Abstract
New ways of recycling fly ash are of great significance for reducing the environmental pollution. In this work, biodegradable hydrophobic poly (L-lactic acid)/fly ash composites for anti-icing application were successfully fabricated via a facile solvent-volatilization-induced phase separation approach. A silane coupling agent of 3-(Trimethoxysilyl) propyl methacrylate was used to decorate a fly ash surface (FA@KH570) for strengthening the interface bonding between fly ash and poly (L-lactic acid). Moreover, FA@KH570 could obviously enhance the crystallinity of poly (L-lactic acid) (PLLA)/FA@KH570 composites, which accelerated the conversion from the liquid-liquid to the liquid-solid phase separation principle. Correspondingly, the controllable surface morphology from smooth to petal-like microspheres was attained simply by adjusting the FA@KH570 content. After coating nontoxic candle grease, the apparent contact angle of 5 wt% PLLA/FA@KH570 composite was significantly increased to an astonishing 151.2°, which endowed the composite with excellent anti-icing property. This strategy paves the way for recycling waste fly ash and manufacturing hydrophobic poly (L-lactic acid) composite for potential application as an anti-icing material for refrigerator interior walls.
1. Introduction
As we all know, poly (L-lactic acid) (PLLA) is a linear aliphatic polyester with excellent biodegradability [1], solvent resistance [2], and biocompatibility [3], and good mechanical properties [4], abundant resource [5], and wide application. Thereby, PLLA can be used as a potential biomass matrix material for anti-icing [6,7], tissue repair [8], organ replacement [9], oil-water separation [10], and so on. There are various methods for preparing hydrophobic PLLA materials, including the template method [11], electroplating method [12], electrospinning method [13], phase separation method [14], sol-gel method [15], chemical grafting method [16], etc. Evidently, the electrospinning process and sol-gel methods are cumbersome and energy-intensive, but phase separation is considered a simple and feasible method for efficient preparation of hydrophobic porous materials [17,18].
Based on the theoretical equation of Huggins:
Refs [7,19], the phase separation effect usually occurred when the Gibbs free energy of a single-phase system was higher than that of the two-phase system, which could be brought about by altering the temperature and/or introducing a certain amount of nonsolvent [20]. Liu et al. reported a strong superhydrophobic inorganic-organic hybrid coating by combining fluorinated ethylene propylene (FEP) and aluminum dihydrogen phosphate (ADP) using a creative two-step phase separation approach, which had potential applications in many fields [21]. Wang et al. proposed a water-assisted thermal shock phase separation method for the first time to prepare a kind of PLA foam with special micro-nano structures and a large contact angle up to 151° [22]. However, the tedious TIPS process consumed a lot of energy. On the contrary, NIPS can be more easily achieved by adding some certain nonsolvent. Zhao et al. prepared petal-shaped PLA composite membranes with adjustable water droplet adhesion performance by the NIPS method [23]. Su et al. used a simple NIPS method to obtain environment-friendly super-hydrophobic three-dimensional stereo-PLA composites by adjusting the thickness and surface roughness [24].
It was easily found that the two major factors for the construction of hydrophobic surfaces were reducing surface energy and constructing micro-nano structures [25]. Natheless, the micro-nano structures constructed by phase separation were relatively isolated. To address this problem, several reports widely indicated that introducing nanoparticles and stacking them on the surface was a common strategy to obtain complex micro-nano structures with a certain roughness for the hydrophobic property [25,26]. Sun et al. introduced zinc oxide modified by KH570 into PLLA and constructed a rough surface with a high contact angle of 146° by NIPS method [27]. Zhang et al. prepared a PLA/γFe2O3 membrane by electrospinning, and the PLA/γFe2O3 membrane possessed high porosity [28]. Wu et al. obtained a superhydrophobic membrane for gas absorption by using hydrophobically modified SiO2 nanoparticles to induce PVDF precipitation [29]. Most of the nanoparticles used for hydrophobic modification were ceramic nanoparticles, which were relatively expensive and high-density. Therefore, choosing a cheap and recyclable particle to fabricate hydrophobic materials has become a hot research topic.
Fly ash (FA) mainly comes from the flue gas emitted by burning coal during the process of thermal power generation [30], metal smelting [31], heating for warmth, and so forth. FA usually exhibited a smooth surface like glass [32], with an average particle size of less than 20 microns [33], and contains a variety of active components such as unburned carbon and metal oxides (SiO2, Al2O3, and Fe2O3 [34,35]). According to previous researches and reports, FA had been effectively and deeply applied in the fields of building materials [36], metallurgy [37], agriculture [38], and chemical industry [39] at home and abroad. However, less than half of FA was recycled as raw material; the residue was simply landfilled or piled up. The long-term stacking of FA not only pollutes soil and water but also does harm to human health [40]. Hence, the recovery and reuse of FA had become an urgent problem to be solved.
New routes of recycling FA were of significance for producing high-value materials and reducing environmental pollution. It is feasible to fabricate functional composites by incorporating FA into polymers [41]. Nonetheless, FA could not be well distributed into polymer matrix due to poor compatibility, which would lead to the deterioration of properties, especially mechanical properties. Thus, FA was normally modified to improve the compatibility and/or to achieve a new function; modification methods included physical modification such as mechanical modification [42], microwave modification [43], high-temperature roasting modification [44], and so on, and chemical modification such as acid modification [45], alkali modification [46], salt modification [47], organic reagent modification [48,49], and so forth. The common silane coupling agent KH570 could be facilely applied to chemically modify FA and graft long-chain alkanes on the surface [50]. As expected, the KH570-modified FA (FA@KH570) particles exhibited excellent compatibility with PLLA.
