Halloysite-TiO2 Nanocomposites for Water Treatment: A Review
Abstract
1. Introduction
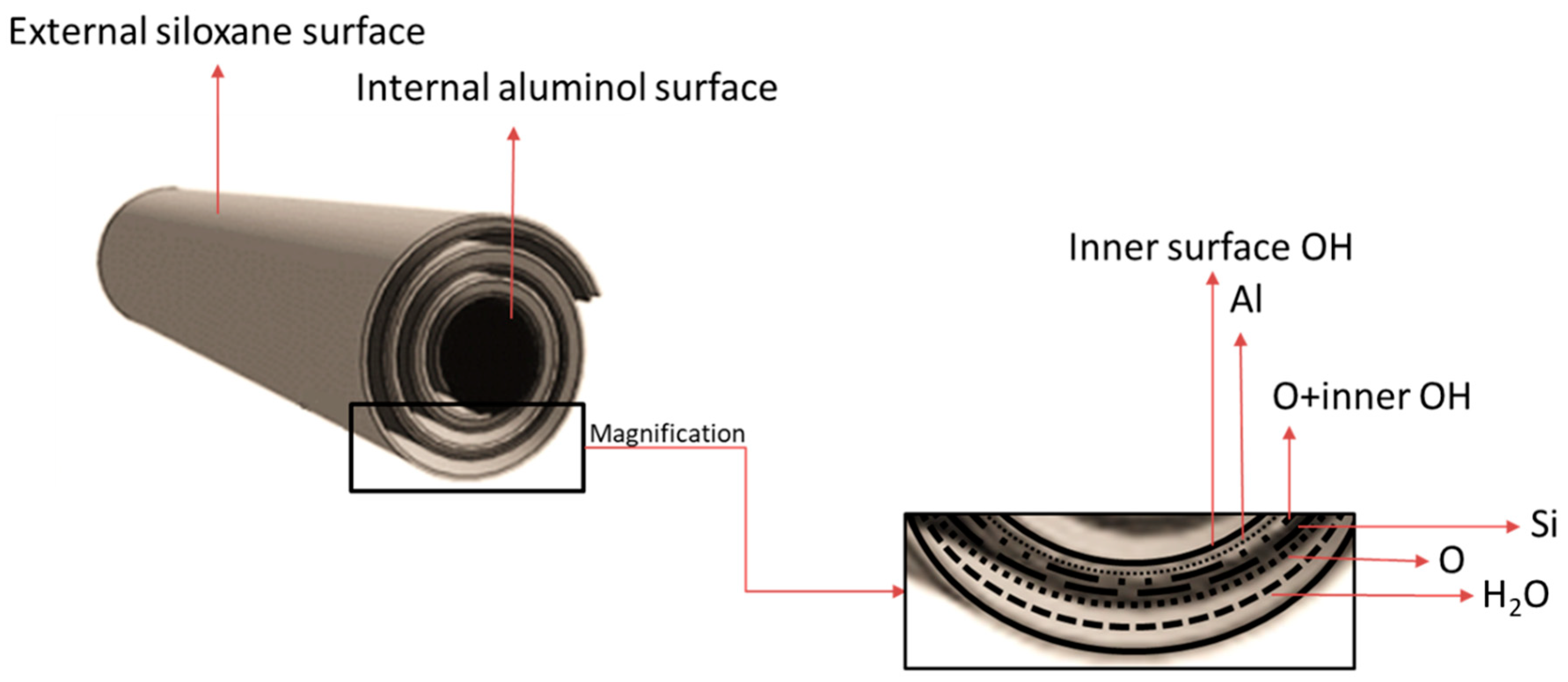
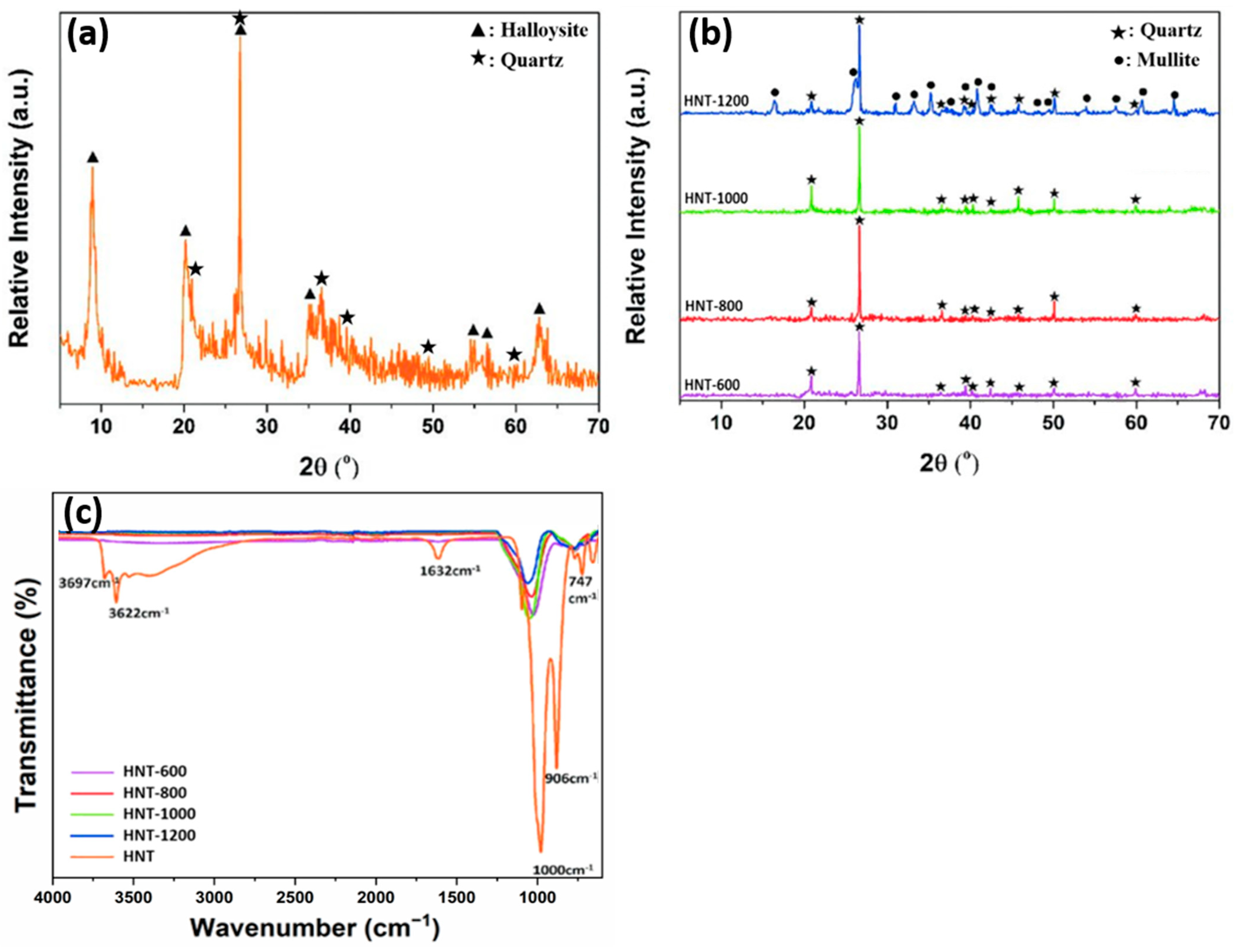
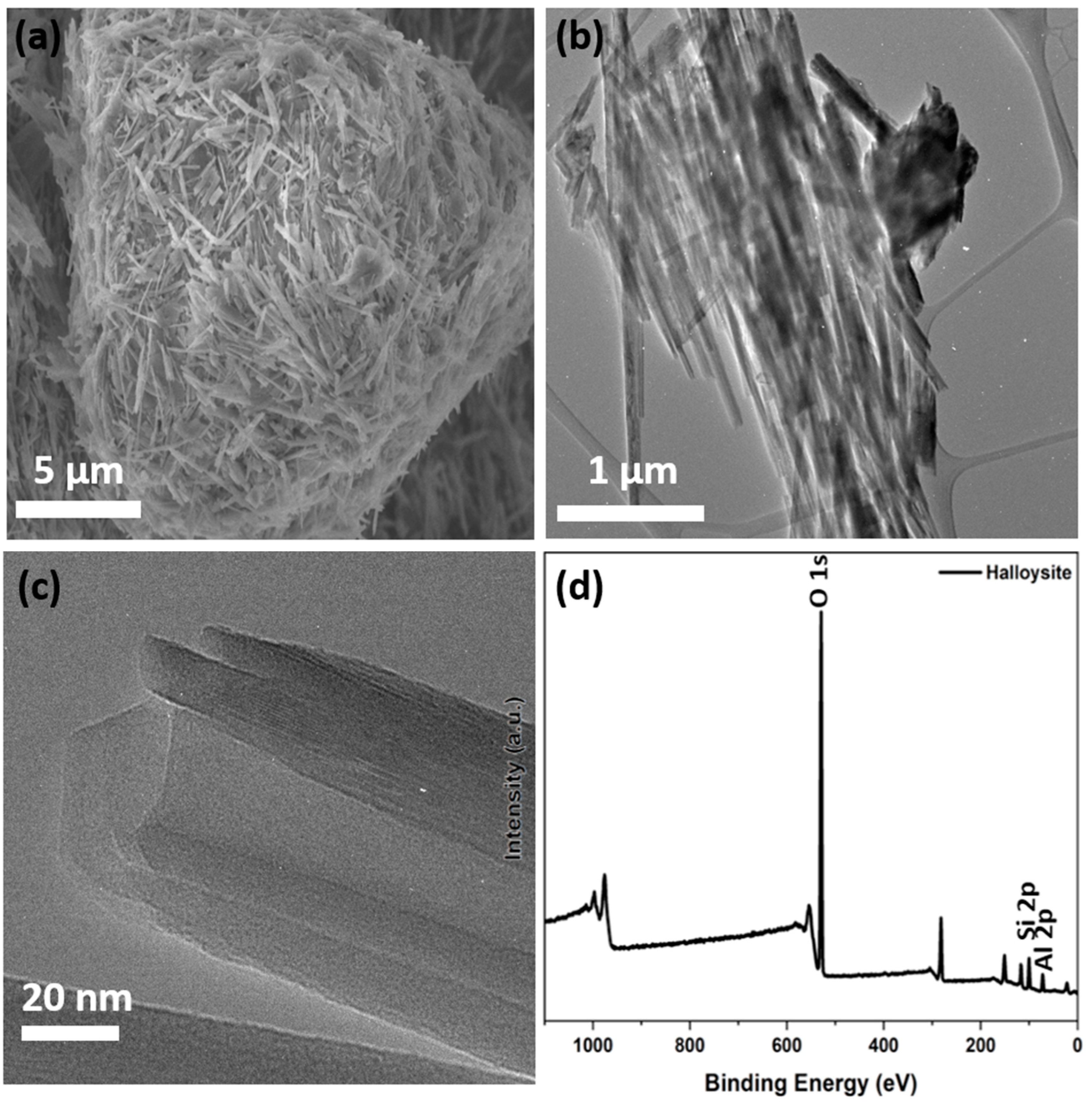
2. Halloysite Structure and Characterization
3. Extraction and Purification of Natural Halloysite
3.1. Sedimentation and Purification
3.2. Base-Treated Purification
3.3. Drying and Ball Milling
3.4. Magnetic Separation
3.5. Acid Leaching and Ball Milling
3.6. Ultrasonic Dispersion
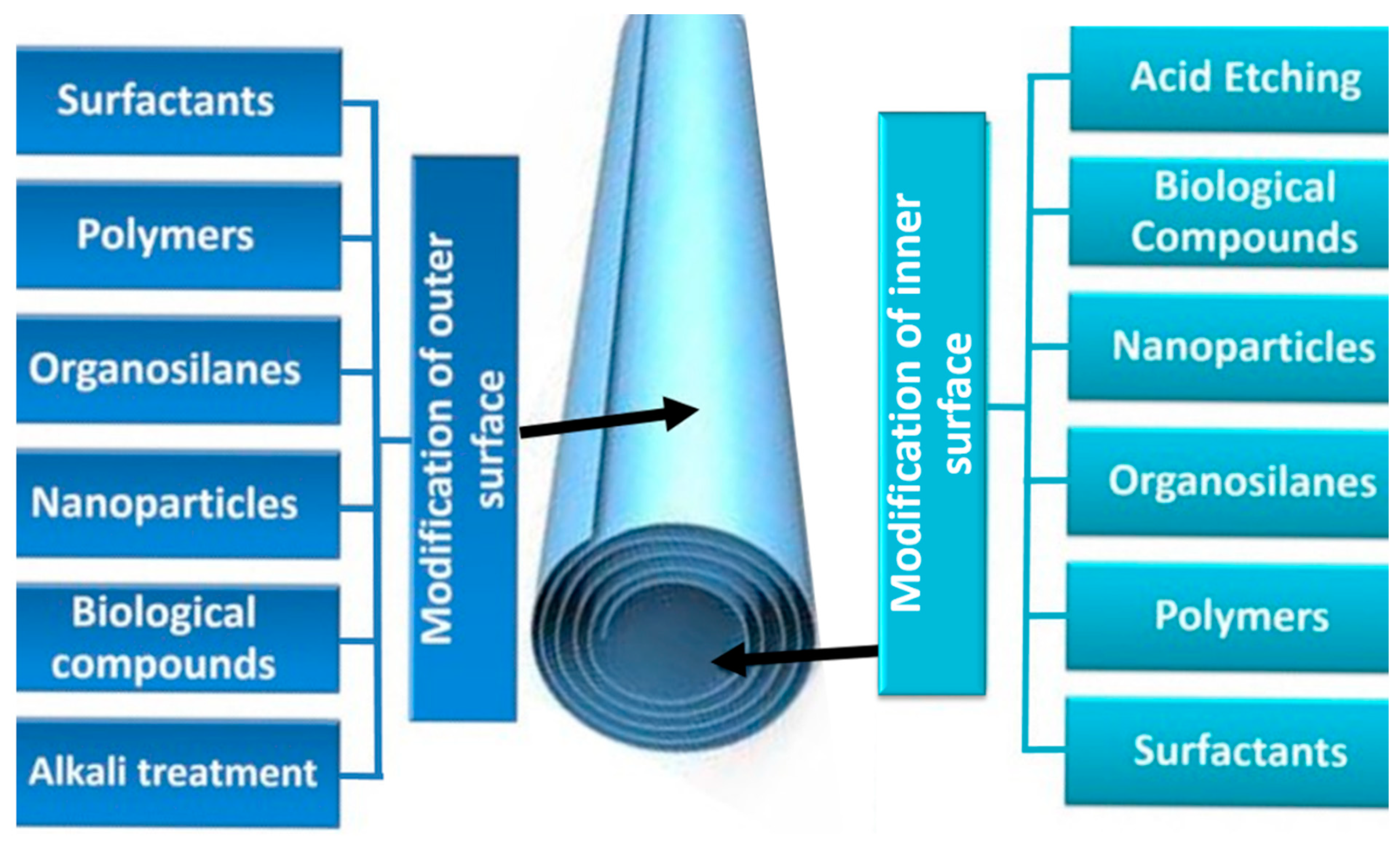
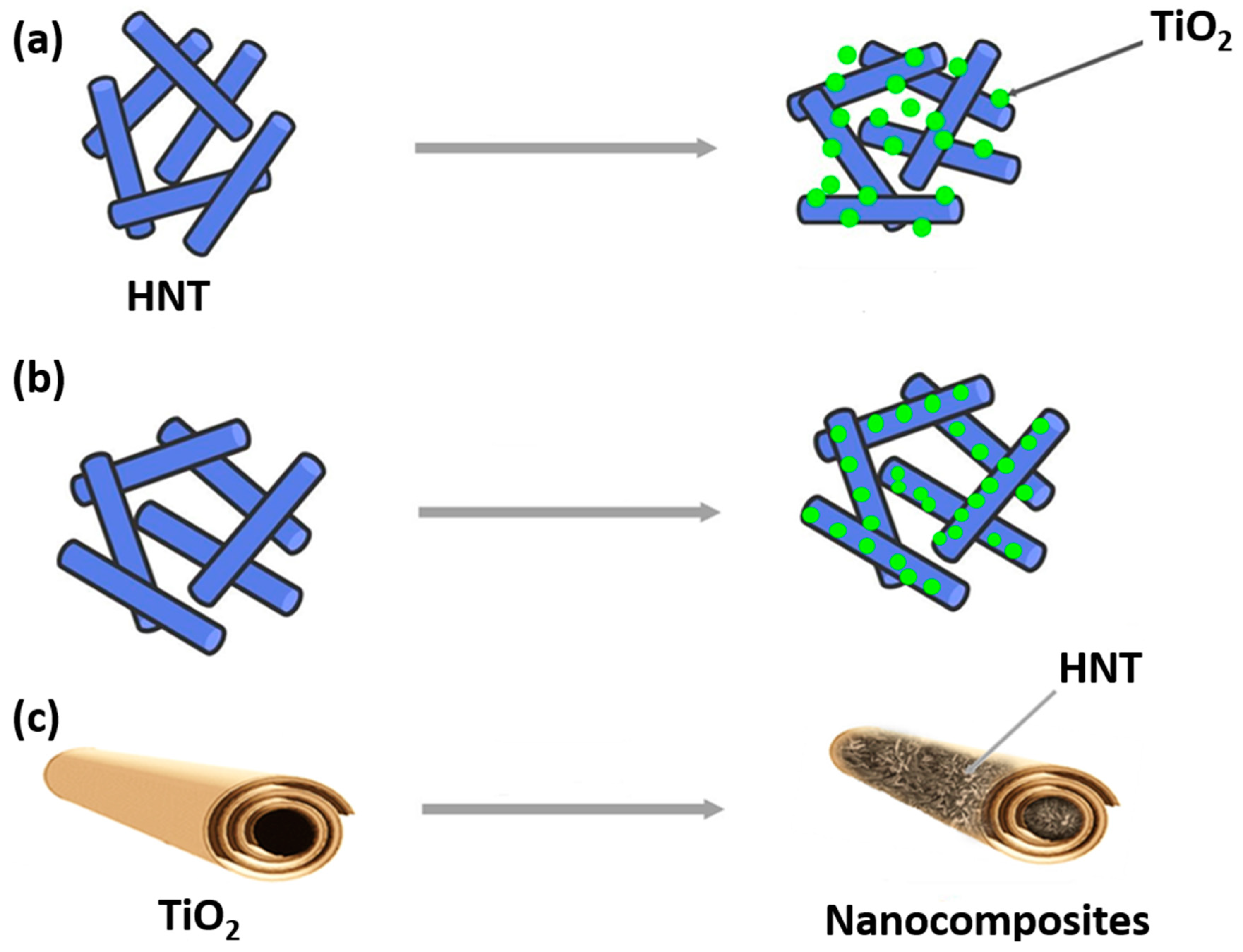
4. Modification of Halloysite Outer and Inner Surfaces
| Modification | Category | Example | Applications | Ref |
|---|---|---|---|---|
| Outer surface | Alkali etching | NaOH | HNT toughening | [57] |
| Biological compounds | Tannic acid | Dye and salt separation | [63] | |
| Nanoparticles | Ru | Preferential oxidation of CO in H2 | [59] | |
| Co3O4 | Enhancement of magnetic properties | [7] | ||
| Fe3O4 | Synthesis of dihydropyrimidinones | [62] | ||
| TiO2 | Photocatalysis | [64] | ||
| ZnO | Antibacterial activity | [61] | ||
| Au | Benzyl alcohol oxidation | [60] | ||
| Ag | Antibacterial activity | [61] | ||
| Organosilanes | 3-aminopropyl-triethoxysilane (APTES) | Immobilization of α-amylase | [65] | |
| 3-(2-aminoethylamino)propyltrimethoxysilane (AEAPTMS) | Antibacterial activity | [66] | ||
| 3-mercaptopropyltrimethoxysilane (MPTS) | New drug delivery system | [67] | ||
| Polymers and biopolymers | Poly(3,4-ethylenedioxythiophene) PEDOT | - | [68] | |
| Polyethylenimine (PEI) | Gene delivery | [34] | ||
| Polyaniline | Sensitive ascorbic acid sensor | [69] | ||
| Pectin | Adsorption | [70] | ||
| Hexadecyltrimethoxysilane (DNA) | - | [71] | ||
| Polydopamine | Catalyst for ammonia borane hydrolysis | [72] | ||
| Chitosan | Drug carrier | [73] | ||
| Surfactants | Alkyltrimethyl ammonium bromide | Reverse micelles for water-in-oil emulsion | [74] | |
| Hexadecyltrimethoxysilane (HDTMS) | Oil water separation | [75] | ||
| Inner surface | Acid etching | H2SO4 | - | [76] |
| HCl | Adsorption and release of ofloxacin | [77] | ||
| Biomolecule | Curcumin | Adsorption | [78] | |
| Nanomaterials | Ag | Photocatalysis | [79] | |
| Cu-Ni | - | [80] | ||
| Carbon nanodots | - | [81] | ||
| Organosilanes | APTES | Adsorption | [82] | |
| Polymers | Polymethyl methacrylate (PMMA) | - | [83] | |
| Polyethylene oxide (PEO) | - | [84] | ||
| Surfactant | Perfluoropentanoic acid (PCF5H) Perfluoroheptanoic acid (PCF7H) Perfluorooctanoic acid (PCF8H) | - | [85] |
5. Nanocomposites Based on HNT and Semiconductors
| Nanocomposite | Synthesis Method | Applications | Ref |
|---|---|---|---|
| Ag-ZnO/HNT | Sol–gel | E. coli antibacterial activity | [61] |
| CeO2-ZnO/HNT | Precipitation in ethanol system | E. coli antibacterial activity | [87] |
| Polymer-Fe3O4-HNT | Surface-initiated precipitation polymerization + computer simulation | Delivery of a cationic drug (norfloxacin) | [98] |
| Core/shell HNT/Fe3O4 | Co-precipitation and modified mussel-inspired co-modification route | Adsorption of methylene blue | [91] |
| KH42@Fe3O4/HNT | In situ growth of Fe3O4 nanoparticles | Removal of heavy metals [Cr(VI), Sb(V)] | [93] |
| Fe3O4-HNT | Chemical precipitation | Removal of cadmium(II) | [94] |
| MHNT@MnO2 | Precipitation and hydrothermal method | Lead(II) removal from aqueous solutions | [96] |
| HNT-TiO2-(solid phase microextraction) | Etching, hydroxylation, amino grafting, and sol–gel | Parathion removal | [97] |
| g-C3N4-ZnO/Hal | Calcination | Degradation of tetracycline under visible light irradiation | [86] |
| g-C3N4/TiO2/Hal | Sol–gel + calcination | Visible light photodegradation of ciprofloxacin | [104] |
| CeO2/AgBr-HNT | Microwave | Degradation of methyl orange | [102] |
| CdS-HNT | Hydrothermal | Degradation of tetracycline | [95] |
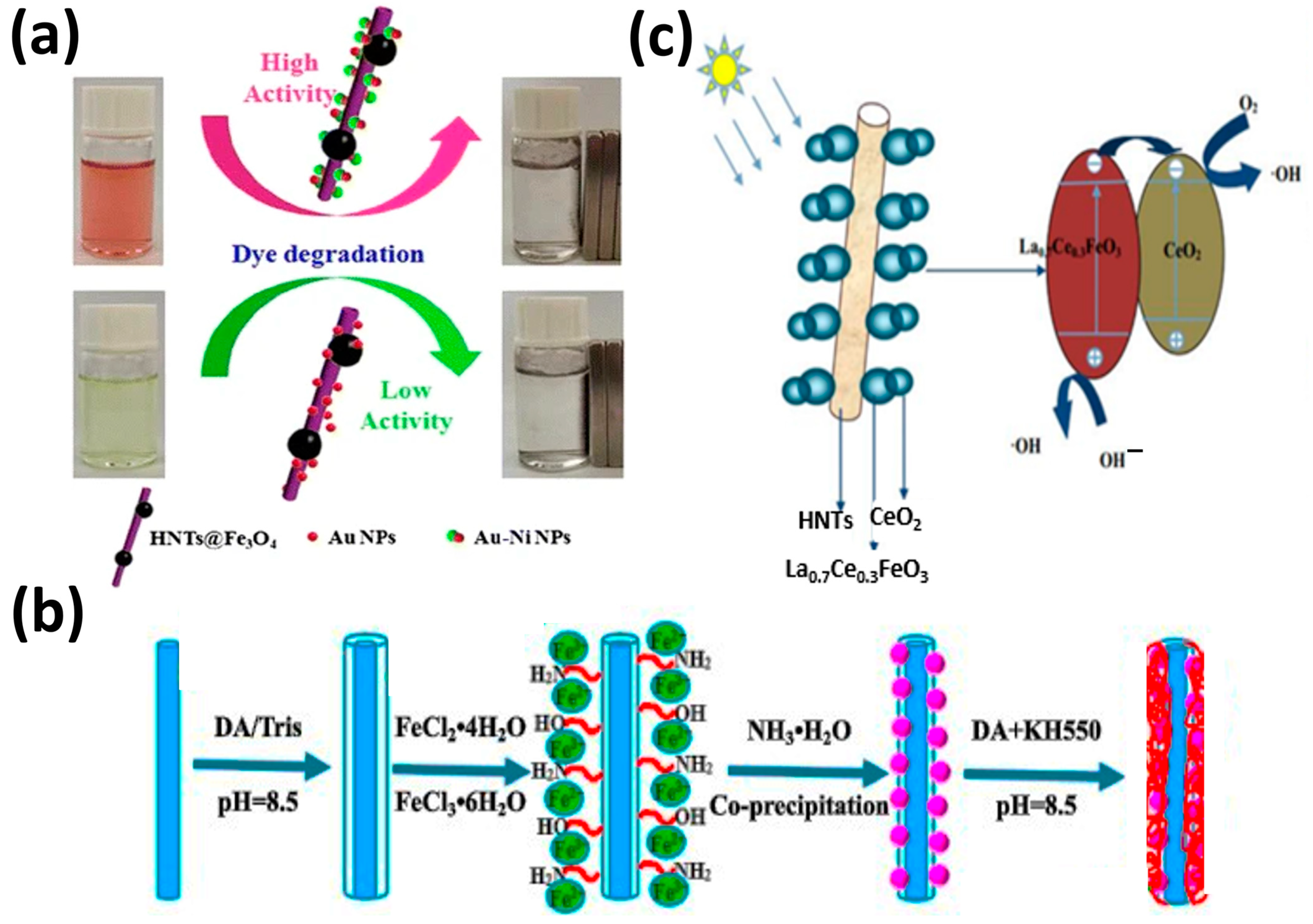
| Au–Ni/Fe3O4-HNT | Impregnation | Degradation of Congo red | [90] |
| Polyaniline–crystalline TiO2-HNT | Impregnation | Degradation of rhodamine B | [101] |
| TiO2-HNT | Impregnation | Degradation of methylene blue | [25] |
| ZnO or TiO2-HNT | Deposition | Degradation of methylene blue | [88] |
| LaFeO3-HNT | Sol–gel | Degradation of chlortetracycline | [92] |
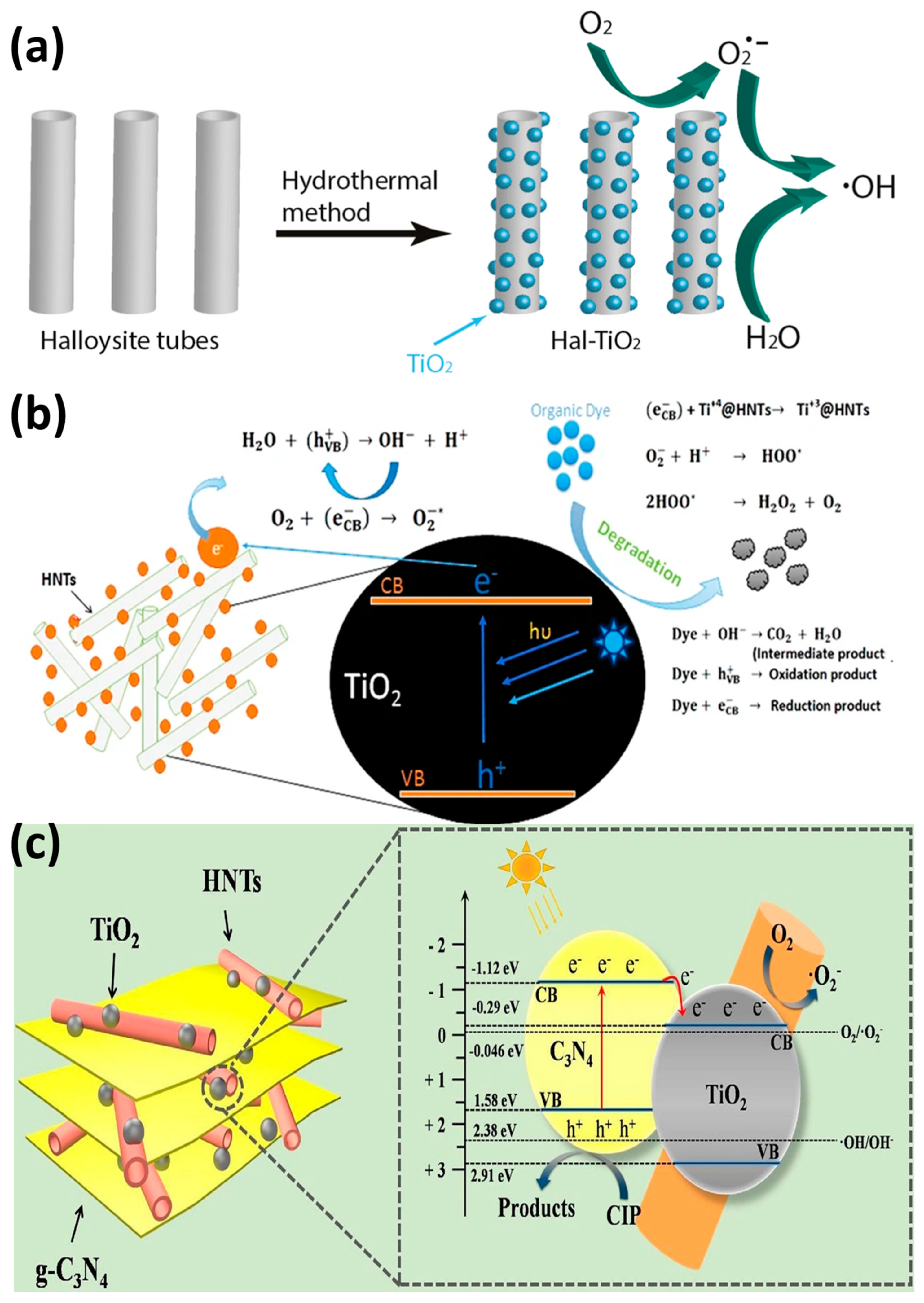
| TiO2/Halloysite | TiO2 sol dispersion and hydrothermal treatment | Decomposition of NOx gases | [103] |
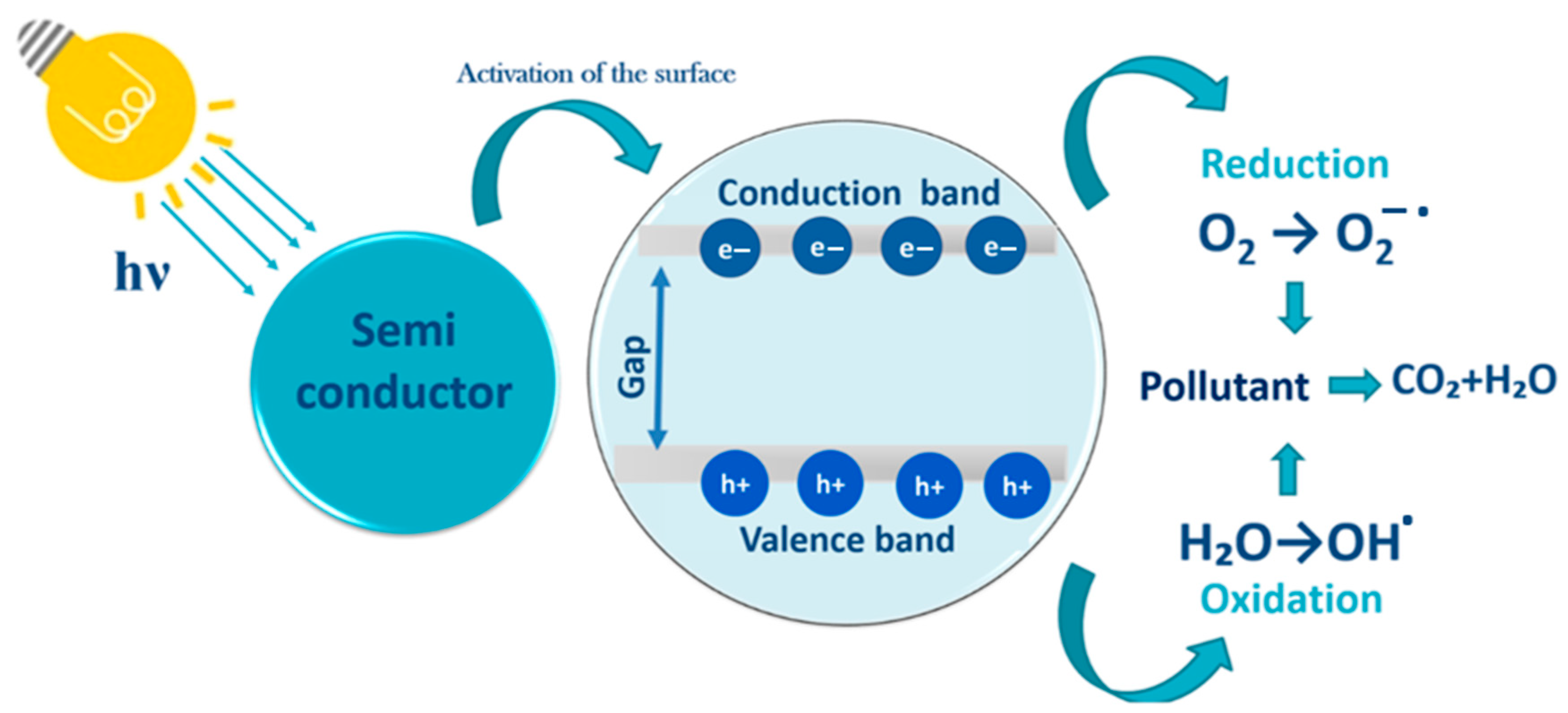
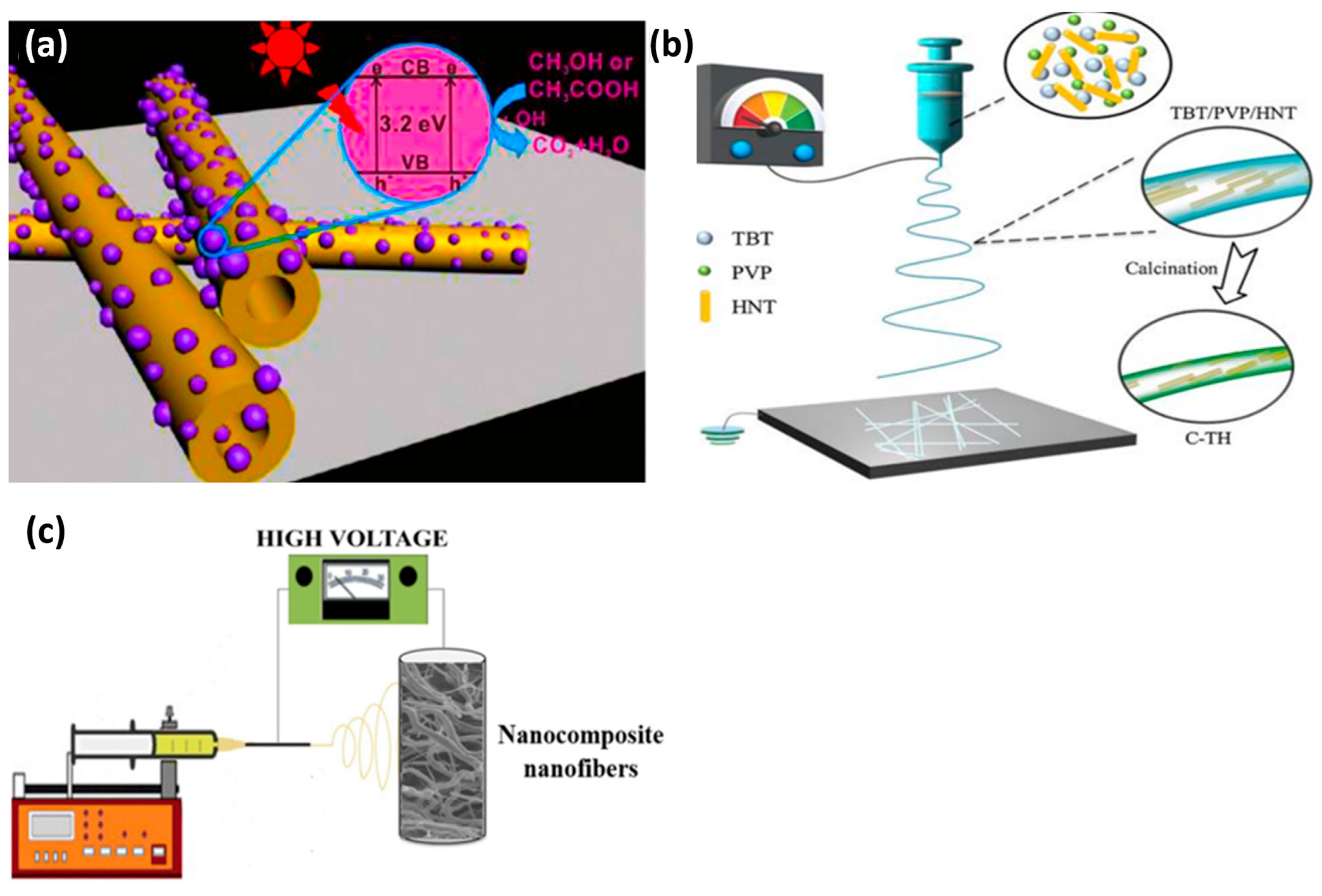
6. Heterogeneous Photocatalysis
