React. Kinet. Mech. Catal. 2024, 137, 1515–1534. [Google Scholar] [CrossRef]
- Ouayloul, L.; El Doukkali, M.; Jiao, M.Y.; Dumeignil, F.; Agirrezabal-Telleria, I. New mechanistic insights into the role of water in the dehydration of ethanol into ethylene over ZSM-5 catalysts at low temperature. Green. Chem. 2023, 25, 3644–3659. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Alonso, A.; Valle, B.; Aguayo, A.T.; Bilbao, J. Selective production of olefins from bioethanol on HZSM-5 zeolite catalysts treated with NaOH. Appl. Catal. B 2010, 97, 299–306. [Google Scholar] [CrossRef]
- Zhai, X.L.; Mao, D.S.; Huangfu, J.J.; Guo, Q.S. Effects of pore structure and acidity of nano-HZSM-5 on its catalytic performance for the conversion of bio-ethanol to propene. Acta Pet. Sin. (Pet. Process. Sect.) 2016, 32, 1212–1220. [Google Scholar] [CrossRef]
- Xia, W.; Wang, J.G.; Wang, L.X.; Qian, C.; Ma, C.; Huang, Y.X.; Fan, Y.; Hou, M.D.; Chen, K. Ethylene and propylene production from ethanol over Sr/ZSM-5 catalysts: A combined experimental and computational study. Appl. Catal. B 2021, 294, 120242. [Google Scholar] [CrossRef]
- Ghazimoradi, M.; Soltanali, S.; Safari, N.; Ghassabzadeh, H. Synthesis of fluorinated ZSM-5 catalysts: Fluoride effect on structure properties and coke resistance in n-hexane catalytic cracking. J. Mater. Sci. 2023, 58, 11551–11567. [Google Scholar] [CrossRef]
- Zhao, R.R.; Li, S.K.; Bi, L.X.; Fu, Q.; Tan, H.Z.; Wang, M.; Cui, H.Y. Enhancement of p-xylene selectivity in the reaction between 2,5-dimethylfuran and ethanol over an ammonium fluoride-modified ZSM-5 zeolite. Catal. Sci. Technol. 2022, 12, 2248–2256. [Google Scholar] [CrossRef]
