Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SuperSomes Process and Plant Description
2.3. Characterization Analyses
3. Results and Discussion
3.1. Production of Unloaded Niosomes
3.2. Production of Vancomycin-Loaded Niosomes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bashkar, L.V.K.S.; Nair, P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Song, M.; Aipire, A.; Dilxat, E.; Li, J.; Xia, G.; Jiang, Z.; Fan, Z.; Li, J. Research progress of polysaccharide-gold nanocomplexes in drug delivery. Pharmaceutics 2024, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Baldino, L.; Reverchon, E. Liposomes, transfersomes and niosomes: Production methods and their applications in the vaccinal field. J. Transl. Med. 2024, 22, 339. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Z.; Ju, X.; Deng, F.; Yang, F.; He, R. Co-Encapsulation of rutinoside and β-carotene in liposomes modified by rhamnolipid: Antioxidant activity, antibacterial activity, Storage stability, and in vitro gastrointestinal digestion. J. Food Sci. 2023, 88, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating materials to increase the stability of liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Pan, Y.; Yang, H.; Han, J.; Liu, J.; Liu, W. Specific surface modification of liposomes for gut targeting of food bioactive agents. Comp. Rev. Food Sci. Food Saf. 2023, 22, 3685–3706. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, D.; Thakur, P.; Devi, P.; Sharma, M.D. A comprehensive review on niosomes; targeted drug delivery system. Ymer 2023, 22, 1969–1987. [Google Scholar]
- Du, X.; Huang, X.; Wang, L.; Mo, L.; Jing, H.; Bai, X.; Wang, H. Nanosized niosomes as effective delivery device to improve the stability and bioaccessibility of goat milk whey protein peptide. Food Res. Int. 2022, 161, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.P.; Singh, R.P.; Jain, S.; Mishra, V.; Mahor, S.; Singh, P.; Gupta, P.N.; Rawat, A.; Dubey, P. Non-ionic surfactant based vesicles (niosomes) for non-invasive topical genetic immunization against hepatitis B. Int. J Pharm. 