Facile Preparation of Magnetically Separable Fe3O4/ZnO Nanocomposite with Enhanced Photocatalytic Activity for Degradation of Rhodamine B
Abstract
1. Introduction
2. Materials and Methods
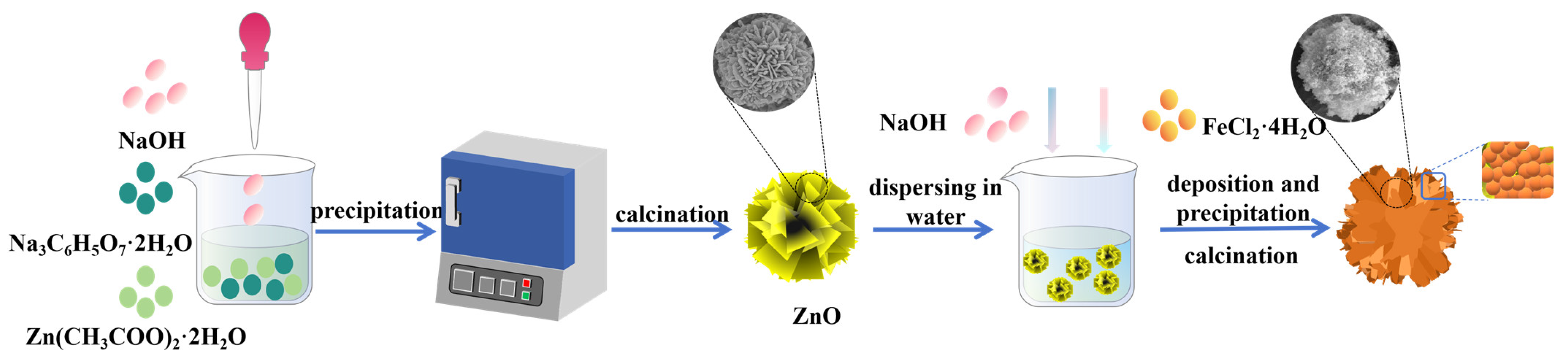
2.1. Synthesis of ZnO
2.2. Synthesis of Fe3O4/ZnO
2.3. Characterization
2.4. Photocatalytic Activity Measurement
3. Results
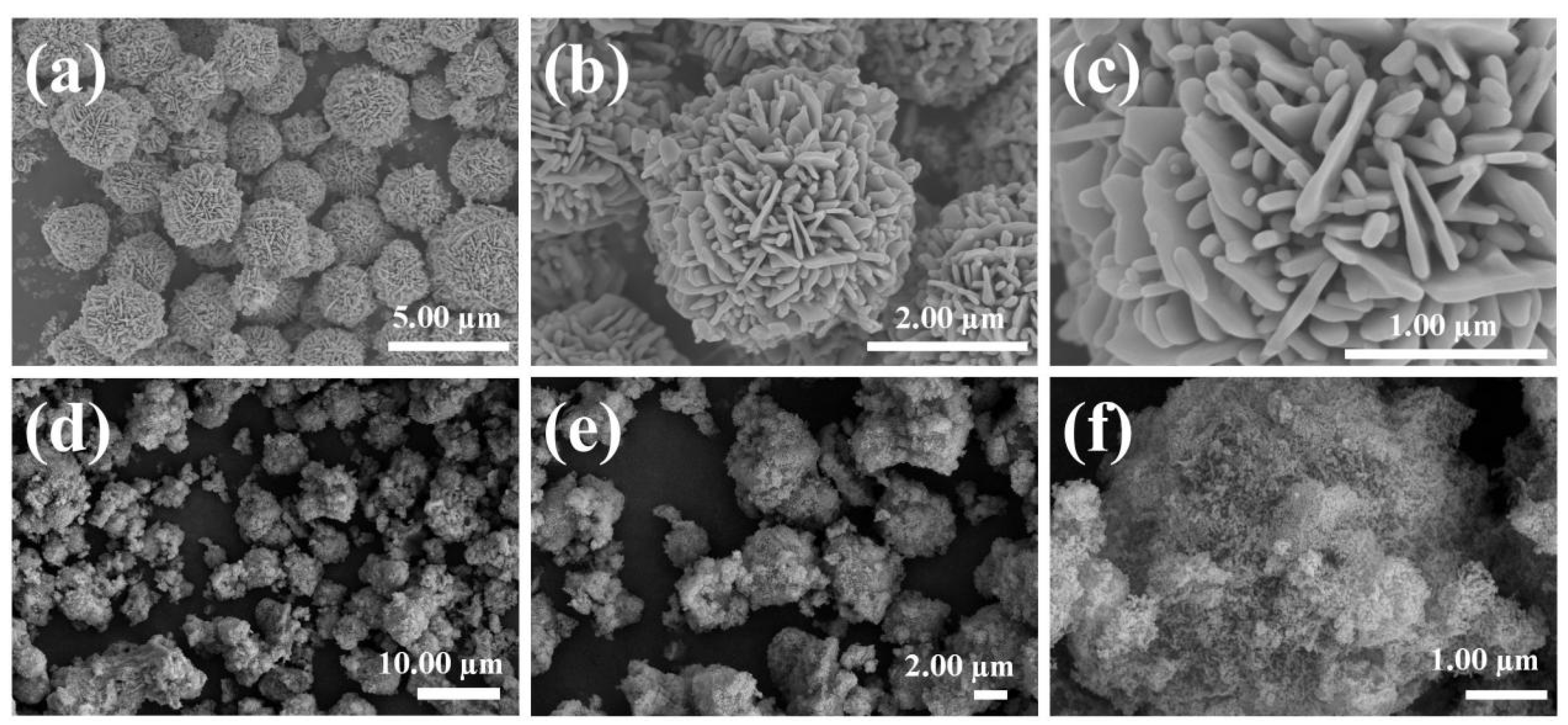
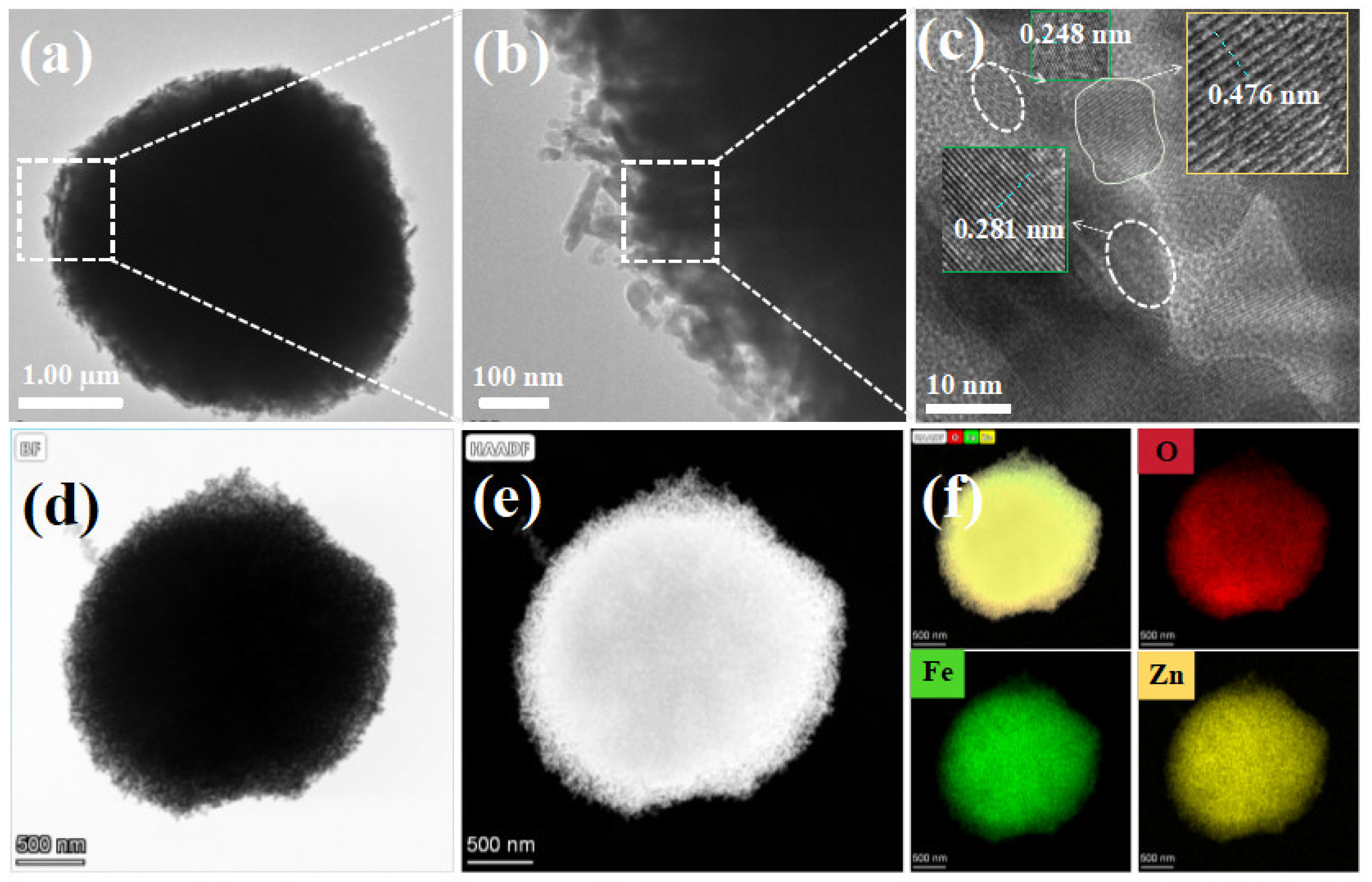
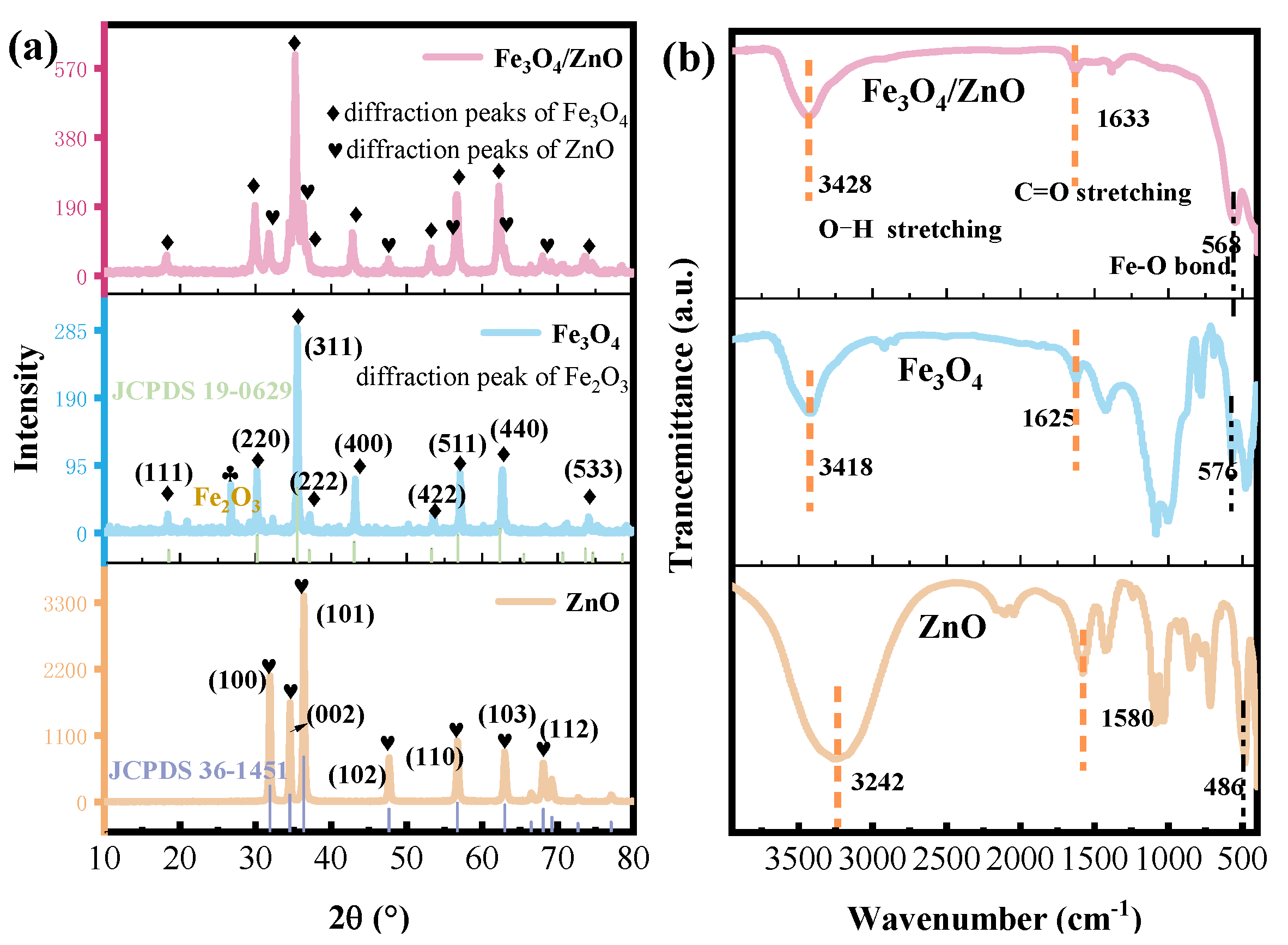
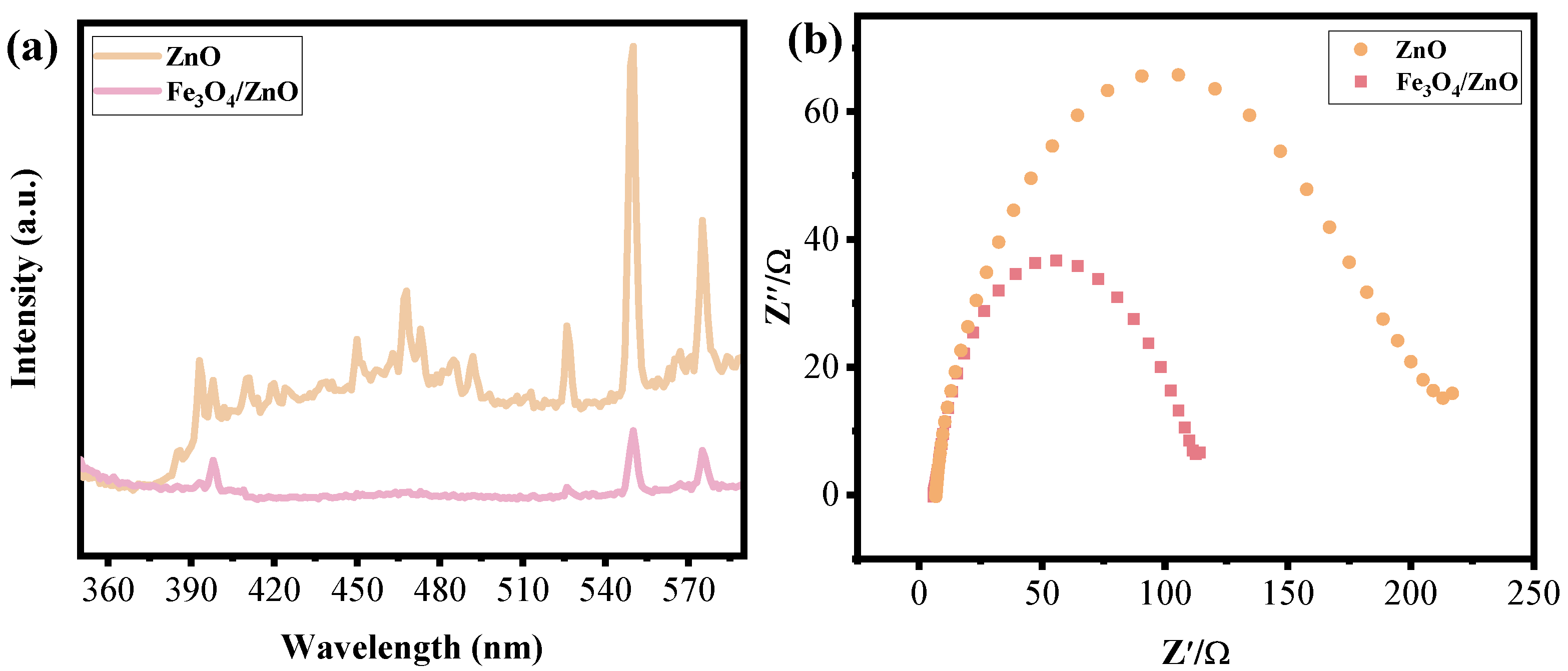
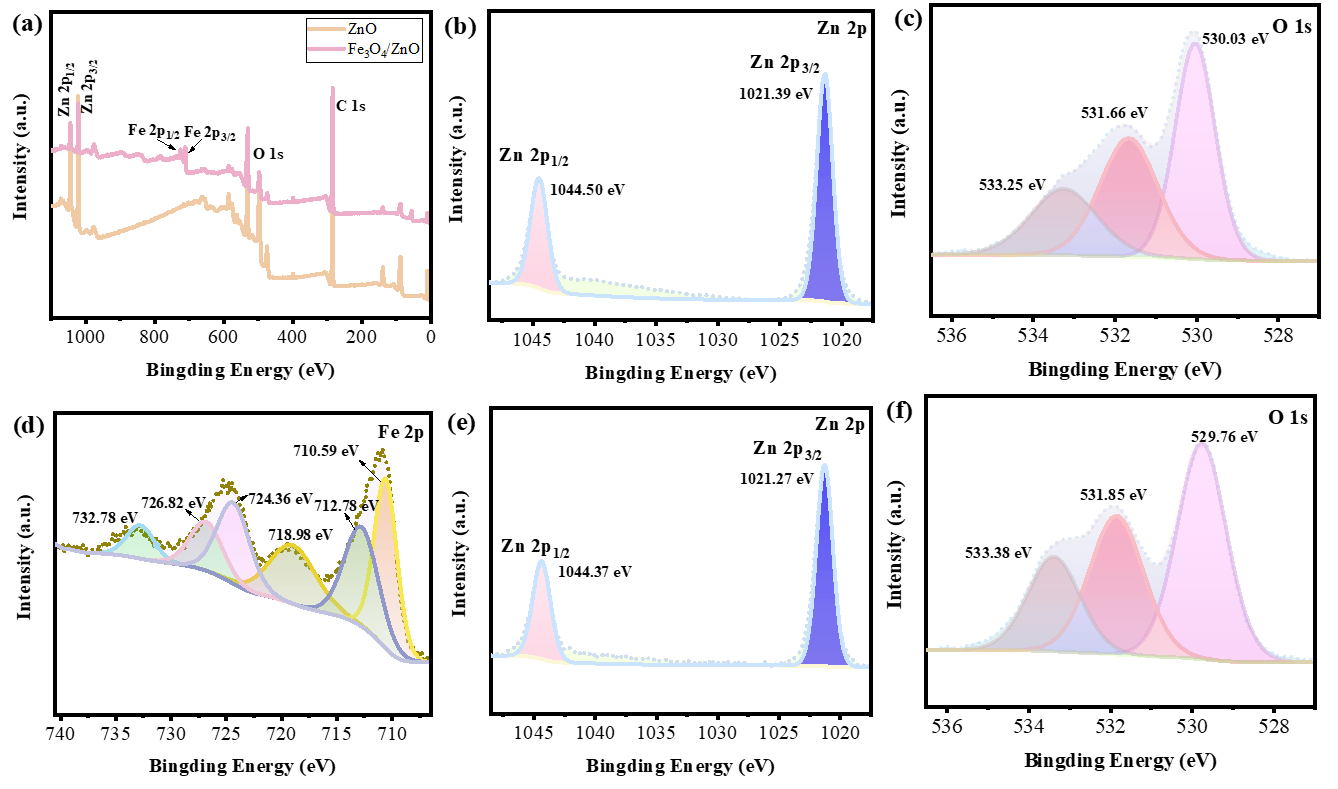
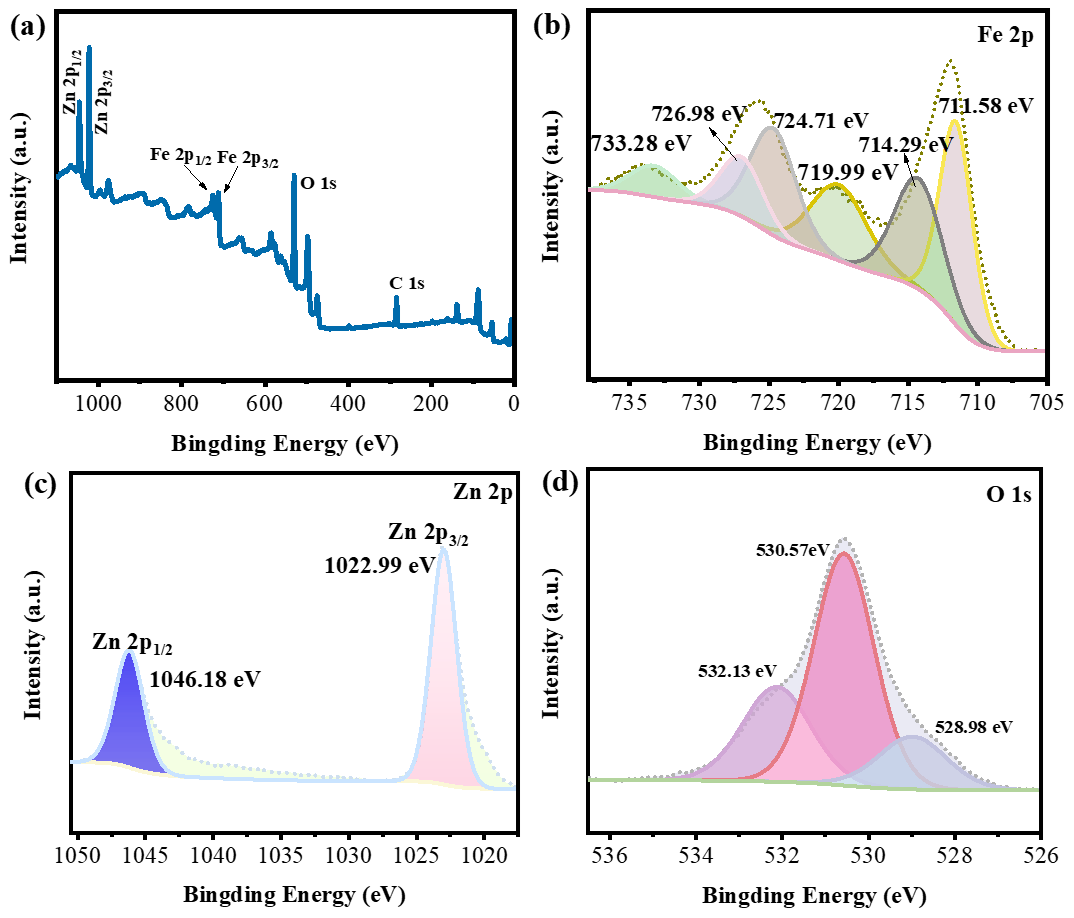
3.1. Microstructure Analysis
3.2. Photocatalytic Performance
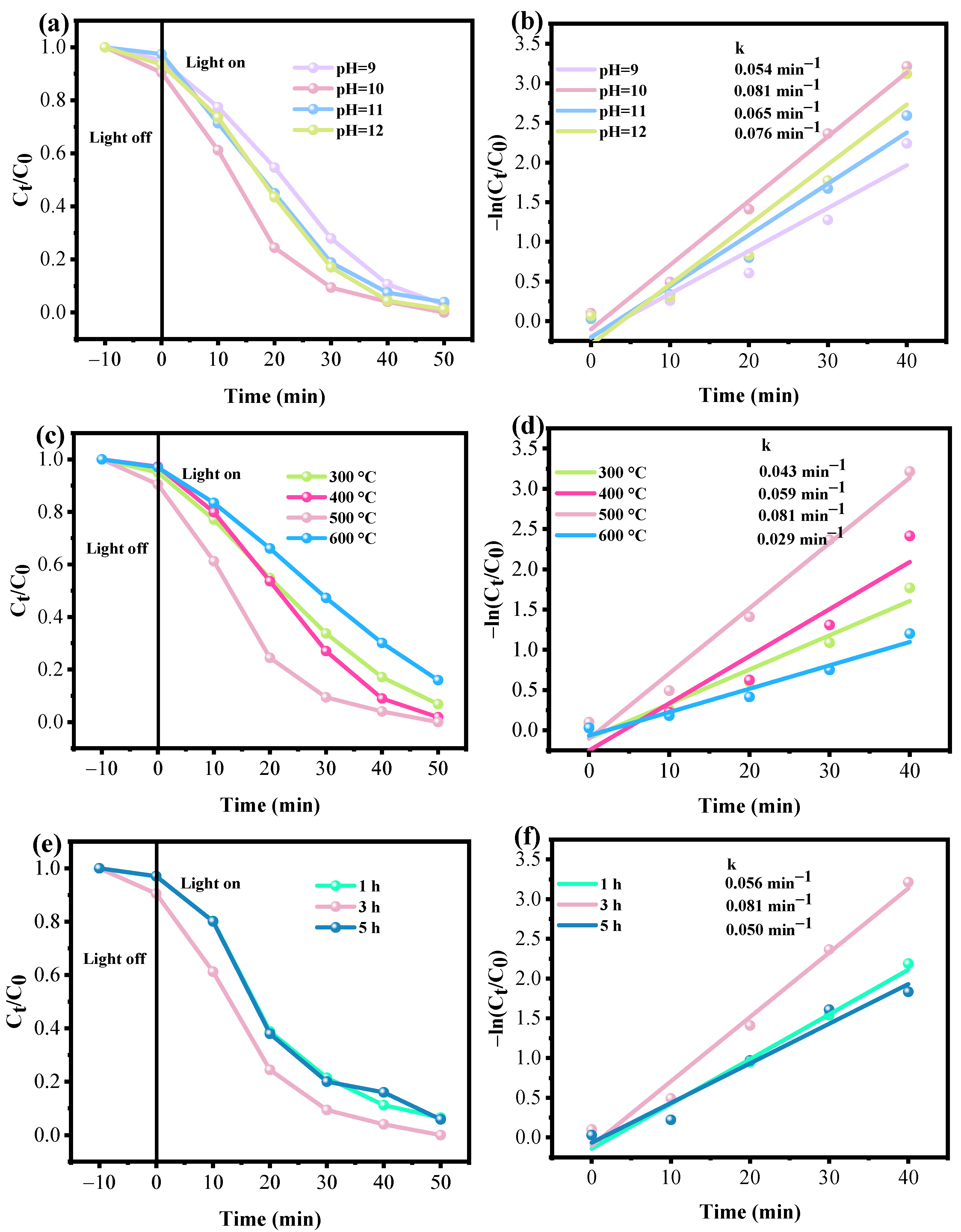
3.2.1. Effect of the pH Value in the Preparation of Fe3O4/ZnO
3.2.2. Effect of Annealing Temperature of Fe3O4/ZnO
3.2.3. Effect of Calcination Time
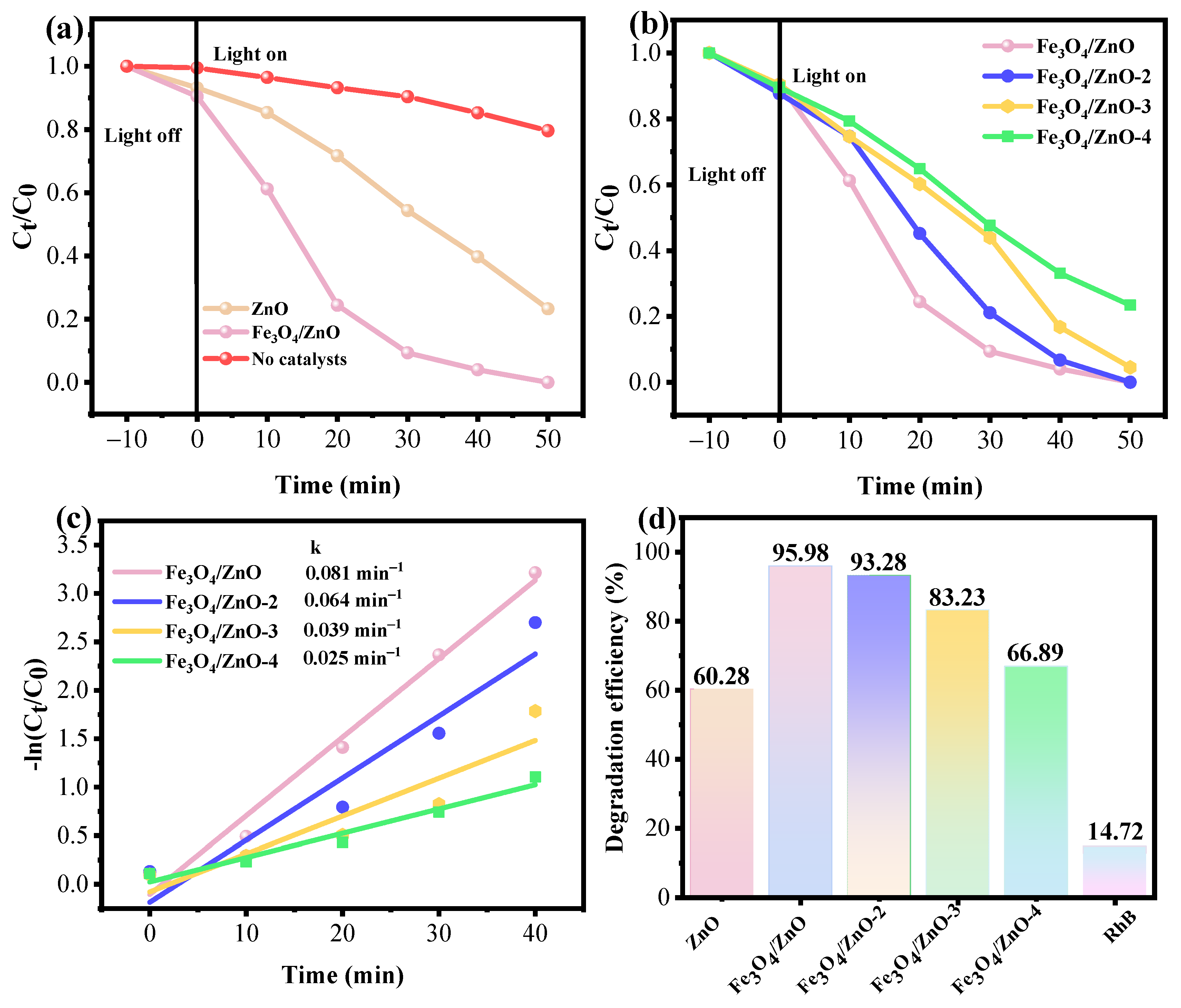
3.2.4. Photocatalytic Activity of Fe3O4/ZnO
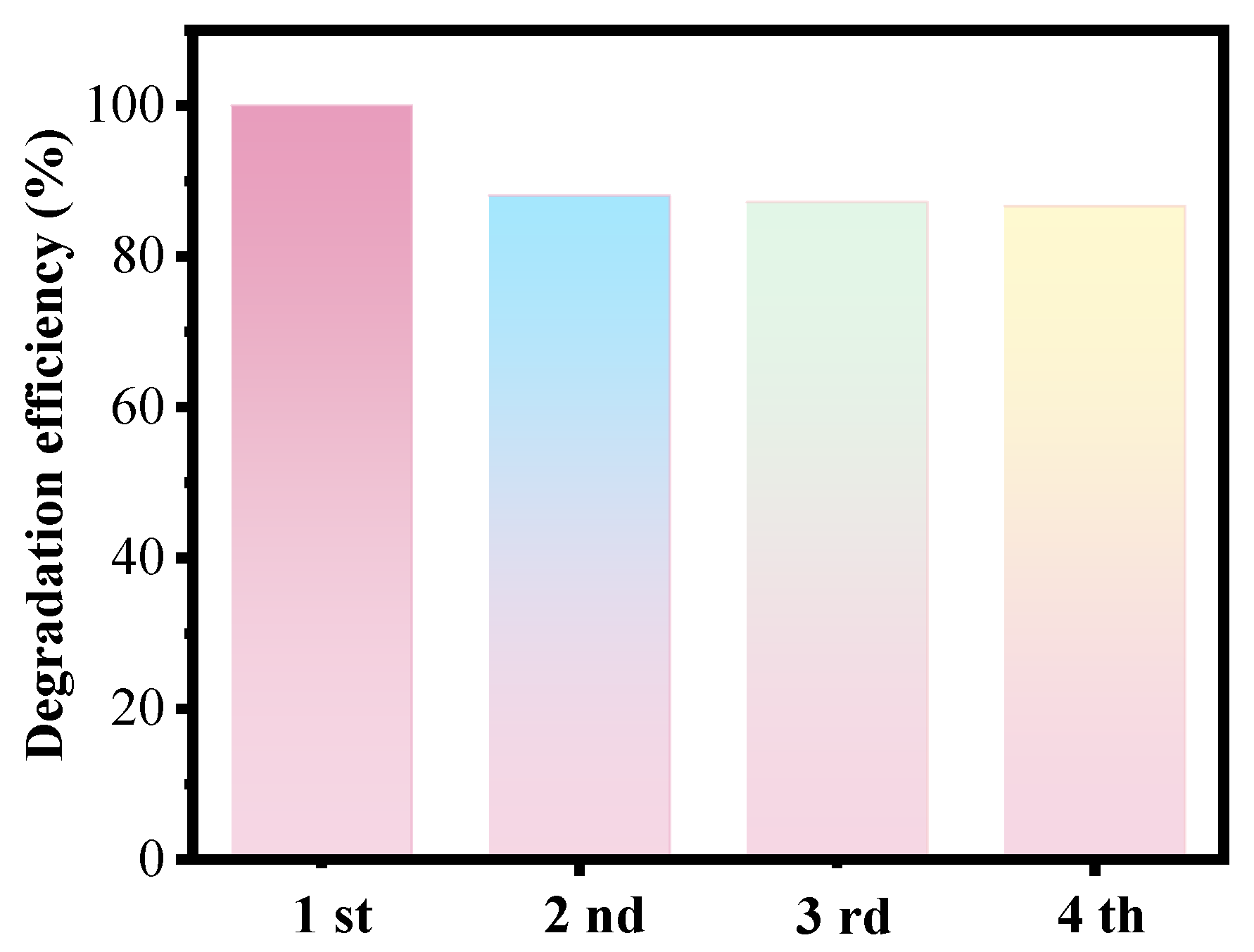
3.3. Cycle and Magnetic Separation Performances
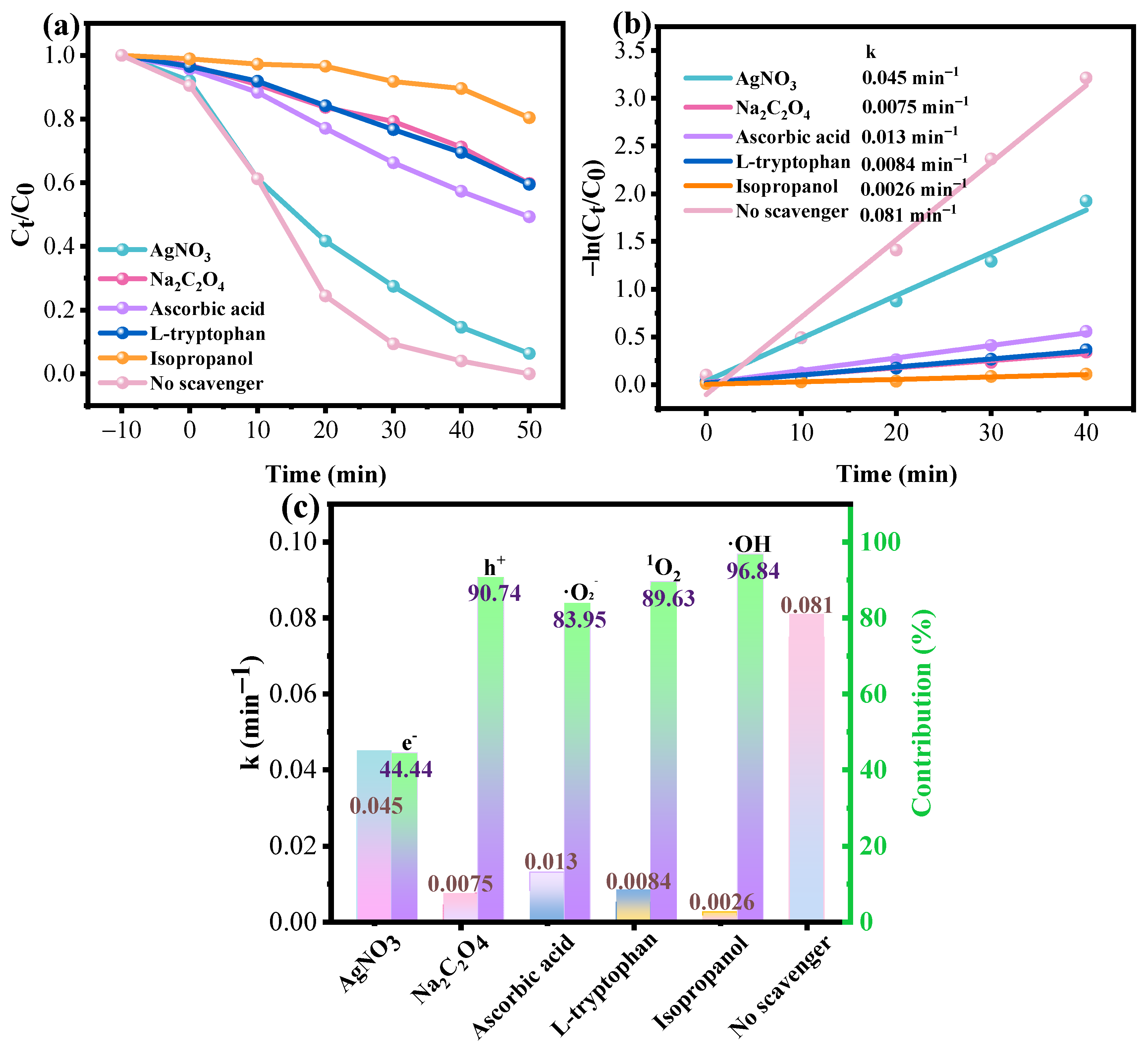
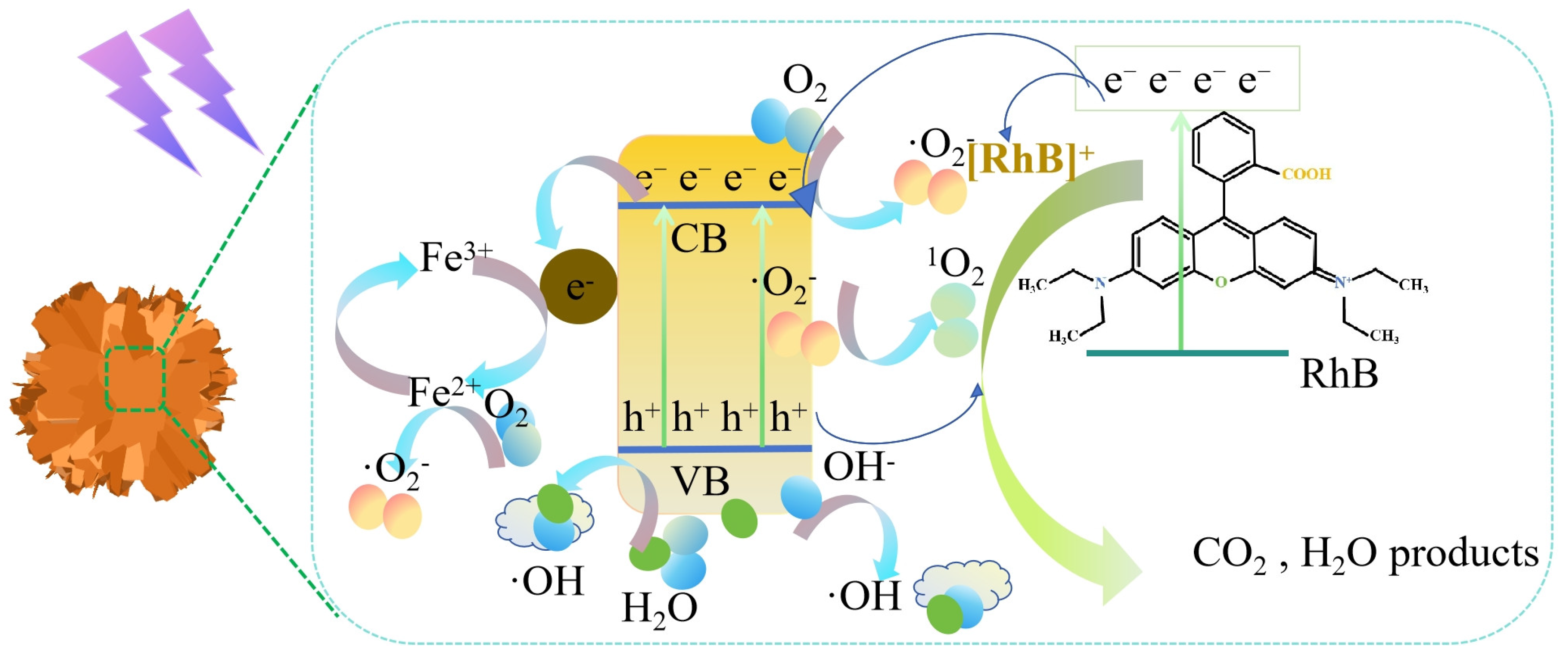
3.4. Photocatalysis Mechanism of Fe3O4/ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohari, M.S.; Yangjeh, A.H. Fe3O4/ZnO/CoWO4 nanocomposites: Novel magnetically separable visiblelight-driven photocatalysts with enhanced activity in degradation of different dye pollutants. Ceram. Int. 2017, 43, 3063–3071. [Google Scholar] [CrossRef]
