Selective Separation of Zr(IV) from Simulated High-Level Liquid Waste by Mesoporous Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Characterization
2.3. Batch Experiments
3. Results and Discussion
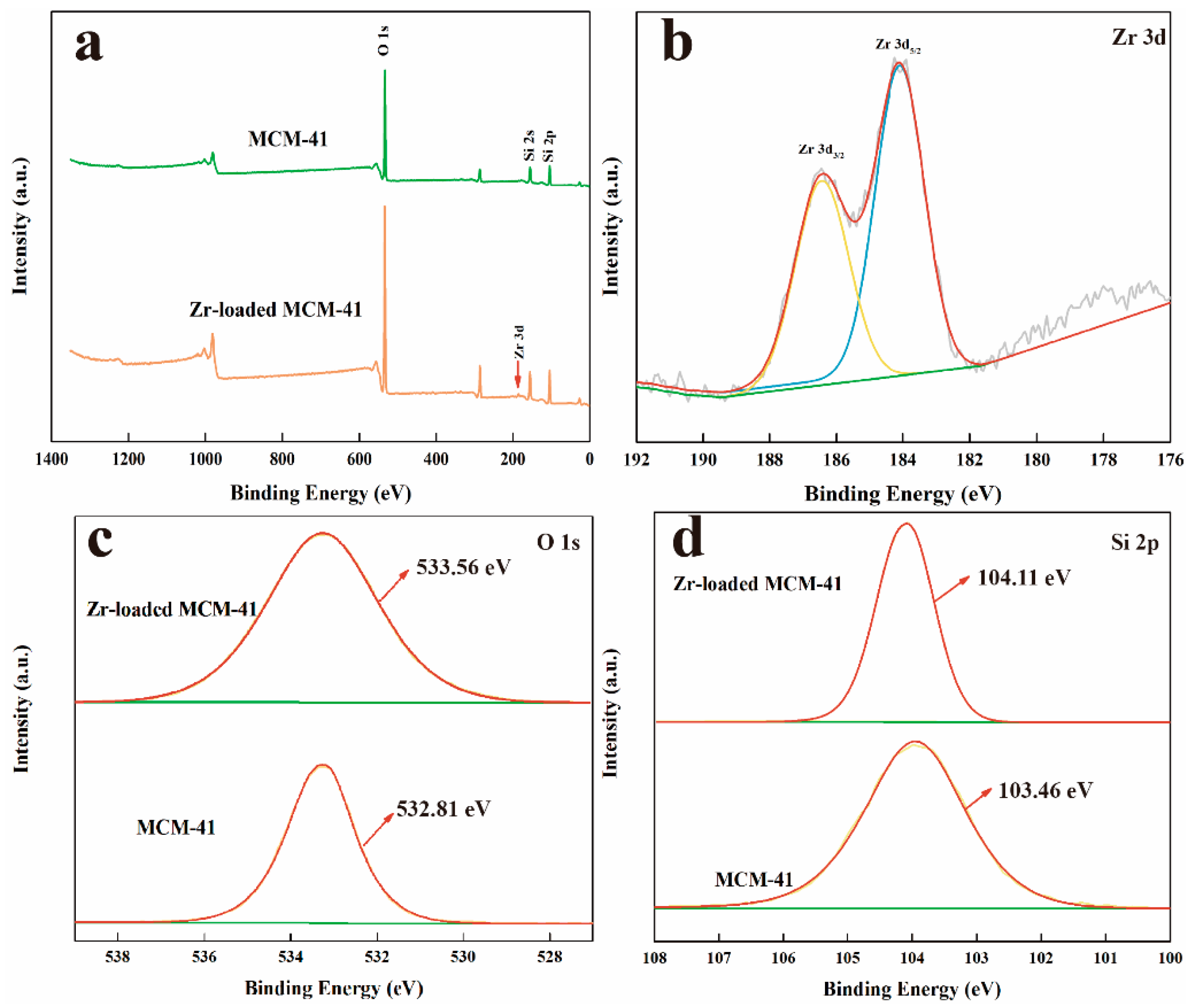
3.1. Characterization
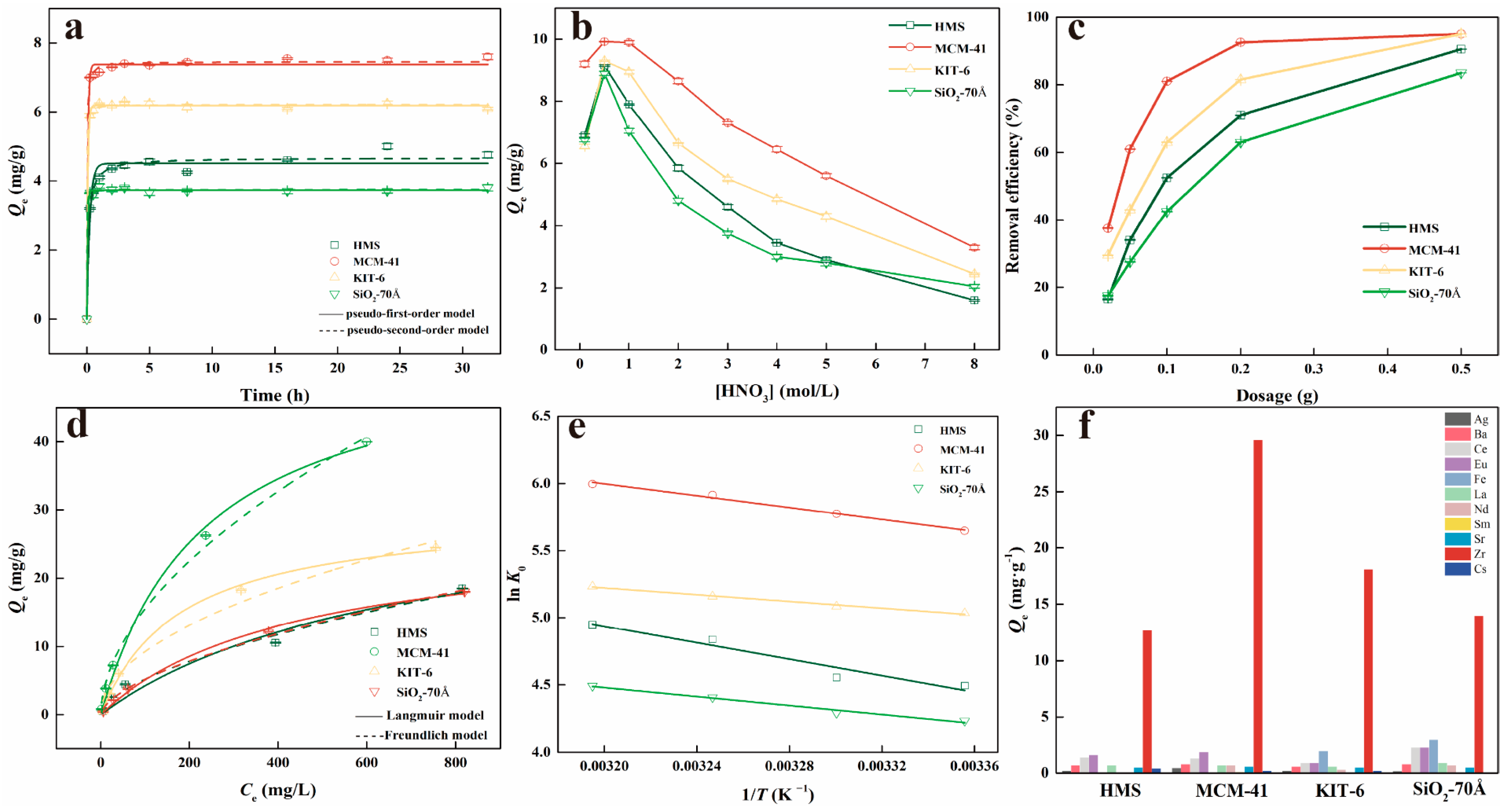
3.2. Kinetics
3.3. Effects of Nitric Acid Acidity
3.4. Effects of Adsorbent Dosage
3.5. Isotherm
3.6. Adsorption Selectivity
3.7. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uetake, N. Precipitation formation of zirconium-dibutyl phosphate complex in PUREX process. J. Nucl. Sci. Technol. 1989, 26, 329–338. [Google Scholar] [CrossRef]
- Miyake, C.; Hirose, M.; Yoshimura, T.; Ikeda, M.; Imoto, S.; Sano, M. “The Third Phase” of extraction processes in fuel reprocessing, (I). J. Nucl. Sci. Technol. 2008, 27, 157–166. [Google Scholar] [CrossRef]
- Sugai, H. Crud in solvent washing process for nuclear fuel reprocessing. J. Nucl. Sci. Technol. 1992, 29, 445–453. [Google Scholar] [CrossRef]
- Zimmer, E.; Borchardt, J. Borchardt, Crud formation in the Purex and Thorex processes. Nucl. Technol. 1986, 75, 332–337. [Google Scholar] [CrossRef]
- Ren, F. Formation of interface dirt in Purex process and measures to reduce its hazards. Nucl. Sci. Eng. 1993, 13, 346–351. [Google Scholar] [CrossRef]
- Hardy, C.; Scargill, D. Studies on mono- and Di-N-Butylphosphoric acids—III the extraction of zirconium from nitrate solution by Di-N-Butylphosphoric acid. J. Inorg. Nucl. Chem. 1961, 17, 337–349. [Google Scholar] [CrossRef]
- Mishra, S.; Soda, A.K.; Sridhar, M.; Mallika, C.; Pandey, N.K.; Mudali, U.K. Identification of diluent degradation products in radiolyzed PUREX solvent. Solvent Extr. Ion Exch. 2018, 36, 54–65. [Google Scholar] [CrossRef]
