Abstract
Organic–inorganic hybrid halides and their analogs that exhibit efficient broadband emission from self-trapped excitons (STEs) offers an unique pathway towards realization of highly efficient white light sources for lighting applications. An appropriate dilution of ns2 ions into a halide host is essential to produce auxiliary emissions. However, the realization of ns2 cation-based halides phosphor that can be excited by blue light-emitting diode (LED) is still rarely reported. In this study, a zero-dimensional Te-based single crystal (C8H20N)2TeCl6 was synthesized, which exhibits a yellow-orange emission centered at 600 nm with a full width at half maximum of 130 nm upon excitation under 437 nm. Intense electron–phonon coupling was confirmed in the (C8H20N)2TeCl6 single crystal and the light emitting mechanism is comprehensively discussed. The results of this study are pertinent to the emissive mechanism of Te-based hybrid halides and can facilitate discovery of unidentified metal halides with broadband excitation features.
1. Introduction
Lead-free perovskite materials—which exhibit strong photoelectric conversion efficiency, exceptional photoluminescence quantum yield (PLQY), high light absorption coefficients, and excellent flexibility—are an active area of research in flexible displays, semiconductor high-energy lasers, X-ray scintillator, light-emitting diodes, and infrared detectors [1,2,3,4]. The general structural formula of perovskites has progressively developed from the primary AB(II)X3 to A2B(I)B(III)X6 (halide double perovskites), A2B(IV)X6 (vacancy-ordered perovskites), A3B(III)2X9 (halide triple perovskites), A4B(II)B(III)2X12 (halide quadruple perovskites) and other configurations [5,6,7]. A is monovalent organic or inorganic cations, such as CH3NH3+, NH2CHNH2+, Cs+, Rb+, K+; B(I), B(II), B(III) and B(IV), respectively, stand for metal cations with different valence states, such as Ag+, Cu+, Sn2+, Zn2+, Sb3+, In3+, Zr4+, Hf4+, Te4+, and Sn4+; X represents monovalent halide anion, such as Cl−, I−, Br− or mixed halide anions [8,9]. Moreover, perovskites and perovskite derivatives can be classified into three-, two-, one-, and zero-dimensional (0D) at the molecular level [10]. Low-dimensional metal halide materials have large forbidden bands and high exciton binding energies, because of their remarkable crystal and electronic structures [11,12]. Introducing organic molecules leads to extraordinary possibilities in structure and performance research of metal halides with various dimensions [13]. Therefore, developing organic–inorganic hybrid, lead-free, low-dimensional metal halides is important for expanding functional applications.
Presently, the exceptional photoelectric performance of organic–inorganic hybrid, lead-free, low-dimensional metal halides is particularly influenced by B-site metal cations [14]. Metal cations containing the outermost electron arrangement as ns2 lone pair electrons are called ns2 ions. 5S2 ions (Sn2+, Sb3+, Te4+) and 6S2 ions (Bi3+, Pb2+) can exhibit their lone pair electron transition characteristics during photoexcitation. Metal halide materials containing ns2 cations have advantages compared with analogous materials that contain other cations, in terms of outstanding self-trapped exciton (STE) emission and other optoelectronic characteristics [15,16,17,18]. For example, the Zhao group reported that (C10H22N)2SbBr5 and (C10H22N)3Sb2Br9 exhibit orange emission with maxima at 640 and 660 nm, respectively, which originates from STE emission of the Sb3+ cation [19]. Nevertheless, currently, the widely investigated Sb-based halides cannot be excited with blue light, which also occurs in, e.g., Bi- or Ge-based halides [20,21]. Mn-based halides can be excited with blue light; however, the luminescence mechanism of Mn-based halides is attributable to the d-d transition of Mn ions, which leads to their lack of bright STE broad-spectrum emission [22,23,24]. In that vein, an immense challenge in STE emitters is finding an STE emitter with ultra-broadband excitation involving blue light regions.
Significantly, tetravalent tellurium ion with a lone-pair 5s2 electronic configuration was found to be an ion accompanied by distinctive STE emission. And the ultra-wide absorption band present in Te-based or Te-doped metal halides may cause them to be excited by blue light [25]. Recently, some progress has been achieved in all-inorganic metal halides-doped Te4+ ions with STE emission that can be excited by blue light. For example, Zhang et al. attributed the luminescence of Te4+-doped Rb2SnCl6 that can be excited by blue light to the STE emission of the [TeCl6]2− octahedron [26]. Li et al. developed 0D Sc(III)-based Te4+-doped Rb2ScCl5·H2O single crystals with a broadband orange STE emission (650 nm) that can be excited by light in the range of 240 nm to 440 nm [27]. Compared with inorganic metal halides doped with Te4+ ions, the STE emission research on Te-based organic–inorganic hybrid metal halides usually show limited light emission efficiency. For instance, Mirochnik et al. discovered the broadband emission of ((CH3)4N)2TeCl6 halide at 77 K, with poor luminescence at room temperature [28]. For the time being, there are few Te-based organic–inorganic hybrid metal halides that can exhibit obvious STE emission at room temperature, although some Te-based halides can exhibit relatively high luminescence intensity at low temperatures. Therefore, there is an urgent need for new low-dimensional Te-based metal halides that exhibit STE emission at room temperature.
