Enhancing the Performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) Perovskites as Catalysts for CO Oxidation by Decreasing the Ba Content
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Catalysts Characterization
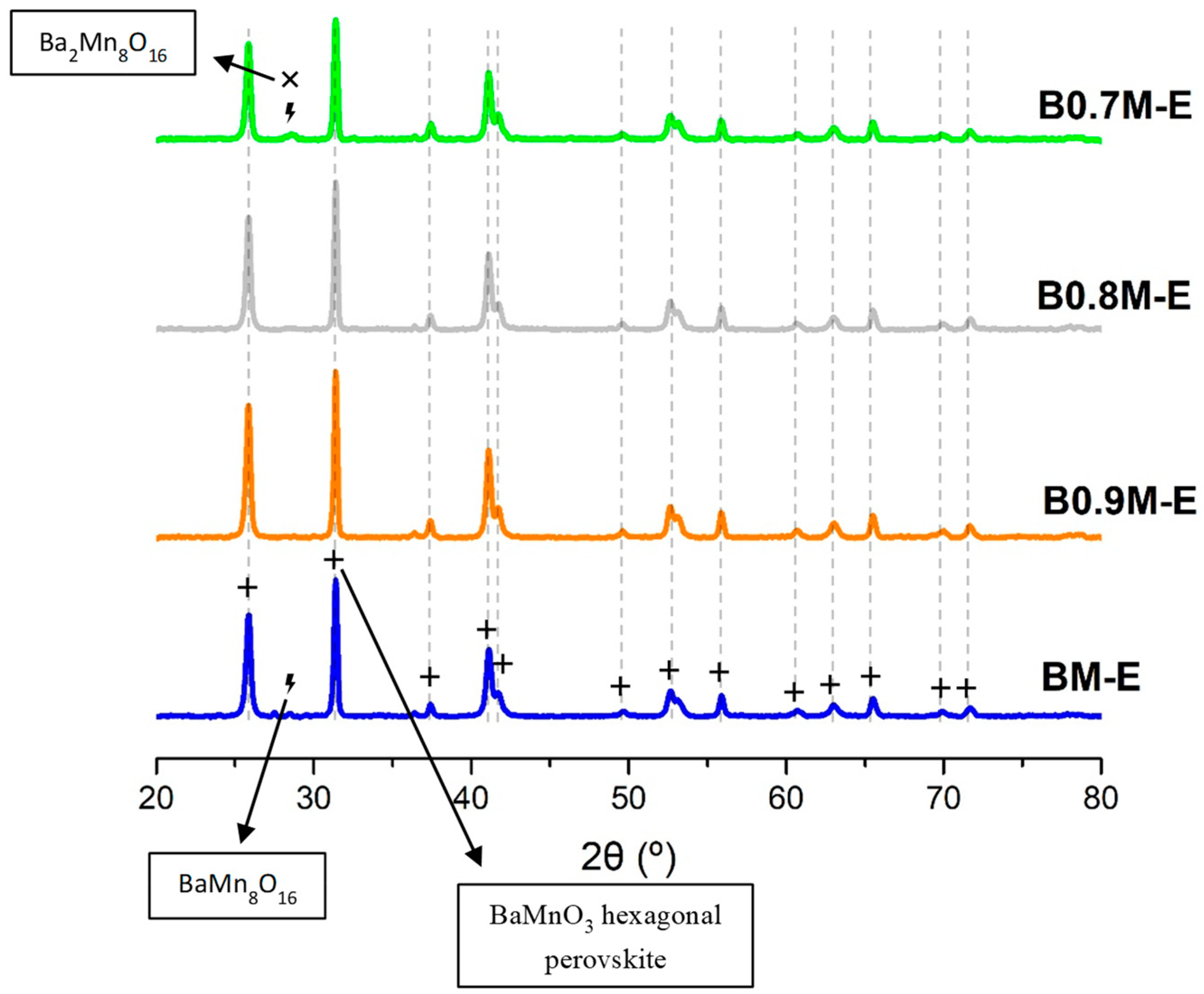
3.1.1. Chemical, Morphological and Structural Properties
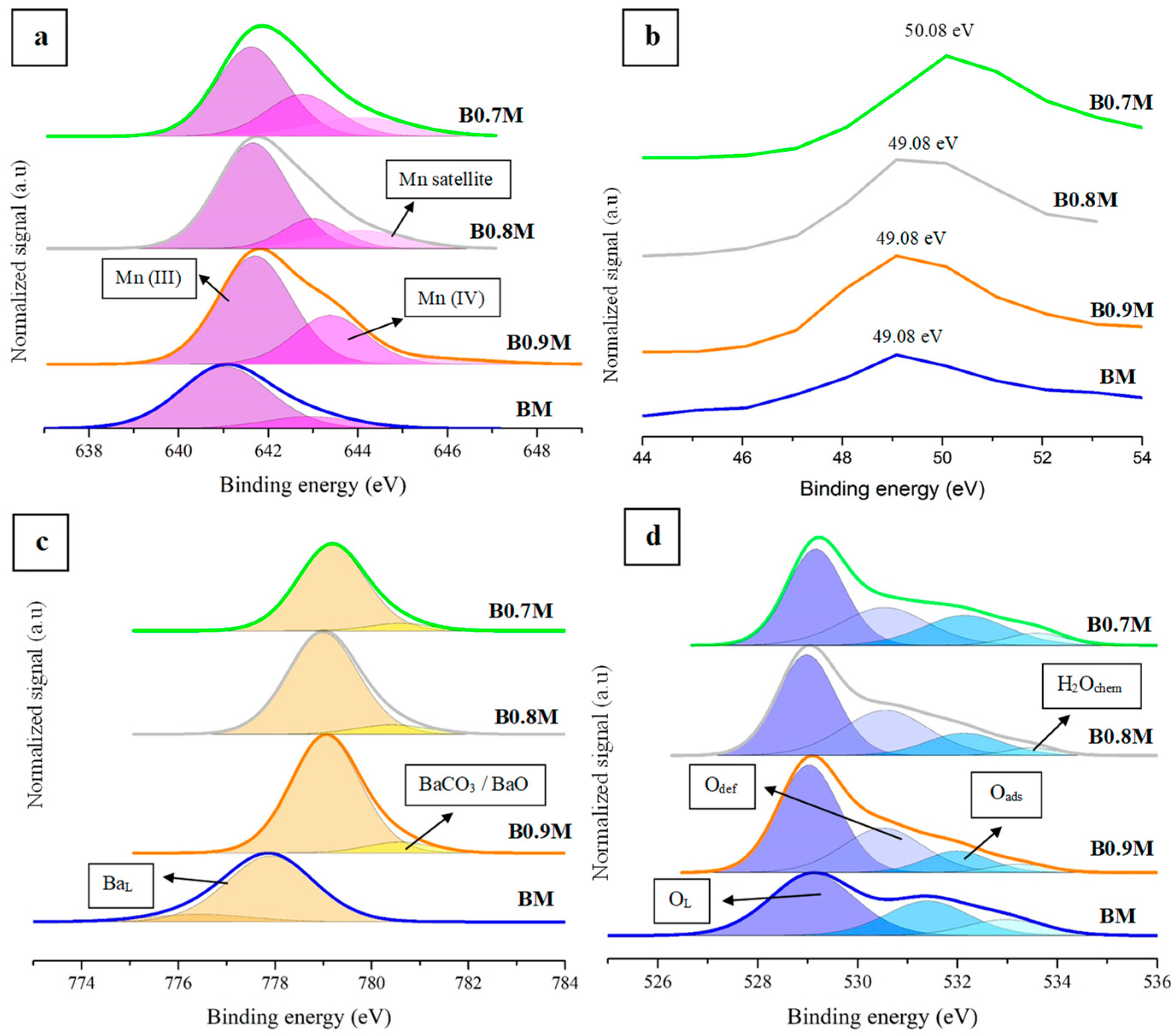
3.1.2. Surface Composition