To the best of our knowledge, the introduction of FA into a PLLA matrix to prepare hydrophobic composites has been rarely reported. Herein, a facile solvent-volatilization induced phase separation approach is proposed to obtain hydrophobic PLLA/FA@KH570 composites. The surface hierarchical microstructures converted from smooth to petal-like microspheres merely by regulating the FA@KH570 loading. In addition, candle grease was melt-coated on the PLLA/FA@KH570 composite surface to further improve the hydrophobic property. PLLA/FA@KH570 composite (5 wt%) exhibited the maximum contact angle of 151.2°, resulting in a fascinating anti-icing property. This investigation opened up a new avenue for recycling waste FA to fabricate highly hydrophobic PLLA composites with a favorable anti-icing property.
2. Materials and Experimental Methods
2.1. Materials
The poly (L-lactic acid) (PLLA) granules (commercial mark: 4032D) with of 11.9 × 104 g/mol and of 6.6 × 104 g/mol were procured from Nature Works (Blair, NE, USA). Fly ash (grade: first-class fly ash, diameter: 8–12 µm, purity: 99%, density: 2.1 × 103 kg/m3, and melting temperature: 1300 °C) was supplied by Jinchuan Stone Factory (Jining, China). Anhydrous ethanol and dichloromethane were acquired from Tianjin Fuyu Fine Chemical Co., Ltd. (Jianjin China). Silane coupling agent 3-(Trimethoxysilyl) propyl methacrylate (KH570) (density: ≈1 g/cm3) was provided by Nanjing You Pu Chemical Co., Ltd. (Nanjing, China). Candles were obtained from supermarkets. Distilled water was produced in the laboratory.
2.2. Modification of FA
FA containing a variety of active functional groups was conducive to modification. Typically, 2 g of FA was firstly distributed in a solution of 45 mL ethanol and 5 mL deionized water, and then transferred into a three-neck flask. Subsequently, 1 mL of KH570 was added into the above dispersion by a pipette under continuous mechanical stirring at 65 °C for 1 h. After that, the dispersion was subjected to ultrasonic treatment for 2 h in an ultrasonic cleaning machine. Finally, the modified FA powders (FA@KH570) were collected by filtration and transferred to an oven for drying at 45 °C for 24 h. After weighing, the powder weight was 1.57 g and the yield was 52.3%.
2.3. Preparation of PLLA/FA@KH570 Composites
As can be seen from Figure 1, PLLA/FA@KH570 composites were prepared via the phase separation induced by solvent evaporation. Firstly, a predesigned quantity of FA@KH570 was added to 20 mL of dichloromethane solution. After ultrasonically dispersing for 30 min, 1 g PLLA was mixed with the FA dispersion under mechanical stirring for 2 h. Then, 5 mL of the mixed solution was poured into a petri dish (diameter: 6 cm). A quantity of 2 mL non-solvent (anhydrous ethanol) was gradually dripped to induce phase separation. Finally, a series of PLLA/FA@KH570 composites were successfully prepared, including 1, 3, 5, and 7 wt%-PLLA/FA@KH570. For further enhancing the hydrophobicity, a small piece of candle (0.05 g) purchased from the supermarket was scraped onto the surface of the prepared PLLA/FA@KH570 composites, and then heated at 60 °C for 20 min to achieve an even distribution of candle grease.
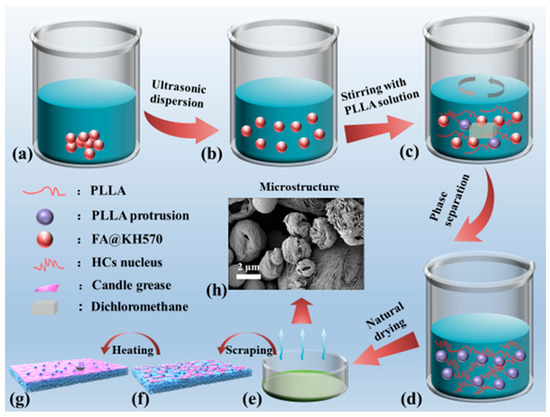
Figure 1.
Schematic diagram of the preparation procedure of PLLA/FA@KH570 composites. (a) FA@KH570 in dichloromethane, (b) FA@KH570 dichloromethane dispersion, (c) PLLA/FA@KH570 dichloromethane solution, (d) PLLA/FA@KH570 gel, (e) PLLA/FA@KH570 composite, (f) candle-scraped PLLA/FA@KH570 composite, (g) heated candle-scraped PLLA/FA@KH570 composite, (h) Microstructure of PLLA/FA@KH570 composite.
2.4. Characterizations
FA and FA@KH570 were tested by Fourier-transform infrared spectroscopy (FTIR, IS50, Thermo Nicolet, St. Bend, OR, USA) to analyze the characteristic groups in the wavelength range from 4000 to 500 cm−1. The surface microstructures of FA and PLLA/FA composites were observed on a scanning electron microscope (SEM, model SU8010, HITACHI, Japan) at an acceleration voltage of 10 kV. The surface of the sample was sprayed with gold to increase the electrical conductivity before observation. The melting/crystallization behaviors of pure PLLA and PLLA/FA composites were explored using a differential scanning calorimeter (DSC, Model Q2000, manufacturer, TA Instruments, Newcastle, DE, USA) under a nitrogen atmosphere. The samples were heated from 10 °C to 200 °C at an ascending rate of 10 °C/min. To determine the crystalline structures, the specimens were scanned from 5° to 80° at a rate of 10°/min using an X-ray diffractometer (XRD, model, XPertPRO manufacturer, PANalytical, Almelo, The Netherlands) at ambient temperature. A contact angle measuring instrument (model JC2000D1 manufacturer, Shanghai Zhong Chen Digital Technology Equipment Co., Ltd., Shanghai, China) was employed to measure the apparent contact angle and rolling angle of the sample. Distilled water was injected into the provided needle tube, and the droplet size was determined by the cross-sectional area of the needle. Five points of each sample were selected for contact angle test, and the average value was taken. The abrasion stability of the contact angle for PLLA/FA@KH570 composites was evaluated by sandpaper abrasion measurement. The composite films were pressed on sandpaper (800 mesh) under a 200 g loading and slipped for 10 cm with an external force. Ultimately, the water contact angle of the abrased films was measured again. The anti-icing properties were characterized by simply measuring the weight evolution and delay time during the icing process. To simulate the frigid natural environment, the samples were placed in a refrigerator (the temperature was accurately retained at −18 °C and the humidity was adapted to 75% by a humidifier). The weight of the samples was obtained on an analytical balance at fixed intervals. In addition, the delay time was also tested on the above conditions. A deionized water droplet of 10 µL was dropped on the sample surface through a pipette. The icing point was defined as the moment that the water droplet changed from translucent to opacified. The average delay time of three tests was taken for each sample.
