Abstract
To reduce and prevent postsurgical adhesions, a variety of scientific approaches have been suggested and applied. This includes the use of advanced therapies like tissue-engineered (TE) biomaterials and scaffolds. Currently, biocompatible antiadhesive constructs play a pivotal role in managing postoperative adhesions and several biopolymer-based products, namely hyaluronic acid (HA) and polyethylene glycol (PEG), are available on the market in different forms (e.g., sprays, hydrogels). TE polymeric constructs are usually associated with critical limitations like poor biocompatibility and mechanical properties. Hence, biocompatible nanocomposites have emerged as an advanced therapy for postoperative adhesion treatment, with hydrogels and electrospun nanofibers among the most utilized antiadhesive nanocomposites for in vitro and in vivo experiments. Recent studies have revealed that nanocomposites can be engineered to generate smart three-dimensional (3D) scaffolds that can respond to different stimuli, such as pH changes. Additionally, nanocomposites can act as multifunctional materials for the prevention of adhesions and bacterial infections, as well as tissue healing acceleration. Still, more research is needed to reveal the clinical potential of nanocomposite constructs and the possible success of nanocomposite-based products in the biomedical market.
1. Introduction
Postoperative adhesion represents one of open surgery’s most common and challenging complications [1]. Post-surgery adhesions can occur anywhere on the body that has undergone an open manipulation, including the abdominopelvic, gynecologic, cardiovascular, and nervous system [2,3,4,5]. However, complications, such as chronic infection and endometriosis, may also lead to tissue adhesion [6]. The morbidity and incidence of postoperative adhesions are higher in abdominopelvic areas (peritoneal cavity) due to the peritoneum’s unique histological characteristics, as well as the multi-organ nature of the peritoneal cavity [7]. To illustrate this, a ten-year prospective research study carried out on 12,584 patients undergoing abdominal surgeries revealed a high prevalence (93%) of adhesion-related complications [8,9]. The high rate of postoperative adhesions and adhesion-related complications not only resulted in a high economic burden on patients and the healthcare system, but also resulted in serious long-term health problems for patients. From a morphological perspective, adhesions represent undigested, non-anatomical fibrous tissue connections (rich in fibroblasts) between the surgery site and adjacent tissues and organs (e.g., intestines) or the abdominopelvic wall [10]. Adhesion symptoms usually present as chronic pain (e.g., abdominal and pelvic pains) and may be followed by severe life-threatening conditions like small bowel obstructions [11]. Secondary infertility in females, organ movement limitation, and dysfunction are other complications associated with postsurgical adhesions [12,13]. Adhesions can be classified as de novo (type I) and secondary (type II), according to their development site and formation cause (surgery or not) [14]. De novo adhesion refers to adhesions that are formed in the body after surgery regardless of their location, whereas secondary adhesions result from either previously performed surgery or complications other than previous surgery (e.g., infections) [10,15]. Three core biological events happen during postoperative adhesion formation: (I) the inhibition of the fibrinolytic system and extracellular matrix (ECM) degradation, (II) induction of an inflammatory reaction, and (III) induction of tissue hypoxia and subsequent promotion of angiogenesis [16].
Up to now, a wide range of approaches have been proposed and employed to reduce and prevent postoperative adhesions, including improved surgical procedures, the use of specific pharmaceutical drugs (anti-inflammatory, anticoagulation, and anti-fibrosis medications), and combined therapies. However, the use of tissue-engineered (TE) constructs (biomaterials and three-dimensional (3D) scaffolds) has gradually become common practice for postoperative adhesion management in the era of modern medicine. In this regard, various biomaterial-based antiadhesive products have been commercialized and clinically used in different forms, including powders, solutions, sprays, injectable hydrogels, sponges, and films [17]. Most antiadhesive products are indeed biopolymer-based constructs and suffer from some inherent limitations, such as poor biocompatibility and mechanical properties [18,19,20]. It is well documented that natural polymers lack sufficient processability, brittleness, and tensile strength, while also undergoing rapid degradation. Using synthetic polymers, however, could remove these obstacles to some extent, but there are still some concerns regarding their biocompatibility like potential toxic effects, inflammation, and so on. To overcome these limitations, it is commonly suggested to fabricate composites by combining both natural and synthetic polymers to enhance their physicomechanical properties [21]. Recently, nanocomposites have received much attention for their use in treating postsurgical adhesions due to their superior physicochemical, mechanical, and biological features (e.g., enhanced biocompatibility and mechanical properties), as compared to their polymeric counterparts [22]. Current research indicates that there are excellent opportunities in utilizing antiadhesive nanocomposites for the treatment of postoperative adhesions, along with managing other complications (e.g., tumor recurrence) [23]. In this regard, nanocomposites can easily be engineered as multifunctional, stimuli-responsive constructs that can facilitate tissue healing acceleration through hemostasis maintenance, microbial infection inhibition, and cell growth stimulation and proliferation [24].
Most developed and employed nanocomposites are indeed organic–organic composites; thus, it may be interesting to explore the usability of organic–inorganic nanocomposites in managing postoperative tissue adhesions. It is worth noting that inorganic materials, including clays, carbon-based nanomaterials, metal oxides (e.g., bioceramics and bioactive glasses), and metallic nanoparticles, can reinforce the mechanical properties, anti-microbial performance, and cellular behavior of antiadhesive nanocomposites [25,26]. For instance, gold nanoparticles demonstrated lower microscopic and macroscopic peritoneal adhesion scores when compared with the untreated group in a rat animal model [27]. Additionally, taking advantage of novel technologies (e.g., 3D printing and customized additive manufacturing) in preparing TE constructs plays a substantial role in the next generation of antiadhesive substances. This paper is a comprehensive review that aims to introduce and criticize various types of antiadhesive biomaterials and scaffolds with a focus on the critical significance of biocompatible nanocomposites in the current and future fields. Accordingly, this work may be helpful for researchers and scientists who are trying to develop and introduce the next generation of antiadhesive products for possible clinical use.
2. Pathophysiology of Adhesions
Internal cavities of the body (e.g., thoracic and abdominopelvic cavities) are covered by a thin monolayer of loosely connected mesothelial cells, also known as the mesothelium, which is uniquely named for each cavity (pleura/peritoneum). The loose connection of monolayer cells and their delicacy have made mesothelial coverings susceptible not only to invasive traumas (e.g., surgery), but also to stressful conditions (e.g., exposure to talc powder of gloves, infection) [13]. Any disruption in mesothelial coverage can expose the basement membrane and underlying connective tissue to other adjacent tissues, which can trigger local inflammation. In the case of laparoscopies and laparotomies, postoperative adhesion can occur at the surgery site and is characterized as the initial and normal response of the body that deals with induced damage (Figure 1). In fact, the body begins the repair process of the injured tissue through the activation of coagulation, inflammation, proliferation, and remodeling pathways (i.e., the normal wound-healing process). In the case of adhesions, an imbalance between fibrin deposition and fibrinolysis arises during the wound-healing process, which leads to downregulated fibrinolysis and, consequently, adhesion development [25]. Providing precise information regarding affected tissues, including their repair process and the mechanisms by which adhesion formation develops, can help in discovering possible effective solutions to reduce and inhibit postoperative adhesions.
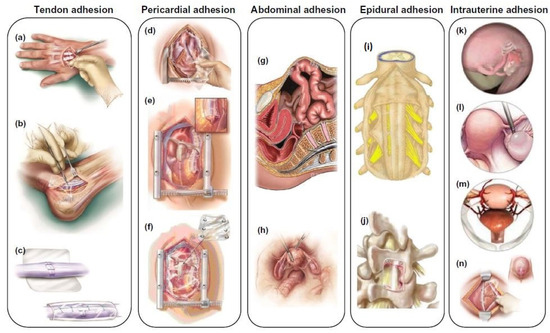
Figure 1.
Schematic illustration of different types of tissue adhesion, including tendon adhesion (a–c), pericardial adhesion (d–f), abdominal adhesion (g,h), epidural adhesion (i,j), and intrauterine adhesion (k–n). Reproduced with permission from [21].
Connective tissue is a rich pool of various cells, including fibroblasts, macrophages, and mast cells. These cells are pioneers in the inflammation cascade, once the peritoneum is damaged [26]. Mesothelial cells secrete peritoneal fluid, which is a rich source of blood histocytes, macrophages, mast cells, erythrocytes, and small amounts of mesothelial cells. The primary function of peritoneal fluid is lubricating and disinfecting the abdominopelvic cavity [27]. Importantly, mesothelial cells can gain profibrotic phenotypes, also known as adhesion phenotypes or myofibroblasts, when exposed to pro-inflammatory factors and undergo a mesothelial-mesenchyme transition (MMT). The MMT enables mesothelial cells to secrete inflammatory factors, chemokines, growth factors, and extracellular matrix (ECM) elements and facilitates the healing process [28]. In the following section, we will describe the underlying mechanisms related to different stages of postoperative adhesion, such as coagulation, inflammation, and fibrinolysis. These phases commonly occur in a parallel and overlapping time frame, in which the activation of each phase can potentially activate others.