- Song, C.; Zhao, S.D.; Xing, B.L.; Shi, C.L.; Meng, W.B.; Zhang, C.X.; Bo, Z. Facile one-pot green synthesis of magnetic separation photocatalyst-adsorbent and its application. J. Water Process Eng. 2022, 47, 102802. [Google Scholar]
- Chen, Q.; Wu, L.N.; Zhao, X.L.; Yang, X.J. Fabrication of Zn-Ti layered double oxide nanosheets with ZnO/ZnTiO3 heterojunction for enhanced photocatalytic degradation of MO, RhB and MB. J. Mol. Liq. 2022, 353, 118794. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Li, J.J.; Guo, H.F.; Lv, B.L.; Zhang, F.W. Graphitic carbon nitride anchored Fe single atom towards the highly efficient degradation of dyes under visible light. Mater. Lett. 2022, 328, 133045. [Google Scholar] [CrossRef]
- Sonu, S.P.; Thakur, S.; Le, Q.V.; Ahamad, T.; Singh, P.; Nguyen, V.H.; Khan, A.A.P.; Hussain, C.M.; Raizada, P. Facile synthesis of Co, Fe-bimetallic MIL-88A/microcrystalline cellulose composites for efficient adsorptive and photo-Fenton degradation of RhB dye. J. Taiwan Inst. Chem. Eng. 2023, 153, 105189. [Google Scholar]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In Situ Charge Transfer at the Ag@ZnO Photoelectrochemical Interface toward the High Photocatalytic Performance of H2 Evolution and RhB Degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Zhang, B.; Liu, E.Z.; Hu, X.Y.; Hao, Q.Q.; Fan, J. Nano composite of CuInS2/ZnO with improved photocatalytic activity of degradation and hydrogen production. Opt. Mater. 2020, 109, 110379. [Google Scholar] [CrossRef]