3. Results and Discussion
3.1. Dispersibility and Morphology of FA@KH570
For the sake of proving the dispersibility of the FA@KH570 microparticles, unmodified FA and FA@KH570 microparticles were dispersed in dichloromethane solution and deionized water under ultrasonication, respectively (Figure 2). After standing for 2 h, it was clearly seen that FA microparticles were in a turbid state in the water (Figure 2a), which was the proof that FA microparticles were dispersed well in the water. However, most of FA microparticles were deposited at the bottom in dichloromethane due to the poor dispersibility and high density (Figure 2a1). FA@KH570 microparticles showed the opposite state: FA@KH570 floated on the surface in aqueous dispersion due to its hydrophobicity (Figure 2b); FA@KH570 was uniformly dispersed in dichloromethane, indicating the excellent compatibility of FA@KH570 with dichloromethane (Figure 2b1). From the optical image in Figure 2c, the water droplet would directly penetrate into FA microparticles, further illustrating the hydrophilicity of FA. On the contrary, FA@KH570 repelled the water droplet, owing to the grafting of long-chain alkanes (Figure 2c1).

Figure 2.
(a) and (a1) the photos of FA dispersion in water and dichloromethane, respectively. (b) and (b1) the photos of FA@KH570 dispersion in water and dichloromethane, respectively. (c) and (c1) the optical pictures of water droplet on the surface of FA@KH570 and FA, respectively.
In addition, FTIR spectroscopy was applied to characterize the chemical structures of FA@KH570 and pure FA (Figure 3a). It could be found that both FA@KH570 and pure FA had a strong peak at 3462 cm−1, which was ascribed to the stretching vibration of -OH. The strong band at 1077 cm−1 was indicative of the asymmetric stretching vibration of Si/Al-O. It was worth noting that a new band was observed at 1725 cm−1, belonging to the stretching absorption peak of C=O. The macromolecules with hydroxyl in the hydrolyzed products of KH570 could form covalent bonds by chemical bonding with –OH on the surface of FA [51]. The stretching absorption peak of C=O existing in FA@KH570 proved that KH570 was successfully grafted on the FA surface. Meanwhile, the XRD tests were applied to explore the crystalline structures of FA and FA@KH570 (Figure 3b and Table S1). It was easy to find that FA exhibited the diffraction peaks located at 18.2° and 26.7°, corresponding to the crystal phase quartz. Therein, the carbon content was low and the characteristic peaks were covered with quartz, which was imperceptible in the pattern. The diffraction peak observed at 40.1° was assigned to the crystal of mullite. Moreover, the crystallization peaks of hematite were prevailingly focused on 33.3°, and the characteristic peak of alumina appeared at 35.4°. It was noteworthy that FA@KH570 illustrated similar diffraction peaks to FA, demonstrating that KH570-modification had little effect on the crystalline structures of FA. As can be seen from the morphology of FA and FA@KH570 (Figure 3c,d), the modification of KH570 also scarcely changed the morphology and microstructures of FA.
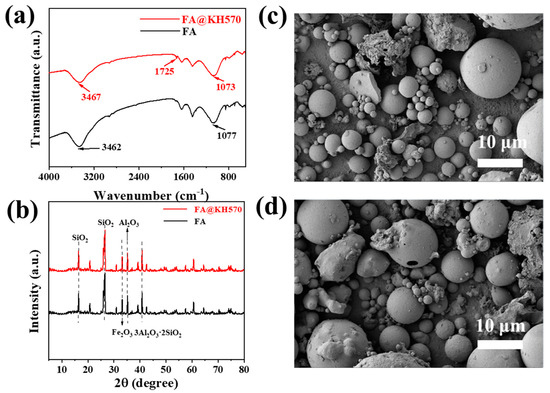
Figure 3.
(a) FTIR of FA and FA@KH570. (b) XRD patterns of FA and FA@KH570. (c) and (d) SEM micrograms of FA and FA@KH570.
3.2. Morphology of PLLA/FA Composites
As can be seen from Figure 4a,a1 and Figure S1a, the surface of pure PLLA was relatively smooth with a small quantity of microspheres (diameter: <2 µm). The morphology of PLLA/FA@KH570 composites exhibited a sequence of changes with the increase in the FA@KH570 fraction from 1 wt% to 7 wt% (Figure 4b–e and Figure S1b–e). In comparison with pure PLLA, 1 wt% PLLA/FA@KH570 possessed more and larger microspheres (diameter ≈ 2.5 µm) on the surface, due to the effective nucleation of FA@KH570 (Figure 4b,b1). For 3 wt% PLLA/FA@KH570, the number of microspheres was continuously increased with the incremental FA@KH570 loading. Strikingly, petal-like microspheres with multilayer nano-wrinkles were observed on the 5 wt% PLLA/FA@KH570 composite surface (Figure 4c,c1). The production of petal-like microspheres was associated with the conduct of two diverse phase separation processes, that is to say, quick liquid-solid phase separation stemming from heterogeneous nucleation of PLLA and FA@KH570, generating many micro-domains where tardy liquid-liquid phase separation was next in progress [52,53]. However, when the content of FA@KH570 was increased to 7 wt%, the number of microspheres began to decline, on account of the severe aggregation of FA@KH570 (Figure 4e,e1). In short, the petal-like microspheres attached on the surface provided multiscale micro-nano structures, which favors hydrophobicity.