Inspired by the low dimensionality and configuration of metal halides, the authors of this paper designed and synthesized an organic–inorganic hybrid, lead-free, 0D (C8H20N)2TeCl6 single crystal. The structural details, optical and decay lifetime characteristics at room temperature, as well as temperature-dependent luminescence of this single crystal are discussed in detail. At room temperature, (C8H20N)2TeCl6 single crystals exhibit a broad-band STE emission (λem = 600 nm) with a full width at half maximum (FWHM) of 130 nm under 437 nm excitation. Temperature-dependent data also demonstrate that the yellow-orange emission is attributable to archetypal STE emission.
2. Experimental Section
2.1. Materials
Tetraethylammonium chloride (C8H20NCl, 98%, Aladdin, Shanghai, China), tellurium tetrachloride (TeCl4, 99%, Meryer, Shanghai, China), anhydrous ethanol (C2H6O, AR, Macklin, Shanghai, China), diethyl ether (C4H10O, AR, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and hydrochloric acid (HCl, Sigma-Aldrich, Shanghai, China) were used as received. During the process of synthesizing (C8H20N)2TeCl6 single crystal, TeCl4 materials can also be replaced with tellurium dioxide (TeO2, 99.99%, Bide Pharmatech Co., Ltd., Shanghai, China).
2.2. Synthesis of (C8H20N)2TeCl6 Single Crystal
Anti-solvent evaporation and traditional solvothermal methods can be used to synthesize single crystals. Regarding the traditional solvothermal method, the solvent is HCl. C8H20NCl (0.6628 g), TeCl4 (0.5388 g) and 16 mL hydrochloric acid were loaded into a 25 mL tetrafluoroethylene reactor, heated at 120 °C for 8 h, and cooled to room temperature within 16 h. Figure S1A shows the schematic process of synthesizing (C8H20N)2TeCl6 single crystals using solvothermal method. It is worth noting that after the reaction completes, yellowish-green single crystals can be obtained in the hydrochloric acid liquid. Then, the prepared single crystals are transferred from the tetrafluoroethylene reactor to a culture dish. In order to remove the hydrochloric acid attached to the surface of the (C8H20N)2TeCl6 single crystals, an appropriate amount of anhydrous ethanol was added to the culture dish to rinse the prepared single crystals. The above flushing process is repeated for at least three times. Finally, the anhydrous ethanol in this culture dish was removed by a dropper and placed in an oven at 65 °C for 20 min of drying treatment. In addition, this experimental process can also replace 0.5388 g of TeCl4 with 0.3192 g of TeO2, while keeping most of the remaining experimental requirements unchanged. When washing (C8H20N)2TeCl6 single crystals prepared using TeO2 as raw material, it is necessary to replace anhydrous ethanol with isopropanol. However, it should be noted that the single crystal luminescence performance synthesized using TeCl4 is superior to that synthesized using TeO2. The luminescence characteristics detected in this article are the properties of single crystals prepared using TeCl4. Regarding anti-solvent evaporation, first, 0.002 mol C8H20NCl (0.3314 g) and 0.001 mol TeCl4 (0.2694 g) were dissolved in a single solvent (1.5 mL C2H6O at 65 °C) to form a clear precursor solution (Figure S1B). Then, the as-formed precursor solution was transferred to an unsealed 10 mL glass bottle, the mouth of this 10 mL glass bottle was sealed with plastic film, and sharp needles were used to puncture several breathable openings. Finally, this small glass bottle was placed into a large sealed weighing bottle with diethyl ether reagent at the bottom. Within 3 d at room temperature, the yellowish-green crystals formed. The (C8H20N)2TeCl6 single crystals synthesized by the anti-solvent evaporation method were washed with anhydrous ethanol. The drying conditions were consistent with those used in the traditional solvothermal method above.
2.3. Characterization
Powder XRD measurements of (C8H20N)2TeCl6 powder were conducted using an X-ray diffractometer (D2 Phaser) with Cu-Kα radiation (λ = 1.5418 Å, 40 kV, 20 mA). The data used for Powder XRD analysis (2θ range 5–60°) was collected in a step-scanning mode with a step size of 0.02° and 0.5 s counting time per step. The single crystal XRD measurements were performed with a Bruker D8 Venture diffractometer. (C8H20N)2TeCl6 crystals were maintained at 193.15 K during data collection. In Olex2, the structure was solved with the olex2.solve structure solution program using charge-flipping, and refined with the XL refinement package using least-squares minimization [29,30,31]. The absorption spectra were collected using a UV-3600i Plus UV–visible–NIR spectrophotometer (Shimadzu, Kyoto, Japan), in which BaSO4 commercial backing plate was used as the reference standard. The photoluminescence excitation (PLE) and photoluminescence (PL) patterns were recorded with FLUOROMAX_PLUS fluorescence spectrometer (HORIBA Scientific, Kyoto, Japan). The correlated color temperature (CCT) and color purity of (C8H20N)2TeCl6 powder were calculated using ColorCalculator v7.77 software. The color purity was calculated based on equation, in which (xi, yi) was the standard white light coordinate, (xm, ym) represented the Commission Internationale de I’Eclairage (CIE) coordinate of the dominant wavelength, and (xr, yr) was the CIE coordinate of (C8H20N)2TeCl6 halide. The PLQY pattern was recorded with an FS5 spectrofluorometer (Edinburgh integrated steady-state transient fluorescence spectrometer) at room temperature. The decay lifetimes were measured using an FS5 instrument equipped with a 405 nm (Edinburgh Instruments, Livingston, Scotland, UK; EPL-405 picosecond pulsed diode laser, 5 mW) laser.