2.1. Coagulation and Inflammation
Once the peritoneum integrity is disrupted, hemostasis and inflammation cascades are overlapping processes that suppress bleeding and prevent potential infections in the damaged areas. The local release of histamine and other signaling molecules (i.e., cytokines and growth factors) stimulates blood vessels for increased permeability, leading to the promoted recruitment and infiltration of inflammatory cells, specifically neutrophils and macrophages, around the wound area. Following local blood vessel injury, the endothelial cell monolayer can be interrupted, leading to von Willebrand factor (VWF) and tissue factor (TF) exposure, both of which exist in the vessel walls. VWF binds to platelets and mediates interactions between platelets and exposed collagen, resulting in platelet aggregation and platelet plug generation. With the initiation of coagulation, fibrinogen in the plasma is exuded, and many prothrombins are activated into thrombin due to platelet-released factors [29,30]. In the meantime, fibrinogen molecules are converted to fibrin through thrombin, consequently coagulating with aggregated platelets to generate a stable clot. Subsequently, the released cytokines from platelets, as well as the products resulting from the clot’s degradation, recruit more macrophages, neutrophils, mast cells, T cells, and mesothelial cells [31,32]. In fact, the fibrin deposition over the injured area can prepare a provisional matrix that facilitates inflammatory cell migration and subsequent inflammation (Figure 2). Immediately after the peritoneal injury, a huge number of inflammatory cells, mainly lymphocytes, monocytes, and neutrophils, infiltrate along with the peritoneal fluid. These cells can secrete various cytokines, including interleukin-1β (IL-1β), IL-6, tumor necrosis factor α (TNF-α), and vascular endothelial growth factor-A (VEGF-A), which are directly associated with the degree of adhesion [32]. IL-1, IL-6, and TNF-α may also be secreted from macrophages that are differentiated from peritoneal fluid monocytes and mesenteric mesenchymal stem cells [33,34,35,36]. Also, peritoneal macrophages may secrete tissue plasminogen activator (tPA), which is involved in the fibrinolysis process. As a matter of fact, there are two macrophage phenotypes, M1 (classical macrophages) and M2 (alternatively activated macrophages). M1 can secrete different cytokines (e.g., IL-1α, IL-1β, and TNF-α) and lead to the elimination of microorganisms, activation of nitric oxide synthase (NOS), and subsequent tissue damage in the case of excessive oxidative stress. In contrast, M2 macrophages have an anti-inflammatory role in the late stages of inflammation and contribute to wound healing and fibrosis. Pro-inflammatory cytokines released from M1 macrophages can bind to the surface receptors of mesothelial cells and activate the NF-κB signaling cascade, which consequently promotes the secretion of subsequent inflammatory molecules. Simultaneously, the activated Rho signaling pathway may cause the overexpression of plasminogen activator inhibitor (PAI-1), and hence suppress the peritoneal fibrinolysis system’s performance [28]. Activated neutrophils and macrophages can lead to increased oxidative stress within the mesothelium and promote adhesion formation [37]. Furthermore, the subsequent extra hydrogen peroxide and superoxide anions generated are cytotoxic and can damage cells, e.g., endothelial cells, platelets, and fibroblasts [35]. Cytolysis and lipid peroxidation of cell membranes can result in enhanced vascular permeability and further exudate leakage, facilitating and expediting the adhesion process. Moreover, hyaluronic acid (HA) secretion from the peritoneal mesothelium can act as a recruitment factor for inflammatory and immune cells, promoting the expression of transforming growth factor-β (TGF-β) and TNF-α [38], which play substantial roles in fibrosis and adhesion development. Several cellular pathways, along with the TGF-β1 signaling pathway, can contribute to fibrosis. These pathways (e.g., PI3K/AKT, Wnt/β-catenin) can proceed with the irreversible conversion of the mesothelium to myofibroblasts [39,40,41]. Myofibroblasts can form adhesions in the peritoneal cavity due to their high-level expression of collagen (type I and III), matrix metalloproteinase-1 (MMP-1), tissue inhibitor of metalloproteinase-1 (TIMP-1), VEGF, and other factors [42,43]. C-X-C motif ligand 1 (CXCL1) and monocyte chemoattractant protein 1 (MCP-1) produced by recruited neutrophils and monocytes can lead to adhesions through the activation of angiogenesis and fibrin deposition [44,45,46]. In conclusion, the inflammation phase can regulate adhesion development through the recruitment of cells and the secretion of chemokines. The final products of this step can stimulate further fibrin and ECM deposition.
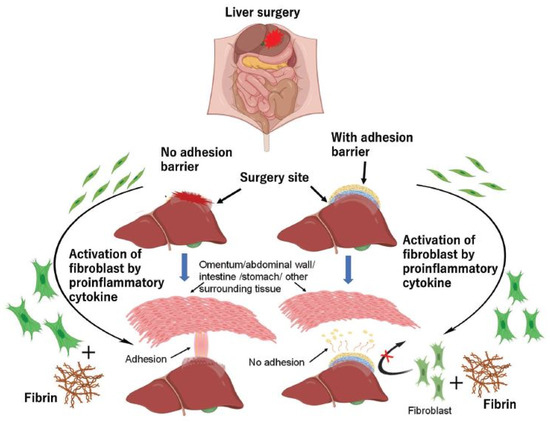
Figure 2.
A schematic representation of the role of fibrin and fibroblasts in the formation and inhibition of tissue adhesion. Reproduced with permission from [47].
2.2. Fibrinolysis
Fibrin serves as a temporary matrix during tissue repair and is removed after the healing process. The appropriate performance of the fibrinolytic system (anticoagulation system), alongside the inflammation and coagulation processes, is required to dissolve the fibrin scaffold. When fibrin does not degrade in the desired time frame, fibroblasts synthesize collagen within the fibrin matrix, leading to permanent adhesion. Hence, it is necessary to dissolve extra fibrin in time. The ideal time for adhesion prevention is five to seven days after injury, except for those affected by infection or foreign-body invasion. The fibrinolytic system’s activation is regulated by intact mesothelial cells and macrophages. The final product of this system is plasmin, the activated form of plasminogen. Plasmin can destroy fibrin clots by breaking down the peptide bonds between arginine and lysine in fibrinogen and fibrin, making them soluble substances [48]. Plasminogen–plasmin conversion is controlled by several factors, including tissue/urokinase plasminogen activators (tPA, uPA), plasminogen activator inhibitors (PAI-1, PAI-2), plasmin inhibitors (PIs), and a series of enzymatic reactions. Moreover, thrombin-activatable fibrinolysis inhibitor (TAFI) and α2-antiplasmin (α2-AP) can inhibit this conversion through the inactivation of plasmin [26]. The tPA and uPA activate the inactive precursor plasminogen into plasmin, while PAI-1 can efficiently suppress this activation. The highest amount of tPA is released from vascular endothelial cells (ECs), mesothelial cells, and macrophages [49]. tPA has a strong affinity for fibrin and forms the fibrin–tPA complex, which can rapidly activate plasminogen in blood clots. Plasminogen activation in the abdominal area is mostly (around 95%) regulated by tPA. The binding of uPA to its receptor urokinase-type plasminogen activator receptor (uPAR) can promptly activate plasminogen, which has a significant role in tissue remodeling. Both fibrinogen and fibrin can promote the production of TNF-α and IL-1β, inciting the secretion of PAI-1 from mesothelial and endothelial cells to prevent plasminogen activation. PAI-1 can in turn enhance adipose tissue development, as well as trigger macrophages to increase inflammation in the damaged area, leading to adhesion [50]. It should be noted that there is a synergistic relationship between fibrinolysis and matrix metalloproteinase (MMPs) in wound repair. The main biological task of MMPs is to degrade the ECM. As a major member of the MMP family, MMP-3 can block PAI-1 and α2-AP to increase plasmin amounts. On the other hand, plasmin can activate proMMP-9 to MMP-9 to remodel the ECM [51,52,53]. In addition, normal mesothelial cells can also contribute to fibrinolysis and restrict adhesion formation. Finally, it is worth noting that any disruption in the balance between coagulation and inflammation with fibrinolysis phases can cause adhesion (Figure 2).
2.3. Others
In normal conditions the fibrin matrix appears temporarily and is degraded through the fibrinolysis event within three to five days. The fibrinolytic process’s balance is varied based on the operative intervention. In a rat animal model study [54], postoperative adhesion formation was evaluated by measuring the levels of tPA and PAI-1 in three distinct groups: the control nonoperative group, open-surgery group, and laparoscopic surgery group. The collected data revealed that tPA levels were significantly reduced in the laparoscopic operation group versus the nonoperative control and open-surgery groups. Furthermore, the mRNA expression of PAI-1 was higher in the open-surgery group when compared with the laparoscopic group, indicating enhanced adhesion in the open group. These results reflect the minimally invasive nature of laparoscopic surgeries, even when prolonged operations, compared to open surgeries. Indeed, hypoxia-inducible factor-1α (HIF-1α) can bind to the oxygen-sensitive promoter regions of the PAI-1 gene, resulting in its upregulation [55]. It has been reported that the tPA/PAI-1 ratio declined in hypoxic conditions [56,57]. Tissue hypoxia with increased generation of oxygen and nitrogen reactive species, ROS and RNS, respectively, can contribute to enhanced oxidative stress, resulting in DNA damage, cell apoptosis, and the overproduction of oxidized proteins [58]. ROS production has been demonstrated to stimulate adhesion formation by upregulating the expression of several factors, including TNF-α, IL6, type I collagen, and VEGF [59]. Moreover, tissue hypoxia can increase the production of superoxides. It was shown that fibroblasts exposed to superoxides can generate pro-fibrogenic factors, like TGF-β and type I collagen [60]. Additionally, it has been revealed that HIF-1α and TGF-β can both increase the levels of VEGF that are primarily released by mast cells, stimulating vascularization and promoting vascular permeability [61]. Angiogenesis is another physiological phenomenon that contributes to the wound-healing process in response to local ischemia. This process is highly intermingled with inflammation, coagulation, and fibrinolytic processes. VEGF can enhance the deposition of a fibrin-rich ECM to provide a substrate for newly formed vasculature to overcome local ischemia [62]. Furthermore, the angiogenesis process can contribute to the organization and maturation of adhesive bands, and can provide the nourishment of residing cells [63]. Alongside this, fibrinolytic system activity is required to facilitate ECM degradation and then its subsequent remodeling [64]. Regulated parallel activity between the aforementioned systems may reduce adhesion during wound healing; however, this is a rare occurrence, and adhesion takes place as a result of excessive fibrin deposition [25].
Despite all efforts, there is no perfect approach to reducing and preventing postsurgical adhesions. For example, adhesiolysis surgery is usually performed to relieve patients with severe conditions; however, it was reported that 47% of patients need secondary surgery [65]. Preventive practices to inhibit postsurgical adhesions can include (I) improved surgical techniques, (II) utilization of mechanical barriers (e.g., solid membranes and films, as well as fluidic hydrogels), and (III) administration of antiadhesive agents that can interfere with various adhesion processes (e.g., anticoagulant, anti-inflammatory, antioxidant) [48] (Table 1). Consequently, findings have demonstrated that performing minimally invasive surgical approaches, such as laparoscopy, may minimize postsurgical adhesions in candidate patients. However, several ineligible patients return with adhesion complications even after laparoscopic surgery [66]. The burden associated with secondary surgery and postsurgical complications has led to the development and use of barrier patches to prevent adhesions. Up to now, many commercialized antiadhesive biomaterials (e.g., Seprafilm®, Spraygel®, Adept®) have been developed in the form of films, gels, sprays, and solutions, to name a few [50,65,67,68]. However, there are some concerns associated with the use of these barriers, including decreased efficacy attributed to their high biodegradation in the abdominal cavity (liquid barriers), dislocation from the targeted place after surgery, and limited capability to properly cover the affected area [50]. In the following sections, we introduce different types of biocompatible materials utilized for the treatment of postoperative adhesions, and then highlight the beneficial role of nanostructured biocomposites in comparison to commonly used polymeric constructs.