2005, 296, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Teeravatcharoenchai, T.; Connell, D.; Niwasabutra, K.; Hussain, M.; Carte, K.; Ferro, V.A. Examination of the effect of niosome preparation methods in encapsulating model antigens on the vesicle characteristics and their ability to induce immune responses. J. Liposome Res. 2021, 31, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Pamornpathomkul, B.; Niyomtham, N.; Yingyongnarongkul, B.-E.; Prasitpuriprecha, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Cationic niosomes for enhanced skin immunization of plasmid DNA-encoding ovalbumin via hollow microneedles. AAPS PharmSciTech 2018, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Pucci, R.; Abruzzo, P.M.; Canaider, S.; Parolin, C.; Vitali, B.; Valle, F.; Brucale, M.; Cerchiara, T.; Luppi, B.; et al. Azithromycin-loaded liposomes and niosomes for the treatment of skin infections: Influence of excipients and preparative methods on the functional properties. Eur. J. Pharm. Biopharm. 2024, 197, 14233. [Google Scholar] [CrossRef] [PubMed]
- Al-Kofahi, T.; Altrad, B.; Amawi, H.; Aljabali, A.A.; Abul-Haija, Y.M.; Obeid, M.A. Paclitaxel-loaded niosomes in combination with metformin: Development, characterization and anticancer potentials. Ther. Deliv. 2024, 15, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Khambalkar, S.M.; Ghuge, A.D.; Deshmukh, S.P.; Jadhav, K.P.; Jaiswal, R.V.; Khune, A.A. Niosomes: A targeted drug delivery system. GCS Biol. Pharm. Sci. 2024, 26, 48–62. [Google Scholar] [CrossRef]
- Priyadarshini, K.; Gopinath, E.; Ganesh, N.S.; Vineeth, C. Niosomes as a potential approach for enhanching topical application. Int. J. Pharm. Sci. Invent. 2024, 2, 35–45. [Google Scholar] [CrossRef]
- Momekova, D.B.; Gugleva, V.E.; Petrov, P.D. Nanoarchitectonics of multifunctional niosomes for advanced drug delivery. ACS Omega 2021, 6, 33265–33273. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: A review of challenges to clinical transition and promising resolution. Drug. Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Maurer, V.; Altin, S.; Ag Seleci, D.; Zarinwall, A.; Temel, B.; Vogt, P.M.; Strauß, S.; Stahl, F.; Scheper, T.; Bucan, V.; et al. In-vitro application of magnetic hybrid niosomes: Targeted siRNA-delivery for enhanced breast cancer therapy. Pharmaceutics 2021, 13, 394. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Hossam, S.E.-S.; Ahmed, M.R.; Fathy, I.A.; Sherif, K.A.E.; Ahmed, E.L.; Hatem, R.I.; Khalid, M.E. Effect of nanovesicular surface-functionalization via chitosan and/or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 2022, 307, 120908. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Muzzalupo, R.; Celia, C.; Citrato, R.; Caponio, D.; Picci, N.; Fresta, M. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed. Microdevices 2009, 11, 1115–1125. [Google Scholar] [CrossRef]
- Kopermsub, P.; Mayen, V.; Warin, C. Potential use of niosomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res. Int. 2011, 44, 605–612. [Google Scholar] [CrossRef]
- Baranei, M.; Taheri, R.A.; Tirgar, M.; Saeidi, A.; Oroojalian, F.; Uzun, L.; Asefnejad, A.; Wurm, F.R.; Goodarzi, V. Anticancer effect of green tea extract (GTE)-loaded pH-responsive niosome coated with PEG against different cell lines. Mater. Today Commun. 2021, 26, 101751. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Supercritical CO2 assisted process for the production of mixed phospholipid nanoliposomes: Unloaded and vitamin D3-loaded vesicles. J. Food Eng. 2022, 316, 110851. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Production of Nanoliposomes by a supercritical CO2 assisted process: Application to cosmetics. Chem. Eng. Trans. 2023, 101, 61–66. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Co-Encapsulation of curcumin and vitamin D3 in mixed phospholipid nanoliposomes using a continuous supercritical CO2 assisted process. J. Taiwan Inst. Chem. Eng. 2022, 132, 104120. [Google Scholar] [CrossRef]