- Rao, P.R.V.; Kolarik, Z. A review of third phase formation in extraction of actinides by neutral organophosphorus extractants. Solvent Extr. Ion Exch. 1996, 14, 955–993. [Google Scholar] [CrossRef]
- Chen, J. Crud in the solvent extraction process for spent fuel reprocessing. At. Energy Sci. Technol. 2004, 38, 343–349. [Google Scholar] [CrossRef]
- Sakr, A.K.; Al-Hamarneh, I.F.; Gomaa, H.; Aal, M.M.A.; Hanfi, M.Y.; Sayyed, M.; Khandaler, M.U.; Cheira, M.F. Removal of uranium from nuclear effluent using regenerated bleaching earth steeped in β–naphthol. Radiat. Phys. Chem. 2022, 200, 110204. [Google Scholar] [CrossRef]
- Sakr, A.K.; Aal, M.M.A.; El-Rahem, K.A.A.; Allam, E.M.; Dayem, S.M.A.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials 2022, 12, 3866. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.-Y.; Usuda, S.; Wei, Y.; Ishii, K. Adsorption and desorption behavior of tetravalent zirconium onto a silica-based macroporous TODGA adsorbent in HNO3 solution. J. Radioanal. Nucl. Chem. 2013, 297, 91–96. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Elshehy, E.A. Extraction and separation of zirconium(IV) and hafnium(IV) from chloride media using magnetic resin with phosphoric acid functionality. J. Dispers. Sci. Technol. 2011, 32, 193–202. [Google Scholar] [CrossRef]
- Qureshi, M.; Husain, K. Quantitative cation exchange separation of zirconium and hafnium in formic acid media. Anal. Chem. 1971, 43, 447–449. [Google Scholar] [CrossRef]
- Xu, Z.-G.; Wu, Y.-K.; Zhang, J.-D.; Zhang, L.; Wang, L.-J. Equilibrium and kinetic data of adsorption and separation for zirconium and hafnium onto MIBK extraction resin. Trans. Nonferrous Met. Soc. China 2010, 20, 1527–1533. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Wadas, T.J. Recent advances in zirconium-89 chelator development. Molecules 2018, 23, 638. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Koyama, S.-I.; Mimura, H. Removal of zirconium from spent fuel solution by alginate gel polymer. Prog. Nucl. Energy 2015, 82, 69–73. [Google Scholar] [CrossRef]
