Mo-Doped Ni/C Catalyst for Improved Simultaneous Production of Hydrogen and Carbon Nanotubes through Ethanol Decomposition
Abstract
1. Introduction
2. Experimental Section
2.1. Catalysts Preparation
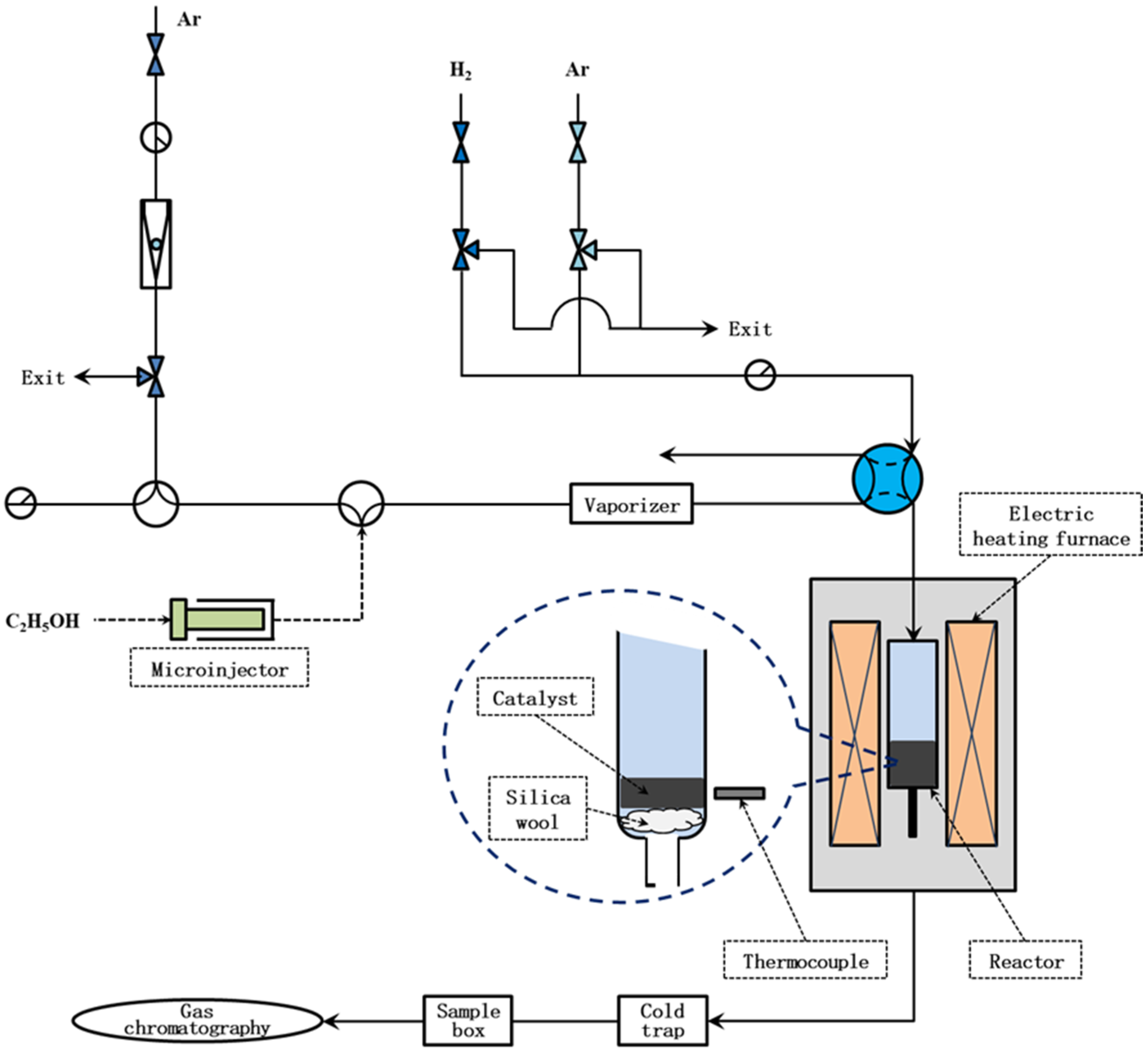
2.2. Decomposition of Ethanol
2.3. Characterization
2.4. Evaluation of Catalysts
3. Results and Discussion
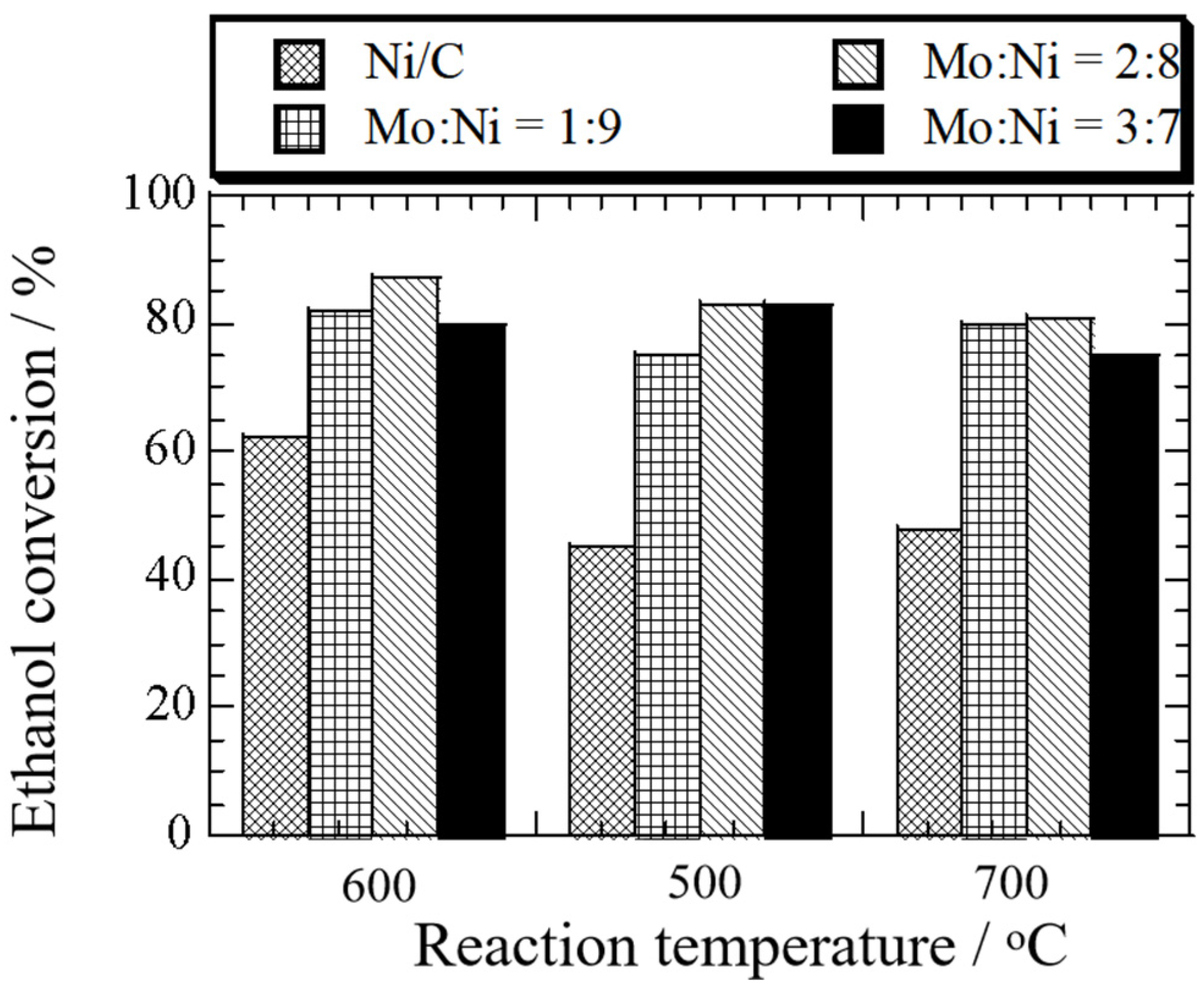
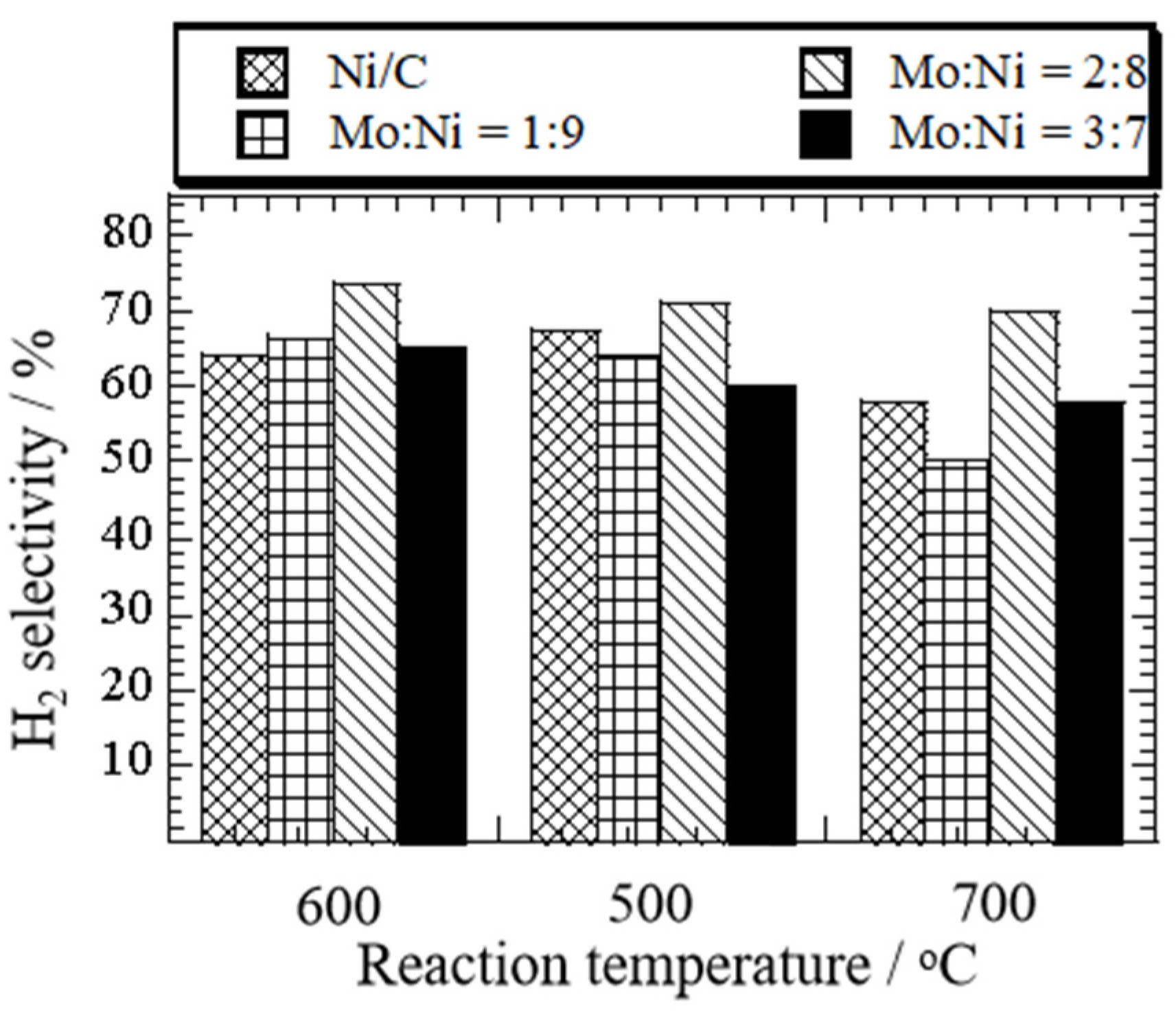
3.1. Effect of Temperature and Amount of Mo Addition on Ethanol Decomposition
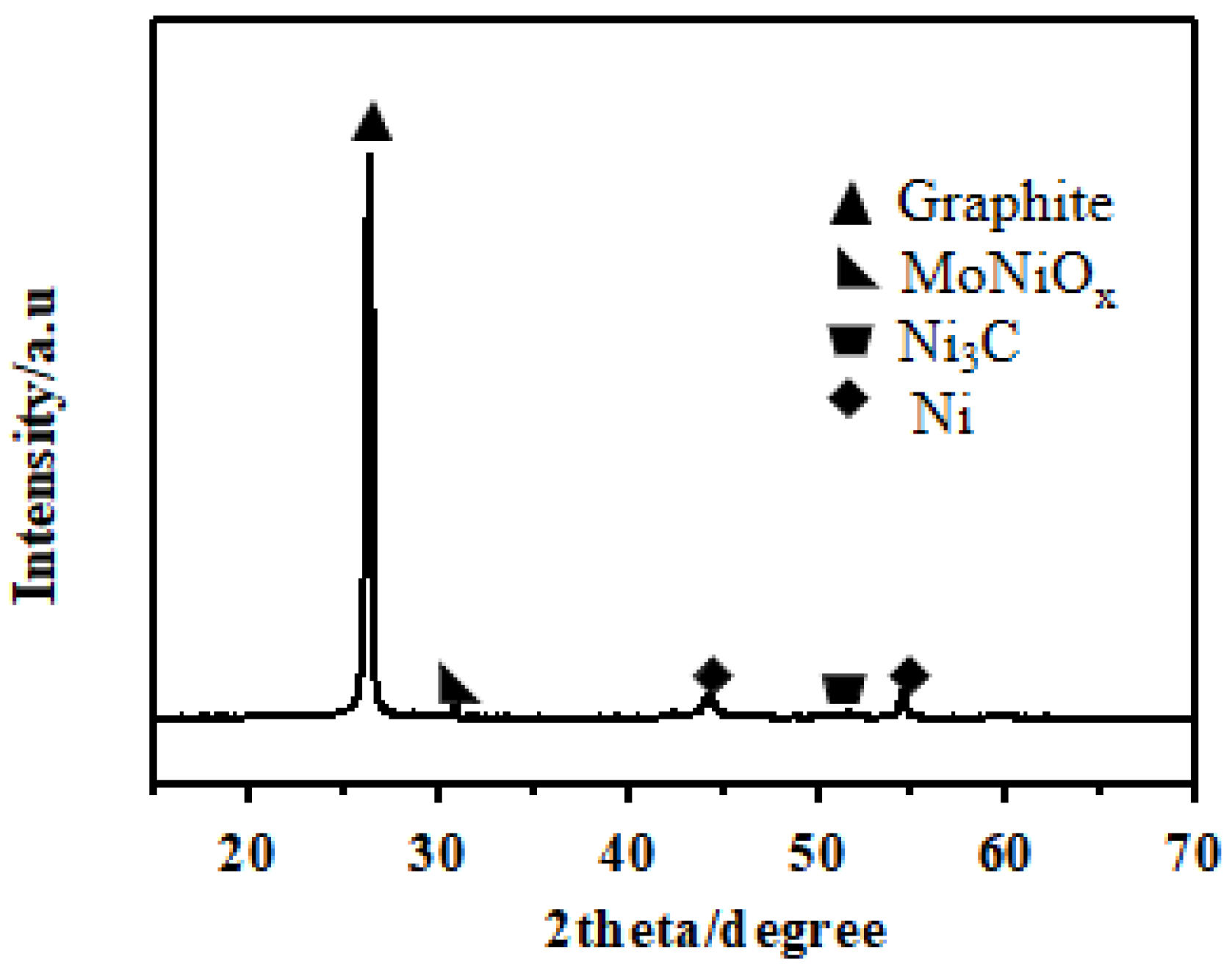
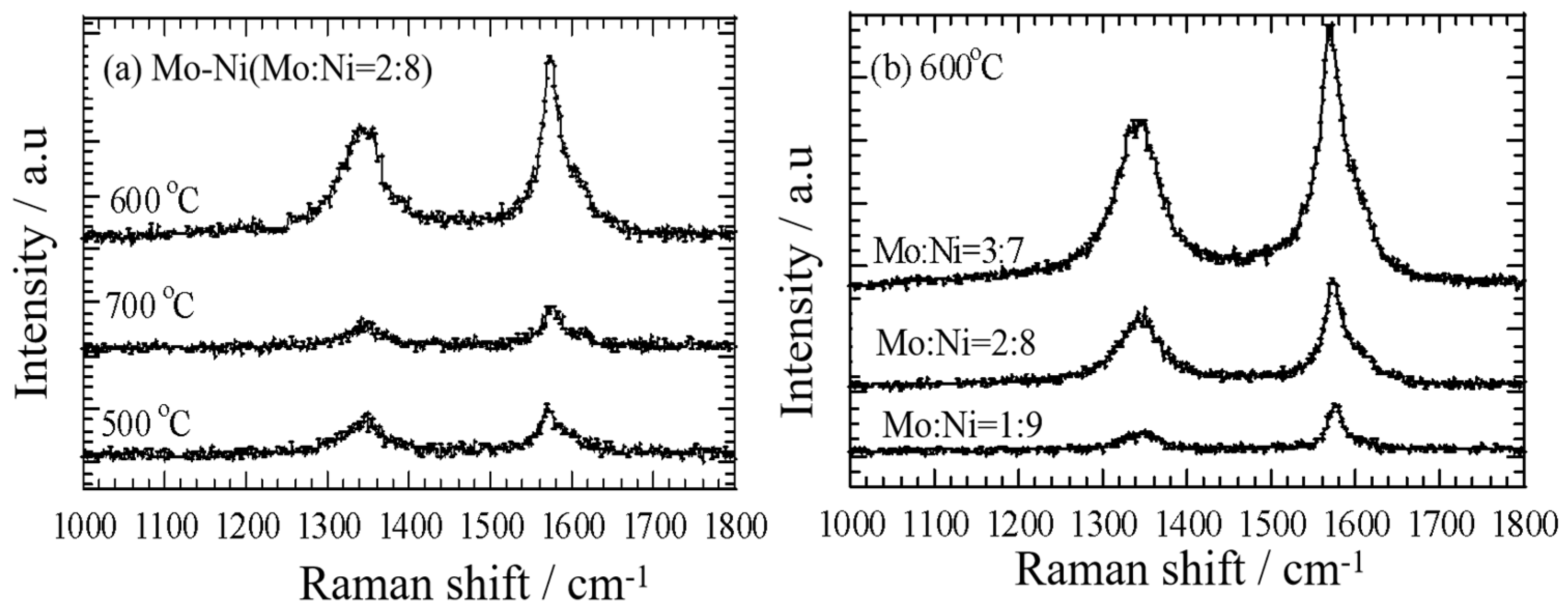
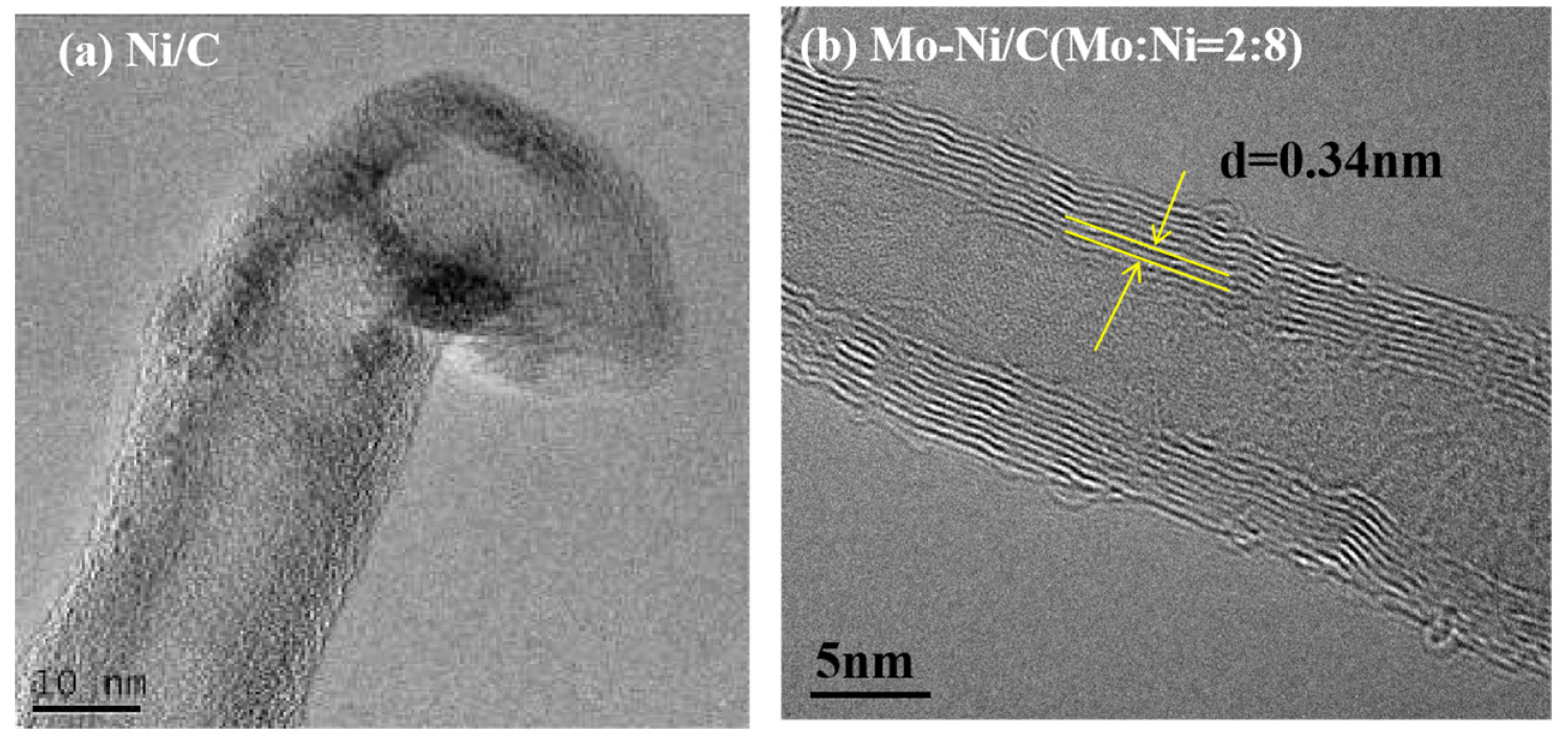
3.2. MWCNT Growth on the Mo-Ni/C Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Li, M.; Zhao, F.; Ji, Y.; Han, F. Status and prospects in technical standards of hydrogen-powered ships for advancing maritime zero-carbon transformation. Int. J. Hydrogen Energy 2024, 62, 925–946. [Google Scholar] [CrossRef]
- Luan, X.; Zheng, Z.; Zhao, S.; Xue, Y.; Li, Y. Controlled growth of the interface of CdWOx/GDY for hydrogen energy conversion. Adv. Funct. Mater. 2022, 32, 2202843. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Gopinath, M.; Marimuthu, R. A review on solar energy-based indirect water-splitting methods for hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 37742–37759. [Google Scholar] [CrossRef]
