Tailored Fabrication of Plasmonic Film Light Filters for Enhanced Microalgal Growth and Biomass Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Nanospheres
2.2. Synthesis of Gold Nanorods
2.3. Preparation of Plasmonic Films Containing Gold Nanorods
2.4. Preparation of Plasmonic Films with Dual Extinction Peaks
2.5. Microalgal Strain and Culture Conditions
2.6. Microalgal Pigment Determination
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of the Plasmonic Films
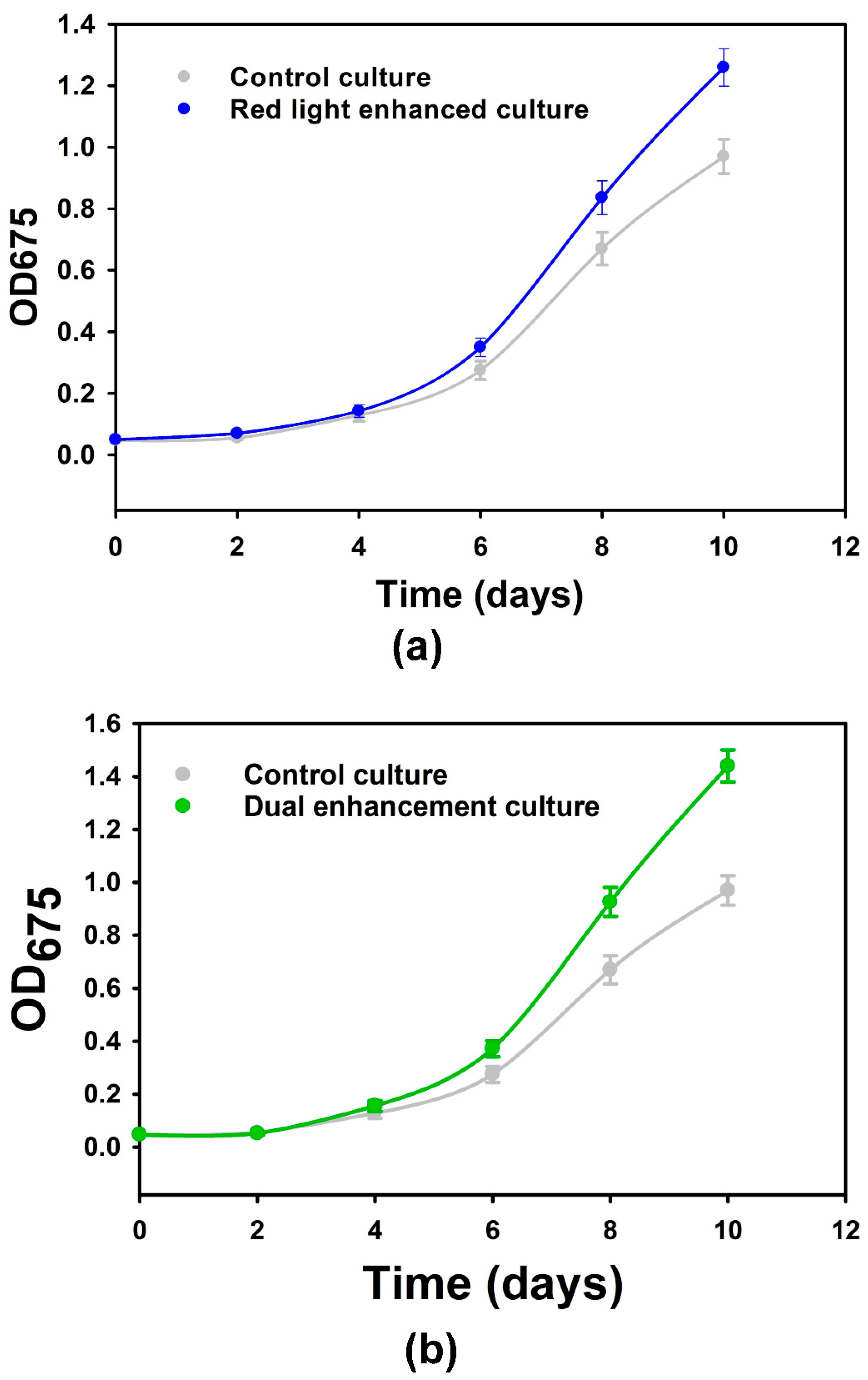
3.2. Microalgal Growth and Biomass Production with Tailored Plasmonic Film Filters
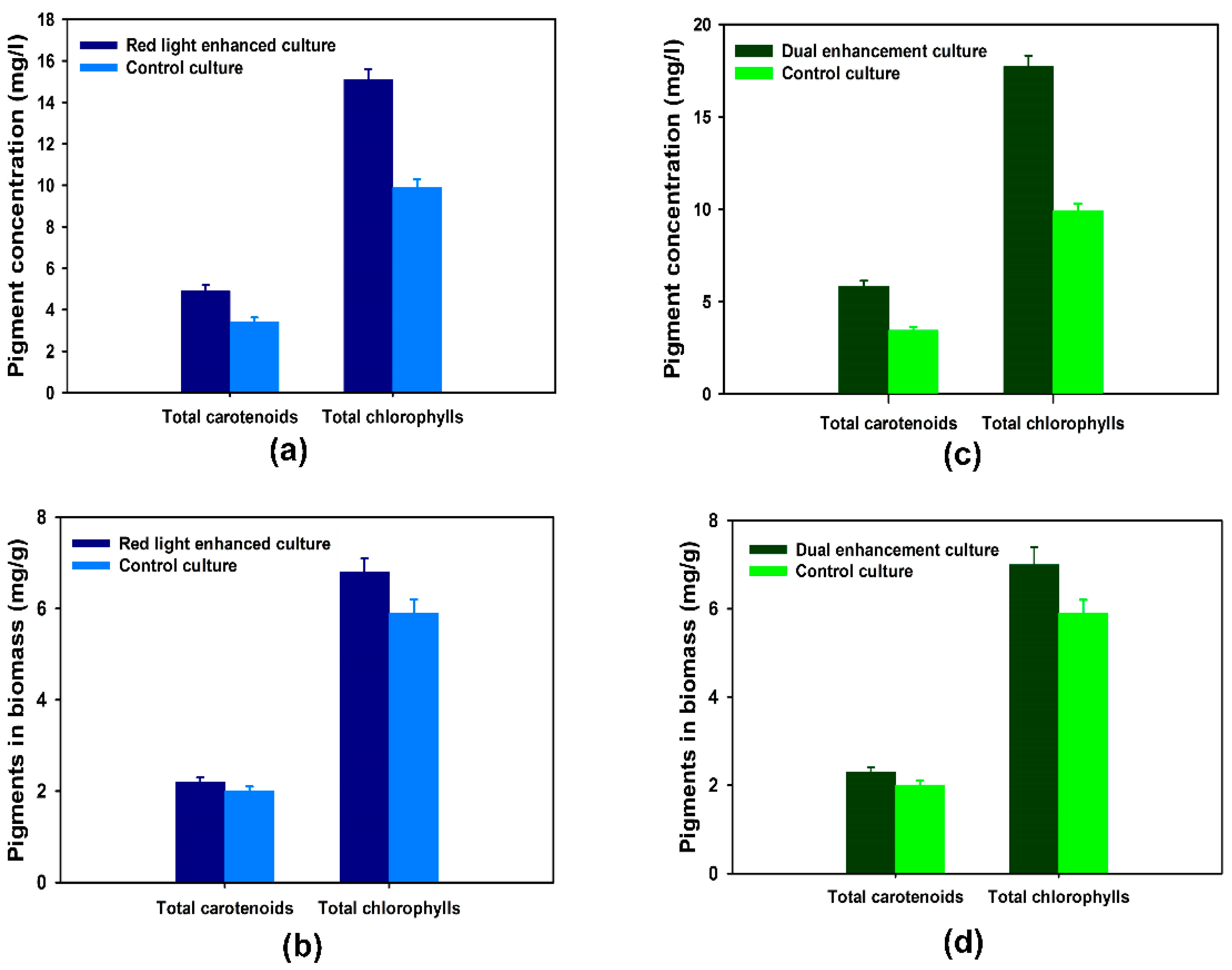
3.3. Specialty Chemicals Production with Plasmonic Film Filters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khurana, K.; Jaggi, N. Localized surface plasmonic properties of Au and Ag nanoparticles for sensors: A review. Plasmonics 2021, 16, 981–999. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Neuzil, P.; Reboud, J. Palm-sized biodetection system based on localized surface plasmon resonance. Anal. Chem. 2008, 80, 6100–6103. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Rho, W.Y.; Lee, S.K.; Kim, S.H.; Hahn, Y.B. TiO2 nanoparticles/nanotubes for efficient light harvesting in perovskite solar cells. Nanomaterials 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ouyang, S.; Ye, J. Gold-Nanorod-Photosensitized Titanium Dioxide with Wide-Range Visible-Light Harvesting Based on Localized Surface Plasmon Resonance. Angew. Chem. Int. Ed. 2013, 52, 6689–6693. [Google Scholar] [CrossRef]
- Cong, T.; Wani, S.N.; Paynter, P.A.; Sureshkumar, R. Structure and optical properties of self-assembled multicom-ponent plasmonic nanogels. Appl. Phys. Lett. 2011, 2010, 99. [Google Scholar]
- Estime, B.; Ren, D.; Sureshkumar, R. Effects of plasmonic film filters on microalgal growth and biomass composition. Algal Res. 2015, 11, 85–89. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.; Chang, J. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Nultsch, W.; Pfau, J.; Martena-Weide, M. Fluence and wavelength dependence of photoinhibition in the brown alga Dictyota dichotoma. Mar. Ecol. Prog. Ser. 1987, 41, 93–97. [Google Scholar] [CrossRef]
- Torkamani, S.; Wani, S.N.; Tang, Y.J.; Sureshkumar, R. Plasmon-enhanced microalgal growth in miniphotobioreactors. Appl. Phys. Lett. 2010, 97, 04703. [Google Scholar] [CrossRef]
