New Approach to Synthesizing Cathode PtCo/C Catalysts for Low-Temperature Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
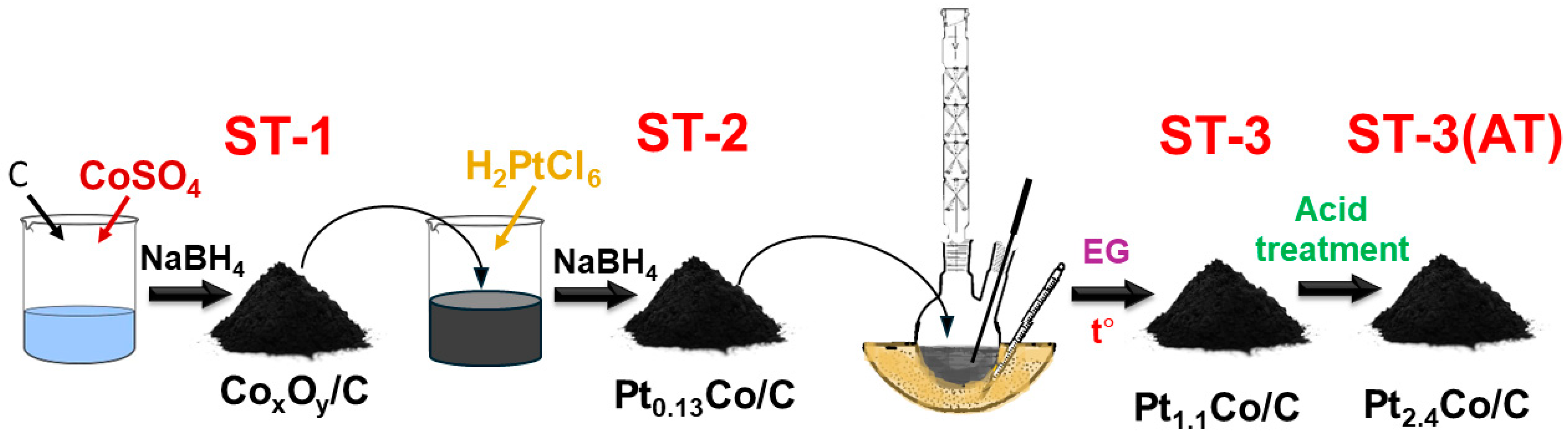
2.2. The Preparation of PtCo/C Catalysis by Multi-Step Synthesis
2.3. Structural Study for Synthesized PtCo/C Catalysts
2.4. Activity Study for Synthesized PtCo/C Catalysts
2.4.1. Preparation of Catalytic Ink
2.4.2. Formation of Catalytic Layer at RDE
2.4.3. ESA Determination
- ERHE—potential value relative to the reversible hydrogen electrode (RHE), V;
- E—is the set value of the potential, V;
- ESCE—potential value of silver chloride electrode (Ag/AgCl), V;
- EpH—correction for solution pH, for 0.1 M HClO4 equal to 0.059 V at 25 °C, V;
- iR—is the ohmic potential drop equal to the product of the current strength and the resistance of the solution layer between the reference electrode and the studied one.
2.4.4. ORR Activity Determination
2.4.5. Assessment of Catalyst Stability
2.5. Activity Study for Catalysts in MEAs
2.5.1. Preparation of Catalysts for MEAs
2.5.2. Preparation of MEAs
2.5.3. Testing of Single Fuel Cells
3. Results
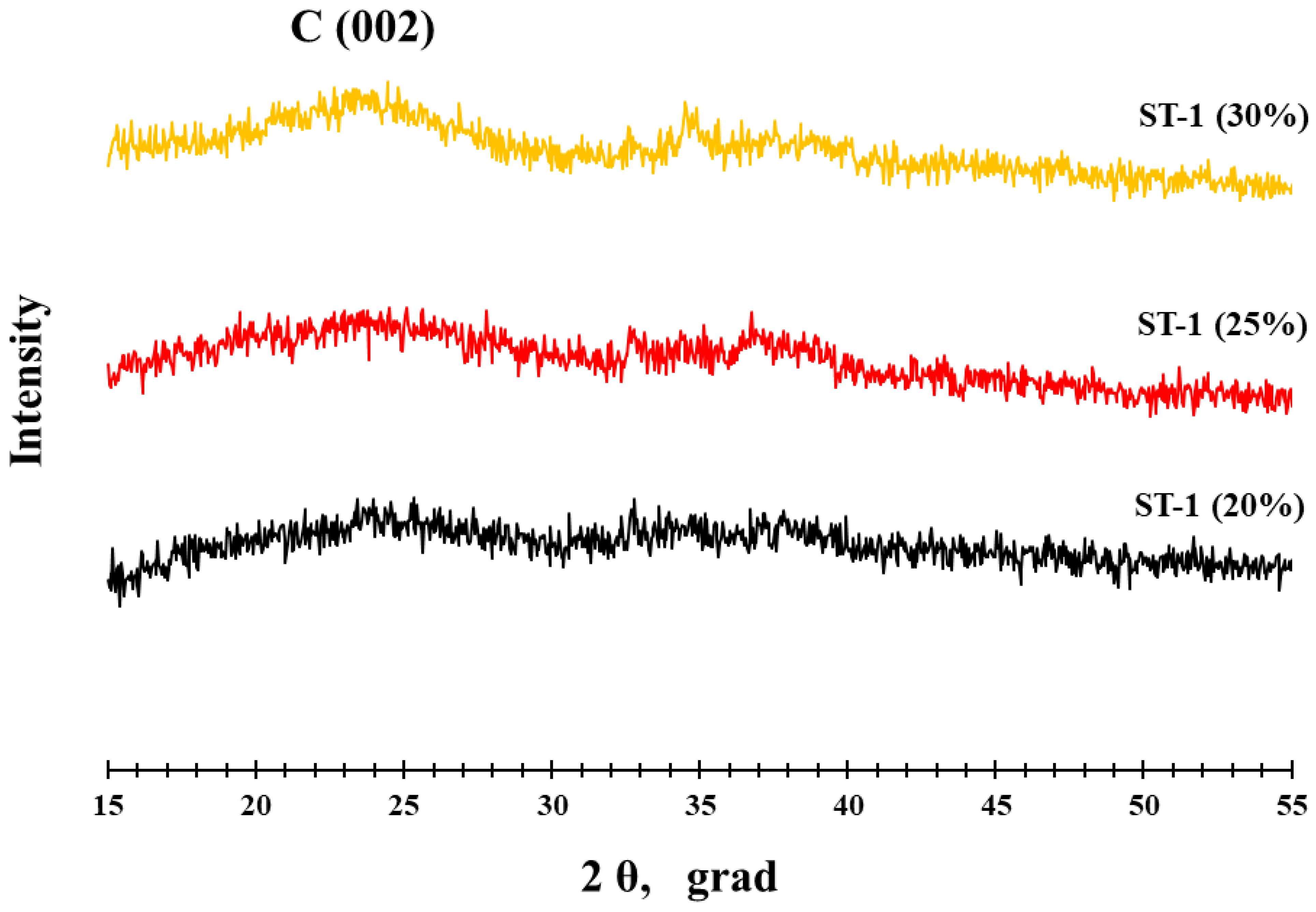
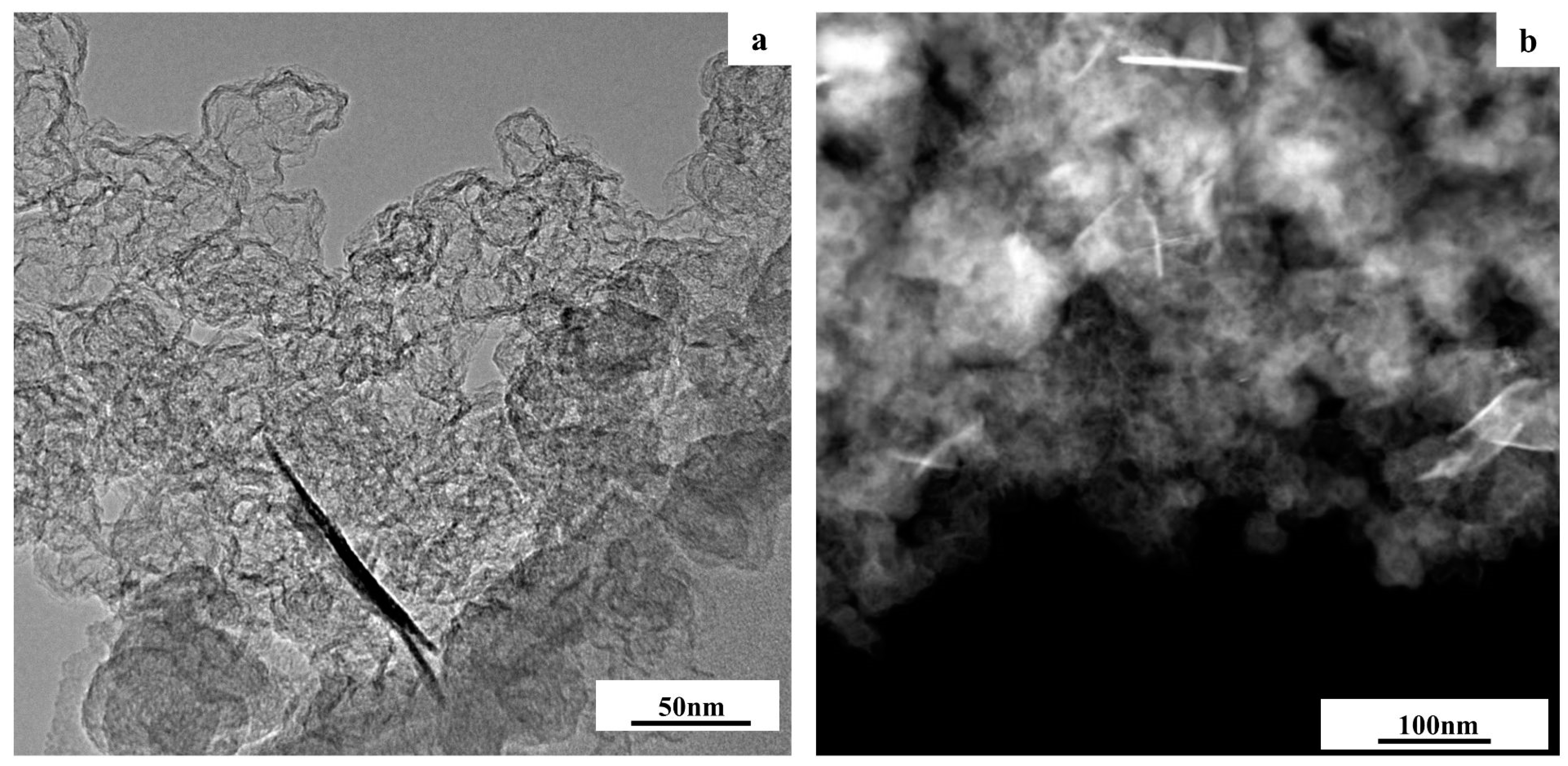
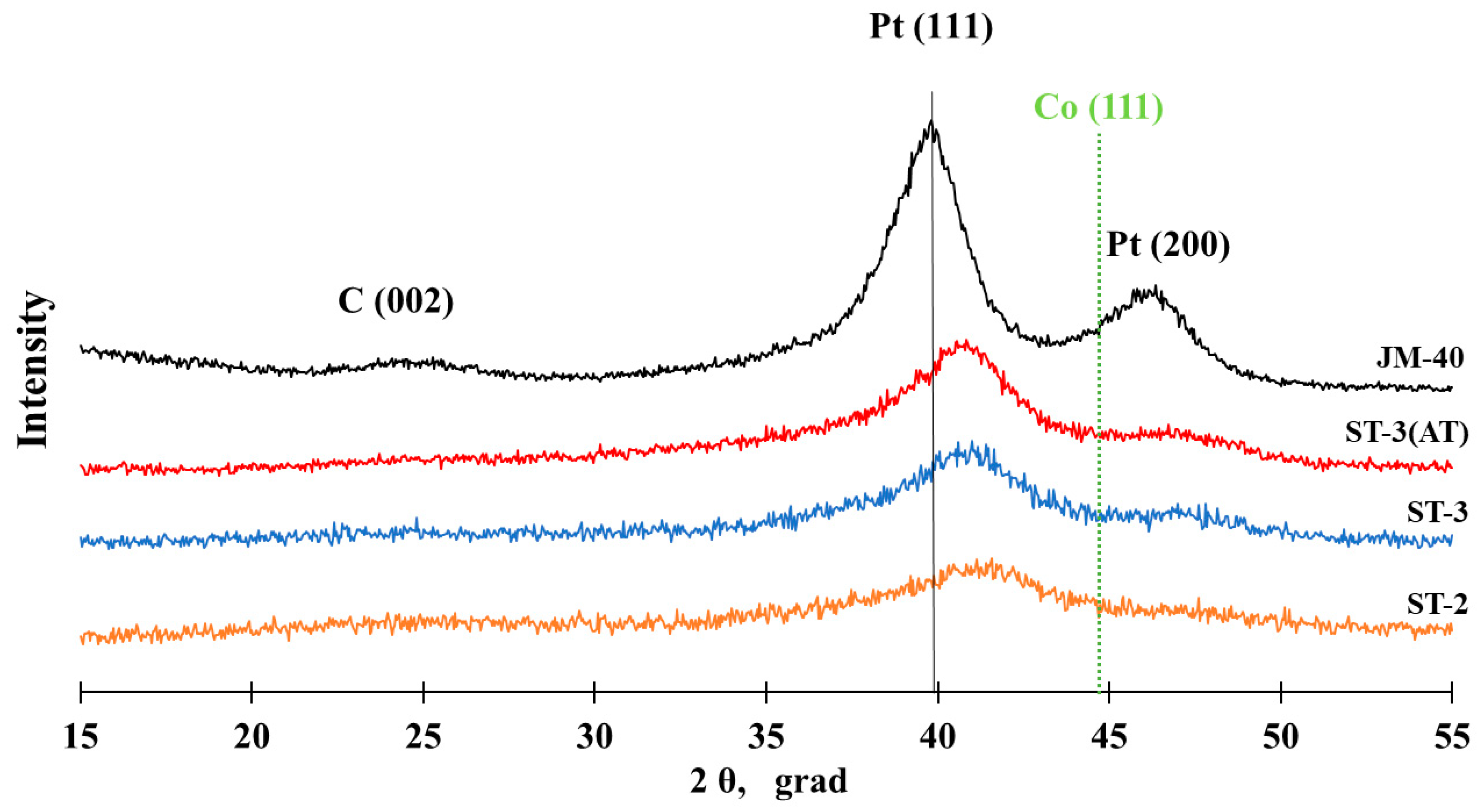
3.1. Structural Characteristics of PtCo/C Catalysts Synthesized with Three-Step Method
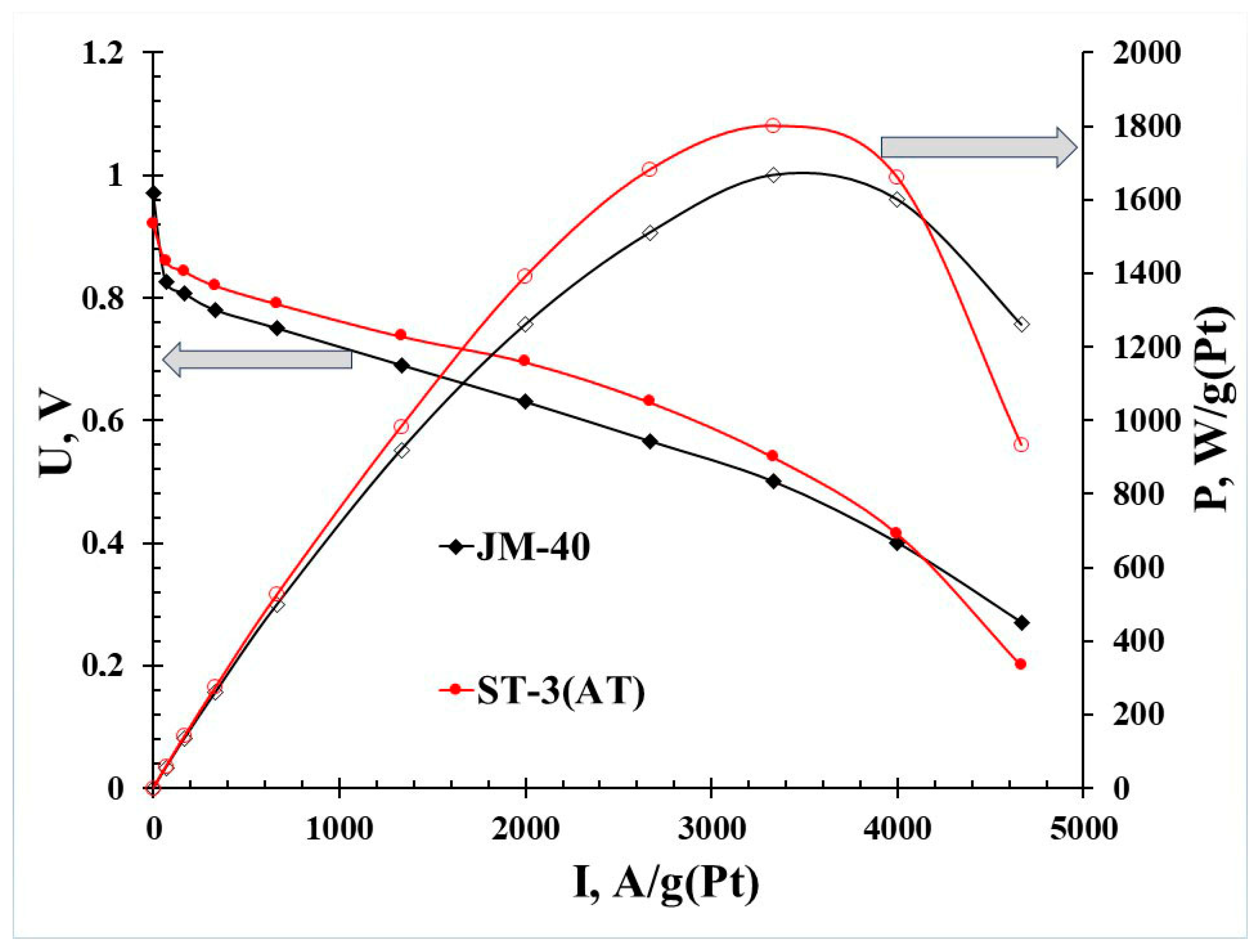
3.2. Catalytic Activity of PtCo/C Catalysts Synthesized with Three-Step Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorensen, B. Hydrogen and Fuel Cells Emerging Technologies and Applications; Academic Press: Oxford, UK; Burlington, NC, USA, 2012; pp. 1–492. [Google Scholar]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Zalitis, C.; Kucernak, A.; Lin, X.; Sharman, J. Electrochemical Measurement of Intrinsic Oxygen Reduction Reaction Activity at High Current Densities as a Function of Particle Size for Pt4−xCox/C (x = 0,1,3) Catalysts. ACS Catal. 