Impact of Nanoparticle Additions on Life Cycle Assessment (LCA) of Ceramic Tiles Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
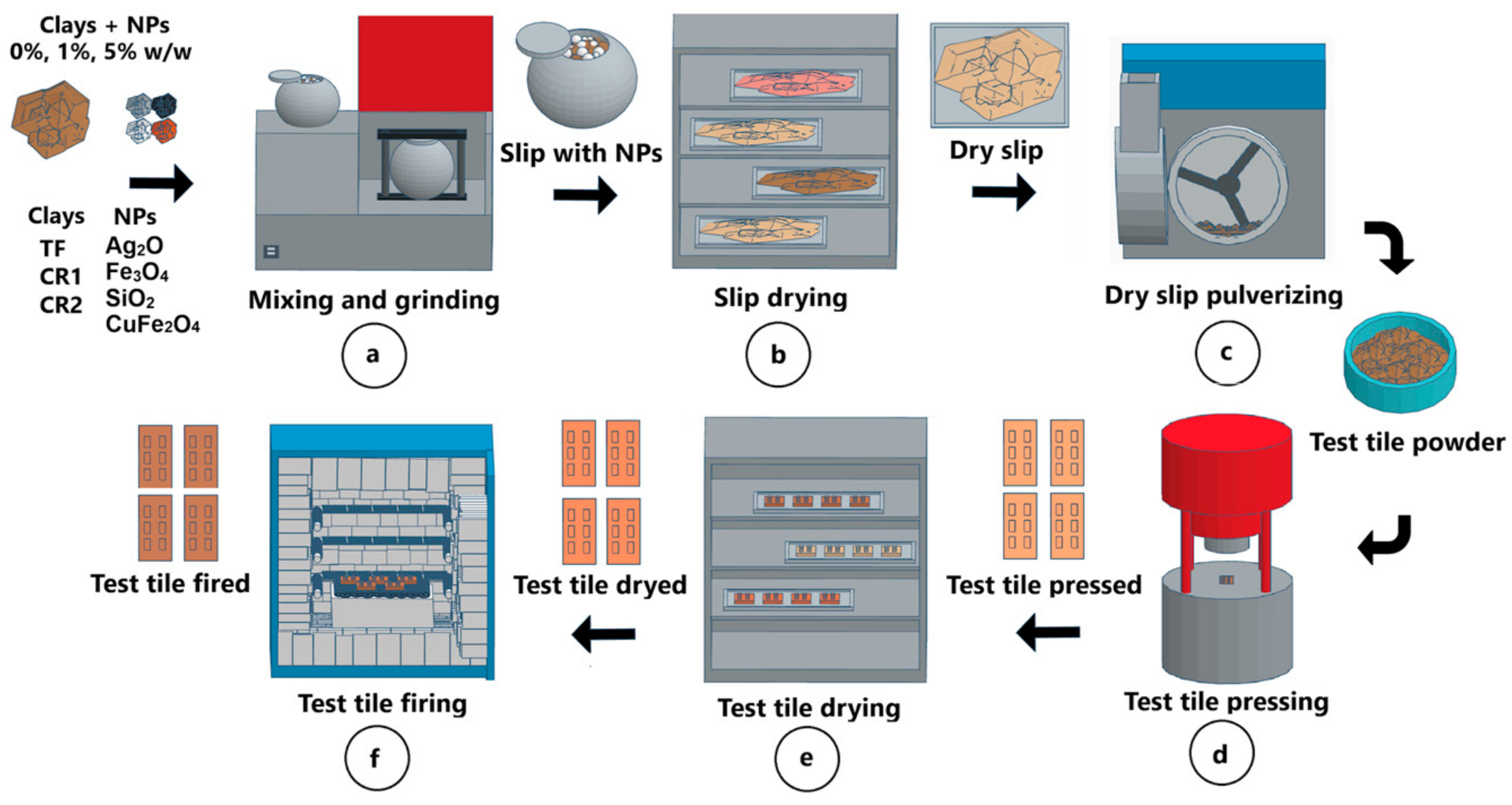
2.3. Test Tiles Manufacturing Process
2.4. Method for Calculating Percentage Linear Shrinkage (%LS), Loss on Fire (%LOF), Water Absorption (%WA) and Flexural Strength
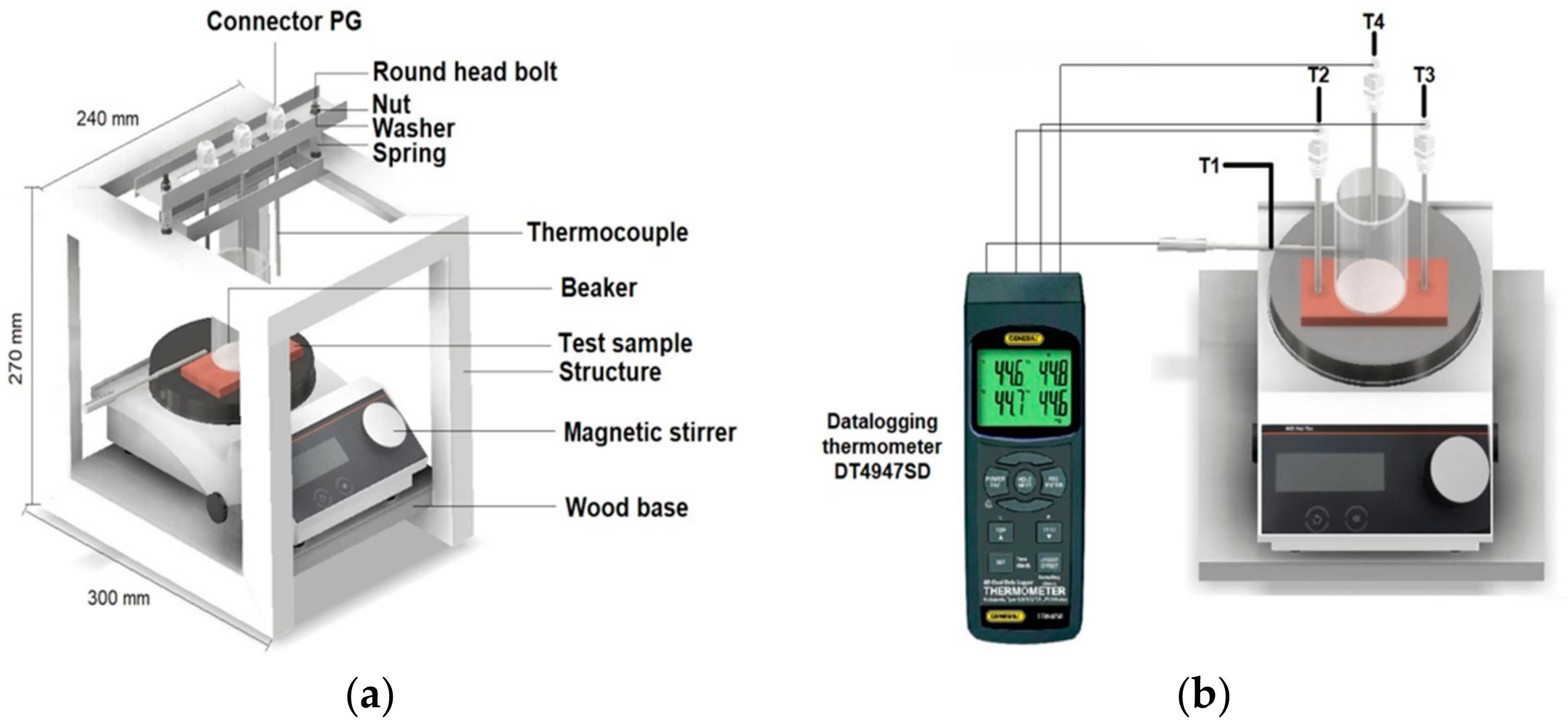
2.5. Apparent Thermal Conductivity Test
2.6. Characterization of Clays
2.7. Study of Nanoparticles Selection
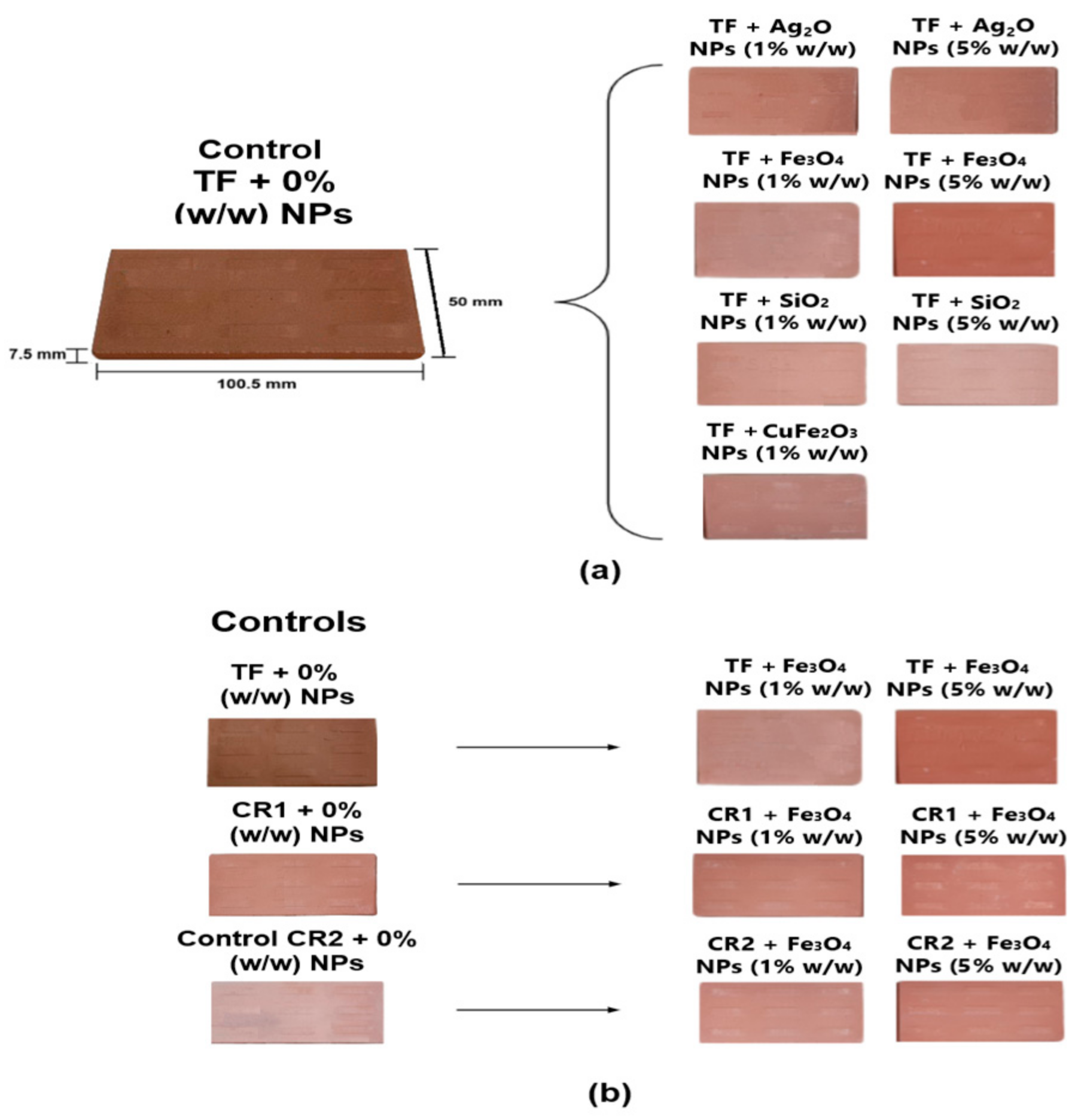
2.8. Study of Clays with Nanoparticles Addition
2.9. Microstructural and Morphological Analysis TF Test Tile with Fe3O4 Nanoparticles Addition
2.10. Life Cycle Assessment
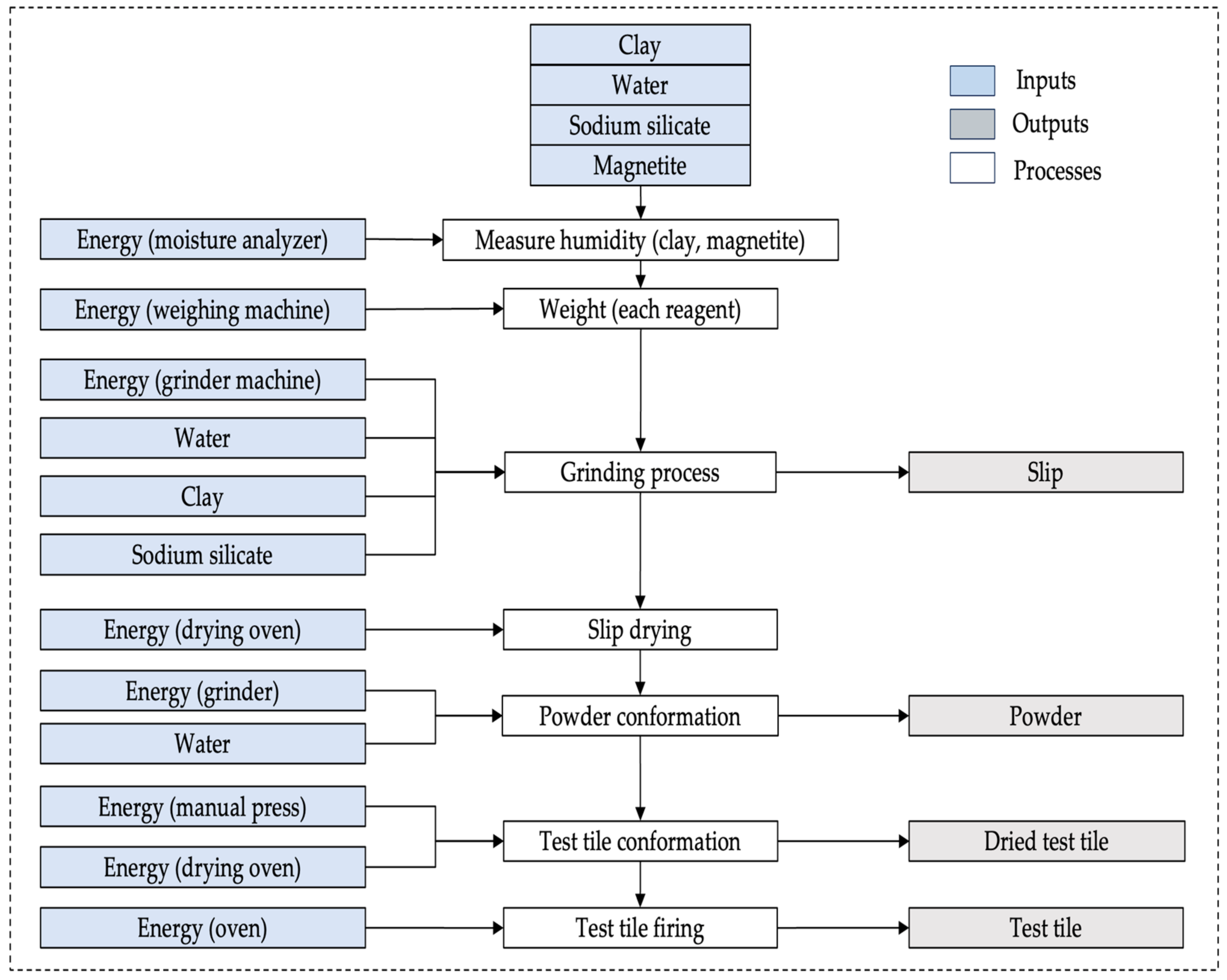
2.11. Life Cycle Inventory (LCI)
3. Results
3.1. Characterization of Clays
3.2. Study of Nanoparticles Selection
3.3. Study of Clays with Nanoparticles Addition
3.4. Microstructural and Morphological Analysis TF Test Tile with Fe3O4 Nanoparticles Addition
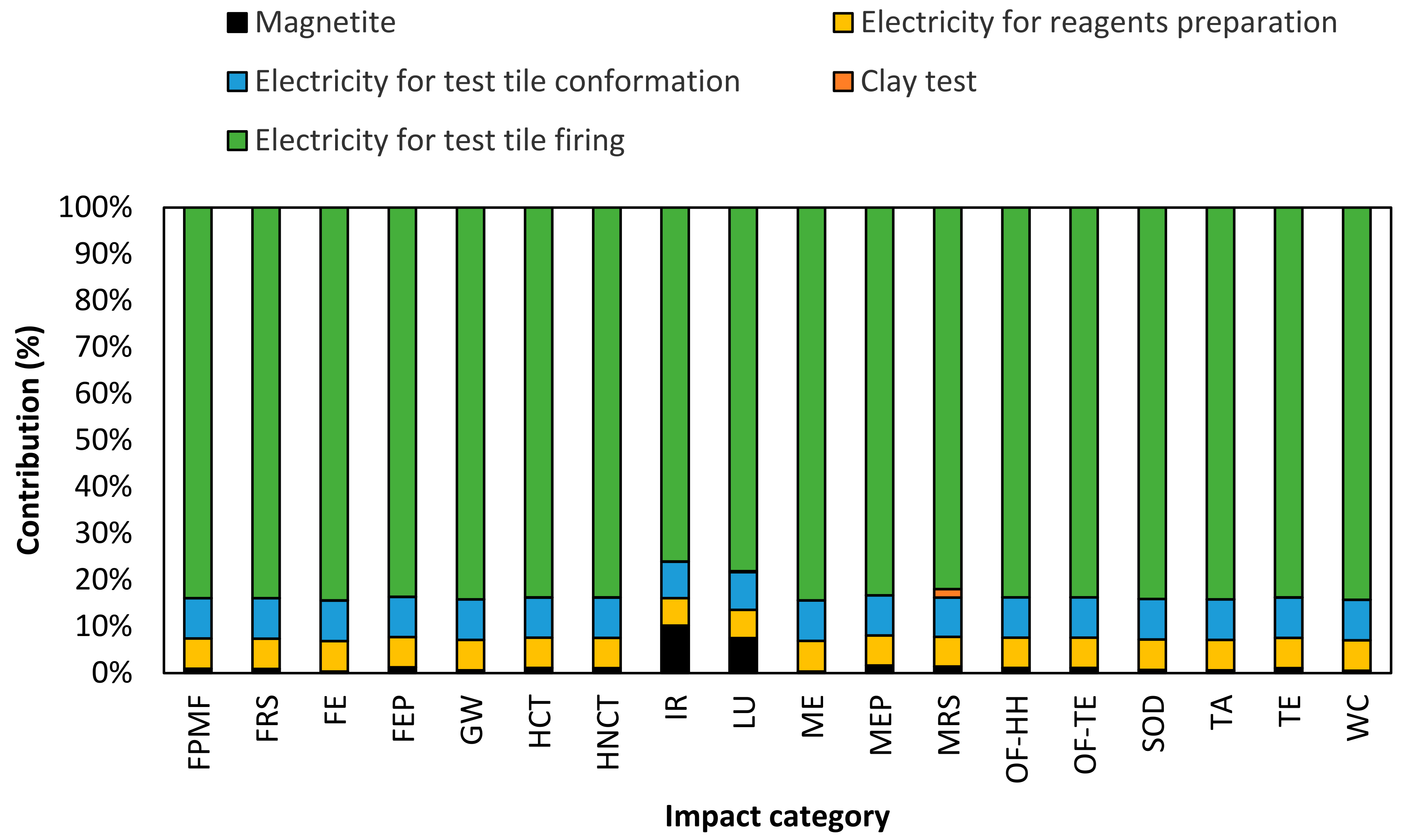
3.5. Life Cycle Assessment of Test Tile Clay Manufacturing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida, M.I.; Dias, A.C.; Demertzi, M.; Arroja, L. Environmental profile of ceramic tiles and their potential for improvement. J. Clean. Prod. 2016, 131, 583–593. [Google Scholar] [CrossRef]
- Boschi, G.; Masi, G.; Bonvicini, G.; Bignozzi, M.C. Sustainability in Italian Ceramic Tile Production: Evaluation of the Environmental Impact. Appl. Sci. 2020, 10, 9063. [Google Scholar] [CrossRef]
- Dondi, M.; Raimondo, M.; Zanelli, C. Clays and bodies for ceramic tiles: Reappraisal and technological classification. Appl. Clay Sci. 2014, 96, 91–109. [Google Scholar] [CrossRef]
- MECS. World Production and Consumption of Ceramic Tiles (2022). 2023. Manufacturing Economic Studies. Available online: https://ceramicworldweb.com/en/economics-and-markets/world-production-and-consumption-ceramic-tiles-2022 (accessed on 12 October 2023).
