Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
3. Results
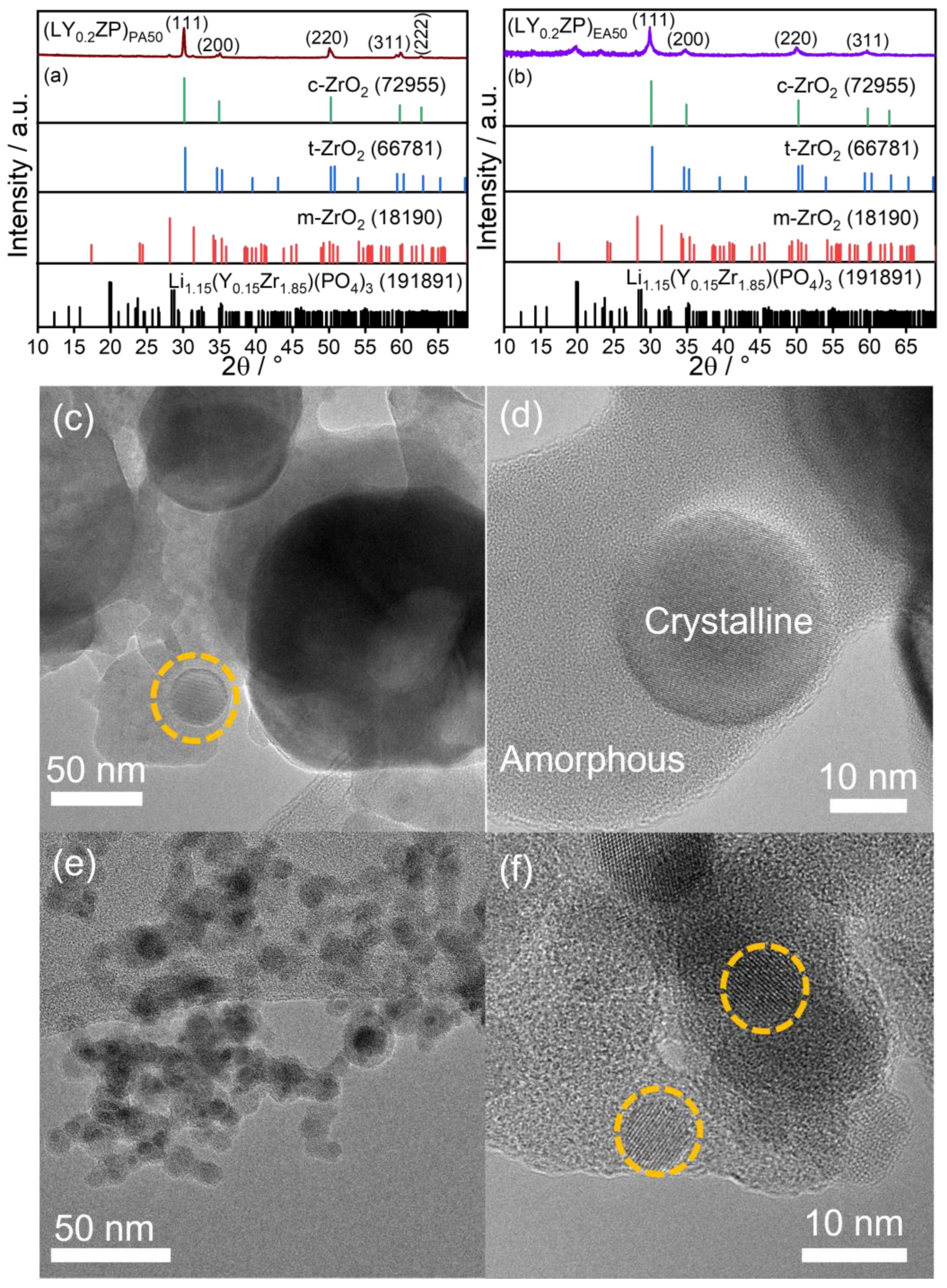
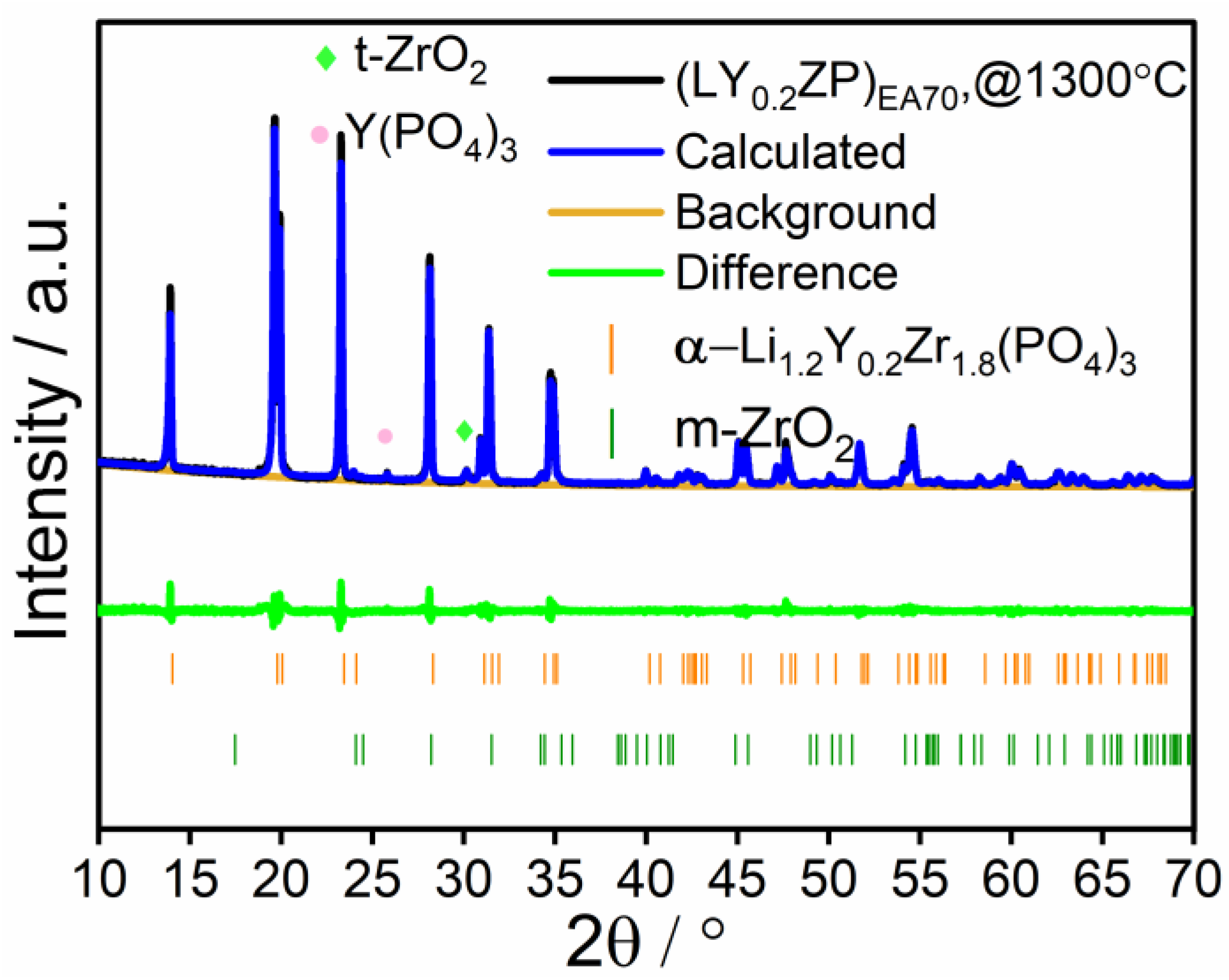
Characterization of (LY0.2ZP)PA50 and (LY0.2ZP)EA50 Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaies, B.; Guesmi, K.; Porcher, T.; Boroumand, R. Financial instability and oil price fluctuations: Evidence from oil exporting developing countries. Eur. J. Comp. Econ. 2020, 17, 55–71. [Google Scholar] [CrossRef]
- Brand, K. Umstrittene Ostsee-Pipeline: Grünes Licht für Nord Stream 2. 2021. Available online: https://www.tagesschau.de/ausland/amerika/nord-stream-2-einigung-103.html (accessed on 2 February 2022).