2020, 10, 4361–4376. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Guo, W.; Xiao, S.; Wang, L.; OuYang, Y.; Gao, P. The mechanism of Co oxyhydroxide nano-islands deposited on a Pt surface to promote the oxygen reduction reaction at the cathode of fuel cells. RSC Adv. 2020, 10, 44719–44727. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, O.; Romar, H.; Lassi, U.; Kallio, T. Co-electrodeposited mesoporous PtM (M = Co, Ni, Cu) as an active catalyst for oxygen reduction reaction in a polymer electrolyte membrane fuel cell. Electrochim. Acta. 2017, 230, 49–57. [Google Scholar] [CrossRef]
- Xiang, L.; Hu, Y.; Zhao, Y.; Cao, S.; Kuai, L. Carbon-Supported High-Loading Sub-4 nm PtCo Alloy Electrocatalysts for Superior Oxygen Reduction Reaction. Nanomaterials 2023, 13, 2367. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; You, L.X.; Zhang, X.F.; Feng, J.J.; Zhang, L.; Wang, A.J. L-proline assisted solvothermal preparation of Cu-rich rhombic dodecahedral PtCu nanoframes as advanced electrocatalysts for oxygen reduction and hydrogen evolution reactions. Electrochim. Acta 2019, 299, 89–97. [Google Scholar] [CrossRef]
- Kwon, T.; Jun, M.; Kim, H.Y.; Oh, A.; Park, J.; Baik, H.; Lee, K. Vertex-Reinforced PtCuCo Ternary Nanoframes as Efficient and Stable Electrocatalysts for the Oxygen Reduction Reaction and the Methanol Oxidation Reaction. Adv. Funct. Mater. 2018, 28, 1706440. [Google Scholar] [CrossRef]
- Oezaslan, M.; Strasser, P. Activity of dealloyed PtCo3 and PtCu3 nanoparticle electrocatalyst for oxygen reduction reaction in polymer electrolyte membrane fuel cell. J. Power Sources 2011, 196, 5240–5249. [Google Scholar] [CrossRef]
- Schmidt, D.; Asara, G.; Baletto, F. A kinetic Monte Carlo-blueprint for oxygen reduction on oxide-supported PtNi nanoalloys. J. Chem. Phys. 2020, 152, 034107. [Google Scholar] [CrossRef]