Table 1.
A summary of proposed and applied treatment approaches for the management of postoperative adhesions.
3. Biocompatible Materials for Postoperative Adhesions
To date, a wide range of natural and synthetic materials have been processed and developed for managing postoperative adhesions in various locations, such as the peritoneum and heart. Polymers stand out among applied biomaterials in terms of postoperative adhesion prevention due to their biocompatibility and tunable characteristics (e.g., controllable biodegradability and ease of functionalization) [84]. Additionally, polymers can be used for the loading and delivery of a broad range of therapeutic cargoes to improve outcomes. It can be concluded from the literature that theadministration of both natural and synthetic polymers has led to the successful prevention of postsurgical adhesions (Table 2). Although naturally occurring biopolymers exhibit excellent compatibility with living organisms, they suffer from poor mechanical properties, lower productibility, and batch-to-batch variations. Collagen, gelatin, chitosan, alginate, hyaluronic acid (HA), silk fibroin (SF), dextran, chondroitin sulfate, carboxymethyl cellulose (CMC), starch, and cellulose are some of the most commonly used natural polymers for surgical purposes [85]. On the other hand, numerous synthetic biopolymers have been suggested and employed to control postsurgical adhesions. Poly ε-caprolactone (PCL), polyethylene glycol (PEG), polylactic acid (PLA), poly lactic-co-glycolic acid (PLGA), polyvinyl alcohol (PVA), and polyvinylpyrrolidone (PVP) are some examples of well-known synthetic biopolymers utilized for the prevention of postoperative adhesions.

Table 2.
Pros and cons of polymers utilized to prevent postoperative adhesions.
It should be highlighted that many of the mentioned biopolymers are now available as commercialized products in the market for clinical use, including Seprafilm®, SeprasprayTM, Hyalobarrier®, Adept®, and SprayGelTM [106] (Table 3). Importantly, each product can be used for specific locations and medical conditions in the body according to the manufacturer’s guidelines. For example, resorbable membranes made of HA and CMC in the form of a film (Seprafilm®, Sanofi/Genzyme) or a woven fabric (Interceed®, Ethicon) were indicated only for the management of abdominal adhesions [107]. Biopolymer-based products are commonly formulated into diverse forms (e.g., films, hydrogels, solutions, sprays, and porous scaffolds) for use as antiadhesive constructs. In this regard, several products, such as polymer-based films, have been commercialized and are available on the market. For instance, Seprafilm®, a mechanical bioresorbable antiadhesive barrier, received FDA market approval in 1996. It is made of HA and CMC, which can be converted to a gel form 24 to 48 hours post-operation and remains in the area for up to seven days. However, research on novel antiadhesive film compositions is in progress, since no significant differences were reported regarding their potential in adhesion prevention [71]. It is important to note that electrospun nanofibers with different compositions were also studied as barrier membranes for antiadhesive strategies. Electrospun nanofibrous scaffolds offer specific advantages for treating postsurgical adhesions, including ease of fabrication and modification, high surface area, and drug-loading capability. Although several electrospun nanofibers were successfully utilized to reduce adhesions, some studies have mentioned that electrospun nanofibers fail to completely prevent adhesion formation. In this regard, polymer degradation may interfere with the antiadhesion process in vivo; for example, PLA degradation was shown to hamper the antiadhesion effectiveness against peritendinous adhesions by promoting M2 macrophage polarization mediated by enhanced STAT6 phosphorylation and activated myofibroblasts [108]. Therefore, optimizing the rate of electrospun nanofiber biodegradation should be considered to reduce possible side effects. In addition, loading therapeutic biomolecules and drugs onto electrospun nanofibers was found to be useful for obtaining satisfying outcomes [109,110].

Table 3.
A summary of commercially available antiadhesive products in clinical administration or development [17].
As naturally derived constructs, decellularized extracellular matrixes have been studied for preventing postsurgical adhesions and promoting tissue repair and regeneration [111,112,113]. To exemplify this, a decellularized tendon matrix (DTM) has been successfully prepared through the decellularization of bovine tendon tissue and employed as an antiadhesive construct [114]. The results showed that DTM could degrade after subcutaneous implantation over 12 weeks without any significant signs of inflammatory reaction. Furthermore, this natural construct could prevent tendon adhesion in the Achilles tendon of rabbits, along with accelerated tendon repair and regeneration.
Injectable hydrogels are considered the most promising tissue-engineered construct in the prevention of postoperative tissue adhesions [107,115]. Hydrogels exhibit outstanding characteristics for use in postsurgical adhesions, including antifouling capability and the possibility of loading and controlled release of different drugs at desired sites. Moreover, hydrogel crosslinking can be performed by using various physical (e.g., interaction between ions, hydrogen bonding) or chemical (e.g., photo-initiated hydrogels, enzymatic reactions, “Click” reactions) approaches to modulate and customize their properties [115]. Stimuli-responsive hydrogels are a specific class of hydrogels that undergo structural, mechanical, and biochemical changes in response to environmental cues (e.g., heat). They are known as useful constructs in drug delivery and tissue-engineering applications [116]. Concerning antiadhesive applications, thermo-responsive hydrogels composed of poly(N-isopropyl acrylamide) (PNIPAM) grafted with chitosan (CS) and hyaluronic acid (HA) were shown to have superior capabilities in preventing postoperative paratendinous adhesion. These hydrogels exist in a free-flowing form before injection, and are then converted to a gel in the body, leading to the separation of the wound surface from adjacent tissues and organs with negligible invasiveness [117]. In some studies, drug-loaded hydrogels were investigated for managing postoperative adhesions in vivo. The obtained data from these studies has been promising in terms of preventing adhesion formation through pharmacological processes [118]. For example, the administration of naproxen-nanoparticle-containing chitosan hydrogels in a rat model of abdominal cecum adhesion led to the prevention of postoperative abdominal adhesions and pain relief without any significant adverse effects to vital organs, including the liver, spleen, heart, lung, and kidneys [119]. Optimal dosage selection and the sustained release of loaded drugs are mentioned among the most challenging issues for the extensive usage of drug-loaded hydrogels.
Recently, porous scaffolds based on droplet microfluidics have attracted huge research interest for the prevention of specific postsurgical adhesion cases, such as intrauterine adhesions. Notably, Cai et al. utilized microfluidics for the production of monodisperse droplet templates of gelatin methacryloyl (GelMA) and sodium alginate (Na-alginate) and then fabricated 3D scaffolds having an external-internal connected pore structure with the assistance of ultraviolet (UV) and calcium chloride solidification [120]. They first investigated different aspects of the produced scaffolds (e.g., biocompatibility, compressibility, swellability, degradation rate, and drug release profile) in vitro. Next, bFGF-loaded scaffolds were transplanted into an IUA rat model to determine their potential in the prevention of postoperative adhesions. The authors observed excellent biocompatibility and biodegradability of the fabricated scaffolds, along with proper compressibility for delivery to the uterus through the vagina. Moreover, the scaffolds regenerated the damaged endometrium in the IUA rat model, providing proof of their value in preventing IUAs. It should be noted that the potential of stem-cell-loaded constructs has been evaluated for preventing tissue adhesions [41,121]. For example, bone-marrow-derived mesenchymal stem cells (BMSCs) were loaded onto scaffolds made of poly (glycerol sebacate) (PGS), a poly(lactic-co-glycolic acid) (PLGA) scaffold, and collagen and subsequently transplanted into a wounded rat uterus model. The reported findings indicate that the administration of BMSC-loaded PGS constructs can result in better regeneration (thicker endometrium with more glands) of the damaged uterus and prevent intrauterine adhesions (IUAs) compared to the other groups. This construct was introduced as a promising alternative to estrogen-containing intrauterine devices that are clinically used for preventing intrauterine adhesions (IUAs) and promoting endometrium regeneration after surgical synechiotomy.
4. Biocompatible Nanocomposites: New Players in Postoperative Adhesions
Compared to bulk materials, the use of nanosized materials (nanomaterials) has led to substantial improvements in biomedical strategies due to their unique characteristics, such as higher surface area and reactivity [122]. Nanocomposites are heterogeneous materials that are composed of matrixes and nanosized fillers. Depending on the incorporated matrix material, they are divided into ceramic matrix nanocomposites (CMNC), polymer matrix nanocomposites (PMNC), and metal matrix nanocomposites (MMNC). The integrated nanosized filler phase can be in any shape (nanofiber, nanoparticle, nanosheet, etc.) and from an organic/inorganic source. Therefore, depending on the contributing phases, nanocomposites can be organic-organic or organic-inorganic [123] (Figure 3). The incorporation of nanoparticles into polymeric matrixes results in biocompatible nanocomposite generation that can be utilized for diverse biomedical applications, such as treating postoperative adhesions. Both naturally occurring substances (e.g., amnion) and synthetic biomaterials (e.g., PLA) have been utilized as polymeric substrates of biocomposites [124,125]. Nanocomposites offer exceptional benefits for treating postoperative adhesions, including improved biocompatibility and enhanced mechanical properties, which can be achieved through various methods, including crosslinking, hybridization/reinforcement with nanoparticles, etc. [126,127,128]. Moreover, they benefit from tunable and controlled biodegradability, which means that various compositions of natural- and/or synthetic-based biomaterials can be designed to generate a biocompatible nanocomposite possessing a controlled degradation rate synchronized with tissue repair [17]. Given adhesion’s role in infectious disease development, it is worth mentioning that antiadhesive nanocomposites can inhibit the adhesion of microorganisms. This can lead to the development of a new generation of anti-microbial nanocomposites for several strains that are resistant to common antibiotics [129]. Based on the literature, it can be stated that hydrogels and electrospun nanofibers form the main types of antiadhesive nanocomposites [130,131,132].
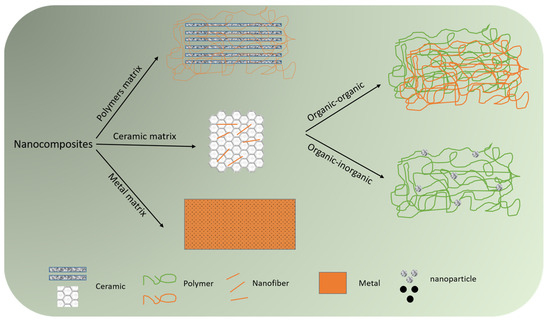
Figure 3.