- Liu, R.J.; Fu, X.N.; Guo, Y.F.; Zhang, J.F.; Tian, W.F. A study on Ag or Ce doped and co-doped ZnO for the photocatalytic degradation of RhB dye. Vacuum 2023, 215, 112337. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, G.J.; Ma, Y.S.; Chen, X.; Ren, L.L. Fe-Doped Alumina Aerogels as a Green Heterogeneous Catalyst for Effcient Degradation of Organic Pollutants. Catal. Lett. 2019, 149, 1874–1887. [Google Scholar] [CrossRef]
- Liu, S.M.; Zhang, J.S.; Chen, M.L.; Wang, X.X. Continuous release of SO4− over g-C3N4/ZnO/Fe(III) systems mediated by persulfates: The Fe(III)/Fe(II) cycling and degradation pathway. Catal. Sci. Technol. 2020, 10, 2203–2212. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Liu, J.J.; Ma, H.C.; Liu, W.Q.; Zuo, S.L.; Yu, Y.C.; Yan, N.; Mushtaq, M.A. Synergistic photocatalytic performance of Fe@Fe2O3 core-shell nanostructures supported on g-C3N4 nanosheets for RhB and phenol decolorization: Fabrication, characterization, and mechanistic insights. J. Water Process Eng. 2023, 53, 103866. [Google Scholar] [CrossRef]
- Shi, W.N.; Fang, W.X.; Wang, J.C.; Qiao, X.; Wang, B.B.; Guo, X.W. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, X.; Han, X.; Sun, Y.; Jin, S.; Liu, R.J. Photocatalytic degradation of tetracycline with Fe3O4/g-C3N4/TiO2 catalyst under visible light. Carbon Lett. 2024, 34, 75–83. [Google Scholar] [CrossRef]
- Huong, V.H.; Nguyen, V.C.; Ha, M.N.; Pham, D.V.; Nguyen, T.B.; Ma, Y.R.; Ngac, A.B.; Loan, T.T. A S-scheme heterojunction Fe-doped TiO2/SnO2 with rich oxygen vacancies for photo-Fenton degradation of Rhodamine B under visible light illumination. Opt. Mater. 2023, 140, 113864. [Google Scholar] [CrossRef]