The time width of the 405 nm laser used for the dynamics of emission decays was 5 µs window, and the single-photon counting capability was 5000 counts (1024 channels). Temperature-dependent PL spectroscopic measurements were conducted using an FS5 equipped with a temperature precision controller and liquid nitrogen input equipment (Beijing Physike Technology Co., Ltd., Beijing, China).
3. Results and Discussion
As-grown (C8H20N)2TeCl6 single crystals were synthesized using the traditional solvothermal method, and single crystal X-ray diffraction (SCXRD) was used to confirm its structure. The crystal structure of (C8H20N)2TeCl6 exhibited a monoclinic space group P21/c; the cell parameters were a = 14.0511 Å, b = 14.6234(15) Å, c = 13.0285(15) Å, and V = 2677.0(5) Å3. The crystallographic information file (CIF) of (C8H20N)2TeCl6 single crystal has been uploaded to the Cambridge Crystallographic Data Centre (CCDC#2304048). Furthermore, Tables S1–S7 (Supporting Information) show the remaining essential crystallographic data as well as relevant parameter analysis, respectively. As shown in Figure 1A, (C8H20N)2TeCl6 exhibited a 0D structure with units of [TeCl6]2− octahedral, and each isolated [TeCl6]2− octahedron was surrounded by extensive organic molecular (C8H20N)+ units [32,33]. Figure 1B shows that each [TeCl6]2− octahedron contains two Te-Cl bond distances (2.5346 Å and 2.5564 Å) and two Cl-Te-Cl bond angles (89.371° and 90.629°). From the same perspective, the phenomenon of two [TeCl6]2− octahedra twisting in opposite directions can be observed (Figure 1C). The simulated SCXRD pattern of (C8H20N)2TeCl6 is highly consistent with the powder XRD pattern (Figure 1D), which indicates that the powder prepared by grinding exhibited an identical structure as the single crystal [34]. Figure S2 shows a locally enlarged XRD pattern, which also demonstrates good consistency between the experimental data and the calculated data.
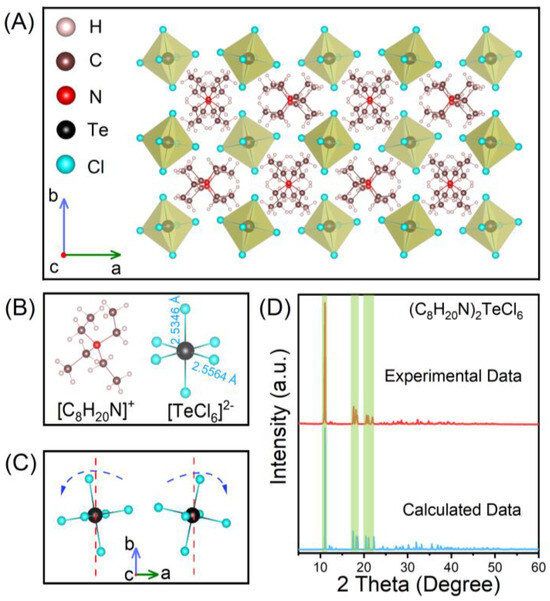
Figure 1.
(A) Structural diagram of (C8H20N)2TeCl6 single crystals. (B) The structure of the individual [C8H20N]+ and [TeCl6]2−. (C) [TeCl6]2− octahedral distortion diagram. (D) XRD pattern of (C8H20N)2TeCl6.
Regarding Pb-free metal halide luminescent systems, the luminescent behavior of ns2 metal ions as visible and near-infrared (NIR) luminescent centers is an active area of research. Te4+, Sb3+, Sn2+ and Bi3+ ions exhibit analogous chemical properties and electronic configurations as significant ions [15,35]. The Te4+ ion containing 5s2 lone pair electrons has a similar ground state electronic structure to the Sb3+ ion that has been extensively studied in the field of metal halide luminescence. Figure S3A shows a schematic of the excitation state of Te4+ in metal halides. The excited states (sp) split into two branches (1P1 and 3Pn) under the influence of spin–orbit coupling (SOC); the excited-state splitting phenomena of 1P1 and 3P1 follows the order of energy from low to high, which is usually attributed to the lattice symmetry breaking caused by the Jahn–Teller effect [36]. Several previously reported papers (Cs2SnCl6:Te4+; Cs2ZrCl6:Te4+) have confirmed the level (1P1 and 3P1) splitting phenomenon of Te4+ ions [37,38]. 1S0–1P1, 1S0–3P2, and 1S0–3P1 transitions are allowed, vibration-induced, and spin–orbit-allowed, respectively. The spin–orbit-allowed behavior can derive from the intense effect of SOC by tellurium atoms [38,39]. Deformation of octahedra is common in [TeCl6]2− octahedra that are susceptible to the Jahn–Teller effect (Figure S3B). The vibrational mode of [TeCl6]2− octahedra in (C8H20N)2TeCl6 belongs to v2 mode [35].