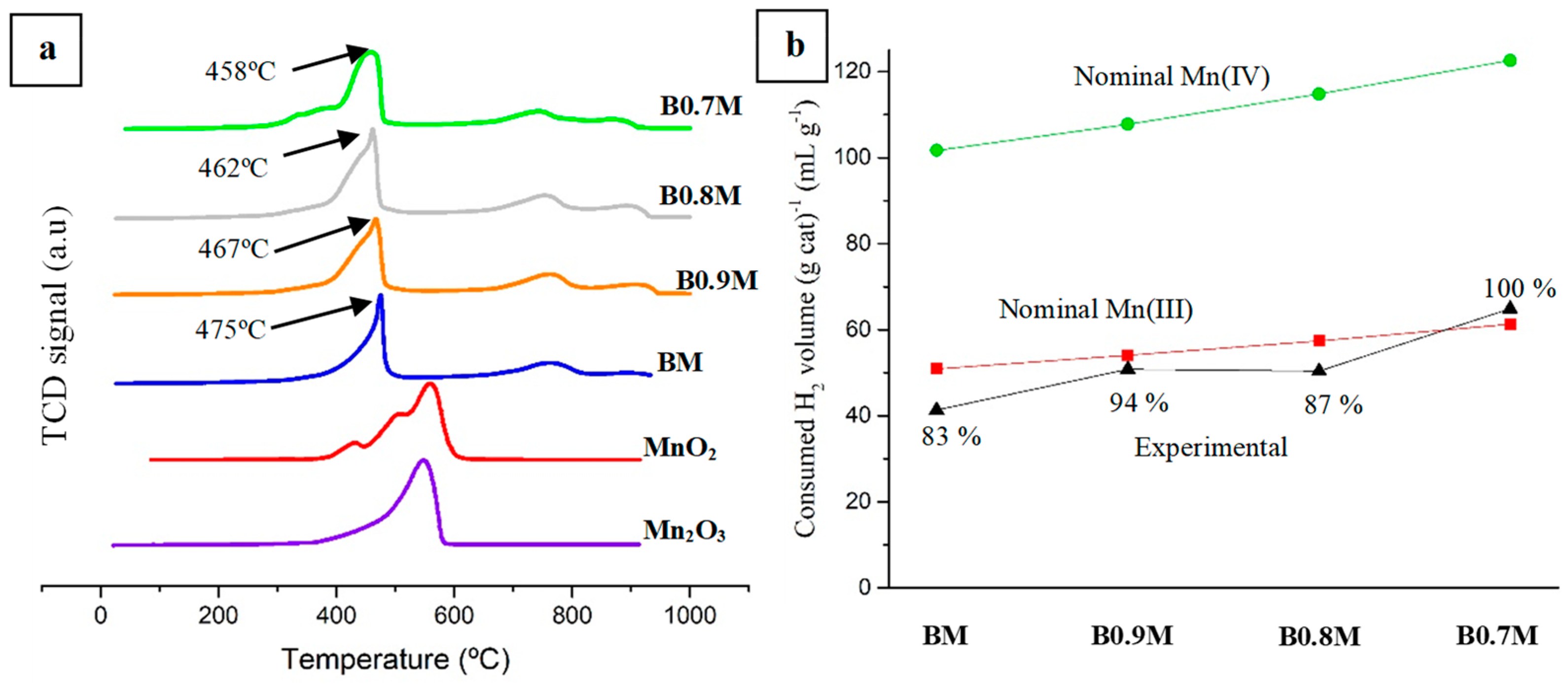
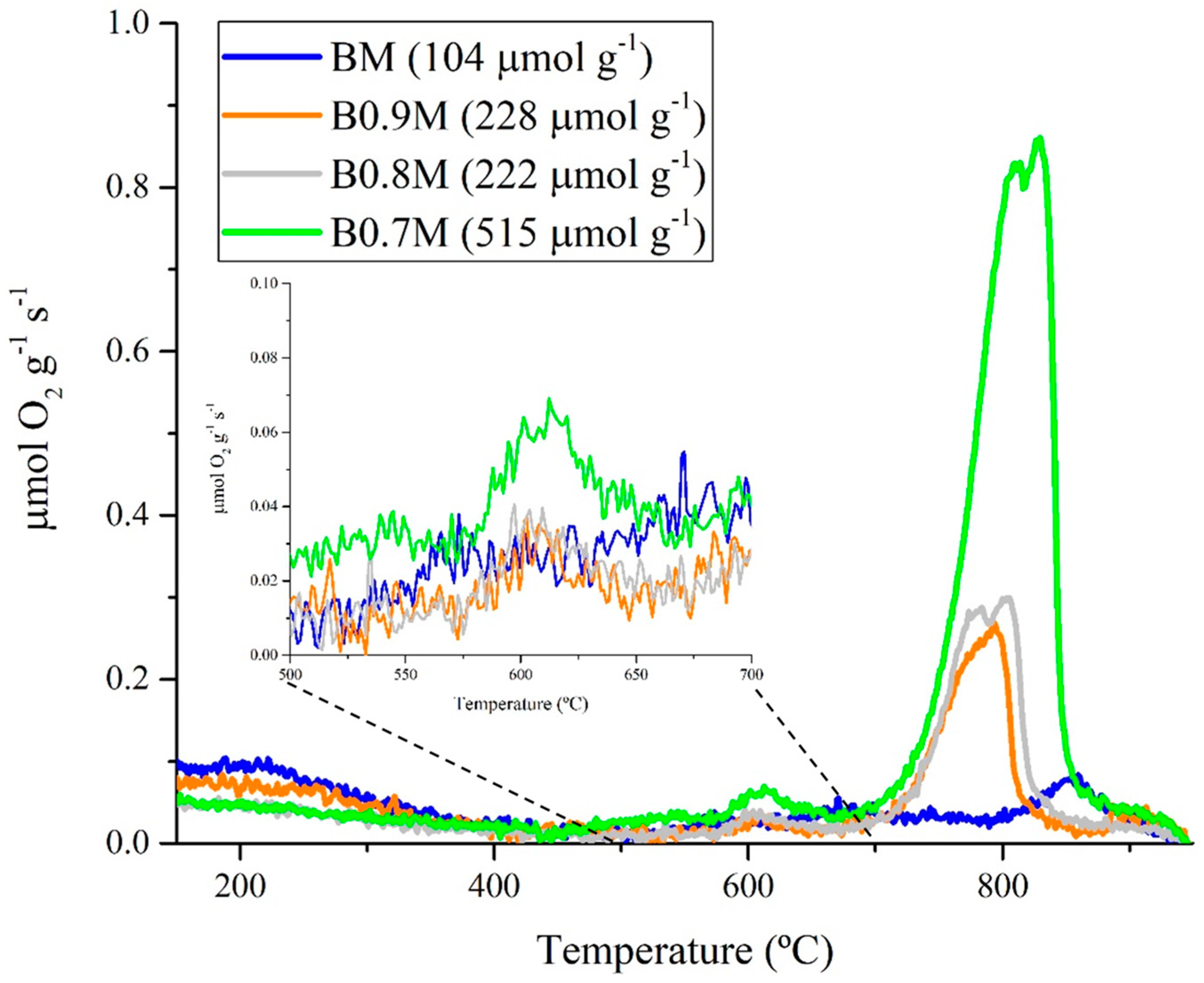
3.1.3. Redox Properties
3.2. Catalytic Activity
4. Conclusions
- The synthesis procedure allowed for achieving a perovskite-type structure, as confirmed by XRD data.
- XPS data substantiated the coexistence of a Mn(IV)/Mn(III) pair on the surface of the samples and, also, the presence of surface oxygen vacancies, being particularly prominent for B0.7M.
- The presence of oxygen vacancies in the Ba-deficient perovskites lattice allows us to increase the reducibility of samples and the oxygen mobility.
- The higher amount of oxygen vacancies and the improved reducibility of the Ba-deficient samples boosted the catalytic activity for the CO oxidation reaction of BM, even though the 1% Pt/Al2O3 reference sample still presents the best performance.