- Mouget, J.L.; Rosa, P.; Tremblin, G. Acclimation of Haslea ostrearia to light of different spectral qualities–confirmation of chromatic adaptation in diatoms. J. Photochem. Photobiol. B Biol. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Napolitano, G.E. The relationship of lipids with light and chlorophyll measurements in freshwater algae and periphyton. J. Phycol. 1994, 30, 943–950. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzin, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon management for augmented photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef]
- Eroglu, E.; Eggers, P.K.; Winslade, M.; Smith, S.M.; Rasto, C.L. Enhanced accumulation of microalgal pigments using metal nanoparticle solutions as light filtering devices. Green Chem. 2013, 15, 3155–3159. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Haxo, F.T.; Blinks, L.R. Photosynthetic action spectra of marine algae. J. Gen. Physiol. 1950, 33, 389–422. [Google Scholar] [CrossRef] [PubMed]
- Metting, F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Borjas Esqueda, A.; Gardarin, C.; Laroche, C. Exploring the diversity of red microalgae for exopolysaccharide production. Mar. Drugs 2022, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Cordeiro, N. Microalgae as sustainable biofactories to produce high-value lipids: Biodiversity, exploitation, and biotechnological applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef] [PubMed]
- Cornet, J.F.; Dussap, C.G.; Dubertret, G. A structured model for simulation of cultures of the cyanobacterium Spirulina platensis in photobioreactors: I. Coupling between light transfer and growth kinetics. Biotechnol. Bioeng. 1992, 40, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Estime, B.; Ren, D.; Sureshkumar, R. Cultivation and energy efficient harvesting of microalgae using thermoreversible sol-gel transition. Sci. Rep. 2017, 7, 40725. [Google Scholar] [CrossRef]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta. J. Photochem. Photobiol. B Biol. 2005, 80, 71–78. [Google Scholar] [CrossRef]