- Skabelund, B.B.; Milcarek, R.J. Review of thermal partial oxidation reforming with integrated solid oxide fuel cell power generation. Renew. Sustain. Energy Rev. 2022, 168, 112852. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Ng, K.H.; Ahmed, S.F.; Nguyen, H.T.; Kumar, P.S.; Tran, H.T.; Rajamohan, N.; Yusuf, M.; Show, P.L.; Balakrishnan, A.; et al. Hydrogen generation by heterogeneous catalytic steam reforming of short-chain alcohols: A review. Environ. Chem. Lett. 2024, 22, 561–583. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.Q. Thermodynamic analysis of steam reforming of ethanol for hydrogen generation. Int. J. Energy Res. 2008, 32, 1432–1443. [Google Scholar] [CrossRef]
- Yuan, L.; Ye, T.; Gong, F.; Guo, Q.; Torimoto, Y.; Yamamoto, M.; Li, Q. Hydrogen production from the current-enhanced reforming and decomposition of ethanol. Energy Fuels 2009, 23, 3103–3112. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ren, Z.; Wang, G.; Bai, J. Co-production of hydrogen and multi-wall carbon nanotubes from ethanol decomposition over Fe/Al2O3 catalysts. Appl. Catal. B Environ. 2008, 84, 433–439. [Google Scholar] [CrossRef]
- Rincón, R.; Marinas, A.; Muñoz, J.; Calzada, M.D. Hydrogen production from ethanol decomposition by microwave plasma TIAGO torch. Int. J. Hydrogen Energy 2014, 39, 11441–11453. [Google Scholar] [CrossRef]