- B0.7M is the most active catalyst from the BxM series, as it presents the highest amount of oxygen vacancies on the surface (XPS), the highest reducibility (H2-TPR) and lattice oxygen mobility (O2-TPD and CO-TPR).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Su, Y.; Deng, P.; Qi, R.; Zhu, J.; Chen, J.; Wang, Z.; Zhou, L.; Guo, X.; Xia, B.Y. Highly selective carbon dioxide electroreduction on structure-evolved copper perovskite oxide toward methane production. ACS Catal. 2020, 10, 4640–4646. [Google Scholar] [CrossRef]
- Xia, C.; Wang, X.; He, C.; Qi, R.; Zhu, D.; Lu, R.; Li, F.M.; Chen, Y.; Chen, S.; You, B.; et al. Highly selective electrocatalytic CO2 conversion to tailored products through precise regulation of hydrogenation and C-C coupling. J. Am. Chem. Soc. 2024, 146, 20530–20538. [Google Scholar] [CrossRef] [PubMed]
- Savioli, G.; Gri, N.; Ceresa, I.F.; Piccioni, A.; Zanza, C.; Longhitano, Y.; Ricevuti, G.; Daccò, M.; Esposito, C.; Candura, S.M. Carbon monoxide poisoning: From occupational health to emergency medicine. J. Clin. Med. 2024, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Cha, X.; Huang, Z.; Hu, S.; Xu, K.; Cai, D.; Xiao, J.; Zhan, G. Green fabrication of hierarchically-structured Pt/bio-CeO2 nanocatalysts using natural pollen templates for low-temperature CO oxidation. Mol. Catal. 2022, 524, 112251. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Zhu, M.; Fu, Y.; Sun, Y.; Meng, Z.; Peng, B.; Nie, L. Incorporation of Ag on stable Pt/CeO2 for low-temperature active and high-temperature stable CO oxidation catalyst. Mol. Catal. 2024, 553, 113704. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Ma, C.; Liu, Y.; Giroux, M.; Chi, M.; Jin, J.; Greeley, J.; Wang, C. Plating precious metals on nonprecious metal nanoparticles for sustainable electrocatalysts. Nano Lett. 2017, 17, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, J.; Liu, T.; Xu, M.; Dong, Y.; Wang, C.A. Constructing morphologically stable supported noble metal catalysts in heterogeneous catalysis: Mechanisms and strategies. Nano Energy 2024, 129, 109975. [Google Scholar] [CrossRef]
- Chai, Y.; Shang, W.; Li, W.; Wu, G.; Dai, W.; Guan, N.; Li, L. Noble metal particles confined in zeolites: Synthesis, characterization, and applications. Adv. Sci. 2019, 6, 1900299. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Concas, A.; Orrù, R.; Cao, G.; Rupprechter, G. Microalgae-derived Co3O4 nanomaterials for catalytic CO oxidation. RSC Adv. 2024, 14, 4575–4586. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Zhao, M.; Xu, S.; Wang, M.; Cao, J.; Zhang, Q.; Chen, J. Engineering defective Mnδ+-OV active structure in α-MnO2 for efficient low-temperature CO oxidation. Appl. Surf. Sci. 2024, 670, 160602. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental understanding and application of Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite in energy storage and conversion: Past, present, and future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Shao, Z.; Qiu, J.; Wang, S.; Liu, S. Perovskite oxide catalysts for advanced oxidation reactions. Adv. Funct. Mater. 2021, 31, 2102089. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning synergy between nickel and iron in Ruddlesden-Popper perovskites through controllable crystal dimensionalities towards enhanced oxygen-evolving activity and stability. Carbon Energy 2024, 6, e465. [Google Scholar] [CrossRef]
- Yang, C.; Grimaud, A. Factors controlling the redox activity of oxygen in perovskites: From theory to application for catalytic reactions. Catalysts 2017, 7, 149. [Google Scholar] [CrossRef]