- Wang, Y.J.; Liu, Y.; Cui, S.; Sun, B.; Gong, X.; Gao, F.; Wang, Z. Comparative life cycle assessment of different fuel scenarios and milling technologies for ceramic tile production: A case study in China. J. Clean. Prod. 2020, 273, 122846. [Google Scholar] [CrossRef]
- Mezquita, A.; Monfort, E.; Ferrer, S.; Gabaldón-Estevan, D. How to reduce energy and water consumption in the preparation of raw materials for ceramic tile manufacturing: Dry versus wet route. J. Clean. Prod. 2017, 168, 1566–1570. [Google Scholar] [CrossRef]
- Monfort, E.; Mezquita, A.; Vaquer, E.; Mallol, G.; Alves, H.J.; Boschi, A.O. Consumo de energía térmica y emisiones de dióxido de carbono en la fabricación de baldosas cerámicas Análisis de las industrias Española y Brasileña. Boletín De La Soc. Española De Cerámica Y Y Vidrio 2012, 51, 275–284. [Google Scholar] [CrossRef]
- Mezquita, A.; Boix, J.; Monfort, E.; Mallol, G. Energy saving in ceramic tile kilns: Cooling gas heat recovery. Appl. Therm. Eng. 2014, 65, 102–110. [Google Scholar] [CrossRef]
- Ros-Dosdá, T.; Celades, I.; Monfort, E.; Fullana-i-Palmer, P. Environmental profile of Spanish porcelain stoneware tiles. Int. J. Life Cycle Assess. 2018, 23, 1562–1580. [Google Scholar] [CrossRef]
- Delpech, B.; Axcell, B.; Jouhara, H. A review on waste heat recovery from exhaust in the ceramics industry. E3S Web Conf. 2017, 22, 00034. [Google Scholar] [CrossRef]
- Delpech, B.; Milani, M.; Montorsi, L.; Boscardin, D.; Chauhan, A.; Almahmoud, S.; Axcell, B.; Jouhara, H. Energy efficiency enhancement and waste heat recovery in industrial processes by means of the heat pipe technology: Case of the ceramic industry. Energy 2018, 158, 656–665. [Google Scholar] [CrossRef]
