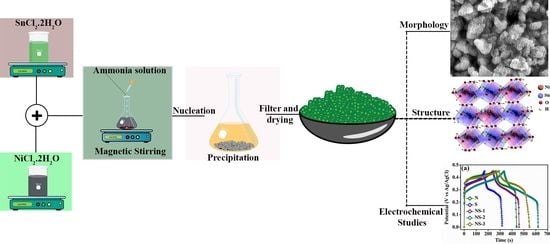
Influence of Ni and Sn Perovskite NiSn(OH)6 Nanoparticles on Energy Storage Applications
Abstract
1. Introduction
2. Experimental and Synthesis Techniques
2.1. Materials
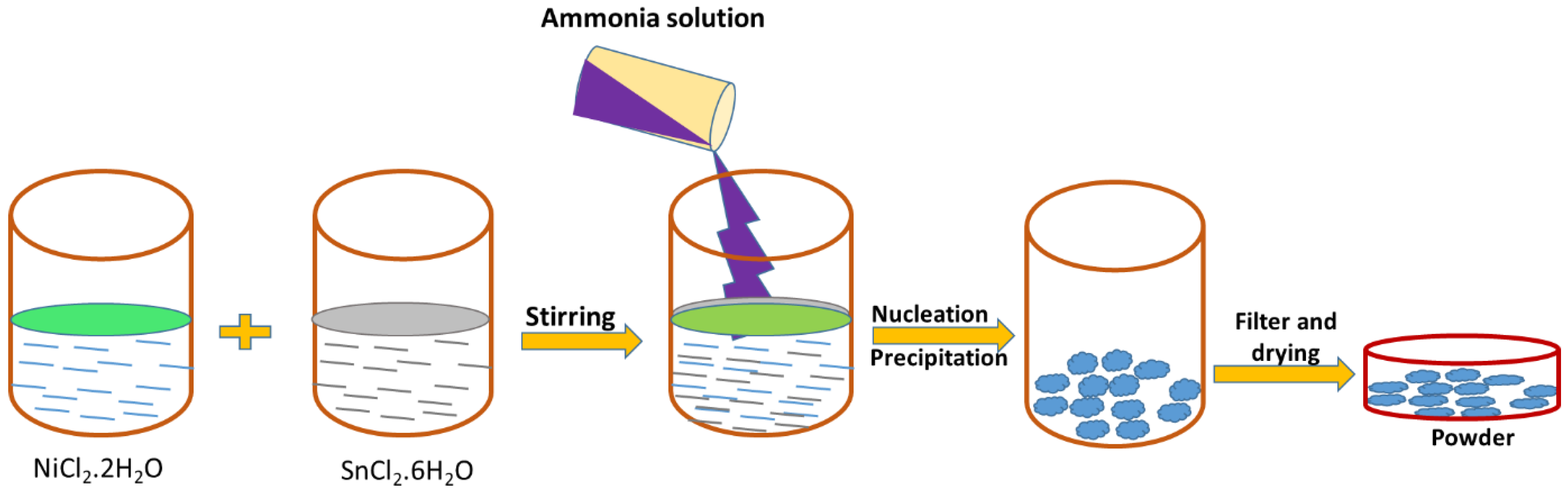
2.2. Synthesis of NiSn(OH)6
2.3. Electrode Fabrication and Capacitance Calculations
2.4. Characterisation
3. Results and Discussions
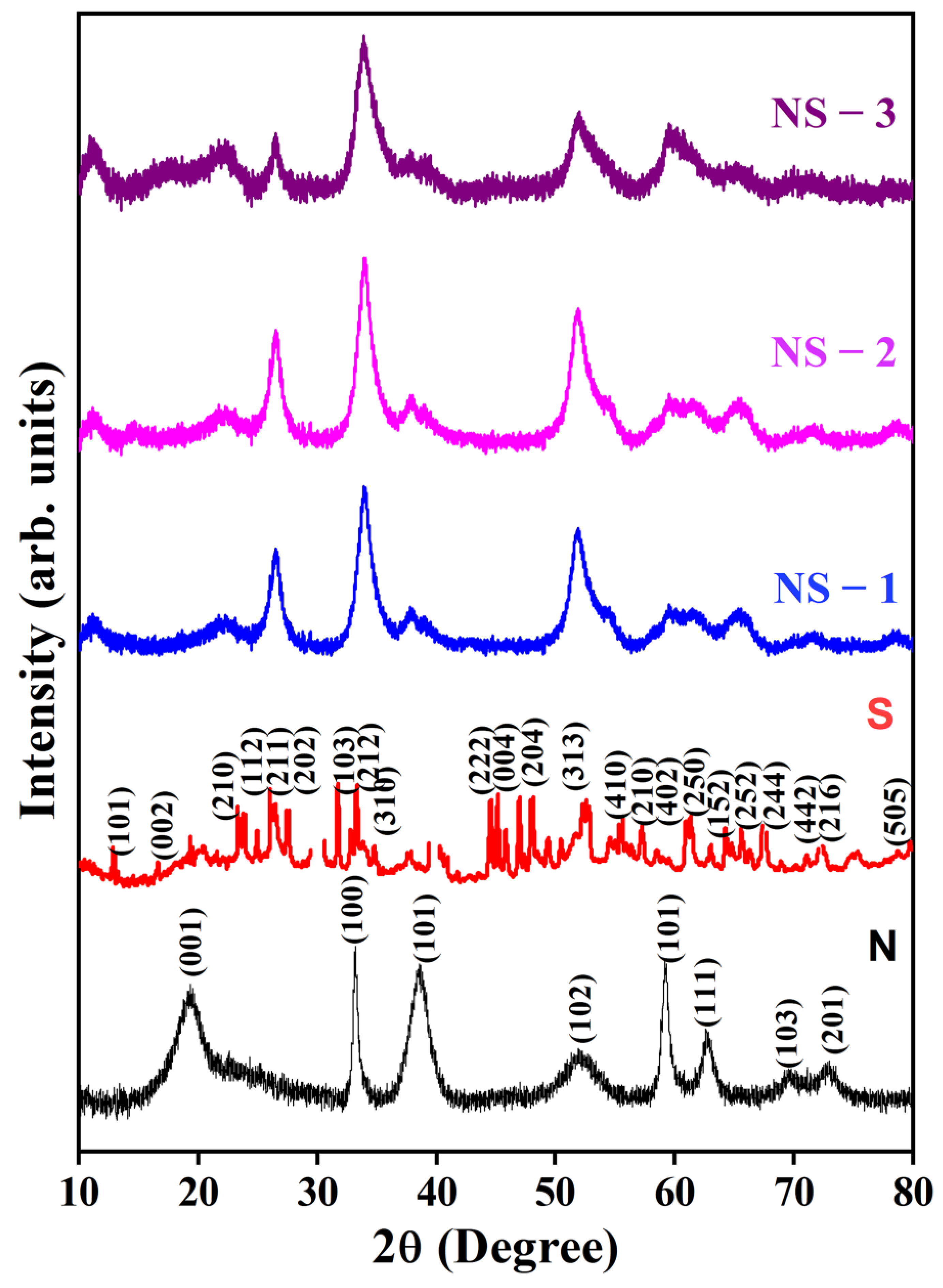
3.1. X-ray Diffraction (XRD)
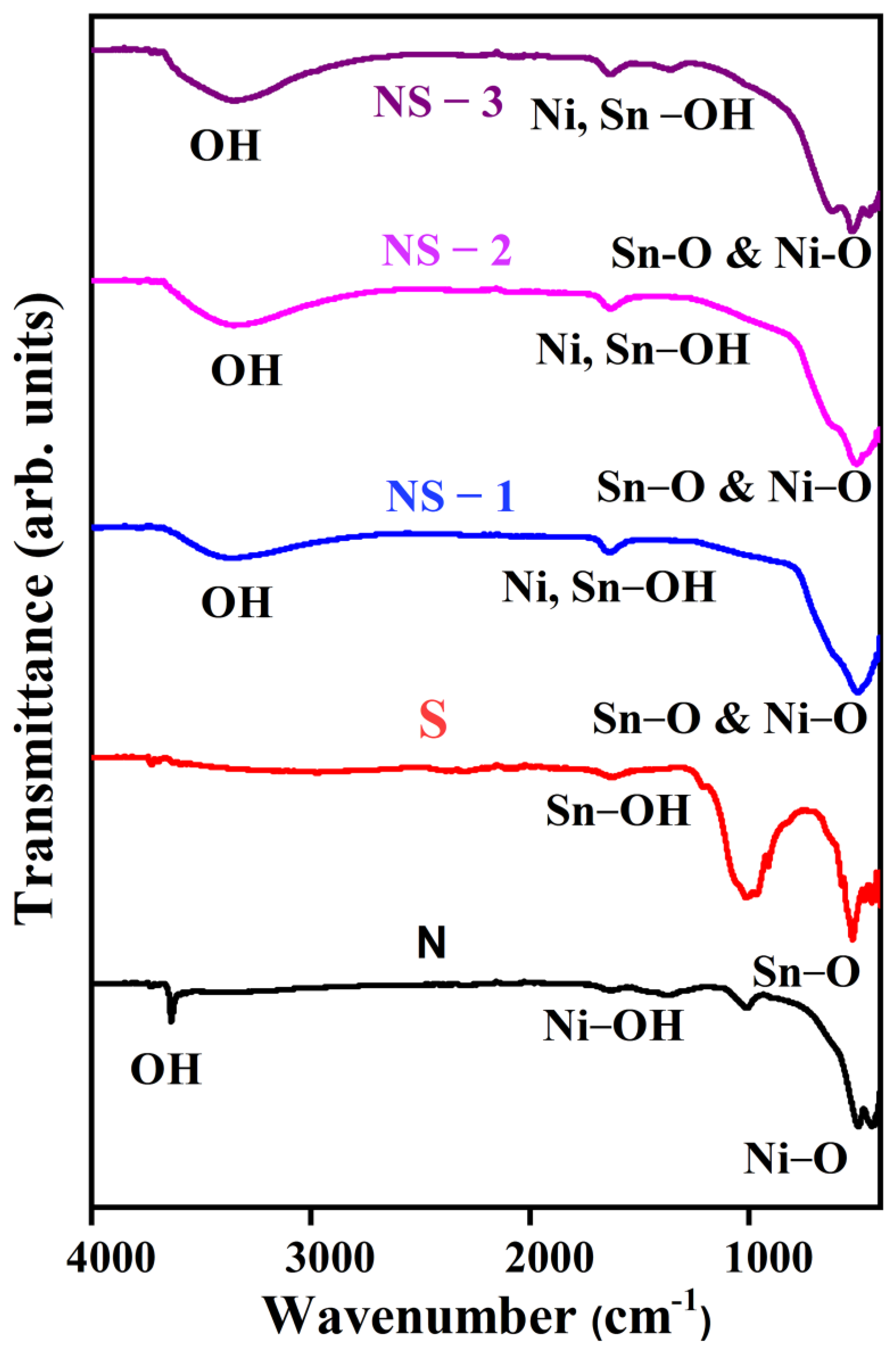
3.2. Fourier Transform Infrared Spectroscopy
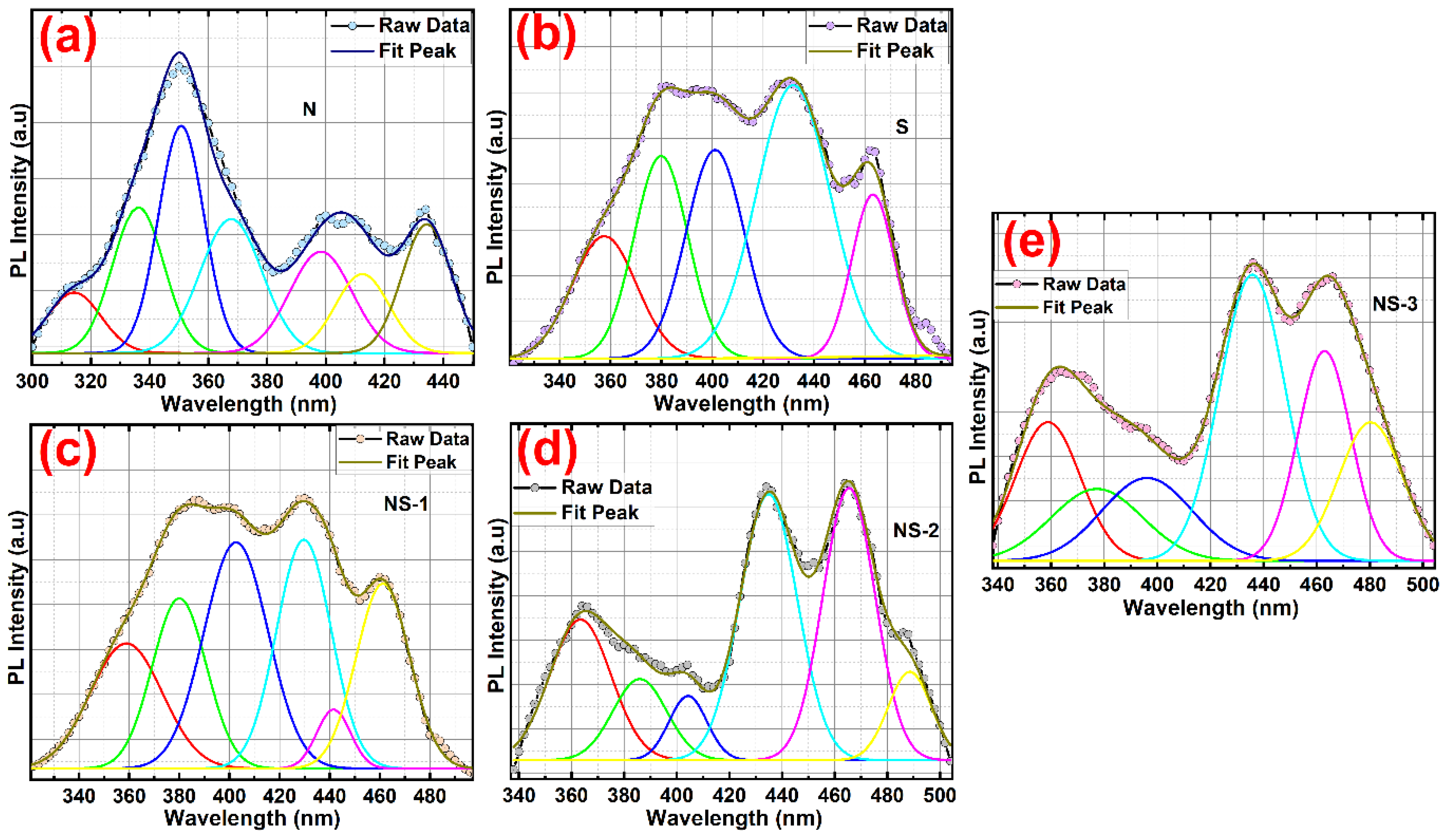
3.3. UV-Vis Spectra and PL Spectra for Optical Studies
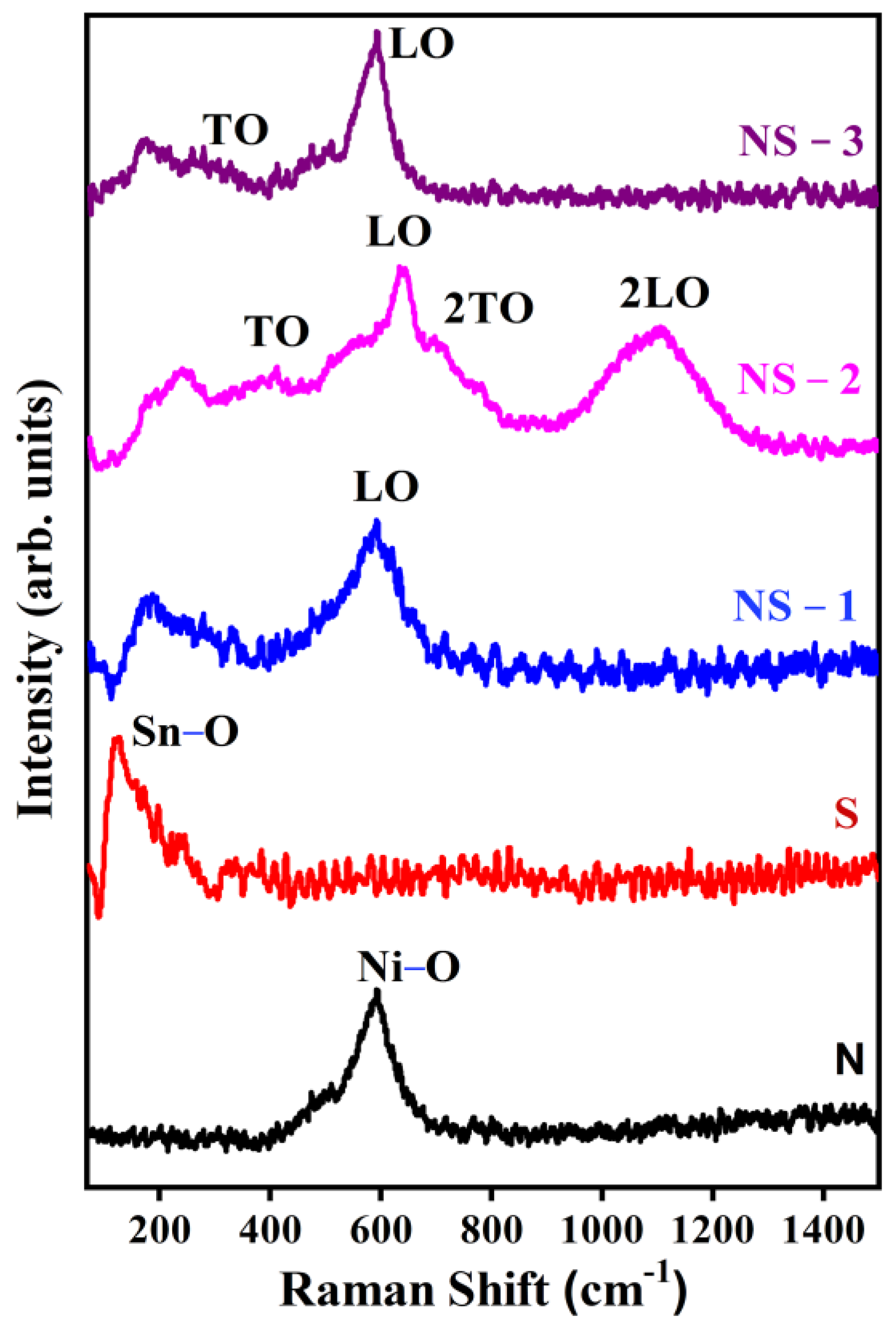
3.4. Raman Spectroscopy
3.5. XPS Analysis
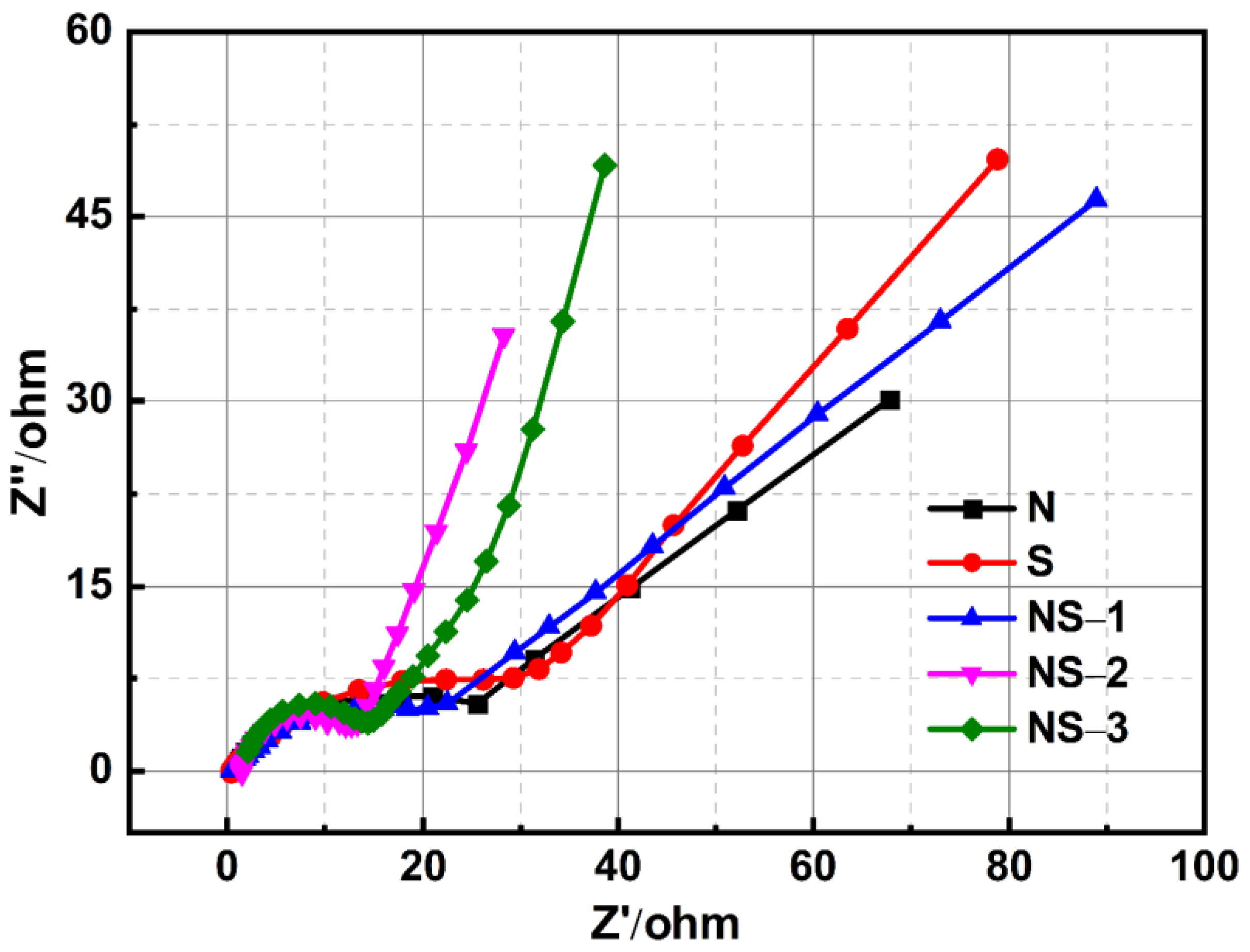
3.6. Electrochemical Studies
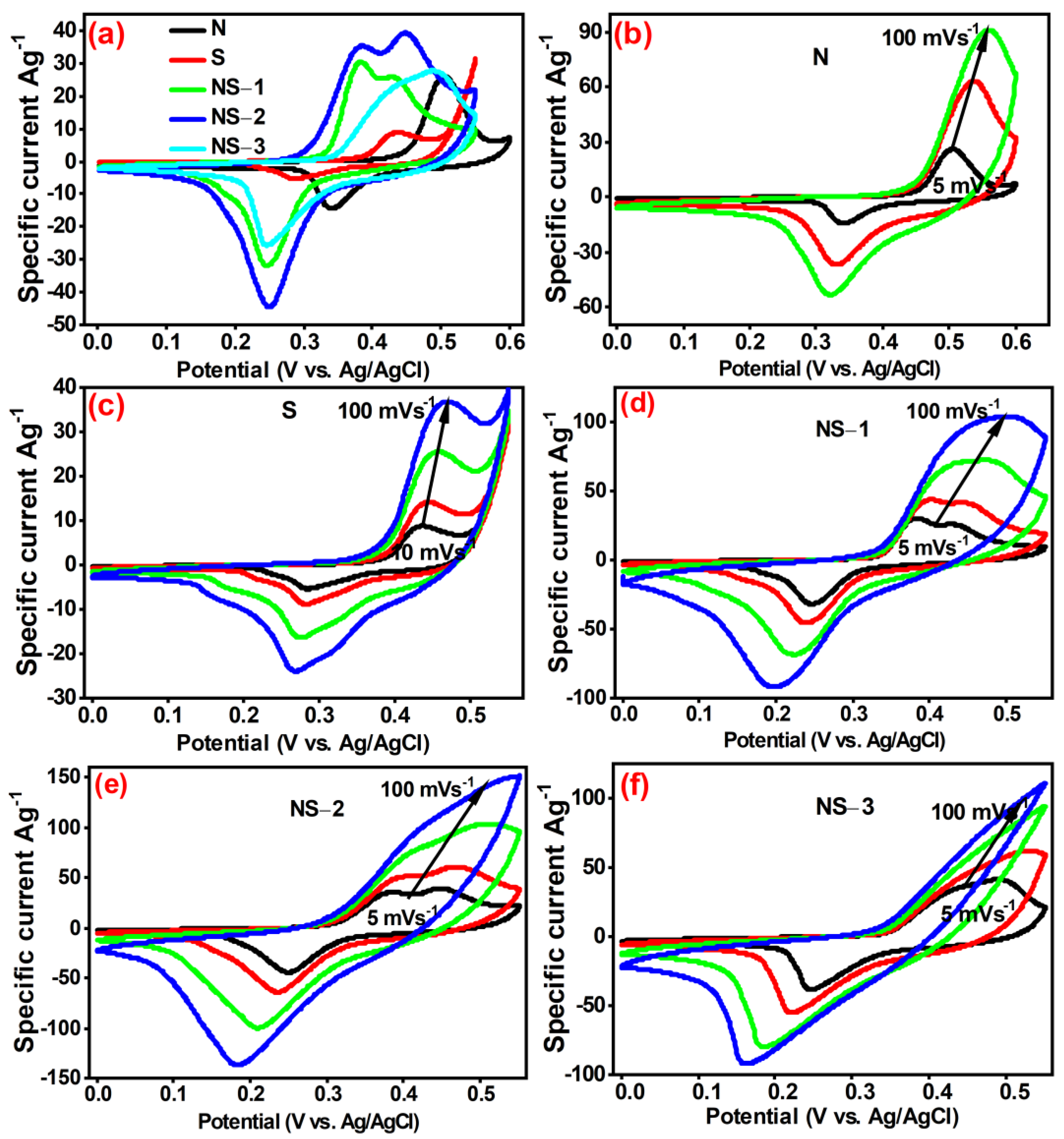
3.6.1. Cyclic Voltammetry Studies
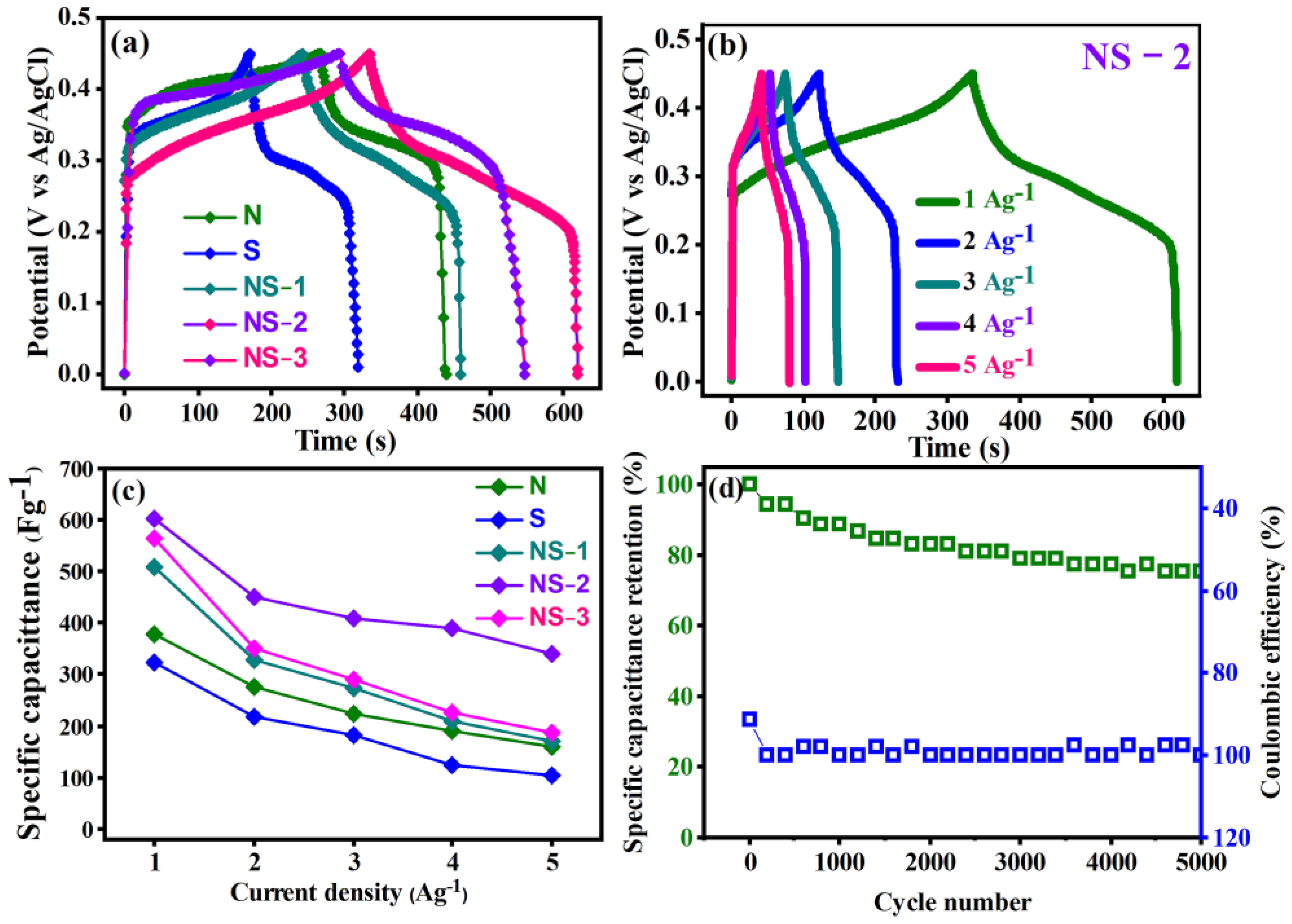
3.6.2. Galvanostatic Charge/Discharge Method
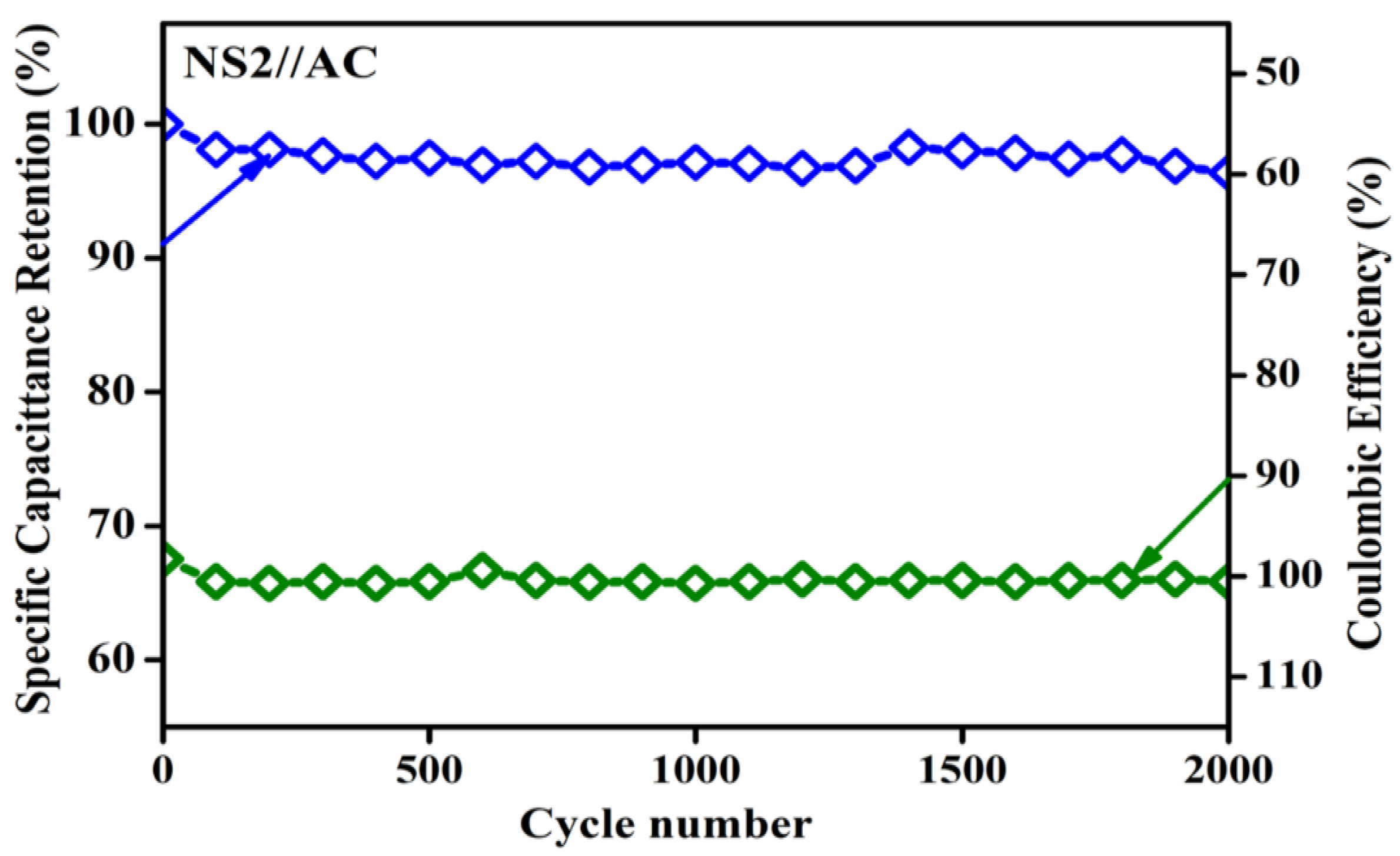
3.6.3. ASC Device Two-Electrode Electrochemical Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. APL Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, D.; Ji, S.; Huo, Y.; Liu, J. Facile construction of CoSn/Co3Sn2@C nanocages as anode for superior lithium-/sodium-ion storage. Carbon Neutralization 2023, 2, 54–62. [Google Scholar] [CrossRef]
- Gu, C.; Cui, Y.; Wang, L.; Sheng, E.; Shim, J.J.; Huang, J. Synthesis of the porous NiO/SnO2 microspheres and microcubes and their enhanced formaldehyde gas sensing performance. Sens. Actuators B Chem. 2017, 241, 298–307. [Google Scholar] [CrossRef]
- Sivaprakash, P.; Kumar, K.A.; Muthukumaran, S.; Pandurangan, A.; Dixit, A.; Arumugam, S. NiF2 as an efficient electrode material with high window potential of 1.8 V for high energy and power density asymmetric supercapacitor. J. Electroanal. Chem. 2020, 873, 114379. [Google Scholar] [CrossRef]
- Sivakumar, A.; Dhas, S.S.J.; Sivaprakash, P.; Raj, A.D.; Kumar, R.S.; Arumugam, S.; Prabhu, S.; Ramesh, R.; Chakraborty, S.; Dhas, S.M.B. Shock wave recovery experiments on α-V2O5 nano-crystalline materials: A potential material for energy storage applications. J. Alloys Compd. 2022, 929, 167180. [Google Scholar] [CrossRef]
- Sivaprakash, P.; Kumar, K.A.; Subalakshmi, K.; Bathula, C.; Sandhu, S.; Arumugam, S. Fabrication of high-performance asymmetric supercapacitors with high energy and power density based on binary metal fluoride. Mater. Lett. 2020, 275, 128146. [Google Scholar] [CrossRef]
- Padmanaban, A.; Padmanathan, N.; Dhanasekaran, T.; Manigandan, R.; Srinandhini, S.; Sivaprakash, P.; Arumugam, S.; Narayanan, V. Hexagonal phase Pt-doped cobalt telluride magnetic semiconductor nanoflakes for electrochemical sensing of dopamine. J. Electroanal. Chem. 2020, 877, 114658. [Google Scholar] [CrossRef]
- Long, C.; Zheng, M.; Xiao, Y.; Lei, B.; Dong, H.; Zhang, H.; Hu, H.; Liu, Y. Amorphous Ni-Co binary oxide with hierarchical porous structure for electrochemical capacitors. ACS Appl. Mater. Interfaces 2015, 7, 24419–24429. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Li, X.; Yu, J.; Sun, X.; Gao, T.; Zhou, G. Pomegranate-like Sn-Ni nanoalloys@N-doped carbon nanocomposites as high-performance anode materials for Li-ion and Na-ion batteries. Appl. Surf. Sci. 2023, 611, 155672. [Google Scholar] [CrossRef]
- Prakash, D.; Manivannan, S. Unusual battery type pseudocapacitive behaviour of graphene oxynitride electrode: High energy solid-state asymmetric supercapacitor. J. Alloy. Compd. 2021, 854, 156853. [Google Scholar] [CrossRef]
- Prakash, D.; Manivannan, S. N, B co-doped and Crumpled Graphene Oxide Pseudocapacitive Electrode for High Energy Supercapacitor. Surf. Interfaces 2021, 23, 101025. [Google Scholar] [CrossRef]
- Ede, S.R.; Anantharaj, S.; Kumaran, K.T.; Mishra, S.; Kundu, S. One step synthesis of Ni/Ni(OH)2 nano sheets (NSs) and their application in asymmetric supercapacitors. RSC Adv. 2017, 7, 5898–5911. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Sivasubramanian, D.; Nithya, A.; Jothivenkatachalam, K.; Maheswari, N.; Gopalan, M.; Dillibabu, S.; Eftekhari, A. Ni-Doped SnO Nanoparticles for Sensing and Photocatalysis. ACS Appl. Nano Mater. 2018, 1, 5823–5836. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Liu, Y.; Lin, Z.; Yan, X. Ultra-small, size-controlled Ni(OH)2 nanoparticles: Elucidating the relationship between particle size and electrochemical performance for advanced energy storage devices. NPG Asia Mater. 2015, 7, 183. [Google Scholar] [CrossRef]
- Chand, P. Effect of pH values on the structural, optical and electrical properties of SnO2 nanostructures. Optik 2019, 181, 768–778. [Google Scholar]
- Srinivas, K.; Rao, S.M.; Reddy, P.V. Structural, electronic and magnetic properties of Sn0.95Ni0.05O2 nanorods. Nanoscale 2011, 3, 642–653. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Bezuidenhoudt, B.C.; van Vuuren, A.J.; Lee, M.; Ntwaeaborwa, O.M. The role of neutral and ionized oxygen defects in the emission of tin oxide nanocrystals for near white light application. Nanotechnology 2015, 26, 295703. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Di, Q.; Zhao, H.; Liang, B.; Yang, J. Mutual Effects of Fluorine Dopant and Oxygen Vacancies on Structural and Luminescence Characteristics of F Doped SnO2 Nanoparticles. Materials 2017, 10, 1398. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, T.H.; Wu, X.L.; Shena, J.C.; Chu, P.K. Identification of oxygen vacancy types from Raman spectra of SnO2 nanocrystals. J. Raman Spectrosc. 2012, 43, 1423–1426. [Google Scholar] [CrossRef]
- Chen, H.; Ding, L.; Sun, W.; Jiang, Q.; Hu, J.; Li, J. Synthesis and characterization of Ni doped SnO2 microspheres with enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 56401–56409. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, S.; Shi, L.; Zhao, Y.; Fang, J. Preparation, characterization and electrical properties of fluorine-doped tin dioxide nanocrystals. J. Colloid Interface Sci. 2010, 346, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-J.; Choi, H.-C.; Park, K.-W.; Kim, S.-B.; Sung, Y.-E. Investigation of the structural and electrochemical properties of size-controlled SnO2 nanoparticles. J. Phys. Chem. B 2004, 108, 9815–9820. [Google Scholar] [CrossRef]
- Kelly, R. On the problem of whether mass or chemical bonding is more important to bombardment-induced compositional changes in alloys and oxides. Surf. Sci. 1980, 100, 85–107. [Google Scholar] [CrossRef]
- Solsona, B.; Nieto, J.M.L.; Concepción, P.; Dejoz, A.; Ivars, F.; Vázquez, M.I. Vázquez. Oxidative dehydrogenation of ethane over Ni–W–O mixed metal oxide catalysts. J. Catal. 2011, 280, 28–39. [Google Scholar] [CrossRef]
- Wang, M.L.; Liu, W.W.; Huang, C.D. Investigation of PdNiO/C catalyst for methanol electrooxidation. Int. J. Hydrog. Energy 2009, 34, 2758–2764. [Google Scholar] [CrossRef]
- Vedrine, J.C.; Hollinger, G.; Duc, T.M. Investigations of antigorite and nickel supported catalysts by X-ray photoelectron spectroscopy. J. Phys. Chem. 1978, 82, 1515–1520. [Google Scholar] [CrossRef]
- Van Veenendaal, M.A.; Sawatzky, G.A. Nonlocal screening effects in 2p X-ray photoemission spectroscopy core-level line shapes of transition metal compounds. Phys. Rev. Lett. 1993, 70, 2459. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhu, Y.R.; Ji, X.B. NiCo2O4-based Materials for Electrochemical Supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile and large-scale chemical synthesis of highly porous secondary submicron/micron-sized NiCo2O4 materials for high-performance aqueous hybrid AC-NiCo2O4 electrochemical capacitors. Electrochim. Acta 2013, 107, 494–502. [Google Scholar] [CrossRef]
- Lu, X.F.; Wu, D.J.; Li, R.Z.; Ye, S.H.; Tong, Y.X.; Li, G.R. Hierarchical NiCo2O4 nanosheets@hollow microrod arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 4706–4713. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X. Superior Asymmetric Supercapacitor Based on Ni-Co Oxide Nanosheets and Carbon Nanorods. Sci. Rep. 2014, 4, 3712. [Google Scholar] [CrossRef]
- Liu, L.; Hu, X.; Zeng, H.Y.; Yi, M.Y.; Shen, S.G.; Xu, S.; Cao, X.; Du, J.Z. Preparation of NiCoFe-hydroxide/polyaniline composite for enhanced-performance supercapacitors. J. Mater. Sci. Technol. 2019, 35, 1691–1699. [Google Scholar] [CrossRef]
- Cao, X.; Zeng, H.Y.; Xu, S.; Yuan, J.; Han, J.; Xiao, G.F. Facile fabrication of the polyaniline/layered double hydroxide nanosheet composite for supercapacitors. Appl. Clay. Sci. 2019, 168, 175–183. [Google Scholar] [CrossRef]
- Dou, Y.; Pan, T.; Zhou, A.; Xu, S.; Liu, X.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. Reversible thermally-responsive electrochemical energy storage based on smart LDH@ P (NIPAM-co-SPMA) films. Chem. Comm. 2013, 49, 8462–8464. [Google Scholar] [CrossRef]
- Li, S.; Cheng, P.; Luo, J.; Zhou, D.; Xu, W.; Li, J.; Li, R.; Yuan, D. High-performance flexible asymmetric supercapacitor based on CoAl-LDH and rGO electrodes. Nanomicro. Lett. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Sivakumar, A.; Sahaya Jude Dhas, S.; Sivaprakash, P.; Almansour, A.I.; Suresh Kumar, R.; Arumugam, N.; Perumal, K.; Arumugam, S.; Martin Britto Dhas, S.A. Raman Spectroscopic and Electrochemical Measurements of Dynamic Shocked MnFe2O4 Nano-crystalline Materials. J. Inorg. Organomet. Polym. Mater. 2022, 32, 344–352. [Google Scholar] [CrossRef]
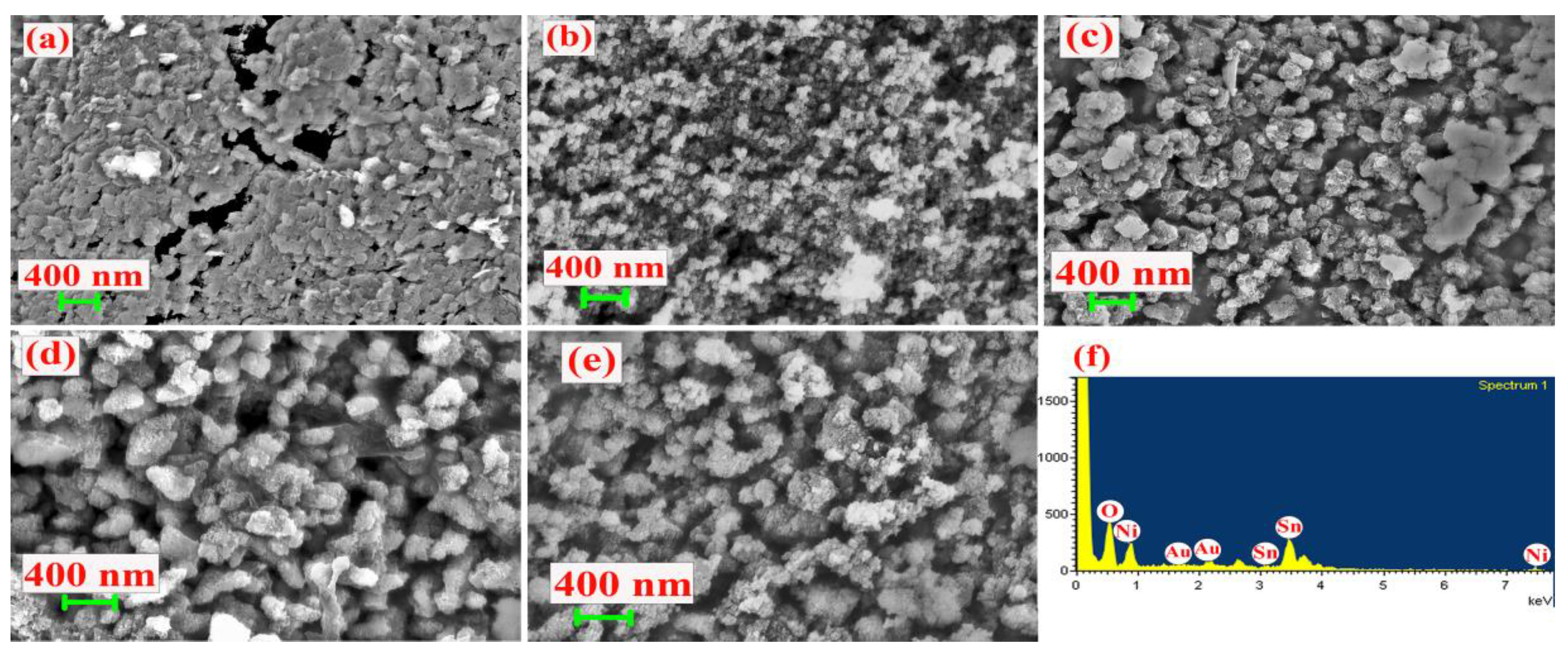


| Material | Ni (at.%) | Sn (at.%) | O (at.%) |
|---|---|---|---|
| N | 52.46 | --- | 47.54 |
| S | --- | 34.66 | 65.34 |
| NS-1 | 10.38 | 18.18 | 71.34 |
| NS-2 | 13.83 | 13.68 | 72.49 |
| NS-3 | 19.12 | 9.94 | 70.94 |
| Electrode Materials | Electrolyte | Specific Capacitance Fg−1 (Ag−1) | Capacitance Retention % (Ag−1) | Cycles | Ref. |
|---|---|---|---|---|---|
| CoAl LDH/graphene foam | 6 m LiOH | 101 (0.5) | 100 (0.5) | 5000 | [32] |
| CoAl LDH/CNTs | 2 m KOH | 873 (0.5) | 79.9 (1.72) | 1000 | [33] |
| NiCoFe-LDH/PANI | 6 m KOH | 408 (2) | 84 (2) | 1000 | [34] |
| MgFe-LDH/PANI | 2 m KOH | 592.5 (2) | 95 (2) | 500 | [35] |
| CoAl-LDH/rGO | 6 m KOH | 616.9 (1) | 95.8 (10) | 2000 | [36] |
| LDH@P(NIPAM-co-SPMA) | 2 m KOH | 505 (1) | — | — | [37] |
| NS-2 (NiSn(OH)6) | 6 m KOH | 607 | 95.8 (5) | 5000 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velmurugan, G.; Ganapathi Raman, R.; Prakash, D.; Kim, I.; Sahadevan, J.; Sivaprakash, P. Influence of Ni and Sn Perovskite NiSn(OH)6 Nanoparticles on Energy Storage Applications. Nanomaterials 2023, 13, 1523. https://doi.org/10.3390/nano13091523
Velmurugan G, Ganapathi Raman R, Prakash D, Kim I, Sahadevan J, Sivaprakash P. Influence of Ni and Sn Perovskite NiSn(OH)6 Nanoparticles on Energy Storage Applications. Nanomaterials. 2023; 13(9):1523. https://doi.org/10.3390/nano13091523
Chicago/Turabian StyleVelmurugan, G., R. Ganapathi Raman, D. Prakash, Ikhyun Kim, Jhelai Sahadevan, and P. Sivaprakash. 2023. "Influence of Ni and Sn Perovskite NiSn(OH)6 Nanoparticles on Energy Storage Applications" Nanomaterials 13, no. 9: 1523. https://doi.org/10.3390/nano13091523
APA StyleVelmurugan, G., Ganapathi Raman, R., Prakash, D., Kim, I., Sahadevan, J., & Sivaprakash, P. (2023). Influence of Ni and Sn Perovskite NiSn(OH)6 Nanoparticles on Energy Storage Applications. Nanomaterials, 13(9), 1523. https://doi.org/10.3390/nano13091523