- Mishra, A.; Galinsky, N.; He, F.; Santiso, E.E.; Li, F. Perovskite-structured AMnxB1−xO3 (A = Ca or Ba; B = Fe or Ni) redox catalysts for partial oxidation of methane. Catal. Sci. Technol. 2016, 6, 4535–4544. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Liao, Y.; Lei, M.; Fang, X.; Xu, X.; Wang, X. La/Mn molar ratio tuning the activity of La-Mn perovskites for CO and propane oxidation. J. Energy Inst. 2024, 114, 101595. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Zainul, R.; Akrami, F.; Yusuf, S.; Alfadhlani, A.; Hasanudin, H.; Delson, D.; Mandeli, R.S.; Putra, H.; Mapanta, J.; Sravan, M.S.B.; et al. Advances in manganese oxide research: Environmental geochemistry to biogeochemical cycling and sustainable catalysis. Appl. Organomet. Chem. 2024, 4, 14–29. [Google Scholar] [CrossRef]
- Ahmed, N. Recent advances and emerging opportunities in mechanism and applications of earth abundant manganese-catalysts for sustainable organic transformations. J. Organomet. Chem. 2024, 1009, 123071. [Google Scholar] [CrossRef]
- Kembo, J.P.N.; Wang, J.; Luo, N.; Gao, F.; Yi, H.; Zhao, S.; Zhou, Y.; Tang, X. A review of catalytic oxidation of carbon monoxide over different catalysts with an emphasis on hopcalite catalysts. New J. Chem. 2023, 47, 20222–20247. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Montilla-Verdú, S.; Torregrosa-Rivero, V.; Illán-Gómez, M.J. Tailoring the composition of BaxBO3 (B = Fe, Mn) mixed oxides as CO or soot oxidation catalysts in simulated GDI engine exhaust conditions. Molecules 2023, 28, 3327. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New nanocomposites derived from cation-nonstoichiometric Bax(Co, Fe, Zr, Y)O3-δ as efficient electrocatalysts for water oxidation in alkaline solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Karoui, L.; Smari, M.; Mnasri, T. The effect of the gelation temperature on the structural, magnetic and magnetocaloric properties of perovskite nanoparticles manufactured using the sol-gel method. RSC Adv. 2024, 14, 11456–11469. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.U.; Ikram, M. Structural stability improvement, Williamson Hall analysis and band-gap tailoring through A-site Sr doping in rare earth based double perovskite La2NiMnO6. Rare Met. 2019, 38, 805–813. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, L.; Ma, R.; Chen, Y.; Guan, Q.; Li, W. CuO nano-island anchored Pt catalyst for CO preferential oxidation in H2-rich stream. Chem. Eng. J. 2024, 495, 153295. [Google Scholar] [CrossRef]
- Cai, L.N.; Guo, Y.; Lu, A.H.; Branton, P.; Li, W.C. The choice of precipitant and precursor in the co-precipitation synthesis of copper manganese oxide for maximizing carbon monoxide oxidation. J. Mol. Catal. A Chem. 2012, 360, 35–41. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; García-García, A. Identifying the nature of the copper entities over ceria-based supports to promote diesel soot combustion: Synergistic effects. Appl. Catal. A Gen. 2017, 542, 226–239. [Google Scholar] [CrossRef]
- Guiotto, M.; Pacella, M.; Perin, G.; Iovino, A.; Michelon, N.; Natile, M.M.; Glisenti, A.; Canu, P. Washcoating vs. direct synthesis of LaCoO3 on monoliths for environmental applications. Appl. Catal. A Gen. 2015, 499, 146–157. [Google Scholar] [CrossRef]
- Park, D.C.; Moon, S.; Song, J.H.; Kim, H.; Lee, E.; Lim, Y.H.; Kim, D.H. Widening the operating window of Pt/ZSM-5 catalysts for efficient NOx removal in H2-SCR: Insights from thermal aging. Catal. Today 2024, 425, 114318. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Jia, J.; Sun, T. Catalytic oxidation of VOCs over SmMnO3 perovskites: Catalyst synthesis, change mechanism of active species, and degradation path of toluene. Inorg. Chem. 2019, 58, 14275–14283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z. Lysisne-aided PMMA-templating preparation and high performance of three-dimensionally ordered microporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene. Appl. Catal. B Environ. 2012, 119–120, 20–31. [Google Scholar] [CrossRef]