- He, L.; Hu, S.; Jiang, L.; Syed-Hassan, S.S.A.; Wang, Y.; Xu, K.; Su, S.; Xiang, J.; Xiao, L.; Chi, H.; et al. Opposite effects of self-growth amorphous carbon and carbon nanotubes on the reforming of toluene with Ni/α-Al2O3 for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 14439–14448. [Google Scholar] [CrossRef]
- Xin, Y.; Sun, B.; Zhu, X.; Yan, Z.; Zhao, X.; Sun, X. Hydrogen production from ethanol decomposition by pulsed discharge with needle-net configurations. Appl. Energy 2017, 206, 126–133. [Google Scholar] [CrossRef]
- Kumar, A.; Mukasyan, A.S.; Wolf, E.E. Combustion synthesis of Ni, Fe and Cu multi-component catalysts for hydrogen production from ethanol reforming. Appl. Catal. A Gen. 2011, 401, 20–28. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Manikandan, N.; Suresh Kumar, V.P.; Rathis, G.; Shabariganesh, T.K. Carbon nanotubes and their properties-The review. Mater. Today Proc. 2021, 47, 4682–4685. [Google Scholar]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-driven carbon nanotube functional materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hu, S.; Yin, X.; Xu, J.; Han, H.; Li, H.; Ren, Q.; Su, S.; Wang, Y.; Xiang, J. Promoting effects of Fe-Ni alloy on co-production of H2 and carbon nanotubes during steam reforming of biomass tar over Ni-Fe/α-Al2O3. Fuel 2020, 276, 118116. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.; Liang, C.; Luo, D.; Li, B.; Ma, J. In-situ exsolution of Fe-Ni alloy catalysts for H2 and carbon nanotube production from microwave plasma-initiated decomposition of plastic wastes. J. Hazard. Mater. 2023, 445, 130609. [Google Scholar] [CrossRef]