- Mezquita, A.; Monfort, E.; Vaquer, E.; Ferrer, S.; Arnal, M.A.; Toledo, J.; Cuesta, M.A. Energy optimization in ceramic tile manufacture by using thermal oil. Bol. De La Soc. Esp. De Ceram. Y Vidr. 2012, 51, 183–190. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Iten, M.; Cruz, P.L.; Monteiro, H. Review on Energy Efficiency Progresses, Technologies and Strategies in the Ceramic Sector Focusing on Waste Heat Recovery. Energies 2020, 13, 6096. [Google Scholar] [CrossRef]
- Utlu, Z.; Hepbasli, A. Exergoeconomic analysis of energy utilization of drying process in a ceramic production. Appl. Therm. Eng. 2014, 70, 748–762. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Innovation in hydrogen production. Int. J. Hydrog. Energy 2017, 42, 14843–14864. [Google Scholar] [CrossRef]
- Martinez Plaza, D.; Cañadas Martinez, I.; Mallol Gasch, G.; Téllez Sufrategui, F.; Rodríguez García, J. A case study of the feasibility of using solar concentrating technologies for manufacturing ceramics. J. Clean. Prod. 2015, 87, 977–991. [Google Scholar] [CrossRef]
- Qian, M.; Li, Z.; Fan, L.G.; Wang, H.; Xu, J.; Zhao, W.; Huang, F. Ultra-Light Graphene Tile-Based Phase-Change Material for Efficient Thermal and Solar Energy Harvest. ACS Appl. Energy Mater. 2020, 3, 5517–5522. [Google Scholar] [CrossRef]
- Royo, P.; Acevedo, L.; Ferreira, V.J.; García-Armingol, T.; López-Sabirón, A.M.; Ferreira, G. High-temperature PCM-based thermal energy storage for industrial furnaces installed in energy-intensive industries. Energy 2019, 173, 1030–1040. [Google Scholar] [CrossRef]
- Bazzocchi, F.; Bertagni, S.; Ciacci, C.; Colonna, E.; Di Naso, V. Mechanical characterisation of a low-thickness ceramic tile cladding subject to ageing phenomena. J. Build. Eng. 2020, 29, 101105. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Feltrin, J.; Dal Bó, M.; Bernardin, A.M.; Hotza, D. Effect of reduction of thickness on microstructure and properties of porcelain stoneware tiles. Ceram. Int. 2014, 40, 14693–14699. [Google Scholar] [CrossRef]
- ISO 13006; Ceramic Tiles. Definitions, Classification, Characteristics and Marking. International Organization for Standardization: Geneva, Switzerland, 2018.