7. Halloysite-TiO2 Nanocomposites for Water Treatment
| Photocatalyst (g/L) | Method | Pollutant (mg/L) | Irradiation Type | Removal Efficiency (%) | Degradation Time (min) | Ref |
|---|---|---|---|---|---|---|
| Pani-TiO2-HNT (0.5 g/L) | Low temperature | Rhodamine B (10 mg/L) | XPA-7 photochemical system (800 W Xe lamp) | 73.49 | 360 | [143] |
| g-C3N4/TiO2/HNT (0.8 g/L) | Sol–gel and calcination | Ciprofloxacin (15 mg/L) | 300 W Xe lamp | 87.00 | 60 | [104] |
| H95T5 (0.5 g/L) | Sol–gel + electrospinning | Acetaminophen (10 mg/L) | Medium-pressure metal halide UV | 100 | 60 | [41] |
| Acetaminophen (10 mg/L) | Halogen linear lamp | 91.10 | 360 | |||
| Methylene blue (6.64 mg/L) | Medium-pressure metal halide UV | 99.98 | 20 | |||
| Methylene blue (6.64 mg/L) | Halogen linear lamp | 96.83 | 180 | |||
| TiO2@HNT (4.2 g/L) | Sol–gel + Phase inversion | Methylene blue 20 mg/L | 125 W UV lamp (254 nm) | 87.47 | 120 | [136] |
| Methylene blue 20 mg/L | 96.87 | 120 | ||||
| TiO2/HNT (0.5 g/L) | Sol–gel | Methylene blue (32 mg/L) | 12 W UV lamp (λ = 365 nm) | 81.60 | 240 | [25] |
| TiO2/HNT (20 mg) | Solvothermal | Acetic acid 5 mL of 5% | 100 W high-pressure lamp | 3488.63 μmol/g | 60 | [64] |
| Methanol 5 mL of 5% | 729.37 μmol/g | 120 | ||||
| Ce-TiO2/HNT (0.5 g/L) | Sol–gel | Tetracycline 20 mg/L | 300 W Xe lamp (λ > 420) | 78.00 | 60 | [145] |
| Ag/TiO2-HNT (0.625 g/L) | Sol–gel | Basic blue 41 (12 mg/L) | Four black light fluorescent of 4 W | 100 | 100 | [141] |
| Carbon-TiO2-HNT (8%) (0.2 g/L) | Sol–gel + Electrospinning | Methylene blue (20 mg/L) | 50 W UV lamps (k < 420 nm) | 81.00 | 90 | [31] |
| Amylose-HNT-TiO2 (1 g/L) | Ball milling + Sol–gel | 4-nitrophenol (10 mg/L) | 12 W UV lamp (λ = 253 nm) | 90.00 | 240 | [99] |
8. Reaction Kinetics of Photocatalytic Pollutant Degradation Using Halloysite-TiO2 Nanocomposites as Photocatalysts
9. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.H. Clay mineral-based catalysts and catalysis. Appl. Clay Sci. 2011, 53, 85–86. [Google Scholar] [CrossRef]
- Zhou, C.H.; Keeling, J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Appl. Clay Sci. 2013, 74, 3–9. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.; Liu, S.; Zhang, L.; Wang, T.; Wang, C.; Su, D. Constructing efficient α-Fe2O3/g-C3N4/HNTs-loaded heterojunction photocatalysts for photocatalytic oxidative desulfurization: Influencing factors, kinetics, and mechanism. Fuel 2023, 332, 126147. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Yu, L.; Wang, H.; Zhang, Y.; Zhang, B.; Liu, J. Recent advances in halloysite nanotube derived composites for water treatment. Environ. Sci. Nano 2015, 3, 28–44. [Google Scholar] [CrossRef]
- Lazzara, G.; Bruno, F.; Brancato, D.; Sturiale, V.; D’Amico, A.G.; Miloto, S.; Pasbakhsh, P.; D’Agata, V.; Saccone, S.; Federico, C. Biocompatibility analysis of halloysite clay nanotubes. Mater. Lett. 2023, 336, 133852. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H. Halloysite nanotubes coated with magnetic nanoparticles. Appl. Clay Sci. 2012, 56, 97–102. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.V.; Lvov, Y.M.; Shchukin, D.G.; Möhwald, H. Application of Inhibitor-Loaded Halloysite Nanotubes in Active Anti-Corrosive Coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Schmitt, H.; Creton, N.; Prashantha, K.; Soulestin, J.; Lacrampe, M.; Krawczak, P. Melt-blended halloysite nanotubes/wheat starch nanocomposites as drug delivery system. Polym. Eng. Sci. 2014, 55, 573–580. [Google Scholar] [CrossRef]
- Kelly, H.; Deasy, P.; Ziaka, E.; Claffey, N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int. J. Pharm. 2004, 274, 167–183. [Google Scholar] [CrossRef]
- Feitosa, S.; Palasuk, J.; Kamocki, K.; Geraldeli, S.; Gregory, R.; Platt, J.; Windsor, L.; Bottino, M.C. Doxycycline-Encapsulated Nanotube-Modified Dentin Adhesives. J. Dent. Res. 2014, 93, 1270–1276. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite Nanotubes as Biomimetic Nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Zheng, J. In-Situ Deposition of Pd Nanoparticles on Tubular Halloysite Template for Initiation of Metallization. J. Nanosci. Nanotechnol. 2005, 5, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Meng, Y.; Zhao, Y.; Xu, L.; Su, J.; Han, J.; Dong, J. Preparation of polyvinylidene fluoride coating modified by well-dispersed MEA-ATO@HNTs and its photocatalytic, antistatic and thermal insulation properties. Ceram. Int. 2023, 49, 20437–20446. [Google Scholar] [CrossRef]