Figure 4.
SEM images of (a,a1) pure PLLA, (b,b1) 1 wt% PLLA/FA@KH570, (c,c1) 3 wt% PLLA/FA@KH570, (d,d1) 5 wt% PLLA/FA@KH570, and (e,e1) 7 wt% PLLA/FA@KH570 composites.
The particular mechanism for the construction of hierarchical microstructures via the solvent-volatilization-induced phase separation approach is vividly modeled in Figure 5. The phase separation would take place when the Gibbs free energy of the PLLA solution violently fluctuated. In this investigation, the solution would be stratified as a result of the density difference between the solvent (dichloromethane, 1.33 g/cm3) and nonsolvent (ethanol, 0.79 g/cm3). Since dichloromethane volatilized much more quickly than ethanol, concentration fluctuation appears at the solvent/nonsolvent interface, resulting from PLLA chain agglomeration induced by the decreasing free energy of this system. Hence, the molecular chains could undergo interphase migration along the concentration gradient direction and trigger the formation of polymer-lean and polymer-rich phases through the diffusion-induced separation. Thanks to the superior volatility of dichloromethane, phase inversion took place while the volume of the polymer-lean phase was elevated to a certain extent, which was accompanied by the transformation of the isolated polymer-lean phase into the continuous phase. The 3D-network polymer-rich phase would be compressed into big slices during phase inversion procedure, as a consequence of the increasing concentration and viscosity. Thereby, the fish-scale skeletons were constructed via the liquid-liquid phase separation. The imprint of polymer-lean phase assembled the pores throughout the 3D connected networks.
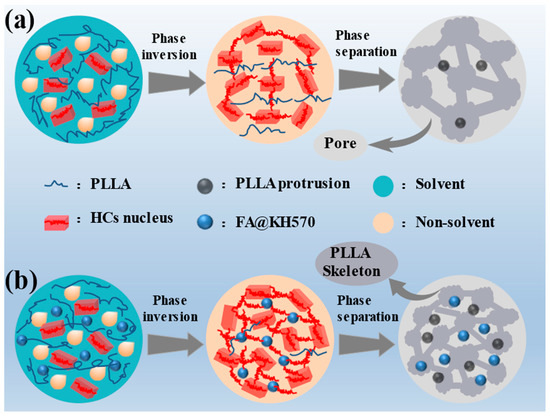
Figure 5.
Mechanism diagram of the growth of hierarchical structures for (a) pure PLLA and (b) PLLA/FA@KH570 composites.
In addition, a small number of homogeneous crystal (HC) nuclei in the polymer-rich phase were already formed by the orderly packing of the closely adjacent pre-ordered polymer chains. These nuclei would grow into microspheres by the stacking of PLLA chains along the phase interface, which was attributed to the increased viscosity and restrained mobility of PLLA chains. Regarding PLLA/FA@KH570 composites, FA@KH570 could effectively improve the heterogeneous nucleation of PLLA and thus generate many more crystal nuclei. After crystal growth, more and larger microspheres were assembled on the composite surface. Notably, at the FA@KH570 content of 7 wt%, the serious aggregation of FA@KH570 largely retarded the movement of PLLA chains and the growth of crystals. Thus, the microspheres on the surface of 7 wt% PLLA/PLLA/FA@KH570 were greatly decreased in number.
3.3. Crystallization Behaviors of PLLA/FA@KH570 Composites
The crystallization properties of the specimens were explored by DSC and XRD systems (Figure 6). The degree of crystallization of PLLA composites could be obtained by melting enthalpy divided by the theoretical enthalpy value for entire crystallization (93.1 J/g) [27,54]. It could be seen from the DSC heating curves of PLLA composites (Figure 6a) that the melting temperature (Tm) of PLLA/FA@KH570 composites exhibited little variation (166.5–167.5 °C). From the glass transition temperature (Tg) point of view, Tg was slightly increased from 55.9 °C for pure PLLA to 60.3 °C for 7 wt% PLLA/FA@KH570, which was mainly owing to the decreasing movement of PLLA chains with the adhesion of FA@KH570. Moreover, the crystallinity of pure PLLA was only 55.1%. The crystallinity of PLLA/FA@KH570 composites was improved from 55.5%, 68.9% to 78.2% with the incremental FA@KH570 content from 1 wt%, to 3 wt%, to 5 wt%. The underlying mechanism was that FA@KH570 could play a role as an excellent heterogeneous nucleating agent to accelerate the crystallization of PLLA composites [55]. Nevertheless, 7 wt% PLLA/FA@KH570 possessed a significantly reduced crystallinity of 65.7%, on account of the agglomeration of FA@KH570, largely reducing the movement of PLLA chains and limiting the growth of crystals.
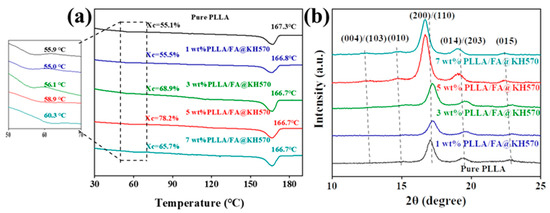
Figure 6.
(a) DSC heating curves of pure PLLA and PLLA/FA@KH570 composites. (b) XRD patterns of pure PLLA and PLLA/FA@KH570 composites.