- Kougias, I. Hydropower—Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021.
- Taylor, N.; Jager-Waldau, A. Photovoltaics: Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021; p. 30504.
- Telsnig, T. Wind Energy Technology Development Report 2020; Publications Office of the European Union: Luxembourg, 2021.
- Yoo, J.; Park, B.; An, K.; Al-Ammar, E.; Khan, Y.; Hur, K.; Kim, J. Look-Ahead Energy Management of a Grid-Connected Residential PV System with Energy Storage under Time-Based Rate Programs. Energies 2012, 5, 1116–1134. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- Meyers, W.F.; Simmons, J.W. Electric Current-Producing Cell with Anhydrous Organic Liquid Electrolyte. U.S. Patent 3,423,242, 21 January 1969. [Google Scholar]
- Brodd, R.J.; Tagawa, K. Lithium-Ion Cell Production Processes. In Advances in Lithium-Ion Batteries; van Schalkwijk, W.A., Scrosati, B., Eds.; Springer: Boston, MA, USA, 2002; pp. 267–288. [Google Scholar]
- Ravdel, B.; Abraham, K.M.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119–121, 805–810. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety focused modeling of lithium-ion batteries: A review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Takada, K.; Nakano, S.; Inada, T.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Solid-State Lithium Batteries with Sulfide-Based Solid Electrolytes. Solid State Ion. 2004, 172, 25–30. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Huang, S.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. Regulating the effects of SnS shrinkage in all-solid-state lithium-ion batteries with excellent electrochemical performance. Chem. Eng. J. 2022, 429, 132424. [Google Scholar] [CrossRef]
- Wang, J.; Okabe, J.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M. The optimized interface engineering of VS2 as cathodes for high performance all-solid-state lithium-ion battery. Sci. China Technol. Sci. 2022, 65, 1859–1866. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Review—Solid Electrolytes for Safe and High Energy Density Lithium-Sulfur Batteries: Promises and Challenges. J. Electrochem. Soc. 2018, 165, A6008. [Google Scholar] [CrossRef]
- Overhoff, G.M.; Ali, M.Y.; Brinkmann, J.-P.; Lennartz, P.; Orthner, H.; Hammad, M.; Wiggers, H.; Winter, M.; Brunklaus, G. Ceramic-in-Polymer Hybrid Electrolytes with Enhanced Electrochemical Performance. ACS Appl. Mater. Interfaces 2022, 14, 53636–53647. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81, 925–929. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.M.; Chen, Z.B. Review-Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [Google Scholar] [CrossRef]
- Duan, H.; Fan, M.; Chen, W.-P.; Li, J.-Y.; Wang, P.-F.; Wang, W.-P.; Shi, J.-L.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Extended Electrochemical Window of Solid Electrolytes via Heterogeneous Multilayered Structure for High-Voltage Lithium Metal Batteries. Adv. Mater. 2019, 31, 1807789. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, S.; Lochala, J.; Desrochers, D.; Liu, B.; Zhang, W.; Yang, J.; Xiao, J. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 2018, 11, 1803–1810. [Google Scholar] [CrossRef]