- Mane, R.; Kim, H.; Han, K.; Kim, H.; Lee, S.S.; Roh, H.S.; Lee, C.; Jeon, Y. Important factors of the A-site deficient Mn perovskites design affecting the CO oxidation activity. Catal. Today 2024, 425, 114347. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, J.; Ma, C.; Yang, J.; Cao, L.; Yang, J. Development of LnMnO3+σ perovskite on low temperature Hg0 removal. J. Environ. Sci. 2022, 113, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, S.; Zhang, L.; Chen, Z.; Wang, M.; Wang, S. A facile method to promote LaMnO3 perovskite catalyst for combustion of methane. Catal. Commun. 2017, 97, 88–92. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between catalytic deactivation and physicochemical properties of LaMnO3 perovskite catalyst during catalytic oxidation of vinyl chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; García-González, L.; Cancino-Gordillo, F.E.; Pal, U. Particle dispersion and lattice distortion induced magnetic behavior of La1−xSrxMnO3 perovskite nanoparticles grown by salt-assisted sol-state synthesis. Mater. Chem. Phys. 2020, 246, 122834. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Copper doped BaMnO3 perovskite catalysts for NO oxidation and NO2-assisted diesel soot removal. RSC Adv. 2017, 7, 35228–35238. [Google Scholar] [CrossRef]
- Shim, K.; Kim, H.W.; Park, S.; Seo, K.D.; Kim, C.Y.; Lee, J.B.; Bae, J.S.; Kim, H.J. A MnS/MnO-coated S,N-doped carbon anode obtained from a Mn(II)-coordinated polymer for long-cycle life Li-ion batteries. Mater. Adv. 2024, 5, 3014–3021. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Cerrato, J.M.; Hochella, M.F.; Knocke, W.R.; Dietrich, A.M.; Cromer, T.F. Use of XPS to identify the oxidation state of Mn in solid surfaces of filtration media oxide samples from drinking water treatment plants. Environ. Sci. Technol. 2010, 44, 5881–5886. [Google Scholar] [CrossRef] [PubMed]
- Padalia, D.; Kumar, U.; Bhandari, P.; Dalal, J.; Ranakoti, L.; Singh, T. Tuning the structural, optical, and dielectric properties of europium-doped barium titanate ceramics. J. Mater. Sci. Mater. Electron. 2024, 35, 1375. [Google Scholar] [CrossRef]
- Garbujo, A.; Pacella, M.; Natile, M.M.; Guiotto, M.; Fabro, J.; Canu, P.; Glisenti, A. On A-doping strategy for tuning the TWC catalytic performance of perovskite based catalysts. Appl. Catal. A Gen. 2017, 544, 94–107. [Google Scholar] [CrossRef]
- Hertzberg, B.; Sviridov, L.; Stach, E.A.; Gupta, T.; Steingart, D. A manganese-doped barium carbonate cathode for alkaline batteries. J. Electrochem. Soc. 2014, 161, A835. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Owolabi, T.O.; Saleh, T.A. X-ray photoelectron spectroscopy of surface-treated TiO2 mesoporous film by 500 eV argon ion beam. J. Mol. Liq. 2024, 393, 123556. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; López-Rodríguez, S.; Villar-García, I.J.; Pérez-Dieste, V.; Bueno-López, A.; González-Velasco, J.R. Molecular elucidation of CO2 methanation over a highly active, selective and stable LaNiO3/CeO2-derived catalyst by in situ FTIR and NAP-XPS. Appl. Catal. B Environ. 2024, 342, 123367. [Google Scholar] [CrossRef]
- Koch, G.; Hävecker, M.; Teschner, D.; Carey, S.J.; Wang, Y.; Kube, P.; Hetaba, W.; Lunkenbein, T.; Auffermann, G.; Timpe, O.; et al. Surface conditions that constrain alkane oxidation on perovskites. ACS Catal. 2020, 10, 7007–7020. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Xing, J.; Zhang, M.; Liao, R.; Wang, C.; Hua, Y.; Ji, H. Novel synergistically effects of palladium-iron bimetal and manganese carbonate carrier for catalytic oxidation of formaldehyde at room temperature. J. Colloid Interface Sci. 2024, 656, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Domingo, N.; Pach, E.; Cordero-Edwards, K.; Pérez-Dieste, V.; Escudero, C.; Verdaguer, A. Water adsorption, dissociation and oxidation on SrTiO3 and ferroelectric surfaces revealed by ambient pressure X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 4920–4930. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Hong, W.T.; Azimi, G.; Giordano, L.; Lee, Y.L.; Crumlin, E.J.; Biegaslki, M.D.; Bluhm, H.; Varanasi, K.K.; Shao-Horn, Y. Reactivity of perovskites with water: Role of hydroxylation in wetting and implications for oxygen electrocatalysis. J. Phys. Chem. C 2015, 119, 18504–18512. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kaliaguine, S. Reduction kinetics of perovskite-based oxygen carriers for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 6946–6955. [Google Scholar] [CrossRef]
