Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Inverse Opal
2.2. Sample Characterization
3. Result and Discussion
3.1. Opal Template as Determined by Thermal Analysis
3.2. Morphological and Compositional Evaluation of the Samples
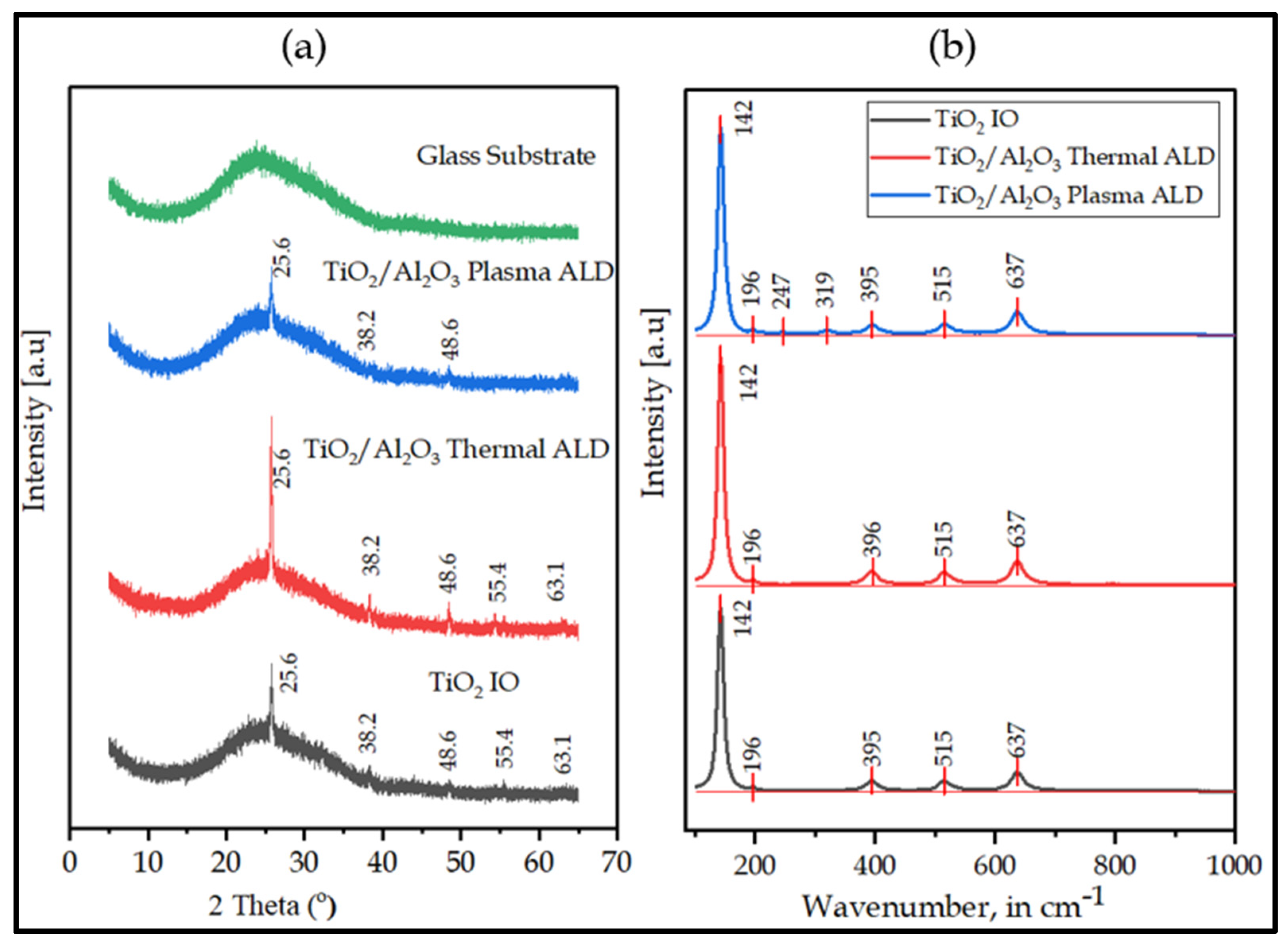
3.3. XRD and Raman Structural Analysis
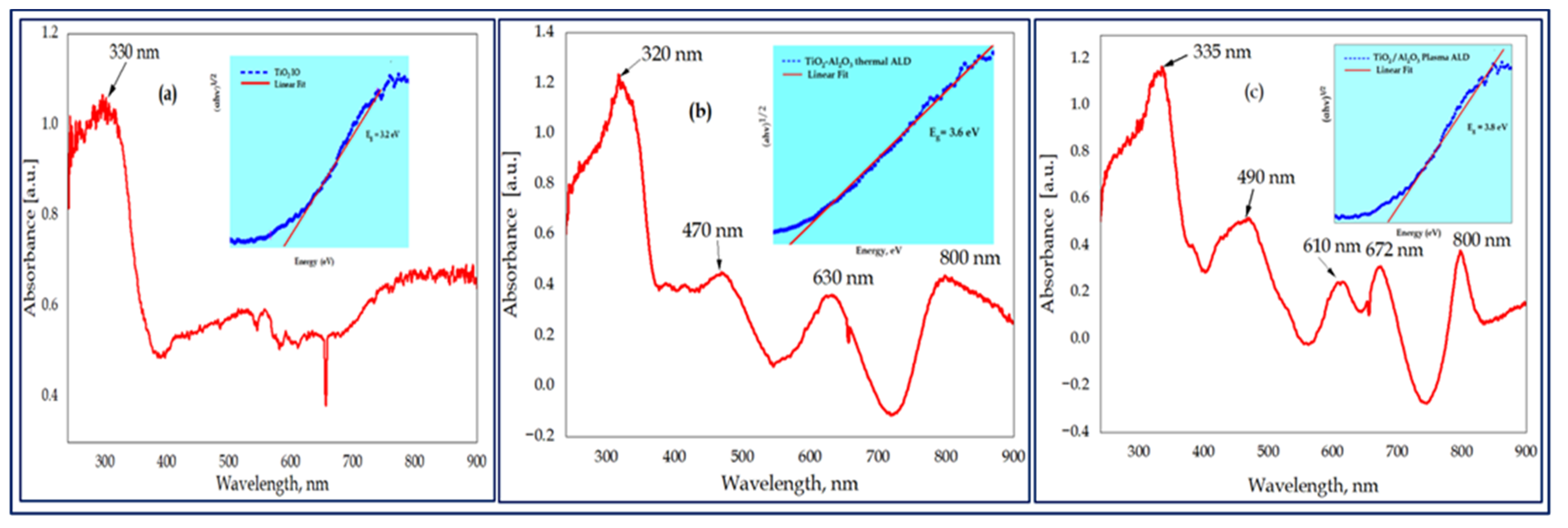
3.4. Optical Analysis
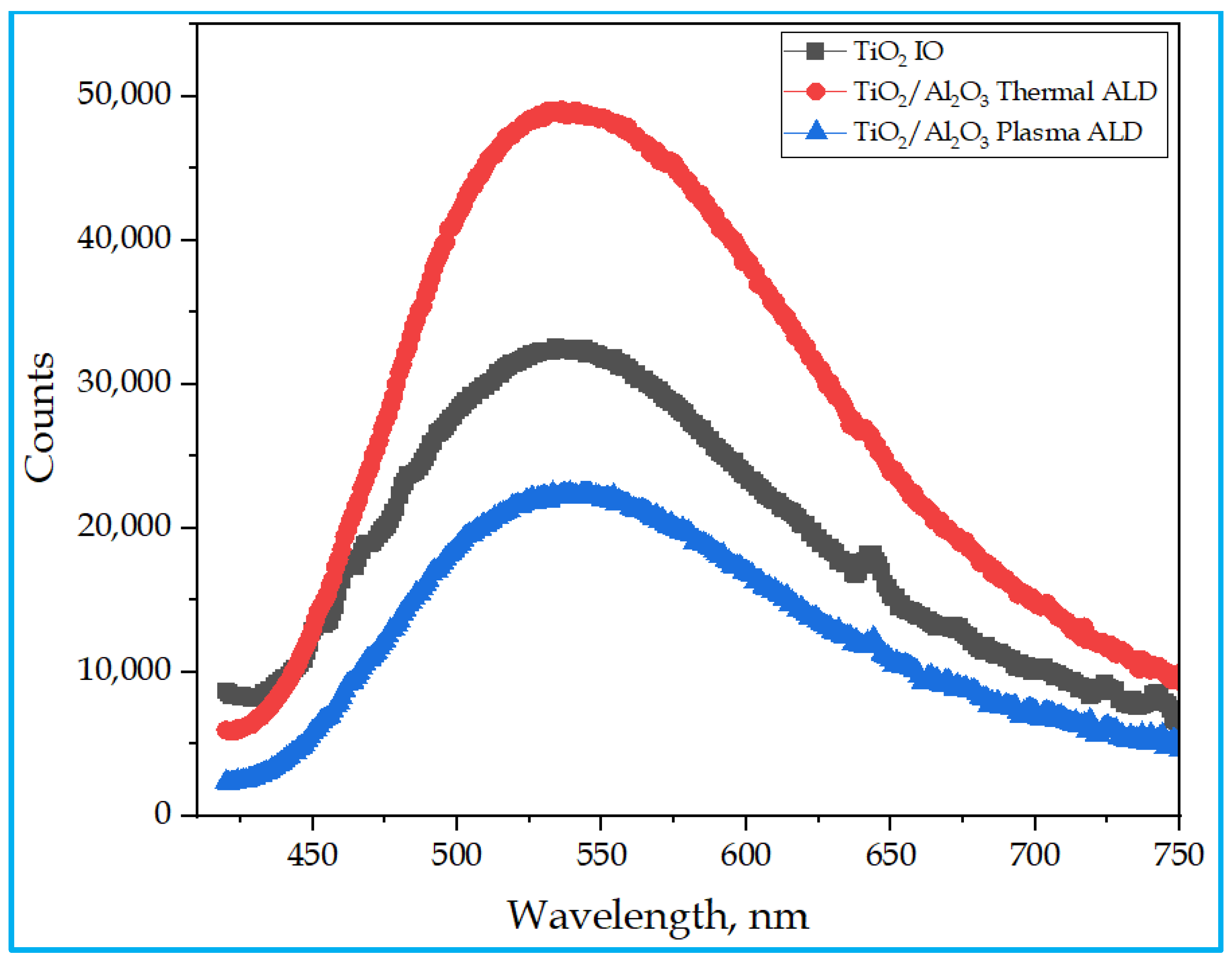
3.5. Photoluminescence (PL) Studies
3.6. Photocatalytic Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Rasheed, T.; Mehmood, S.; Tang, H.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Mitigation of Environmentally-Related Hazardous Pollutants from Water Matrices Using Nanostructured Materials—A Review. Chemosphere 2020, 253, 126770. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Review of Recent Occurrences, Sources, Fate and Effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of Advanced Materials in Sonophotocatalytic Processes for the Remediation of Environmental Pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Gao, J.; Tian, W.; Zhang, H.; Wang, S. Engineered Inverse Opal Structured Semiconductors for Solar Light-Driven Environmental Catalysis. Nanoscale 2022, 14, 14341–14367. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Temerov, F.; Saarinen, J.J. Multicompound Inverse Opal Structures with Gold Nanoparticles for Visible Light Photocatalytic Activity. Mater. Des. 2020, 194, 108886. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Taveira, R.J.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Optical Band Gaps of Organic Semiconductor Materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Xu, X. Photon Localization in Amorphous Photonic Crystal. Appl. Phys. B Lasers Opt. 2007, 86, 467–471. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Yuan, X.; Wang, Y.; Li, F. Enhanced Visible-Light Photocatalytic Activity of a TiO2 Hydrosol Assisted by H2O2: Surface Complexation and Kinetic Modeling. J. Mol. Catal. A Chem. 2016, 414, 122–129. [Google Scholar] [CrossRef]