- Akatsu, E.; Ono, R.; Tsukuechi, K.; Uchiyama, H. Radiochemical study of adsorption behavior of inorganic ions on zirconium phosphate, silica gel and charcoal. J. Nucl. Sci. Technol. 1965, 2, 141–148. [Google Scholar] [CrossRef]
- Milonjić, S.K.; Čokeša, D.M.; Stevanović, R.V. Dynamic adsorption of uranium(VI) and zirconium(IV) on silica gel. J. Radioanal. Nucl. Chem. 1992, 158, 79–90. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, R.S.; Lin, C.S.; Zhang, X.Y. Selective separation behaviour of silica gel for zirconium in simulated high level radioactive liquid waste. J. Radioanal. Nucl. Chem. 2001, 247, 205–208. [Google Scholar] [CrossRef]
- Liu, X.G.; Liang, J.F.; Xu, J.M. Study on the adsorption behavior of silica gel for zirconium, plutonium and other fission products in high-level liquid waste. J. Radioanal. Nucl. Chem. 2007, 273, 49–54. [Google Scholar] [CrossRef]
- Lin, H.C.; Ting, G. The separation of 95Zr and 95Nb from irradiated uranium by silica gel adsorption. Radiochim. Acta. 1976, 23, 1–5. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Daher, A.M.; Desouky, O.A.; Elshehy, E.A. Extraction and separation of Zr(IV) from hydrochloric acid solution using modified silica gel produced from waste solution of sodium silicate. Sep. Sci. Technol. 2011, 46, 1329–1336. [Google Scholar] [CrossRef]
- Zhang, A.; Wei, Y.; Kumagai, M. Properties and mechanism of molybdenum and zirconium adsorption by a macroporous silica-based extraction resin in the MAREC process. Solvent Extr. Ion Exch. 2003, 21, 591–611. [Google Scholar] [CrossRef]