As is well known, the crystal form of PLLA is dependent on the crystallization conditions, and there are generally the four crystal forms of α, α’, β, and γ. According to XRD image of PLLA composites (Figure 6b), the crystallization peaks were located at 2θ angles of 12.5°, 14.8°, 16.7°, 18.9°, and 22.4°, corresponding to (004)/(103), (010), (200)/(110), (014)/(203), and (015) crystal planes, respectively. Noticeably, the corresponding 2θ shifted to a slightly lower angle with the incremental FA@KH570 loading. This phenomenon could be explained by the fact that the increased FA@KH570 particles would lead to the larger unit cell parameters and interplanar spacing. In addition, the crystallization peak intensity was enhanced with the increase of FA@KH570 loading from 1 wt% to 5 wt%, and then decreased at a FA@KH570 loading of 7 wt%, in good agreement with DSC analyses. All in all, a small amount of FA@KH570 had little impact on the crystalline structures of PLLA.
3.4. Surface Wettability of PLLA/FA@KH570 Composites
The surficial wettability of composite materials heavily depended on the geometrical structure and surface energy. Nevertheless, a large quantity of polar ester groups was located in PLLA chains, leading to unsatisfactory hydrophobicity. Therefore, constructing proper surface microstructures with high roughness and reducing surface energy were keys to enable PLLA materials with hydrophobic properties. In this study, the rough surface of PLLA composites with crystalline microspheres and FA@KH570 particles was successfully assembled via a straightforward solvent-volatilization-induced phase separation method. Afterward, the PLLA/FA@KH570 composites were coated with candle grease by a simple melt-coating method to reduce the surface energy, finally exhibiting surface super-hydrophobicity [56].
The contact angle tests were carried out to explore the surface wetting property of the PLLA/FA@KH570 composites. As displayed in Figure 7a, the apparent contact angle of the PLLA/FA@KH570 composites was raised from 124.2° to 144.7° with an increase in the FA@KH570 content from 0 to 5 wt%. Surface wettability evolution was primarily dominated by the surface roughness of the PLLA/FA@KH570 composites. A gas cushion was built under the water droplet by a mass of entrapped air in the rough PLLA composite surface, which made the solid-liquid contact surface transform into a three-phase surface consisting of air, water, and solid. Thus, spherical droplets appeared on the PLLA/FA@KH570 composite surface, exhibiting greatly improved hydrophobicity. At the incremental FA@KH570 loading of 7 wt%, the apparent contact angle was exceptionally reduced to 131.6°. The underlying principle was that the surface roughness decreased, accompanied by a reduced number of microspheres on the 7 wt% PLLA/FA@KH570 surface, which had been confirmed by the SEM results.
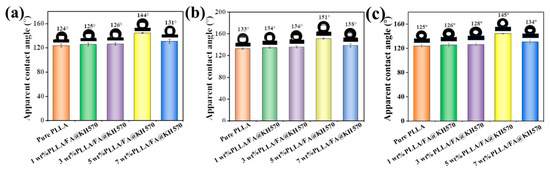
Figure 7.
(a) Contact angles of neat PLLA and PLLA/FA@KH570 composites. (b) Contact angles of neat PLLA and PLLA/FA@KH570 composites with candle grease coating. (c) Contact angles of neat PLLA and PLLA/FA@KH570 composites after abrasion.
In order to achieve hydrophobic PLLA/FA@KH570 composites, commercially available and nontoxic candle grease, as a common low-surface energy substance, was coated on the PLLA/FA@KH570 surface by a facile melt-coating approach to reduce the surface energy. The apparent contact angle of PLLA/FA@KH570 composites after candle grease coating is depicted in detail in Figure 7b and Figure S2. It was obvious that the contact angle of PLLA/FA@KH570 composites was highly enhanced after candle grease coating at the same FA@KH570 loading fraction. For instance, the contact angle of 5 wt% PLLA/FA@KH570 composite after candle grease coating was elevated to the maximum value of 151.2°, compared with that of 5 wt% PLLA/FA@KH570 composite without candle grease coating (144.7°). PLLA/FA@KH570 composites after candle grease coating could show astonishing hydrophobicity. As shown in Figure 7c, the apparent contact angle of PLLA/FA@KH570 composites after sandpaper abrasion was slightly decreased. For example, the contact angle of 5 wt% PLLA/FA@KH570 was moderately reduced from 151° to 145° after abrasion, which still exhibited great hydrophobicity. The results indicated the excellent abrasion stability of PLLA/FA@KH570 composites.
According to the adhesion theory, rose petal-like surfaces were not only hydrophobic but also somewhat adherent. As could be seen from Figure 4, the surface of the 5 wt% PLLA/FA@KH570 composite had a petal-like structure. Correspondingly, we conducted a rolling angle test and the results were as expected (Figures S3–S7). As shown in Figure S6, the advancing and receding contact angles of 5 wt% PLLA/FA@KH570 at a tilt angle of 60° are 155.4° and 108.2°, respectively. Thus, the contact angle hysteresis up to 47.2° indicates the excellent adhesion of the water droplet on the composite surface, in spite of the high static contact angle of 151°.
3.5. Anti-Icing Property
The highly hydrophobic PLLA/FA@KH570 composites opened up a promising application in the anti-icing field. To estimate the anti-icing properties, the weight increase (φ) and delay time during icing process (−18 °C and 75% RH) were measured in detail (Figure 8). The weight increase (φ) was calculated according to the following equation:
where Wi is the weight after icing at designed intervals and W0 is the primal weight of the specimen.
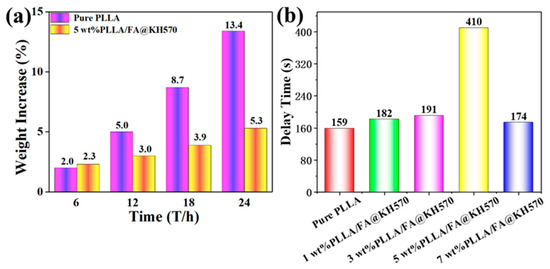
Figure 8.