- Khalakhan, I.; Vega, L.; Vorokhta, M.; Skála, T.; Viñes, F.; Yakovlev, Y.V.; Matolínová, I. Irreversible structural dynamics on the surface of bimetallic PtNi alloy catalyst under alternating oxidizing and reducing environments. Appl. Catal. B Environ. 2019, 264, 118476. [Google Scholar] [CrossRef]
- Jia, X.; Liu, S.; Huang, L.; Devaraji, P.; Walekar, L.S.; Chen, W.; Mao, L. PtNixCoy Concave Nanocubes: Synthesis, Application in Photocatalytic Hydrogen Generation. Catal. Sci. Technol. 2020, 10, 113–123. [Google Scholar] [CrossRef]
- Travitsky, N.; Ripenbein, T.; Golodnitsky, D.; Rosenberg, Y.; Burshtein, L.; Peled, E. Pt-, PtNi- and PtCo-supported catalysts for oxygen reduction in PEM fuel cells. J. Power Sources 2006, 161, 782–789. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Schmidt, T.J.; Ross, P.N.; Markovic, N.M. Surface Composition Effects in Electro- catalysis: Kinetics of Oxygen Reduction on Well-De- fined Pt3Ni and Pt3Co Alloy Surfaces. J. Phys. Chem. 2002, 106, 11970. [Google Scholar] [CrossRef]
- Xiong, L.; Kannan, A.M.; Manthiram, A. Pt–M (M = Fe, Co, Ni and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells. Electrochem. Commun. 2002, 4, 898. [Google Scholar] [CrossRef]
- Munoz, M.; Ponce, S.; Zhang, G.R.; Etzold, B.J.M. Size-controlled PtNi nanoparticles as highly efficient catalyst for hydrodechlorination reactions. Appl. Catal. B Env. 2016, 192, 1–7. [Google Scholar] [CrossRef]
- Konno, N.; Mizuno, S.; Nakaji, H.; Ishikawa, Y. Development of compact and high- performance fuel cell stack. SAE Int. J. Altern. Powertains 2015, 4, 123–129. [Google Scholar] [CrossRef]
- Xu, W.C.; Zhang, Z.M.; Yang, C.H.; Zhao, K.M.; Wang, Y.; Tian, N.; Zhou, Z.Y.; Sun, S.G. Promotion mechanism of PtCo intermetallic ordered alloys in oxygen reduction reaction and its application in fuel cells. Electrochem. Commun. 2023, 152, 107516. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Jayakumar, A.; Palanichamy, K. Evaluation of Pt-Co Nano-Catalyzed Membranes for Polymer Electrolyte Membrane Fuel Cell Applications. Energies 2023, 16, 7713. [Google Scholar] [CrossRef]