- Chen, R.F.; Yin, C.C.; Liu, H.; Wei, Y. Degradation of rhodamine B during the formation of Fe3O4 nanoparticles by air oxidation of Fe(OH)2. J. Mol. Catal. A Chem. 2015, 397, 114–119. [Google Scholar] [CrossRef]
- Kohzadi, S.; Maleki, A.; Bundschuh, M.; Vahabzadeh, Z.; Johari, S.A.; Rezaee, R.; Shahmoradi, B.; Marzban, N.; Amini, N. Doping zinc oxide (ZnO) nanoparticles with molybdenum boosts photocatalytic degradation of Rhodamine B (RhB): Particle characterization, degradation kinetics and aquatic toxicity testing. J. Mol. Liq. 2023, 385, 122412. [Google Scholar] [CrossRef]
- Pandey, S.K.; Mishra, P.K.; Tiwary, D. Enhanced photocatalytic performance of NiS/ZnO nanocomposite for the remediation of PNP and RhB dye. J. Environ. Chem. Eng. 2022, 10, 107459. [Google Scholar] [CrossRef]
- Ning, X.E.; Hao, A.Z.; Chen, R.Q.; Khan, M.F.; Jia, D.Z. Constructing of GQDs/ZnO S-scheme heterojunction as efficient piezocatalyst for environmental remediation and understanding the charge transfer mechanism. Carbon 2024, 218, 118772. [Google Scholar] [CrossRef]
- Cheng, J.X.; Zhao, Y.X.; Feng, X.; Huang, Q.L.; Huang, Y.; Guo, M.J.; Cheng, B.W. Near-field electrospinning of PTFE/ZnO membrane with regular pore geometry and its photocatalytic performance. J. Environ. Chem. Eng. 2024, 12, 111745. [Google Scholar] [CrossRef]