- Santamaria, A.; Méndez-A, C.; Gallego, J. LaMn1−xCoxO3 perovskite-like doped and impregnated with Cu for the enhancement of toluene total oxidation. Appl. Surf. Sci. 2024, 669, 160587. [Google Scholar] [CrossRef]
- Kobayashi, M.; Naniwa, S.; Goto, K.; Matsuo, H.; Iguchi, S.; Tsunehiro, T.; Teramura, K. Promoting effect of Pd nanoparticles on SrTi0.8Mn0.2O3 in the reverse-gas shift reaction via the mars-van krevelen mechanism. ChemCatChem 2024, e202400871. [Google Scholar] [CrossRef]
- Osti, A.; Rizzato, L.; Cavazzani, J.; Glisenti, A. Optimizing citrate combustion synthesis of A-site deficient La,Mn-based perovskites: Application for catalytic CH4 combustion in stoichiometric conditions. Catalysts 2023, 13, 1177. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kleitz, F.; Kaliaguine, S. Novel oxygen carriers for chemical looping combustion: La1−xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ. Sci. 2011, 4, 4258–4269. [Google Scholar] [CrossRef]
- Hussain, S.K.; Bang, J.H. Overview of the oxygen vacancy effect in bimetallic spinel and perovskite oxide electrode materials for high-performance supercapacitors. Phys. Chem. Chem. Phys. 2023, 25, 11892–11907. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, C.; Guo, Y.; Lu, G.; Wang, Y. Effects of surface area and oxygen vacancies on ceria in CO oxidation: Differences and relationships. J. Mol. Catal. A Chem. 2010, 316, 59–64. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Li, L.; Wang, A.; Zhang, W.; Wang, X.; Zhang, T. Activation of an Ir-in-CeO2 catalyst by pulses of CO: The role of oxygen vacancy and carbonates in CO oxidation. Catal. Today 2012, 180, 155–160. [Google Scholar] [CrossRef]
- Wu, R.; Li, L.; Lu, Z.; Sun, C.; Cheng, L.; Ye, R.; Zhang, R.; Li, Q.; Feng, G. DFT insights into doping and oxygen vacancy effects on CO and CO2 adsorptions over CuAl2O4 spinel surfaces. J. Catal. 2024, 434, 115502. [Google Scholar] [CrossRef]
- Ghani, F.; Haidry, A.A.; Raza, A.; Fatima, Q.; Weng, Y.; Sajjad, M.; Albaqami, M.D.; Mohammad, S. Anisotropic CO adsorption and enhanced O2 activation on defective TiS2 monolayer: A DFT study. Mater. Today Commun. 2024, 40, 109680. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Niu, X.; Yuan, F.; Fu, H. Preparation of La-Mn-O perovskite catalyst by microwave irradiation method and its application to methane combustion. Catal. Lett. 2010, 135, 152–158. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the highly catalytic performance of spinel CoMn2O4 for toluene oxidation: Involvement and replenishment of oxygen species using in situ designed-TP techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Popczun, E.J.; Baltrus, J.P.; Wang, K.; Serna, P.; Liu, S.; Meyer, R.; Lekse, J.W. Investigation of AFeO3 (A = Ba, Sr) perovskites for the oxidative dehydrogenation of light alkanes under chemical looping conditions. ChemPlusChem 2024, 89, e202300596. [Google Scholar] [CrossRef] [PubMed]
- García-García, F.J.; Sayagués, M.J.; Gotor, F.J. A novel, simple and highly efficient route to obtain PrBaMn2O5+δ double perovskite: Mechanochemical synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, J. Carbon monoxide: A mild and efficient reducing agent towards atomically precise gold nanoclusters. Chem. Rec. 2016, 16, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; An, K.; Li, S.; Zhang, Z.; Sun, R.; Liu, Y. Bi-active sites of stable and highly dispersed platinum and oxygen vacancy constructed by reducing a loaded perovskite-type oxide for CO oxidation. Appl. Surf. Sci. 2020, 532, 147455. [Google Scholar] [CrossRef]