- Kaewsai, D.; Piumsomboon, P.; Pruksathorn, K.; Hunsom, M. Synthesis of polyaniline-wrapped carbon nanotube-supported PtCo catalysts for proton exchange membrane fuel cells: Activity and stability tests. RSC Adv. 2017, 7, 20801–20810. [Google Scholar] [CrossRef]
- Koh, S.; Toney, M.F.; Strasser, P. Activity–stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR). Electrochim. Acta 2007, 52, 2765–2774. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, L.; Zhu, F.; Cheng, X.; Yang, F.; Shen, S.; Zhang, J. Degradation of core-shell Pt3Co catalysts in proton exchange membrane fuel cells (PEMFCs) studied by mathematical modeling. Electrochim. Acta 2019, 323, 134751. [Google Scholar] [CrossRef]
- Chen, S.; Sheng, W.; Yabuuchi, N.; Ferreira, P.J.; Allard, L.F.; Shao-Horn, Y. Origin of Oxygen Reduction Reaction Activity on “Pt3Co” Nanoparticles: Atomically Resolved Chemical Compositions and Structures. J. Phys. Chem. C 2008, 113, 1109–1125. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Mi, B.; Wang, H. Self-Assembly of Pt3Co Superlattice as a Catalyst for Oxygen Reduction Reaction. Catalysts 2023, 13, 406. [Google Scholar] [CrossRef]
- Mondal, A.; De, A.; Datta, J. Selective methodology for developing PtCo NPs and performance screening for energy efficient electro-catalysis in direct ethanol fuel cell. Int. J. Hydrog. Energy 2019, 44, 10996–11011. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Gao, F.; Blunier, B.; Miraoui, A. Proton Exchange Membrane Fuel Cell Modeling; ISTE: London, UK; Hoboken, NJ, USA, 2012; pp. 1–239. [Google Scholar]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhu, X.Y.; Feng, J.J.; Wang, A.J. Solvothermal synthesis of N-doped graphene supported PtCo nanodendrites with highly catalytic activity for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 428, 798–808. [Google Scholar] [CrossRef]