- Yang, X.Y.; Wu, L.; Zhang, B.G.; Li, J.W.; Shen, Y.F.; Liu, Y.; Hu, Y. Fabrication of a P-Si/ZnO heterojunction based on galvanic cell driven and the complete degradation of RhB via fast charge transfer. Nanoscale 2023, 15, 16323. [Google Scholar] [CrossRef] [PubMed]
- Sarvalkar, P.D.; Kamble, S.S.; Powar, P.S.; Kakade, S.S.; Jamadar, A.S.; Thounaojam, P.; Patil, M.S.; Kalake, S.V.; Nimbalkar, M.S.; Sharma, K.K.K. Synthesized rGO/f-MWCNT-architectured 1-D ZnO nanocomposites for azo dyes adsorption, photocatalytic degradation, and biological applications. Catal. Commun. 2024, 187, 106846. [Google Scholar] [CrossRef]
- Chuaicham, C.; Rizki, I.N.; Sekar, K.; Shenoy, S.; Srikhaow, A.; Trakulmututa, J.; Sasaki, K. Bio-reduced Ag nanoparticle decorated on ZnO for enhancement of photocatalytic reduction of hexavalent chromium and photocatalytic degradation of rhodamine B. J. Alloys Compd. 2023, 939, 168797. [Google Scholar] [CrossRef]
- Shen, Z.F.; Zhang, Q.L.; Yin, C.C.; Kang, S.F.; Jia, H.Y.; Li, X.; Li, X.; Wang, Y.G.; Cui, L.F. Facile synthesis of 3D flower-like mesoporous Ce-ZnO at room temperature for the sunlight-driven photocatalytic degradations of RhB and phenol. J. Colloid Interface Sci. 2019, 556, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, E.; Eden, C.; Canpolat, N.; Cirak, C.; Yilmaz, M. Optimizing the structural and photocatalytic performance of Ag-decorated ZnO/Zn(OH)2 nanoparticles for RhB degradation. Int. J. Appl. Ceram. Technol. 2024, 21, 2011–2023. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Olaru, N.; Airinei, A. Photocatalytic Activity of ZnO-SnO2 Ceramic Nanofibers for RhB Dye Degradation: Experimental Design, Modeling, and Process Optimization. Phys. Status Solidi B 2019, 256, 1800474. [Google Scholar] [CrossRef]