- Squittieri, R.; Baldino, L.; Reverchon, E. Production of antioxidant transfersomes by a supercritical CO2 assisted process for transdermal delivery applications. Nanomater. 2023, 13, 1812. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Reverchon, E. Niosomes formation using a continuous supercritical CO2 assisted process. J. CO2 Util. 2021, 52, 101669. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E. Continuous supercritical CO2 assisted process for the production of nano-niosomes loaded with a second-generation antibiotic for ocular therapy. J. Supercrit. Fluids 2022, 188, 1056–1073. [Google Scholar] [CrossRef]
- Coleman, K.J.; Roos, K.L. Acute bacterial infections of the central nervous system. In Aminoff’s Neurology and General Medicine, 6th ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 683–701. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, V.; Kashyap, S.; Singh, A.V.; Kishore, V.; Sitti, M.; Saxena, P.S.; Srivastava, A. graphene oxide synergistically enhances antibiotic efficacy in vancomycin-resistant staphylococcus aureus. ACS App. Bio Mater. 2019, 2, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Alfano, P.; Muzzalupo, R.; de Cindio, B. Niosomes vs microemulsions: New carriers for topical delivery of capsaicin. Colloids Surf. B Biointerfaces 2011, 87, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Malviya, K.; Soni, P.; Omray, L.K. Application of ethanol-injection technique for formulation of vitexin loaded noisomes and characterization of the vesicles. J. Popl. Ther. Clin. Pharmacol. 2023, 30, 2850–2858. [Google Scholar] [CrossRef]
- Tyagi, R.; Waheed, A.; Kumar, N.; Ahad, A.; Bin Jardan, Y.A.; Mujeeb, M.; Kumar, A.; Naved, T.; Madan, S. Formulation and Evaluation of plumbagin-loaded niosomes for an antidiabetic study: Optimization and in vitro evaluation. Pharmaceuticals 2023, 16, 1169. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Portela, R.; Baeza, P.; Alcolea-Rodriguez, V.; Villarroel, M.; Avila, P. Zeta potential as a tool for functional materials development. Catal. Today 2022, 423, 113862. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of surfactant type, cholesterol, and various downsizing methods on the particle size of niosomes. Iran. J. Pharm. Res. 2018, 17, 1–11. [Google Scholar] [PubMed]