- Meng, H.B.; Zhang, X.F.; Pu, Y.L.; Chen, X.L.; Feng, J.J.; Han, D.M.; Wang, A.J. One-pot solvothermal synthesis of reduced graphene oxide-supported uniform PtCo nanocrystals for efficient and robust electrocatalysis. J. Colloid Interface Sci. 2019, 543, 17–24. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Z.; Chen, H.; Wang, S.; Li, X.; Zhang, X.; Shen, P.K. Ultrathin PtCo nanorod assemblies with self-optimized surface for oxygen reduction reaction. J. Electroanal. Chem. 2020, 870, 114194–114201. [Google Scholar] [CrossRef]
- Yan, W.; Sun, P.; Luo, C.; Xia, X.; Liu, Z.; Zhao, Y.; Zhang, S.; Sun, L.; Du, F. PtCo-based nanocatalyst for oxygen reduction reaction: Recent highlights on synthesis strategy and catalytic mechanism. Chin. J. Chem. Eng. 2023, 53, 101–123. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, B.; Jiang, K.; Cai, W.B. Surfactant-Free Synthesis of Carbon-Supported Palladium Nanoparticles and Size-Dependent Hydrogen Production from Formic Acid–Formate Solution. ACS Appl. Mater. Interfaces 2017, 9, 24678–24687. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, C.; Yang, Y.; Liu, G.; Cai, W.B. An alternate aqueous phase synthesis of the Pt3Co/C catalyst towards efficient oxygen reduction reaction. Chin. J. Catal. 2019, 40, 1895–1903. [Google Scholar] [CrossRef]
- Grolleau, C.; Coutanceau, C.; Pierre, F.; Leger, J.M. Optimization of a surfactant free polyol method for the synthesis of platinum–cobalt electrocatalysts using Taguchi design of experiments. J. Power Sources 2010, 195, 1569–1576. [Google Scholar] [CrossRef]
- Miyatake, K.; Shimizu, Y. Pt/Co Alloy Nanoparticles Prepared by Nanocapsule Method Exhibit a High Oxygen Reduction Reaction Activity in the Alkaline Media. ACS Omega 2017, 2, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Rong, C.; Chu, D. Surface coverage of Pt atoms on PtCo nanoparticles and catalytic kinetics for oxygen reduction. Electrochim. Acta 2011, 56, 2532–2540. [Google Scholar] [CrossRef]