- Melnikov, D.; Reshetina, M.; Novikov, A.; Cherednichenko, K.; Stavitskaya, A.; Stytsenko, V.; Vinokurov, V.; Huang, W.; Glotov, A. Strategies for palladium nanoparticles formation on halloysite nanotubes and their performance in acetylene semi-hydrogenation. Appl. Clay Sci. 2023, 232, 106763. [Google Scholar] [CrossRef]
- Abid, M.; Sayegh, S.; Tanos, F.; Belaid, H.; Iatsunskyi, I.; Coy, E.; Cretin, M.; Lesage, G.; Amara, A.B.H.; Bechelany, M. A novel BN/TiO2/HNT nanocomposite for photocatalytic applications fabricated by electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131043. [Google Scholar] [CrossRef]
- Abid, M.; Makhoul, E.; Tanos, F.; Iatsunskyi, I.; Coy, E.; Lesage, G.; Cretin, M.; Cornu, D.; Amara, A.B.H.; Bechelany, M. N-Doped HNT/TiO2 Nanocomposite by Electrospinning for Acetaminophen Degradation. Membranes 2023, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, P.; Ratnam, C.T.; Manickam, S. Development of silane grafted halloysite nanotube reinforced polylactide nanocomposites for the enhancement of mechanical, thermal and dynamic-mechanical properties. Appl. Clay Sci. 2017, 135, 583–595. [Google Scholar] [CrossRef]
- Selli, N.T.; Basaran, N. Controlling the hardness and wear resistance of opaque white glaze by addition of halloysite clay in the composition. Boletín de la Sociedad Española de Cerámica y Vidrio, 2022; in press. [Google Scholar] [CrossRef]
- Rozynek, Z.; Zacher, T.; Janek, M.; Čaplovičová, M.; Fossum, J.O. Electric-field-induced structuring and rheological properties of kaolinite and halloysite. Appl. Clay Sci. 2013, 77–78, 1–9. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Liu, Q.; Chen, Y.; Jin, S.; Xiao, K.; Zhu, H.; Zhang, X.; Wu, X. Simultaneous promotion of mechanical and electrical properties of hot-pressing halloysite-based mullite ceramics through carbon incorporation. Appl. Clay Sci. 2023, 232, 106766. [Google Scholar] [CrossRef]
- Maleki, S.T.; Sadati, S.J. Synthesis and investigation of hyperthermia properties of Fe3O4/HNTs magnetic nanocomposite. Inorg. Chem. Commun. 2022, 145, 110000. [Google Scholar] [CrossRef]
- Mei, D.; Zhang, B.; Liu, R.; Zhang, Y.; Liu, J. Preparation of capric acid/halloysite nanotube composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 2772–2777. [Google Scholar] [CrossRef]
- Du, Y.; Zheng, P. Adsorption and photodegradation of methylene blue on TiO2-halloysite adsorbents. Korean J. Chem. Eng. 2014, 31, 2051–2056. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Singer, A.; Zarei, M.; Lange, F.; Stahr, K. Halloysite characteristics and formation in the northern Golan Heights. Geoderma 2004, 123, 279–295. [Google Scholar] [CrossRef]
- Hashemifard, S.; Ismail, A.; Matsuura, T. Mixed matrix membrane incorporated with large pore size halloysite nanotubes (HNTs) as filler for gas separation: Morphological diagram. Chem. Eng. J. 2011, 172, 581–590. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Y.; Liu, T. Enhanced visible-light photocatalytic performance of electrospun carbon-doped TiO2/halloysite nanotube hybrid nanofibers. J. Colloid Interface Sci. 2015, 439, 62–68. [Google Scholar] [CrossRef]
- Rawtani, D.; Agrawal, Y.K. Study the Interaction of DNA with Halloysite Nanotube-Gold Nanoparticle Based Composite. J. Bionanoscience 2012, 6, 95–98. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, C.; Wei, J.; Tjiu, W.; Pan, J.; Chen, Y.; Liu, T. Surface modifications of halloysite nanotubes with superparamagnetic Fe3O4 nanoparticles and carbonaceous layers for efficient adsorption of dyes in water treatment. Chem. Res. Chin. Univ. 2014, 30, 971–977. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, J.; Shen, Y.; Zhou, C.; Liu, M. Polyethyleneimine grafted short halloysite nanotubes for gene delivery. Mater. Sci. Eng. C 2017, 81, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite Nanotubes and Halloysite-Based Composites for Environmental and Biomedical Applications. J. Mol. Liquids 2020, 309, 113077. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Xu, Y.; Zhang, S.-Q.; Dai, L.-Y.; Wang, Y. Fabricating a Fe3O4@HNTs nanoreactor to expedite heterogeneous Fenton-like reactions. Mater. Lett. 2023, 337, 133985. [Google Scholar] [CrossRef]
- Selli, N.T.; Aker, I.M.; Basaran, N.; Kabakci, E. Influence of calcined halloysite on technological & mechanical properties of wall tile body. J. Asian Ceram. Soc. 2021, 9, 1331–1344. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2018, 168, 164–174. [Google Scholar] [CrossRef]
- Abid, M.; Sayegh, S.; Iatsunskyi, I.; Coy, E.; Lesage, G.; Ramanavicius, A.; Amara, A.B.H.; Bechelany, M. Design of halloysite-based nanocomposites by electrospinning for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129696. [Google Scholar] [CrossRef]