- Javani, R.; Hashemi, F.S.; Ghanbarzadeh, B.; Hamishehkar, H. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT 2021, 141, 110865. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Chen, X.; Gao, J.; Liang, W. PEGylated synthetic surfactant vesicles (niosomes): Novel carriers for oligonucleotides. J. Mater. Sci. Mater. Med. 2020, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf. B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Wolfram, J.; Suri, K.; Yang, Y.; Shen, J.; Celia, C.; Fresta, M.; Zhao, Y.; Shen, H.; Ferrari, M. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf. B Biointerfaces 2014, 114, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Elsewedy, H.S.; Shehata, T.M.; Tratrat, C.; Dhubiab, A.; Venugopala, K.N.; Almostafa, M.M.; Kochkar, H.; Elnahas, H.M. Significant of injectable brucine PEGylated niosomes in treatment of MDA cancer cells. J. Drug. Deliv. Sci. Technol. 2022, 71, 103322. [Google Scholar] [CrossRef]
- Asghari Lalami, Z.; Tafvizi, F.; Naseh, V.; Salehipour, M. Fabrication, optimization, and characterization of pH-responsive PEGylated nanoniosomes containing gingerol for enhanced treatment of breast cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3867–3886. [Google Scholar] [CrossRef] [PubMed]
- Mahsa, B.; Nazanin, K.; Noorbazargan, H.; Mohammad, T.Y.; Zahra, A.L.; Iman, A.; Faten, E.Y.; Aghigh, D.; Fatmeh, M.Y.; Yen, N.T. Evaluation of anti-cancer and anti-metastatic effects of folate-PEGylated niosomes for co-delivery of letrozole and ascorbic acid on breast cancer cells. Mol. Syst. Des. Eng. 2022, 7, 1102–1118. [Google Scholar] [CrossRef]
- Park, S.-J.; Choi, S.G.; Davaa, E.; Park, J.-S. Encapsulation enhancement and stabilization of insulin in cationic liposomes. Int. J. Pharm. 2011, 415, 267–272. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Zarepour, A.; Zarrabi, A. Empowering cancer therapy: Comparing PEGylated and non-PEGylated niosomes loaded with curcumin and doxorubicin on MCF-7 cell Line. Bioengineering 2023, 10, 1159. [Google Scholar] [CrossRef]