- Notomi, M. Negative Refractive Optics in Photonic Crystals. Phys. Simul. Optoelectron. Devices IX 2001, 4283, 428. [Google Scholar] [CrossRef]
- Baba, T. Slow Light in Photonic Crystals. Nat. Photonics 2008, 2, 465–473. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. TiO2 Inverse Opal Photonic Crystals: Synthesis, Modification, and Applications—A Review. J. Alloys Compd. 2018, 769, 740–757. [Google Scholar] [CrossRef]
- Kosaka, H.; Kawashima, T. Superprism Phenomena in Photonic Crystals. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 58, R10096–R10099. [Google Scholar] [CrossRef]
- Meng, S.; Li, D.; Zheng, X.; Wang, J.; Chen, J.; Fang, J.; Shao, Y.; Fu, X. ZnO Photonic Crystals with Enhanced Photocatalytic Activity and Photostability. J. Mater. Chem. A 2013, 1, 2744–2747. [Google Scholar] [CrossRef]
- Zhang, X.; John, S. Enhanced Photocatalysis by Light-Trapping Optimization in Inverse Opals. J. Mater. Chem. A 2020, 8, 18974–18986. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.H.; Jang, K.; Lee, S.; Kim, J.H.; Yoon, C.S.; Kim, J.; Kim, D.H.; Ahn, H. Periodically Ordered Inverse Opal TiO2/Polyaniline Core/Shell Design for Electrochemical Energy Storage Applications. J. Alloys Compd. 2017, 694, 111–118. [Google Scholar] [CrossRef]
- Armstrong, E.; O’Dwyer, C. Artificial Opal Photonic Crystals and Inverse Opal Structures-Fundamentals and Applications from Optics to Energy Storage. J. Mater. Chem. C 2015, 3, 6109–6143. [Google Scholar] [CrossRef]
- Justin Kunene, T.; Kwanda Tartibu, L.; Ukoba, K.; Jen, T.C. Review of Atomic Layer Deposition Process, Application and Modeling Tools. Mater. Today Proc. 2022, 62, S95–S109. [Google Scholar] [CrossRef]
- Fathi, F.; Monirinasab, H.; Ranjbary, F.; Nejati-Koshki, K. Inverse Opal Photonic Crystals: Recent Advances in Fabrication Methods and Biological Applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103377. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Katwal, R.; Kothari, R.; Pathania, D. An Overview on Degradation Kinetics of Organic Dyes by Photocatalysis Using Nanostructured Electrocatalyst; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 195–213. ISBN 9780128218419. [Google Scholar] [CrossRef]
- Von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up Assembly of Photonic Crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of Polystyrene Spheres in Different Particle Sizes and Assembly of the PS Colloidal Crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Subban, C.V.; Smith, I.C.; Disalvo, F.J. Interconversion of Inverse Opals of Electrically Conducting Doped Titanium Oxides and Nitrides. Small 2012, 8, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Cai, J.; Sun, Q.; Meng, X. Novel Nanostructured Materials by Atomic and Molecular Layer Deposition. AIMS Mater. Sci. 2018, 5, 957–999. [Google Scholar] [CrossRef]
- Eswar, N.K.R.; Singh, S.A.; Heo, J. Atomic Layer Deposited Photocatalysts: Comprehensive Review on Viable Fabrication Routes and Reactor Design Approaches for Photo-Mediated Redox Reactions. J. Mater. Chem. A 2019, 7, 17703–17734. [Google Scholar] [CrossRef]
- Inui, C.; Tsuge, Y.; Kura, H.; Fujihara, S.; Shiratori, S.; Sato, T. Preparation of One-Dimensional Photonic Crystals by Sol-Gel Process for Magneto-Optical Materials. Thin Solid Films 2008, 516, 2454–2459. [Google Scholar] [CrossRef]
- Galusha, J.W.; Tsung, C.K.; Stucky, G.D.; Bartl, M.H. Optimizing Sol-Gel Infiltration and Processing Methods for the Fabrication of High-Quality Planar Titania Inverse Opals. Chem. Mater. 2008, 20, 4925–4930. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Chen, X.Y.; Leung, Y.H. Recent Progress in Hydrothermal Synthesis of Zinc Oxide Nanomaterials. Recent Pat. Nanotechnol. 2012, 6, 124–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, H.; Cai, Y.; Jurado-Sánchez, B.; Dong, R. Recent Advances in One-Dimensional Micro/Nanomotors: Fabrication, Propulsion and Application. Nano-Micro Lett. 2023, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Knoops, H.C.M.; Potts, S.E.; Bol, A.A.; Kessels, W.M.M. Atomic Layer Deposition, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 3, ISBN 9780444633057. [Google Scholar]
- Justh, N.; Bakos, L.P.; Hernádi, K.; Kiss, G.; Réti, B.; Erdélyi, Z.; Parditka, B.; Szilágyi, I.M. Photocatalytic Hollow TiO2 and ZnO Nanospheres Prepared by Atomic Layer Deposition. Sci. Rep. 2017, 7, 4337. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Ma, W.; Li, R.; Li, Y.; Du, H. Preparation and Photocatalytic Properties of TiO2-Al2O3 Composite Loaded Catalysts. J. Chem. 2015, 2015, 806568. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Zi, T.Q.; Zhao, X.R.; Liu, C.; Ren, Q.; Fang, J.-B.; Li, W.M.; Li, A.D. Enhanced Visible Light Photocatalytic Activity of Fe2O3 Modified TiO2 Prepared by Atomic Layer Deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-mayouf, A.; Adil, S.F. Editorial Article Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Halim, W.; Moumen, A.; Ouaskit, S.; Errien, N. Photocatalytic Properties of TiO2/ZnO Thin Film. Mol. Cryst. Liq. Cryst. 2016, 627, 49–55. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Warwick, M.E.A.; Kaunisto, K.; Gasparotto, A.; Gombac, V.; Sada, C.; Turner, S.; Van Tendeloo, G.; Maccato, C.; et al. Fe2O3-TiO2 Nanosystems by a Hybrid PE-CVD/ALD Approach: Controllable Synthesis, Growth Mechanism, and Photocatalytic Properties. CrystEngComm 2015, 17, 6219–6226. [Google Scholar] [CrossRef]
- Karunakaran, C.; Magesan, P.; Gomathisankar, P.; Vinayagamoorthy, P. Photocatalytic Degradation of Dyes by Al2O3-TiO2 and ZrO2-TiO2 Nanocomposites. Mater. Sci. Forum 2013, 734, 325–333. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, G.; Liu, H.; Han, S.; Chen, S.; Kong, Q.; Feng, W. One-Step Hydrothermal Synthesis and Characterization of Cu-Doped TiO2 Nanoparticles/Nanobucks/Nanorods with Enhanced Photocatalytic Performance under Simulated Solar Light. J. Mater. Sci. Mater. Electron. 2019, 30, 13826–13834. [Google Scholar] [CrossRef]
- Lei, J.; Chen, B.; Lv, W.; Zhou, L.; Wang, L.; Liu, Y.; Zhang, J. An Inverse Opal TiO2/g-C3N4 Composite with a Heterojunction for Enhanced Visible Light-Driven Photocatalytic Activity. Dalt. Trans. 2019, 48, 3486–3495. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, X.; Yuan, X.; Wei, W.; Xiao, Y.; Cao, S. Constructing TiO2@Bi2O3 Multi-Heterojunction Hollow Structure for Enhanced Visible-Light Photocatalytic Performance. J. Clean. Prod. 2022, 375, 134112. [Google Scholar] [CrossRef]
- Liu, J.; Sun, C.; Fu, M.; Long, J.; He, D.; Wang, Y. Enhanced Photochemical Catalysis of TiO2 Inverse Opals by Modification with ZnO or Fe2O3 using ALD and the Hydrothermal Method. Mater. Res. Express 2018, 5, 025509. [Google Scholar] [CrossRef]
- Kinnunen, S.; Arstila, K.; Sajavaara, T. Al2O3 ALD Films Grown Using TMA + rare Isotope 2H216O and 1H218O Precursors. Appl. Surf. Sci. 2021, 546, 2–8. [Google Scholar] [CrossRef]
- Guo, J.; van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; van Ommen, J.R. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Sridharan, K.; Park, Y.M.; Park, T.J. Eliminated Phototoxicity of TiO2 Particles by an Atomic-Layer-Deposited Al2O3 Coating Layer for UV-Protection Applications. Chem. Eur. J. 2016, 22, 12022–12026. [Google Scholar] [CrossRef]
- Shenouda, S.S.; Hussien, M.S.A.; Parditka, B.; Csík, A.; Takats, V.; Erdélyi, Z. Novel Amorphous Al-Rich Al2O3 Ultra-Thin Films as Active Photocatalysts for Water Treatment from Some Textile Dyes. Ceram. Int. 2020, 46, 7922–7929. [Google Scholar] [CrossRef]
- Worzakowska, M. Thermal and Mechanical Properties of Polystyrene Modified with Esters Derivatives of 3-Phenylprop-2-En-1-Ol. J. Therm. Anal. Calorim. 2015, 121, 235–243. [Google Scholar] [CrossRef]
- Manikandan Nair, K.C.; Thomas, S.; Groeninckx, G. Thermal and Dynamic Mechanical Analysis of Polystyrene Composites Reinforced with Short Sisal Fibres. Compos. Sci. Technol. 2001, 61, 2519–2529. [Google Scholar] [CrossRef]
- Yew, R.; Karuturi, S.K.; Liu, J.; Tan, H.H.; Wu, Y.; Jagadish, C. Exploiting Defects in TiO2 Inverse Opal for Enhanced Photoelectrochemical Water Splitting. Opt. Express 2019, 27, 761. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Dey, A.; Mukhopadhyay, A.K. Loading Rate Effect on Nanohardness of Soda-Lime-Silica Glass. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 1301–1312. [Google Scholar] [CrossRef]
- Šćepanović, M.J.; Grujič-Brojčin, M.U.; Dohčević-Mitrović, Z.D.; Popović, Z.V. Effects of Confinement, Strain and Nonstoichiometry on Raman Spectra of Anatase TiO2 Nanopowders. Mater. Sci. Forum 2006, 518, 101–106. [Google Scholar] [CrossRef]
- Ginter, J.; Piwoński, I. Evaluation of the Synergistic Effect between Slow Photons and Electron Trapping on the Photocatalytic Properties of TiO2 Photonic Crystals. Mater. Res. Bull. 2018, 107, 100–110. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic Crystal-Assisted Visible Light Activated TiO2 Photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Greenwood, D.A. Amorphous and Liquid Semiconductors. Opt. Acta Int. J. Opt. 1973, 17, 952. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical Properties of Amorphous Al2O3 Thin Films Prepared by a Sol-Gel Process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Shi, S.; Qian, S.; Hou, X.; Mu, J.; He, J.; Chou, X. Structural and Optical Properties of Amorphous Al2O3 Thin Film Deposited by Atomic Layer Deposition. Adv. Condens. Matter Phys. 2018, 2018, 7598978. [Google Scholar] [CrossRef]
- Costina, I.; Franchy, R. Band Gap of Amorphous and Well-Ordered Al2O3 on Ni3Al(100). Appl. Phys. Lett. 2001, 78, 4139–4141. [Google Scholar] [CrossRef]
- Jin, C.; Liu, B.; Lei, Z.; Sun, J. Structure and Photoluminescence of the TiO2 Films Grown by Atomic Layer Deposition Using Tetrakis-Dimethylamino Titanium and Ozone. Nanoscale Res. Lett. 2015, 10, 95. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Narayanan, S.; Kennedy, L.J.; Vijaya, J.J. Pure and Mg-Doped Self-Assembled ZnO Nano-Particles for the Enhanced Photocatalytic Degradation of 4-Chlorophenol. J. Environ. Sci. 2013, 25, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Atomic Layer Deposition-Confined Nonstoichiometric TiO2 Nanocrystals with Tunneling Effects for Solar Driven Hydrogen Evolution. J. Phys. Chem. Lett. 2016, 7, 1173–1179. [Google Scholar] [CrossRef] [PubMed]





| Sample | Deposited Oxides | Temp. °C | No. of ALD Cycles | Pulse/Purge Time (s) for 1 Cycle | Thickness (nm) | Pressure (mbar) | |
|---|---|---|---|---|---|---|---|
| (Chamber) | (Reactor) | ||||||
| TiO2 | TiO2 | 52.9 | 671 | 0.3 s TiCl4-3 s N2/0.3 s H2O-3 s N2 | 51.6 | 6.8 | 1.3 |
| TiO2/Al2O3 Thermal | Al2O3 | 52.9 | 7 | 0.15 s TMA-0.5 s N2/0.15 s H2O-0.5 s N2 | 5 | 6.8 | 1.4 |
| TiO2/Al2O3 Plasma | Al2O3 | 52.9 | 9 | 0.15 s TMA-2 s N2/2 s O2-N2 Plasma/2 s N2 | 5 | 7.0 | 1.4 |
| Sample | % w/w Ti | % w/w Al | % w/w O | % w/w C | % w/w Si | % w/w Ca | % w/w Cl | % w/w Na |
|---|---|---|---|---|---|---|---|---|
| TiO2 | 23.4 | - | 64.0 | 3.2 | 8.0 | - | - | 1.4 |
| TiO2/Al2O3 Thermal | 18.1 | 2.6 | 64.3 | 3.8 | 7.9 | 1.4 | 0.3 | 1.6 |
| TiO2/Al2O3 Plasma | 19.0 | 2.5 | 65.6 | 2.8 | 7.8 | 1.4 | 0.3 | 1.6 |
| Sample | R2 | Rate Constant, K (×10−2 min−1) |
|---|---|---|
| TiO2 | 0.9957 | 0.24 |
| TiO2/Al2O3 Thermal | 0.9981 | 0.16 |
| TiO2/Al2O3 Plasma | 0.9932 | 0.089 |
| MB | 0.994 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemago, H.H.; Addin, F.S.; Kárajz, D.A.; Igricz, T.; Parditka, B.; Erdélyi, Z.; Hessz, D.; Szilágyi, I.M. Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications. Nanomaterials 2023, 13, 1314. https://doi.org/10.3390/nano13081314
Lemago HH, Addin FS, Kárajz DA, Igricz T, Parditka B, Erdélyi Z, Hessz D, Szilágyi IM. Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications. Nanomaterials. 2023; 13(8):1314. https://doi.org/10.3390/nano13081314
Chicago/Turabian StyleLemago, Hamsasew Hankebo, Feras Shugaa Addin, Dániel Atilla Kárajz, Tamás Igricz, Bence Parditka, Zoltán Erdélyi, Dóra Hessz, and Imre Miklós Szilágyi. 2023. "Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications" Nanomaterials 13, no. 8: 1314. https://doi.org/10.3390/nano13081314
APA StyleLemago, H. H., Addin, F. S., Kárajz, D. A., Igricz, T., Parditka, B., Erdélyi, Z., Hessz, D., & Szilágyi, I. M. (2023). Synthesis of TiO2/Al2O3 Double-Layer Inverse Opal by Thermal and Plasma-Assisted Atomic Layer Deposition for Photocatalytic Applications. Nanomaterials, 13(8), 1314. https://doi.org/10.3390/nano13081314