Figure 2A shows the UV–visible absorption spectrum of (C8H20N)2TeCl6 halide. The absorption curve of (C8H20N)2TeCl6 halide exhibits an ultra-wide absorption band in the wavelength range of 200–500 nm, which is similar to reports for (C13H22N)2TeCl6 and (C20H20N)2TeCl6 [40,41]. The ultrabroad absorption bands are attributable to the dipole-allowed, vibration-allowed, and spin–orbit-allowed transitions of Te4+ ions. And the inset shows the optical images of (C8H20N)2TeCl6 under the illumination of daylight and 365 nm UV light. (C8H20N)2TeCl6 single crystal appears yellowish-green and transparent in daylight; however, when exposed to UV light at 365-nm, it displays yellow-orange fluorescence. In addition, the same emission occurs in the (C8H20N)2TeCl6 powder. Figure 2B shows the normalized room-temperature PL emission and excitation spectra of (C8H20N)2TeCl6. The PLE spectrum (monitored at 600 nm) consists of an ultrabroad band in the UV and blue regions with a peak at 437 nm, indicating that this halide could be excited with available blue light-emitting diode chips. Upon excitation at 437 nm, (C8H20N)2TeCl6 exhibited a strong-broad yellow-orange emission ranging from 450 to 800 nm, with a FWHM of 130 nm and a peak at 600 nm. In addition, there was a large Stokes shift of 163 nm. Figure 2C shows the pseudo-color map of a PL/PLE of (C8H20N)2TeCl6 sample at room temperature, in which the characteristics of emission spectra under variable excitation and STE emission characteristics of Te4+ ions can have a precise correspondence [42]. To gain a deeper understanding of the luminous mechanism in the (C8H20N)2TeCl6 system, further information is obtained from wavelength-scanning time-correlated decay experiments. Under 405 nm laser excitation, the lifetime monitored at 600 nm in (C8H20N)2TeCl6 can be fitted using a single exponential equation: I = Ae−t/τ, in which I is the PL intensity, A refers to the constant, and τ represents the fitting decay time. The fitting lifetime of (C8H20N)2TeCl6 halide was 236.43 ns, and the value of R2 (R2 = 0.999) indicates that this fitting is reliable (Figure 2D). The decay-mapping and fitting results are shown in Figure 2E and Table S8 (Supporting Information). The emission peaks located from 540 nm to 660 nm represent similar fitting lifetimes, indicating that there was only one PL component. The multiple lifetimes fitted by (C8H20N)2TeCl6 sample are similar to the previously reported lifetime characteristics of STE luminescence behavior related to Te4+ ion doping (Rb2ZrCl6:Te4+, 235.62 ns; Cs2ZnCl4:Te4+, 53.2 ns) or Te-based materials ((Ph3S)2TeCl6, 42.7 ns) [35,42,43]. The lifetimes of hundreds of nanoseconds are attributable to STE emission of [TeCl6]2− octahedrons. The CIE coordinate of (C8H20N)2TeCl6 halide is (0.52, 0.46) with a CCT of 2376 K and a color purity of 95.2% (Figure 2F), which is higher than that of Cs2SnCl6:Te4+ (84.69%) [44]. Regarding Te-based halides, a constraint is that the PLQY at room temperature is low, which can cause STE luminescence emission that is insubstantial to the unaided eye. Here, the PLQY at room temperature was 10.12% (Figure S4).
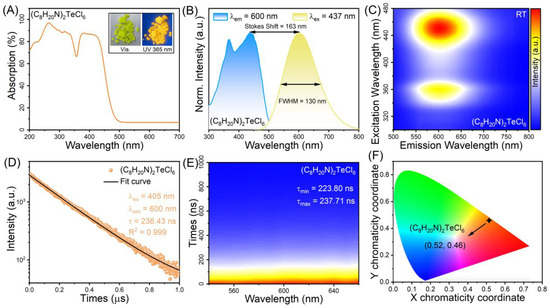
Figure 2.
(A) Absorption spectra of (C8H20N)2TeCl6. The inset shows the optical images of (C8H20N)2TeCl6 under the illumination of daylight and 365 nm UV light. (B) PLE and PL spectra of (C8H20N)2TeCl6 at RT. (C) Pseudo color map of PL/PLE of (C8H20N)2TeCl6. (D) Emission wavelength scanning decay of (C8H20N)2TeCl6 excited by 405 nm. (E) Emission wavelength scanning decay of (C8H20N)2TeCl6 excited by 405 nm. (F) CIE color coordinate of (C8H20N)2TeCl6 sample.