- Ooms, M.D.; Jeyaram, Y.; Sinton, D. Wavelength-selective plasmonics for enhanced cultivation of microalgae. Appl. Phys. Lett. 2015, 106, 063902. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Gordon, H.T.; Bauernfeind, J.C.; Furia, T.E. Carotenoids as food colorants. CRC Crit. Rev. Food Sci. 1982, 18, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Microalgae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kightlinger, W.; Chen, K.; Pourmir, A.; Crunkleton, D.W.; Price, G.L.; Johannes, T.W. Production and characterization of algae extract from Chlamydomonas reinhardtii. Electron. J. Biotechnol. 2014, 17, 14–18. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Parlevliet, D.A.; Laird, D.W.; Alameh, K.; Moheimani, N.R. Light management technologies for increasing algal photobioreactor efficiency. Algal Res. 2019, 39, 101433. [Google Scholar] [CrossRef]
- Kirnev, P.C.S.; Carvalho, J.C.; Vandenberghe, L.P.S.; Karp, S.G.; Soccol, C.R. Technological mapping and trends in photobioreactors for the production of microalgae. World J. Microbiol. Biotechnol. 2020, 36, 42. [Google Scholar] [CrossRef]
- Song, X.; You, X.; Ren, X.; Zhang, X.; Tang, D.; Li, X. Vertically aligned Ag-decorated MoS2 nanosheets supported on polyvinyl alcohol flexible substrate enable high-sensitivity and self-cleaning SERS devices. J. Environ. Chem. Eng. 2023, 11, 109437. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Panahi, H.K.S.; Dehhaghi, M.; Orooji, Y.; Shahbeik, H.; Mahian, O.; Tabatabaei, M. Nanomaterials and their role in advancing biodiesel feedstock production: A comprehensive review. Biofuel Res. J. 2023, 10, 1901–1932. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estime, B.; Ren, D.; Sureshkumar, R. Tailored Fabrication of Plasmonic Film Light Filters for Enhanced Microalgal Growth and Biomass Composition. Nanomaterials 2024, 14, 44. https://doi.org/10.3390/nano14010044
Estime B, Ren D, Sureshkumar R. Tailored Fabrication of Plasmonic Film Light Filters for Enhanced Microalgal Growth and Biomass Composition. Nanomaterials. 2024; 14(1):44. https://doi.org/10.3390/nano14010044
Chicago/Turabian StyleEstime, Bendy, Dacheng Ren, and Radhakrishna Sureshkumar. 2024. "Tailored Fabrication of Plasmonic Film Light Filters for Enhanced Microalgal Growth and Biomass Composition" Nanomaterials 14, no. 1: 44. https://doi.org/10.3390/nano14010044
APA StyleEstime, B., Ren, D., & Sureshkumar, R. (2024). Tailored Fabrication of Plasmonic Film Light Filters for Enhanced Microalgal Growth and Biomass Composition. Nanomaterials, 14(1), 44. https://doi.org/10.3390/nano14010044