- Zhang, A.; Kuraoka, E.; Kumagai, M. Removal of Pd(II), Zr(IV), Sr(II), Fe(III), and Mo(VI) from simulated high level liquid waste by extraction chromatography utilizing the macroporous silica-based polymeric materials. Sep. Purif. Technol. 2006, 50, 35–44. [Google Scholar] [CrossRef]
- Yuezhou YX, W.; Jianhua, Z.U. Adsorption behavior of Zr(IV) and Hf(IV) on a silica-based macroporous TODGA adsorbent. Nucl. Sci. Tech. 2013, 4, 9–16. [Google Scholar] [CrossRef]
- Qin, W.; Xu, K.; Wang, J.; Cui, X.; Zhang, J.; Weng, Y. Phosphorous-functionalized PAMAM dendrimers supported on mesoporous silica for Zr(IV) and Hf(IV) separation. Rsc. Adv. 2021, 11, 34754. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kim, S.-Y.; Miwa, M.; Matsuyama, S. Synergistic adsorption behavior of a mesoporous silica toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J. Hazard. Mater. 2021, 411, 125136. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, W.; Wang, W.; Zhang, Y.; Chen, Q.; Yan, W.; Yan, T. Selective separation of Zr(iv) from simulated high-level liquid wastes by zeolites. Solvent Extr. Ion Exch. 2023, 41, 767–788. [Google Scholar] [CrossRef]
- Wenhui, Y.; Congqiang, L. Sorption of Zr4+ and Hf4+ onto hydrous ferric oxide and their fractionation behaviors: An experimental study. Acta. Geol. Sin-Engl. 2005, 79, 343–348. [Google Scholar] [CrossRef]
- Tan, J.; Su, W.; Dou, T.; Fan, X. Adsorption of Ce(IV) ions from aqueous solution by silica aerogels. Sep. Sci. Technol. 2012, 47, 1149–1155. [Google Scholar] [CrossRef]
- Gallas, J.-P.; Goupil, J.-M.; Vimont, A.; Lavalley, J.-C.; Gil, B.; Gilson, J.-P.; Miserque, O. Quantification of Water and Silanol Species on Various Silicas by Coupling IR Spectroscopy and in-Situ Thermogravimetry. Langmuir 2009, 25, 5825–5834. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Theoretical description of the kinetics of solute adsorption at heterogeneous solid/solution interfaces. Appl. Surf. Sci. 2007, 253, 5827–5840. [Google Scholar] [CrossRef]