- Hou, F.; Zhao, H.; Song, H.; Chou, L.; Zhao, J.; Yang, J.; Yan, L. Effect of impregnation strategy on catalytic hydrogenation behavior of PtCo catalysts supported on La2O2CO3 nanorods. J. Rare Earths 2018, 36, 965–973. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Wang, J.C.; Liang, X. PtCo/MWCNTs Prepared by a Microwave-assisted Polyol Method for Selective Cinnamaldehyde Hydrogenation. ChemNanoMat 2023, 9, e202300294. [Google Scholar] [CrossRef]
- Skibina, L.M.; Mauer, D.K.; Volochaev, V.A.; Guterman, V.E. Nanostructured Cobalt-Containing Carbon Supports for New Platinum Catalysts. Russ. J. Electrochem. 2019, 55, 438–448. [Google Scholar] [CrossRef]
- Mauer, D.; Belenov, S.; Guterman, V.; Nikolsky, A.; Kozakov, A.; Nikulin, A.; Alexeenko, D.; Safronenko, O. Gram-Scale Synthesis of CoO/C as Base for PtCo/C High-Performance Catalysts for the Oxygen Reduction Reaction. Catalysts 2021, 11, 1539. [Google Scholar] [CrossRef]
- Dutta, I.; Carpenter, M.K.; Balogh, M.P.; Ziegelbauer, J.M.; Moylan, T.E.; Atwan, M.H.; Irish, N.P. Electrochemical and structural study of a chemically dealloyed PtCu oxygen reduction catalyst. J. Phys. Chem. C 2010, 114, 16309–16320. [Google Scholar] [CrossRef]
- Sohn, Y.; Park, J.H.; Kim, P.; Joo, J.B. Dealloyed PtCu catalyst as an efficient electrocatalyst in oxygen reduction reaction. Curr.Appl. Phys. 2015, 15, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; O’Malley, R.; Theobald, B.; Strasser, P. Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 2013, 13, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures from 376 Polycrystalline and Amorphous Materials; John Wiley: New York, NY, USA, 1974. [Google Scholar]