- Lobiak, E.V.; Shlyakhova, E.V.; Bulusheva, L.G.; Plyusnin, P.E.; Shubin, Y.V.; Okotrub, A.V. Ni-Mo and Co-Mo alloy nanoparticles for catalytic chemical vapor deposition synthesis of carbon nanotubes. J. Alloys Compd. 2015, 621, 351–356. [Google Scholar] [CrossRef]
- He, L.; Hu, S.; Jiang, L.; Liao, G.; Zhang, L.; Han, H.; Chen, X.; Wang, Y.; Xu, K.; Su, S.; et al. Co-production of hydrogen and carbon nanotubes from the decomposition/reforming of biomass-derived organics over Ni/α-Al2O3 catalyst: Performance of different compounds. Fuel 2017, 210, 307–314. [Google Scholar] [CrossRef]
- Yudasaka, M.; Kikuchi, R.; Matsui, T.; Ohki, Y.; Yoshimura, S.; Ota, E. Specific conditions for Ni catalyzed carbon nanotube growth by chemical vapor deposition. Appl. Phys. Lett. 1995, 67, 2477–2479. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Sol-gel synthesis of titanium oxide supported nickel catalysts for hydrogen and carbon production by methane decomposition. J. Power Sources 2015, 280, 467–475. [Google Scholar] [CrossRef]