To obtain deep insights into the mechanism of STE emission of Te-based halides, temperature-dependent PL spectroscopic measurements of (C8H20N)2TeCl6 halide were implemented in the range of 90 K to 300 K. Figure 3A shows the temperature-dependent PL spectra of (C8H20N)2TeCl6. The test temperature was negatively correlated with the PL intensity. As the test temperature continued to increase from cryogenic to room temperature, an inconspicuous blue shift of the emission band was observed, which originates from electron–phonon coupling interactions and thermal expansion of the metal halide lattice [45]. According to the currently recognized theory of STE luminescence in metal halides, the STE emissions are affected by the strength of exciton–phonon coupling. Figure 3B shows a plot of the FWHM (obtained using Gaussian fitting) and integrated PL intensity against the temperature. Conventional theories use the Huang–Rhys factor (S), dimensionless, to characterize the strength of electron–phonon coupling, which exhibits a close correlation with the FWHM. The Huang–Rhys factor can be calculated using the fitting formula [46]:
where ħωphonon, T, and kB represent the phonon frequency, practical temperature, and the Boltzmann constant, respectively. Through multiple derivations and simplifications of Formula (1), Figure 3C shows the functional relationship between FWHM2 vs. 2kT as well as the linear fitting line (red line). Regarding the (C8H20N)2TeCl6 halide, the phonon frequency and Huang–Rhys factor were ca. 0.01303 eV, and 38.71, respectively. In general, there is a certain correlation between the S value and the ease of STE formation. For STE emissions of metal halides, the appropriate value of S is generally between 10 and 40 [47]. For instance, pure inorganic Sb3+-doped Rb4CdCl6 sample with typical STE emission has an S of 34.94, which is extremely similar to the 38.71 of (C8H20N)2TeCl6 halide [48]. The Huang–Rhys factor of (C8H20N)2TeCl6 halide is evidently higher than the previously reported halides of Cs2Sn0.89Te0.11Cl6 (16.0), (CH3CH2CH2)4NCuCl2 (16.4), and Sb3+-doped Cs3Cd2Cl7 (30.06) with emblematic STE emissions, indicative of intense exciton–phonon coupling and Jahn–Teller distortion in (C8H20N)2TeCl6 [48,49,50]. Moreover, according to the STE emission calculation formula (EPL = Eg − Eb − ESTE − Ed), the exciton binding energy (Eb) is a pertinent physical parameter that is used to deduce STE emission. Figure 3D shows the temperature-dependent integrated PL intensity; the Eb values were determined using the Arrhenius expression:
where I(T) and I0 represent the integrated intensity at the actual measurement temperature and the integrated intensity at 0 K, respectively. The Eb of (C8H20N)2TeCl6 was ca. 338.27 meV, which is comparable with the previously reported all-inorganic low-dimensional metal halides of Rb2ScCl5·H2O:Te4+ (384 meV), and CsCu2I3 (346 meV) [27]. The fitted exciton binding energy and Huang–Rhys factor can both prove that this yellow-orange broadband emission at 600 nm in (C8H20N)2TeCl6 halide is attributed to STEs of [TeCl6]2− octahedral [36,37]. Overall, the luminescence mechanism for (C8H20N)2TeCl6 halide (Figure 4A) can be summarized as follows: UV or blue-light excitation leads to the electron excitation from the 1S0 state to the excited states of Te–Cl. Then, the free carriers (FCs) become free excitons (FEs) through coulombic interactions. In addition, the low-dimensional (0D) structure of the (C8H20N)2TeCl6 halide is beneficial for promoting the presence of a soft lattice in this single crystal, thus easily forming STE emission. FEs are subjected to intense exciton–phonon interactions, resulting in lattice distortion of the [TeCl6]2− octahedral and trapping in the STE state. Therefore, FEs ultimately fall into the newly formed STE state, leading to yellow-orange STE emission. Moreover, as shown in Figure 4B, the PXRD peak pattern of the (C8H20N)2TeCl6 sample under long-term air exposure conditions exhibit significant consistency, with several major PXRD peaks corresponding to each other in the highlighted yellow area. And the PL intensity of the (C8H20N)2TeCl6 powder after continuous exposure to 437 nm blue light for 70 h is not significantly different from the original PL intensity (Figure 4C). Hence, the above XRD and spectral results indicate that the as-synthesized (C8H20N)2TeCl6 samples have a reliable stability.
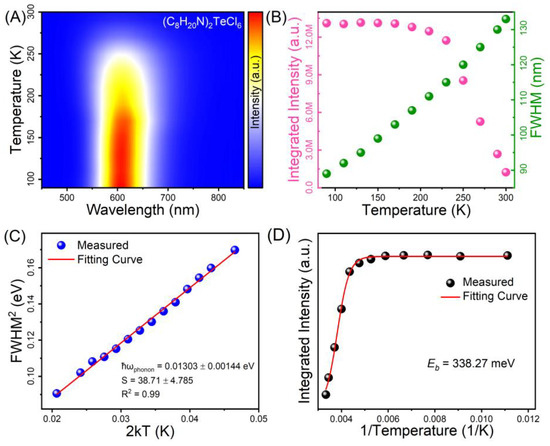
Figure 3.
(A) Temperature-dependent PL spectrum of (C8H20N)2TeCl6. (B) Integrated intensity and FWHM as functions of temperature. (C) FWHM2 as functions of 2 kT. (D) Integrated PL intensity as functions of 1/T.
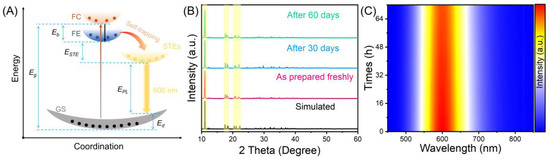
Figure 4.
(A) Configuration coordinate diagram demonstrating the photophysical process in (C8H20N)2TeCl6. (B) PXRD patterns of (C8H20N)2TeCl6 stored in the atmosphere for 60 d. (C) The PL map of (C8H20N)2TeCl6 sample under different blue light excitation times.