- Tang, W.; Cao, J.P.; Yang, F.L.; Feng, X.B.; Ren, J.; Wang, J.X.; Zhao, X.Y.; Zhao, M.; Cui, X.; Wei, X.Y. Highly active and stable HF acid modified HZSM-5 supported Ni catalysts for steam reforming of toluene and biomass pyrolysis tar. Energy Convers. Manag. 2020, 212, 112799. [Google Scholar] [CrossRef]
- Shang, Q.H.; Xu, G.L.; Tang, N.F.; Wu, C.T.; Chen, S.; Cong, Y. Fluoride-modified ZSM-5 for endothermic catalytic cracking of n-decane. Microporous Mesoporous Mater. 2019, 288, 109616. [Google Scholar] [CrossRef]
- Wang, J.X.; Cao, J.P.; Zhao, X.Y.; Liu, S.N.; Ren, X.Y.; Zhao, M.; Cui, X.; Chen, Q.; Wei, X.Y. Enhancement of light aromatics from catalytic fast pyrolysis of cellulose over bifunctional hierarchical HZSM-5 modified by hydrogen fluoride and nickel/hydrogen fluoride. Bioresour. Technol. 2019, 278, 116–123. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, D.S.; Zhai, X.L. Selective conversion of bio-ethanol to propene over nano-HZSM-5 zeolite: Remarkably enhanced catalytic performance by fluorine modification. Fuel Process. Technol. 2017, 167, 50–60. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, N.; Xue, Z.T.; Meng, T.; Guo, Q.S.; Mao, D.S. Selective catalytic conversion of bio-ethanol to propene by hydrofluoric acid modified nano-ZSM-5 zeolites. Acta Pet. Sin. (Pet. Process. Sect.) 2024, 40, 1203–1212. [Google Scholar] [CrossRef]
- Meng, T.; Mao, D.S.; Guo, Q.S.; Lu, G.Z. The effect of crystal sizes of HZSM-5 zeolites in ethanol conversion to propylene. Catal. Commun. 2012, 21, 52–57. [Google Scholar] [CrossRef]