- Luo, W.; Gong, Y.; Zhu, Y.; Fu, K.K.; Dai, J.; Lacey, S.D.; Wang, C.; Liu, B.; Han, X.; Mo, Y.; et al. Transition from Superlithiophobicity to Superlithiophilicity of Garnet Solid-State Electrolyte. J. Am. Chem. Soc. 2016, 138, 12258–12262. [Google Scholar] [CrossRef]
- Lu, J.Y.; Li, Y. Perovskite-type Li-ion solid electrolytes: A review. J. Mater. Sci.-Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- DeWees, R.; Wang, H. Synthesis and Properties of NaSICON-type LATP and LAGP Solid Electrolytes. Chemsuschem 2019, 12, 3713–3725. [Google Scholar] [CrossRef]
- Hood, Z.D.; Wang, H.; Pandian, A.S.; Keum, J.K.; Liang, C.D. Li2OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes. J. Am. Chem. Soc. 2016, 138, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, J.; Vogel, S.; Wang, C.-A. The reaction of Li6.5La3Zr1.5Ta0.5O12 with water. Solid. State. Ion. 2014, 269, 57–61. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Toda, A.; Zhang, T.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Synthesis of Garnet-Type Li7−xLa3Zr2O12−1/2x and Its Stability in Aqueous Solutions. Solid State Ion. 2011, 183, 48–53. [Google Scholar] [CrossRef]
- Schroeder, D.J.; Hubaud, A.A.; Vaughey, J.T. Stability of the solid electrolyte Li3OBr to common battery solvents. Mater. Res. Bull. 2014, 49, 614–617. [Google Scholar] [CrossRef]
- Dashjav, E.; Ma, Q.; Xu, Q.; Tsai, C.-L.; Giarola, M.; Mariotto, G.; Tietz, F. The influence of water on the electrical conductivity of aluminum-substituted lithium titanium phosphates. Solid State Ion. 2018, 321, 83–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef]
- Bronowski, J. CHAPTER 6—D.C. CONDUCTIVITY. In Structural Chemistry of Glasses; Rao, K.J., Ed.; Elsevier Science Ltd.: Oxford, UK, 2002; pp. 203–261. [Google Scholar]
- Li, Y.; Liu, M.; Liu, K.; Wang, C.-A. High Li+ conduction in NASICON-type Li1+xYxZr2−x(PO4)3 at room temperature. J. Power Sources 2013, 240, 50–53. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, X.; Xie, Y.; Lei, C.; Wei, T.; Zhang, Y. Advanced NASICON-type LiTi2(PO4)3 as electrode materials for lithium-ion batteries. Chem. Phys. Lett. 2022, 806, 140010. [Google Scholar] [CrossRef]
- Khatua, S.; Rao, Y.B.; Achary, K.R.; Patro, L.N. Li-ion transport studies of NASICON-type LiZr2(PO4)3 solid electrolyte crystallizing in rhombohedral structure at room temperature. Surf. Interfaces 2023, 41, 103212. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Shen, Y.; Lin, Y.; Nan, C.-W. Enhanced lithium-ion conductivity in a LiZr2(PO4)3 solid electrolyte by Al doping. Ceram. Int. 2017, 43, S598–S602. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, B.; Chien, P.-H.; Li, Y.; Huang, B.; Wu, N.; Xu, H.; Grundish, N.S.; Hu, Y.-Y.; Goodenough, J.B. NASICON Li1.2Mg0.1Zr1.9(PO4)3 Solid Electrolyte for an All-Solid-State Li-Metal Battery. Small Methods 2020, 4, 2000764. [Google Scholar] [CrossRef]
- Catti, M.; Comotti, A.; Di Blas, S. High-Temperature Lithium Mobility in α-LiZr2(PO4)3 NASICON by Neutron Diffraction. Chem. Mater. 2003, 15, 1628–1632. [Google Scholar] [CrossRef]