Classification of nanocomposites based on constituent matrix (polymer, ceramic, or metal) and incorporated phases.
Polysaccharide-based composite hydrogels made of N, O-carboxymethyl chitosan (N, O-CS) and oxidized dextran (ODA) were previously developed as self-healing injectable antiadhesion barriers with antibacterial and hemostatic capacities [133]. In fact, carboxymethyl was added to the N and O positions of glucosamine and N-acetylglucosamine units of chitosan to improve its solubility under physiological conditions. On the other hand, the crosslinking of the ODA aldehyde functional group with the N, O-CS amino functional group modulated by the Schiff base resolved the need for any toxic crosslinking agents or radiation sources. Accordingly, the fabricated hydrogels showed excellent biocompatibility and hemocompatibility in vitro and in vivo with an optimal degradation rate. After 14 days of surgery, these hydrogels could inhibit the adhesion of fibroblasts to the injured abdominal wall in rats, thereby preventing tissue adhesion formation even better than commercial carboxymethyl chitosan hydrogels. Recent progress in the field has led to the introduction of a new generation of hydrogels that show unique characteristics for postoperative adhesions as compared to conventional hydrogels (covalently crosslinked hydrogels). In this regard, dynamically crosslinked supramolecular polymer–nanoparticle hydrogels were produced from hydrophobically modified HPMC-C12 and PEG-PLA nanoparticles as an effective postoperative pericardial adhesion barrier [107]. The hydrogels were shear-thinning and self-healing with viscoelastic flow properties that could be sprayed with standard equipment. The results from the in vivo study performed on a rat model with severe pericardial adhesions indicated that the hydrogels could adhere to tissue due to their adhesive part (HPMC-C12), as well as reduce the severity of pericardial adhesions even better than two commercial products, Seprafilm® (film) and Interceed® (fabric). Moreover, the fabricated hydrogel nanocomposites could successfully decrease the severity of cardiac adhesions in a cardiopulmonary-bypass model in sheep compared to untreated animals.
Recently, the development and utilization of multifunctional composite hydrogels have become a trend for the simultaneous prevention of postoperative adhesions and tumor recurrence [134]. For example, pH-responsive nanocomposite hydrogels were prepared using collagen (Col) and recombinant albumin nanoparticles (HHD NPs) crosslinked with aldehydeylated polyethylene glycol (APG6K) as a potential construct for overcoming two major problems in the postoperative treatment of abdominal tumors (i.e., abdominal adhesion and tumor recurrence [23] (see Figure 4)). One side of the hydrogel surface was coated with zwitterionic cysteine (Cys) to form a hydration layer that hinders protein and cell attachment, subsequently reducing adhesion between tissues. The other side of the construct served as an adhesive surface due to the presence of collagen. This hydrogel system released HHD NPs under acidic conditions, which is commonly observed in the cancer microenvironment. The released NPs led to a sharp decrease in the survival rate of cancer cells (HeLa cell line) compared to normal cells (NIH 3T3 cell line). The results of this in vivo study showed that hydrogels could prevent intraperitoneal adhesion in the lateral wall defect of a cecal abrasion rat model. This drug-loaded system could inhibit tumor growth in female Balb/c-nu nude mice, as the tumor’s volume was eight times smaller than that in the control group on the fifteenth day, with minimal side effects on the animals’ organs (e.g., heart and liver).
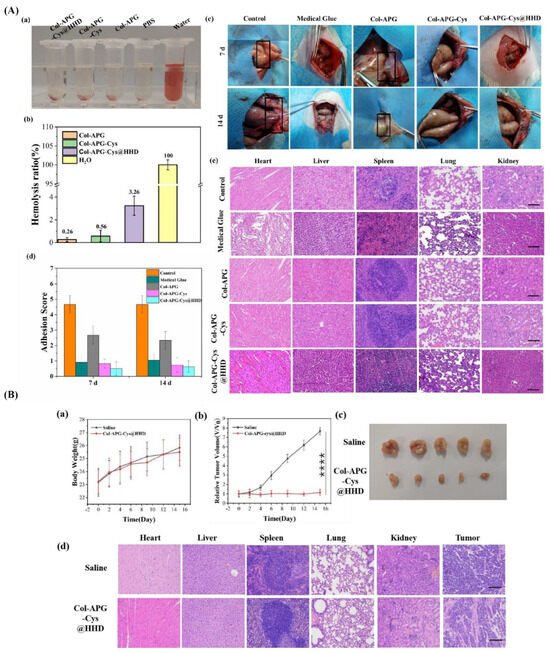
Figure 4.
Antiadhesive and anti-cancer properties of COL-APG_Cys@HHD hydrogel. (A) (a) Optical image of the hemolysis interaction between blood and five distinct groups, namely, Col-APG-Cys@HHD, Col-APG-Cys, Col-APG, PBS, and water. (b) Quantitative representation of the hemolysis ratio of relevant samples. (c) Macroscopic observation of the control group and the antiadhesive effect of medical glue, Col-APG, Col-APG-Cys, and Col-APG-Cys@HHD hydrogels on tissue. (d) Distribution of adhesion scores on days 7 and 14 after surgery in the corresponding groups. (e) Histopathological evaluation of normal organ sections collected from animals treated with saline, medical glue, Col-APG, Col-APG-Cys, and Col-APG-Cys@HHD hydrogels after sacrifice through H&E staining. The scale bar shows 100 µm. (B). (a) Variations in body weight of tumor-bearing mice for the duration of therapy. Data are shown as mean ± SD, n = 5. (b) Tumor growth curves after tail IV injection of saline and Col-APG-Cys@HHD. Data are shown as mean ± SD, n = 5. (**** p < 0.0001) (c) Macroscopic images of the tumor size stripped from mice in control and Col-APG-Cys@HHD groups. (d) H&E staining of normal organs and tumor sections collected from animals treated with saline and Col-APG-Cys@HHD at the end of the experiment. The scale bar shows 100 µm. Reproduced with permission from [23].
Electrospun nanofibrous nanocomposites have shown great promise as barrier membranes in postsurgical adhesion management [44,135,136,137]. However, commercially available membranes suffer from some restrictions; for example, SeprafilmTM and InterceedTM are considered to have poor mechanical properties and performance with blood contact, respectively. Accordingly, researchers have made huge efforts to develop novel formulations of electrospun mats with improved mechanical and biological features. In this regard, gelatin and CMC were previously added to PCL to fabricate tricomposite nanofiber barrier membranes with improved hydrophilicity, mechanical stability, and biocompatibility [138]. Another crucial issue in using electrospun mats is associated with their surface characteristics, which commonly provide a suitable surface for cell attachment and growth. This can be a critical challenge once adjacent tissue cells attach and grow on the membrane, resulting in severe tissue adhesion. Therefore, electrospun membrane surface modification has been suggested as a reasonable approach to improve their performance in vivo. In this regard, the surface of electrospun nanocomposites made of PLA/photo-initiator Irgacure-2959 underwent nanoscale coating to generate a super-lubricated nano-skin (SLNS) on the surface of nanofibers [139]. The surface-modified constructs exhibited ideal tensile properties and biocompatibility, and in vivo studies in rat tendon and abdominal adhesion models supported their acceptable antiadhesive performance.
Membrane nanocomposites fabricated by coaxial electrospinning techniques are generally known as appropriate vehicles for the delivery of various therapeutic cargoes to desired locations. Concerning postsurgical adhesions, a series of core-shell nanofibrous membranes were prepared in which HA/platelet-rich plasma (PRP) and PCL serve as the core and shell of electrospun nanocomposites, respectively [140]. PRP is a rich source of bioactive molecules (e.g., growth factors TGF-β1, PDGF, FGF-2) that can help accelerate wound healing (e.g., tendon recovery after tendon injury). The shell of the nanocomposites was prepared by solving PCL in ethylene chloride and N, N′-dimethylformamide (volume ratio = 4:1) to obtain a PCL polymer solution (8%(w/v)). The optimized core solution had a composition of 1.75% (w/w) PRP, 1.75% (w/w) HA, and 0.5% PEO at a 1/1 HA/PRP mass ratio. The PRP-loaded membrane scaffolds prevented penetration and decreased the attachment/focal adhesion of NIH/3T3 mouse embryonic fibroblasts while promoting tenocyte migration in vitro. In addition, the implantation of drug-loaded nanocomposites reduced tendon adhesion formation and inflammation and improved the healing process in the rabbit flexor tendon rupture model. The use of organic-inorganic nanocomposites made of polymers and nanoparticles offers another interesting class of constructs for treating postsurgical adhesions. On this matter, core-sheath nanofiber membranes were previously prepared by embedding silver nanoparticles (Ag NPs) in a PLA nanofiber sheath, with HA in the nanofiber core, and used to prevent postoperative tendon adhesions in peritendinous antiadhesion rabbit models [134]. The thicknesses measured for a thick sheath (Tk) and thin sheath (Tn) without Ag NPs and a thick sheath (Tk+) and thin sheath (Tn+) containing Ag NPs were 643 ± 119, 680 ± 167, 692 ± 165, and 725 ± 226 nm, respectively. The in vitro experiments demonstrated that nanocomposites with thin sheaths containing Ag NPs possess suitable antibacterial activity and could prevent fibroblast penetration and attachment with negligible cytotoxicity. Moreover, in vivo data indicate that this nanocomposite can meaningfully prevent peritendinous adhesions and moderate inflammatory reactions in comparison to its thin-sheath counterparts and the PLA-based commercial adhesion barrier film (SurgiWrap®). Another interesting class of nanocomposites used to prevent postoperative adhesion includes multilayer membranes made of electrospun nanofibers and naturally occurring substances. In this regard, electrospun PCL-amniotic membrane composites were successfully developed and evaluated for preventing postsurgical tendon adhesions [141]. For instance, fresh amnions were first freeze- and vacuum-dried, with both sides then coated with PCL nanofibers using electrospinning techniques. In fact, PCL nanofibers were assumed to act as the outer layer and mimic the function of a natural tendon sheath to prevent the invasion of fibroblasts and other tissues. The amniotic membrane was assumed to serve as the intermediate layer for promoting the tendon’s endogenous healing via the release of bioactive molecules (e.g., TGF-β1, bFGF, PDGF, and VEGF). Subsequently, diffusion of the aforementioned molecules from nanofiber pores into the injured site can lead to improved tenocyte proliferation and collagen synthesis. The in vitro assessments of this bioactive-molecule-enriched nanocomposite showed that the upregulation of the phosphorylation of ERK1/2 and SMAD2/3 resulted in improved tenocyte proliferation and enhanced collagen synthesis. Furthermore, in vivo administration of the nanocomposite in the rabbit tendon repair model confirmed that this system can effectively inhibit exogenous adhesion and enhance endogenous tendon healing.