- Firoozi, M.; Baghalha, M.; Asadi, M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction. Catal. Commun. 2009, 10, 1582–1585. [Google Scholar] [CrossRef]
- Chen, D.; Moljord, K.; Fuglerud, T.; Holmen, A. The effect of crystal size of SAPO-34 on the selectivity and deactivation of the MTO reaction. Microporous Mesoporous Mater. 1999, 29, 191–203. [Google Scholar] [CrossRef]
- Iadrat, P.; Prasertsab, A.; Limlamthong, M.; Choi, J.; Park, H.E.; Wattanakit, C.; Yip, A.C. Modification of zeolite morphology via NH4F etching for catalytic bioalcohol conversion. ChemCatChem 2024, 16, e202301208. [Google Scholar] [CrossRef]
- Onfroy, T.; Qin, Z.; Casale, S.; Valtchev, V. Optimization of ammonium fluoride route to hierarchical ZMS-5 zeolites. Microporous Mesoporous Mater. 2023, 362, 112760. [Google Scholar] [CrossRef]
- Zhang, C.X.; Lin, M.; Zhou, S.L.; Li, Y.X.; Shu, X.T. A green route of isobutane-butene alkylation catalyzed by Y zeolite modified with synergistic F-containing solution. Microporous Mesoporous Mater. 2024, 364, 112874. [Google Scholar] [CrossRef]
- Qin, Z.; Lakiss, L.; Gilson, J.P.; Thomas, K.; Goupil, J.M.; Fernandez, C.; Valtchev, V. Chemical equilibrium controlled etching of MFI-type zeolite and its influence on zeolite structure, acidity, and catalytic activity. Chem. Mater. 2013, 25, 2759–2766. [Google Scholar] [CrossRef]
- Luo, C.W.; Li, A. Synthesis of 3-picoline from acrolein dimethyl acetal and ammonia over NH4F-HF treated ZSM-5. React. Kinet. Mech. Catal. 2018, 125, 365–380. [Google Scholar] [CrossRef]