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.; Petit, C.; Apostolopoulou, A.; Stathatos, E.; Komarneni, S.; Koukouvelas, I. Halloysite and sepiolite –TiO2 nanocomposites: Synthesis characterization and photocatalytic activity in three aquatic wastes. Mater. Sci. Semicond. Process. 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhou, Z.; Zhang, Y.; Yang, H. High morphological stability and structural transition of halloysite (Hunan, China) in heat treatment. Appl. Clay Sci. 2014, 101, 16–22. [Google Scholar] [CrossRef]
- Prishchenko, D.A.; Zenkov, E.V.; Mazurenko, V.V.; Fakhrullin, R.F.; Lvov, Y.M.; Mazurenko, V.G. Molecular dynamics of the halloysite nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 5841–5849. [Google Scholar] [CrossRef]
- Ferrante, F.; Armata, N.; Lazzara, G. Modeling of the Halloysite Spiral Nanotube. J. Phys. Chem. C 2015, 119, 16700–16707. [Google Scholar] [CrossRef]
- Guimarães, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, Electronic, and Mechanical Properties of Single-Walled Halloysite Nanotube Models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]
- Hu, X.L.; Michaelides, A. Ice formation on kaolinite: Lattice match or amphoterism? Surf. Sci. 2007, 601, 5378–5381. [Google Scholar] [CrossRef]
- Hu, X.L.; Michaelides, A. Water on the hydroxylated (001) surface of kaolinite: From monomer adsorption to a flat 2D wetting layer. Surf. Sci. 2008, 602, 960–974. [Google Scholar] [CrossRef]
- Fares, M.L.; Athmani, M.; Khelfaoui, Y.; Khettache, A. An investigation into the effects of conventional heat treatments on mechanical characteristics of new hot working tool steel. IOP Conf. Series Mater. Sci. Eng. 2012, 28, 012042. [Google Scholar] [CrossRef]
- Cheng, C.; Song, W.; Zhao, Q.; Zhang, H. Halloysite nanotubes in polymer science: Purification, characterization, modification and applications. Nanotechnol. Rev. 2020, 9, 323–344. [Google Scholar] [CrossRef]
- Ben Rhaiem, H.; Tessier, D.; Amara, A.B.H. Mineralogy of the <2 μm fraction of three mixed-layer clays from southern and central Tunisia. Clay Miner. 2000, 35, 375–381. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Wu, Y.; Song, H.; Ba, X. High-efficiency grafting of halloysite nanotubes by using π-conjugated polyfluorenes via “click” chemistry. J. Mater. Sci. 2015, 50, 4387–4395. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutynski, M.; Soltys, J.; Pytlinski, A. Purification of halloysite by magnetic separation. Physicochem. Probl. Miner. Process. 2016, 52, 991–1001. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Lutynski, M.A. Purification of Dunino halloysite by H2SO4leaching and magnetic separation. E3S Web Conf. 2016, 8, 1032. [Google Scholar] [CrossRef]
- Rong, R.; Xu, X.; Zhu, S.; Li, B.; Wang, X.; Tang, K. Facile preparation of homogeneous and length controllable halloysite nanotubes by ultrasonic scission and uniform viscosity centrifugation. Chem. Eng. J. 2016, 291, 20–29. [Google Scholar] [CrossRef]
- Tharmavaram, M.; Pandey, G.; Rawtani, D. Surface modified halloysite nanotubes: A flexible interface for biological, environmental and catalytic applications. Adv. Colloid Interface Sci. 2018, 261, 82–101. [Google Scholar] [CrossRef]
- Zeng, S.; Reyes, C.; Liu, J.; Rodgers, P.A.; Wentworth, S.H.; Sun, L. Facile hydroxylation of halloysite nanotubes for epoxy nanocomposite applications. Polymer 2014, 55, 6519–6528. [Google Scholar] [CrossRef]
- Rawtani, D.; Pandey, G.; Tharmavaram, M.; Pathak, P.; Akkireddy, S.; Agrawal, Y. Development of a novel ‘nanocarrier’ system based on Halloysite Nanotubes to overcome the complexation of ciprofloxacin with iron: An in vitro approach. Appl. Clay Sci. 2017, 150, 293–302. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Ge, L.; Rudolph, V.; Zhu, Z. Halloysite Nanotube Supported Ru Nanocatalysts Synthesized by the Inclusion of Preformed Ru Nanoparticles for Preferential Oxidation of CO in H2-Rich Atmosphere. J. Phys. Chem. C 2013, 117, 4141–4151. [Google Scholar] [CrossRef]