- Kludpantanapan, T.; Nantapong, P.; Rattanaamonkulchai, R.; Srifa, A.; Koo-Amornpattana, W.; Chaiwat, W.; Sakdaronnarong, C.; Assabumrungrat, S.; Wongsakulphasatch, S.; Sudoh, M.; et al. Simultaneous production of hydrogen and carbon nanotubes from biogas: On the effect of Ce addition to CoMo/MgO catalyst. Int. J. Hydrogen Energy 2021, 46, 38175–38190. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Impact of Co/Mo ratio on the activity of CoMo/MgO catalyst for production of high-quality multi-walled carbon nanotubes from polyethylene waste. Mater. Chem. Phys. 2019, 238, 121879. [Google Scholar] [CrossRef]
- Ramasubramanian, V.; Ramsurn, H.; Price, G.L. Hydrogen production by catalytic decomposition of methane over Fe-based bi-metallic catalysts supported on CeO2-ZrO2. Int, J. Hydrogen Energy 2020, 45, 12026e36. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Tang, Z.; Li, W.; Bai, J. Simultaneous production of hydrogen and multi-walled carbon nanotubes by ethanol decomposition over Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2009, 88, 142–151. [Google Scholar] [CrossRef]
- Ahmad, W.; Mehmood, U.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Aslam, M.Z.; Kamal, M.S.; Shawabkeh, R.A. Synthesis of zinc oxide/titanium dioxide (ZnO/TiO2) nanocomposites by wet incipient wetness impregnation method and preparation of ZnO/TiO2 paste using poly (vinylpyrrolidone) for efficient dye-sensitized solar cells. Electrochim. Acta 2016, 222, 473–480. [Google Scholar] [CrossRef]