- Meng, F.J.; Wang, X.; Wang, S.H.; Wang, Y.Q. Fluoride-treated HZSM-5 as a highly stable catalyst for the reaction of methanol to gasoline. Catal. Today 2017, 298, 226–233. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Atutxa, A.; Olazar, M.; Bilbao, J. Catalyst deactivation by coke in the transformation of aqueous ethanol into hydrocarbons. Kinetic modeling and acidity deterioration of the catalyst. Ind. Eng. Chem. Res. 2002, 41, 4216–4224. [Google Scholar] [CrossRef]
- Ramesh, K.; Jie, C.; Han, Y.F.; Borgna, A. Synthesis, characterization, and catalytic activity of phosphorus modified H-ZSM-5 catalysts in selective ethanol dehydration. Ind. Eng. Chem. Res. 2010, 49, 4080–4090. [Google Scholar] [CrossRef]
- Guo, Q.S.; Mao, D.S.; Lao, Y.P.; Lu, G.Z. The effect of fluorine modification on catalytic performance of nanosized HZSM-5 zeolite for conversion of methanol to propene. Chin. J. Catal. 2009, 30, 1248–1254. [Google Scholar] [CrossRef]
- Huangfu, J.J.; Mao, D.S.; Zhai, X.L.; Guo, Q.S. Remarkably enhanced stability of HZSM-5 zeolite co-modified with alkaline and phosphorous for the selective conversion of bio-ethanol to propylene. Appl. Catal. A 2016, 520, 99–104. [Google Scholar] [CrossRef]
- Wu, E.L.; Lawton, S.L.; Olson, D.H.; Rohrman, A.C.; Kokotailo, G.T. ZSM-5-type materials. Factors affecting crystal symmetry. J. Phys. Chem. 1979, 83, 2777–2781. [Google Scholar] [CrossRef]
- Kolasinski, K.W. The mechanism of Si etching in fluoride solutions. Phys. Chem. Chem. Phys. 2003, 5, 1270–1278. [Google Scholar] [CrossRef]