- Yu, R.Y.; Shang, Y.X.; Zhang, X.; Liu, J.T.; Zhang, F.D.; Du, X.; Sun, H.M.; Zeng, J.B. Self-templated synthesis of core-shell Fe3O4@ZnO@ZIF-8 as an efficient visible-light-driven photocatalyst. Catal. Commun. 2023, 174, 106583. [Google Scholar] [CrossRef]
- Mane, V.A.; Dake, D.V.; Raskar, N.D.; Sonpir, R.B.; Dole, B.N. Synergistic Enhancement of Photocatalytic and Antifungal Activities in Microwave-Assisted ZnO/Fe-Doped Bi2O3 Nanocomposites. Phys. Status Solidi A 2024, 221, 2300954. [Google Scholar] [CrossRef]
- Ji, S.Y.; Yang, Y.L.; Zhou, Z.W.; Li, X.; Liu, Y.K. Photocatalysis-Fenton of Fe-doped g-C3N4 catalyst and its excellent degradation performance towards RhB. J. Water Process Eng. 2021, 40, 101804. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chang, M.J.; Li, W.J.; Bai, G.; Xie, H.X.; Li, X.; Du, H.L.; Song, S.J. Facile preparation of core/shell Ag/Fe3O4@MIL-100(Fe) with Ag plasmon-enhanced photo-Fenton activity towards RhB degradation. J. Mateials Sci. 2023, 58, 12596–12610. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Ren, Y.Q.; Huang, L.; Chen, Y.; Bian, Z.F. In situ Fe(III)-doped TiO2 mesocrystals catalyzed visible light photo-Fenton system. Catal. Today 2023, 410, 309–316. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.C.; Bao, W.H.; Gao, H.; Liang, D.X.; Li, J. Facile hydrothermal synthesis of Fe3O4@cellulose aerogel nanocomposite and its application in Fenton-like degradation of Rhodamine B. Carbohydr. Polym. 