In addition to injectable hydrogels and electrospun nanofibrous membranes, other forms of nanocomposites were also developed and employed as antiadhesive barriers; for example, 3D PCL scaffolds were fabricated using a salt-leaching technique and loaded with biphasic calcium phosphate and silver nitrate to serve as bioactive and bioresorbable as well as antibacterial composites, respectively. The obtained data revealed that the silver-functionalized scaffold could significantly decrease the adhesion and growth of staphylococci on the biocomposite. Moreover, polydopamine (PDA)-human keratinocyte growth factor (KGF) nanoparticles (PDA-KGF NPs) combined with hyaluronate (HA) have been examined for preventing postoperative abdominal adhesion formation in rats [142]. The published results indicated that nanoparticles (150 to 210 nm) could not only effectively prevent the incidence of abdominal adhesions in the receiving animals, but also improved mesothelial cell repair in the injured peritoneum. This nanocomposite could decrease collagen deposition and fibrosis in the injured peritoneum and inhibit inflammatory reactions. In another study, Andrew Z. Wang’s research group developed a photo-crosslinkable nanopatch and studied its effectiveness in a rat parietal peritoneum excision (PPE) model [143]. The nanopatch was composed of two nanoparticles: PEG-PLGA functionalized with a collagen IV-targeting peptide (Col-PEG-PLGA) (namely, NP-A) and PLGA-PEG covered with a branched polyethyleneimine (PEI) shell (namely, NP-B). They encapsulated dexamethasone 21-Palmitate (Dex-Pal) with negatively charged NA-A at a concentration of 65.2 ± 4.02 µg mg−1 to prevent adhesion formation. Positively charged NP-B was surface-functionalized with diazirine groups to provide photo-induced crosslinking among the nanoparticles. NP-A was administered to the injured site to investigate its potential for specific binding to the basement membrane, which is exposed following mesothelial damage. Then, an NP-B-containing suspension was administered to the damaged site to rapidly form a dense layer mediated by ionic adsorption between the oppositely charged nanoparticles. Finally, the injury site was exposed to UV light at a 365 nm wavelength to initiate the crosslinking process between the two nanoparticles, forming a nanopatch as a specific and dense biological barrier between the injured peritoneal surfaces. After two weeks of treatment, histological observations confirmed the improved performance of the developed nanopatch in preventing postsurgical peritoneal adhesions compared to other groups (phosphate-buffered saline (PBS), only NP-A, NP-A without targeting ligand and NP-B, NP-A without dexamethasone 21-palmitate, and Seprafilm®) (Figure 5).
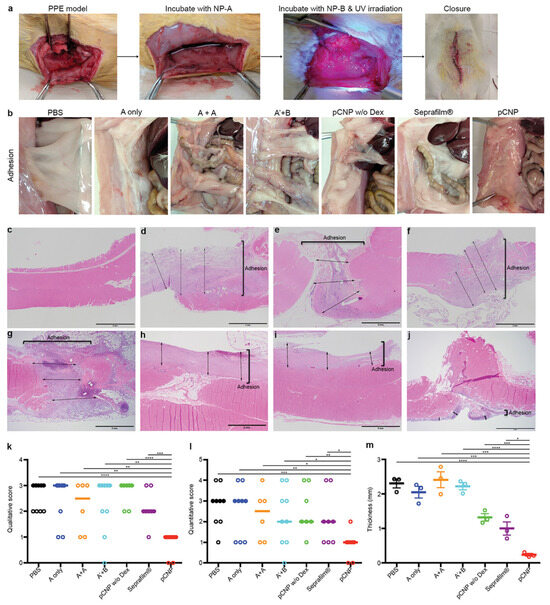
Figure 5.
Photo-crosslinkable nanopatch (pCNP) prevents postoperative peritoneal adhesion in a parietal peritoneal excision (PPE) model in rats. (a) Macroscopic images representing PPE and the administration of pCNP. (b) Representative images of postsurgical adhesions in rats after 14 days of treatment with PBS (the injured site was incubated with saline followed by UV irradiation); A only (the injured site was incubated with NP-A followed by saline under UV irradiation); A + A (the injured site was incubated with NP-A followed by NP-A again under UV irradiation); A′ + B (the injured site was incubated with NP-A′ (NP-A without targeting ligand) followed by NP-B under UV irradiation); pCNP w/o Dex (the injured site was incubated with NP-A without dexamethasone 21-palmitate followed by NP-B under UV irradiation); Seprafilm® (the injured site was incubated with saline under UV irradiation, the saline was wiped out, and the injured site was covered with Seprafilm®); and pCNP (the injured area was incubated with NP-A followed by NP-B under UV irradiation). (All the incubation and irradiation times were 10 min.) (c–j) H&E staining photographs representing the thickness of adhesion/fibrosis in untreated animals (c) and rats that underwent surgery and were further treated with PBS (d), NP-A only (e), NP-A + NP-A (f), NP-A′ + NP-B (g), pCNP without dexamethasone 21-palmitate (h), Seprafilm® (i), and pCNP (j). The scale bar shows 2 mm. (k,l) Qualitative (k) and quantitative (l) scoring analysis of postoperative adhesions in rats 14 days after treatment. Data presented as scatter dot plot with median line (for A + A, n = 6; for pCNP w/o Dex, n = 7; for Seprafilm®, n = 8; for other groups, n = 9). (m) Quantitative evaluation of the adhesion/fibrosis thickness in (d–j). Data are shown as mean ± standard error of the mean (SEM), n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Reproduced with permission from [143].
5. Conclusions and Future Directions
The term “composite” denotes materials made of two or more components (organic or inorganic) with substantially different physical or chemical properties. On the other hand, the term “nanocomposite” is applied once the size of one of the composite’s structural constituents is in the range of 1 to 100 nm. Over the years, biocompatible nanocomposites have been found to be beneficial substances for medical applications, such as the treatment of postoperative adhesions. Compared to conventional types of materials (e.g., biopolymers), nanocomposites offer extraordinary advantages for use in postoperative adhesions, including excellent biocompatibility and enhanced mechanical characteristics. A wide range of nanosized organic and inorganic substances can be mixed to generate diverse nanocomposites with distinct physicochemical, physical, and biological properties; this provides opportunities for the management of various adhesions occurring in different parts of the body. According to the literature, the most developed and used nanocomposites are indeed organic-organic composites (nanostructured polymeric constructs). Hence, different types of organic-inorganic nanocomposites can be explored for their potential to reduce and prevent postsurgical adhesions and accelerate tissue healing. For instance, the carbon family (e.g., carbon nanotubes (CNTs)) may be considered a part of novel formulations of nanocomposites due to their excellent characteristics, such as superior mechanical properties, tissue regeneration, and drug delivery. Still, the toxicity and high manufacturing cost of this kind of material should be considered when developing nanocomposites based on carbon family members. Apart from their composition, newly developed nanocomposites should have the ability to modulate the biological phenomena involved in postoperative adhesions in favor of improved tissue healing and repair, such as coagulation, inflammation, fibrinolysis, and angiogenesis. Moreover, other characteristics of nano-engineered biomaterials and scaffolds can affect the rate of tissue healing in damaged sites. This includes applied nanocomposites’s hydrophilicity and their ability to exchange body fluids, as well as their wound generation potential.
Current research has indicated that nanocomposites can be formulated and fabricated as smart scaffolds (e.g., hydrogels) to release therapeutic cargoes in response to various stimuli (e.g., changes in pH level). This provides a desirable condition for injury management with minimal side effects on the repair and regeneration of damaged tissues. In this sense, biocompatible nanocomposite scaffolds made of stimuli-responsive polymers (e.g., polypyrrole (PPy)) can be beneficial for the acceleration of tissue healing and adhesion management. Future research may focus on the fabrication and utilization of 3D-printed constructs by using nanocomposites as raw materials. These constructs can be defined as the next generation of antiadhesive constructs in the concept of precision medicine. Ultimately, the cost of nanocomposite products should be considered an important factor to compete with the available products in the market.
Author Contributions
All authors contributed to the conceptualization, literature search, methodology, investigation, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through research grant NIH NIGMS 5R01GM130590.
Data Availability Statement
No new data was created.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to impact this work.
References
- Broek, R.P.G.T.; Issa, Y.; van Santbrink, E.J.P.; Bouvy, N.D.; Kruitwagen, R.F.P.M.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 2013, 347, f5588. [Google Scholar] [CrossRef] [PubMed]
- Dizerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Update 2001, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, J.; Satta, J.; Lähde, S.; Suramo, I.; Nissinen, J.; Pokela, R.; Juvonen, T. Computed tomographic evaluation of retrosternal adhesions after pericardial substitution. Ann. Thorac. Surg. 1998, 66, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Mormino, M.A.; Gross, R.M.; McCarthy, J.A. Captured shoulder: A complication of rotator cuff surgery. Arthroscopy 1996, 12, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Pierro, L.; Brancato, R.; Calori, G. Clinical and ultrasound study of peripheral vitreoretinal adhesions. Retina 1997, 17, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Freeman, M.L. Clinical implications of postsurgical adhesions. Hum. Reprod. Update 2001, 7, 567–576. [Google Scholar] [CrossRef]
- Maciver, A.H.; McCall, M.; Shapiro, A.J. Intra-abdominal adhesions: Cellular mechanisms and strategies for prevention. Int. J. Surg. 2011, 9, 589–594. [Google Scholar] [CrossRef]
- Parker, M.C.; Ellis, H.; Moran, B.J.; Thompson, J.N.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’Brien, F. Postoperative adhesions: Ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis. Colon Rectum 2001, 44, 822–829. [Google Scholar] [CrossRef]
- Ellis, H.; Moran, B.J.; Thompson, J.N.; Parker, M.C.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’Brien, F.; et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet 1999, 353, 1476–1480. [Google Scholar] [CrossRef]
- Alpay, Z.; Saed, G.M.; Diamond, M.P. Postoperative adhesions: From formation to prevention. Proc. Semin. Reprod. Med. 2008, 26, 313–321. [Google Scholar] [CrossRef]
- Reddy, S.R.R.; Cappell, M.S. A systematic review of the clinical presentation, diagnosis, and treatment of small bowel obstruction. Curr. Gastroenterol. Rep. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Becker, V.M.; Silver, S.; Seufert, R.; Muensterer, O.J. The association of appendectomy, adhesions, tubal pathology, and female infertility. JSLS 2019, 23, e2018.00099. [Google Scholar]
- Liakakos, T.; Thomakos, N.; Fine, P.M.; Dervenis, C.; Young, R.L. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance: Recent advances in prevention and management. Dig. Surg. 2001, 18, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Berman, M.L.; Montz, F. Adhesions after extensive gynecologic surgery: Clinical significance, etiology, and prevention. Am. J. Obstet. Gynecol. 1994, 170, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Nezhat, F. Adhesions after resection of ovarian endometriomas. Fertil. Steril. 1993, 59, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Ansaloni, L.; Manfredi, R.; Campanati, L.; Poiasina, E.; Bertoli, P.; Capponi, M.G.; Sartelli, M.; Di Saverio, S.; Cucchi, M.; et al. Peritoneal adhesion index (PAI): Proposal of a score for the “ignored iceberg” of medicine and surgery. World J. Emerg. Surg. 2013, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, e2000395. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable self-healing natural biopolymer-based hydrogel adhesive with thermoresponsive reversible adhesion for minimally invasive surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, S.; Gong, X.; King, J.A.; Wang, Y.; Zhang, J.; Yang, X.; Wang, Q.; Zhang, Y.; Zhai, G.; et al. Salt sensitive purely zwitterionic physical hydrogel for prevention of postoperative tissue adhesion. Acta Biomater. 2023, 158, 239–251. [Google Scholar] [CrossRef]
- Yeo, Y.; Ito, T.; Bellas, E.; Highley, C.B.; Marini, R.; Kohane, D.S. In Situ cross-linkable hyaluronan hydrogels containing polymeric nanoparticles for preventing postsurgical adhesions. Ann. Surg. 2007, 245, 819–824. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, R.; das Neves, J.; Tang, J.; Xiao, J.; Ni, Q.; Liu, X.; Pan, G.; Li, D.; Cui, W.; et al. Advances in biomaterials for preventing tissue adhesion. J. Control. Release 2017, 261, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Esparza, G.U.; Wang, X.; Zhang, X.; Jimenez-Vazquez, S.; Diaz-Gomez, L.; Lavoie, A.-M.; Afewerki, S.; Fuentes-Baldemar, A.A.; Parra-Saldivar, R.; Jiang, N.; et al. Nanoengineered Shear-Thinning Hydrogel Barrier for Preventing Postoperative Abdominal Adhesions. Nano-Micro Lett. 2021, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, H.; Chen, H.; Ling, Y.; Xi, Z.; Lv, M.; Chen, J. pH-responsive nanocomposite hydrogel for simultaneous prevention of postoperative adhesion and tumor recurrence. Acta Biomater. 2023, 158, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic nanomaterials in tissue engineering. Pharmaceutics 2022, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Urie, R.; Ghosh, D.; Ridha, I.; Rege, K. Inorganic nanomaterials for soft tissue repair and regeneration. Annu. Rev. Biomed. Eng. 2018, 20, 353–374. [Google Scholar] [CrossRef]
- Mohammadpour, A.H.; Tavassoli, A.; Khakzad, M.R.; Zibaee, E.; Afshar, M.; Hashemzaei, M.; Karimi, G. Effect of gold nanoparticles on postoperative peritoneal adhesions in rats. Nanomedicine 2015, 2, 211–216. [Google Scholar]
- Capella-Monsonís, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.J.; Kooistra, T. Pathogenesis of postoperative adhesion formation. Br. J. Surg. 2011, 98, 1503–1516. [Google Scholar] [CrossRef]
- Goodman, J.W. On the origin of peritoneal fluid cells. Blood 1964, 23, 18–26. [Google Scholar] [CrossRef]
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Birnie, K.; Lansley, S.; Herrick, S.; Elim, C.B.; PrãLe, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Benz, E.J.; Silberstein, L.E.; Heslop, H.; Weitz, J.; Salama, M.E.; Abutalib, S.A. Hematology: Basic Principles and Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Levi, M.; van der Poll, T.; Buller, H.R. Bidirectional relation between inflammation and coagulation. Circulation 2004, 109, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-C.; Chou, T.-H.; Huang, J.-W.; Lee, C.-C.; Chen, S.-C. The small molecule inhibitor QLT-0267 decreases the production of fibrin-induced inflammatory cytokines and prevents post-surgical peritoneal adhesions. Sci. Rep. 2018, 8, 9481. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Fletcher, N.M.; Diamond, M.P. The creation of a model for Ex Vivo development of postoperative adhesions. Reprod. Sci. 2016, 23, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wu, Y.; Gao, Q.; Zhou, C.; Wang, K.; Shen, C.; Wang, G.; Wang, K.; Sun, X.; Li, X. Effect of emodin on preventing postoperative intra-abdominal adhesion formation. Oxid. Med. Cell. Longev. 2017, 2017, 1740317. [Google Scholar] [CrossRef]
- Torres, K.; Pietrzyk, Ł.; Plewa, Z.; Załuska-Patel, K.; Majewski, M.; Radzikowska, E.; Torres, A. TGF-β and inflammatory blood markers in prediction of intraperitoneal adhesions. Adv. Med. Sci. 2018, 63, 220–223. [Google Scholar] [CrossRef]
- Biondo-Simões, M.d.L.P.; Oda, M.H.; Pasqual, S.; Robes, R.R. Comparative study of polyglactin 910 and simple catgut in the formation of intraperitoneal adhesions. Acta Cir. Bras. 2018, 33, 102–109. [Google Scholar] [CrossRef]
- D’Agostino, A.; Stellavato, A.; Corsuto, L.; Diana, P.; Filosa, R.; La Gatta, A.; De Rosa, M.; Schiraldi, C. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydr. Polym. 2017, 157, 21–30. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, W.; Lei, D.; Huang, J.; Yin, Y.; Zhu, Y.; You, Z.; Wang, F.; Sun, S. PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthc. Mater. 2019, 8, 1801455. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Xu, Y.; Shen, Z.; Cheng, H.; Cheng, F.; Liu, X.; Wang, R. RhoA/Rho-kinase triggers epithelial-mesenchymal transition in mesothelial cells and contributes to the pathogenesis of dialysis-related peritoneal fibrosis. Oncotarget 2018, 9, 14397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, G.; Wang, F.; Zhang, C.; Ge, Z.; Zheng, X.; Deng, H.; Yuan, C.; Zhou, B.; Tao, X.; et al. Aspirin inhibits adipogenesis of tendon stem cells and lipids accumulation in rat injury tendon through regulating PTEN/PI3K/AKT signalling. J. Cell. Mol. Med. 2019, 23, 7535–7544. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, L.; Xiao, L.; Gou, R.; Fang, Y.; Liang, Y.; Wang, R.; Li, N.; Liu, F.; Tang, L. Aberrant WNT/beta-catenin pathway activation in dialysate-induced peritoneal fibrosis. Front. Pharmacol. 2017, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. TGF-β system: The principal profibrotic mediator of peritoneal adhesion formation. Semin. Reprod. Med. 2008, 26, 298–312. [Google Scholar] [CrossRef]
- Hassanabad, A.F.; Zarzycki, A.N.; Jeon, K.; Deniset, J.F.; Fedak, P.W.M. Post-operative adhesions: A comprehensive review of mechanisms. Biomedicines 2021, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.M.; Shoham, M.; Fernhoff, N.B.; George, B.M.; Marjon, K.D.; McCracken, M.N.; Kao, K.S.; Sinha, R.; Volkmer, A.K.; Miyanishi, M.; et al. Neutrophil and monocyte kinetics play critical roles in mouse peritoneal adhesion formation. Blood Adv. 2019, 3, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Catar, R.A.; Bartosova, M.; Kawka, E.; Chen, L.; Marinovic, I.; Zhang, C.; Zhao, H.; Wu, D.; Zickler, D.; Stadnik, H.; et al. Angiogenic role of mesothelium-derived chemokine CXCL1 during unfavorable peritoneal tissue remodeling in patients receiving peritoneal dialysis as renal replacement therapy. Front. Immunol. 2022, 13, 821681. [Google Scholar] [CrossRef]
- Herrick, S.E.; Wilm, B. Post-surgical peritoneal scarring and key molecular mechanisms. Biomolecules 2021, 11, 692. [Google Scholar] [CrossRef]
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Katz, S.; Zsiros, V.; Dóczi, N.; Kiss, A.L. Inflammation-induced epithelial-to-mesenchymal transition and GM-CSF treatment stimulate mesenteric mesothelial cells to transdifferentiate into macrophages. Inflammation 2018, 41, 1825–1834. [Google Scholar] [CrossRef]
- Moris, D.; Chakedis, J.; Rahnemai-Azar, A.A.; Wilson, A.; Hennessy, M.M.; Athanasiou, A.; Beal, E.W.; Argyrou, C.; Felekouras, E.; Pawlik, T.M. Postoperative abdominal adhesions: Clinical significance and advances in prevention and management. J. Gastrointest. Surg. 2017, 21, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Pilpel, Y.; Pines, G.; Birkenfeld, A.; Bornstein, S.R.; Miller, R. Metabolic syndrome is a risk factor for post-operative adhesions: Need for novel treatment strategies. Horm. Metab. Res. 2019, 51, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Matrix Metalloproteinases and cellular fibrinolytic activity. Biochemistry 2002, 67, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Hinoi, T.; Ikeda, S.; Adachi, T.; Kawaguchi, Y.; Tokunaga, M.; Sasada, T.; Egi, H.; Tanabe, K.; Okajima, M.; et al. Preservation of peritoneal fibrinolysis owing to decreased transcription of plasminogen activator inhibitor-1 in peritoneal mesothelial cells suppresses postoperative adhesion formation in laparoscopic surgery. Surgery 2013, 153, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T.; Samoylenko, A.; Roth, U.; Jungermann, K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood 2003, 101, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Abu-Soud, H.M.; Diamond, M.P. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil. Steril. 2004, 82, 1198–1205. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-β1 in human peritoneal fibroblasts. Fertil. Steril. 2002, 78, 144–147. [Google Scholar] [CrossRef]
- Wassilev, W.; Wedel, T.; Michailova, K.; Kühnel, W. A scanning electron microscopy study of peritoneal stomata in different peritoneal regions. Ann. Anat.—Anat. Anz. 1998, 180, 137–143. [Google Scholar] [CrossRef]
- Książek, K. Mesothelial cell: A multifaceted model of aging. Ageing Res. Rev. 2013, 12, 595–604. [Google Scholar] [CrossRef]
- Yung, S.; Tak Mao, C. Mesothelial cells. Perit. Dial. Int. 2007, 27, 110–115. [Google Scholar] [CrossRef]
- Cahill, R.A.; Wang, J.H.; Soohkai, S.; Redmond, H.P. Mast cells facilitate local VEGF release as an early event in the pathogenesis of postoperative peritoneal adhesions. Surgery 2006, 140, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Molinas, C.R.; Binda, M.M.; Koninckx, P.R. Angiogenic factors in peritoneal adhesion formation. Gynecol. Surg. 2006, 3, 157–167. [Google Scholar] [CrossRef]
- Esposito, A.J.; Heydrick, S.J.; Cassidy, M.R.; Gallant, J.; Stucchi, A.F.; Becker, J.M. Substance P is an early mediator of peritoneal fibrinolytic pathway genes and promotes intra-abdominal adhesion formation. J. Surg. Res. 2013, 181, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; Broek, R.P.G.T. Adhesion-related readmissions after open and laparoscopic surgery: A retrospective cohort study (SCAR update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. Peritoneal molecular environment, adhesion formation and clinical implication. Front. Biosci. 2002, 7, 91–115. [Google Scholar]
- Tingstedt, B.; Isaksson, J.; Andersson, R. Long-term follow-up and cost analysis following surgery for small bowel obstruction caused by intra-abdominal adhesions. J. Br. Surg. 2007, 94, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Sanbhal, N.; Li, Y.; Yu, C.; Wang, F.; Guidoin, R.; Gao, J.; Wang, L. Chitosan functionalised poly(ε-caprolactone) nanofibrous membranes as potential anti-adhesive barrier films. React. Funct. Polym. 2019, 143, 104319. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, Y.; Li, H.; Yan, T.; Wei, X.; Wu, G.; He, J.; Huang, Y. Biodegradable N, O-carboxymethyl chitosan/oxidized regenerated cellulose composite gauze as a barrier for preventing postoperative adhesion. Carbohydr. Polym. 2018, 207, 180–190. [Google Scholar] [CrossRef]
- Allègre, L.; Le Teuff, I.; Leprince, S.; Warembourg, S.; Taillades, H.; Garric, X.; Letouzey, V.; Huberlant, S. A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model. PLoS ONE 2018, 13, e0202285. [Google Scholar] [CrossRef]
- Ward, B.C.; Panitch, A. Abdominal Adhesions: Current and Novel Therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Reduction of fibrous adhesion formation by a copolymer possessing an affinity for anionic surfaces. J. Biomed. Mater. Res. 1998, 42, 55–65. [Google Scholar] [CrossRef]
- de Virgilio, C.; Elbassir, M.; Hidalgo, A.; Schaber, B.; French, S.; Amin, S.; Stabile, B.E. Fibrin glue reduces the severity of intra-abdominal adhesions in a rat model. Am. J. Surg. 1999, 178, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Arikan, S.; Adas, G.; Barut, G.; Toklu, A.S.; Kocakusak, A.; Uzun, H.; Kemik, O.; Daduk, Y.; Aydin, S.; Purisa, S. An evaluation of low molecular weight heparin and hyperbaric oxygen treatment in the prevention of intra-abdominal adhesions and wound healing. Am. J. Surg. 2005, 189, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Almamar, A.; Schlachta, C.M.; Alkhamesi, N.A. The systemic effect and the absorption rate of aerosolized intra-peritoneal heparin with or without hyaluronic acid in the prevention of postoperative abdominal adhesions. Surg. Endosc. 2019, 33, 2517–2520. [Google Scholar] [CrossRef] [PubMed]
- Legrand, E.K.; Rodgers, K.E.; Girgis, W.; Campeau, J.D.; Dizerega, G.S. Comparative efficacy of nonsteroidal anti-inflammatory drugs and anti-thromboxane agents in a rabbit adhesion-prevention model. J. Investig. Surg. 1995, 8, 187–194. [Google Scholar] [CrossRef] [PubMed]
- de la Portilla, F.; Ynfante, I.; Bejarano, D.; Conde, J.; Fernández, A.; Ortega, J.M.; Carranza, G. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin e: An experimental study in rats. Dis. Colon Rectum 2004, 47, 2157–2161. [Google Scholar] [CrossRef]
- Hemadeh, O.; Chilukuri, S.; Bonet, V.; Hussein, S.; Chaudry, I.H. Prevention of peritoneal adhesions by administration of sodium carboxymethyl cellulose and oral vitamin E. Surgery 1993, 114, 907–910. [Google Scholar]
- Kuru, S.; Bozkirli, O.B.; Barlas, A.M.; Duymus, M.E.; Senes, M.; Yumusak, N.; Yilmaz, C.; Kismet, K. The preventive effect of dexmedetomidine against postoperative intra-abdominal adhesions in rats. Int. Surg. 2015, 100, 87–95. [Google Scholar] [CrossRef]
- Lai, H.-S.; Chen, Y.; Chang, K.-J.; Chen, W.-J. Effects of octreotide on epidermal growth factor receptor, tissue plasminogen activator, and plasminogen activator inhibitor during intraperitoneal adhesion formation. J. Gastroenterol. 2003, 38, 555–560. [Google Scholar] [CrossRef]
- Bianchi, E.; Boekelheide, K.; Sigman, M.; Lamb, D.J.; Hall, S.J.; Hwang, K. Ghrelin inhibits post-operative adhesions via blockage of the TGF-β signaling pathway. PLoS ONE 2016, 11, e0153968. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Li, S.; Chen, E.; Garlick, B.; Kim, K.-S.; Fang, D.; Chiu, J.; Zimmerman, T.; Brathwaite, C.; Hsiao, B.S.; et al. Prevention of Postsurgery-Induced Abdominal Adhesions by Electrospun Bioabsorbable Nanofibrous Poly(lactide-co-glycolide)-Based Membranes. Ann. Surg. 2004, 240, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for Surgical Applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Bajpai, M.; Shukla, P.; Bajpai, S. Ca (II)+ Ba (II) ions crosslinked alginate gels prepared by a novel diffusion through dialysis tube (DTDT) approach and preliminary BSA release study. Polym. Degrad. Stab. 2016, 134, 22–29. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Day, J.; Shikanov, A. Immunoisolation to prevent tissue graft rejection: Current knowledge and future use. Exp. Biol. Med. 2016, 241, 955–961. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Chung, P.K.; Yoo, J.C. Effect of sodium hyaluronate/carboxymethyl cellulose (Guardix-sol) on retear rate and postoperative stiffness in arthroscopic rotator cuff repair patients: A prospective cohort study. J. Orthop. Surg. 2017, 25, 2309499017718908. [Google Scholar] [CrossRef]
- Eriksson, S.; Fraser, J.E.; Laurent, T.C.; Pertoft, H.; Smedsrød, B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp. Cell Res. 1983, 144, 223–228. [Google Scholar] [CrossRef]
- Tammi, R.H.; Rilla, K.; Pienimäki, J.-P.; MacCallum, D.K.; Hogg, M.; Luukkonen, M.; Hascall, V.C.; Tammi, M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 2003, 278, 7742. [Google Scholar] [CrossRef]
- Mais, V.; Bracco, G.; Litta, P.; Gargiulo, T.; Melis, G. Reduction of postoperative adhesions with an auto-crosslinked hyaluronan gel in gynaecological laparoscopic surgery: A blinded, controlled, randomized, multicentre study. Hum. Reprod. 2006, 21, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Hellio, D.; Djabourov, M. Physically and chemically crosslinked gelatin gels. Macromol. Symp. 2006, 241, 23–27. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Park, Y.; Kang, H.; Lee, D. Development of a Lidocaine-Loaded Alginate/CMC/PEO electrospun nanofiber film and application as an anti-adhesion barrier. Polymers 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Lim, S.; Kim, T.E.; Jeon, I.O.; Choi, Y.S. Preparation of in situ injectable chitosan/gelatin hydrogel using an acid-tolerant tyrosinase. Biotechnol. Bioprocess Eng. 2018, 23, 500–506. [Google Scholar] [CrossRef]
- Wei, C.-Z.; Hou, C.-L.; Gu, Q.-S.; Jiang, L.-X.; Zhu, B.; Sheng, A.-L. A thermosensitive chitosan-based hydrogel barrier for post-operative adhesions’ prevention. Biomaterials 2009, 30, 5534–5540. [Google Scholar] [CrossRef] [PubMed]
- Çipe, G.; Köksal, H.M.; Yildirim, S.; Celayir, M.F.; Baykan, A. Efficacy of hyaluronic acid—Carboxymethyl cellulose membrane (Seprafilm®) and polylactic acid barrier film (Surgiwrap™) for the prevention of adhesions after thyroid surgery: An experimental model. Turk. J. Med. Sci. 2011, 41, 73–79. [Google Scholar] [CrossRef]
- Urano, H.; Iwatsuki, K.; Yamamoto, M.; Ohnisi, T.; Kurimoto, S.; Endo, N.; Hirata, H. Novel anti-adhesive CMC-PE hydrogel significantly enhanced morphological and physiological recovery after surgical decompression in an animal model of entrapment neuropathy. PLoS ONE 2016, 11, e0164572. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Lu, R.; Tao, L.; Shi, G.; Li, X.; Qin, C. Synthesis of poly(vinyl alcohol-graft-lactic acid) copolymer and its application as medical anti-tissue adhesion thin film. Polym. Bull. 2015, 72, 1515–1529. [Google Scholar] [CrossRef]
- Lo, H.; Kuo, H.; Huang, Y. Application of polycaprolactone as an anti-adhesion biomaterial film. Artif. Organs 2010, 34, 648–653. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jastrzębska, M.; Janik, H. Biodegradation of polycaprolactone in sea water. React. Funct. Polym. 1998, 38, 27–30. [Google Scholar] [CrossRef]
- Freytag, C.; Odermatt, E.K. Standard biocompatibility studies do not predict all effects of PVA/CMC anti-adhesive gel in vivo. Eur. Surg. Res. 2016, 56, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Z.; Li, Z.; Fan, J.M.; Meng, X.H.; Shi, K.; Qu, Y.; Yang, L.L.; Wu, J.B.; Fan, J.; Luo, F.; et al. Biodegradable and Thermosensitive Monomethoxy Poly(ethylene glycol)–Poly(lactic acid) Hydrogel as a Barrier for Prevention of Post-Operative Abdominal Adhesion. J. Biomed. Nanotechnol. 2014, 10, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Leitner, K.; Odermatt, E.; Worthley, D.L.; Angele, M.K.; Jauch, K.-W.; Lang, R.A. PVA gel as a potential adhesion barrier: A safety study in a large animal model of intestinal surgery. Langenbeck’s Arch. Surg. 2014, 399, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to prevent post-operative adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, L.M.; Steele, A.N.; Wang, H.; Hernandez, H.L.; Yu, A.C.; Paulsen, M.J.; Smith, A.A.A.; Roth, G.A.; Thakore, A.D.; Lucian, H.J.; et al. Use of a supramolecular polymeric hydrogel as an effective post-operative pericardial adhesion barrier. Nat. Biomed. Eng. 2019, 3, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, M.; Cao, Y.; Tao, Z.; Sun, Z.; Liu, X.; Liu, J.; Liu, S. Degradative polylactide nanofibers promote M2 macrophage polarization via STAT6 pathway in peritendinous adhesion. Compos. Part B Eng. 2023, 253, 110520. [Google Scholar] [CrossRef]
- Kheilnezhad, B.; Hadjizadeh, A. Ibuprofen-Loaded Electrospun PCL/PEG Nanofibrous Membranes for Preventing Postoperative Abdominal Adhesion. ACS Appl. Bio Mater. 2022, 5, 1766–1778. [Google Scholar] [CrossRef]
- Shin, Y.C.; Yang, W.J.; Lee, J.H.; Oh, J.-W.; Kim, T.W.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. PLGA nanofiber membranes loaded with epigallocatechin-3-O-gallate are beneficial to prevention of postsurgical adhesions. Int. J. Nanomed. 2014, 9, 4067–4078. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Bai, J.; Tian, D.; Liu, G. Experimental study of tendon sheath repair via decellularized amnion to prevent tendon adhesion. PLoS ONE 2018, 13, e0205811. [Google Scholar] [CrossRef]
- Park, D.Y.; Yun, H.; Lim, S.; Truong, M.; Yin, X.Y.; Park, J.; Kim, B.K.; Shin, D.I.; Li, X.G.; Chung, J.Y.; et al. Cross-linked cartilage acellular matrix film decreases postsurgical peritendinous adhesions. Artif. Organs 2020, 44, E136–E149. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Yun, H.; Park, S.Y.; Choi, S.J.; Park, I.; Min, B.; Kim, J.K. Effect of glutaraldehyde-crosslinked cartilage acellular matrix film on anti-adhesion and nerve regeneration in a rat sciatic nerve injury model. J. Tissue Eng. Regen. Med. 2021, 15, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Liang, F.; He, J.; Ye, W.; Javed, R.; Wang, W.; Yu, T.; Fan, J.; Tian, X.; Wang, X.; et al. Decellularized tendon matrix membranes prevent post-surgical tendon adhesion and promote functional repair. Acta Biomater. 2021, 134, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guo, J.; Wang, S. Application of polymer hydrogels in the prevention of postoperative adhesion: A review. Gels 2023, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chen, C.-H.; Chen, S.-H.; Fong, Y.T.; Chen, J.-P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef]
- Ji, J.; Cheng, J.; Chen, C.; Lu, Y.; Chen, X.; Zhang, F. Pirfenidone-loaded hyaluronic acid methacryloyl hydrogel for preventing epidural adhesions after laminectomy. Drug Deliv. Transl. Res. 2022, 13, 770–781. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, X.; Luo, J.; Wen, Q.; Wu, Z.; Ding, Q.; Zhao, L.; Yang, L.; Wang, B.; Fu, S. Naproxen nanoparticle-loaded thermosensitive chitosan hydrogel for prevention of postoperative adhesions. ACS Biomater. Sci. Eng. 2019, 5, 1580–1588. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, F.; Yu, Y.; Liu, Y.; Shao, C.; Gu, H.; Li, M.; Zhao, Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019, 84, 222–230. [Google Scholar] [CrossRef]
- Kou, L.; Jiang, X.; Xiao, S.; Zhao, Y.-Z.; Yao, Q.; Chen, R. Therapeutic options and drug delivery strategies for the prevention of intrauterine adhesions. J. Control. Release 2020, 318, 25–37. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Bai, J.; Liu, C.; Kong, L.; Tian, S.; Yu, K.; Tian, D. Electrospun Polycaprolactone (PCL)-Amnion Nanofibrous Membrane Promotes Nerve Regeneration and Prevents Fibrosis in a Rat Sciatic Nerve Transection Model. Front. Surg. 2022, 9, 842540. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, X.; Shi, J.; Liu, T.; Ding, J. Porous polylactide film plus atorvastatin-loaded thermogel as an efficient device for peritoneal adhesion prevention. ACS Omega 2018, 3, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hu, S.; Yu, B.; Cai, Y.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Transglutaminase-catalyzed preparation of crosslinked carboxymethyl chitosan/carboxymethyl cellulose/collagen composite membrane for postsurgical peritoneal adhesion prevention. Carbohydr. Polym. 2018, 201, 201–210. [Google Scholar] [CrossRef]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly (N, N-dimethylacrylamide) and clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Nejad, Z.M.; Torabinejad, B.; Davachi, S.M.; Zamanian, A.; Garakani, S.S.; Najafi, F.; Nezafati, N. Synthesis, physicochemical, rheological and in-vitro characterization of double-crosslinked hyaluronic acid hydrogels containing dexamethasone and PLGA/dexamethasone nanoparticles as hybrid systems for specific medical applications. Int. J. Biol. Macromol. 2019, 126, 193–208. [Google Scholar] [CrossRef]
- Furtat, I.; Lupatsii, M.; Murlanova, T.; Vakuliuk, P.; Gaidai, A.; Biliayeva, O.; Sobczuk, H.; Golub, A. Nanocomposites with ornidazole—Antibacterial and antiadhesive agents against Gram-positive and Gram-negative bacteria. Appl. Nanosci. 2020, 10, 3193–3203. [Google Scholar] [CrossRef]
- Song, L.; Li, L.; He, T.; Wang, N.; Yang, S.; Yang, X.; Zeng, Y.; Zhang, W.; Yang, L.; Wu, Q.; et al. Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model. Sci. Rep. 2016, 6, 37600. [Google Scholar] [CrossRef]
- Sultana, T.; Van Hai, H.; Abueva, C.; Kang, H.J.; Lee, S.-Y.; Lee, B.-T. TEMPO oxidized nano-cellulose containing thermo-responsive injectable hydrogel for post-surgical peritoneal tissue adhesion prevention. Mater. Sci. Eng. C 2019, 102, 12–21. [Google Scholar] [CrossRef]
- Zhang, E.; Guo, Q.; Ji, F.; Tian, X.; Cui, J.; Song, Y.; Sun, H.; Li, J.; Yao, F. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, X.; Yi, X.; Tang, S.; He, J.; Huang, Y.; Cheng, F. Antibacterial, hemostasis, adhesive, self-healing polysaccharides-based composite hydrogel wound dressing for the prevention and treatment of postoperative adhesion. Mater. Sci. Eng. C 2021, 123, 111978. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Cheng, Y.-H.; Chen, S.-H.; Chuang, A.D.-C.; Chen, J.-P. Functional hyaluronic acid-polylactic acid/silver nanoparticles core-sheath nanofiber membranes for prevention of post-operative tendon adhesion. Int. J. Mol. Sci. 2021, 22, 8781. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Wu, H.; Kong, Q. Superhydrophilic PLGA-Graft-PVP/PC Nanofiber Membranes for the Prevention of Epidural Adhesion. Int. J. Nanomed. 2022, 17, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wang, S.; Hu, D.; Fu, W.; Wu, J.; Hong, H.; Domian, I.J.; Li, F.; Liu, J. Bioresorbable electrospun gelatin/polycaprolactone nanofibrous membrane as a barrier to prevent cardiac postoperative adhesion. Acta Biomater. 2019, 83, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, O.; Pekkoc-Uyanik, K.C.; Findik, S.; Avci, A.; Altuntas, Z. Prevention of peritendinous adhesions with electrospun poly (lactic acid-co-glycolic acid) (PLGA) bioabsorbable nanofiber: An experimental study. Colloids Surfaces B Biointerfaces 2021, 209, 112181. [Google Scholar] [CrossRef] [PubMed]
- Sathish, P.; Narmadha, R.; Selvakumar, R. Synthesis and characterization of anti-adhesion tricomposite electrospun nanofiber barrier membrane for use in post-surgical adhesion conditions. Mater. Lett. 2021, 285, 129038. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhai, W.; Zhang, Z.; Liu, Y.; Cheng, S.; Zhang, H. In-situ growth of robust superlubricated nano-skin on electrospun nanofibers for post-operative adhesion prevention. Nat. Commun. 2022, 13, 5056. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Chuang, A.D.-C.; Darshan, T.; Chen, J.-P. Hyaluronic acid/platelet rich plasma-infused core-shell nanofiber membrane to prevent postoperative tendon adhesion and promote tendon healing. Int. J. Biol. Macromol. 2023, 231, 123312. [Google Scholar] [CrossRef]
- Liu, C.; Tian, S.; Bai, J.; Yu, K.; Liu, L.; Liu, G.; Dong, R.; Tian, D. Regulation of ERK1/2 and SMAD2/3 pathways by using multi-layered electrospun PCL–amnion nanofibrous membranes for the prevention of post-surgical tendon adhesion. Int. J. Nanomed. 2020, 15, 927–942. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Z.; Liu, R.; Zhou, C.; Li, E.; Shen, T.; Wang, X.; Wu, Y.; Li, X. A combination of hybrid polydopamine-human keratinocyte growth factor nanoparticles and sodium hyaluronate for the efficient prevention of postoperative abdominal adhesion formation. Acta Biomater. 2022, 138, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Yang, F.; Bloomquist, C.; Xia, Y.; Sun, B.; Qi, Y.; Wagner, K.; Montgomery, S.A.; Zhang, T.; Wang, A.Z. Biologically Targeted Photo-Crosslinkable Nanopatch to Prevent Postsurgical Peritoneal Adhesion. Adv. Sci. 2019, 6, 1900809. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).