(a) Weight increase of pure PLLA and 5 wt% PLLA/FA@KH570 composite. (b) Delay times of neat PLLA and PLLA/FA@KH570 composites during the icing procedure.
After an icing time of 24 h, the weight of both pure PLLA and PLLA/FA@KH570 composite changed little. The weight evolution of the samples was recorded within 24 h. As seen from Figure 8a and Table S2, the weight of pure PLLA was sharply increased from 0.299 g to 0.339 g at 24 h, exhibiting a weight increase up to 13.4%. However, 5 wt% PLLA/FA@KH570 composite indicated a much lower weight increase of only 5.3%. Moreover, the delay time of 5 wt% PLLA/FA@KH570 composite was significantly prolonged to 410 s, in comparison with that of pure PLLA (merely 159 s). The improved anti-icing property stemmed from the enhanced hydrophobicity of 5 wt% PLLA/FA@KH570 composite, of which the apparent contact angle appreciably reached to the maximum value of 151.2°. On the PLLA/FA@KH570 composite surface, the water droplet trapped a lot of air in the micro-nanostructures to elicit the formation of an air cushion, immensely decreasing the contact area between the PLLA composite surface and the water droplet, largely weakening the surface adhesion, and effectively lessening the heat transfer. Considering all the above analyses, one could make a conclusion that highly hydrophobic PLLA/FA@KH570 composites are a great prospect in anti-icing smart materials. Concretely, it could be applied to the outer walls of electronic devices to prevent the corrosion of parts by water.
4. Conclusions
In summary, reusing FA is crucial to reducing environment pollution and producing high-value materials. First, FA was modified by KH570 (FA@KH570) to reinforce the interfacial bonding between FA@KH570 and PLLA. Then, PLLA/FA@KH570 composites were successfully fabricated via a facile solvent-volatilization-induced phase separation approach. FA@KH570 could play a role as an excellent heterogeneous nucleating agent to accelerate the crystallization of PLLA composites. Correspondingly, the crystallinity of the PLLA/FA@KH570 composites was increased from 55.1 to 78.2% with the slightly incremental content of FA@KH570 from 0 to 5 wt%. Thus, the surface morphology of PLLA/FA@KH570 composites could transform from smooth to petal-like microsphere by merely modulating the FA@KH570 loading. In addition, the surface of the PLLA/FA@KH570 composites was melt-coated with commercial candle grease to reduce the surface energy. It was worth noting that 5 wt% PLLA/FA@KH570 composite exhibited a maximum contact angle of 151.2°, due to the increased surface roughness and the reduced surface energy, according to Cassie wetting theory. In addition, the contact angle hysteresis of 5 wt% PLLA/FA@KH570 composite was up to 47.2°, indicating the excellent adhesion of the water droplet on the rose petal surface. The water droplet trapped air to create an air cushion on the rough PLLA composite surface, contributing to the greatly reduced contact area of the water droplet with the PLLA composite surface. Predictably, 5 wt% PLLA/FA@KH570 composite displayed an excellent anti-icing property, and deviated from the minimum weight increase of 5.3% and the maximum delay time of 410 s during the icing process. The FA@KH570-controlled surface morphology strategy represents a significant step towards recycling waste FA and fabricating green hydrophobic PLLA composites, which has tremendous implications for anti-icing materials and environmental protection. The PLLA/FA@KH570 composite films have great potential as a promising material for refrigerator interior walls.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13071230/s1, Figure S1: Surface SEM images of (a) pure PLLA, (b) 1 wt% PLLA/FA@KH570, (c) 3 wt% PLLA/FA@KH570, (d) 5 wt% PLLA/FA@KH570, and (e) 7 wt% PLLA/FA@KH570 composites; Figure S2: Static contact angles of pure PLLA (a) and 5 wt% PLLA/FA@KH570 composite (b), respectively; Figure S3: Rolling angle photos of pure PLLA at the tilt angle of 30° (a) and 60° (b), respectively; Figure S4: Rolling angle photos of 1 wt% PLLA/FA@KH570 composite at the tilt angle of 30° (a) and 60° (b), respectively; Figure S5: Rolling angle photos of 3 wt% PLLA/FA@KH570 composite at the tilt angle of 30° (a) and 60° (b), respectively; Figure S6: Rolling angle photos of 5 wt% PLLA/FA@KH570 composite at the tilt angle of 30° (a) and 60° (b), respectively; Figure S7: Rolling angle photos of 7 wt% PLLA/FA@KH570 composite at the tilt angle of 30° (a) and 60° (b), respectively; Table S1: XRD data of FA; Table S2: Data analysis tables for pure PLLA and 5 wt% PLLA/FA@KH570 composite.