- Moguchikh, E.A.; Alekseenko, A.A.; Pankov, I.V.; Alekseenko, D.V.; Guterman, V.E. Changes in the Microstructure and Electrochemical Behavior of Pt/C Electrocatalysts under Various Stress Testing Conditions. Nanobiotechnol. Rep. 2023, 18, S301–S315. [Google Scholar] [CrossRef]
- Pavlets, A.S.; Alekseenko, A.A.; Pankov, I.V.; Belenov, S.V.; Guterman, V.E. Memory Effect: How the Initial Structure of Nanoparticles Affects the Performance of De-Alloyed PtCu Electrocatalysts? Energies 2022, 15, 9643. [Google Scholar] [CrossRef]
- Moguchikh, E.; Paperzh, K.; Pankov, I.; Belenov, S.; Alekseenko, A. Durability of Commercial Catalysts within Relevant Stress Testing Protocols. Catalysts 2023, 13, 923. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cardona, J.; Sirés, I.; Alcaide, F.; Brillas, E.; Centellas, F.; Cabot, P.L. Electrochemical Performance of Carbon-Supported Pt(Cu) Electrocatalysts for Low-Temperature Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 20582–20593. [Google Scholar] [CrossRef]
- Favilla, P.C.; Acosta, J.J.; Schvezov, C.E.; Sercovich, D.J.; Collet-Lacos, J.R. Size control of carbon-supported platinum nanoparticles made using polyol method for low temperature fuel cells. Chem. Eng. Sci. 2013, 101, 27–34. [Google Scholar] [CrossRef]
- Chattot, R.; Martens, I.; Scohy, M.; Herranz, J.; Drnec, J.; Maillard, F.; Dubau, L. Disclosing Pt-Bimetallic Alloy Nanoparticle Surface Lattice Distortion with Electrochemical Probes. ACS Energy Lett. 2020, 5, 162–169. [Google Scholar] [CrossRef]
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115. [Google Scholar] [CrossRef]
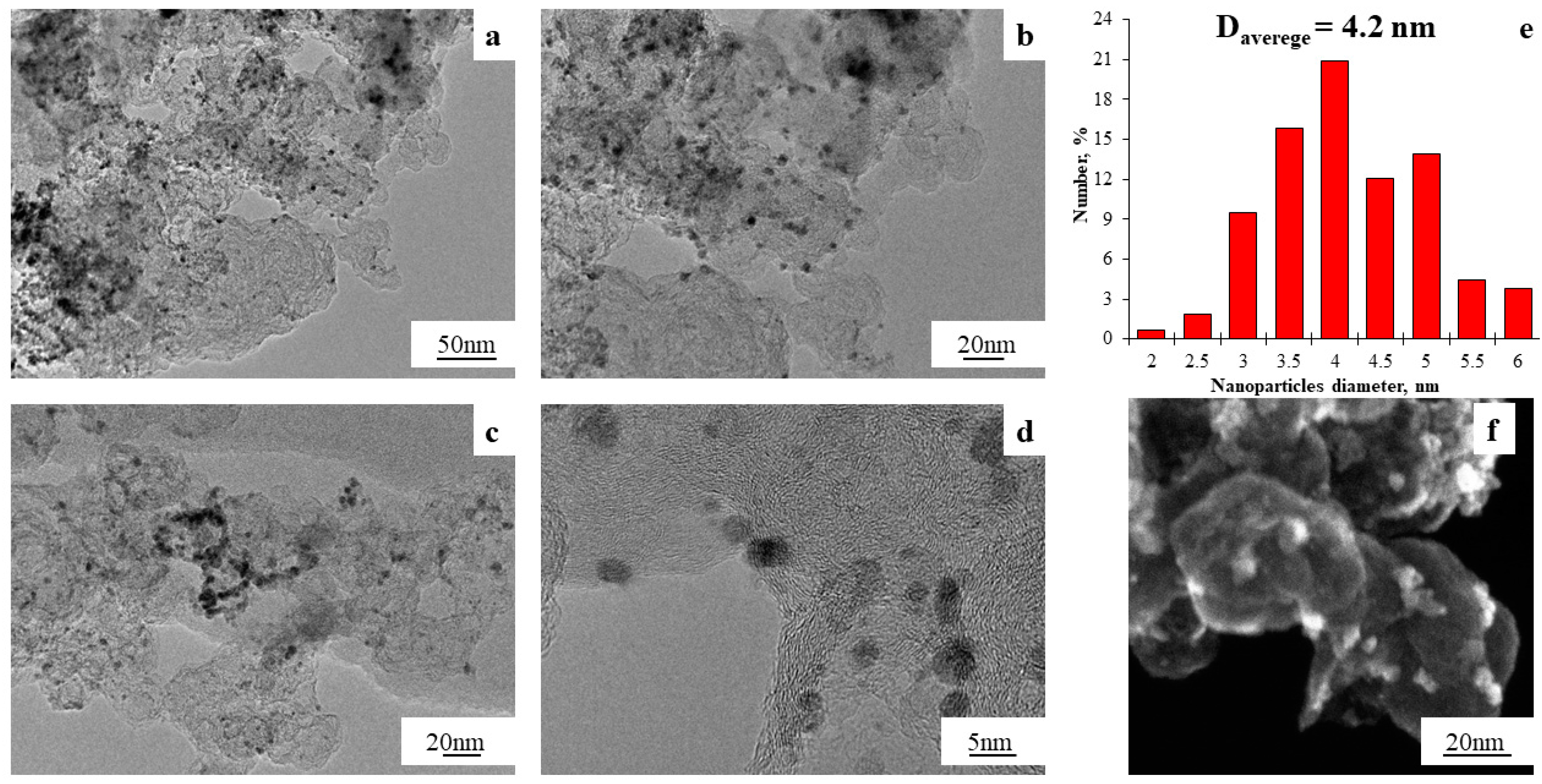
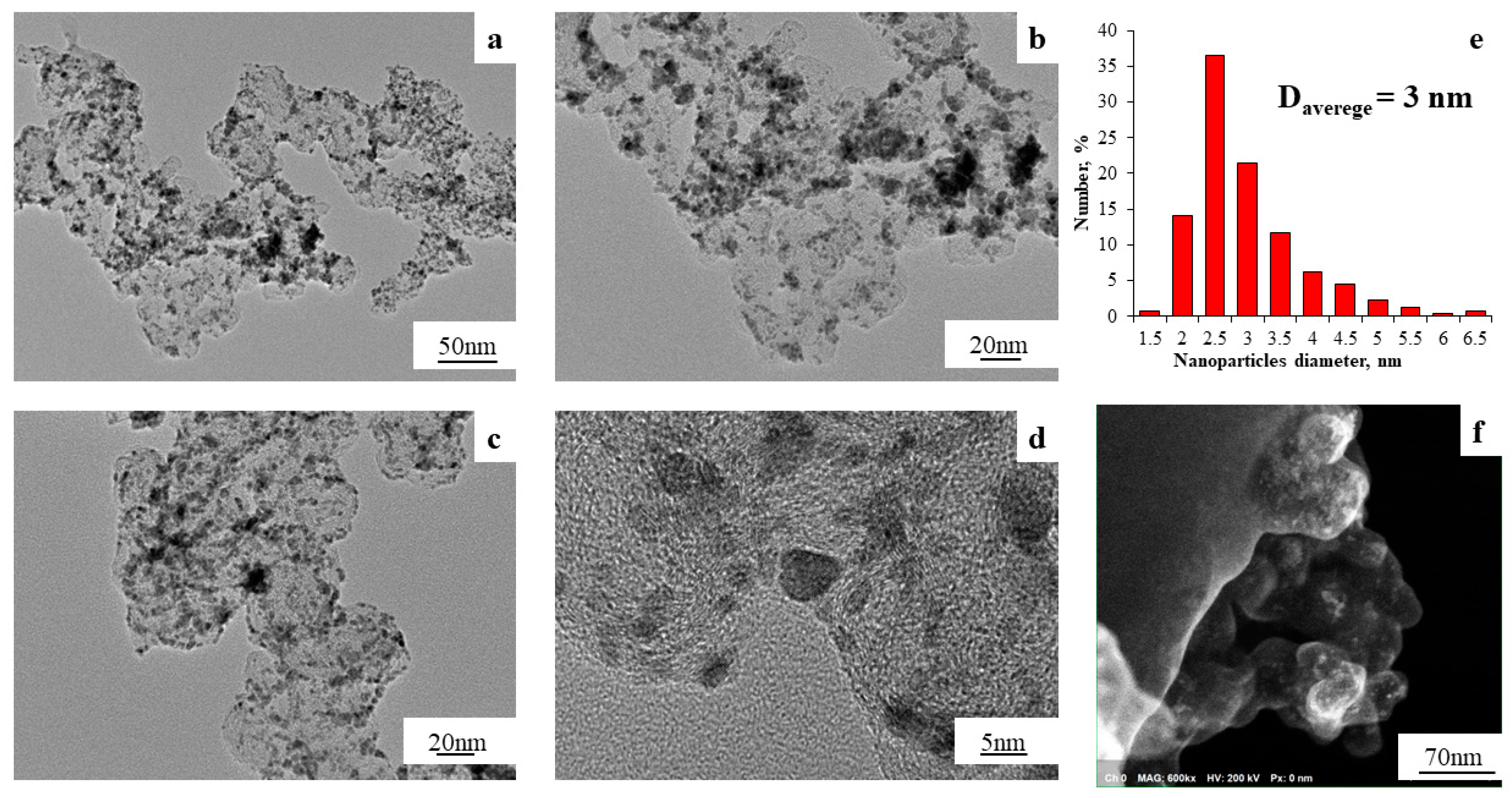
| Designation | Chemical Composition (Theoretical) | Chemical Composition (XRF) | Chemical Composition (XRD) | Pt Mass Fraction (Theoretical), % | Mass Fraction of Metals (Gravimetry), % | Average Crystallite Size (XRD), nm | Average Size of Bimetallic NPs (TEM), nm | Lattice Parameter, Å |