- La Torre, V.; Rambaldi, E.; Masi, G.; Nici, S.; Ghezzi, D.; Cappelletti, M.; Bignozzi, M.C. Validation of Antibacterial Systems for Sustainable Ceramic Tiles. Coatings 2021, 11, 1409. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Dondi, M.; Raimondo, M.; Hotza, D. Photocatalytic ceramic tiles: Challenges and technological solutions. J. Eur. Ceram. Soc. 2018, 38, 1002–1017. [Google Scholar] [CrossRef]
- Berto, A.M. Ceramic tiles: Above and beyond traditional applications. J. Eur. Ceram. Soc. 2007, 27, 1607–1613. [Google Scholar] [CrossRef]
- Cramer, C.L.; Ionescu, E.; Graczyk-Zajac, M.; Nelson, A.T.; Katoh, Y.; Haslam, J.J.; Wondraczek, L.; Aguirre, T.G.; LeBlanc, S.; Wang, H.; et al. Additive manufacturing of ceramic materials for energy applications: Road map and opportunities. J. Eur. Ceram. Soc. 2022, 42, 3049–3088. [Google Scholar] [CrossRef]
- Majumdar, S.D.; Kumar, S.; Mathew, V.T.; Saha, S. Functional Ceramic Tiles. Trans. Indian Ceram. Soc. 2010, 69, 37–44. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Dondi, M.; Hotza, D. Self-cleaning ceramic tiles coated with Nb2O5-doped-TiO2 nanoparticles. Ceram. Int. 2017, 43, 11986–11991. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Martinez, V.P.; Yabarrena, J.M.S.C.; Simões, M.B.; Perissinotto, A.P.; Wender, H.; de Vicente, F.S.; Noeske, P.-L.M.; Ribeiro, S.J.L.; et al. Thermally stable SiO2@TiO2 core@shell nanoparticles for application in photocatalytic self-cleaning ceramic tiles. Mater. Adv. 2021, 2, 2085–2096. [Google Scholar] [CrossRef]
- Djellabi, R.; Basilico, N.; Delbue, S.; D’alessandro, S.; Parapini, S.; Cerrato, G.; Laurenti, E.; Falletta, E.; Bianchi, C.L. Oxidative Inactivation of SARS-CoV-2 on Photoactive AgNPs@TiO2 Ceramic Tiles. Int. J. Mol. Sci. 2021, 22, 8836. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Cerrato, G.; Bresolin, B.M.; Djellabi, R.; Rtimi, S. Digitally Printed AgNPs Doped TiO2 on Commercial Porcelain-Gres Tiles: Synergistic Effects and Continuous Photocatalytic Antibacterial Activity. Surfaces 2020, 3, 11–25. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.R.; Relinque, E.J.; Manzano-Ramírez, A.; Castellote, M.; Romero-Pérez, M. Optimising processing conditions for the functionalisation of photocatalytic glazes by ZnO nanoparticle deposition. Mater. De Constr. 2021, 71, e261. [Google Scholar] [CrossRef]
- Ke, S.; Tian, W.; Meng, Z.; Fan, W. Antibacterial and Antistatic Laboratory Ceramic Countertop and Preparation Method Therefor. WO Patent WO 2022/252931 A1, 8 December 2022. [Google Scholar]
- Liu, C. Method for Preparing Anion Glazed Porcelain Tiles. CN Patent CN 108530030 A, 14 September 2018. [Google Scholar]
- Ryan, D.; Pillai, S.; Carroll, J. A Surface Coating. EP Patent EP 2908646 B1, 22 January 2020. [Google Scholar]
- Hayashi, H.; Kumazawa, T. Inkjet Ink for Ceramic Substrate. US Patent US 2020/0199389 A1, 25 June 2020. [Google Scholar]
- Wang, Q.; Chai, L.; Zhong, L.; Shi, M.; Jiang, K.; Li, Q. Antibacterial Ceramic, Antibacterial Ceramic Glaze Slip and Preparation Method Thereof. CN Patent CN 114315138 A, 12 April 2022. [Google Scholar]
- Ferreira-Neto, E.P.; Worsley Marcus, A.; Filho Ubirajara Pereira, R. Preparation of SiO2-TiO2 Composite Aerogels and SiO2@TiO2 Core-Shell Aerogels with High Thermal Stability and Enhanced Photocatalysis. US Patent US 2020/0180969 A1, 11 June 2020. [Google Scholar]
- Kim, J.; Kim, U.; Han, K.; Choi, J. Antibacterial persistence of hydrophobically glazed ceramic tiles. J. Korean Ceram. Soc. 2022, 59, 920–928. [Google Scholar] [CrossRef]
- Chen, L.; Lin, D.F. Applications of sewage sludge ash and nano-SiO2 to manufacture tile as construction material. Constr. Build. Mater. 2009, 23, 3312–3320. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Halbas, A.M.; El-Sharkawy, R.M. Ceramic tiles doped with lead oxide nanoparticles: Their fabrication, physical, mechanical characteristics and γ-ray shielding performance. Radiat. Phys. Chem. 2021, 189, 109780. [Google Scholar] [CrossRef]
- De la Garza, D.A.; Guzmán, A.M.; Gómez-Rodríguez, C.; Martínez, D.I.; Elizondo, N. Influence of Al2O3 and SiO2 nanoparticles addition on the microstructure and mechano-physical properties of ceramic tiles. Ceram. Int. 2022, 48, 12712–12720. [Google Scholar] [CrossRef]
- Nawaukkaratharnant, N.; Thueploy, A.; Khunthon, S.; Nilpairach, S.; Theerapapvisetpong, A. Improving the technological properties of red stoneware tiles derived from Ratchaburi red clay by the addition of iron oxide. Cases Stud. Constr. Mater. 2022, 16, e00983. [Google Scholar] [CrossRef]
- Fix Fierro, C.; Acosta Gonzalez Guillermo, E.; Aguilera Bustos Monica, I.; Gutiérrez Antonio, J. Preparation Method of Nanometric Size Metal Oxide Additives that Reduce the Temperature of Sinterized and/or Increase Productivity in the Manufacture of Ceramic Parts, Improving Mechanical Properties without Affecting the Gresification Properties of Ceramic Bodies, Tiles or Coatings. US Patent US 11339098 B2, 24 May 2022. [Google Scholar]
- Vieira, A.W.; Rosso, L.S.; Demarch, A.; Pasini, D.; Ruzza, S.P.; Arcaro, S.; Ribeiro, M.J.; Angioletto, E. Life cycle assessment in the ceramic tile industry: A review. J. Mater. Res. Technol. 2023, 23, 3904–3915. [Google Scholar] [CrossRef]
- Cucchi, M.; Volpi, L.; Ferrari, A.M.; García-Muiña, F.E.; Settembre-Blundo, D. Industry 4.0 real-world testing of dynamic organizational life cycle assessment (O-LCA) of a ceramic tile manufacturer. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.M.; Volpi, L.; Settembre-Blundo, D.; García-Muiña, F.E. Dynamic life cycle assessment (LCA) integrating life cycle inventory (LCI) and Enterprise resource planning (ERP) in an industry 4.0 environment. J. Clean. Prod. 2021, 286, 125314. [Google Scholar] [CrossRef]
- Quinteiro, P.; Almeida, M.I.; Serra, J.; Arroja, L.; Dias, A.C. Life cycle assessment of ceramic roof tiles: A temporal perspective. J. Clean. Prod. 2022, 363, 132568. [Google Scholar] [CrossRef]
- Caglayan, H.; Caliskan, H. Life cycle assessment based exergoenvironmental analysis of a cogeneration system used for ceramic factories. Sustain. Energy Technol. Assess. 2022, 52, 102078. [Google Scholar] [CrossRef]
- Barbosa, M.Z.; Dias, J.D.; Marvila, M.T.; de Azevedo, A.R.G. Life cycle approach applied to the production of ceramic materials incorporated with ornamental stone wastes. Environ. Sci. Pollut. Res. 2022, 29, 9957–9970. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Forcina, A.; Silvestri, C.; Ioppolo, G. Life cycle assessment of sanitaryware production: A case study in Italy. J. Clean. Prod. 2020, 251, 119708. [Google Scholar] [CrossRef]
- Monteiro, H.; Cruz, P.L.; Moura, B. Integrated environmental and economic life cycle assessment of improvement strategies for a ceramic industry. J. Clean. Prod. 2022, 345, 131173. [Google Scholar] [CrossRef]
- Del Rio, D.D.F.; Sovacool, B.K.; Foley, A.M.; Griffiths, S.; Bazilian, M.; Kim, J.; Rooney, D. Decarbonizing the ceramics industry: A systematic and critical review of policy options, developments and sociotechnical systems. Renew. Sustain. Energy Rev. 2022, 157, 112081. [Google Scholar] [CrossRef]
- Türkmen, B.A.; Özbilen, S.K.; Duhbaci, T.B. Improving the sustainability of ceramic tile production in Turkey. Sustain. Prod. Consum. 2021, 27, 2193–2207. [Google Scholar] [CrossRef]
- ASTM E11; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM International: West Conshohocken, PA, USA, 2022.