Author Contributions
Conceptualization, B.X.; methodology, B.X. and L.X.; investigation, Z.J.; formal analysis, X.M.; Data curation, Z.J. and L.Z.; writing—original draft preparation, Z.J.; writing—review and editing, B.X.; Visualization, C.W. and L.Z.; supervision, Q.Z.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 51963003 and 52263003), Guizhou Provincial Science and Technology Project (No. ZK [2022]Maj019 and [2020]1Z044).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campardelli, R.; Oleandro, E.; Reverchon, E. Supercritical assisted injection in a liquid antisolvent for PLGA and PLA microparticle production. Powder Technol. 2016, 287, 12–19. [Google Scholar] [CrossRef]
- Xie, Y.; Lan, X.R.; Bao, R.Y. High-performance porous polylactide stereocomplex crystallite scaffolds prepared by solution blending and salt leaching. Mater. Sci. Eng. C 2018, 90, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Gonc, C.; Pinto, A.; Machado, A.V.; Moreira, O. Biocompatible Reinforcement of Poly (Lactic Acid) With Graphene Nanoplatelets. Polym. Compos. 2018, 39, E308–E320. [Google Scholar]
- Zhou, S.Q.; Cheng, X.C.; Jin, Y.L. Molecular dynamics simulation on interacting and mechanical properties of polylactic acid and attapulgite. J. Appl. Polym. Sci. 2013, 128, 3043–3049. [Google Scholar] [CrossRef]
- Wang, X.C.; Song, Y.L.; Wang, Y.M.; Huang, C.P.; Li, Y.X.; Chen, B.H. Preparation of Lactic Acid by Polymer-Catalyzed Conversion of Maltose in Aqueous Alkaline Media. Adv. Mater. Res. 2014, 3381, 947–953. [Google Scholar] [CrossRef]
- Li, H.G.; Guo, X.J.; Ding, Y.R.; Liu, B.Y.; An, Q.F. Superhydrophobic anti-icing coatings with self-deicing property using melanin nanoparticles from cuttlefish juice. Chem. Eng. J. 2021, 424, 130553. [Google Scholar]
- Sun, X.; Yang, S.D.; Xue, B.; Xie, L.; Zheng, Q. Super-hydrophobic poly (lactic acid) by controlling the hierarchical structure and polymorphic transformation. Chem. Eng. J. 2020, 397, 12529. [Google Scholar] [CrossRef]
- Roca, F.G.; Santos, L.G.; Roig, M.M.; Medina, L.M.; Martínez-Ramos, C.; Pradas, M.M. Novel Tissue-Engineered Multimodular Hyaluronic Acid-Polylactic Acid Conduits for the Regeneration of Sciatic Nerve Defect. Biomedicines 2022, 10, 963. [Google Scholar] [CrossRef]
- Butt, M.S.; Bai, J.; Wan, X.F.; Chu, C.L.; Xue, F.; Ding, H.Y.; Zhou, G.H. Mg alloy rod reinforced biodegradable poly-lactic acid composite for load bearing bone replacement. Surf. Coat. Tech. 2017, 309, 471–479. [Google Scholar] [CrossRef]
- Chhajed, M.; Verma, C.; Sathawane, M.; Singh, S.; Maji, P.K. Mechanically durable green aerogel composite based on agricultural lignocellulosic residue for organic liquids/oil sorption. Mar. Pollut. Bull. 2022, 180, 113790. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.P.; Ke, Q.P. Fabrication of microcavity-array superhydrophobic surfaces using an improved template method. J. Colloid Interf. Sci. 2013, 395, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Aghaee, M.M.; Hassan, K.; Mohammad, T. Investigation of wettability of copper plate with nickel-graphene oxide coating produced by electroplating method. Appl. Phys. A Mater. 2022, 143, 128–143. [Google Scholar]
- Makowskia, T.; Svyntkivska, M.; Piorkowskaa, M.; Kregiel, D. Multifunctional polylactide nonwovens with 3D network of multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 527, 146898. [Google Scholar] [CrossRef]
- Liu, W.L.; Huang, N.L.; Yang, J.J. Characterization and application of porous polylactic acid films prepared by nonsolvent-induced phase separation method. Food. Chem. 2021, 373, 131525. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.Y.; Jang, J.; Jeong, Y.G.; Lyoo, W.S.; Min, B.G. Superhydrophobic PLA fabrics prepared by UV photo-grafting of hydrophobic silica particles possessing vinyl groups. J. Colloid Interface Sci. 2010, 344, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Y.; Wang, S.H.; Zhang, J.h.; Liu, Y. Chemical grafting of the superhydrophobic surface on copper with hierarchical microstructure and its formation mechanism. Appl. Surf. Sci. 2018, 436, 950–956. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, X.d.; Chen, Q. A simple superhydrophobic/superhydrophilic Janus-paper with enhanced biocompatibility by PDMS and candle soot coating for actuator. Chem. Eng. J. 2021, 406, 126532. [Google Scholar] [CrossRef]
- Li, E.; Pan, Y.M.; Liu, X.H. Asymmetric Superhydrophobic Textiles for Electromagnetic Interference Shielding, Photothermal Conversion, and Solar Water Evaporation. ACS. Appl. Mater. Inter. 2021, 13, 28996–29007. [Google Scholar] [CrossRef]
- Sun, X.; Guo, Y.F.; Wang, R.; Qin, S.H. Flexure-resistant and additive-free poly (L-lactic acid) hydrophobic membranes fabricated by slow phase separation. Int. J. Biol. Macromol. 2022, 209, 1605–1612. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Adams, P.M.W.; Drew, R.A.L. Isothermal ternary phase diagram of the polylactic acid-dichloromethane-hexane system. Polymer 2014, 55, 3100–3106. [Google Scholar] [CrossRef]
- Liu, Z.J.; Ren, L.N.; Jing, J.; Wang, C.J. Fabrication of robust superhydrophobic organic-inorganic hybrid coating through a novel two-step phase separation method. Prog. Org. Coat. 2021, 157, 106320. [Google Scholar] [CrossRef]