- Lister, B.A.J.; McDonald, L.A. 827. Some aspects of the solution chemistry of zirconium. J. Chem. Soc. 1952, 4315–4330. [Google Scholar] [CrossRef]
- Ekberg, C.; Källvenius, G.; Albinsson, Y.; Brown, P.L. Studies on the hydrolytic behavior of zirconium(IV). J. Solut. Chem. 2004, 33, 47–79. [Google Scholar] [CrossRef]
- Walther, C.; Rothe, J.; Fuss, M.; Büchner, S.; Koltsov, S.; Bergmann, T. Investigation of polynuclear Zr(IV) hydroxide complexes by nanoelectrospray mass-spectrometry combined with XAFS. Anal. Bioanal. Chem. 2007, 388, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, C.; Kessler, V.; Persson, I. Structure of the hydrated, hydrolysed and solvated zirconium(IV) and hafnium(IV) ions in water and aprotic oxygen donor solvents. A crystallographic, EXAFS spectroscopic and large angle X-ray scattering study. Dalton Trans. 2004, 2142–2151. [Google Scholar] [CrossRef]
- Jurinak, J.J. The hydrolysis of cations. Soil Sci. Soc. Am. J. 1976, 40, vi. [Google Scholar] [CrossRef]
- Zielen, A.J.; Connick, R.E. The hydrolytic polymerization of zirconium in perchloric acid solutions. J. Am. Chem. Soc. 1956, 78, 5785–5792. [Google Scholar] [CrossRef]
- Busca, G. Chapter 7—Zeolites and other structurally microporous solids as acid–base materials. In Heterogeneous Catalytic Materials; Busca, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 197–249. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- LeVan, M.D.; Vermeulen, T. Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J. Phys. Chem. 1981, 85, 3247–3250. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; El-Boraey, H.A.; Mabrouk, D.H. Adsorption of Ag(I) on glycidyl methacrylate/N,N′-methylene bis-acrylamide chelating resins with embedded iron oxide. Sep. Purif. Technol. 2006, 48, 281–287. [Google Scholar] [CrossRef]
- Bu, N.; Liu, X.; Song, S.; Liu, J.; Yang, Q.; Li, R.; Zheng, F.; Yan, L.; Zhen, Q.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zhang, J.; Wu, F.; Liu, F.; Zhao, H.; Hu, X.; Zhao, X.; Li, J.; Ju, X.; et al. Stabilization of lead in waste water and farmland soil using modified coal fly ash. J. Clean. Prod. 2021, 314, 127957. [Google Scholar] [CrossRef]
- Niu, W.; Wu, P.; Jiang, L.; Shang, Z.; Ye, Q.; Wu, J.; Li, Y.; Chen, M.; Rehman, S.; Zhu, N. Transformation from halloysite nanotubes to gobbinsite zeolite improves the fixation capacity of Cd(II). Microporous Mesoporous Mater. 2022, 335, 111781. [Google Scholar] [CrossRef]
- Branda, M.; Montani, R.; Castellani, N. The distribution of silanols on the amorphous silica surface: A Monte Carlo simulation. Surf. Sci. 2000, 446, L89–L94. [Google Scholar] [CrossRef]
- Maciel, G.E.; Sindorf, D.W. Silicon-29 NMR study of the surface of silica gel by cross polarization and magic-angle spinning. J. Am. Chem. Soc. 1980, 102, 7606–7607. [Google Scholar] [CrossRef]
- Sindorf, D.W.; Maciel, G.E. Silicon-29 NMR study of dehydrated/rehydrated silica gel using cross polarization and magic-angle spinning. J. Am. Chem. Soc. 1983, 105, 1487–1493. [Google Scholar] [CrossRef]
- Miller, M.; Linton, R.; Maciel, G.; Hawkins, B. Characterization of silanol reactivity and acidity on octadecyl-bonded chromatographic supports by 29 Si solidstate nuclear magnetic resonance spectroscopy and surface titration. J. Chromatogr. A 1985, 319, 9–21. [Google Scholar] [CrossRef]
- Murray, D.K. Differentiating and characterizing geminal silanols in silicas by Si-29 NMR spectroscopy. J. Colloid Interface Sci. 2010, 352, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chatterjee, P.; Slowing, I.I.; Kobayashi, T. In Situ 29Si solid-state NMR study of grafting of organoalkoxysilanes to mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2022, 339, 112019. [Google Scholar] [CrossRef]
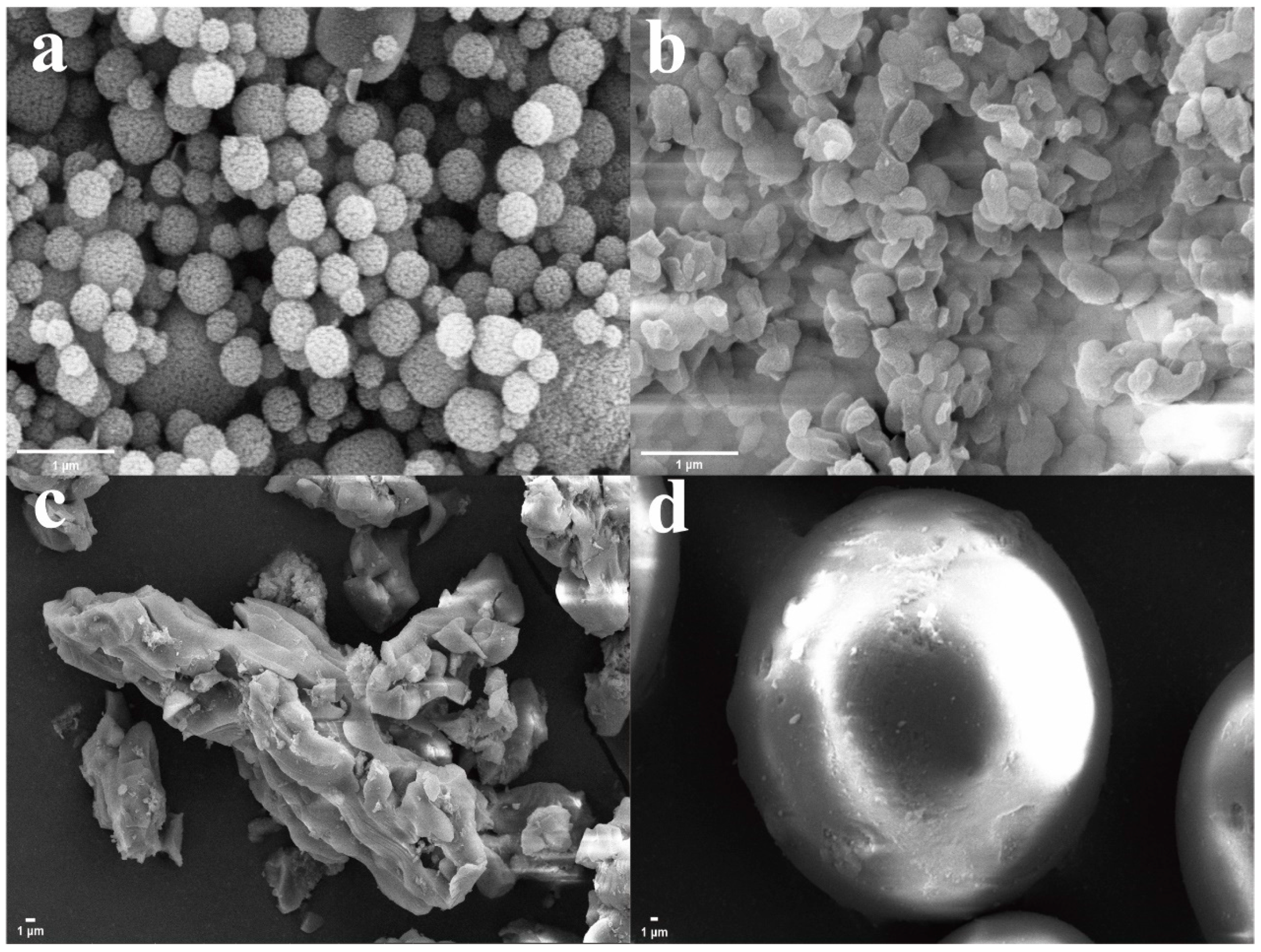
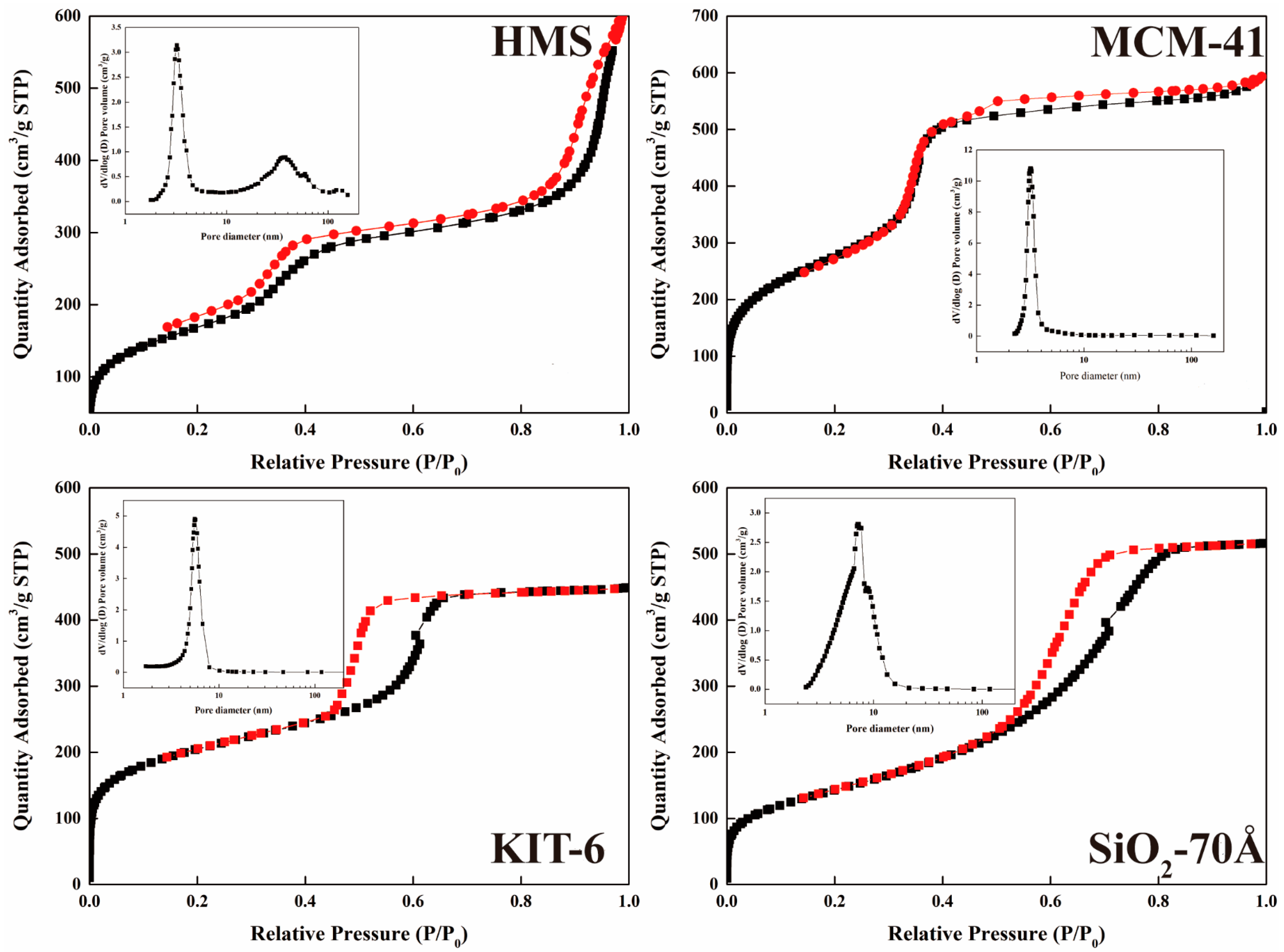
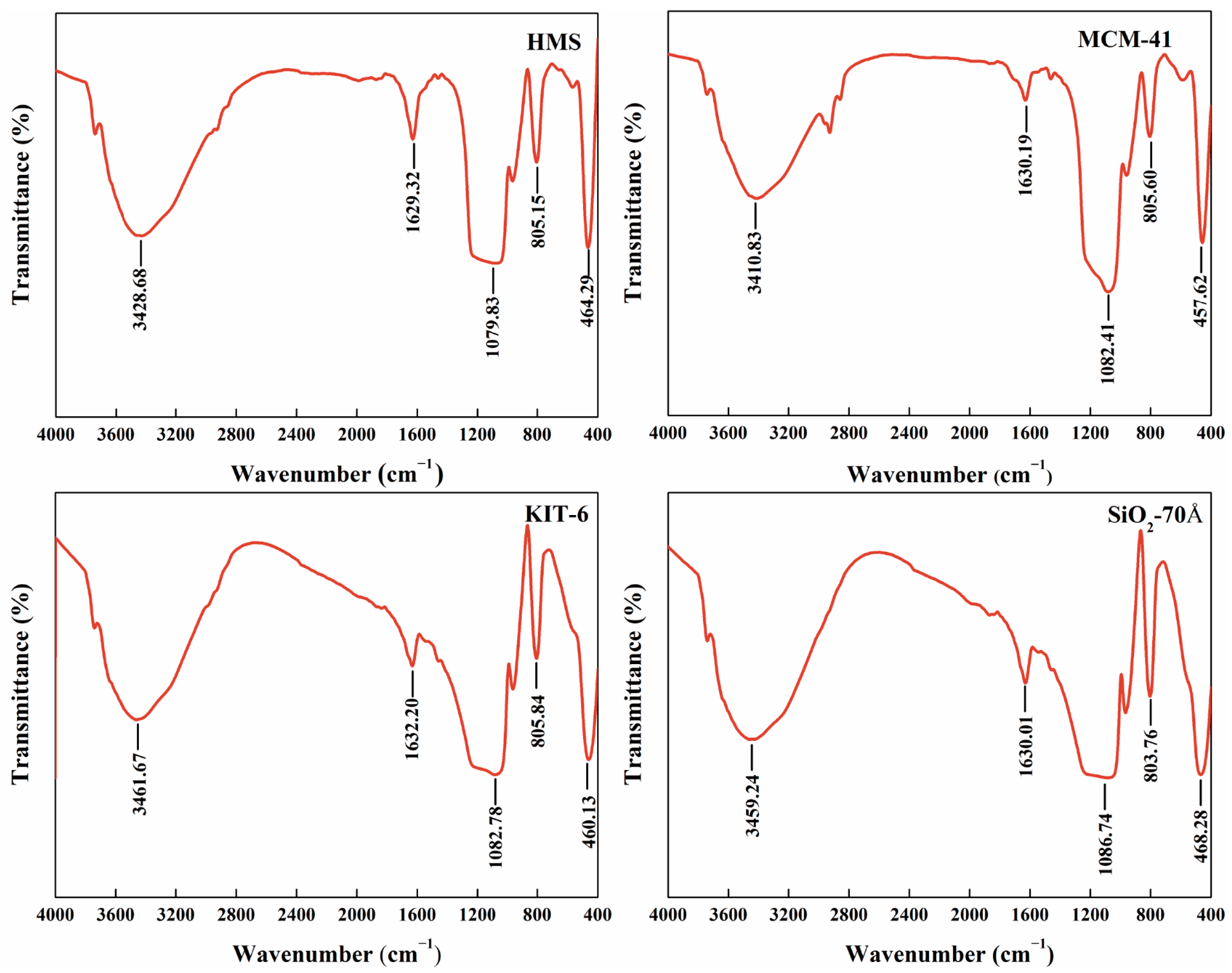
| Name | BET Specific Surface Area (m²/g) | Average Pore Size (Å) |
|---|---|---|
| HMS | 612.21 | 59.71 |
| MCM-41 | 1014.69 | 36.16 |
| KIT-6 | 733.30 | 37.88 |
| SiO2-70 Å | 518.06 | 61.60 |
| Name of Material | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 (min−1) | R2 | χ2 | Qe (mg/g) | k2 (min−1) | R2 | χ2 | |
| HMS | 4.504 | 4.204 | 0.960 | 7.64 × 10−2 | 4.666 | 1.611 | 0.984 | 3.08 × 10−2 |
| MCM-41 | 7.379 | 11.533 | 0.994 | 2.81 × 10−2 | 7.457 | 6.863 | 0.998 | 1.07 × 10−2 |
| KIT-6 | 6.186 | 12.133 | 0.998 | 6.28 × 10−3 | 6.215 | 18.097 | 0.998 | 6.20 × 10−3 |
| SiO2-70 Å | 3.729 | 15.155 | 0.996 | 5.57 × 10−3 | 3.741 | 34.794 | 0.996 | 5.21 × 10−3 |
| Adsorbents | Temperature (K) | Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm (mg/g) | KL | R2 | χ2 | KF | 1/n | R2 | χ2 | ||
| HMS | 298 K | 32.78 | 1.47 × 10−3 | 0.945 | 2.94 | 0.337 | 0.593 | 0.985 | 0.811 |
| MCM-41 | 298 K | 54.91 | 4.24 × 10−3 | 0.993 | 2.06 | 1.273 | 0.542 | 0.994 | 1.644 |
| KIT-6 | 298 K | 29.84 | 5.54 × 10−3 | 0.996 | 0.396 | 0.914 | 0.502 | 0.977 | 2.513 |
| SiO2-70 Å | 298 K | 27.24 | 2.28 × 10−3 | 0.995 | 0.258 | 0.326 | 0.600 | 0.996 | 0.243 |
| Name of Material | Temperature | ∆H0 (kJ/mol) | ∆S0 (J/mol·K) | ∆G0 (kJ/mol) |
|---|---|---|---|---|
| HMS | 298 K | 25.533 | 147.697 | −18.481 |