- Judge, J.S. A study of the dissolution of SiO2 in acidic fluoride solutions. J. Electrochem. Soc. 1971, 118, 1772–1775. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, R.J.; Yang, X.X. Effect of alkaline treatment on pore structure and acidity of HZSM-5 in the synthesis of ethyl mercaptan. Catal. Commun. 2015, 60, 32–36. [Google Scholar] [CrossRef]
- Groen, J.C.; Pérez-Ramıírez, J. Critical appraisal of mesopore characterization by adsorption analysis. Appl. Catal. A 2004, 268, 121–125. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Tarrío, A.M.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Kinetic modelling of the transformation of aqueous ethanol into hydrocarbons on a HZSM-5 zeolite. Ind. Eng. Chem. Res. 2001, 40, 3467–3474. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Alonso, A.; Valle, B.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Kinetic modelling for the transformation of bioethanol into olefins on a hydrothermally stable Ni-HZSM-5 catalyst considering the deactivation by coke. Chem. Eng. J. 2011, 167, 262–277. [Google Scholar] [CrossRef]
- Song, Z.X.; Takahashi, A.; Mimura, N.; Fujitani, T. Production of propylene from ethanol over ZSM-5 zeolites. Catal. Lett. 2009, 131, 364–369. [Google Scholar] [CrossRef]
- Meng, T.; Mao, D.S.; Guo, Q.S.; Ma, Z. Effect of the Si/Al ratios of nanocrystalline HZSM-5 zeolite on the performance in catalytic conversion of ethanol to propylene. J. Nanosci. Nanotechnol. 2017, 17, 3779–3785. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Tarrío, A.M.; Atutxa, A.; Bilbao, J. Study of operating variables in the transformation of aqueous ethanol into hydrocarbons on an HZSM-5 zeolite. J. Chem. Technol. Biotechnol. 2002, 77, 211–216. [Google Scholar] [CrossRef]
