Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests
Abstract
1. Introduction
2. Infectious Diseases
2.1. Viral Infections
2.2. Bacterial Infections
2.3. Parasite-Caused Infections
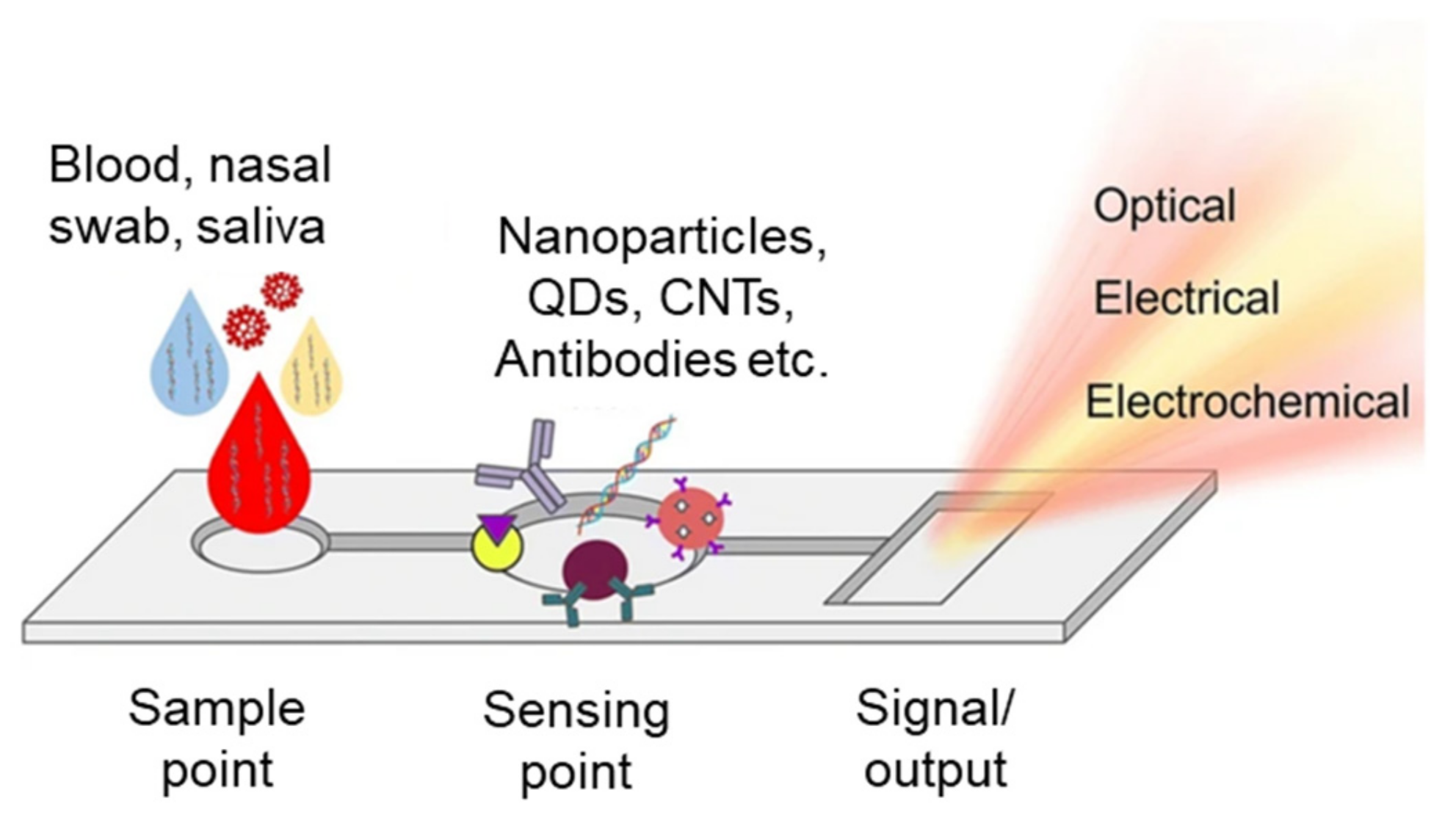
3. Point-of-Care (POCT) Assays for Infectious Diseases
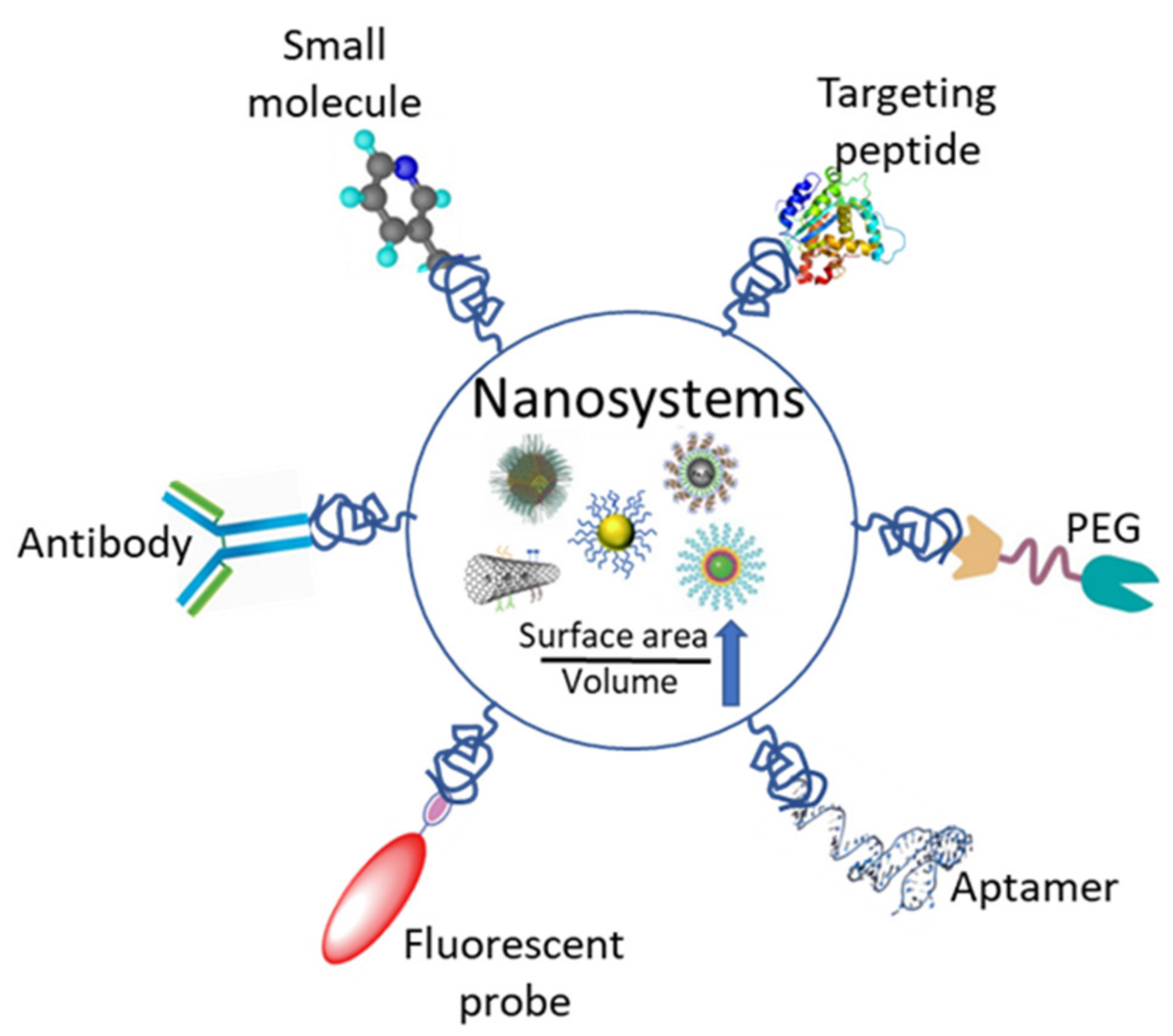
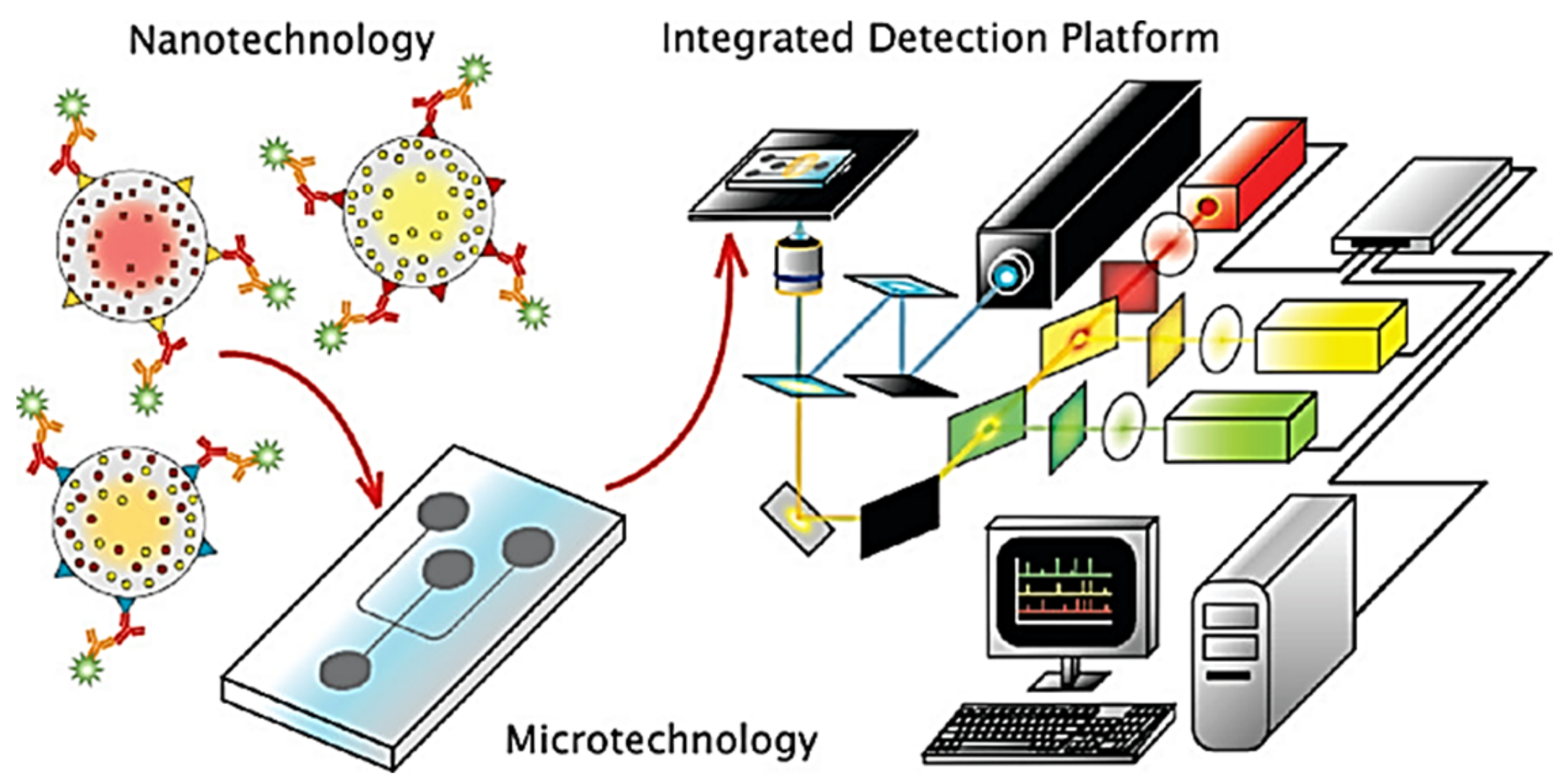
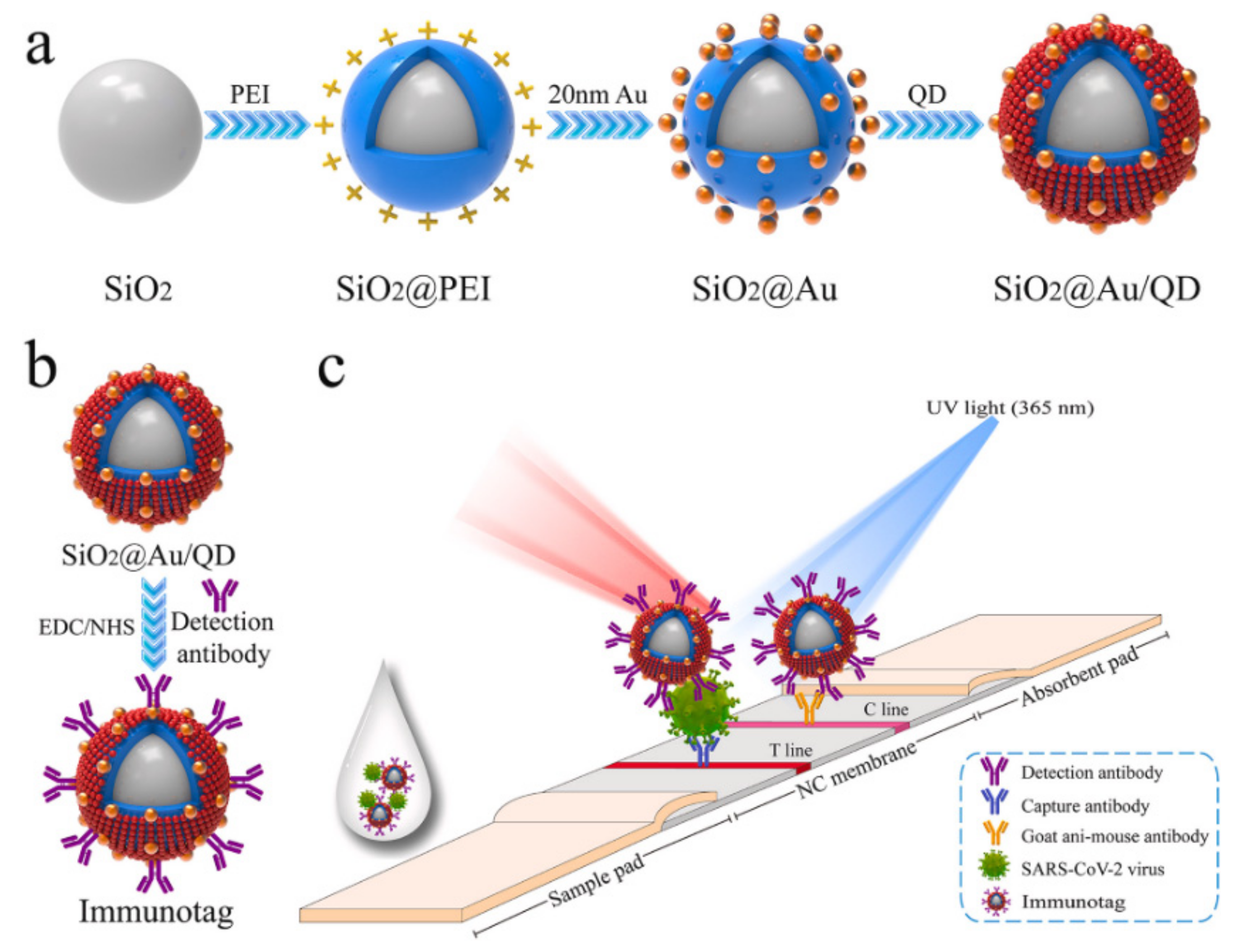
3.1. Nanoparticles
3.2. Microfluidic Devices
3.2.1. Lab-on-Chip (LOC)
3.2.2. Laboratory on a Cartridge Chip (LOCC)
3.3. Lateral Flow Immunoassay Chips
4. Non-Infectious Diseases
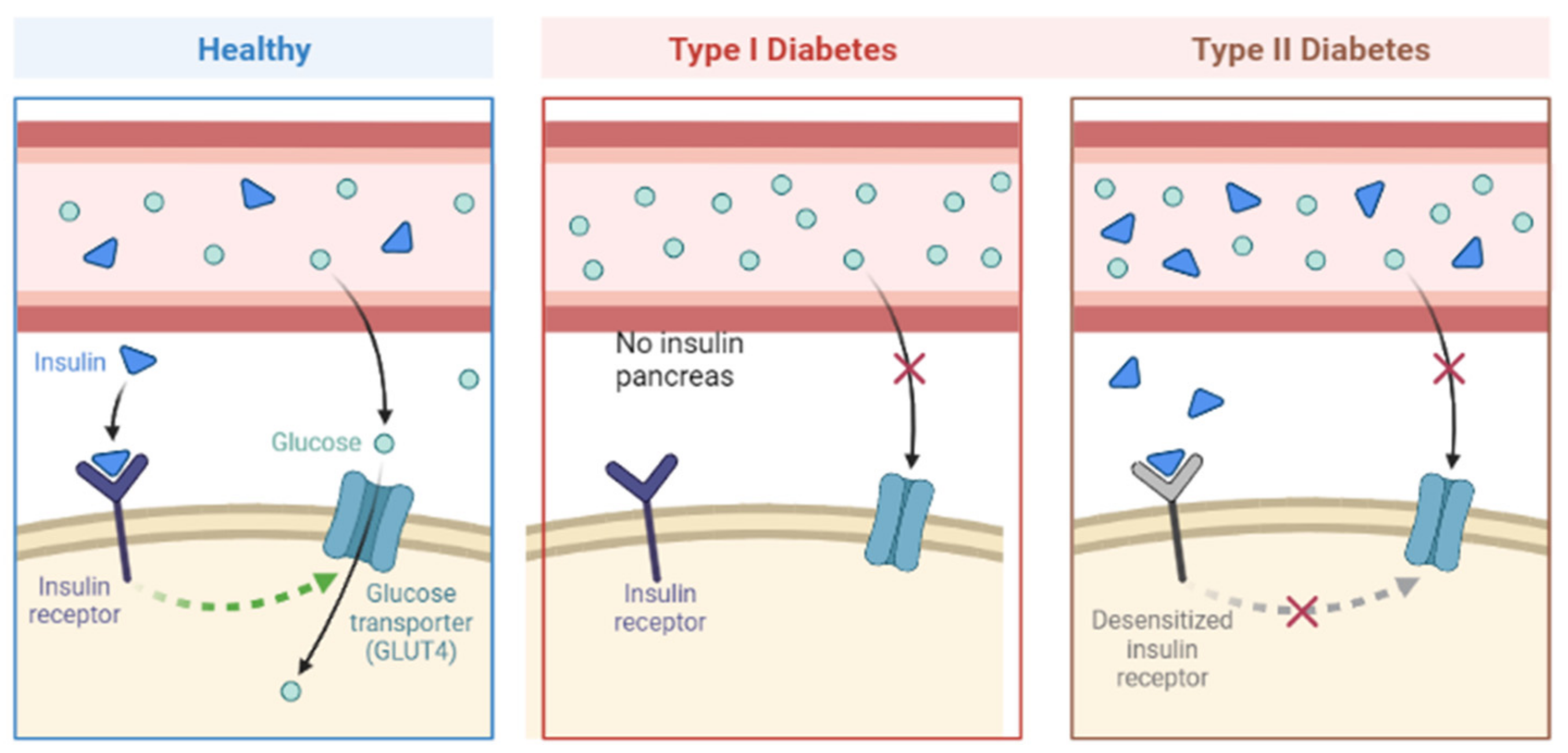
4.1. Diabetes Mellitus
4.2. Point-of-Care Testing Assays
4.3. Nanomaterials
4.4. Wearable Devices
5. Emerging Trends and Future Outlooks
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Yu, L.; Kong, X.; Sun, L. Application of nanodiagnostics in point-of-care tests for infectious diseases. Int. J. Nanomed. 2017, 12, 4789–4803. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Ikbal, A.; Sahu, R.; Bhattacharjee, B.; Paul, T.; Deka, B.; Fattepur, S.; Widyowati, R.; Vijaya, J.; Al Mohaini, M.; et al. Nanotechnology Integration for SARS-CoV-2 Diagnosis and Treatment: An Approach to Preventing Pandemic. Nanomaterials 2021, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Dukle, A.; Nathanael, A.J.; Panchapakesan, B.; Oh, T.-H. Role of Paper-Based Sensors in Fight against Cancer for the Developing World. Biosensors 2022, 12, 737. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Park, J.; Le, H.T.N.; Santhosh, M.; Kadam, A.N.; Cho, S. Recent advances in microfluidic paper-based electrochemiluminescence analytical devices for point-of-care testing applications. Biosens. Bioelectron. 2019, 126, 68–81. [Google Scholar] [CrossRef]
- Liu, W.; Yue, F.; Lee, L.P. Integrated Point-of-Care Molecular Diagnostic Devices for Infectious Diseases. Acc. Chem. Res. 2021, 54, 4107–4119. [Google Scholar] [CrossRef]
- Nanotechnology for infectious diseases. Nat. Nanotechnol. 2021, 16, 1. [CrossRef]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for Diagnosis and Treatment of Infectious Diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef]
- Cassedy, A.; Parle-McDermott, A.; O’Kennedy, R. Virus Detection: A Review of the Current and Emerging Molecular and Immunological Methods. Front. Mol. Biosci. 2021, 8, 76. [Google Scholar] [CrossRef]
- Rudolph, D.L.; Sullivan, V.; Owen, S.M.; Curtis, K.A. Detection of Acute HIV-1 Infection by RT-LAMP. PLoS ONE 2015, 10, e0126609. [Google Scholar] [CrossRef]
- Kim, S.-K.; Oh, Y.-H.; Ko, D.-H.; Sung, H.; Oh, H.-B.; Hwang, S.-H. Nanoparticle-Based Visual Detection of Amplified DNA for Diagnosis of Hepatitis C Virus. Biosensors 2022, 12, 744. [Google Scholar] [CrossRef]
- Huang, W.E.; Lim, B.; Hsu, C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef]
- Park, M.; Won, J.; Choi, B.Y.; Lee, C.J. Optimization of primer sets and detection protocols for SARS-CoV-2 of coronavirus disease 2019 (COVID-19) using PCR and real-time PCR. Exp. Mol. Med. 2020, 52, 963–977. [Google Scholar] [CrossRef]
- Kim, H.; Chung, D.-R.; Kang, M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst 2019, 144, 2460–2466. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Saei, A.A.; Walker, E.D.; Conley, B.; Omidi, Y.; Lee, K.; Mahmoudi, M. Nanotechnology for Targeted Detection and Removal of Bacteria: Opportunities and Challenges. Adv. Sci. 2021, 8, 2100556. [Google Scholar] [CrossRef]
- Zumla, A.; Al-Tawfiq, J.A.; Enne, V.I.; Kidd, M.; Drosten, C.; Breuer, J.; Muller, M.A.; Hui, D.; Maeurer, M.; Bates, M.; et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Dube, D.; Agrawal, G.P.; Vyas, S.P. Tuberculosis: From molecular pathogenesis to effective drug carrier design. Drug Discov. Today 2012, 17, 760–773. [Google Scholar] [CrossRef]
- Sudha, S. Tuberculosis diagnosis—An overview to the conventional diagnostic methodology and need for nanodiagnosis. Int. J. Med. Eng. Inform. 2016, 8, 27. [Google Scholar] [CrossRef]
- Oyegoke, O.O.; Maharaj, L.; Akoniyon, O.P.; Kwoji, I.; Roux, A.T.; Adewumi, T.S.; Maharaj, R.; Oyebola, B.T.; Adeleke, M.A.; Okpeku, M. Malaria diagnostic methods with the elimination goal in view. Parasitol. Res. 2022, 121, 1867–1885. [Google Scholar] [CrossRef]
- Berzosa, P.; De Lucio, A.; Romay-Barja, M.; Herrador, Z.; González, V.; García, L.; Fernández-Martínez, A.; Santana-Morales, M.; Ncogo, P.; Valladares, B.; et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea 11 Medical and Health Sciences 1108 Medical Microbiology. Malar. J. 2018, 17, 333. [Google Scholar] [CrossRef]
- Nichols, J.H. Point-of-care testing. In Contemporary Practice in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 323–336. [Google Scholar] [CrossRef]
- Noah, N.M.; Ndangili, P.M. Current Trends of Nanobiosensors for Point-of-Care Diagnostics. J. Anal. Methods Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Colino, C.I.; Millán, C.G.; Lanao, J.M. Nanoparticles for Signaling in Biodiagnosis and Treatment of Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1627. [Google Scholar] [CrossRef] [PubMed]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated Polymer Nanoparticles for Bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Abbas, A.; Hao, Y.; Malik, A.H.; Tian, X.; Song, H.; Gao, R. Conjugated polymer nanoparticles and their nanohybrids as smart photoluminescent and photoresponsive material for biosensing, imaging, and theranostics. Microchim. Acta 2022, 189, 83. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Zhang, X.; Xing, S.; Lu, Q.; Yao, J.-S.; Liu, Q.-Z.; Qiao, C.-D.; Xie, R.-X.; Ding, B. Conjugated Polymer Nanoparticles Based on Carbazole for Detecting Ferric Ion (III) with a Large Stokes Shift and High Sensitivity and the Application in Cell; Elsevier: Amsterdam, The Netherlands, 2019; Available online: https://www.sciencedirect.com/science/article/pii/S0143720819300919 (accessed on 24 March 2023).
- Jin, W.; Park, D.-H. Functional Layered Double Hydroxide Nanohybrids for Biomedical Imaging. Nanomaterials 2019, 9, 1404. [Google Scholar] [CrossRef]
- Luciano, K.; Wang, X.; Liu, Y.; Eyler, G.; Qin, Z.; Xia, X. Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts. Bioengineering 2022, 9, 666. [Google Scholar] [CrossRef]
- Jackson, T.C.; Patani, B.O.; Ekpa, D.E. Nanotechnology in Diagnosis: A Review. Adv. Nanoparticles 2017, 06, 93–102. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Omidifar, N.; Lai, C.W.; Rao, N.V.; Gholami, A.; Chiang, W.-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals 2022, 15, 880. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Xiang, Q.; Farcas, G.A.; Lee, J.A.; Rhee, A.; Lafferty, E.I.; Perrault, S.D.; Kain, K.C.; Chan, W.C.W. Convergence of Quantum Dot Barcodes with Microfluidics and Signal Processing for Multiplexed High-Throughput Infectious Disease Diagnostics. Nano Lett. 2007, 7, 2812–2818. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Chen, J.; Han, H.; Ma, W. Simple and Sensitive Detection of HBsAg by Using a Quantum Dots Nanobeads Based Dot-Blot Immunoassay. Theranostics 2014, 4, 307–315. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Bardhan, N.M.; Jansen, P.; Belcher, A.M. Graphene, Carbon Nanotube and Plasmonic Nanosensors for Detection of Viral Pathogens: Opportunities for Rapid Testing in Pandemics like COVID-19. Front. Nanotechnol. 2021, 3, 733126. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Carbon nanotubes in COVID-19: A critical review and prospects. Colloid Interface Sci. Commun. 2022, 46, 100544. [Google Scholar] [CrossRef]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.C.; Cheng, Y.W.; Sun, H.Y.; et al. Rapid SARS-CoV-2 Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. medRxiv 2020. [Google Scholar] [CrossRef]
- He, Y.; Hu, C.; Li, Z.; Wu, C.; Zeng, Y.; Peng, C. Multifunctional carbon nanomaterials for diagnostic applications in infectious diseases and tumors. Mater. Today Bio 2022, 14, 100231. [Google Scholar] [CrossRef]
- Jain, S. Development of an Antibody Functionalized Carbon Nanotube Biosensor for Foodborne Bacterial Pathogens. J. Biosens. Bioelectron. 2012, 11, 2. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A. Microfluidics: Recent Advances Toward Lab-on-Chip Applications in Bioanalysis. Adv. Eng. Mater. 2021, 24, 2100738. [Google Scholar] [CrossRef]
- Wang, X.; Yi, L.; Mukhitov, N.; Schrell, A.M.; Dhumpa, R.; Roper, M.G. Microfluidics-to-Mass Spectrometry: A Review of Coupling Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2015; Available online: https://www.sciencedirect.com/science/article/pii/S0021967314016112 (accessed on 16 February 2023).
- Li, X.; Fan, X.; Li, Z.; Shi, L.; Liu, J.; Luo, H.; Wang, L.; Du, X.; Chen, W.; Guo, J.; et al. Application of Microfluidics in Drug Development from Traditional Medicine. Biosensors 2022, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Volpetti, F.; Garcia-Cordero, J.L.; Maerkl, S.J. A Microfluidic Platform for High-Throughput Multiplexed Protein Quantitation. PLoS ONE 2015, 10, e0117744. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, O. XXIX. An experimental investigation of the circumstances which determine whether the motion of water shall be direct or sinuous, and of the law of resistance in parallel channels. Philos. Trans. R. Soc. Lond. 1883, 174, 935–982. [Google Scholar] [CrossRef]
- Pumera, M. Nanomaterials meet microfluidics. Chem. Commun. 2011, 47, 5671–5680. [Google Scholar] [CrossRef]
- Mauk, M.G.; Chiou, R.; Carr, M.E.; Gillander, J.B.; Newton, J.C.; Reid, K.R. Point-of-Care Medical Tests Devices and Their Value as Educational Projects for Engineering Students. 2014. Available online: https://peer.asee.org/point-of-care-medical-tests-devices-and-their-value-as-educational-projects-for-engineering-students (accessed on 17 February 2023).
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Mejia-Salazar, J.R.; Rodrigues Cruz, K.; Materon Vasques, E.M.; Novais de Oliveira, O., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for Ehealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Reis, N.M. A critical insight into the development pipeline of microfluidic immunoassay devices for the sensitive quantitation of protein biomarkers at the point of care. Analyst 2017, 142, 858–882. [Google Scholar] [CrossRef]
- Duffy, D.C.; Gillis, H.L.; Lin, J.; Sheppard, N.F.; Kellogg, G.J. Microfabricated Centrifugal Microfluidic Systems: Characterization and Multiple Enzymatic Assays. Anal. Chem. 1999, 71, 4669–4678. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yeo, W.-H.; Shu, Z.; Soelberg, S.D.; Inoue, S.; Kalyanasundaram, D.; Ludwig, J.; Furlong, C.E.; Riley, J.J.; Weigel, K.M.; et al. Immunosensor towards low-cost, rapid diagnosis of tuberculosis. Lab Chip 2012, 12, 1437–1440. [Google Scholar] [CrossRef]
- Kim, J.-H. Micro- and Nanostructured Immuno-Tip Sensors for Point-of-Care Diagnosis of Tuberculosis. July 2013. Available online: https://digital.lib.washington.edu:443/researchworks/handle/1773/23556 (accessed on 17 February 2023).
- Sun, F.; Ganguli, A.; Nguyen, J.; Brisbin, R.; Shanmugam, K.; Hirschberg, D.L.; Cunningham, B.T. Smartphone-based multiplex 30-minute nucleic acid test of live virus from nasal swab extract. Lab Chip 2020, 20, 1621–1627. [Google Scholar] [CrossRef]
- Kang, B.-H.; Lee, Y.; Yu, E.-S.; Na, H.; Kang, M.; Huh, H.J.; Jeong, K.-H. Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics. ACS Nano 2021, 15, 10194–10202. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Biji, P.; Panthalingal, M.K.; Krishna, C.M.; Rajkumar, S.; Joshi, D.S.; Sundaram, N. Development of integrated microfluidic platform coupled with Surface-enhanced Raman Spectroscopy for diagnosis of COVID-19. Med. Hypotheses 2021, 146, 110356. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Guo, Y. Recent Trends in Nanomaterial-Based Biosensors for Point-of-Care Testing. Front. Chem. 2020, 8, 586702. [Google Scholar] [CrossRef]
- Wang, D.; He, S.; Wang, X.; Yan, Y.; Liu, J.; Wu, S.; Liu, S.; Lei, Y.; Chen, M.; Li, L.; et al. Rapid lateral flow immunoassay for the fluorescence detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1150–1158. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Liu, Z.; Zhao, B.; Xiao, R. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens. Actuators B Chem. 2022, 351, 130897. [Google Scholar] [CrossRef]
- Noninfectious Diseases—Biology LibreTexts. Available online: https://bio.libretexts.org/Bookshelves/Human_Biology/Book%3A_Human_Biology_(Wakim_and_Grewal)/21%3A_Disease/21.6%3A_Noninfectious_Diseases (accessed on 22 February 2023).
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 February 2023).
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Ji, W.; Yao, H.; Lin, L.; Cui, H.; Santos, H.A.; Pan, G. Emerging Theranostic Nanomaterials in Diabetes and Its Complications. Adv. Sci. 2021, 9, 2102466. [Google Scholar] [CrossRef]
- Young, E. Non-invasive glucose monitoring for diabetes: Five strategies under development. Pharm. J. 2017, 299. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Yu, Q.; Ye, L.; Yang, L.; Cui, Y. Review of point-of-care platforms for diabetes: (1) sensing. Sens. Actuators Rep. 2022, 4, 100113. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-Invasive Diabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2019, 7, 5963–5974. [Google Scholar] [CrossRef]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-Oxide Nanomaterials Synthesis and Applications in Flexible and Wearable Sensors. ACS Nanosci. Au 2022, 2, 64–92. [Google Scholar] [CrossRef]
- Singh, S.U.; Chatterjee, S.; Lone, S.A.; Ho, H.-H.; Kaswan, K.; Peringeth, K.; Khan, A.; Chiang, Y.-W.; Lee, S.; Lin, Z.-H. Advanced wearable biosensors for the detection of body fluids and exhaled breath by graphene. Microchim. Acta 2022, 189, 236. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Li, G.; Wen, D. Sensing nanomaterials of wearable glucose sensors. Chin. Chem. Lett. 2020, 32, 221–228. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.-E. Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-Body Sweat Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wu, F.; Cao, X.; Li, Z.; Alharbi, M.; Abbas, A.N.; Amer, M.R.; Zhou, C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano 2018, 12, 1170–1178. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, T.; Shen, L.; Ma, J.; Ma, H.; Sun, T.; Lv, F.; Zhu, N. Facet-Dependent Cu2O Electrocatalysis for Wearable Enzyme-Free Smart Sensing. ACS Catal. 2021, 11, 2949–2955. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Ng, B.Y.C.; Xiao, W.; West, N.P.; Wee, E.J.H.; Wang, Y.; Trau, M. Rapid, Single-Cell Electrochemical Detection of Mycobacterium tuberculosis Using Colloidal Gold Nanoparticles. Anal. Chem. 2015, 87, 10613–10618. [Google Scholar] [CrossRef]
- Chunduri, L.A.A.; Kurdekar, A.; Haleyurgirisetty, M.K.; Bulagonda, E.P.; Kamisetti, V.; Hewlett, I.K. Femtogram Level Sensitivity achieved by Surface Engineered Silica Nanoparticles in the Early Detection of HIV Infection. Sci. Rep. 2017, 7, 7149. [Google Scholar] [CrossRef]
- Castilho, M.D.S.; Laube, T.; Yamanaka, H.; Alegret, S.; Pividori, M.I. Magneto Immunoassays for Plasmodium falciparum Histidine-Rich Protein 2 Related to Malaria based on Magnetic Nanoparticles. Anal. Chem. 2011, 83, 5570–5577. [Google Scholar] [CrossRef]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A Critical Review of Glucose Biosensors Based on Carbon Nanomaterials: Carbon Nanotubes and Graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef]
- Feng, T.; Feng, D.; Shi, W.; Li, X.; Ma, H. A graphene oxide-peptide fluorescence sensor for proteolytically active prostate-specific antigen. Mol. Biosyst. 2012, 8, 1441–1445. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Markiewicz, K.; Wilczewska, A.; Car, H. Magnetic Nanoparticles as New Diagnostic Tools in Medicine; Elsevier: Amsterdam, The Netherlands, 2012; Available online: https://www.sciencedirect.com/science/article/pii/S1896112614600754 (accessed on 24 March 2023).
- Tang, C.; He, Z.; Liu, H.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; Chen, Z.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Falkowski, P.; Lukaszewski, Z.; Gorodkiewicz, E. Potential of surface plasmon resonance biosensors in cancer detection. J. Pharm. Biomed. Anal. 2020, 194, 113802. [Google Scholar] [CrossRef]
- Pramanik, A.; Gao, Y.; Patibandla, S.; Mitra, D.; McCandless, M.G.; Fassero, L.A.; Gates, K.; Tandon, R.; Ray, P.C. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv. 2021, 3, 1588–1596. [Google Scholar] [CrossRef]
- Xu, H.; Tang, F.; Dai, J.; Wang, C.; Zhou, X. Ultrasensitive and rapid count of Escherichia coli using magnetic nanoparticle probe under dark-field microscope. BMC Microbiol. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wang, Y.; Li, R.; Liu, S.; Yao, J.; Pei, Q.; Cui, X.; Tu, Y.; Tang, D.; Huang, J. Circular exponential amplification of photoinduced electron transfer using hairpin probes, G-quadruplex DNAzyme and silver nanocluster-labeled DNA for ultrasensitive fluorometric determination of pathogenic bacteria. Microchim. Acta 2018, 185, 168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Qi, P.; Zhang, D. DNA-templated fluorescent silver nanoclusters for sensitive detection of pathogenic bacteria based on MNP-DNAzyme-AChE complex. Sens. Actuators B Chem. 2018, 276, 42–47. [Google Scholar] [CrossRef]
- Hossein-Nejad-Ariani, H.; Kim, T.; Kaur, K. Peptide-Based Biosensor Utilizing Fluorescent Gold Nanoclusters for Detection of Listeria monocytogenes. ACS Appl. Nano Mater. 2018, 1, 3389–3397. [Google Scholar] [CrossRef]
- Santopolo, G.; Doménechdoménech-Sánchezsánchez, A.; Russell, S.M.; De La Rica, R. Ultrafast and Ultrasensitive Naked-Eye Detection of Urease-Positive Bacteria with Plasmonic Nanosensors. ACS Sens. 2019, 4, 961–967. [Google Scholar] [CrossRef]
- Alba-Patiño, A.; Russell, S.M.; Borges, M.; Pazos-Pérez, N.; Álvarez-Puebla, R.A.; de la Rica, R. Nanoparticle-based mobile biosensors for the rapid detection of sepsis biomarkers in whole blood. Nanoscale Adv. 2020, 2, 1253–1260. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Kumar, A.; Panda, U. Microfluidics-based devices and their role on point-of-care testing. In Biosensor Based Advanced Cancer Diagnostics: From Lab to Clinics; Academic Press: Cambridge, MA, USA, 2021; pp. 197–224. [Google Scholar] [CrossRef]
- Singh, J.A. Artificial Intelligence and global health: Opportunities and challenges. Emerg. Top. Life Sci. 2019, 3, 741–746. [Google Scholar] [CrossRef]

| Nanomaterial | Description | Potential Applications |
|---|---|---|
| Quantum dots |  Quantum dots are semi-conductor nanocrystals that possess excellent optical and electrical properties. Quantum dots are semi-conductor nanocrystals that possess excellent optical and electrical properties. Bright and photostable for the detection and detection of biomarkers. | Optical detection of proteins Tumor visualization |
| Carbon nanotubes |  Carbon nanotubes are made of a layer of graphene that is rolled into a cylinder. Carbon nanotubes are made of a layer of graphene that is rolled into a cylinder. Functionalized/decorated with different moieties for biomedical applications. | DNA mutations detectors Disease protein biomarker detection |
| Nanoparticles |  Metallic, polymeric, and layered double hydroxide nanoparticles. Metallic, polymeric, and layered double hydroxide nanoparticles. Properties tailored by size, shape, composition, and surface modification. | Disease protein biomarker detection Detection of DNA mutations Targeted drug delivery Imaging agents |
| Nanoshells |  Spherical nanoparticles made of a di-electric core that is capped by a thin metallic shell (gold). Nanoshells have a quasiparticle known as a plasmon. Spherical nanoparticles made of a di-electric core that is capped by a thin metallic shell (gold). Nanoshells have a quasiparticle known as a plasmon. | Tumor Imaging or Visualization |
| Type of Nanomaterial | Detection Analyte | Detection Methodology | Targeted Disease | Reference |
|---|---|---|---|---|
| Gold nanoparticles | Mycobacterium tuberculosis | Electrochemical | Tuberculosis | [79] |
| Silica nanoparticles | HIV-1 p24 antigen | Photochemical | HIV/AIDS | [80] |
| Magnetic nanoparticles | Plasmodium falciparum | Electrochemical | Malaria | [81] |
| Gold nanoparticles | SARS-CoV-2/N gene RNA | Colorimetric | COVID-19 | [82] |
| Carbon nanotubes | Glucose | Amperometric | Diabetes | [83] |
| Graphene oxide | Prostate-specific antigen | Fluorescence | Prostate cancer | [84] |
| Magnetic nanoparticles | DNA | Electrochemical | Genetic disorders | [85,86] |
| Quantum dots | Carcinoembryonic antigen | Optical | Colon cancer | [87] |
| Gold nanoparticles | SARS-CoV-2 pseudo virus | Colorimetric | COVID-19 | [88] |
| Magnetic nanoparticle | E. coli | Microscopy | Foodborne disease by E. coli | [89] |
| Silver nanoclusters | S. typhimurium | Fluorescence | Typhoid | [90] |
| Silver nanoclusters | E. coli | Fluorescence | Bacterial infection | [91] |
| Gold nanoparticles | Listeria monocytogenes | Fluorescence | listeriosis | [92] |
| Gold nanoparticles | Proteus mirabilis in urine | Colorimetric | Urease producing bacteria | [93] |
| Gold nanoparticles | IL-6 in blood | Colorimetric | Sepsis | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. https://doi.org/10.3390/nano13071247
Thwala LN, Ndlovu SC, Mpofu KT, Lugongolo MY, Mthunzi-Kufa P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials. 2023; 13(7):1247. https://doi.org/10.3390/nano13071247
Chicago/Turabian StyleThwala, Lungile Nomcebo, Sphumelele Colin Ndlovu, Kelvin Tafadzwa Mpofu, Masixole Yvonne Lugongolo, and Patience Mthunzi-Kufa. 2023. "Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests" Nanomaterials 13, no. 7: 1247. https://doi.org/10.3390/nano13071247
APA StyleThwala, L. N., Ndlovu, S. C., Mpofu, K. T., Lugongolo, M. Y., & Mthunzi-Kufa, P. (2023). Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials, 13(7), 1247. https://doi.org/10.3390/nano13071247





