Atomistic Insights on Surface Quality Control via Annealing Process in AlGaN Thin Film Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Interatomic Potential
2.2. Simulation Details
3. Results and Discussion
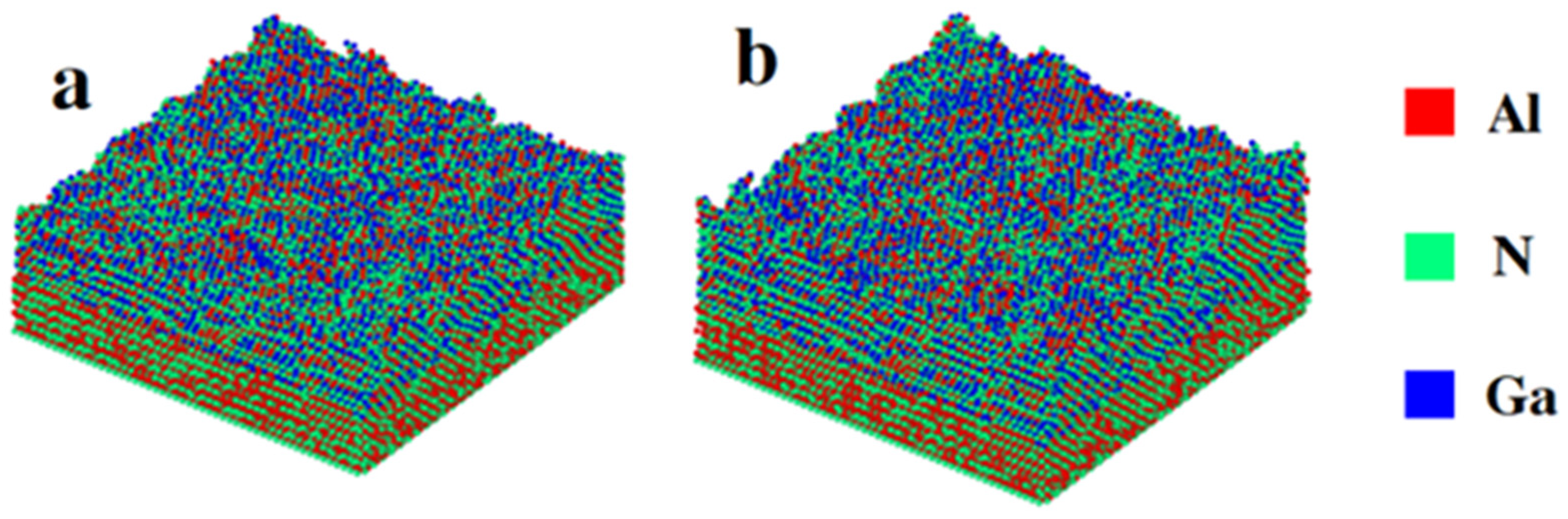
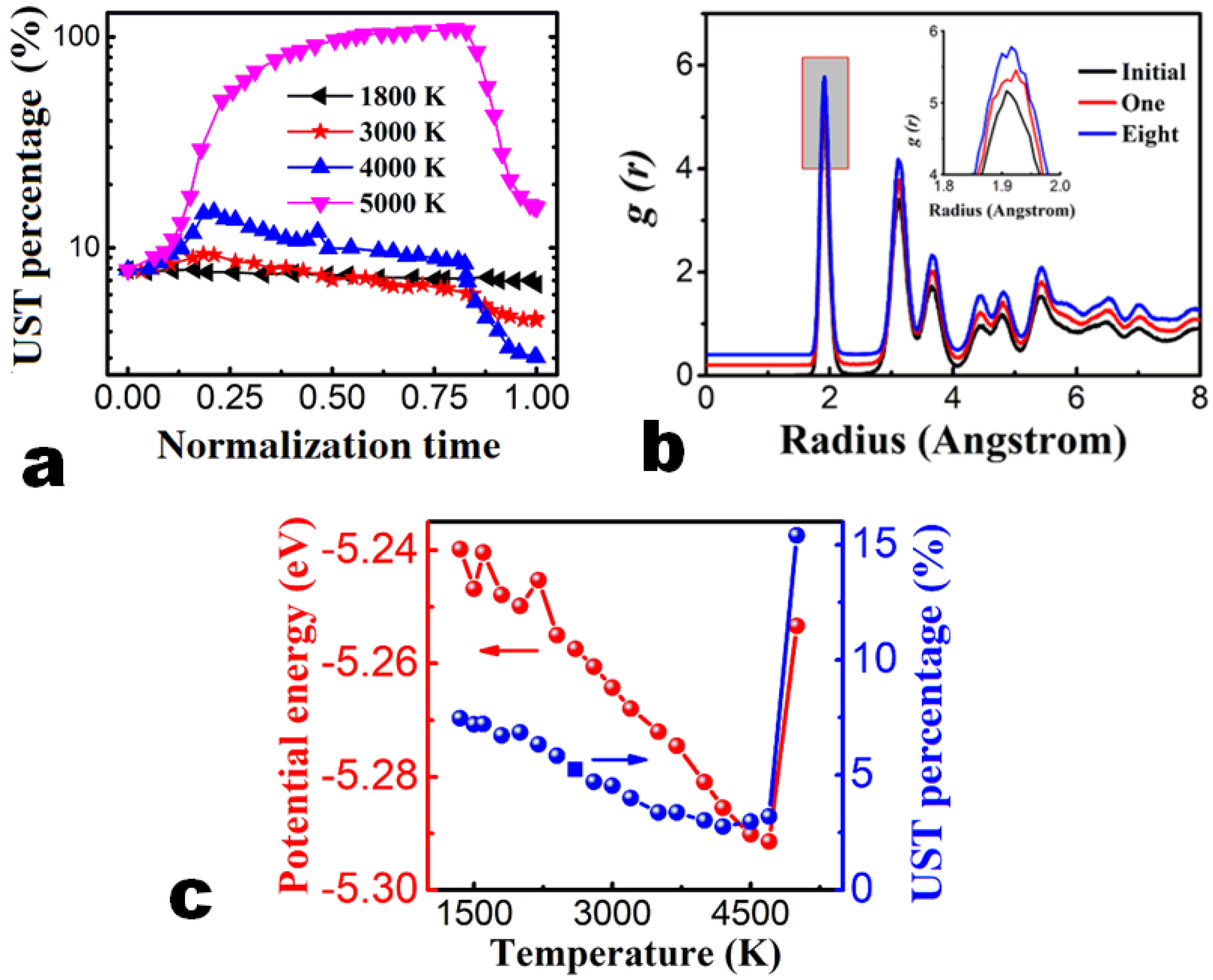
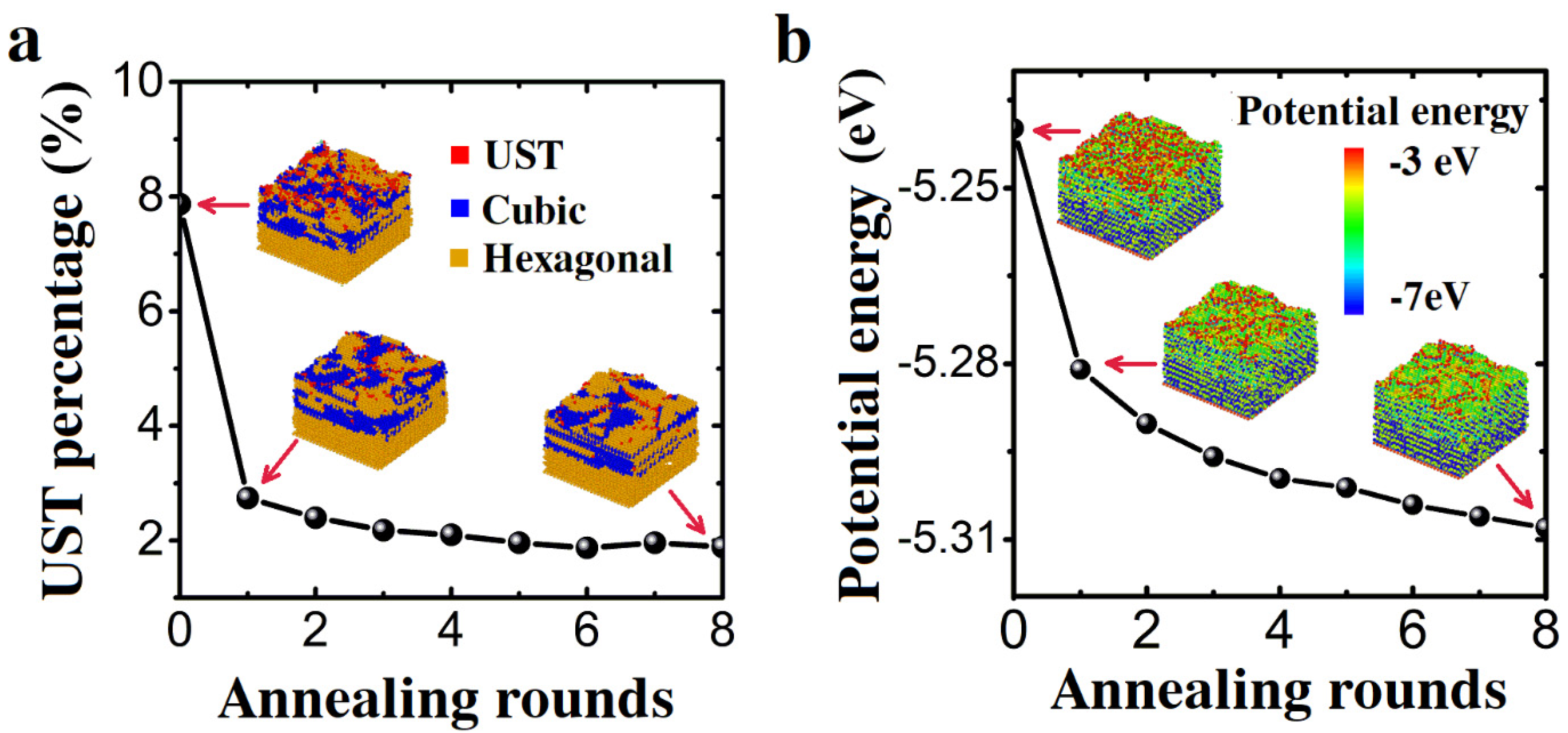
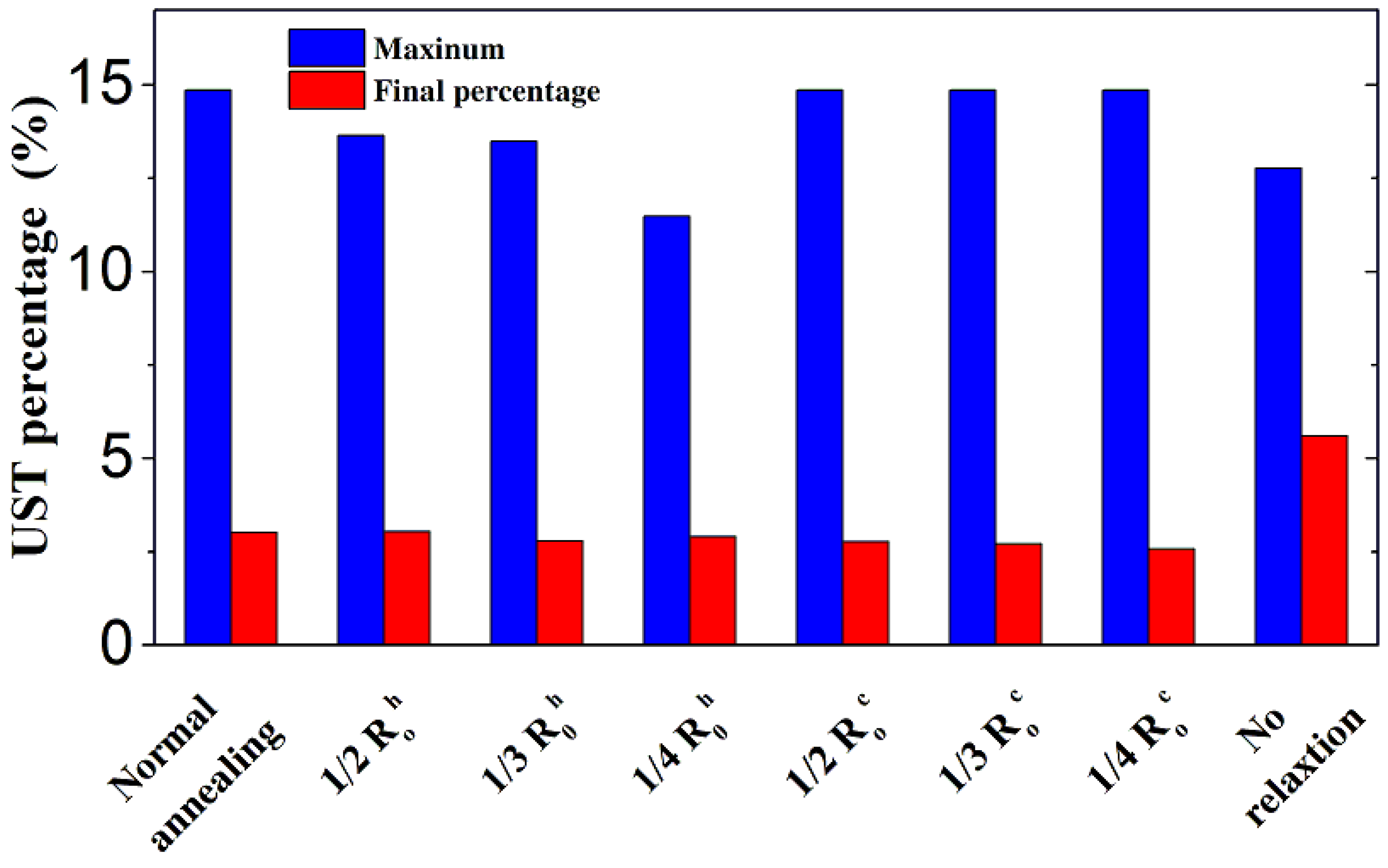
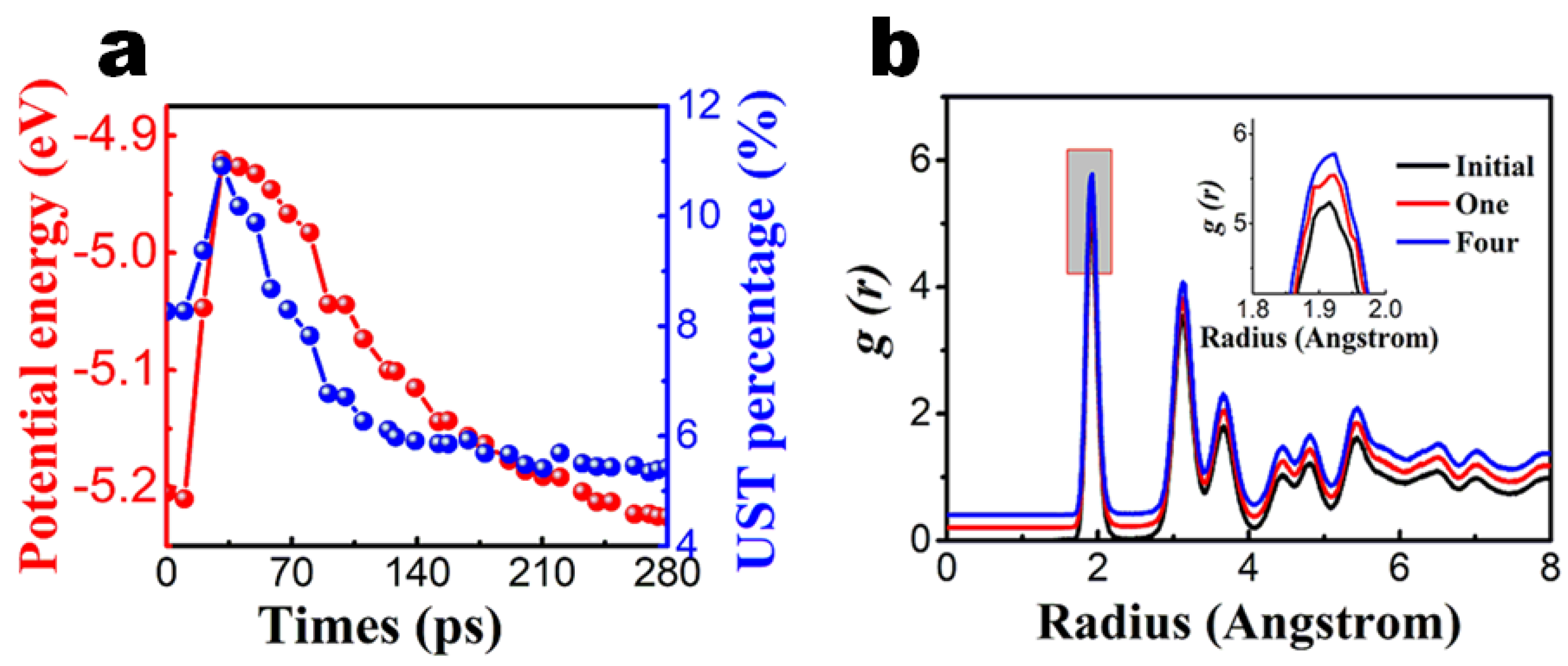
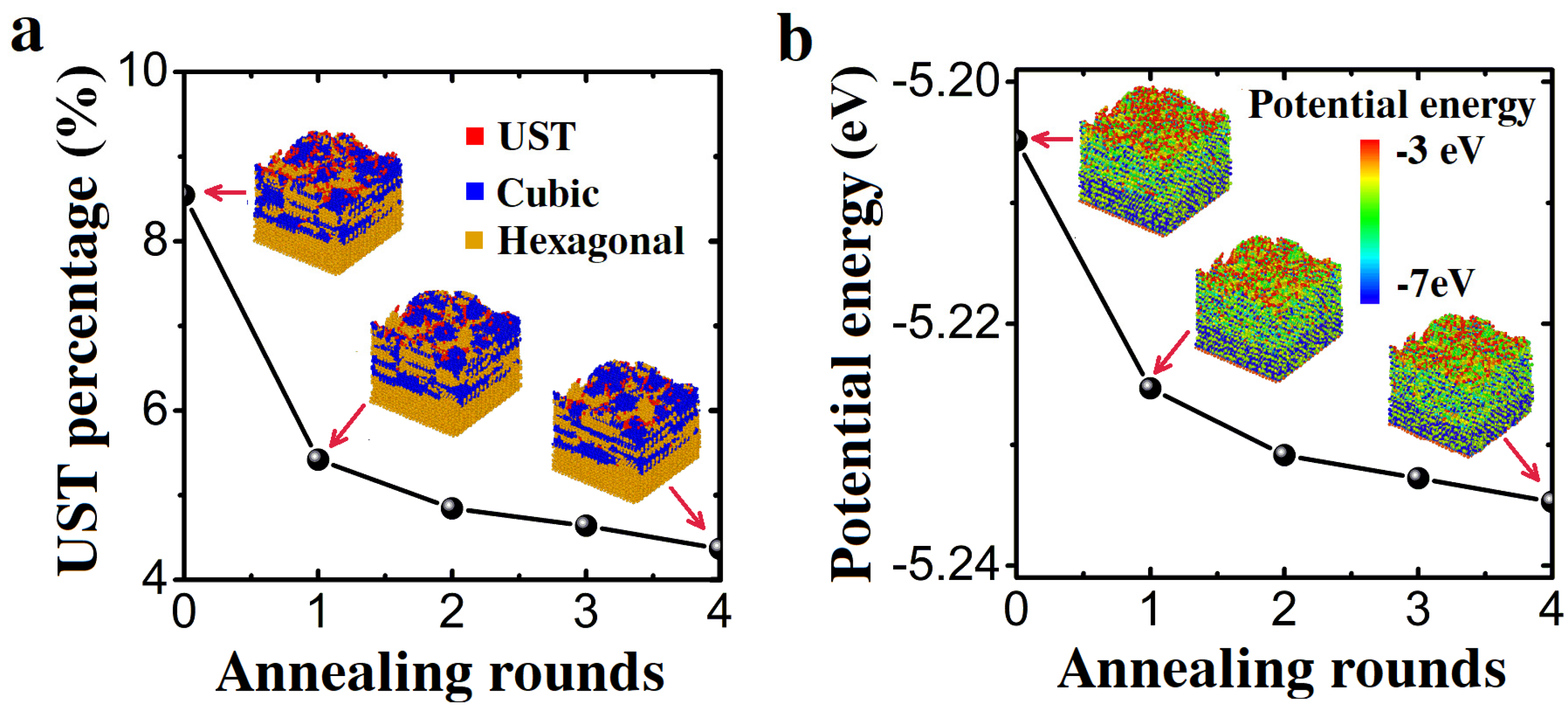
3.1. Constant Temperature Annealing
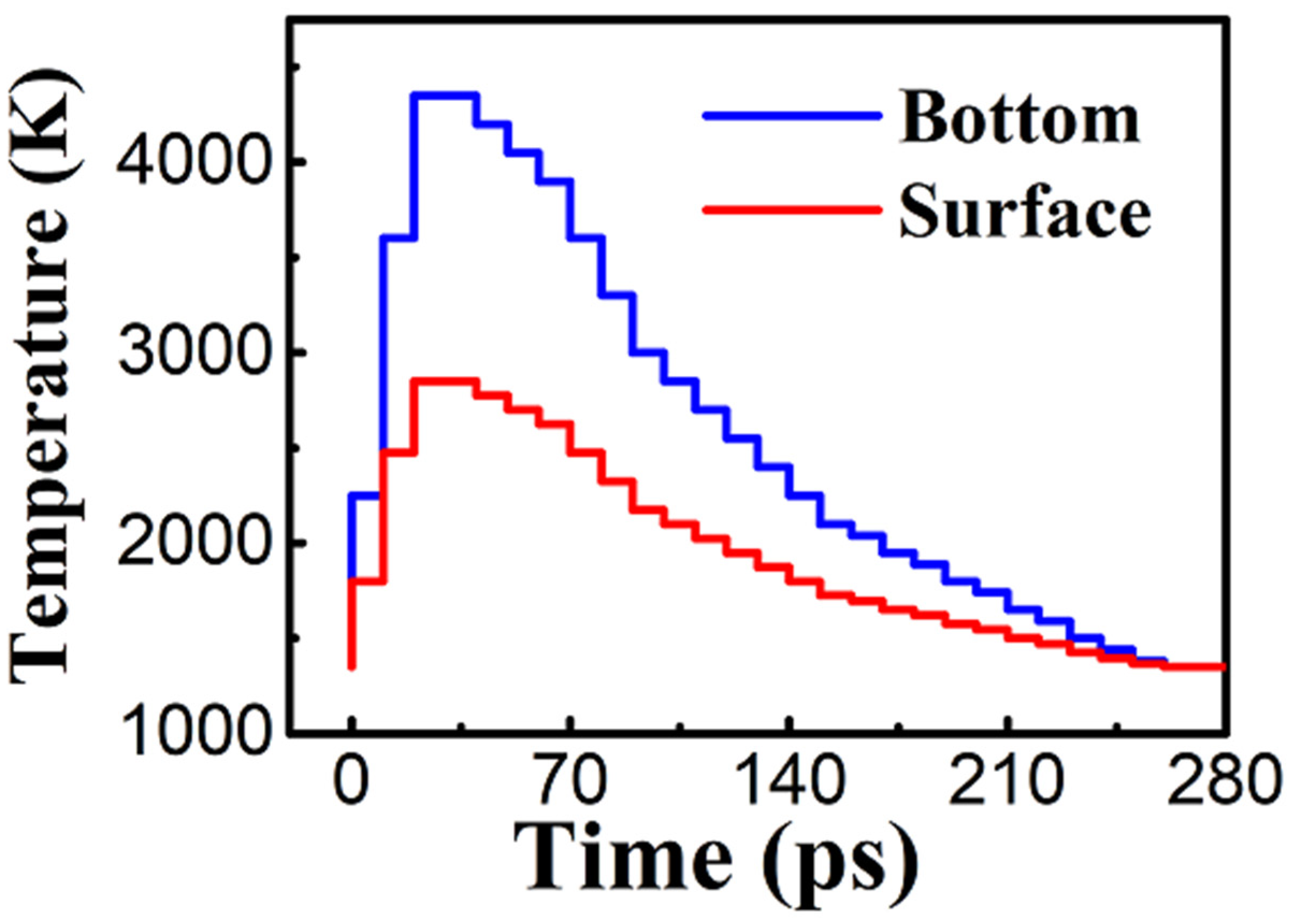
3.2. Laser Thermal Annealing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.P.; Wang, H.M.; Gaevski, M.E.; Chen, C.Q.; Fareed, Q.; Yang, J.W.; Simin, G.; Khan, M.A. Crack-Free Thick AlGaN Grown on Sapphire Using AlN/AlGaN Superlattices for Strain Management. Appl. Phys. Lett. 2002, 80, 3542–3544. [Google Scholar] [CrossRef]
- Xiang, H.G.; Li, H.T.; Fu, T.; Zhao, Y.B.; Huang, C.; Zhang, G.; Peng, X.H. Molecular Dynamics Simulation of AlN Thin Films under Nanoindentation. Ceram. Int. 2016, 43, 4068–4075. [Google Scholar] [CrossRef]
- Xiang, H.G.; Li, H.T.; Fu, T.; Huang, C.; Peng, X.H. Formation of Prismatic Loops in AlN and GaN under Nanoindentation. Acta Mater. 2017, 138, 131–139. [Google Scholar] [CrossRef]
- Jayathilaka, W.; Chinnappan, A.; Ji, D.X.; Ghosh, R.; Tran, T.; Ramakrishna, S. Facile and Scalable Electrospun Nanofiber-Based Alternative Current Electroluminescence (ACEL) Device. ACS Appl. Electron. Mater. 2021, 3, 267–276. [Google Scholar] [CrossRef]
- Satter, M.M.; Lochner, Z.; Ryou, J.H.; Shen, S.C.; Dupuis, R.D.; Yoder, P.D. Polarization Matching in AlGaN-Based Multiple-Quantum-Well Deep Ultraviolet Laser Diodes on AlN Substrates Using Quaternary AlInGaN Barriers. J. Light. Technol. 2012, 30, 3017–3025. [Google Scholar] [CrossRef]
- Xie, Z.L.; Liu, B.; Nie, C.; Jiang, R.L.; Ji, X.L.; Zhao, H.; Han, P.; Xiu, X.Q.; Zhang, R.; Zheng, Y.D.; et al. AlGaN-Based 330 nm Resonant-Cavity-Enhanced P-I-N Junction Ultraviolet Photodetectors Using AlN/AlGaN Distributed Bragg Reflectors. Phys. Status Solidi C 2010, 7, 7–8. [Google Scholar] [CrossRef]
- Shen, L.; Heikman, S.; Moran, B.; Coffie, R.; Zhang, N.Q.; Buttari, D.; Smorchkova, I.P.; Keller, S.; DenBaars, S.P.; Mishra, U.K. AlGaN/AlN/GaN High-Power Microwave HEMT. Electron Device Lett. IEEE 2001, 22, 457–459. [Google Scholar] [CrossRef]
- Wang, X.L.; Hu, G.X.; Ma, Z.Y.; Ran, J.X.; Wang, C.M.; Mao, H.L.; Tang, H.; Li, H.P.; Wang, J.X.; Zeng, Y.P.; et al. AlGaN/AlN/GaN/SiC HEMT Structure with High Mobility GaN Thin Layer as Channel Grown by MOCVD. J. Cryst. Growth 2007, 298, 835–839. [Google Scholar] [CrossRef]
- Hirayama, H.; Fujikawa, S.; Noguchi, N.; Norimatsu, J.; Takano, T.; Tsubaki, K.; Kamata, N. 222–282 nm AlGaN and InAlGaN-Based Deep-UV LEDs Fabricated on High-Quality AlN on Sapphire. Phys. Status Solidi 2009, 206, 1176–1182. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Sun, Q.; Kwon, S.Y.; Han, J.; Davitt, K.; Song, Y.K.; Nurmikko, A.V.; Liu, W.; Smart, J.; Schowalter, L. AlGaN Deep Ultraviolet LEDs on Bulk AlN Substrates. Phys. Status Solidi C 2007, 4, 2482–2485. [Google Scholar] [CrossRef]
- Mastro, M.A.; Eddy, C.R.; Gaskill, D.K.; Bassim, N.D.; Casey, J.; Rosenberg, A.; Holm, R.T.; Henry, R.L.; Twigg, M.E. MOCVD Growth of Thick AlN and AlGaN Superlattice Structures on Si Substrates. J. Cryst. Growth 2006, 287, 610–614. [Google Scholar] [CrossRef]
- Koukitu, A.; Seki, H. Unstable Region of Solid Composition in Ternary Nitride Alloys Grown by Metalorganic Vapor-Phase Epitaxy. Jpn. J. Appl. Phys. 1996, 35, 1638–1640. [Google Scholar] [CrossRef]
- Lu, D.C.; Duan, S. Quasi-Thermodynamic Analysis of MOVPE of AlGaN. J. Cryst. Growth 2000, 208, 73–78. [Google Scholar] [CrossRef]
- Lobanova, A.V.; Mazaev, K.M.; Talalaev, R.A.; Leys, M.; Boeykens, S.; Cheng, K.; Degroote, S. Effect of V/III Ratio in AlN and AlGaN MOVPE. J. Cryst. Growth 2006, 287, 601–604. [Google Scholar] [CrossRef]
- Liu, D.M.; Kim, H.; Lu, W. Post-Annealing Effects on Device Performance of AlGaN/GaN HFETs. Solid-State Electron. 2004, 48, 1855–1859. [Google Scholar]
- Hou, M.C.; Xie, G.; Sheng, K. Improved Device Performance in AlGaN/GaN HEMT by Forming Ohmic Contact with Laser Annealing. IEEE Electron Device Lett. 2018, 39, 1137–1140. [Google Scholar] [CrossRef]
- Duong, H.M.; Tran, T.Q.; Kopp, R.; Myint, S.M.; Peng, L. Chapter 1—Direct Spinning of Horizontally Aligned Carbon Nanotube Fibers and Films from the Floating Catalyst Method. In Nanotube Superfiber Materials: Science, Manufacturing, Commercialization; William Andrew: Norwich, NY, USA, 2019; pp. 3–29. [Google Scholar]
- Peng, Q.; Meng, F.J.; Yang, Y.Z.; Lu, C.Y.; Deng, H.Q.; Wang, L.M.; De, S.; Gao, F. Shockwave generates <100> dislocation loops in bcc iron. Nat. Commun. 2018, 9, 4880. [Google Scholar] [PubMed]
- Zhou, X.W.; Murdick, D.A.; Gillespie, B.; Wadley, H.N.G. Atomic assembly during GaN film growth: Molecular dynamics simulations. Phys. Rev. B 2006, 73, 045337. [Google Scholar] [CrossRef]
- Leathersich, J.; Suvarna, P.; Tungare, M.; Shahedipour-Sandvik, F.S. Homoepitaxial growth of non-polar AlN crystals using molecular dynamics simulations. Surf. Sci. 2013, 617, 36–41. [Google Scholar] [CrossRef]
- Gruber, J.; Zhou, X.W.; Jones, R.E.; Lee, S.R.; Tucker, G.J. Molecular dynamics studies of defect formation during heteroepitaxial growth of InGaN alloys on (0001) GaN surfaces. J. Appl. Phys. 2017, 121, 195301. [Google Scholar] [CrossRef]
- Chu, K.; Gruber, J.; Zhou, X.W.; Jones, R.E.; Lee, S.R.; Tucker, G.J. Molecular dynamics studies of InGaN growth on nonpolar (1120) GaN surfaces. Phys. Rev. Mater. 2018, 2, 013402. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Zhou, X.W.; Jones, R.E.; Kimmer, C.J.; Duda, J.C.; Hopkins, P.E. Relationship of thermal boundary conductance to structure from an analytical model plus molecular dynamics simulations. Phys. Rev. B 2013, 87, 094303. [Google Scholar] [CrossRef]
- Zhou, X.W.; Jones, R.E.; Duda, J.C.; Hopkins, P.E. Molecular dynamics studies of material property effects on thermal boundary conductance. Phys. Chem. Chem. Phys. 2013, 15, 11078–11087. [Google Scholar] [CrossRef]
- Nord, J.; Albe, K.; Erhart, P.; Nordlund, K. Modelling of compound semiconductors: Analytical bond-order potential for gallium, nitrogen and gallium nitride. J. Phys. Condens. Matter 2003, 15, 5649–5662. [Google Scholar] [CrossRef]
- Béré, A.; Serra, A. On the atomic structures, mobility and interactions of extended defects in GaN: Dislocations, tilt and twin boundaries. Philos. Mag. 2006, 86, 2159–2192. [Google Scholar] [CrossRef]
- Tungare, M.; Shi, Y.; Tripathi, N.; Suvarna, P.; Shahedipour-Sandvik, F.S. A Tersoff-based interatomic potential for wurtzite AlN. Phys. Status Solidi A 2011, 208, 1569–1572. [Google Scholar] [CrossRef]
- Vashishta, P.; Kalia, R.K.; Nakano, A.; Rino, J.P. Interaction potential for aluminum nitride: A molecular dynamics study of mechanical and thermal properties of crystalline and amorphous aluminum nitride. J. Appl. Phys. 2011, 109, 033514. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Zhu, G.; Liu, S.; Gan, Z.; Zhang, Z. Effect of Substrate Surface on Deposition of AlGaN: A Molecular Dynamics Simulation. Crystals 2018, 8, 279. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wang, Y.; Suo, Y.; Liu, S.; Gan, Z. Atomic simulation of AlGaN film deposition on AlN template. Mol. Phys. 2019, 118, 1–8. [Google Scholar] [CrossRef]
- Susilo, N.; Hagedorn, S.; Jaeger, D.; Miyake, H.; Zeimer, U.; Reich, C.; Neuschulz, B.; Sulmoni, L.; Guttmann, M.; Mehnke, F.; et al. AlGaN-based deep UV LEDs grown on sputtered and high temperature annealed AlN/sapphire. Appl. Phys. Lett. 2018, 112, 041110. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Li, S.M.; Gao, H.W.; Wang, W.H.; Sun, Q.; Peng, Q.; Gui, C.Q.; Qian, Z.F.; Liu, S. Stress evolution in AlN and GaN grown on Si(111): Experiments and theoretical modeling. J. Mater. Sci. Mater. Electron. 2016, 27, 2. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Li, S.M.; Sun, Q.; Peng, Q.; Gui, C.Q.; Zhou, Y.; Liu, S. Properties of AlN film grown on Si(111). J. Cryst. Growth 2016, 435, 76–83. [Google Scholar] [CrossRef]
- Dai, Y.Q.; Wang, W.H.; Gui, C.Q.; Wen, X.D.; Peng, Q.; Liu, S. A first-principles study of the mechanical properties of AlN with Raman verification. Comput. Mater. Sci. 2016, 112, 342–346. [Google Scholar] [CrossRef]
- Lorazo, P.; Lewis, L.J.; Meunier, M. Molecular-Dynamics simulations of picosecond pulsed laser ablation and desorption of silicon. Laser Plasma Gener. Diagn. 2000, 3935, 66–75. [Google Scholar]
- Fisicaro, G.; Magna, A.L. Modeling of laser annealing. J. Comput. Electron. 2014, 13, 70–94. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Maras, E.; Trushin, O.; Stukowski, A.; AlaNissila, T.; Jónsson, H. Global transition path search for dislocation formation in Ge on Si (001). Comput. Phys. Commun. 2016, 205, 13–21. [Google Scholar] [CrossRef]
- Makaram, P.; Joh, J.; del Alamo, J.A.; Palacios, T.; Thompson, C.V. Evolution of Structural Defects Associated with Electrical Degradation in AlGaN/GaN High Electron Mobility Transistors. Appl. Phys. Lett. 2010, 96, 233509. [Google Scholar] [CrossRef]
- Neugebauer, J. Abinitio Analysis of Surface Structure and Adatom Kinetics of Group-III Nitrides. Phys. Status Solidi B 2001, 227, 93–114. [Google Scholar] [CrossRef]
- Xie, L.; An, H.J.; Peng, Q.; Qin, Q.; Zhang, Y. Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth. Materials 2018, 11, 2548. [Google Scholar] [CrossRef]
- Utz, M.; Peng, Q.; Nandagopal, M. Athermal Simulation of Plastic Deformation in Amorphous Solids at Constant Pressure. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2057–2065. [Google Scholar] [CrossRef]
- Sun, Z.H.; Liu, B.S.; He, C.W.; Xie, L.; Peng, Q. Shift of Creep Mechanism in Nanocrystalline NiAl Alloy. Materials 2018, 12, 2508. [Google Scholar] [CrossRef]
- Qin, Q.; He, W.; Xie, L.; Deng, J.C.; Zhu, X.H.; Peng, Q. Nonlinear Diffusion, Bonding, and Mechanics of the Interface between Austenitic Steel and Iron. Phys. Chem. Chem. Phys. 2019, 21, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Lorazo, P.; Lewis, L.J.; Meunier, M. Short-Pulse Laser Ablation of Solids: From Phase Explosion to Fragmentation. Phys. Rev. Lett. 2003, 91, 225502. [Google Scholar] [CrossRef]
- Lietoila, A.; Gibbons, J.F. Computer Modeling of the Temperature Rise and Carrier Concentration Induced in Silicon by Nanosecond Laser Pulses. J. Appl. Phys. 1982, 53, 3207–3213. [Google Scholar] [CrossRef]
- Pronko, P.P.; Dutta, S.K.; Du, D.; Singh, R.K. Thermophysical Effects in Laser Processing of Materials with Picosecond and Femtosecond Pulses. J. Appl. Phys. 1995, 78, 6233–6240. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Kodali, P.B.S.; Garrison, B.J. Molecular Dynamics Model for Laser Ablation and Desorption of Organic Solids. J. Phys. Chem. B 1997, 101, 8624. [Google Scholar] [CrossRef]
- Seifert, N.; Betz, G. Computer Simulations of Laser-Induced Ejection of Droplets. Appl. Surf. Sci. 1998, 133, 189–194. [Google Scholar] [CrossRef]
- Stampfli, P.; Bennemann, K.H. Time Dependence of the Laser-Induced Femtosecond Lattice Instability of Si and GaAs: Role of Longitudinal Optical Distortions. Phys. Rev. B 1994, 49, 7299–7305. [Google Scholar] [CrossRef]
- Silvestrelli, P.L.; Alavi, A.; Parrinello, M.; Frenkel, D. Structural, Dynamical, Electronic, and Bonding Properties of Laser-Heated Silicon: An ab Initio Molecular-Dynamics Study. Phys. Rev. B 1997, 56, 3806–3812. [Google Scholar] [CrossRef]
| Parameter | GaGa | NN | AlAl | GaN | GaAl | NAl |
|---|---|---|---|---|---|---|
| ε (eV) | 1.2000 | 1.2000 | 0.5650 | 2.1700 | 0.5223 | 2.2614 |
| σ (Å) | 2.1000 | 1.3000 | 2.6674 | 1.6950 | 2.7322 | 1.7103 |
| α | 1.60 | 1.80 | 1.55 | 1.80 | 1.55 | 1.80 |
| λ | 32.5 | 32.5 | 0.0 | 32.5 | 0.0 | 40.5 |
| γ | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| A | 7.9170 | 7.9170 | 17.8118 | 7.9170 | 17.8118 | 7.917 |
| B | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Ma, Z.; Cai, S.; Zhao, S.; Chen, X.; Cao, Q. Atomistic Insights on Surface Quality Control via Annealing Process in AlGaN Thin Film Growth. Nanomaterials 2023, 13, 1382. https://doi.org/10.3390/nano13081382
Peng Q, Ma Z, Cai S, Zhao S, Chen X, Cao Q. Atomistic Insights on Surface Quality Control via Annealing Process in AlGaN Thin Film Growth. Nanomaterials. 2023; 13(8):1382. https://doi.org/10.3390/nano13081382
Chicago/Turabian StylePeng, Qing, Zhiwei Ma, Shixian Cai, Shuai Zhao, Xiaojia Chen, and Qiang Cao. 2023. "Atomistic Insights on Surface Quality Control via Annealing Process in AlGaN Thin Film Growth" Nanomaterials 13, no. 8: 1382. https://doi.org/10.3390/nano13081382
APA StylePeng, Q., Ma, Z., Cai, S., Zhao, S., Chen, X., & Cao, Q. (2023). Atomistic Insights on Surface Quality Control via Annealing Process in AlGaN Thin Film Growth. Nanomaterials, 13(8), 1382. https://doi.org/10.3390/nano13081382