- Sousa, Z.S.; Cesar, D.V.; Henriques, C.A.; da Silva, V.T. Bioethanol conversion into hydrocarbons on HZSM-5 and HMCM-22 zeolites: Use of in situ DRIFTS to elucidate the role of the acidity and of the pore structure over the coke formation and product distribution. Catal. Today 2014, 234, 182–191. [Google Scholar] [CrossRef]
- Sousa, Z.S.; Veloso, C.O.; Henriques, C.A.; da Silva, V.T. Ethanol conversion into olefins and aromatics over HZSM-5 zeolite: Influence of reaction conditions and surface reaction studies. J. Mol. Catal. A Chem. 2016, 422, 266–274. [Google Scholar] [CrossRef]
- Song, Z.X.; Liu, W.; Chen, C.; Takahashi, A.; Fujitani, T. Production of propylene from ethanol over ZSM-5 co-modified with zirconium and phosphorus. React. Kinet. Mech. Catal. 2013, 109, 221–231. [Google Scholar] [CrossRef]
- Inoue, K.; Okabe, K.; Inaba, M.; Takahara, I.; Murata, K. Metal modification effects on ethanol conversion to propylene by H-ZSM-5 with Si/Al2 ratio of 150. React. Kinet. Mech. Catal. 2010, 101, 477–489. [Google Scholar] [CrossRef]
- Yin, J.B.; Guo, X.X.; Sun, Y.X.; Han, S.; Li, Q.G. Understanding the nanoconfinement effect on the ethanol-to-propene mechanism catalyzed by acidic ZSM-5 and FAU zeolites. J. Phys. Chem. C 2021, 125, 310–334. [Google Scholar] [CrossRef]
- Sheng, Q.T.; Ling, K.C.; Li, Z.R.; Zhao, L.F. Effect of steam treatment on catalytic performance of HZSM-5 catalyst for ethanol dehydration to ethylene. Fuel Process. Technol. 2013, 110, 73–78. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Alonso, A.; Valle, B.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Hydrothermal stability of HZSM-5 catalysts modified with Ni for the transformation of bioethanol into hydrocarbons. Fuel 2010, 89, 3365–3372. [Google Scholar] [CrossRef]
- Alonso, A.; Valle, B.; Atutxa, A.; Gayubo, A.G.; Aguayo, A. Development of alternative catalysts based on HZSM-5 zeolite for the BTO process. Int. J. Chem. Reactor Eng. 2007, 5, A61. [Google Scholar] [CrossRef]
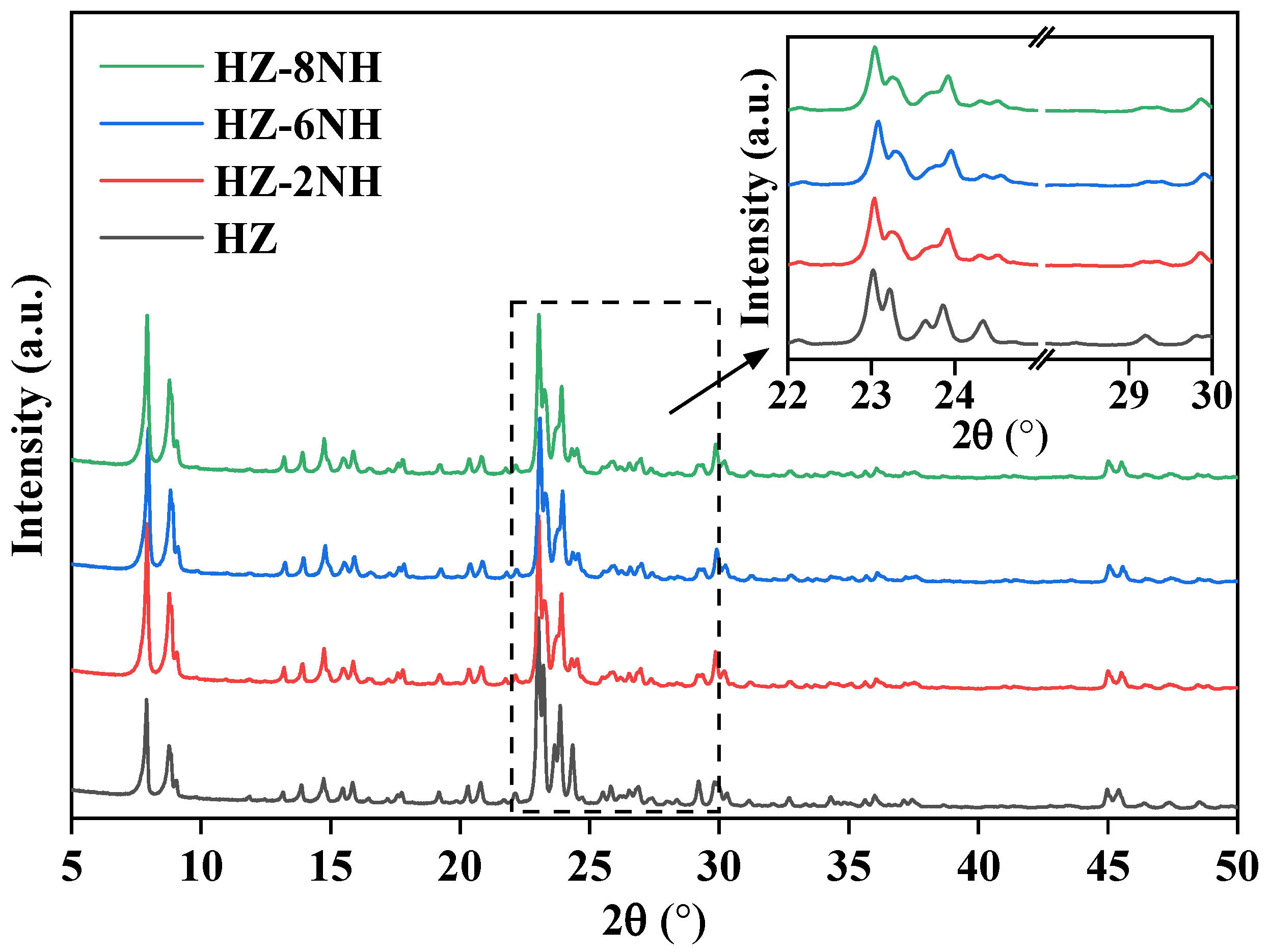
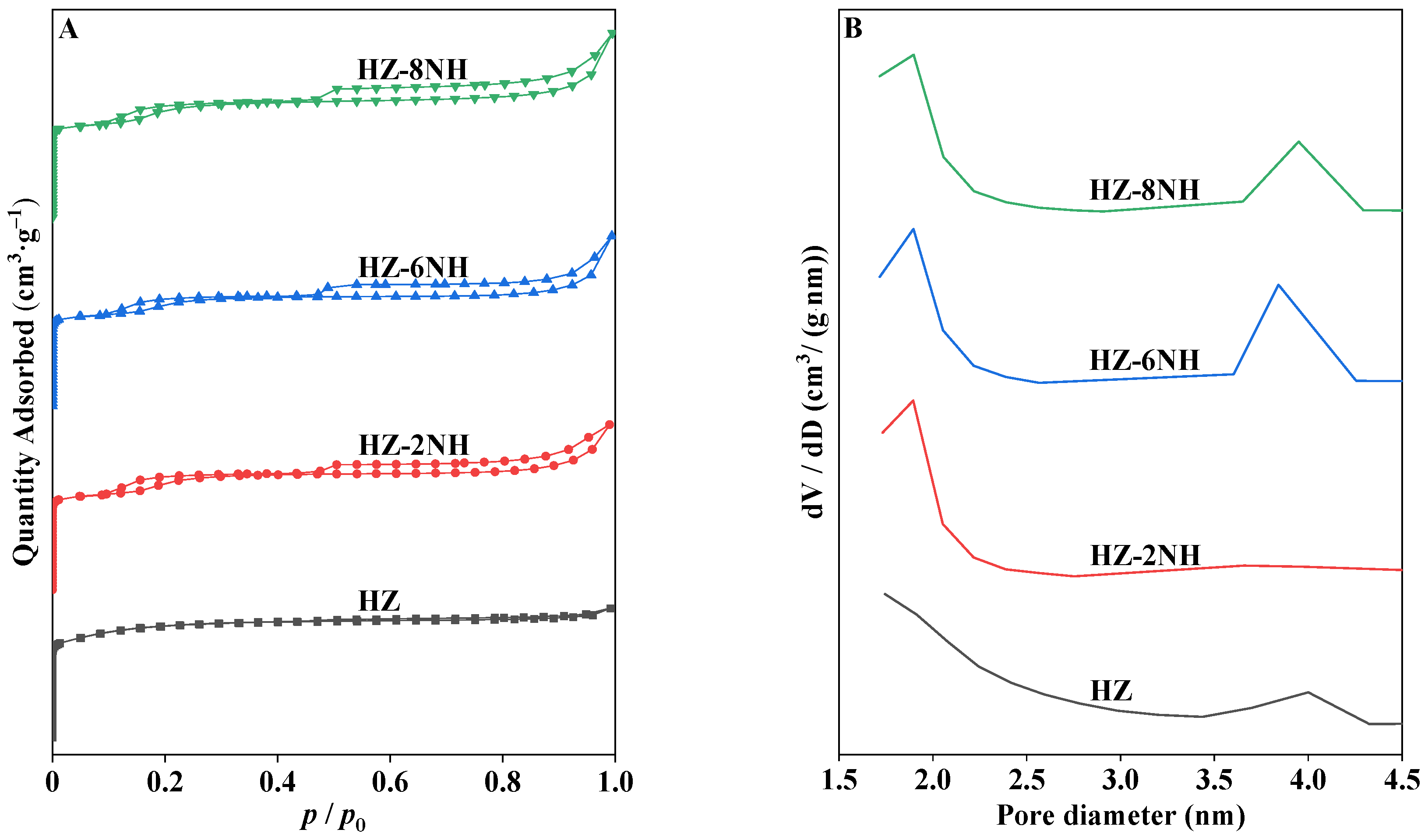
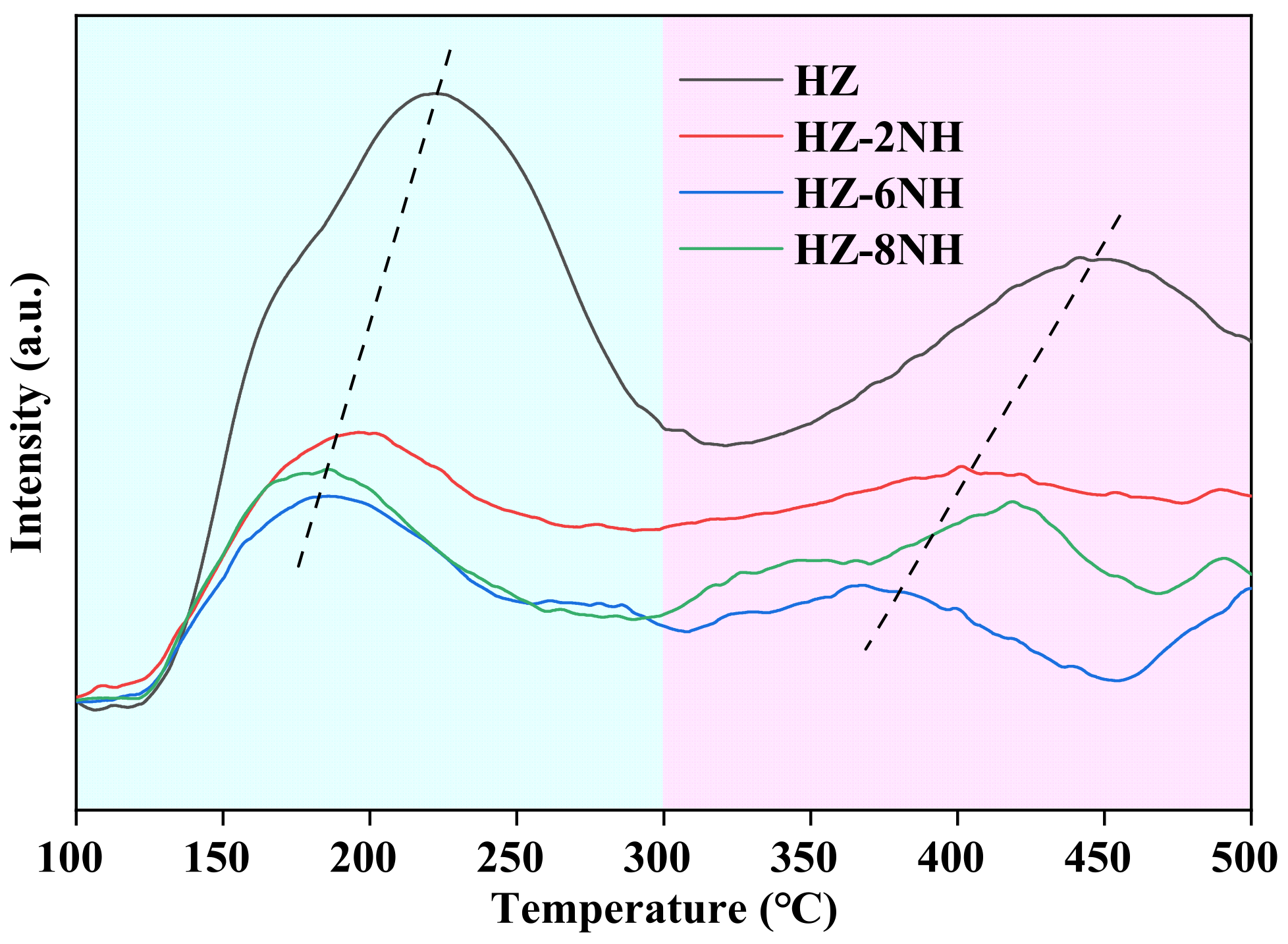
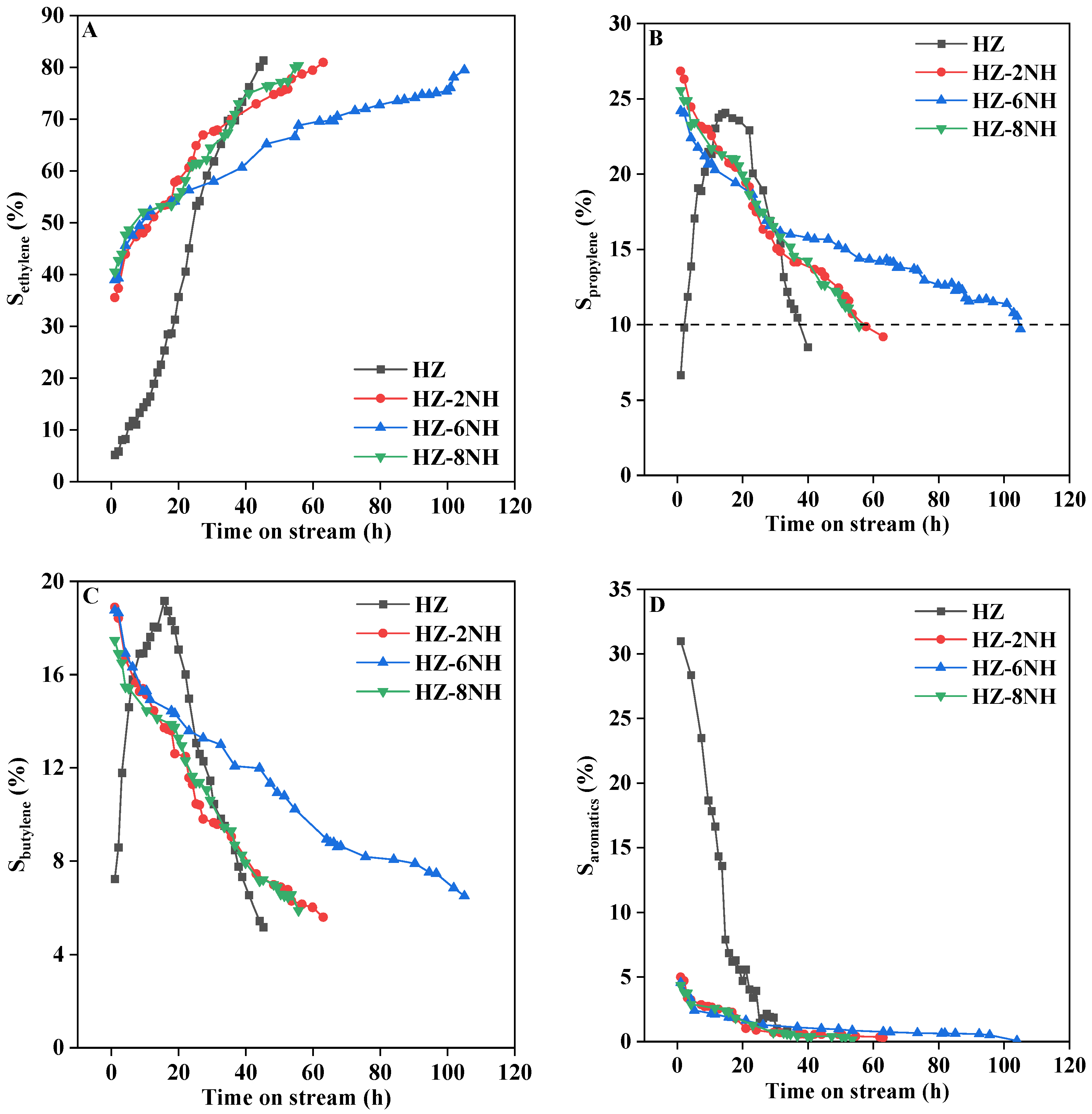
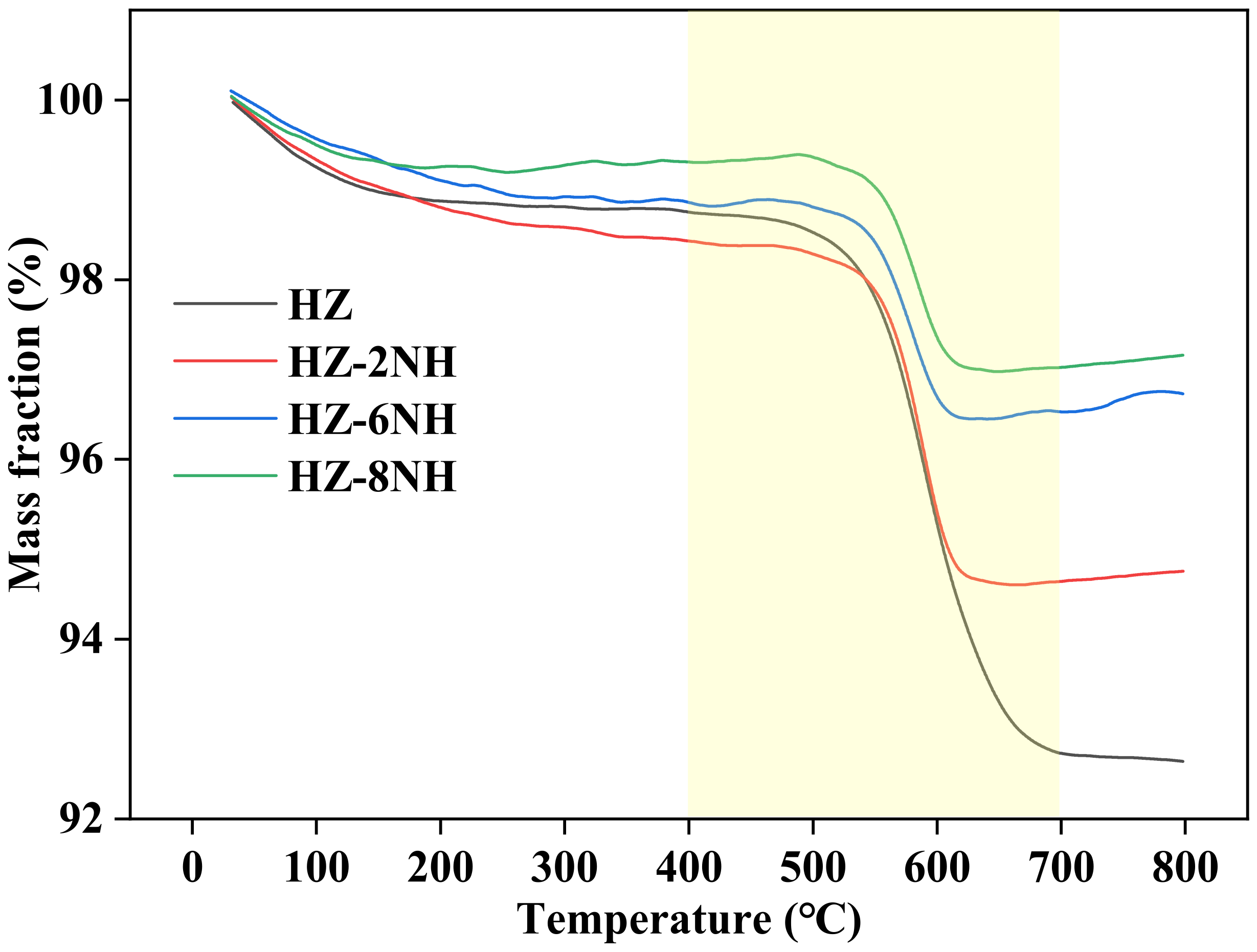
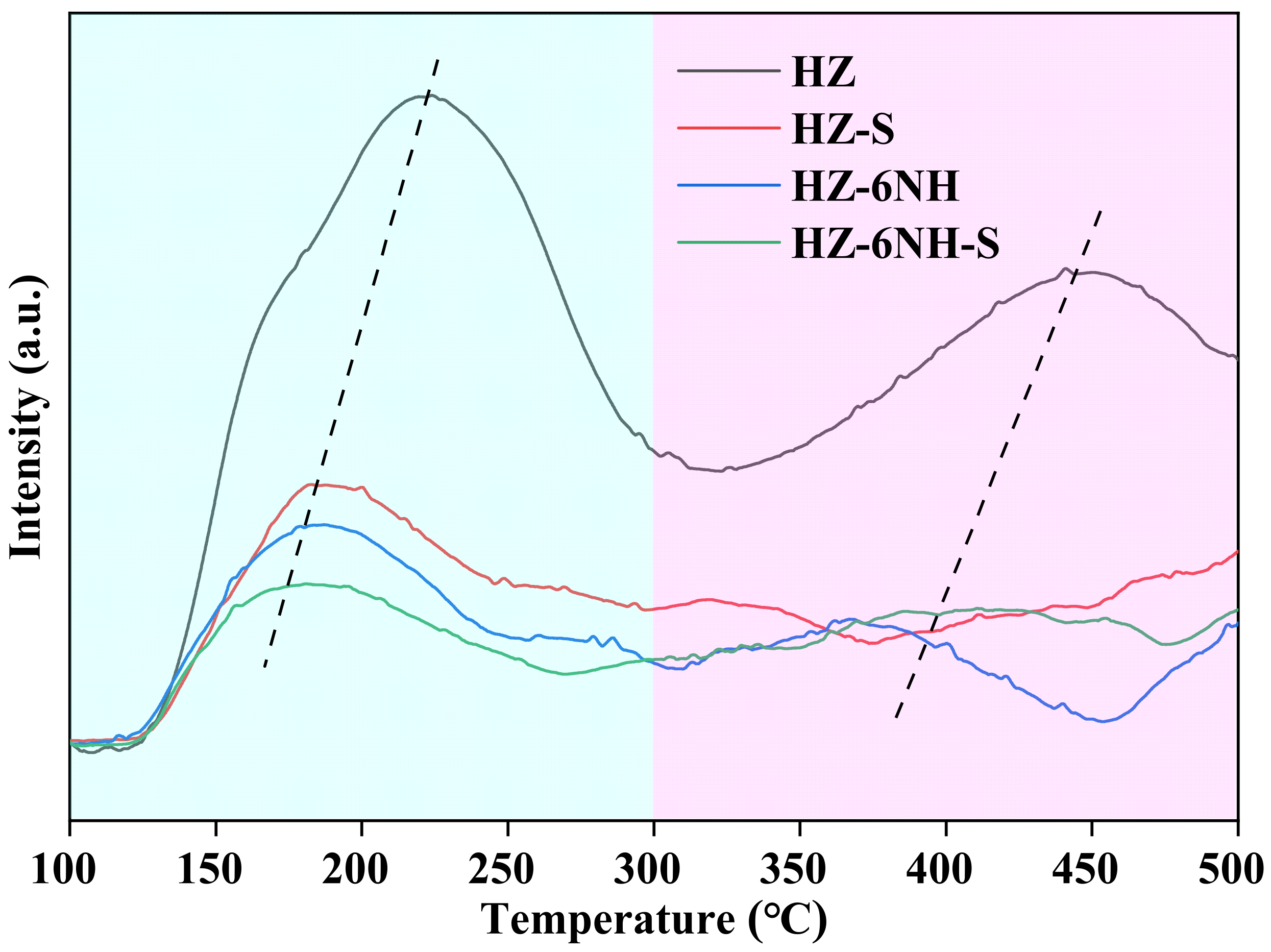
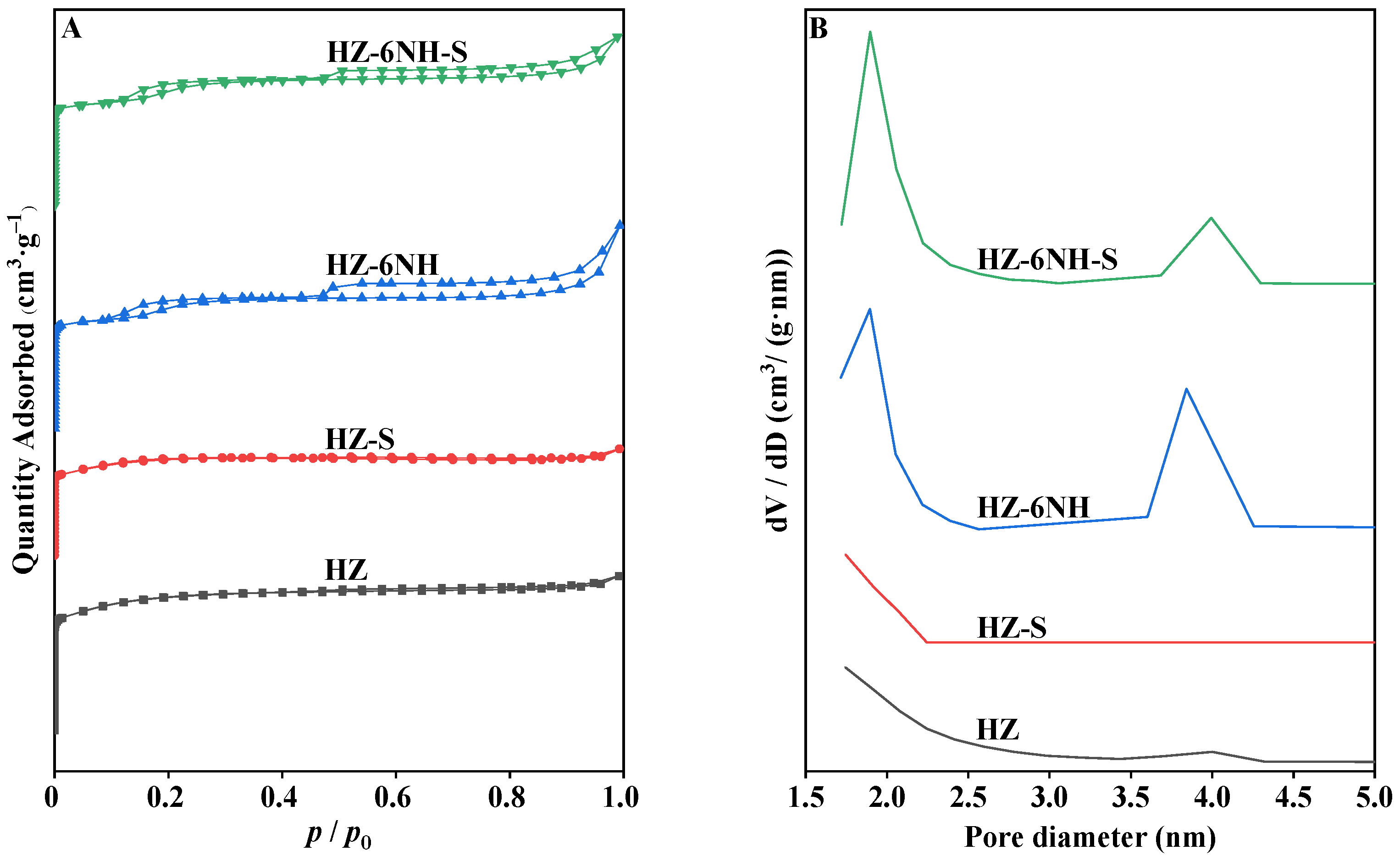
| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | DP a (nm) | RC b (%) | Si/Al Molar Ratio c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Micropore | External | Total | Micropore | Mesopore | ||||
| HZ | 326 | 226 | 100 | 0.19 | 0.12 | 0.07 | 2.30 | 100 | 16.9 |
| HZ-2NH | 336 | 80 | 256 | 0.24 | 0.05 | 0.19 | 2.92 | 79 | 17.0 |
| HZ-6NH | 318 | 92 | 226 | 0.25 | 0.05 | 0.20 | 3.18 | 77 | 16.1 |
| HZ-8NH | 337 | 64 | 273 | 0.27 | 0.04 | 0.23 | 3.24 | 76 | 16.5 |
| Samples | Distribution of Main Products (%) | |||||
|---|---|---|---|---|---|---|
| Ethylene | Propylene | Butylene | Paraffins (C1–C4) | Aromatics | Others | |
| HZ | 5.2 | 6.0 | 7.2 | 41.9 | 31.0 | 8.7 |
| HZ-2NH | 35.5 | 26.8 | 18.9 | 12.4 | 5.0 | 1.4 |
| HZ-6NH | 38.9 | 24.2 | 18.8 | 12.1 | 4.6 | 1.4 |
| HZ-8NH | 40.5 | 25.6 | 17.5 | 11.1 | 4.4 | 0.9 |