2018, 189, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yangjeh, A.H.; Gohari, M.S. Fe3O4/ZnO/Ag3VO4/AgI nanocomposites: Quaternary magnetic photocatalysts with excellent activity in degradation of water pollutants under visible ligh. Sep. Purif. Technol. 2016, 166, 63–72. [Google Scholar] [CrossRef]
- Goshtasb, Z.K.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z. Magnetic Ag3PO4/Ag2CrO4/Fe/Fe3O4 quaternary composite for improved solar-driven photocatalytic degradation of cationic dyes under natural solar radiation. J. Photochem. Photobiol. A Chem. 2022, 428, 113856. [Google Scholar]
- Althabaiti, S.A.; Khan, Z.; Bawaked, S.M.; Sheheri, S.Z.A.; Mokhtar, M.; Malik, M.A.; Narasimharao, K. PtOx deposited Fe3O4-ZnO/TiO2 nanocomposites for photocatalytic H2 production under visible light. J. Environ. Chem. Eng. 2023, 11, 110615. [Google Scholar] [CrossRef]
- Zalfani, M.; Schueren, B.V.D.; Mahdouani, M.; Bourguiga, R.; Yuc, W.B.; Wuc, M.; Deparis, O.; Li, Y.; Su, B.L. ZnO quantum dots decorated 3DOM TiO2 nanocomposites: Symbiose of quantum size effects and photonic structure for highly enhanced photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2016, 199, 187–198. [Google Scholar] [CrossRef]
- Zhu, D.D.; Huang, Y.; Li, R.; Peng, S.Q.; Wang, P.G.; Cao, J.J. Constructing Active Cu2+−O−Fe3+ Sites at the CuO−Fe3O4 Interface to Promote Activation of Surface Lattice Oxygen. Environ. Sci. Technol. 2023, 57, 17598–17609. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.S.; Zhang, Y.; Liu, P.F.; Yang, H.G.; Zhang, X.; Wei, S.H. Interface-confined intermediate phase in TiO2 enables efficient photocatalysis. Proc. Natl. Acad. Sci. USA 2024, 121, 2318341121. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Wang, S.; Liu, Y.; Zhao, P.; Tian, J.; Zhu, B.; Zhang, S.; Xie, W.; Yu, H. Facile Preparation of Magnetically Separable Fe3O4/ZnO Nanocomposite with Enhanced Photocatalytic Activity for Degradation of Rhodamine B. Nanomaterials 2024, 14, 926. https://doi.org/10.3390/nano14110926
Qi L, Wang S, Liu Y, Zhao P, Tian J, Zhu B, Zhang S, Xie W, Yu H. Facile Preparation of Magnetically Separable Fe3O4/ZnO Nanocomposite with Enhanced Photocatalytic Activity for Degradation of Rhodamine B. Nanomaterials. 2024; 14(11):926. https://doi.org/10.3390/nano14110926
Chicago/Turabian StyleQi, Li, Siyu Wang, Yun Liu, Peng Zhao, Jing Tian, Baolin Zhu, Shoumin Zhang, Wenqi Xie, and Huanhuan Yu. 2024. "Facile Preparation of Magnetically Separable Fe3O4/ZnO Nanocomposite with Enhanced Photocatalytic Activity for Degradation of Rhodamine B" Nanomaterials 14, no. 11: 926. https://doi.org/10.3390/nano14110926
APA StyleQi, L., Wang, S., Liu, Y., Zhao, P., Tian, J., Zhu, B., Zhang, S., Xie, W., & Yu, H. (2024). Facile Preparation of Magnetically Separable Fe3O4/ZnO Nanocomposite with Enhanced Photocatalytic Activity for Degradation of Rhodamine B. Nanomaterials, 14(11), 926. https://doi.org/10.3390/nano14110926