4. Conclusions
In summary, we successfully synthesized a 0D (C8H20N)2TeCl6 single crystal through various methods and investigated the STE mechanism of broadband luminescence. The crystal structure of (C8H20N)2TeCl6 was identified in the space group P21/c and comprised [TeCl6]2− octahedral and charge-balancing C8H20N+ cations. (C8H20N)2TeCl6 single crystals exhibited a yellow-orange emission (≈600 nm) with a large FWHM of 130 nm. And it exhibits a unique ultrabroad excitation band containing a blue excitation region. The color purity of (C8H20N)2TeCl6 halide is 95.2%. The temperature-dependent optical properties demonstrate that the Huang–Rhys factor and exciton binding energy were 38.71 and 338.27 meV, respectively, indicative of strong electron–phonon coupling. Therefore, we attributed the origin of the yellow-orange emission band to STE emission of [TeCl6]2− octahedral. Our achievement in (C8H20N)2TeCl6 single crystals offers a strategy for designing and synthesizing Pb-free, low-dimensional, luminescent metal halides with broad blue-light excitation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano14010046/s1, Figure S1: Diagram of synthesis of (C8H20N)2TeCl6; Figure S2: PXRD patterns of (C8H20N)2TeCl6; Figure S3: Scheme of the energy level of (C8H20N)2TeCl6 and the v2 vibration mode of [TeCl6]2− octahedra; Figure S4: PLQY of (C8H20N)2TeCl6; Tables S1−S7: crystallographic data; Table S8: PL lifetimes of (C8H20N)2TeCl6.
Author Contributions
X.Y.: conceptualization, methodology, investigation, data curation, writing—original draft. J.N.: investigation, writing—review and editing. H.H. and H.Z.: writing—review and editing. D.X., Y.S. and H.L.: supervision, investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 61874074), Science and Technology Project of Shenzhen (Grant No. JCYJ20220531100815034), and Guangdong Basic and Applied Basic Research Foundation (General Program, Grant No. 2022A1515012055).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Luo, J.J.; Wang, X.M.; Li, S.R.; Liu, J.; Guo, Y.M.; Niu, G.D.; Yao, L.; Fu, Y.H.; Gao, L.; Dong, Q.S.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef]
- Tan, Z.F.; Li, J.H.; Zhang, C.; Li, Z.; Hu, Q.S.; Xiao, Z.W.; Kamiya, T.; Hosono, H.; Niu, G.D.; Lifshitz, E.; et al. Highly efficient blue-emitting Bi-doped Cs2SnCl6 perovskite variant: Photoluminescence induced by impurity doping. Adv. Funct. Mater. 2018, 28, 1801131. [Google Scholar] [CrossRef]
- Yan, J.K.; Li, H.J.; Aldamasy, M.H.; Frasca, C.; Abate, A.; Zhao, K.; Hu, Y. Advances in the synthesis of halide perovskite single crystals for optoelectronic applications. Chem. Mater. 2023, 35, 2683–2712. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Liu, X.; Hu, Y.F.; Lou, Z.D.; Hou, Y.B.; Teng, F. Property modulation of two-dimensional lead-free perovskite thin films by aromatic polymer additives for performance enhancement of field-effect transistors. ACS Appl. Mater. Interfaces 2021, 13, 24272–24284. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.F.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.L.; Chen, J.; Lin, J. Solution and solid-phase growth of bulk halide perovskite single crystals. Chin. J. Chem. 2021, 39, 1353–1363. [Google Scholar] [CrossRef]
- Yao, J.S.; Wang, J.J.; Yang, J.N.; Yao, H.B. Modulation of metal halide structural units for light emission. Acc. Chem. Res. 2021, 54, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Luo, S.L.; Wang, X.J.; Zhang, L.J. Alternative lone-pair ns2-cation-based semiconductors beyond lead halide perovskites for optoelectronic applications. Adv. Mater. 2021, 33, 2008574. [Google Scholar] [CrossRef]
- McCall, K.M.; Morad, V.; Benin, B.M.; Kovalenko, M.V. Efficient lone-pair-driven luminescence: Structure–property relationships in emissive 5s2 metal halides. ACS Mater. Lett. 2020, 2, 1218–1232. [Google Scholar] [CrossRef]
- Huang, T.; Zou, B.S. Luminescent Behavior of Sb3+-Activated Luminescent Metal Halide. Nanomaterials 2023, 13, 2867. [Google Scholar] [CrossRef]