- Cho, J.; Kim, M.; Yang, I.; Park, K.T.; Rhee, C.H.; Park, H.W.; Jung, J.C. Oxygen vacancy engineering for tuning the catalytic activity of LaCoO3 perovskite. J. Rare Earths 2024, 42, 506–514. [Google Scholar] [CrossRef]
- Drosou, C.; Nikolaraki, E.; Nikolaou, V.; Koilia, E.; Artemakis, G.; Stratakis, A.; Evdou, A.; Charisiou, N.D.; Goula, M.A.; Zaspalis, V.; et al. Activity and thermal aging stability of La1−xSrxMnO3 (x = 0.0, 0.3, 0.5, 0.7) and Ir/La1−xSrxMnO3 catalysts for CO oxidation with excess O2. Nanomaterials 2023, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Ding, J.; Ma, M.; Yuan, S.; Yang, Q.; Wang, Z.; Peng, Y.; Sun, C.; Zhou, H.; et al. Engineering active surface oxygen sites of cubic perovskite cobalt oxides toward catalytic oxidation reactions. ACS Catal. 2023, 13, 6338–6350. [Google Scholar] [CrossRef]
- Cui, X.; Liu, J.; Yan, X.; Yang, Y.; Xiong, B. Exploring reaction mechanism of CO oxidation over SrCoO3 catalyst: A DFT study. Appl. Surf. Sci. 2021, 570, 151234. [Google Scholar] [CrossRef]


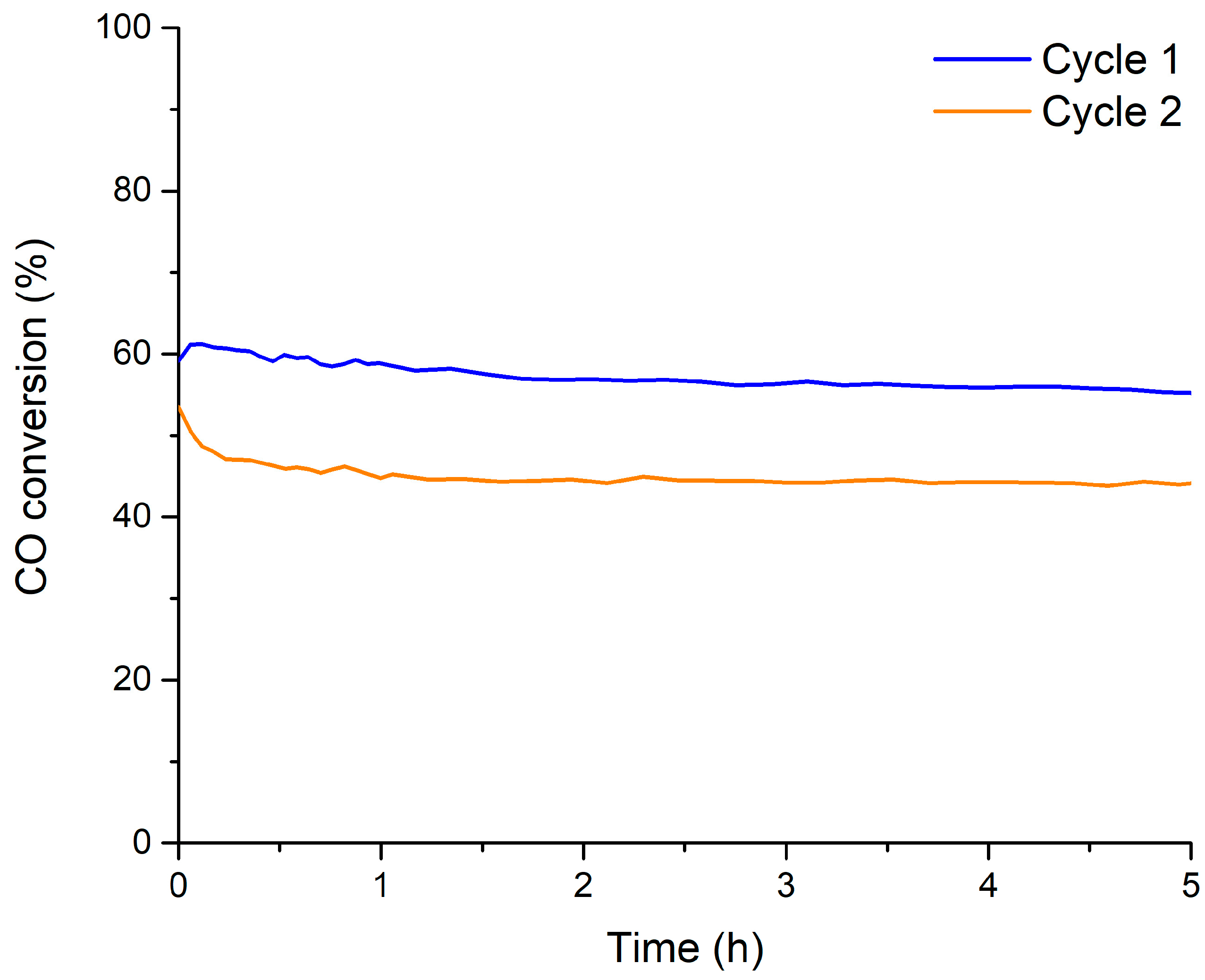
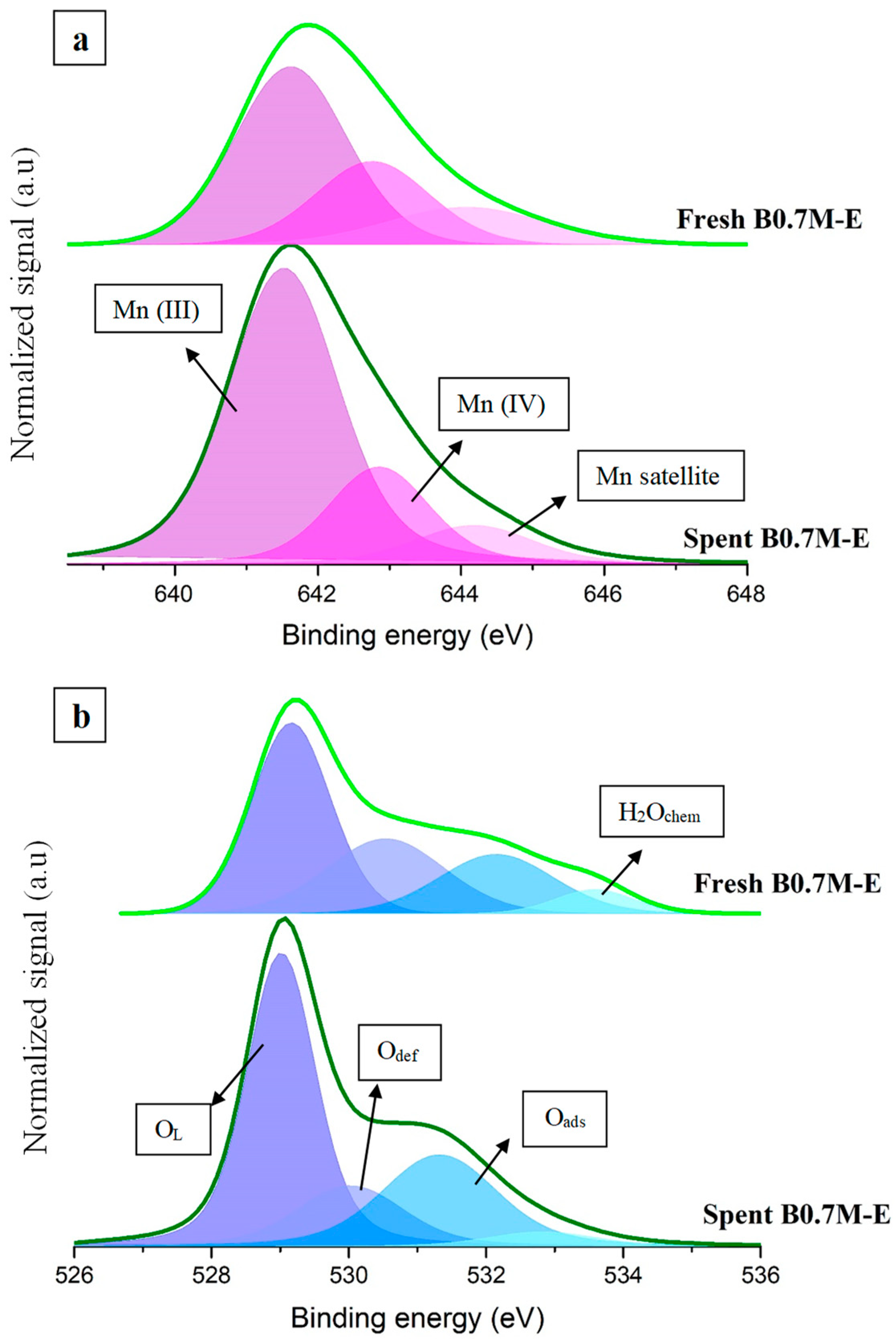
| Molecular Formula | Nomenclature | BET Surface Area (m2 g−1) | Chemical Composition (%) | Cell Parameters (Å) 1 | Average Crystallite Size (nm) | Lattice Strain | |||
|---|---|---|---|---|---|---|---|---|---|
| Ba | Mn | O | a | c | |||||
| BaMnO3 | BM | 9 | 62 | 24 | 14 | 5.7 | 4.8 | 24.8 | 3 × 10−4 |
| Ba0.9MnO3 | B0.9M | 8 | 58 | 27 | 15 | 5.7 | 4.8 | 27.2 | 3 × 10−5 |
| Ba0.8MnO3 | B0.8M | 10 | 56 | 29 | 15 | 5.7 | 4.8 | 26.7 | 4 × 10−5 |
| Ba0.7MnO3 | B0.7M | 11 | 55 | 30 | 15 | 5.7 | 4.8 | 26.2 | 2 × 10−4 |
| Catalyst | Mn (IV)/Mn (III) | Mn (III)s (%) | Mn (IV)s (%) | XPS OL/(Ba + Mn) (Nominal) |
|---|---|---|---|---|
| BM | 0.2 | 20 | 4 | 1.2 (1.5) |
| B0.9M | 0.4 | 19 | 8 | 1.0 (1.6) |
| B0.8M | 0.4 | 21 | 8 | 1.0 (1.7) |
| B0.7M | 0.5 | 20 | 10 | 1.1 (1.8) |
| Catalyst | Amount of O2 Consumed (μmol O2 (g cat)−1) | Percentage of O2 Consumed (%) |
|---|---|---|
| BM | 845 | 14 |
| B0.9M | 1040 | 16 |
| B0.8M | 1030 | 15 |
| B0.7M | 1330 | 18 |
| Catalyst | O2 Involved (μmol O2 (g cat)−1) 1 | Percentage of O2 Involved (%) 2 | CO Converted (%) 3 | |||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |
| BM | 1678 | 1855 | 27 | 30 | 8 | 9 |
| B0.9M | 1601 | 1501 | 24 | 23 | 8 | 7 |
| B0.8M | 1164 | 1029 | 17 | 15 | 5 | 5 |
| B0.7M | 1763 | 1652 | 23 | 22 | 8 | 7 |
| Catalyst | T50% (°C) | ΔT50%,CO (°C) 1 | ΔT50%,O2 (°C) 2 | ||
|---|---|---|---|---|---|
| 0.1%CO/1%O2/He | 1%CO/1%O2/He | 1%CO/10%O2/He | |||
| BM | 364 | 446 | 364 | 82 | −82 |
| B0.9M | 276 | 343 | 352 | 67 | 9 |
| B0.8M | 313 | 423 | 377 | 110 | −46 |
| B0.7M | 249 | 307 | 303 | 58 | −4 |
| 1% Pt/Al2O3 | 164 | 212 | 204 | 48 | −8 |
| Sample | Mn (IV)/Mn (III) | XPS OL/(Ba + Mn) | Mn 3p Position (eV) |
|---|---|---|---|
| Fresh B0.7M | 0.5 | 1.1 | 50 |
| Spent B0.7M | 0.3 | 0.2 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Verde, Á.; Illán-Gómez, M.J. Enhancing the Performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) Perovskites as Catalysts for CO Oxidation by Decreasing the Ba Content. Nanomaterials 2024, 14, 1334. https://doi.org/10.3390/nano14161334
Díaz-Verde Á, Illán-Gómez MJ. Enhancing the Performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) Perovskites as Catalysts for CO Oxidation by Decreasing the Ba Content. Nanomaterials. 2024; 14(16):1334. https://doi.org/10.3390/nano14161334
Chicago/Turabian StyleDíaz-Verde, Á., and M. J. Illán-Gómez. 2024. "Enhancing the Performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) Perovskites as Catalysts for CO Oxidation by Decreasing the Ba Content" Nanomaterials 14, no. 16: 1334. https://doi.org/10.3390/nano14161334
APA StyleDíaz-Verde, Á., & Illán-Gómez, M. J. (2024). Enhancing the Performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) Perovskites as Catalysts for CO Oxidation by Decreasing the Ba Content. Nanomaterials, 14(16), 1334. https://doi.org/10.3390/nano14161334