- de Lima, S.M.; Silva, A.M.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Ethanol decomposition and steam reforming of ethanol over CeZrO2 and Pt/CeZrO2 catalyst: Reaction mechanism and deactivation. Appl. Catal. A Gen. 2009, 352, 95–113. [Google Scholar] [CrossRef]
- Saconsint, S.; Srifa, A.; Koo-Amornpattana, W.; Assabumrungrat, S.; Sano, N.; Fukuhara, C.; Ratchahat, S. Development of Ni-Mo carbide catalyst for production of syngas and CNTs by dry reforming of biogas. Sci. Rep. 2023, 13, 12928. [Google Scholar] [CrossRef]
- Lobiak, E.V.; Kuznetsova, V.R.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Structure, functional composition and electrochemical properties of nitrogen-doped multi-walled carbon nanotubes synthesized using Co-Mo, Ni-Mo and Fe-Mo catalysts. Mater. Chem. Phys. 2020, 255, 123563. [Google Scholar] [CrossRef]
- Rattanaamonkulchai, R.; Kludpantanapan, T.; Srifa, A.; Koo-Amornpattana, W.; Chaiwat, W.; Sakdaronnarong, C.; Ratchahat, S. Simultaneous production of hydrogen and carbon nanotubes from biogas over mono-and bimetallic catalyst. J. Environ. Chem. Eng. 2022, 10, 107910. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G.; Hu, H.; Ma, X. Hydrogen production by catalytic methane decomposition: Carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Kazakova, M.A.; Kuznetsov, V.L.; Bokova-Sirosh, S.N.; Krasnikov, D.V.; Golubtsov, G.V.; Romanenko, A.I.; Prosvirin, I.P.; Ishchenko, A.V.; Orekhov, A.S.; Chuvilin, A.L.; et al. Fe-Mo and Co-Mo Catalysts with Varying Composition for Multi-Walled Carbon Nanotube Growth. Phys. Status Solidi (b) 2018, 255, 1700260. [Google Scholar] [CrossRef]
- Ji, Z.H.; Zhang, L.; Tang, D.M.; Zhao, Y.M.; Zou, M.K.; Xie, R.H.; Chang, L.; Cheng, H.M. Statistical patterns in high-throughput growth of single-wall carbon nanotubes from Co/Pt/Mo ternary catalysts. Carbon 2023, 210, 118073. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Aboul-Gheit, A.K. Effect of progressive Co loading on commercial Co-Mo/Al2O3 catalyst for natural gas decomposition to COx-free hydrogen production and carbon nanotubes. Energy Convers. Manag. 2014, 77, 143–151. [Google Scholar] [CrossRef]
- Haripriya, M.; Manimekala, T.; Dharmalingam, G.; Minakshi Sundaram, M.; Sivasubramanian, R. Asymmetric Supercapacitors Based on ZnCo2O4 Nanohexagons and Orange Peel Derived Porous Carbon Electrodes. Chem. Asian J. 2024, 19, e202400202. [Google Scholar] [CrossRef] [PubMed]
- Sugime, H.; Noda, S.; Maruyama, S.; Yamaguchi, Y. Multiple “optimum” conditions for Co-Mo catalyzed growth of vertically aligned single-walled carbon nanotube forests. Carbon 2009, 47, 234–241. [Google Scholar] [CrossRef]
- Kukovitsky, E.F.; L’vov, S.G.; Sainov, N.A.; Shustov, V.A.; Chernozatonskii, L.A. Correlation between metal catalyst particle size and carbon nanotube growth. Int. J. Hydrogen Energy 2002, 355, 497–503. [Google Scholar] [CrossRef]
- Algadri, N.A.; Hassan, Z.; Ibrahim, K. Effect of ferrocene catalyst particle size on structural and morphological characteristics of carbon nanotubes grown by microwave oven. J. Mater. Sci. 2017, 52, 12772–12782. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, J.; Liu, X.; Wang, X.; Zhang, Y.; Yue, J.; Wang, H. Mo-Doped Ni/C Catalyst for Improved Simultaneous Production of Hydrogen and Carbon Nanotubes through Ethanol Decomposition. Nanomaterials 2024, 14, 1205. https://doi.org/10.3390/nano14141205
Diao J, Liu X, Wang X, Zhang Y, Yue J, Wang H. Mo-Doped Ni/C Catalyst for Improved Simultaneous Production of Hydrogen and Carbon Nanotubes through Ethanol Decomposition. Nanomaterials. 2024; 14(14):1205. https://doi.org/10.3390/nano14141205
Chicago/Turabian StyleDiao, Jinxiang, Xiaojie Liu, Xianmeng Wang, Yuzhu Zhang, Jingkai Yue, and Hui Wang. 2024. "Mo-Doped Ni/C Catalyst for Improved Simultaneous Production of Hydrogen and Carbon Nanotubes through Ethanol Decomposition" Nanomaterials 14, no. 14: 1205. https://doi.org/10.3390/nano14141205
APA StyleDiao, J., Liu, X., Wang, X., Zhang, Y., Yue, J., & Wang, H. (2024). Mo-Doped Ni/C Catalyst for Improved Simultaneous Production of Hydrogen and Carbon Nanotubes through Ethanol Decomposition. Nanomaterials, 14(14), 1205. https://doi.org/10.3390/nano14141205