- Fu, Y.P.; Jin, S.; Zhu, X.Y. Stereochemical expression of ns2 electron pairs in metal halide perovskites. Nat. Rev. Chem. 2021, 5, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.M.; Zeng, H.B. Lead-free halide double perovskites: Structure, luminescence, and applications. Small Struct. 2021, 2, 2000071. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Cai, W.S.; Wang, H.X.; Zang, Z.G.; Chen, J.Z. All-inorganic lead-free perovskite(-like) single crystals: Synthesis, properties, and applications. Small Methods 2021, 5, 2001308. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Luo, J.J.; Liu, J.; Tang, J. Self-trapped excitons in all-inorganic halide perovskites: Fundamentals, status, and potential applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.H.; Zhou, B.; Fang, S.F.; Wang, Y.; Wang, Y.; Tian, B.B.; Hu, H.L.; Zhong, H.Z.; Li, H.N.; Shi, Y.M. Chemical doping of lead-free metal-halide-perovskite related materials for efficient white-light photoluminescence. Mater. Today Phys. 2023, 31, 100992. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Z.Y.; Zhang, F.; Yin, H.; Xu, X.; Chen, J.S.; Chen, Z.; Luo, J.H.; Han, K.L.; Zhang, R.L. All-inorganic zero-dimensional Sb3+-doped Rb2ScCl5(H2O) perovskite single crystals: Efficient self-trapped exciton emission and X-ray detection. J. Phys. Chem. Lett. 2023, 14, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.Y.; Liu, Y.; Zhao, J.; Xia, Z.G. Sb3+ doping-induced triplet self-trapped excitons emission in lead-free Cs2SnCl6 nanocrystals. J. Phys. Chem. Lett. 2019, 10, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.P.; Liu, S.B.; Teng, Z.W.; Li, H.; Chen, W.Q.; Zhou, W.; Qiu, J.B.; Yu, X.; Wang, S.; Xu, X.H. High quality lead-free perovskites toward white light emitting diodes and X-ray imaging. J. Mater. Chem. C 2022, 10, 16294–16300. [Google Scholar] [CrossRef]
- Li, M.Y.; Lin, J.W.; Wang, N.; Liu, K.J.; Fan, L.B.; Guo, Z.N.; Yuan, W.X.; Zhao, J.; Liu, Q.L. Synthetic-method-dependent antimony bromides and their photoluminescent properties. Inorg. Chem. 2022, 61, 15016–15022. [Google Scholar] [CrossRef]
- Sun, C.; Deng, Z.Y.; Li, Z.Y.; Chen, Z.W.; Zhang, X.Y.; Chen, J.; Lu, H.P.; Canepa, P.; Chen, R.; Mao, L.L. Achieving near-unity photoluminescence quantum yields in organic-inorganic hybrid antimony (III) chlorides with the [SbCl5] geometry. Angew. Chem. Int. Ed. 2023, 62, e202216720. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Y.; Liang, P.; Zhou, T.L.; Wang, L.; Xie, R.J. Dual-band luminescent lead-free antimony chloride halides with near-unity photoluminescence quantum efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- Li, M.Z.; Zhou, J.; Molokeev, M.S.; Jiang, X.X.; Lin, Z.S.; Zhao, J.; Xia, Z.G. Lead-free hybrid metal halides with a green-emissive [MnBr4] unit as a selective turn-on fluorescent sensor for acetone. Inorg. Chem. 2019, 58, 13464–13470. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lu, H.; Yue, C.Y.; Fei, H.H.; Wu, S.F.; Wang, S.H.; Lei, X.W. Multiple light source-excited organic manganese halides for water-jet rewritable luminescent paper and anti-counterfeiting. ACS Appl. Mater. Interfaces 2022, 14, 56176–56184. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Sakhatskyi, K.; Jin, J.C.; Zhang, Q.Y.; Kovalenko, M.V.; Xia, Z.G. Seed-crystal-induced cold sintering toward metal halide transparent ceramic scintillators. Adv. Mater. 2022, 34, 2110420. [Google Scholar] [CrossRef] [PubMed]
- Zi, L.; Xu, W.; Song, Z.J.; Sun, R.; Liu, S.; Xie, T.Y.; Zhu, J.Y.; Lu, S.Y.; Song, H.W. Highly efficient and stable Cs2TeCl6:Cr3+ perovskite microcrystals for white light emitting diodes. J. Mater. Chem. C 2023, 11, 2695–2702. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhang, W.J.; Wen, T.Z.; Wen, X.; Ding, C.; Li, Z.F.; Yan, W.B. Excitation-dependent tunable white light of ns2 ions doped Rb2SnCl6 vacancy ordered double perovskite. J. Phys. Chem. Lett. 2022, 13, 11143–11152. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.K.; Sun, H.Y.; Li, L.H.; Li, R.F.; Ye, H.Y.; Li, J.R. Te4+-doping rubidium scandium halide perovskite single crystals enabling optical thermometry. J. Phys. Chem. C 2022, 126, 21689–21698. [Google Scholar] [CrossRef]
- Sedakova, T.V.; Mirochnik, A.G. Structure and luminescent properties of complex compounds of tellurium(IV) with ammonium bases. Opt. Spectrosc. 2015, 119, 54–58. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Cryst. 2015, 71, 59–75. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.K.; Ma, Z.M.; Qiu, Y.X.; Li, Z.W.; Fu, X.H.; Jiang, H.; Ma, Z.Y. Excitation-dependent luminescence of 0D ((CH3)4N)2ZrCl6 across the full visible region. J. Phys. Chem. Lett. 2022, 13, 7553–7560. [Google Scholar] [CrossRef]
- Yan, S.Y.; Tian, W.L.; Chen, H.; Tang, K.X.; Lin, T.T.; Zhong, G.Y.; Qiu, L.Z.; Pan, X.Y.; Wang, W.Z. Synthesis of 0D manganese-based organic–inorganic hybrid perovskite and its application in lead-free red light-emitting diode. Adv. Funct. Mater. 2021, 31, 2100855. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Zhang, Z.H.; Wang, X.X.; Huang, T.; Dong, T.T.; Xiao, Y.H.; Wang, J.P.; Zou, B.S. Bulk assembly of zero-dimensional organic copper bromide hybrid with bright self-trapped exciton emission and high antiwater stability. J. Phys. Chem. C 2021, 125, 20014–20021. [Google Scholar] [CrossRef]