- Nomura, K.; Ikeda, S.; Ito, K.; Einaga, H. Ionic conduction behavior in zirconium phosphate framework. Solid State Ion. 1993, 61, 293–301. [Google Scholar] [CrossRef]
- Arbi, K.; Ayadi-Trabelsi, M.; Sanz, J. Li mobility in triclinic and rhombohedral phases of the Nasicon-type compound LiZr2(PO4)3 as deduced from NMR spectroscopy. J. Mater. Chem. 2002, 12, 2985–2990. [Google Scholar] [CrossRef]
- Li, Q.H.; Xu, C.; Huang, B.; Yin, X. Rhombohedral Li1+xYxZr2−x(PO4)3 Solid Electrolyte Prepared by Hot-Pressing for All-Solid-State Li-Metal Batteries. Materials 2020, 13, 035930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wu, L.-R.; Ma, J.; Cui, G. Nanotechnology in solid state batteries, what’s next? Next Nanotechnol. 2023, 2, 100011. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Figueiredo, F.; Marques, F.; Franco, J. Influence of microstructure on the electrical properties of NASICON materials. Solid State Ion. 2001, 140, 173–179. [Google Scholar] [CrossRef]
- Fuentes, R.; Figueiredo, F.; Marques, F.; Franco, J. Processing and Electrical Properties of NASICON Prepared from Yttria-Doped Zirconia Precursors. J. Eur. Ceram. Soc. 2001, 21, 737–743. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Bassoli, M.; Buscaglia, V. Solid-state synthesis of nanocrystalline BaTiO3: Reaction kinetics and powder properties. J. Am. Ceram. Soc. 2008, 91, 2862–2869. [Google Scholar] [CrossRef]
- Clabel, J.L.; Awan, I.T.; Pinto, A.H.; Nogueira, I.C.; Bezzon, V.D.N.; Leite, E.R.; Balogh, D.T.; Mastelaro, V.R.; Ferreira, S.O.; Marega, E. Insights on the mechanism of solid state reaction between TiO2 and BaCO3 to produce BaTiO3 powders: The role of calcination, milling, and mixing solvent. Ceram. Int. 2020, 46, 2987–3001. [Google Scholar] [CrossRef]
- Kotobuki, M.; Koishi, M. Preparation of Li1.5Al0.5Ti1.5(PO4)(3) solid electrolyte via a sol-gel route using various Al sources. Ceram. Int. 2013, 39, 4645–4649. [Google Scholar] [CrossRef]
- Ugemuge, N.; Parauha, Y.R.; Dhoble, S.J. Chapter 15—Synthesis and luminescence study of silicate-based phosphors for energy-saving light-emitting diodes. In Energy Materials; Dhoble, S.J., Kalyani, N.T., Vengadaesvaran, B., Kariem Arof, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 445–480. [Google Scholar]
- Takada, K.; Fujimoto, K.; Inada, T.; Kajiyama, A.L.; Kouguchi, M.; Kondo, S.; Watanabe, M. Sol-gel preparation of Li+ ion conductive thin film. Appl. Surf. Sci. 2002, 189, 300–306. [Google Scholar] [CrossRef]
- Xu, X.X.; Wen, Z.Y.; Wu, J.G.; Yang, X.L. Preparation and electrical properties of NASICON-type structured Li1.4Al0.4Ti1.6(PO4)(3) glass-ceramics by the citric acid-assisted sol-gel method. Solid State Ion. 2007, 178, 29–34. [Google Scholar] [CrossRef]
- Ulrich, G.D. Flame Synthesis of Fine Particles. Chem. Eng. News 1984, 62, 22–29. [Google Scholar] [CrossRef]
- Guo, J.Z.; Goodings, J.M.; Hayhurst, A.N.; Taylor, S.G. A simple method for measuring positive ion concentrations in flames and the calibration of a nebulizer/atomizer. Combust. Flame 2003, 133, 335–343. [Google Scholar] [CrossRef]