| Sample | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | DP a (nm) | RC b (%) | Si/Al Molar Ratio c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Micropore | External | Total | Micropore | Mesopore | ||||
| HZ | 326 | 226 | 100 | 0.19 | 0.12 | 0.07 | 2.30 | 100 | 16.9 |
| HZ-S | 243 | 193 | 50 | 0.13 | 0.10 | 0.03 | 2.22 | – | – |
| HZ-6NH | 318 | 92 | 226 | 0.25 | 0.05 | 0.20 | 3.18 | 77 | 16.1 |
| HZ-6NH-S | 305 | 71 | 234 | 0.21 | 0.04 | 0.17 | 2.76 | – | – |
| Sample | Mesopore Volume (cm3 g−1) | Average Pore Diameter (nm) | Average Coke Deposition Rate (g (gcat·h)−1) | Maximum Propylene Selectivity (%) | Working Life (h) |
|---|---|---|---|---|---|
| HZ | 0.07 | 2.30 | 1.4 × 10−3 | 24.1 | 36.8 |
| ZSM-5-6HF [28] | 0.14 | 2.30 | 2.9 × 10−4 | 24.0 | 95.0 |
| HZ-6NH | 0.20 | 3.18 | 2.3 × 10−4 | 24.2 | 105.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, N.; Meng, T.; Guo, Q.; Xue, Z.; Mao, D. Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene. Nanomaterials 2024, 14, 1558. https://doi.org/10.3390/nano14191558
Zhou J, Zhang N, Meng T, Guo Q, Xue Z, Mao D. Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene. Nanomaterials. 2024; 14(19):1558. https://doi.org/10.3390/nano14191558
Chicago/Turabian StyleZhou, Jian, Ni Zhang, Tao Meng, Qiangsheng Guo, Zhaoteng Xue, and Dongsen Mao. 2024. "Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene" Nanomaterials 14, no. 19: 1558. https://doi.org/10.3390/nano14191558
APA StyleZhou, J., Zhang, N., Meng, T., Guo, Q., Xue, Z., & Mao, D. (2024). Fluoride-Treated Nano-HZSM-5 Zeolite as a Highly Stable Catalyst for the Conversion of Bioethanol to Propylene. Nanomaterials, 14(19), 1558. https://doi.org/10.3390/nano14191558