- Zhou, J.; Rong, X.M.; Molokeev, M.S.; Wang, Y.L.; Yun, X.Y.; Xu, D.H.; Li, X. Alloying Cs+ into Rb2ZrCl6:Te4+ toward highly efficient and stable perovskite variant. Mater. Chem. Front. 2021, 5, 4997–5003. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhou, B.; Fang, S.F.; Nie, J.H.; Zhong, H.Z.; Hu, H.L.; Li, H.N.; Shi, Y.M. Modulation of the excitation states in all-inorganic halide perovskites via Sb3+ and Bi3+ cooping. J. Phys. Chem. Lett. 2023, 14, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, W.; Li, L.Y.; Huang, P.; Gong, Z.L.; Zhou, Z.W.; Sun, J.Y.; Yu, Y.; Chen, X.Y. Dual-Band-Tunable White-Light Emission from Bi3+/Te4+ Emitters in Perovskite-Derivative Cs2SnCl6 Microcrystals. Angew. Chem. Int. Ed. 2022, 61, e202116085. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Juan, Z.L.; Sun, X.; Zhang, W.G.; Zeng, H.B.; Li, X.M. Efficient, Stable, and Tunable Cold/Warm White Light from Lead-Free Halide Double Perovskites Cs2Zr1-xTexCl6. Adv. Opt. Mater. 2021, 9, 2100815. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.X.; Fang, S.F.; Nie, J.H.; Zhong, H.Z.; Hu, H.L.; Li, H.N.; Shi, Y.M. Emission mechanism of self-trapped excitons in Sb3+-doped all-inorganic metal-halide perovskites. J. Phys. Chem. Lett. 2022, 13, 9140–9147. [Google Scholar] [CrossRef]
- Biswas, A.; Bakthavatsalam, R.; Bahadur, V.; Biswas, C.; Mali, B.P.; Raavi, S.S.K.; Gonnade, R.G.; Kundu, J. Lead-free zero dimensional tellurium(IV) chloride-organic hybrid with strong room temperature emission as a luminescent material. J. Mater. Chem. C 2021, 9, 4351–4358. [Google Scholar] [CrossRef]
- Mao, Y.L.; Zhang, J.; Ren, Q.Q.; Molokeev, M.S.; Zhou, G.J.; Zhang, X.M. Unveiling the uncommon blue-excitable broadband yellow emission from self-trapped excitons in a zero-dimensional hybrid tellurium-based double perovskite. J. Mater. Chem. C 2022, 10, 17638–17645. [Google Scholar] [CrossRef]
- Liu, X.X.; Peng, C.D.; Zhang, L.J.; Guo, D.Y.; Pan, Y.X. Te4+-doped zero-dimensional Cs2ZnCl4 single crystals for broadband yellow light emission. J. Mater. Chem. C 2022, 10, 204–209. [Google Scholar] [CrossRef]
- Luo, Z.S.; Liu, Y.J.; Liu, Y.L.; Li, C.; Li, Y.W.; Li, Q.; Wei, Y.; Zhang, L.M.; Xu, B.; Chang, X.Y.; et al. Integrated Afterglow and Self-Trapped Exciton Emissions in Hybrid Metal Halides for Anti-Counterfeiting Applications. Adv. Mater. 2022, 34, 2200607. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Nie, K.; Wang, X.Y.; Duan, X.Q.; Zhou, R.R.; Wu, M.Y.; Ma, X.X.; Zhang, X.D.; Wang, L.X.; Mei, L.F.; et al. Facile synthesis strategy for cesium tin halide perovskite crystals toward light emitting devices and anti-counterfeiting flexible fiber. Nanoscale 2023, 15, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.F.; Wei, Q.L.; Lin, W.C.; Huang, T.; Ge, S.G.; Yu, Z.M.; Zou, B.S. Efficient yellow self-trapped exciton emission in Sb3+-doped RbCdCl3 metal halides. Inorg. Chem. 2022, 61, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Su, B.B.; Li, M.Z.; Song, E.H.; Xia, Z.G. Sb3+-doping in cesium zinc halides single crystals enabling high-efficiency near-infrared emission. Adv. Funct. Mater. 2021, 31, 2105316. [Google Scholar] [CrossRef]
- Jin, J.C.; Peng, Y.H.; Xu, Y.T.; Han, K.; Zhang, A.R.; Yang, X.B.; Xia, Z.G. Bright Green Emission from Self-Trapped Excitons Triggered by Sb3+ Doping in Rb4CdCl6. Chem. Mater. 2022, 34, 5717–5725. [Google Scholar] [CrossRef]
- Tan, Z.F.; Chu, Y.M.; Chen, J.X.; Li, J.H.; Ji, G.Q.; Niu, G.D.; Gao, L.; Xiao, Z.W.; Tang, J. Lead-free perovskite variant solid solutions Cs2Sn1–xTexCl6: Bright luminescence and high anti-water stability. Adv. Mater. 2020, 32, 2002443. [Google Scholar] [CrossRef]
- Peng, H.; Tian, Y.; Wang, X.X.; Dong, T.T.; Yu, Z.M.; Xiao, Y.H.; Zhang, Z.H.; Wang, J.P.; Zou, B.S. Highly efficient broadband green emission of (TPA)CuCl2 single crystals: Understanding the formation of self-trapped states. J. Phys. Chem. C 2022, 126, 8545–8552. [Google Scholar] [CrossRef]
- Dai, Y.R.; Wei, Q.L.; Chang, T.; Zhao, J.L.; Cao, S.; Zou, B.S.; Zeng, R.S. Efficient self-trapped exciton emission in ruddlesden–popper Sb-doped Cs3Cd2Cl7 perovskites. J. Phys. Chem. C 2022, 126, 11238–11245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).