- Sacmi Group. Ceramic Tile Technology, 3rd ed; Imola, S., Ed.; Copyright Sacmi Imola, SC: Imola, Italy, 2022; Chapter 1; pp. 33–34. [Google Scholar]
- Temga, J.P.; Mache, J.R.; Madi, A.B.; Nguetnkam, J.P.; Bitom, D.L. Ceramics applications of clay in Lake Chad Basin, Central Africa. Appl. Clay Sci. 2019, 171, 118–132. [Google Scholar] [CrossRef]
- Rat, E.; Martínez-Martínez, S.; Sánchez-Garrido, J.A.; Pérez-Villarejo, L.; Garzón, E.; Sánchez-Soto, P.J. Characterization, thermal and ceramic properties of clays from Alhabia (Almería, Spain). Ceram. Int. 2023, 49, 14814–14825. [Google Scholar] [CrossRef]
- ISO 10545-3; Ceramic Tiles Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and bulk Density. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 10545-4; Ceramic Tiles Part 4: Determination of Modulus of Rupture and Breaking Strength. International Organization for Standardization: Geneva, Switzerland, 2019.
- Asadi, I.; Shafigh, P.; Abu Hassan, Z.F.B.; Mahyuddin, N.B. Thermal conductivity of concrete—A review. J. Build. Eng. 2018, 20, 81–93. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- El hammouti, A.; Charai, M.; Channouf, S.; Horma, O.; Nasri, H.; Mezrhab, A.; Karkri, M.; Tankari, M.A. Laboratory-testing and industrial scale performance of different clays from eastern Morocco for brick manufacturing. Constr. Build. Mater. 2023, 370, 130624. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment. Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Garcia-Valles, M.; Cuevas, D.; Alfonso, P.; Martínez, S. Thermal behaviour of ceramics obtained from the kaolinitic clays of Terra Alta, Catalonia, Spain. J. Therm. Anal. Calorim. 2022, 147, 5303–5312. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Pérez-Villarejo, L.; Garzón, E.; Sánchez-Soto, P.J. Influence of firing temperature on the ceramic properties of illite-chlorite-calcitic clays. Ceram. Int. 2023, 49, 24541–24557. [Google Scholar] [CrossRef]
- Kurmus, H.; Mohajerani, A. Energy savings, thermal conductivity, micro and macro structural analysis of fired clay bricks incorporating cigarette butts. Constr. Build. Mater. 2021, 283, 122755. [Google Scholar] [CrossRef]
- Leyton-Vergara, M.; Pérez-Fargallo, A.; Pulido-Arcas, J.; Cárdenas-Triviño, G.; Piggot-Navarrete, J. Influence of Granulometry on Thermal and Mechanical Properties of Cement Mortars Containing Expanded Perlite as a Lightweight Aggregate. Materials 2019, 12, 4013. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, C.; Sengul, O.; Tasdemir, M.A. A comparative study on the thermal conductivities and mechanical properties of lightweight concretes. Energy Build. 2017, 151, 469–475. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Nabiałek, M.; Sandu, A.V.; Szmidla, J.; Jurczyńska, A.; Razak, R.A.; A Aziz, I.H.; Jamil, N.H.; et al. Assessment of the Suitability of Ceramic Waste in Geopolymer Composites: An Appraisal. Materials 2021, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.B.; Zaccaron, A.; Nandi, V.d.S.; Inocente, J.M.; Muller, T.G.; Bo, A.G.D.; Bernardin, A.M.; Peterson, M. Measurement of apparent sintering activation energy for densification of clays. Clay Miner. 2021, 56, 299–305. [Google Scholar] [CrossRef]
- Wang, T.; Ma, S.; Wang, X.; Hong, T.; Luo, Y. A 100% high-aluminum fly ash-based high-density mullite ceramic with a triple microstructure: Preparation and mechanical characterization. Constr. Build. Mater. 2020, 239, 117761. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Zhao, L.; Cang, D. Novel ceramics prepared from inferior clay rich in CaO and Fe2O3: Properties, crystalline phases evolution and densification process. Appl. Clay Sci. 2017, 143, 199–204. [Google Scholar] [CrossRef]
- Chalouati, Y.; Mannai, F.; Bennour, A.; Srasra, E. Firing behavior of argillites from northern Tunisia as raw materials for ceramic applications. Int. J. Appl. Ceram. Technol. 2021, 18, 2323–2335. [Google Scholar] [CrossRef]
- Slimanou, H.; Baziz, A.; Bouzidi, N.; Quesada, D.E.; Tahakourt, A. Thermal, physical, mechanical and microstructural properties of dredged sediment-based ceramic tiles as substituent of kaolin. Environ. Sci. Pollut. Res. 2022, 29, 26792–26809. [Google Scholar] [CrossRef] [PubMed]
- Abou Elmaaty, T.M.; Sayed-Ahmed, K.; El Gohari, M.M.; Noaman, R. Enhancing the properties of bone China ceramics by treatment with microporous SiO2 nanoparticles. Chem. Pap. 2022, 76, 5879–5891. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhang, X.C.; Cao, Z.; Liu, Z.J.; Lu, Z.; Liu, Y.S. Towards the progress of ecological restoration and economic development in China’s Loess Plateau and strategy for more sustainable development. Sci. Total Environ. 2021, 756, 143676. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Karamian, E. Impact of Titania nanoparticles addition on the microstructure and properties of MgO-C refractories. Ceram. Int. 2017, 43, 15472–15477. [Google Scholar] [CrossRef]
- Deng, T.; Li, J. Study on preparation of thermal storage ceramic by using clay shale. Ceram. Int. 2016, 42, 18128–18135. [Google Scholar] [CrossRef]
- Molinari, C.; Alaya, Y.; Pasti, L.; Guarini, G.; Dondi, M.; Zanelli, C. Assessing white clays from Tabarka (Tunisia) in the production of porcelain stoneware tiles. Appl. Clay Sci. 2023, 231, 106741. [Google Scholar] [CrossRef]
- Harizanova, R.; Mihailova, I.; Cherkezova-Zheleva, Z.; Paneva, D.; Georgieva, M.; Tzankov, D.; Avdeev, G.; Rüssel, C. Glass–crystalline materials with high iron oxide concentration: Phase composition, redox ratio and magnetic properties. Boletín De La Soc. Española De Cerámica y Vidr. 2024, 63, 23–32. [Google Scholar] [CrossRef]
- Xu, K.; Huang, W.; Zhang, L.; Fu, S.; Chen, M.; Ding, S.; Han, B. Mechanical properties of low-carbon ultrahigh-performance concrete with ceramic tile waste powder. Constr. Build. Mater. 2021, 287, 123036. [Google Scholar] [CrossRef]
- Nascimento de Souza, B.; Dal Bó, M.; Hotza, D.; Fredel, M.C. Chemical modification of porcelain tile surface to optimize flexural strength and Weibull modulus through the ion exchange process. J. Build. Eng. 2022, 56, 104735. [Google Scholar] [CrossRef]
- De Sousa, I.V.; Jaramillo Nieves, L.J.; Dal-Bó, A.G.; Bernardin, A.M. Valorization of porcelain tile polishing residue in the production of cellular ceramics. Clean. Eng. Technol. 2022, 6, 100381. [Google Scholar] [CrossRef]
- Pérez, J.M.; Romero, M. Microstructure and technological properties of porcelain stoneware tiles moulded at different pressures and thicknesses. Ceram. Int. 2014, 40, 1365–1377. [Google Scholar] [CrossRef]
- Tamsu Selli, N. Relationship between microstructure and the impact resistance of porcelain stoneware tiles. Boletín De La Soc. Española De Cerámica y Vidr. 2022, 61, 210–219. [Google Scholar] [CrossRef]
- Eren Gültekin, E. The effect of heating rate and sintering temperature on the elastic modulus of porcelain tiles. Ultrasonics 2018, 83, 120–125. [Google Scholar] [CrossRef]
- Piccolo, P.d.O.; Zaccaron, A.; Teixeira, L.B.; de Moraes, E.G.; Montedo, O.R.K.; de Oliveira, A.P.N. Development of translucent ceramic tiles from modified porcelain stoneware tile paste. J. Build. Eng. 2022, 45, 103543. [Google Scholar] [CrossRef]
- Ciacco, E.F.S.; Rocha, J.R.; Coutinho, A.R. The energy consumption in the ceramic tile industry in Brazil. Appl. Therm. Eng. 2017, 113, 1283–1289. [Google Scholar] [CrossRef]
- Ferrer, S.; Mezquita, A.; Aguilella, V.M.; Monfort, E. Beyond the energy balance: Exergy analysis of an industrial roller kiln firing porcelain tiles. Appl. Therm. Eng. 2019, 150, 1002–1015. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.L.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tian, Z.; Cui, W.; Gao, L.; Liu, Z.; Diao, X.; Liu, G. Effects of Y2O3 and yttrium aluminates as sintering additives on the thermal conductivity of AlN ceramic substrates. Ceram. Int. 2018, 44, 20556–20559. [Google Scholar] [CrossRef]
- Pini, M.; Ferrari, A.M.; Gamberini, R.; Neri, P.; Rimini, B. Life cycle assessment of a large, thin ceramic tile with advantageous technological properties. Int. J. Life Cycle Assess. 2014, 19, 1567–1580. [Google Scholar] [CrossRef]
- De Souza, W.J.V.; Scur, G.; Hilsdorf, W.D. Eco-innovation practices in the brazilian ceramic tile industry: The case of the Santa Gertrudes and Criciuma clusters. J. Clean. Prod. 2018, 199, 1007–1019. [Google Scholar] [CrossRef]
- Cui, H.W.; Li, D.S.; Fan, Q. Using Nano Hexagonal Boron Nitride Particles and Nano Cubic Silicon Carbide Particles to Improve the Thermal Conductivity of Electrically Conductive Adhesives. Electron. Mater. Lett. 2013, 9, 1–5. [Google Scholar] [CrossRef]
- Settembre-Blundo, D.; García-Muiña, F.E.; Pini, M.; Volpi, L.; Siligardi, C.; Ferrari, A.M. Sustainability as source of competitive advantages in mature sectors: The case of Ceramic District of Sassuolo (Italy). Smart Sustain. Built Environ. 2019, 8, 53–79. [Google Scholar] [CrossRef]
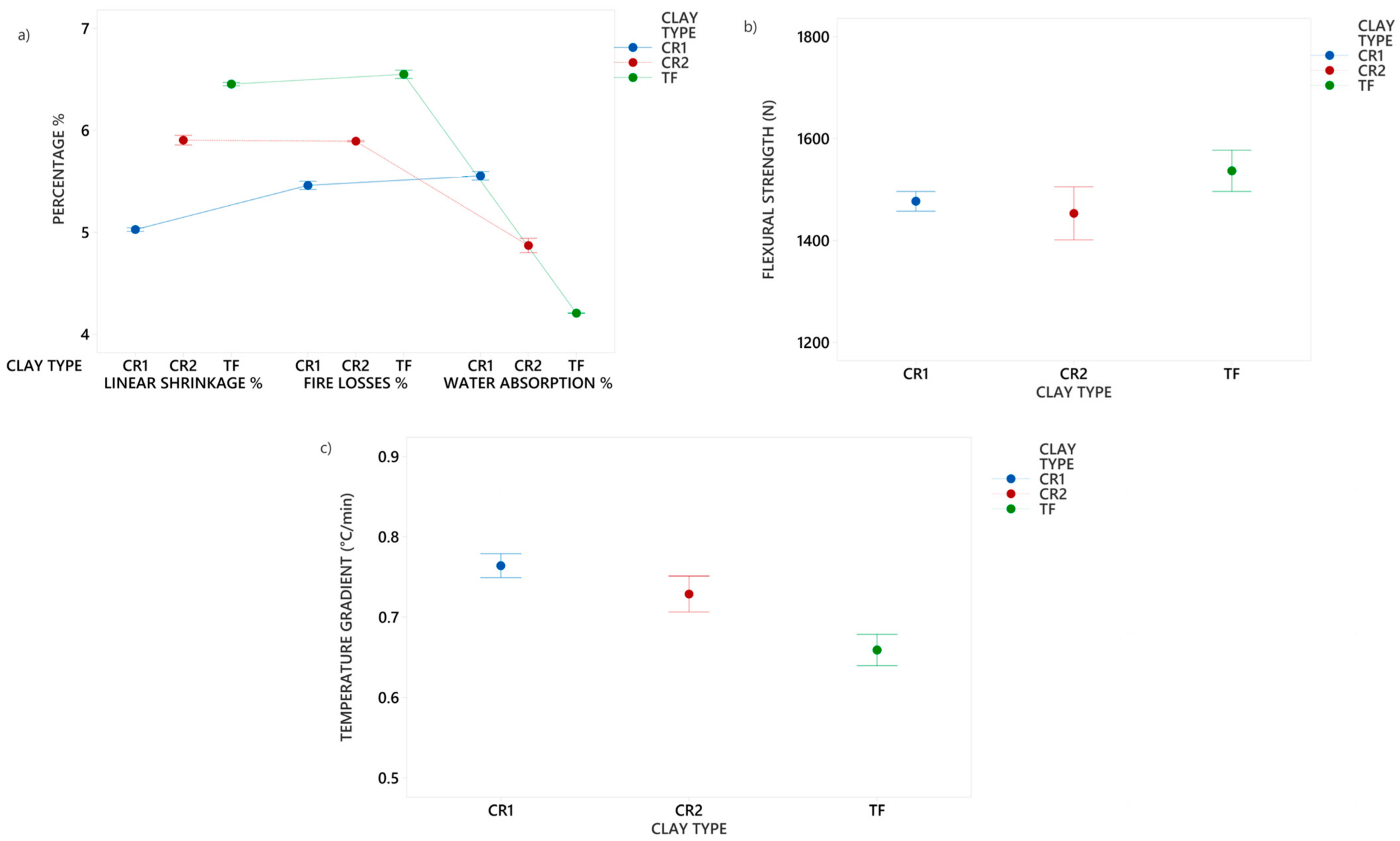
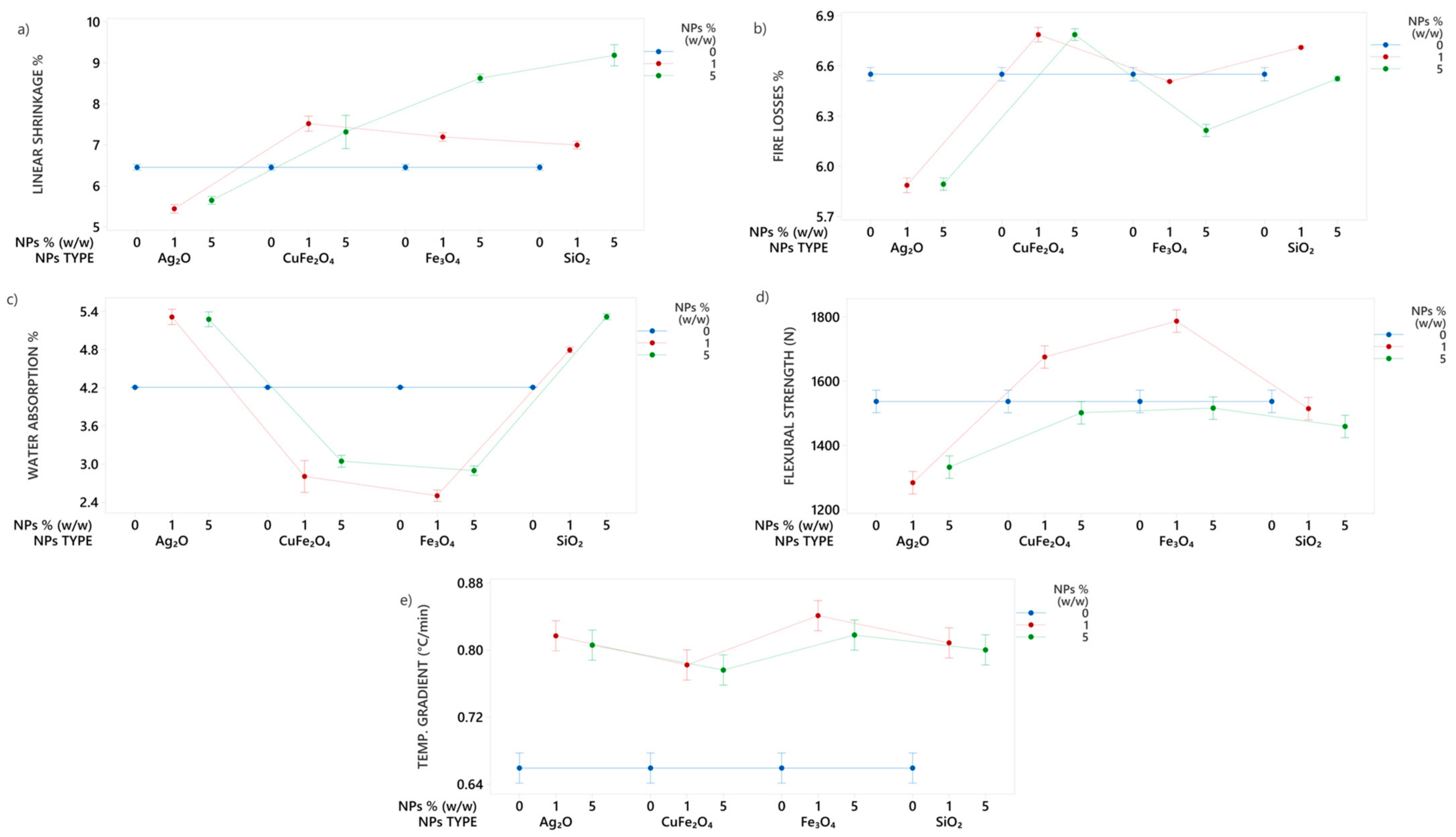


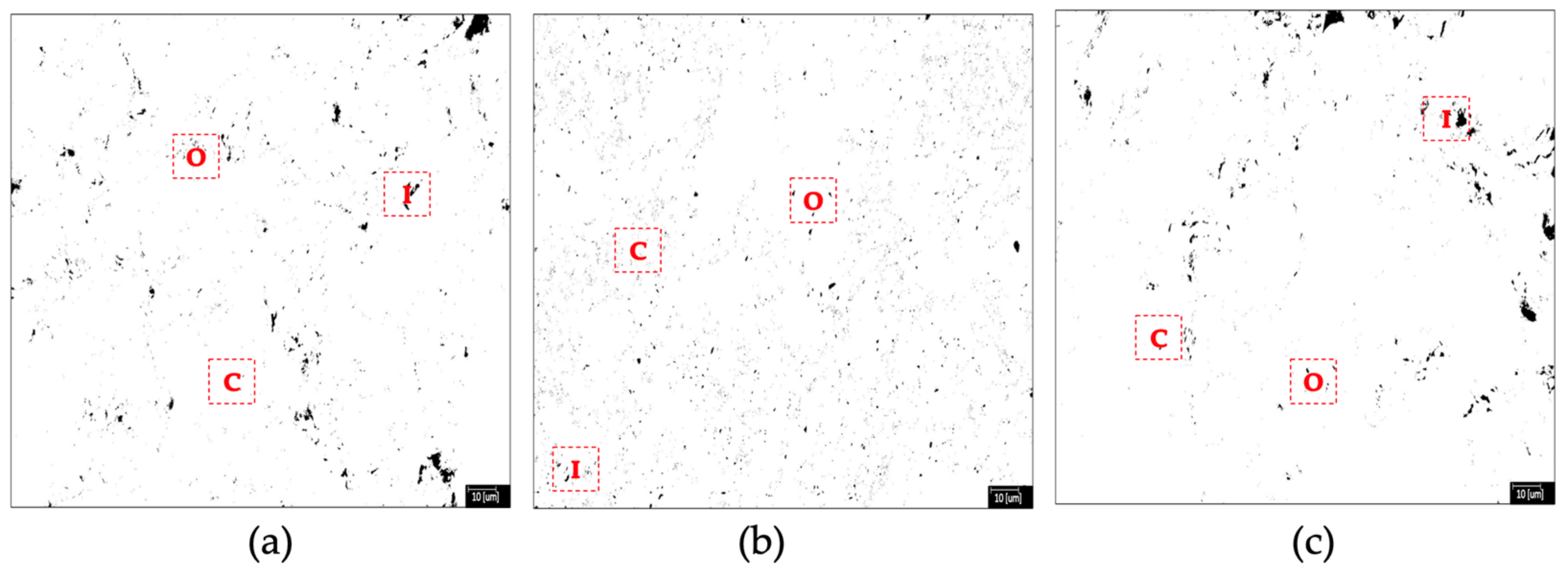

| Clays | |||
|---|---|---|---|
| CR | CR2 | TF | |
| Particle size Oxide Compound (wt.%) | 12.7 mm > 22.0% > 7.9 mm | ||
| 7.9 mm > 4.6% > 6.4 mm | |||
| 6.4 mm > 7.8% > 4.0 mm | |||
| 4.0 mm > 33.6%> 1.0 mm | |||
| 1.0 mm < 32.0% | |||
| SiO2 | 68.05 | 65.47 | 63.84 |
| Al2O3 | 17.73 | 19.33 | 20.76 |
| CaO | 0.23 | 0.23 | 0.39 |
| MgO | 0.64 | 0.68 | 0.70 |
| Na2O | 0.25 | 0.20 | 0.19 |
| K2O | 1.80 | 1.90 | 2.05 |
| Fe2O3 | 4.33 | 4.41 | 4.05 |
| TiO2 | 0.94 | 0.97 | 0.88 |
| SO3 | 0.04 | 0.03 | 0.04 |
| V2O5 | 0.03 | 0.04 | 0.05 |
| P2O5 | 0.09 | 0.10 | 0.09 |
| ZrO2 | 0.05 | 0.04 | 0.04 |
| SrO | 0.04 | 0.04 | 0.04 |
| Mn0 | 0.04 | 0.04 | 0.02 |
| ZnO | 0.03 | 0.03 | 0.04 |
| Cr2O3 | 0.02 | 0.02 | 0.02 |
| LOI | 5.67 | 6.49 | 6.79 |
| Method | Inventory | Amount | Unit | |
|---|---|---|---|---|
| Laboratory-scale process | Reagents preparation | Inputs | ||
| Clay | 500 | g | ||
| Water | 269 | g | ||
| Sodium silicate | 4 | g | ||
| Magnetite | 5 | g | ||
| Electricity (Weighing machine) | 0.0001 | kWh | ||
| Electricity (Grinder Speedy) | 0.026 | kWh | ||
| Electricity (Drying oven) | 1.840 | kWh | ||
| Powder conformation | Water | 25.20 | g | |
| Electricity (Grinder ct-12061) | 0.01 | kWh | ||
| Test tile conformation | Electricity (Manual press) | 0.24 | kWh | |
| Electricity (Drying oven) | 2.484 | kWh | ||
| Test tile firing | Electricity (Oven) | 24.29 | kWh | |
| Outputs | ||||
| Test tile | 420 | g |
| Mineral Phases wt.% | Clays | ||
|---|---|---|---|
| CR1 | CR2 | TF | |
| Quartz | 42 | 41 | 36 |
| Kaolinite 1A | 24 | 27 | 45 |
| Microcline | 2 | 2 | 2 |
| Anatase | <1.0 | 2 | 1 |
| Nacrita-2M2 | 6 | 4 | 3 |
| Muscovite-2M1, ferric | 23 | 23 | 11 |
| Albite | 2 | 1 | 1 |
| Hematite | <1.0% | <1.0% | <1.0% |
| Clay + Fe3O4 NPs | Total Porosity % | Average Size µm |
|---|---|---|
| TF—0% | 1.420 | 0.220 |
| TF—1% | 1.246 | 0.068 |
| TF—5% | 1.384 | 0.304 |
| Impact Category | Abbreviation | Reference Unit | Clays at 0% (w/w) Fe3O4 NPs | Clays at 1%(w/w) Fe3O4 NPs |
|---|---|---|---|---|
| Fine particulate matter formation | FPMF | kg PM2.5 eq | 0.30 | 0.24 |
| Fossil resource scarcity | FRS | kg oil eq | 39.30 | 31.57 |
| Freshwater ecotoxicity | FE | kg 1,4-DCB | 33.31 | 26.54 |
| Freshwater eutrophication | FEP | kg P eq | 0.04 | 0.04 |
| Global warming | GW | kg CO2 eq | 216.51 | 173.20 |
| Human carcinogenic toxicity | HCT | kg 1,4-DCB | 10.38 | 8.37 |
| Human non-carcinogenic toxicity | HNCT | kg 1,4-DCB | 187.36 | 150.88 |
| Ionizing radiation | IR | kBq Co-60 eq | 0.95 | 0.87 |
| Land use | LU | m2a crop eq | 0.39 | 0.35 |
| Marine ecotoxicity | ME | kg 1,4-DCB | 40.74 | 32.47 |
| Marine eutrophication | MEP | kg N eq | 0.00 | 0.00 |
| Mineral resource scarcity | MRS | kg Cu eq | 0.56 | 0.45 |
| Ozone formation, Human health | OF-HH | kg NOx eq | 0.32 | 0.26 |
| Ozone formation, Terrestrial ecosystems | OF-TE | kg NOx eq | 0.33 | 0.26 |
| Stratospheric ozone depletion | SOD | kg CFC11 eq | 0.00 | 0.00 |
| Terrestrial acidification | TA | kg SO2 eq | 0.90 | 0.72 |
| Terrestrial ecotoxicity | TE | kg 1,4-DCB | 849.10 | 683.75 |
| Water consumption | WC | m3 | 4.21 | 3.363 |
| Type of Nanoparticles | Applications | Particle Size (nm) | w/w % | Results | Author | |
|---|---|---|---|---|---|---|
| Body Tile | Coating | |||||
| TiO2 | Self-cleaning ceramic tiles | 9–67 | 5 | Self-cleaning ceramic tiles | Da Silva et al. [28] | |
| SiO2-TiO2 | Self-cleaning ceramic tiles | 5 | 3.4 | Self-cleaning coatings and photocatalytic ceramic | Ferreira-Neto et al. [29] | |
| Ag-TiO2 | Photoactive tiles | 110–130 | 8 | Oxidate inactivation of SARS-CoV-2 | Djellabi et al. [30] | |
| Ag-TiO2 | Photocatalytic antibacterial tile | 110–130 | 1, 4 and 8 | Photocatalytic ability | Bianchi et al. [31] | |
| ZnO | Glazed ceramic tiles. | 38 | 10 | Photocatalytic activity | Guzman-Carrillo et al. [32] | |
| SnO2, ZnO, Sb2O3 | Glazed ceramic countertop. | 50–100 | 0.5–6 | Excellent antibacterial and antistatic properties | Ke et al. [33] | |
| SiO2, Ge | Glazed porcelain tiles | Not available | 2–3 | Odorless and free toxics | Liu et al. [34] | |
| Cu(NO3)2 | Anti-microbial coatings | Not available | 0.03 to 3 | Stable at temperatures up to at 1350 °C | Darragh et al. [35] | |
| Au, Ag, Cu, Pl, Ti | Inkjet ink for a ceramic substrate | 5.0–90 | 0.05 to 0.8 | Better fixed on a ceramic substrate | Hiromichi et al. [36] | |
| Mg | Photocatalytic antibacterial tile | 50 | 0.5–5 | Level 0 anti-mildew | Wang et al. [37] | |
| SiO2, TiO2 | Photocatalytic antibacterial tile | 10 | 50–90 | Excellent photocatalytic activity even after 1000 °C | Ferreira-Neto et al. [38] | |
| Cu | Glazed ceramic tiles. | 30–90 | 3 | Antibacterial efficiency of 99.9% | Kim et al. [39] | |
| SiO2 | Pottery and porcelain clays | 10 | 0–3 | Increase in flexural strength 0.5–2.5 Mpa | Chen et al. [40] | |
| PbO | Ceramic tiles for γ-ray shielding | 29.64 | 0–10 | Increase in modulus of rupture 2.674 Mpa | Mahmoud et al. [41] | |
| Al2O3 and SiO2 | Porcelain stoneware | 20–30 | 0.25–1 | Compressive strength 55.37 and 65.27 Mpa | De la Garza et al. [42] | |
| Fe2O3 | Red stoneware | Not available | 3–7 | Flexural strength 30 Mpa | Nawaukkaratharnant et al. [43] | |
| Si, Ca, Na, Mg, B, Zn, Al, P | Red and porcelain stoneware | 50–500 | 0.2–5 | Sintering temperature reduction up to 13% | Fix-Fierro et al. [44] | |
| AgO2 | Red stoneware | 15 | 1–5 | Increase water absorption up to 26%, decrease flexural strength up to 13% and increase of temperature gradient up to 24%. | This study | |
| CuFe2O4 | Red stoneware | 100 | 1–5 | Decrease water absorption up to 33%, increase flexural strength up to 9% and increase temperature gradient up to 18%. | This study | |
| Fe3O4 | Red stoneware | 88.59 | 1–5 | Decrease water absorption up to 40%, increase flexural strength up to 16% and increase temperature gradient up to 32%. | This study | |
| SiO2 | Red stoneware | 20 | 1–5 | Increase water absorption up to 28%, decrease flexural strength up to 16% and increase of temperature gradient up to 21%. | This study | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra, E.L.; Osma, J.F. Impact of Nanoparticle Additions on Life Cycle Assessment (LCA) of Ceramic Tiles Production. Nanomaterials 2024, 14, 910. https://doi.org/10.3390/nano14110910
Saavedra EL, Osma JF. Impact of Nanoparticle Additions on Life Cycle Assessment (LCA) of Ceramic Tiles Production. Nanomaterials. 2024; 14(11):910. https://doi.org/10.3390/nano14110910
Chicago/Turabian StyleSaavedra, Euler L., and Johann F. Osma. 2024. "Impact of Nanoparticle Additions on Life Cycle Assessment (LCA) of Ceramic Tiles Production" Nanomaterials 14, no. 11: 910. https://doi.org/10.3390/nano14110910
APA StyleSaavedra, E. L., & Osma, J. F. (2024). Impact of Nanoparticle Additions on Life Cycle Assessment (LCA) of Ceramic Tiles Production. Nanomaterials, 14(11), 910. https://doi.org/10.3390/nano14110910