- Wang, X.L.; Pan, Y.M.; Liu, X.H. Facile Fabrication of Superhydrophobic and Eco-Friendly Poly (lactic acid) Foam for Oil−Water Separation via Skin Peeling. ACS. Appl. Mater. Inter. 2019, 11, 14362–14367. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Yang, Q.; Fu, Y.Y. Facile preparation of patterned petal-like PLA surfaces with tunable water micro-droplet adhesion properties based on stereo-complex co-crystallization from non-solvent induced phase separation processes. J. Mater. Chem. A 2016, 4, 12058. [Google Scholar]
- Su, Y.Z.; Zhao, Y.Q.; Zheng, W.G. Asymmetric Sc-PLA Membrane with Multi-scale Microstructures: Wettability, Antifouling, and Oil−Water Separation. ACS Appl. Mater. Inter. 2020, 12, 55520–55526. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Y.; Zhao, X.L.; Li, Y.D.; Weng, Y.X.; Zeng, J.B. Robust and nanoparticle-free superhydrophobic cotton fabric fabricated from all biological resources for oil/water separation. Int. J. Biol. Macromol. 2019, 140, 1175–1182. [Google Scholar] [CrossRef]
- Sethi, S.K.; Singh, M.; Manik, G. A multi-scale modeling and simulation study to investigate the effect of roughness of a surface on its self-cleaning performance. Mol. Syst. Des. Eng. 2020, 5, 1277. [Google Scholar] [CrossRef]
- Sun, X.; Xue, B.; Yang, S.D.; Huo, K.W.; Liao, X.Y. Structural conversion of PLLA/ZnO composites facilitated by interfacial crystallization to potential application in oil-water separation. Appl. Surf. Sci. 2020, 517, 14613. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.Z.; Huang, T.; Zhang, N. Electrospun Fibrous Membranes with Dual-Scaled Porous Structure: Super Hydrophobicity, Super Lipophilicity, Excellent Water Adhesion, and Anti-Icing for Highly Efficient Oil Adsorption/Separation. ACS Appl. Mater. Inter. 2019, 11, 5073–5083. [Google Scholar] [CrossRef]
- Wu, X.N.; Zhao, B.; Wang, L.; Zhang, Z.H. Superhydrophobic PVDF membrane induced by hydrophobic SiO2 nanoparticles and its use for CO2 absorption. Sep. Purif. Technol. 2018, 190, 108–115. [Google Scholar] [CrossRef]
- Rani, R.; Manish, K. Effect of bottom ash at different ratios on hydraulic transportation of fly ash during mine fill. Powder Technol. 2017, 315, 309–317. [Google Scholar] [CrossRef]
- Shu, J.C.; Lei, T.Y. Metal mobility and toxicity of reclaimed copper smelting fly ash and smelting slag. RSC. Adv. 2021, 11, 6877–6884. [Google Scholar] [CrossRef]
- Sulaiman, A.; Ibrahim, A. Treatment of fly ash from power plants using thermal plasma. Beilstein. J. Nanotech. 2017, 8, 1043–1048. [Google Scholar]
- Venkatanarayanan, H.K.; Rangaraju, P.R. Decoupling the effects of chemical composition and fineness of fly ash in mitigating alkali-silica reaction. Cem. Concr. Compos. 2013, 43, 54–68. [Google Scholar] [CrossRef]
- Ming, X.C.; Zhou, K.; Liu, J. Effect of Fly ash Content on Mechanical Properties of Cement-Fly ash Stabilized crushed stones. Appl. Mech. Mater. 2014, 548, 228–232. [Google Scholar]
- Li, C.Y.; Geng, H.B.; Zhou, S.Y. Experimental Study on Preparation and Performance of Concrete with Large Content of Fly-ash. Front. Mater. 2022, 8, 764820. [Google Scholar] [CrossRef]
- Krishnaraj, L.; Ravichandran, P.T. Characterisation of ultra-fine fly ash as sustainable cementitious material for masonry construction. Ain. Shams. Eng. J. 2021, 12, 259–269. [Google Scholar] [CrossRef]
- Rayzman, V.L.; Shcherban, S.A.; Dworkin, R.S. Technology for Chemical-Metallurgical Coal Ash Utilization. Energy Fuels 1997, 11, 761–773. [Google Scholar] [CrossRef]
- Flores, C.G.; Schneider, H.; Marcilio, N.R.; Ferret, L. Potassic zeolites from Brazilian coal ash for use as a fertilizer in agriculture. Waste Manag. Res. 2017, 70, 263–271. [Google Scholar] [CrossRef]
- Ge, X.L.; Zhai, J.W.; Feng, Y.L. The comprehensive utilization of fly ash. Adv. Mater. Res. 2011, 347, 1362–1365. [Google Scholar] [CrossRef]
- Verma, C.; Verma, R. Leaching Behaviour of Fly Ash: A Review, Nature Environment & Polution. Technology 2019, 18, 2. [Google Scholar]
- Xiao, P.; Liang, Y.; He, J.; Zhang, L.; Chen, T. Hydrophilic/Hydrophobic Interphase Mediated Bubble-like Stretchable Janus Ultrathin Films toward Self-Adaptive and Pneumatic Multifunctional Electronics. ACS Nano 2019, 13, 4368–4378. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Yang, G.; Zhang, Y.S. Supercritical CO2 coupled with mechanical force to enhance carbonation of fly ash and heavy metal solidification. Fuel 2022, 315, 123–154. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Ibrahimi, B.E.; Oualid, H.A.; Kassab, Z.; Quintal-Franco, C. Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As(V) ions: Experimental and theoretical studies. Chem. Eng. J. 2021, 421, 129909. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G.; Chai, B.B. Control of Endogenous Phosphorus Release at the Sediment–Water Interface by Lanthanum-Modified Fly Ash. Coatings 2022, 12, 719. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.T. Study on the phosphate removal from aqueous solution using modified fly ash. Fuel 2010, 89, 3668–3674. [Google Scholar] [CrossRef]
- Rashad, A.M.; Ouda, A.S. An investigation on alkali-activated fly ash pastes modified with quartz powder subjected to elevated temperatures. Constr. Build. Mater. 2016, 122, 417–425. [Google Scholar] [CrossRef]
- Xie, Q.; Lin, Y.; Wu, D.Y. Performance of surfactant modified zeolite/hydrous zirconium oxide as a multi-functional adsorbent. Fuel 2017, 203, 411–418. [Google Scholar] [CrossRef]
- