Synthesis, Photoluminescent Characteristics and Eu3+-Induced Phase Transitions in Sr3Zr2O7:Eu3+ Red Phosphors
Abstract
1. Introduction
2. Materials and Methods
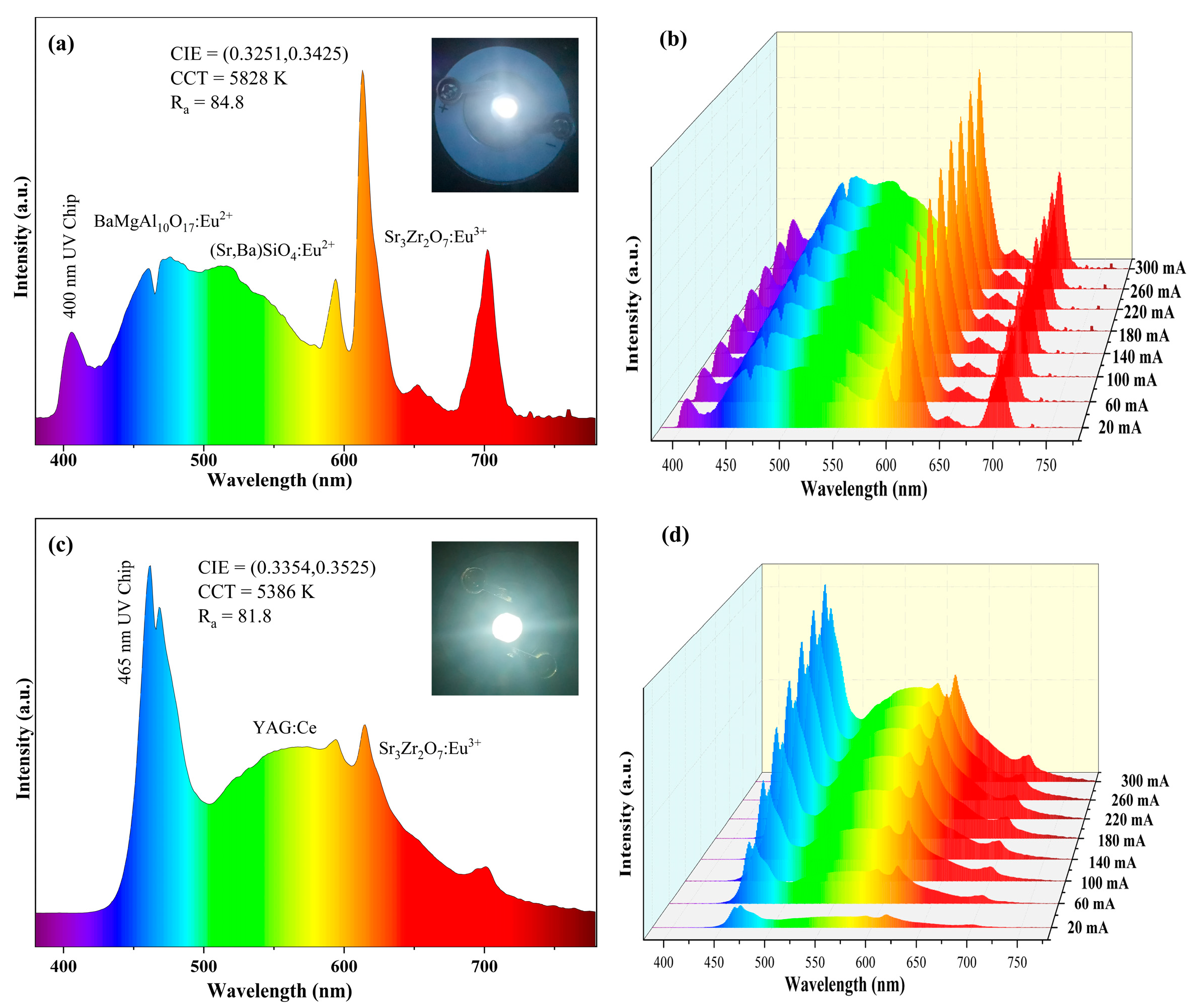
2.1. Fabrication of Warm w-LED Devices
2.2. Phosphor Characterization
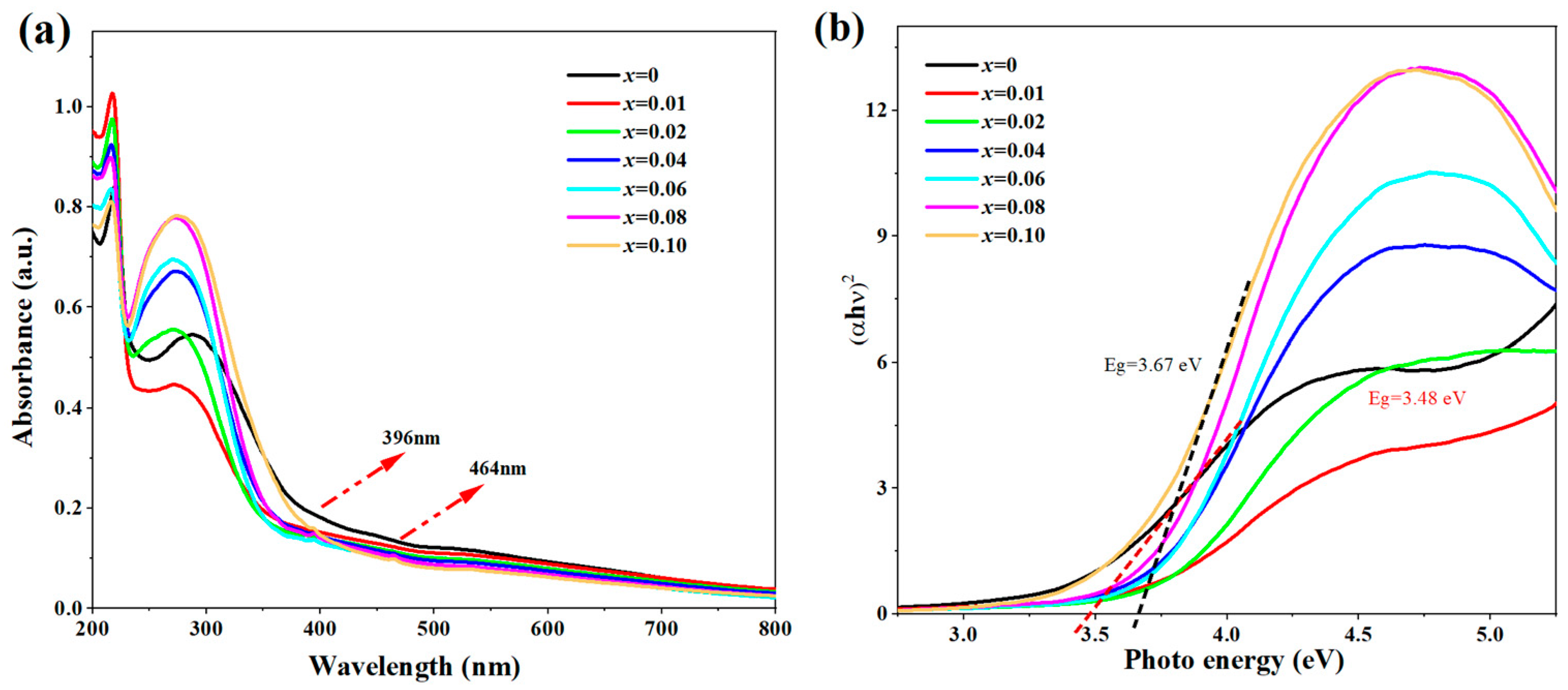
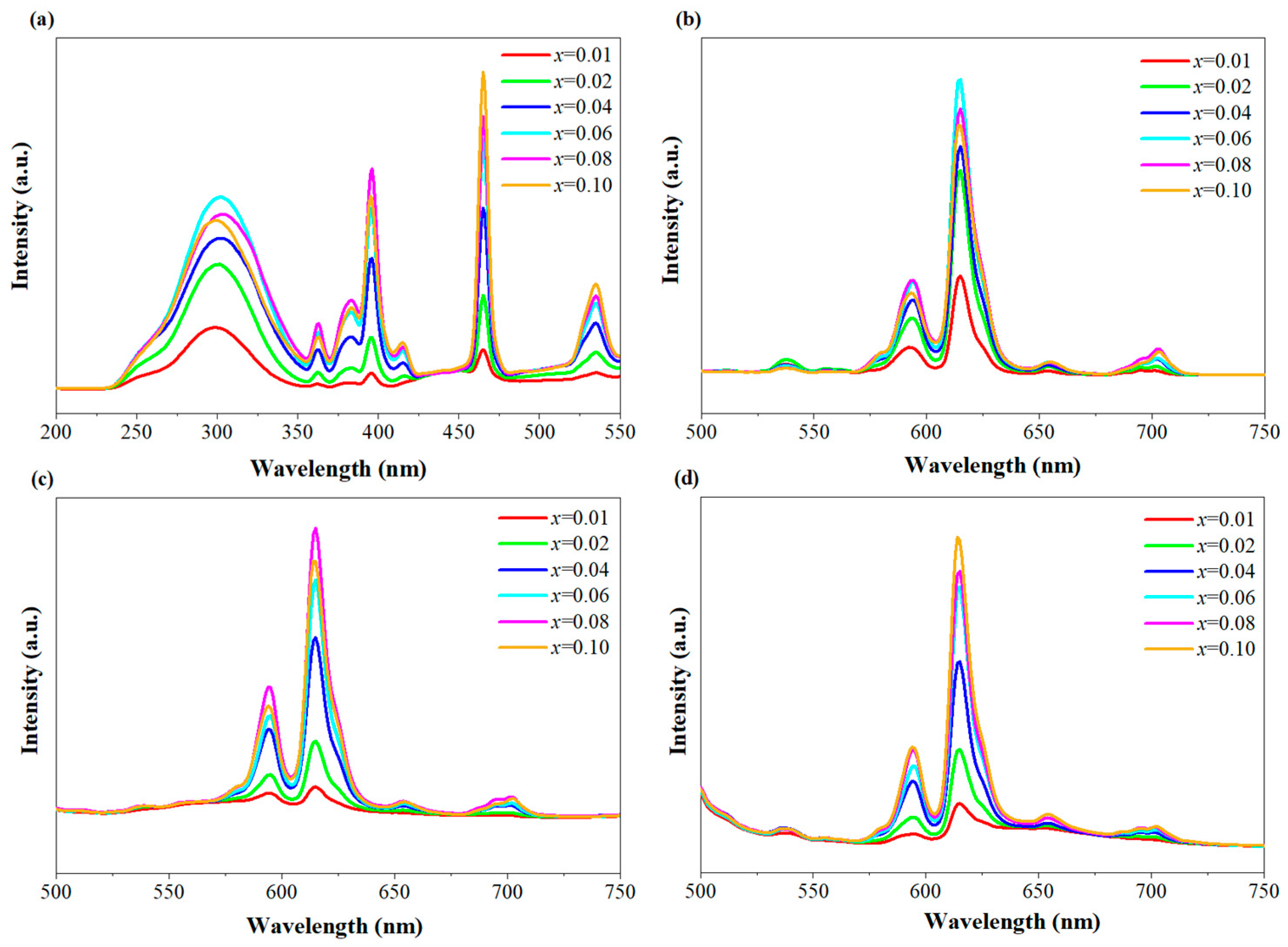
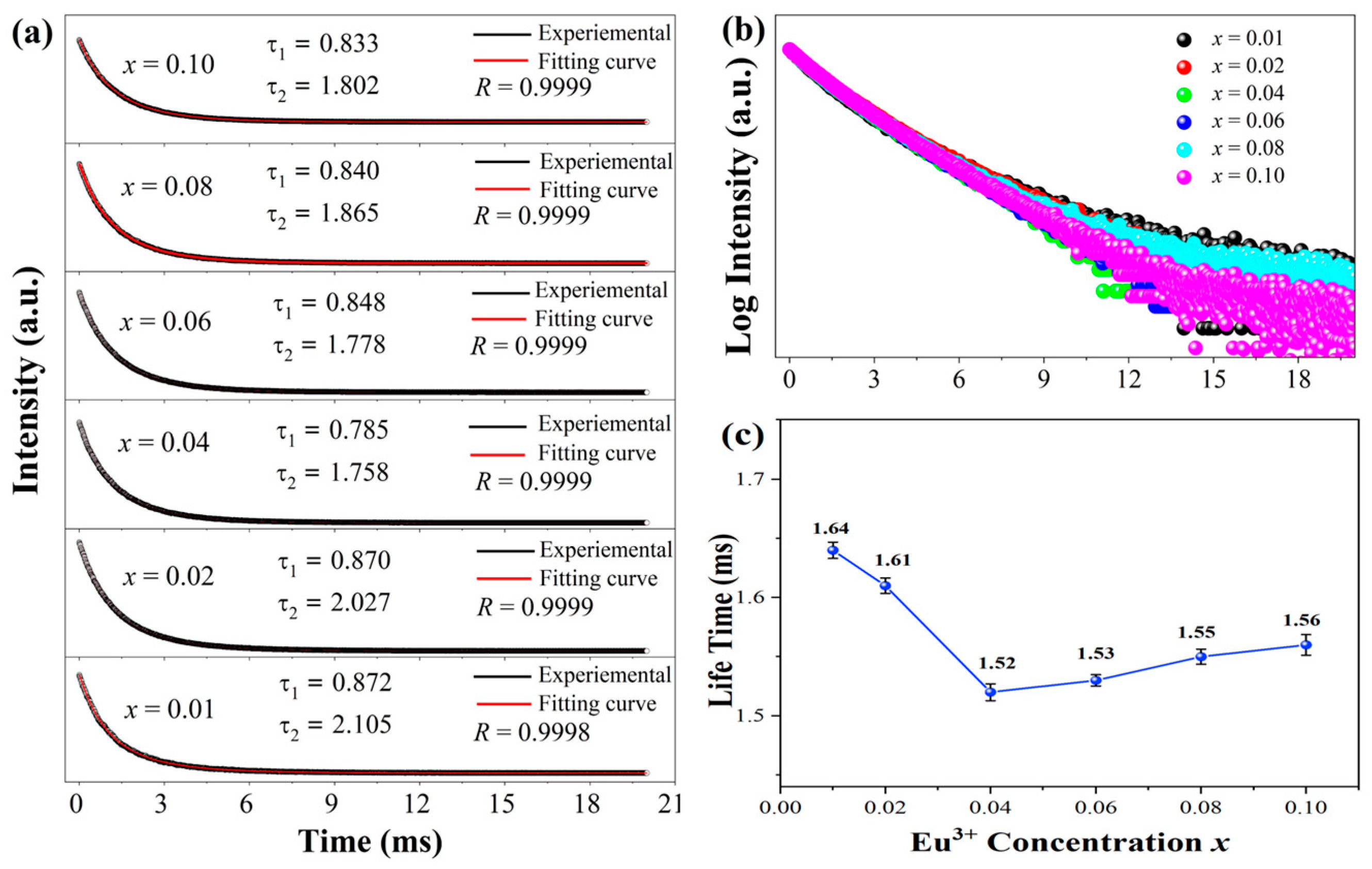
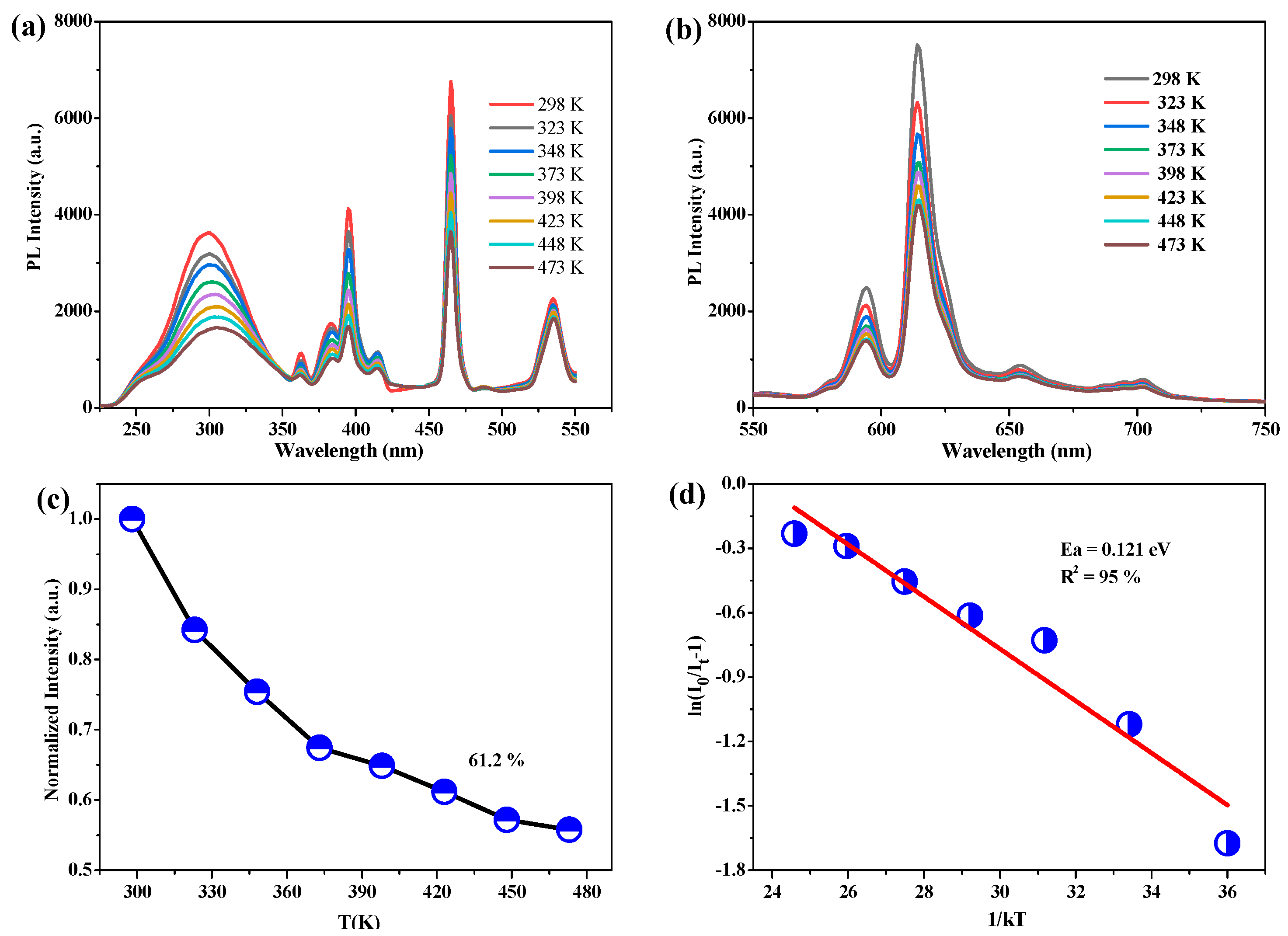
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Wang, S.Y.; Sun, L.L.; Liang, J.; Devakumar, B.; Huang, X.Y. Achieving full-visible-spectrum LED lighting via employing an efficient Ce3+-activated cyan phosphor. Mater. Today Energy 2020, 17, 100448. [Google Scholar] [CrossRef]
- Yu, H.; Deng, D.; Zhou, D.; Yuan, W.; Zhao, Q.; Hua, Y.; Zhao, S.; Huang, L.; Xu, S. Ba2Ca(PO4)2: Eu2+ emission-tunable phosphor for solid-state lighting: Luminescent properties and application as white light emitting diodes. J. Mater. Chem. C 2013, 1, 5577–5582. [Google Scholar] [CrossRef]
- Ran, W.G.; Noh, H.M.; Moon, B.K.; Park, S.H.; Jeong, J.H.; Kim, J.H.; Liu, G.Z.; Shi, J.S. Crystal structure, electronic structure and photoluminescence properties of KLaMgWO6: Eu3+ phosphors. J. Lumin. 2018, 197, 270276. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Sun, L.L.; Devakumar, B.; Annadurai, G.; Liang, J.; Wang, S.Y.; Sun, Q.; Huang, X.Y. Synthesis and photoluminescence properties of a new blue-light-excitable red phosphor Ca2LaTaO6: Eu3+ for white LEDs. J. Lumin. 2020, 222, 117173. [Google Scholar] [CrossRef]
- Rajendran, M.; Vaidyanathan, S. Zero-concentration quenching: A novel Eu3+ based red phosphor with non-layered crystal structure for white LEDs and NaSrY (MoO4)3: Sm3+ based deep-red LEDs for plant growth. Dalton Trans. 2020, 49, 9239–9253. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.R.; Zhou, X.H.; Liu, Y.L.; Lei, B.F. A dual-emitting core-shell carbon dot-silica-phosphor composite for LED plant grow light. Rsc. Adv. 2017, 7, 16662–16667. [Google Scholar] [CrossRef]
- Li, J.H.; Yan, J.; Wen, D.W.; Khan, W.U.; Shi, J.X.; Wu, M.M.; Su, Q.; Tanner, P.A. Advanced red phosphors for white light-emitting diodes. J. Mater. Chem. C 2016, 4, 8611–8623. [Google Scholar] [CrossRef]
- Li, X.H.; Milicevic, B.; Dramicanin, M.D.; Jing, X.P.; Tang, Q.; Shi, J.X.; Wu, M.M. Eu3+-Activated Sr3ZnTa2O9 single-component white light phosphors: Emission intensity enhancement and color rendering improvement. J. Mater. Chem. C 2019, 7, 2596–2603. [Google Scholar] [CrossRef]
- Qiao, J.W.; Ning, L.X.; Molokeev, M.S.; Chuang, Y.C.; Zhang, Q.Y.; Poeppelmeier, K.R.; Xia, Z.G. Site-selective occupancy of Eu2+ toward blue-light-excited red emission in a Rb3YSi2O7: Eu phosphor. Angew. Chem. Int. Ed. 2019, 58, 11521–11526. [Google Scholar] [CrossRef]
- Dai, S.J.; Zhao, D.; Zhang, R.J.; Jia, L.; Yao, Q.X. Enhancing luminescence intensity and improving thermostability of red phosphors Li3Ba2La3(WO4)8: Eu3+ by co-doping with Sm3+ ions. J. Alloys Compd. 2022, 891, 161973. [Google Scholar] [CrossRef]
- Chang, Y.C.; Liang, C.H.; Yan, S.A.; Chang, Y.S. Synthesis and photoluminescence characteristics of high color purity and brightness Li3Ba2Gd3(MoO4)8: Eu3+ red phosphors. J. Phys. Chem. C 2010, 114, 3645–3652. [Google Scholar] [CrossRef]
- Baur, F.; Jüstel, T. Warm-white LED with ultra high luminous efficacy due to sensitisation of Eu3+ photoluminescence by the uranyl moiety in K4(UO2)Eu2(Ge2O7)2. J. Mater. Chem. C 2018, 6, 6966–6974. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wu, C.C.; Li, H.H.; Yang, C.F. Synthesis and Luminescence Properties of Eu2+-Doped Sr3MgSi2O8 Blue Light-Emitting Phosphor for Application in Near-Ultraviolet Excitable White Light-Emitting Diodes. Nanomaterials 2022, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wu, X.; Zhang, H.; Qin, S. Pressure-induced phase transition of La2Zr2O7 and La0.5Gd1.5Zr2O7 pyrochlore. RSC Adv. 2019, 9, 18954–18962. [Google Scholar] [CrossRef]
- Gul, S.R.; Khan, M.; Zeng, Y.; Wu, B. Theoretical investigations of electronic and thermodynamic properties of Ce doped La2Zr2O7 pyrochlore. Mater. Res. Express 2019, 6, 085210. [Google Scholar] [CrossRef]
- Nandi, S.; Jana, Y.M.; Gupta, H.C. Lattice dynamical investigation of the Raman and infrared wave numbers and heat capacity properties of the pyrochlores R2Zr2O7 (R = La, Nd, Sm, Eu). J. Phys. Chem. Solids 2018, 15, 347–354. [Google Scholar] [CrossRef]
- Zotov, N.; Guignard, A.; Mauer, G.; Vaßen, R. Effect of plasma enthalpy on the structure of La2Zr2O7 coatings prepared by suspension plasma spraying. J. Am. Ceram. Soc. 2016, 99, 1086–1091. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Gao, Y.; Tao, S.; Luo, H. Design, preparation, and characterization of graded YSZ/La2Zr2O7 thermal barrier coatings. J. Am. Ceram. Soc. 2010, 93, 1732–1740. [Google Scholar]
- Min, X.; Sun, Y.K.; Kong, L.T.; Guan, M.; Fang, M.H.; Liu, Y.G.; Wu, X.W.; Huang, Z.H. Novel pyrochlore-type La2Zr2O7: Eu3+ red phosphors: Synthesis, structural, luminescence properties and theoretical calculation. Dyes Pigments 2018, 157, 47–54. [Google Scholar] [CrossRef]
- Zhu, H.M.; Lin, C.C.; Luo, W.Q.; Shu, S.T.; Liu, Z.G.; Liu, Y.S.; Kong, J.T.; Ma, E.; Cao, Y.G.; Liu, R.S.; et al. highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Commun. 2014, 5, 4312. [Google Scholar] [CrossRef]
- Pan, Z.F.; Castaing, V.; Yan, L.P.; Zhang, L.L.; Zhang, C.; Shao, K.; Zheng, Y.F.; Duan, C.K.; Liu, J.H.; Richard, C.; et al. Facilitating low energy activation in the near-infrared persistent luminescent phosphor Zn1+xGa2-2xSnxO4: Cr3+ via crystal field strength modulations. J. Phys. Chem. C 2020, 124, 8347–8358. [Google Scholar] [CrossRef]
- Yoshida, S.; Fujita, K.; Akamatsu, H.; Hernandez, O.; Gupta, A.S.; Brown, F.G.; Padmanabhan, H.; Gibbs, A.S.; Kuge, T.; Tsuji, R. Ferroelectric Sr3Zr2O7: Competition between Hybrid Improper Ferroelectric and Antiferroelectric Mechanisms. Adv. Funct. Mater. 2018, 28, 1801856. [Google Scholar] [CrossRef]
- Zhu, H.E.; Zhang, X.Y.; Zhang, M.; Li, Y.; Qi, X.W. Structure and electrical properties of SrZrO3-modified (K, Na, Li) (Nb, Ta) O3 lead-free piezoelectric ceramics. J. Mater. Sci.-Mater. El. 2018, 29, 905–3911. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of inter-atomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, L.; Gao, C.G.; Zhao, Y.X. Synthesis of ZrO2–SiO2 mixed oxide by alcohol-aqueous heating method. J. Sol-Gel Sci. Technol. 2011, 58, 572–579. [Google Scholar] [CrossRef]
- Carvalho, E.V.; de Paula, D.M.; Neto, D.M.A.; Costa, L.S.; Dias, D.F.; Feitosa, V.P.; Fechine, P.B.A. Radiopacity and mechanical properties of dental adhesives with strontium hydroxyapatite nanofillers. J. Mech. Behav. Biomed. 2020, 101, 103447. [Google Scholar] [CrossRef]
- Thieme, C.; Herrmann, A.; Kracker, M.; Patzig, C.; Hoche, T.; Russel, C. Microstructure investigation and fluorescence properties of europium-doped scheelite crystals in glass-ceramics made under different synthesis conditions. J. Lumin. 2021, 238, 118244. [Google Scholar] [CrossRef]
- Li, Y.C.; Yu, B.; Wang, H.; Wang, Y.J. Structural and optical characteristics of novel rare-earth-free red-emitting BaSn(PO4)2: Mn4+ phosphor. J. Mol. Struct. 2021, 1299, 129839. [Google Scholar] [CrossRef]
- Huang, Y.L.; Qin, J.; Fan, Z.T.; Wei, D.L.; Seo, H.J. Photoenergy conversion behaviors of photoluminescence and photocatalysis in silver-coated LiBaPO4: Eu2+. Inorg. Chem. 2019, 58, 13161–13169. [Google Scholar] [CrossRef]
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Gillani, S.S.A.; Ahmad, R.; Zeba, I.; Islah-u-din; Rizwan, M.; Rafique, M.; Shakil, M.; Jabbar, S.; Siddique, M. Structural stability of SrZrO3 perovskite and improvement in electronic and optical properties by Ca and Ba doping for optoelectronic applications: A DFT approach. Philos. Mag. 2019, 99, 3133–3145. [Google Scholar] [CrossRef]
- Borja-Urby, R.; Diaz-Torres, L.A.; Salas, P.; Vega-Gonzalez, M.; Angeles-Chavez, C. Blue and red emission in wide band gap BaZrO3: Yb3+, Tm3+. Mater. Sci. Eng. B-Adv. 2010, 174, 169–173. [Google Scholar] [CrossRef]
- Khajuria, P.; Mahajan, R.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Spectral and optical properties of Ruddlesden-Popper-type Ba3Zr2O7 phosphors doped with Eu3+ ion. Appl. Phys. A 2021, 127, 801. [Google Scholar] [CrossRef]
- Ratnakaram, Y.C.; Prasad, V.R.; Babu, S.; Kumar, V.V.R.K. Luminescence performance of Eu3+-doped lead-free zinc phosphate glasses for red emission. Bull. Mater. Sci. 2016, 39, 1065–1072. [Google Scholar] [CrossRef]
- Wang, P.; Mao, J.; Wei, X.; Qiu, L.; Jiang, B.; Chi, F.; Yin, M.; Chen, Y. Spontaneous-reduction and photoluminescence tuning in singly-doped Ba5-yCay(PO4)3Cl: Eu2+/Eu3+ phosphors. J. Alloys Compd. 2021, 869, 159277. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yang, Z.P.; Yu, Q.M. Preparation and its luminescent properties of AlPO4: Eu3+ phosphor for w-LED applications. J. Alloys Compd. 2011, 509, L199–L202. [Google Scholar] [CrossRef]
- Zeng, S.Y.; Tang, K.B.; Li, T.W.; Liang, Z.H. 3D flower-like Y2O3: Eu3+ nanostructures: Template-free synthesis and its luminescence properties. J. Colloid Interface Sci. 2007, 316, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Du, P.; Ran, W.G.; Li, W.P.; Luo, L.H.; Huang, X.Y. Morphology evolution of Eu3+-activated NaTbF4 nanorods: A highly-efficient near-ultraviolet light-triggered red-emitting platform towards application in white light-emitting diode. J. Mater. Chem. C 2019, 7, 10802–10809. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Philips. Res. Rep. 1969, 24, 131–144. [Google Scholar] [CrossRef]
- Liu, D.J.; Dang, P.P.; Yun, X.H.; Li, G.G.; Lian, H.Z.; Lin, J. Luminescence color tuning and energy transfer properties in (Sr,Ba)2LaGaO5: Bi3+, Eu3+ solid solution phosphors: Realization of single-phased white emission for WLEDs. J. Mater. Chem. C 2019, 7, 13536–13547. [Google Scholar] [CrossRef]
- Ou, J.H.; Yang, X.L.; Xiao, S.G. Luminescence performance of Cr3+ doped and Cr3+, Mn4+ co-doped La2ZnTiO6 phosphors. Mater. Res. Bull. 2020, 124, 11076. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.H.; Zhang, Z.W.; Wen, D.W.; Liang, Q.Y.; Zhou, J.B.; Yan, J.; Shi, J.X. Delayed Concentration Quenching of Luminescence Caused by Eu3+-Induced Phase Transition in LaSc3(BO3). Chem. Mater. 2022, 32, 6958–6967. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, Q.Q.; Takeda, T.; Hirosaki, N.; Xie, R.J. A promising thermally robustblue-green Li-α-sialon: Ce3+ for ultraviolet LED-driven white LEDs. J. Alloys Compd. 2019, 805, 1004–1012. [Google Scholar] [CrossRef]
- Qiao, J.W.; Zhou, G.J.; Zhou, Y.Y.; Zhang, Q.Y.; Xia, Z.G. Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes. Nat. Commun. 2019, 10, 5267. [Google Scholar] [CrossRef]
- Luo, J.B.; Sun, Z.S.; Zhu, Z.P.; Zhang, X.G.; Wu, Z.C.; Mo, F.W. Synthesis, structure and luminescence of a high-purity and thermal-stable Sr9LiMg(PO4)7: Eu3+ red phosphor. Ceram. Int. 2020, 46, 11994–12000. [Google Scholar] [CrossRef]
- Li, M.C.; Zhang, X.J.; Zheng, Y.J.; Xu, Y.L.; Zhang, H.R.; Liu, Y.L.; Lei, B.F. F enhanced luminescence performance of SrLu2O4: Ce3+ glass ceramic for superior high-power artificial horticultural LEDs. Ceram. Int. 2020, 46, 21560–21568. [Google Scholar] [CrossRef]
- Panigrahi, K.; Saha, S.; Sain, S.; Chatterjee, R.; Das, A.; Ghorai, U.K.; Das, N.S.; Chattopadhyay, K.K. White light emitting MgAl2O4: Dy3+, Eu3+ nanophosphor for multifunctional applications. Dalton Trans. 2018, 47, 12228–12242. [Google Scholar] [CrossRef]
- Nair, G.B.; Kumar, A.; Swart, H.C.; Dhoble, S.J. Improved steady-state photo-luminescence derived from the compensation of the charge-imbalance in Ca3Mg3(PO4)4: Eu3+ phosphor. Ceram. Int. 2019, 45, 21709–21715. [Google Scholar] [CrossRef]
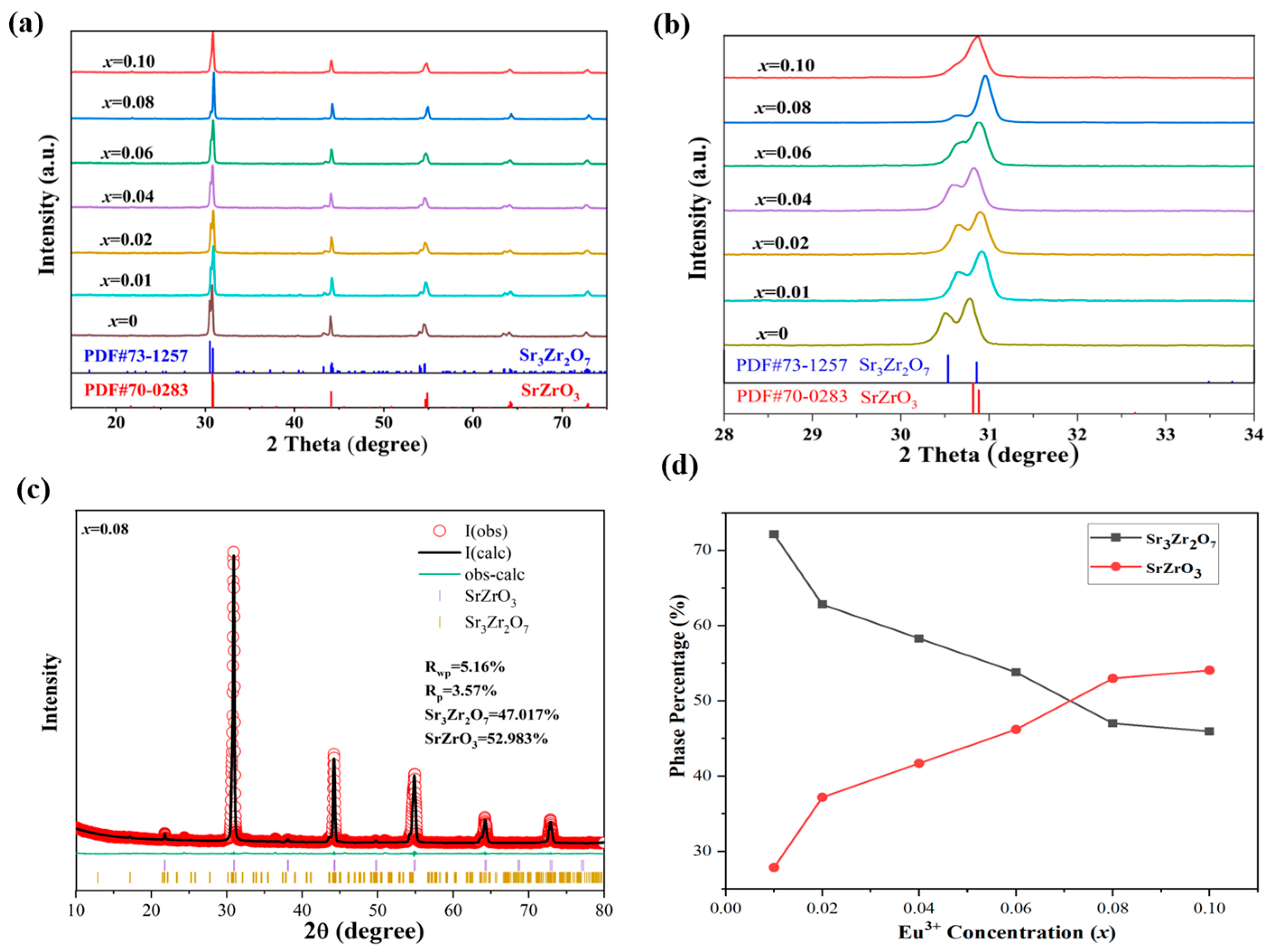
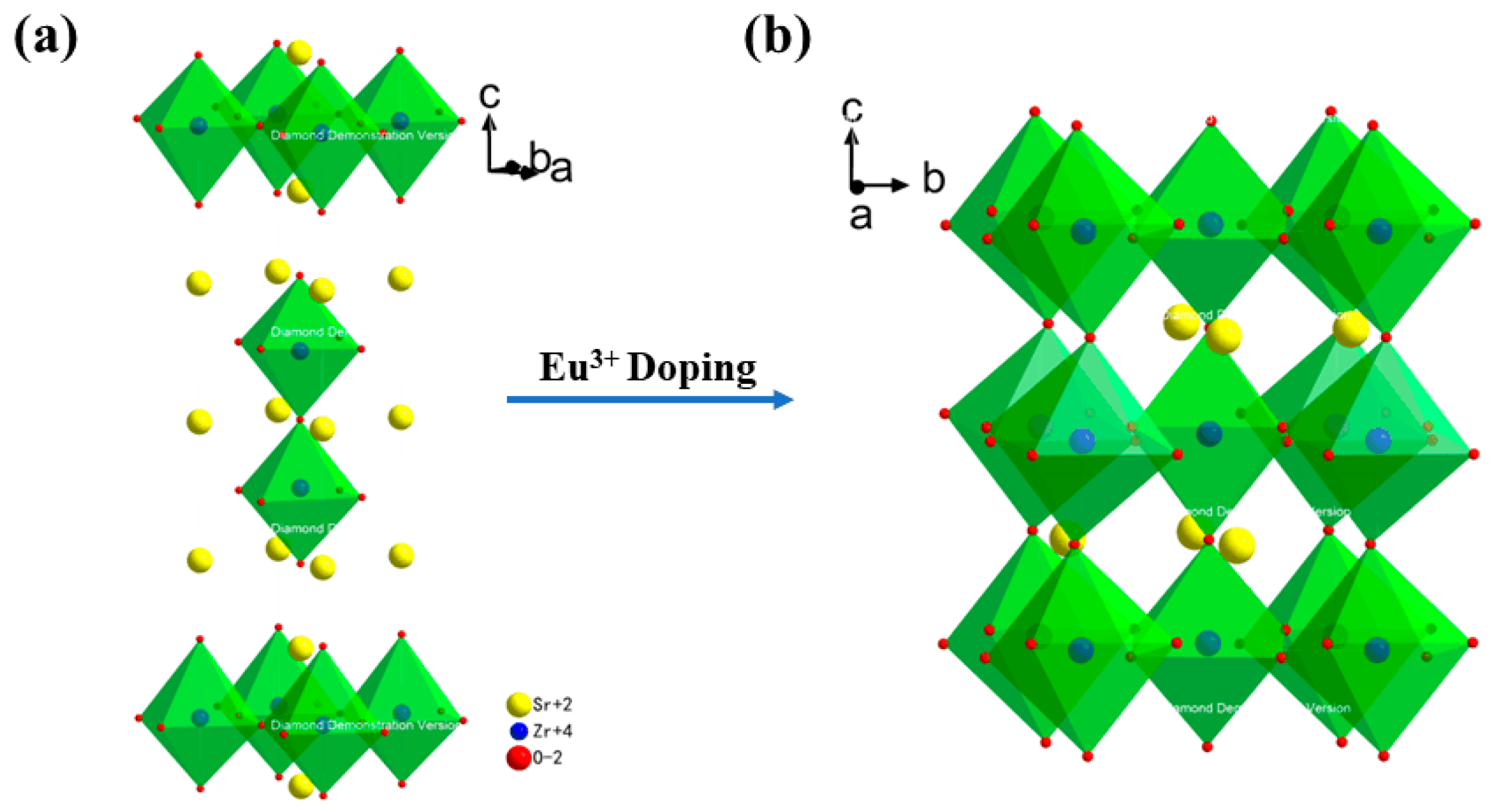
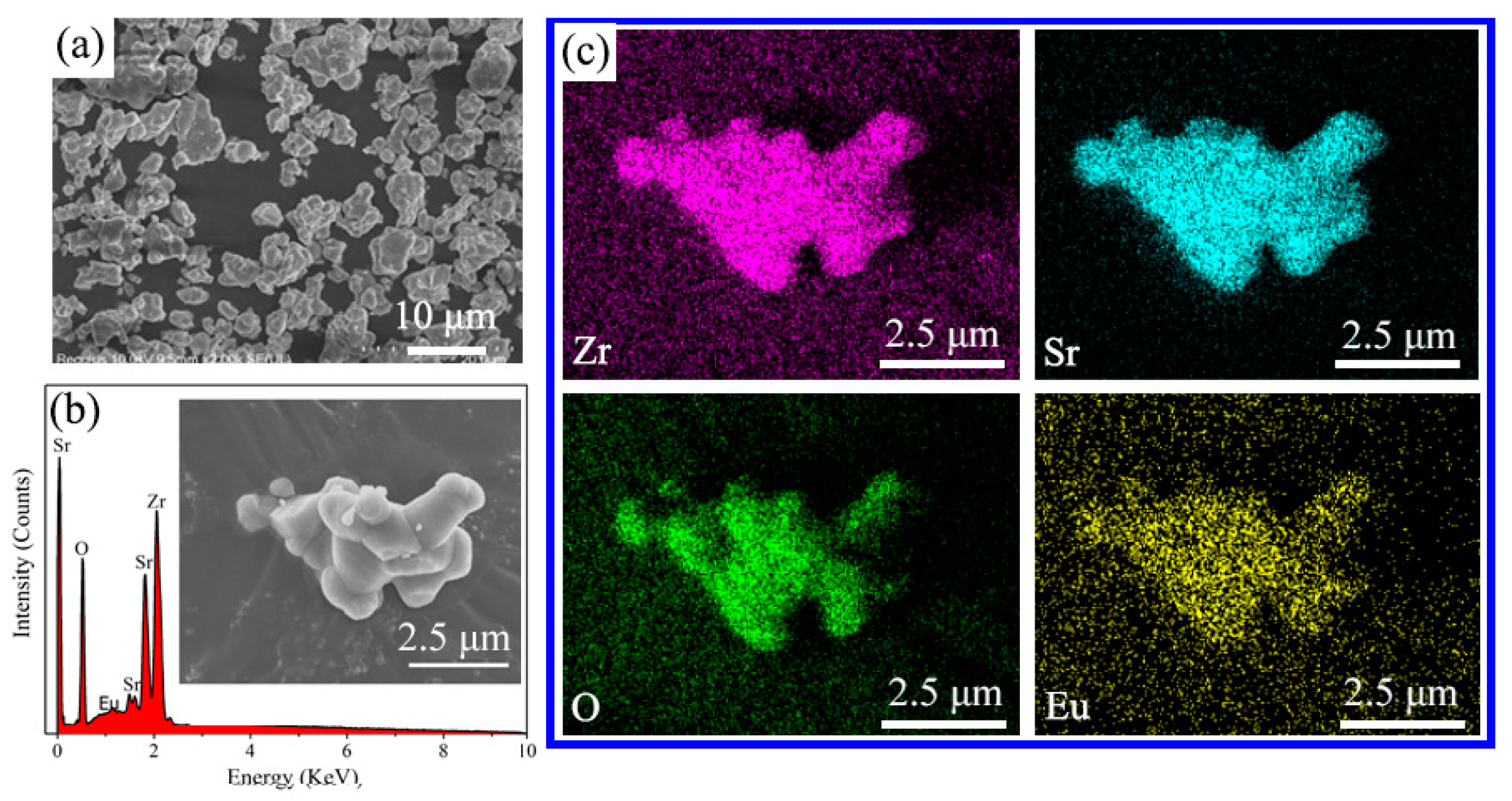
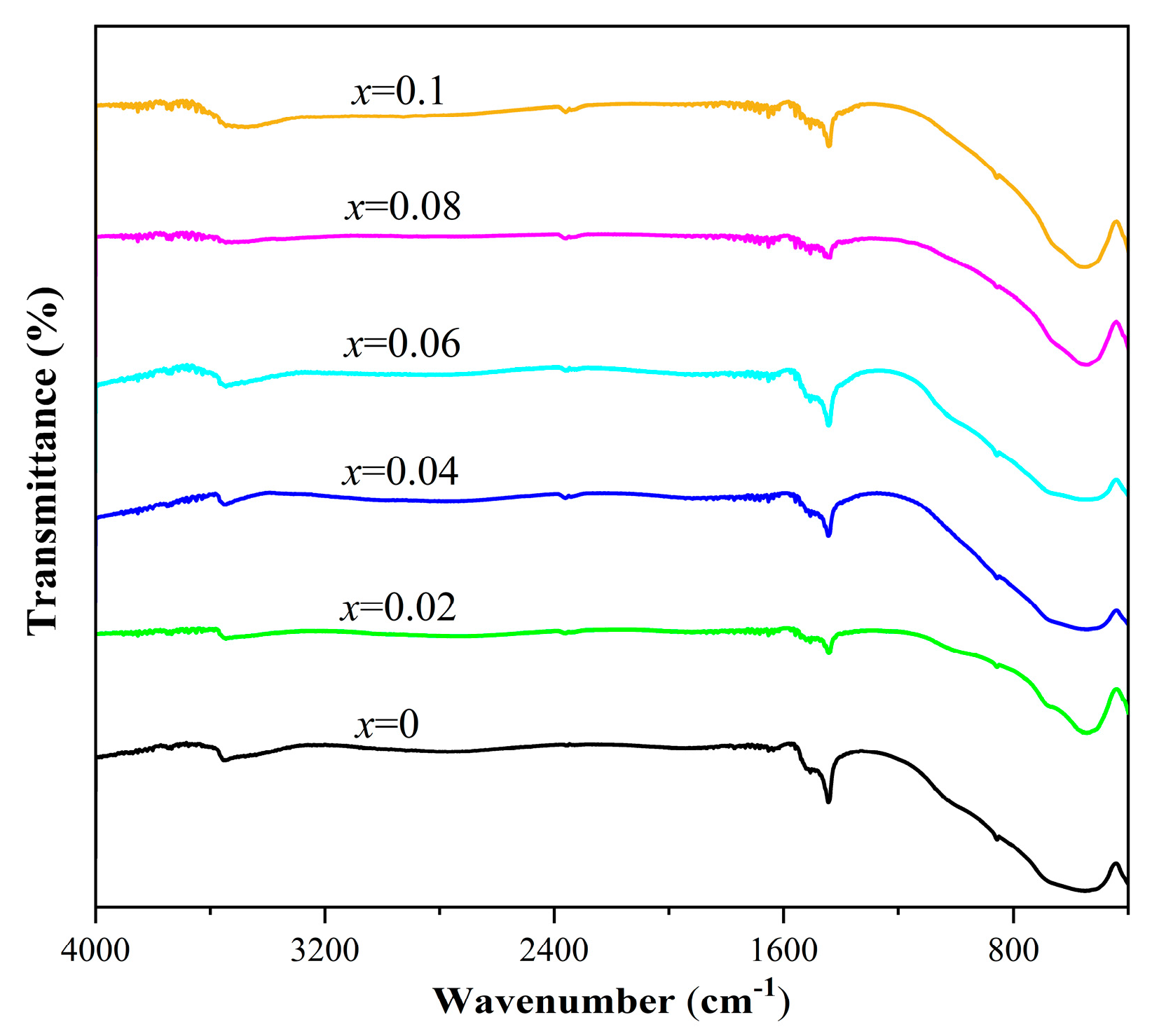
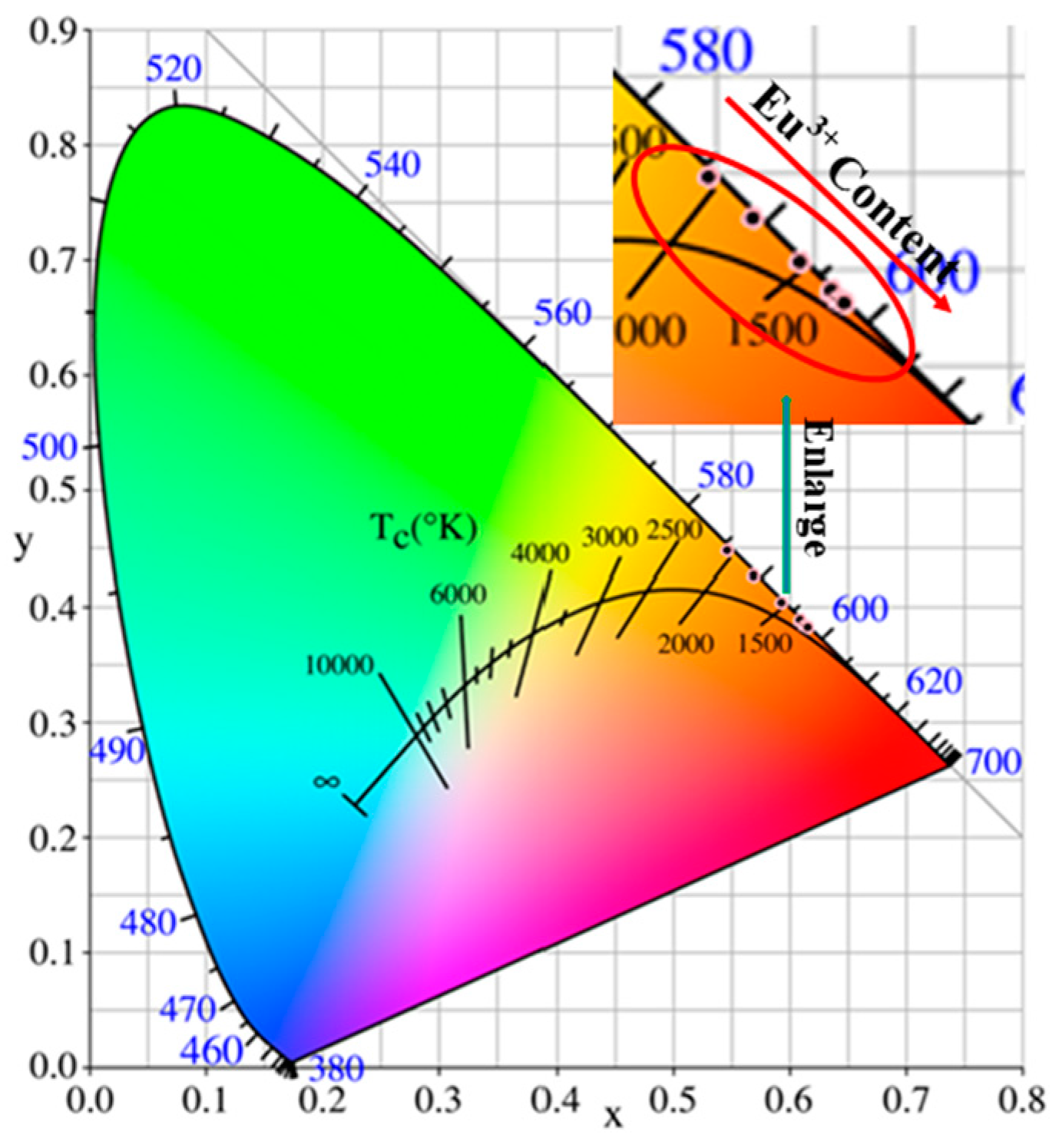
| Sr3Zr2O7:xEu3+ | 1% | 2% | 4% | 6% | 8% | 10% |
|---|---|---|---|---|---|---|
| CIE x | 0.5969 | 0.5936 | 0.6099 | 0.6092 | 0.6136 | 0.6155 |
| CIE y | 0.4267 | 0.4038 | 0.388 | 0.3888 | 0.3846 | 0.3827 |
| CCT (K) | 1724 | 1711 | 1752 | 1747 | 1778 | 1796 |
| CP (%) | 88.60 | 85.54 | 88.87 | 88.72 | 89.68 | 90.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Wang, Y.; Li, L.; Geng, L.; Zhang, M. Synthesis, Photoluminescent Characteristics and Eu3+-Induced Phase Transitions in Sr3Zr2O7:Eu3+ Red Phosphors. Nanomaterials 2023, 13, 1446. https://doi.org/10.3390/nano13091446
Chen N, Wang Y, Li L, Geng L, Zhang M. Synthesis, Photoluminescent Characteristics and Eu3+-Induced Phase Transitions in Sr3Zr2O7:Eu3+ Red Phosphors. Nanomaterials. 2023; 13(9):1446. https://doi.org/10.3390/nano13091446
Chicago/Turabian StyleChen, Nianmin, Yunjian Wang, Longfeng Li, Lei Geng, and Maolin Zhang. 2023. "Synthesis, Photoluminescent Characteristics and Eu3+-Induced Phase Transitions in Sr3Zr2O7:Eu3+ Red Phosphors" Nanomaterials 13, no. 9: 1446. https://doi.org/10.3390/nano13091446
APA StyleChen, N., Wang, Y., Li, L., Geng, L., & Zhang, M. (2023). Synthesis, Photoluminescent Characteristics and Eu3+-Induced Phase Transitions in Sr3Zr2O7:Eu3+ Red Phosphors. Nanomaterials, 13(9), 1446. https://doi.org/10.3390/nano13091446