- Madler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Dasgupta, M.; Fortugno, P.; Wiggers, H. Plasma-assisted gas-phase synthesis and in-line coating of silicon nanoparticles. Plasma Process Polym. 2020, 17, 1900245. [Google Scholar] [CrossRef]
- Chrystie, R.S.M.; Ebertz, F.L.; Dreier, T.; Schulz, C. Absolute SiO concentration imaging in low-pressure nanoparticle-synthesis flames via laser-induced fluorescence. Appl. Phys. B-Lasers Opt. 2019, 125, 29. [Google Scholar] [CrossRef]
- Abdali, A.; Moritz, B.; Gupta, A.; Wiggers, H.; Schulz, C. Hybrid microwave-plasma hot-wall reactor for synthesis of silica nanoparticles under well-controlled conditions. J. Optoelectron. Adv. Mater. 2010, 12, 440–444. [Google Scholar]
- Schneider, F.; Suleiman, S.; Menser, J.; Borukhovich, E.; Wlokas, I.; Kempf, A.; Wiggers, H.; Schulz, C. SpraySyn-A standardized burner configuration for nanoparticle synthesis in spray flames. Rev. Sci. Instrum. 2019, 90, 085108. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.; Tapia, J.D.; Gallego, J.; Hagemann, U.; Wiggers, H. Spray-Flame Synthesis of LaMnO3+δ Nanoparticles for Selective CO Oxidation (SELOX). Energy Fuels 2021, 35, 4367–4376. [Google Scholar] [CrossRef]
- Alkan, B.; Cychy, S.; Varhade, S.; Muhler, M.; Schulz, C.; Schuhmann, W.; Wiggers, H.; Andronescu, C. Spray-Flame-Synthesized LaCo1−xFexO3 Perovskite Nanoparticles as Electrocatalysts for Water and Ethanol Oxidation. ChemElectroChem 2019, 6, 4266–4274. [Google Scholar] [CrossRef]
- Ali, M.Y.; Orthner, H.; Wiggers, H. Spray Flame Synthesis (SFS) of Lithium Lanthanum Zirconate (LLZO) Solid Electrolyte. Materials 2021, 14, 3472. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, H.; Kōmoto, K.; Miyayama, M.; Yamada, H. The Chemistry of Ceramics; Wiley: Chichester, UK, 1996. [Google Scholar]
- Hund, F. Anomale Mischkristalle im System ZrO2Y2O3 Kristallbau der Nernst-Stifte. Z. Elektrochem. Und Angew. Phys. Chem. 1951, 55, 363–366. [Google Scholar] [CrossRef]
- Yamada, T.; Kubota, Y.; Makinose, Y.; Suzuki, N.; Nakata, K.; Terashima, C.; Matsushita, N.; Okada, K.; Fujishima, A.; Katsumata, K.-i. Single Crystal ZrO2 Nanosheets Formed by Thermal Transformation for Solid Oxide Fuel Cells and Oxygen Sensors. ACS Appl. Nano Mater. 2019, 2, 6866–6873. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Greaves, C.; Janek, J. Sol–gel synthesis and room-temperature properties of α-LiZr2(PO4)3. RSC Adv. 2015, 5, 17054–17059. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, Z.; Jiang, L.; Hao, X.; Jia, M.; Wang, L.; Liu, F. Rapid sintering of ceramic solid electrolytes LiZr2(PO4)3 and Li1.2Ca0.1Zr1.9(PO4)3 using a microwave sintering process at low temperatures. Ceram. Int. 2019, 45, 11068–11072. [Google Scholar] [CrossRef]
- Lieber, C.M.; Wang, Z.L. Functional Nanowires. MRS Bull. 2007, 32, 99–108. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- PubChem Compound Summary for CID 26251, Zirconium Nitrate; National Library Medicine: Bethesda, MD, USA, 2021.
- Angel, S.; Neises, J.; Dreyer, M.; Friedel Ortega, K.; Behrens, M.; Wang, Y.; Arandiyan, H.; Schulz, C.; Wiggers, H. Spray-flame synthesis of La(Fe, Co)O3 nano-perovskites from metal nitrates. AIChE J. 2020, 66, e16748. [Google Scholar] [CrossRef]
- Wang, D.; Kou, R.; Ren, Y.; Sun, C.-J.; Zhao, H.; Zhang, M.-J.; Li, Y.; Huq, A.; Ko, J.Y.P.; Pan, F.; et al. Synthetic Control of Kinetic Reaction Pathway and Cationic Ordering in High-Ni Layered Oxide Cathodes. Adv. Mater. 2017, 29, 1606715. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Wilson, H.; Zhao, F.; Manthiram, A. Y-Doped NASICON-type LiZr2(PO4)3 Solid Electrolytes for Lithium-Metal Batteries. Chem. Mater. 2017, 29, 7206–7212. [Google Scholar] [CrossRef]
- Chraska, T.; King, A.H.; Berndt, C.C.; Karthikeyan, J. Phase Transformation as a Function of Particle Size in Nanocrystalline Zirconia. MRS Online Proc. Libr. 1997, 481, 613–617. [Google Scholar] [CrossRef]
- Ramirez, L.; Mecartney, M.L.; Krumdieck, S.P. Nanocrystalline ZrO2 thin films on silicon fabricated by pulsed-pressure metalorganic chemical vapor deposition (PP-MOCVD). J. Mater. Res. 2008, 23, 2202–2211. [Google Scholar] [CrossRef]
- Srinivasan, R.; Rice, L.; Davis, B. Critical Particle Size and Phase Transformation in Zirconia: Transmission Electron Microscopy and X-Ray Diffraction Studies. J. Am. Ceram. Soc. 2005, 73, 3528–3530. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef] [PubMed]
- Egger, P.; Dirè, S.; Ischia, M.; Campostrini, R. Pyrolysis study of sol-gel derived zirconia by TG-GC-MS. J. Therm. Anal. Calorim. 2005, 81, 407–415. [Google Scholar] [CrossRef]
- Shi, L.; Qu, T.; Liu, D.; Deng, Y.; Yang, B.; Dai, Y. Process of Thermal Decomposition of Lithium Carbonate. In Proceedings of the Materials Processing Fundamentals 2020, Cham, Switzerland, 9 January 2020; pp. 107–116. [Google Scholar]
- Geiculescu, A.C.; Spencer, H.G. Thermal Decomposition and Crystallization of Aqueous Sol-Gel Derived Zirconium Acetate Gels: Effects of the Additive Anions. J. Sol-Gel Sci. Technol. 2000, 17, 25–35. [Google Scholar] [CrossRef]
- Efaw, C.M.; Vandegrift, J.L.; Reynolds, M.; McMurdie, S.; Jaques, B.J.; Hu, H.; Xiong, H.; Hurley, M.F. Characterization of zirconium oxides part I: Raman mapping and spectral feature analysis. Nucl. Mater. Energy 2019, 21, 100707. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J.; Yang, I.-S. Raman Spectroscopy of Tetragonal Zirconia Solid Solutions. J. Am. Ceram. Soc. 1993, 76, 2106–2108. [Google Scholar] [CrossRef]
- Colbea, C.; Avram, D.; Cojocaru, B.; Negrea, R.; Ghica, C.; Kessler, V.G.; Seisenbaeva, G.A.; Parvulescu, V.; Tiseanu, C. Full Tetragonal Phase Stabilization in ZrO2 Nanoparticles Using Wet Impregnation: Interplay of Host Structure, Dopant Concentration and Sensitivity of Characterization Technique. Nanomaterials 2018, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A. Infrared and Raman characteristic group frequencies. Tables and charts George Socrates John Wiley and Sons, Ltd., Chichester, Third Edition, 2001. Price £135. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Effect of solvent composition on oxide morphology during flame spray pyrolysis of metal nitrates. Phys. Chem. Chem. Phys. 2011, 13, 92469252. [Google Scholar] [CrossRef] [PubMed]
- Stodt, M.F.B.; Groeneveld Jan, D.; Mädler, L.; Kiefer, J.; Fritsching, U. Microexplosions of multicomponent drops in spray flames. Combust. Flame 2022, 240, 112043. [Google Scholar] [CrossRef]
- Wang, J.; He, T.; Yang, X.; Cai, Z.; Wang, Y.; Lacivita, V.; Kim, H.; Ouyang, B.; Ceder, G. Design principles for NASICON super-ionic conductors. Nat. Commun. 2023, 14, 5210. [Google Scholar] [CrossRef] [PubMed]





| Nomenclature | Precursors | Solvents | ||||
|---|---|---|---|---|---|---|
| Li | Y | Zr | P | (A) | (B) | |
| LiNO3 (50% excess Li) | Y(NO3)3 ·6H2O | ZP | TBP | Propanol/Propionic Acid (PrOH/PA) V/V | Ethanol/2-Ethylhexanoic Acid (EtOH/2-EHA) V/V | |
| (LZP)EA50 | ✓ | 0 | ✓ | ✓ | × | 1:1 |
| (LY0.1ZP)EA50 | ✓ | 0.1 | ✓ | ✓ | × | 1:1 |
| (LY0.2ZP)PA50 | ✓ | 0.2 | ✓ | ✓ | 1:1 | × |
| (LY0.2ZP)EA50 | ✓ | 0.2 | ✓ | ✓ | × | 1:1 |
| (LY0.2ZP)EA70 | ✓ | 0.2 | ✓ | ✓ | × | 3:7 |
| Operating Parameters | ||||||
|---|---|---|---|---|---|---|
| Dispersion CH4 [slm] | Dispersion O2 [slm] | Pilot Flame CH4 [slm] | Pilot Flame O2 [slm] | Quench Gas Air [slm] | Coaxial Sheath Air [slm] | Reactor Pressure [mbar] |
| 1 | 9 | 2 | 16 | 240 | 140 | 800–820 |
| Status | Content of Phase [%] | ||||
|---|---|---|---|---|---|
| t-ZrO2 | m-ZrO2 | α-LYZP | β-LYZP | ||
| (LY0.2ZP)PA50 | As-synthesized | 34.0 | 39.9 | / | / |
| @1300 °C | 0.6 | 27.6 | 31.5 | 40.3 | |
| (LY0.2ZP)EA50 | As-synthesized | 21.8 | 63.5 | 14.7 | / |
| @ 1300 °C | 0.5 | 14.2 | 49.6 | 35.6 | |
| Nomenclature | Solvent Mixture | Composition [wt%] | ||
|---|---|---|---|---|
| Propanol/Propionic Acid (1:1 by Volume) | α-LYZP | β-LYZP | m-ZrO2 | |
| (LY0.2ZP)PA50 | Ethanol/2-EHA (1:1 by volume) | 31.5 | 40.3 | 27.6 |
| (LY0.2ZP)EA50 | Ethanol/2-EHA (3:7 by volume) | 49.6 | 35.6 | 14.2 |
| (LY0.2ZP)EA70 | Propanol/propionic acid (1:1 by volume) | 94.7 | / | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Chen, T.; Orthner, H.; Wiggers, H. Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes. Nanomaterials 2024, 14, 1278. https://doi.org/10.3390/nano14151278
Ali MY, Chen T, Orthner H, Wiggers H. Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes. Nanomaterials. 2024; 14(15):1278. https://doi.org/10.3390/nano14151278
Chicago/Turabian StyleAli, Md Yusuf, Tianyu Chen, Hans Orthner, and Hartmut Wiggers. 2024. "Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes" Nanomaterials 14, no. 15: 1278. https://doi.org/10.3390/nano14151278
APA StyleAli, M. Y., Chen, T., Orthner, H., & Wiggers, H. (2024). Spray-Flame Synthesis of NASICON-Type Rhombohedral (α) Li1+xYxZr2−x(PO4)3 [x = 0–0.2] Solid Electrolytes. Nanomaterials, 14(15), 1278. https://doi.org/10.3390/nano14151278