- Alemi, A.; Zavar Reza, J.; Haghiralsadat, F.; Zarei Jaliani, H.; Haghi, K.M.; Hosseini, S.A.; Haghi, K.S. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J. Nanobiotechnol. 2018, 28, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Biopharm. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S.; Singh, S.K. Vesicular elastic liposomes for transdermal delivery of rifampicin: In-vitro, in-vivo and in silico GastroPlusTM prediction studies. Eur. J. Pharm. Sci. 2020, 151, 105411. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Ahmed, L.; Atif, R.; Eldeen, T.S.; Yahya, I.; Omara, A.; Eltayed, M. Study the using of nanoparticles as drug delivery system based on mathematical models for controlled release. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2019, 8, 52–56. [Google Scholar]
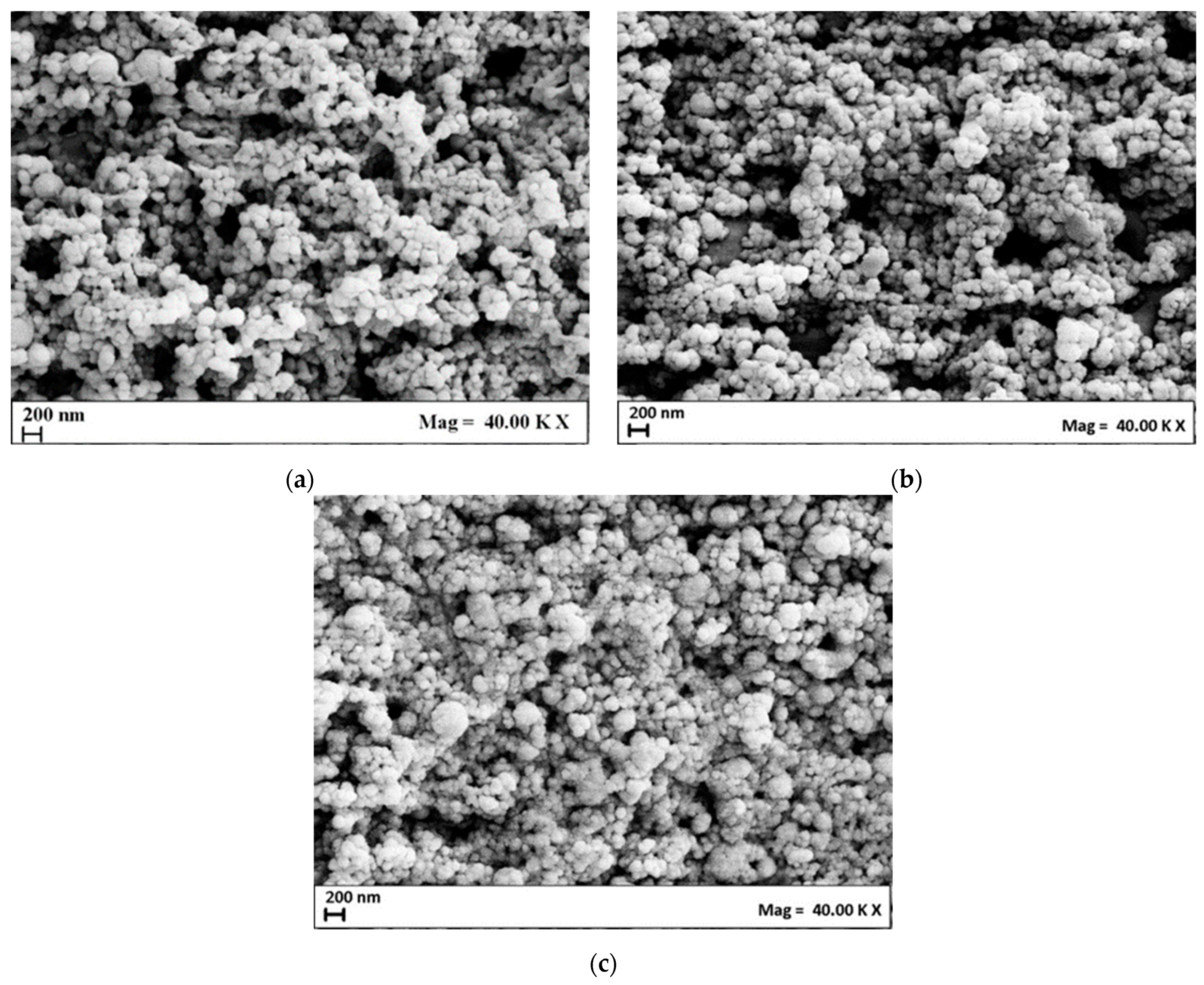

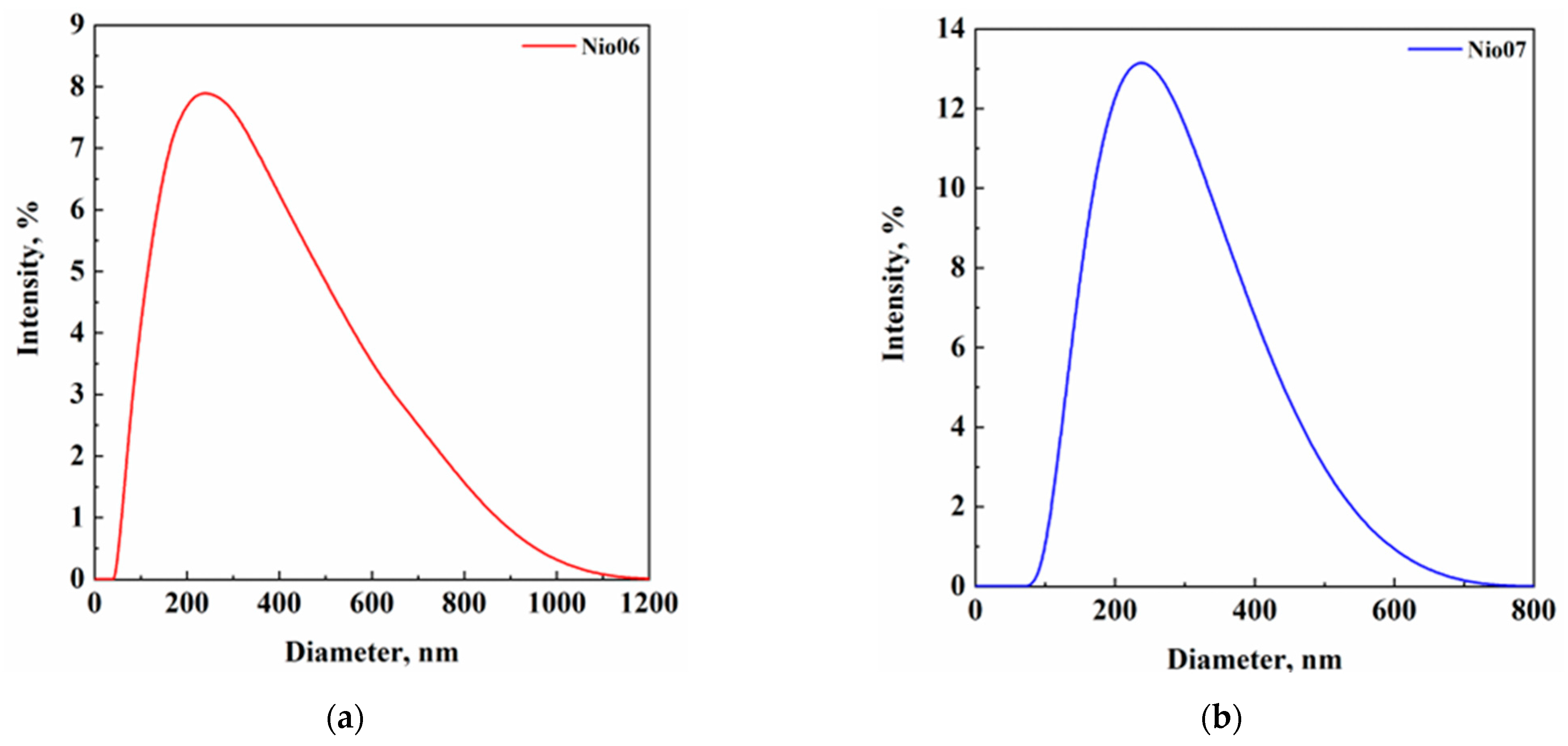

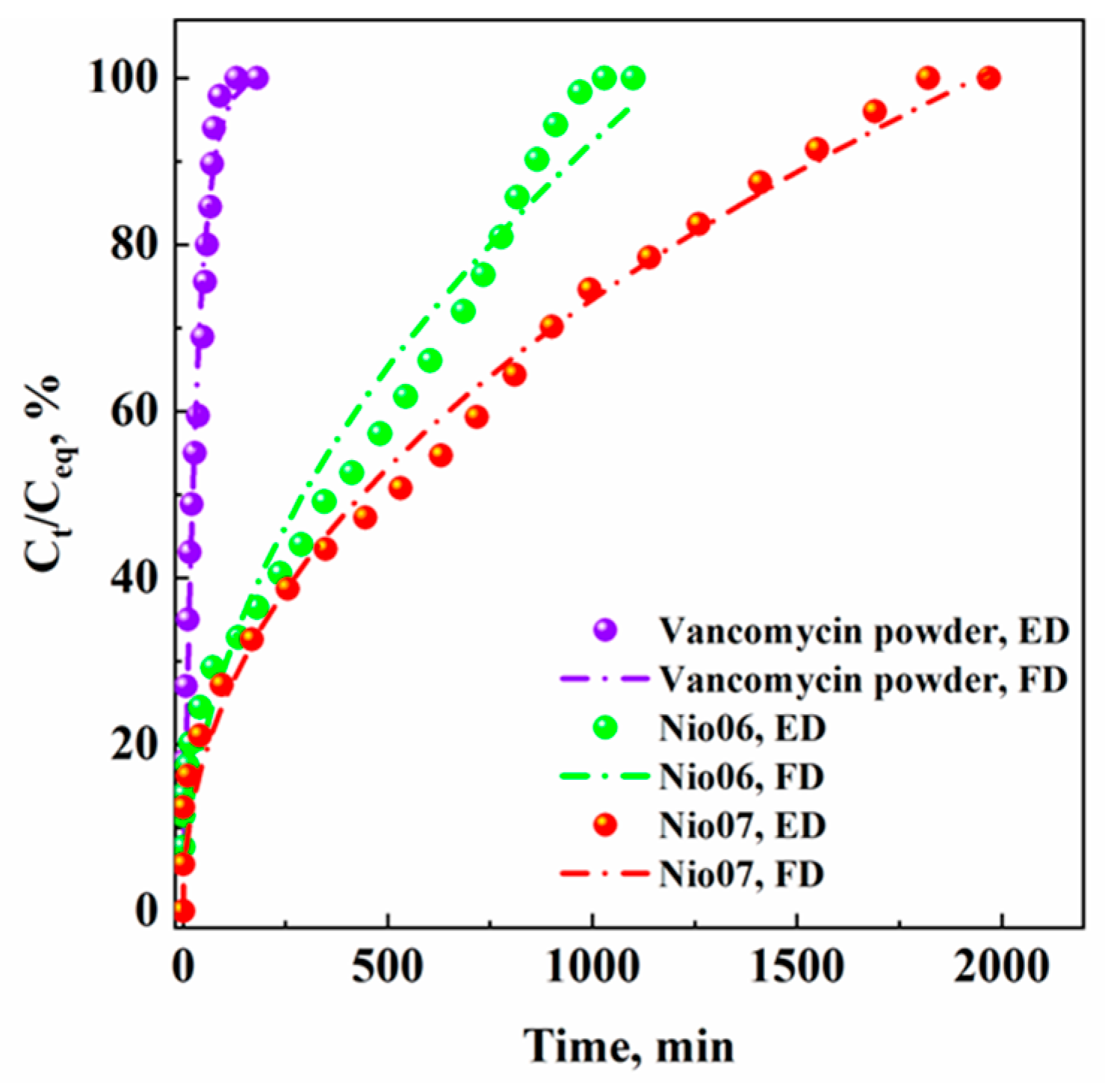
| Set of Experiments | Span 80/Tween 80 | HLB | Span 80, mg | Tween 80, mg | Surfactant/Cholesterol, mol/mol | PEG400, % w/v |
|---|---|---|---|---|---|---|
| Nio01 | 95:5 | 4.84 | 1900 | 100 | - | - |
| Nio02 | 90:10 | 5.37 | 1800 | 200 | - | - |
| Nio03 | 80:20 | 6.44 | 1600 | 400 | - | - |
| Nio04 | 80:20 | 6.44 | 1600 | 400 | 4 | - |
| Nio05 | 80:20 | 6.44 | 1600 | 400 | 4 | 1 |
| Sample | Stability, Days | MHD, nm | PDI | ζ-Potential, mV |
|---|---|---|---|---|
| Nio01 | 0 | 181 ± 55 | 0.365 | −16.2 ± 2.5 |
| 15 | 180 ± 49 | 0.297 | −16.1 ± 3.6 | |
| 30 | 181 ± 54 | 0.362 | −17.1 ± 2.7 | |
| 60 | 185 ± 51 | 0.301 | −15.0 ± 2.2 | |
| Nio02 | 0 | 191 ± 47 | 0.242 | −19.8 ± 2.5 |
| 15 | 190 ± 50 | 0.276 | −19.8 ± 3.1 | |
| 30 | 188 ± 52 | 0.302 | −17.4 ± 3.0 | |
| 60 | 189 ± 56 | 0.348 | −15.3 ± 2.0 | |
| Nio03 | 0 | 248 ± 52 | 0.182 | −23.1 ± 2.3 |
| 15 | 248 ± 58 | 0.222 | −21.0 ± 2.7 | |
| 30 | 247 ± 58 | 0.228 | −19.7 ± 2.4 | |
| 60 | 245 ± 56 | 0.210 | −18.5 ± 2.5 | |
| Nio04 | 0 | 118 ± 33 | 0.318 | −27.4 ± 3.0 |
| 15 | 118 ± 34 | 0.325 | −27.4 ± 3.0 | |
| 30 | 118 ± 35 | 0.357 | −25.7 ± 4.0 | |
| 60 | 119 ± 39 | 0.424 | −23.1 ± 3.1 | |
| Nio05 | 0 | 203 ± 62 | 0.372 | −30.6 ± 2.4 |