| 303 K | −19.219 | |||
| 308 K | −19.958 | |||
| 313 K | −20.696 | |||
| MCM-41 | 298 K | 18.433 | 108.849 | −14.004 |
| 303 K | −14.548 | |||
| 308 K | −15.092 | |||
| 313 K | −15.637 | |||
| KIT-6 | 298 K | 10.457 | 76.878 | −12.453 |
| 303 K | −12.837 | |||
| 308 K | −13.221 | |||
| 313 K | −13.606 | |||
| SiO2-70 Å | 298 K | 13.856 | 81.588 | −10.457 |
| 303 K | −10.865 | |||
| 308 K | −11.273 | |||
| 313 K | −11.681 |
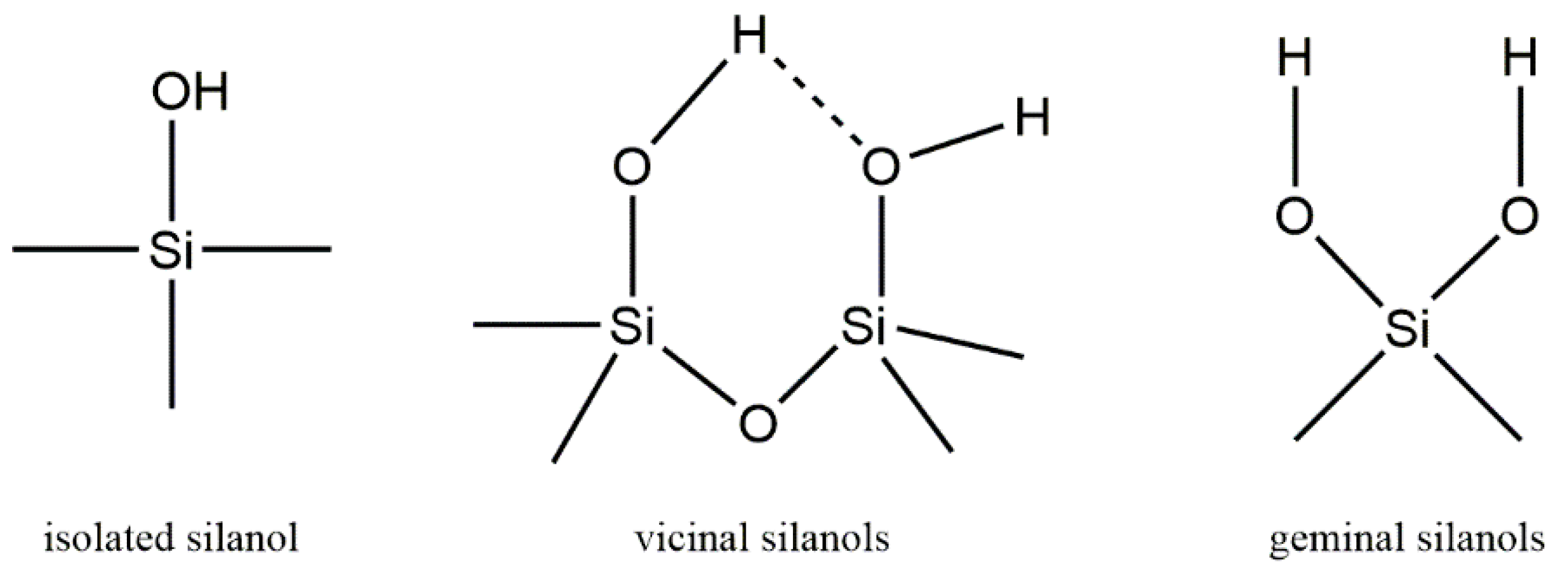
| Isolated Silanol Areas | 29Si NMR Resonance Chemical Shift | |||
|---|---|---|---|---|
| (%I) | (%I OH) | Isolated Silanols (ppm) | Geminal Silanols (ppm) | |
| HMS | 90.31% | 82.33% | −100.43 | −90.86 |
| MCM-41 | 95.72% | 91.79% | −100.41 | −90.4 |
| KIT-6 | 95.64% | 91.65% | −101.58 | −92.13 |
| SiO2-70 Å | 87.45% | 77.69% | −100.71 | −91.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Bai, X.; Chen, Y.; Wang, W.; Chen, Q.; Cao, Z.; Yan, T. Selective Separation of Zr(IV) from Simulated High-Level Liquid Waste by Mesoporous Silica. Nanomaterials 2024, 14, 13. https://doi.org/10.3390/nano14010013
Hu Y, Bai X, Chen Y, Wang W, Chen Q, Cao Z, Yan T. Selective Separation of Zr(IV) from Simulated High-Level Liquid Waste by Mesoporous Silica. Nanomaterials. 2024; 14(1):13. https://doi.org/10.3390/nano14010013
Chicago/Turabian StyleHu, Yifu, Xue Bai, Yan Chen, Wentao Wang, Qi Chen, Zhi Cao, and Taihong Yan. 2024. "Selective Separation of Zr(IV) from Simulated High-Level Liquid Waste by Mesoporous Silica" Nanomaterials 14, no. 1: 13. https://doi.org/10.3390/nano14010013
APA StyleHu, Y., Bai, X., Chen, Y., Wang, W., Chen, Q., Cao, Z., & Yan, T. (2024). Selective Separation of Zr(IV) from Simulated High-Level Liquid Waste by Mesoporous Silica. Nanomaterials, 14(1), 13. https://doi.org/10.3390/nano14010013