- Philip, A.; Lihavainen, J.; Keinänen, M.; Pakkanen, T.T. Gold nanoparticle-decorated halloysite nanotubes–Selective catalysts for benzyl alcohol oxidation. Appl. Clay Sci. 2017, 143, 80–88. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Hajizadeh, Z.; Firouzi-Haji, R. Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Microporous Mesoporous Mater. 2018, 259, 46–53. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; Isloor, A.M.; Moslehyani, A.; Ismail, A. Bio-inspired, fouling resistant, tannic acid functionalized halloysite nanotube reinforced polysulfone loose nanofiltration hollow fiber membranes for efficient dye and salt separation. J. Water Process. Eng. 2017, 20, 138–148. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, G.; Ding, Y.; Wang, Y.; Sun, X.; Wang, X.; Chen, W. Photocatalytic Activity of Heterostructures Based on TiO2 and Halloysite Nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4154–4158. [Google Scholar] [CrossRef]
- Pandey, G.; Munguambe, D.M.; Tharmavaram, M.; Rawtani, D.; Agrawal, Y. Halloysite nanotubes—An efficient ‘nano-support’ for the immobilization of α-amylase. Appl. Clay Sci. 2016, 136, 184–191. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Li, X.; Chen, W. Synthesis of vegetable oil-based waterborne polyurethane/silver-halloysite antibacterial nanocomposites. Compos. Sci. Technol. 2016, 126, 86–93. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Meo, P.L.; Noto, R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Functionalized halloysite multivalent glycocluster as a new drug delivery system. J. Mater. Chem. B 2014, 2, 7732–7738. [Google Scholar] [CrossRef]
- Rosas-Aburto, A.; Gabaldón-Saucedo, I.A.; Espinosa-Magaña, F.; Ochoa-Lara, M.; Roquero-Tejeda, P.; Hernández-Luna, M.; Revilla-Vázquez, J. Intercalation of poly(3,4-ethylenedioxythiophene) within halloysite nanotubes: Synthesis of composites with improved thermal and electrical properties. Microporous Mesoporous Mater. 2015, 218, 118–129. [Google Scholar] [CrossRef]
- Shao, L.; Wang, X.; Yang, B.; Wang, Q.; Tian, Q.; Ji, Z.; Zhang, J. A Highly Sensitive Ascorbic Acid Sensor Based on Hierarchical Polyaniline Coated Halloysite Nanotubes Prepared by Electrophoretic Deposition. Electrochim. Acta 2017, 255, 286–297. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Shamsi, M.H.; Geckeler, K.E. The first biopolymer-wrapped non-carbon nanotubes. Nanotechnology 2008, 19, 075604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, H.; Zhang, J.; Zhao, Y.; Yang, J.-H.; Zhang, B. Polydopamine-coated halloysite nanotubes supported AgPd nanoalloy: An efficient catalyst for hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2018, 43, 2754–2762. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Hydrophobically Modified Halloysite Nanotubes as Reverse Micelles for Water-in-Oil Emulsion. Langmuir 2015, 31, 7472–7478. [Google Scholar] [CrossRef]
- Feng, K.; Hung, G.-Y.; Liu, J.; Li, M.; Zhou, C.; Liu, M. Fabrication of high performance superhydrophobic coatings by spray-coating of polysiloxane modified halloysite nanotubes. Chem. Eng. J. 2018, 331, 744–754. [Google Scholar] [CrossRef]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of Halloysite Clay Nanotube Lumen by Selective Etching of Aluminum Oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zheng, Y.; Wang, A. Adsorption and release of ofloxacin from acid- and heat-treated halloysite. Colloids Surf. B Biointerfaces 2013, 113, 51–58. [Google Scholar] [CrossRef]
- Sabahi, H.; Khorami, M.; Rezayan, A.H.; Jafari, Y.; Karami, M.H. Surface functionalization of halloysite nanotubes via curcumin inclusion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 834–840. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Wang, H.; Yang, Y. Catalytically active silver nanoparticles loaded in the lumen of halloysite nanotubes via electrostatic interactions. J. Mater. Sci. 2017, 52, 8391–8400. [Google Scholar] [CrossRef]
- Sanchez-Ballester, N.M.; Ramesh, G.V.; Tanabe, T.; Koudelkova, E.; Liu, J.; Shrestha, L.K.; Lvov, Y.; Hill, J.P.; Ariga, K.; Abe, H. Activated interiors of clay nanotubes for agglomeration-tolerant automotive exhaust remediation. J. Mater. Chem. A 2015, 3, 6614–6619. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, M.; Guo, B.; Zhang, L.; Lvov, Y. Nanodot-Loaded Clay Nanotubes as Green and Sustained Radical Scavengers for Elastomer. ACS Sustain. Chem. Eng. 2017, 5, 1775–1783. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.; Liu, Z.; Kepert, C. Organosilane functionalization of halloysite nanotubes for enhanced loading and controlled release. Nanotechnology 2012, 23, 375705. [Google Scholar] [CrossRef] [PubMed]
- Yah, W.O.; Xu, H.; Soejima, H.; Ma, W.; Lvov, Y.; Takahara, A. Biomimetic Dopamine Derivative for Selective Polymer Modification of Halloysite Nanotube Lumen. J. Am. Chem. Soc. 2012, 134, 12134–12137. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.; Jiao, Y.; Liu, Y.; Ji, C.; Zhang, Y. New insight into PEO modified inner surface of HNTs and its nano-confinement within nanotube. J. Mater. Sci. 2014, 49, 4270–4278. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Ye, Z.; Wang, H.; Ma, C.; Huo, P.; Yan, Y. Enhanced photocatalytic activity of g-C3N4–ZnO/HNT composite heterostructure photocatalysts for degradation of tetracycline under visible light irradiation. RSC Adv. 2015, 5, 91177–91189. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Ouyang, J.; Yang, H. Characterization and synergetic antibacterial properties of ZnO and CeO2 supported by halloysite. Appl. Surf. Sci. 2017, 420, 833–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, J.; Yang, H. Metal oxide nanoparticles deposited onto carbon-coated halloysite nanotubes. Appl. Clay Sci. 2014, 95, 252–259. [Google Scholar] [CrossRef]
- Rostami, M.; Zamani, R.M.; Aghajanzadeh, K.M.; Danafar, H. Sol–gel synthesis and characterization of zinc ferrite–graphene nano-hybrids for photo-catalytic degradation of the paracetamol. J. Pharm. Investig. 2017, 48, 657–664. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, T.; Xu, J.; Li, X.; Dong, K.; Huang, J.; Xu, Z. The Enhanced Catalytic Activities of Asymmetric Au-Ni Nanoparticle Decorated Halloysite-Based Nanocomposite for the Degradation of Organic Dyes. Nanoscale Res. Lett. 2016, 11, 72. [Google Scholar] [CrossRef]
- Wan, X.; Zhan, Y.; Long, Z.; Zeng, G.; He, Y. Core@double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem. Eng. J. 2017, 330, 491–504. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Yan, X.; Lu, X.; Yao, C.; Ni, C. Hierarchical La0.7Ce0.3FeO3/halloysite nanocomposite for photocatalytic degradation of antibiotics. Appl. Phys. A 2016, 122, 723. [Google Scholar] [CrossRef]
- Zhu, K.; Duan, Y.; Wang, F.; Gao, P.; Jia, H.; Ma, C.; Wang, C. Silane-modified halloysite/Fe3O4 nanocomposites: Simultaneous removal of Cr(VI) and Sb(V) and positive effects of Cr(VI) on Sb(V) adsorption. Chem. Eng. J. 2017, 311, 236–246. [Google Scholar] [CrossRef]
- Amjadi, M.; Samadi, A.; Manzoori, J.L. A composite prepared from halloysite nanotubes and magnetite (Fe3O4) as a new magnetic sorbent for the preconcentration of cadmium(II) prior to its determination by flame atomic absorption spectrometry. Microchim. Acta 2015, 182, 1627–1633. [Google Scholar] [CrossRef]
- Xing, W.; Ni, L.; Huo, P.; Lu, Z.; Liu, X.; Luo, Y.; Yan, Y. Preparation high photocatalytic activity of CdS/halloysite nanotubes (HNTs) nanocomposites with hydrothermal method. Appl. Surf. Sci. 2012, 259, 698–704. [Google Scholar] [CrossRef]
- Afzali, D.; Fayazi, M. Deposition of MnO2 nanoparticles on the magnetic halloysite nanotubes by hydrothermal method for lead(II) removal from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- Saraji, M.; Jafari, M.T.; Mossaddegh, M. Chemically modified halloysite nanotubes as a solid–phase microextraction coating. Anal. Chim. Acta 2017, 964, 85–95. [Google Scholar] [CrossRef]
- Fizir, M.; Richa, A.; He, H.; Touil, S.; Brada, M.; Fizir, L. A mini review on molecularly imprinted polymer based halloysite nanotubes composites: Innovative materials for analytical and environmental applications. Rev. Environ. Sci. Bio/Technol. 2020, 19, 241–258. [Google Scholar] [CrossRef]
- Zheng, P.; Du, Y.; Chang, P.R.; Ma, X. Amylose–halloysite–TiO2 composites: Preparation, characterization and photodegradation. Appl. Surf. Sci. 2015, 329, 256–261. [Google Scholar] [CrossRef]
- Moslehyani, A.; Farnood, R.; Tabe, S.; Matsuura, T.; Ismail, A.F. Novel Nanocomposite HNT-TiO2/PVDF Adsorptive Nanofiber Membranes for Arsenic (III) Removal. J. Membr. Sci. Res. 2020, 6, 416–423. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Zhu, T.; Li, X. Enhanced visible light photocatalytic activity of polyaniline–crystalline TiO2–halloysite composite nanotubes by tuning the acid dopant in the preparation. RSC Adv. 2015, 5, 98482–98491. [Google Scholar] [CrossRef]
- Li, X.; Yao, C.; Lu, X.; Hu, Z.; Yin, Y.; Ni, C. Halloysite–CeO2–AgBr nanocomposite for solar light photodegradation of methyl orange. Appl. Clay Sci. 2015, 104, 74–80. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Guan, J.; Liu, C.; Zhao, X.; Zhu, Z.; Ma, C.; Huo, P.; Li, C.; Yan, Y. Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTs loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 2018, 64, 206–218. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds—Natural Sources, Importance and Applications; InTech Open: London, UK, 2017; pp. 419–443. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, N.; Gul, S.; Khan, S.; Khan, H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry; InTechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K. Environmental contamination by heavy Metals. Heavy Met. 2018, 10, 115–132. [Google Scholar] [CrossRef]