| 15 | 203 ± 62 | 0.372 | −30.6 ± 2.4 | |
| 30 | 204 ± 63 | 0.381 | −30.7 ± 2.4 | |
| 60 | 205 ± 67 | 0.430 | −30.8 ± 2.7 |
| Set of Experiments | Span 80/Tween 80 | Span 80, mg | Tween 80, mg | Surfactant/Cholesterol, mol/mol | PEG400, % w/v | Drug to Lipid Ratio, % w/w |
|---|---|---|---|---|---|---|
| Nio06 | 80:20 | 1600 | 400 | 4 | - | 5 |
| Nio07 | 80:20 | 1600 | 400 | 4 | 1 | 5 |
| Sample | Stability, Days | MHD, nm | PDI | ζ-Potential, mV |
|---|---|---|---|---|
| Nio06 | 0 | 214 ± 59 | 0.300 | −31.1 ± 2.5 |
| 15 | 214 ± 57 | 0.300 | −31.1 ± 2.5 | |
| 30 | 214 ± 65 | 0.372 | −31.1 ± 2.3 | |
| 60 | 214 ± 65 | 0.370 | −31.0 ± 2.0 | |
| Nio07 | 0 | 254 ± 73 | 0.330 | −27.7 ± 2.0 |
| 15 | 254 ± 73 | 0.330 | −27.7 ± 2.0 | |
| 30 | 250 ± 66 | 0.280 | −27.6 ± 2.4 | |
| 60 | 250 ± 65 | 0.270 | −27.3 ± 2.0 |
| Vancomycin Powder | Nio06 | Nio07 | ||
|---|---|---|---|---|
| First order | a | 100.18 | 118.3 | 103.42 |
| b | 0.0298 | 0.0016 | 0.0014 | |
| R2adj | 0.965 | 0.921 | 0.941 | |
| AIC | 70.05 | 118.83 | 98.71 | |
| Korsmeyer–Peppas | kKP | 15.78 | 2.40 | 2.95 |
| n | 0.38 | 0.53 | 0.47 | |
| R2adj | 0.950 | 0.960 | 0.985 | |
| AIC | 70.76 | 95.61 | 62.68 | |
| Higuchi | kH | 9.57 | 2.92 | 2.31 |
| R2adj | 0.899 | 0.961 | 0.983 | |
| AIC | 79.54 | 93.55 | 62.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, L.; Riccardi, D.; Reverchon, E. Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process. Nanomaterials 2024, 14, 846. https://doi.org/10.3390/nano14100846
Baldino L, Riccardi D, Reverchon E. Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process. Nanomaterials. 2024; 14(10):846. https://doi.org/10.3390/nano14100846
Chicago/Turabian StyleBaldino, Lucia, Domenico Riccardi, and Ernesto Reverchon. 2024. "Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process" Nanomaterials 14, no. 10: 846. https://doi.org/10.3390/nano14100846
APA StyleBaldino, L., Riccardi, D., & Reverchon, E. (2024). Production of PEGylated Vancomycin-Loaded Niosomes by a Continuous Supercritical CO2 Assisted Process. Nanomaterials, 14(10), 846. https://doi.org/10.3390/nano14100846