|---|---|---|---|---|---|---|---|---|
| ST-1 | Co | Co | - | - | 24.9 | - | - | - |
| ST-2 | PtCo4.1 | PtCo7.4 | Pt1.3Co | 20 | 45.6 | 1.4 | 4.2 | 3.748 |
| ST-3 | Pt1Co | Pt0.9Co | Pt2.4Co | 40 | 52.9 | 1.8 | 3.0 | 3.815 |
| ST-3(AT) | Pt1Co | Pt2.4Co | Pt3.1Co | 40 | - | 2.6 | 2.7 | 3.836 |
| JM40 | Pt | Pt | Pt | 40 | 40 | 3.2 | 3.3 [48] | 3.923 |
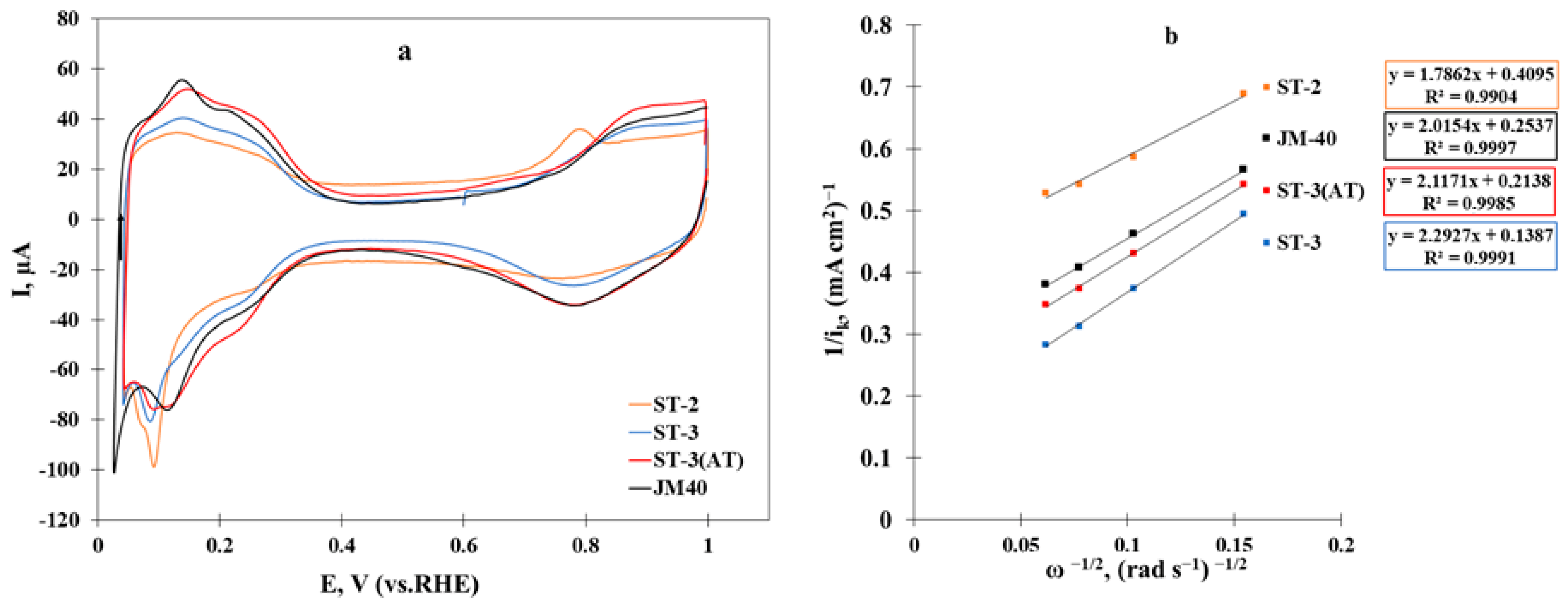
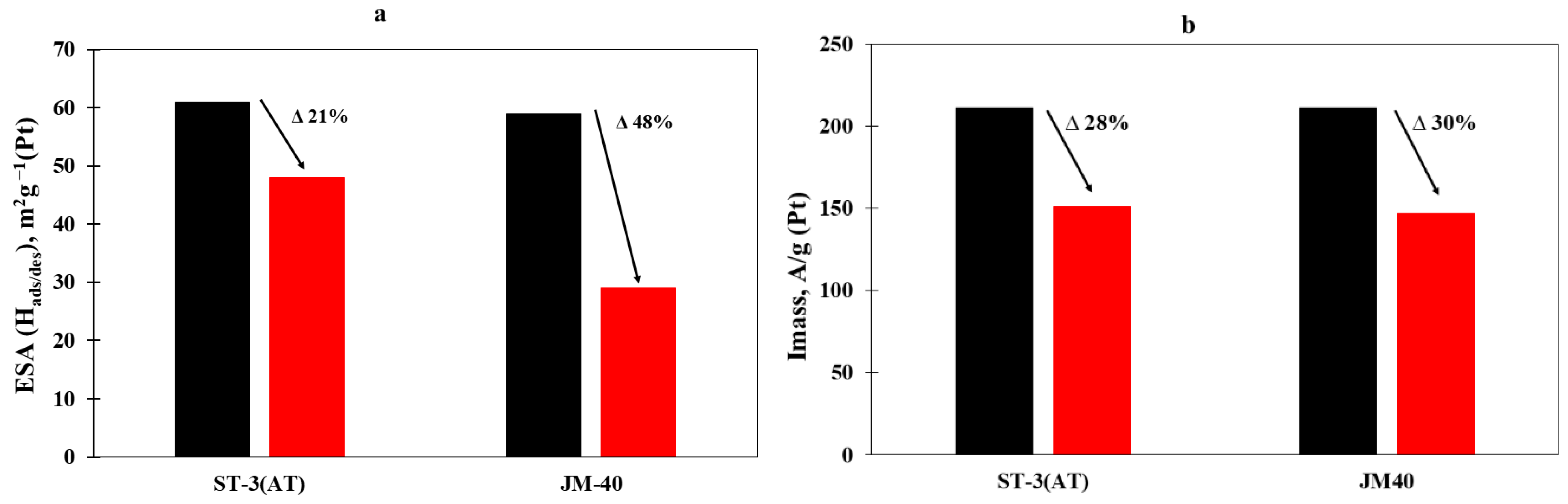
| Material | ESA, m2/g(Pt) | Ik, mA | Imass, A/g(Pt) | Ispec, A/m2(Pt) |
|---|---|---|---|---|
| ST-2 | 37 | 0.48 | 130 | 3.5 |
| ST-3 | 55 | 1.42 | 369 | 6.7 |
| ST-3(AT) | 61 | 0.92 | 211 | 3.5 |
| JM40 | 56 | 0.82 | 211 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belenov, S.; Mauer, D.; Moguchikh, E.; Gavrilova, A.; Nevelskaya, A.; Beskopylny, E.; Pankov, I.; Nikulin, A.; Alekseenko, A. New Approach to Synthesizing Cathode PtCo/C Catalysts for Low-Temperature Fuel Cells. Nanomaterials 2024, 14, 856. https://doi.org/10.3390/nano14100856
Belenov S, Mauer D, Moguchikh E, Gavrilova A, Nevelskaya A, Beskopylny E, Pankov I, Nikulin A, Alekseenko A. New Approach to Synthesizing Cathode PtCo/C Catalysts for Low-Temperature Fuel Cells. Nanomaterials. 2024; 14(10):856. https://doi.org/10.3390/nano14100856
Chicago/Turabian StyleBelenov, Sergey, Dmitriy Mauer, Elizabeth Moguchikh, Anna Gavrilova, Alina Nevelskaya, Egor Beskopylny, Ilya Pankov, Aleksey Nikulin, and Anastasia Alekseenko. 2024. "New Approach to Synthesizing Cathode PtCo/C Catalysts for Low-Temperature Fuel Cells" Nanomaterials 14, no. 10: 856. https://doi.org/10.3390/nano14100856
APA StyleBelenov, S., Mauer, D., Moguchikh, E., Gavrilova, A., Nevelskaya, A., Beskopylny, E., Pankov, I., Nikulin, A., & Alekseenko, A. (2024). New Approach to Synthesizing Cathode PtCo/C Catalysts for Low-Temperature Fuel Cells. Nanomaterials, 14(10), 856. https://doi.org/10.3390/nano14100856