- Snyder, S.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Stehle, S.; Schulz, R. Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 5750–5755. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.-S.; Di Serio, M. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 15. [Google Scholar] [CrossRef]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 2016, 33, 491–522. [Google Scholar] [CrossRef]
- Huang, Y.; Su, W.; Wang, R.; Zhao, T. Removal of Typical Industrial Gaseous Pollutants: From Carbon, Zeolite, and Metal-organic Frameworks to Molecularly Imprinted Adsorbents. Aerosol Air Qual. Res. 2019, 19, 2130–2150. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2012, 219, 499–511. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, H.; Liu, Y.; Lu, Y.; Fan, W.; Qin, W.; Huo, M. Enhanced photoelectrocatalytic degradation of acetaminophen using a bifacial electrode of praseodymium-polyethylene glycol-PbO2//Ti//TiO2-nanotubes. Chem. Eng. J. 2020, 410, 128337. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Kuzhalosai, V.; Subash, B.; Shanthi, M. A novel sunshine active cerium loaded zinc oxide photocatalyst for the effective degradation of AR 27 dye. Mater. Sci. Semicond. Process. 2014, 27, 924–933. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A critical review of metal-doped TiO2 and its structure–physical properties–photocatalytic activity relationship in hydrogen production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Coy, E.; Siuzdak, K.; Pavlenko, M.; Załęski, K.; Graniel, O.; Ziółek, M.; Balme, S.; Miele, P.; Weber, M.; Bechelany, M.; et al. Enhancing photocatalytic performance and solar absorption by schottky nanodiodes heterojunctions in mechanically resilient palladium coated TiO2/Si nanopillars by atomic layer deposition. Chem. Eng. J. 2019, 392, 123702. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. Photocatal.-Appl. Attrib. 2019, 18, 10-5772. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Bikiaris, D.N.; Lambropoulou, D.A. Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation. Macromol 2021, 1, 201–233. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Rao, K.S.; Subrahmanyam, M.; Boule, P. Immobilized TiO2 photocatalyst during long-term use: Decrease of its activity. Appl. Catal. B Environ. 2004, 49, 239–249. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. From a naturally occurring-clay mineral to the production of porous ceramic membranes. Microporous Mesoporous Mater. 2018, 271, 52–58. [Google Scholar] [CrossRef]
- Manova, E.; Aranda, P.; Martín-Luengo, M.A.; Letaïef, S.; Ruiz-Hitzky, E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010, 131, 252–260. [Google Scholar] [CrossRef]
- Rainer, D.N.; Morris, R.E. New avenues for mechanochemistry in zeolite science. Dalton Trans. 2021, 50, 8995–9009. [Google Scholar] [CrossRef]
- García-Romero, E.; Suárez, M. Sepiolite–palygorskite: Textural study and genetic considerations. Appl. Clay Sci. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Álvarez, A.; Santarén, J.; Esteban-Cubillo, A. Advanced Materials and New Applications of Sepiolite and Palygorskite. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 393–452. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Mishra, G.; Mukhopadhyay, M. TiO2 decorated functionalized halloysite nanotubes (TiO2@HNTs) and photocatalytic PVC membranes synthesis, characterization and its application in water treatment. Sci. Rep. 2019, 9, 4345. [Google Scholar] [CrossRef] [PubMed]
- Mohtor, N.H.; Othman, M.H.D.; Abu Bakar, S.; Kurniawan, T.A.; Dzinun, H.; Norddin, M.N.A.M.; Rajis, Z. Synthesis of nanostructured titanium dioxide layer onto kaolin hollow fibre membrane via hydrothermal method for decolourisation of reactive black 5. Chemosphere 2018, 208, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Henych, J.; Štengl, V. Feasible Synthesis of TiO2 Deposited on Kaolin for Photocatalytic Applications. Clays Clay Miner. 2013, 61, 165–176. [Google Scholar] [CrossRef]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Rapsomanikis, A.; Papoulis, D.; Panagiotaras, D.; Kaplani, E.; Stathatos, E. Nanocrystalline TiO2 and Halloysite clay mineral composite films prepared by sol-gel method: Synergistic effect and the case of silver modification to the photocatalytic degradation of Basic Blue- 41 azo dye in water. Glob. Nest J. 2014, 16, 485–498. [Google Scholar] [CrossRef]
- Yao, P.; Zhong, S.; Shen, Z. TiO2/Halloysite Composites Codoped with Carbon and Nitrogen from Melamine and Their Enhanced Solar-Light-Driven Photocatalytic Performance. Int. J. Photoenergy 2015, 2015, 605690. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Guo, H.; Ding, S. Low temperature synthesis of polyaniline–crystalline TiO2–halloysite composite nanotubes with enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2015, 458, 1–13. [Google Scholar] [CrossRef]
- Yu, X.; Lu, Z.; Si, N.; Zhou, W.; Chen, T.; Gao, X.; Song, M.; Yan, Y.; Huo, P.; Yan, C. Preparation of rare earth metal ion/TiO2Hal-conducting polymers by ions imprinting technique and its photodegradation property on tetracycline. Appl. Clay Sci. 2014, 99, 125–130. [Google Scholar] [CrossRef]
- Wang, H.; Wu, D.; Li, X.; Huo, P. Ce doping TiO2/halloysite nanotubes photocatalyst for enhanced electrons transfer and photocatalytic degradation of Tetracycline. J. Mater. Sci. Mater. Electron. 2019, 30, 19126–19136. [Google Scholar] [CrossRef]
- Szczepanik, B.; Rogala, P.; Słomkiewicz, P.M.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. Synthesis, characterization and photocatalytic activity of TiO2-halloysite and Fe2O3-halloysite nanocomposites for photodegradation of chloroanilines in water. Appl. Clay Sci. 2017, 149, 118–126. [Google Scholar] [CrossRef]
- Bekiari, V.; Stathatos, E.; Papoulis, D.; Panagopoulos, G.; Kalarakis, A.; Iliopoulos, I.; Kourkouta, E.; Mavrokota, P. Use of halloysite–TiO2 nanocomposites for the decomposition of tebuconazole fungicide in water. Desalin. Water Treat. 2018, 127, 132–139. [Google Scholar] [CrossRef]
- Chen, J.; Liao, W.; Jiang, Y.; Yu, D.; Zou, M.; Zhu, H.; Zhang, M.; Du, M. Facile Fabrication of ZnO/TiO2 Heterogeneous Nanofibres and Their Photocatalytic Behaviour and Mechanism towards Rhodamine B. Nanomater. Nanotechnol. 2016, 6, 9. [Google Scholar] [CrossRef]
- Konstantinou, I.K. Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: Intermediates and degradation pathways. Appl. Catal. B Environ. 2003, 42, 319–335. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Sayegh, S.; Tanos, F.; Nada, A.; Lesage, G.; Zaviska, F.; Petit, E.; Rouessac, V.; Iatsunskyi, I.; Coy, E.; Viter, R.; et al. Tunable TiO2–BN–Pd nanofibers by combining electrospinning and atomic layer deposition to enhance photodegradation of acetaminophen. Dalton Trans. 2022, 51, 2674–2695. [Google Scholar] [CrossRef]
- Sayegh, S.; Abid, M.; Tanos, F.; Cretin, M.; Lesage, G.; Zaviska, F.; Petit, E.; Navarra, B.; Iatsunskyi, I.; Coy, E.; et al. N-doped TiO2 nanotubes synthesized by atomic layer deposition for acetaminophen degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130213. [Google Scholar] [CrossRef]
- Dikdim, J.M.D.; Gong, Y.; Noumi, G.B.; Sieliechi, J.M.; Zhao, X.; Ma, N.; Yang, M.; Tchatchueng, J.B. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 2018, 217, 833–842. [Google Scholar] [CrossRef]


| Photocatalyst (g/L) | Pollutant (mg/L) | Irradiation Type | Kobs (min−1) | Kobs/(%TiO2) | Ref |
|---|---|---|---|---|---|
| H95T5 (0.5 g/L) | Acetaminophen (10 mg/L) | UV | 0.09719 | 1.9438 | [41] |
| Acetaminophen (10 mg/L) | Halogen linear lamp | 0.00641 | 0.1282 | ||
| Methylene blue (6.64 mg/L) | 0.0175 | 0.3504 | |||
| Methylene blue (6.64 mg/L) | UV | 0.1714 | 3.4284 | ||
| TiO2@HNT (4.2 g/L) | Methylene blue 20 mg/L | 125 W UV | 0.0073 | 0.0075 | [136] |
| Rhodamine B 20 mg/L | 0.0024 | 0.0025 | |||
| Ag/TiO2-HNT (0.625 g/L) | Basic blue 41 (12 mg/L) | Fluorescent black light, 4 W | 0.0282 | 0.0471 | [141] |
| Carbon-TiO2-HNT (0.2 g/L) | Methylene blue (20 mg/L) | 50 W UV | 0.0184 | 0.0200 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abid, M.; Ben Haj Amara, A.; Bechelany, M. Halloysite-TiO2 Nanocomposites for Water Treatment: A Review. Nanomaterials 2023, 13, 1578. https://doi.org/10.3390/nano13091578
Abid M, Ben Haj Amara A, Bechelany M. Halloysite-TiO2 Nanocomposites for Water Treatment: A Review. Nanomaterials. 2023; 13(9):1578. https://doi.org/10.3390/nano13091578
Chicago/Turabian StyleAbid, Mahmoud, Abdesslem Ben Haj Amara, and Mikhael Bechelany. 2023. "Halloysite-TiO2 Nanocomposites for Water Treatment: A Review" Nanomaterials 13, no. 9: 1578. https://doi.org/10.3390/nano13091578
APA StyleAbid, M., Ben Haj Amara, A., & Bechelany, M. (2023). Halloysite-TiO2 Nanocomposites for Water Treatment: A Review. Nanomaterials, 13(9), 1578. https://doi.org/10.3390/nano13091578








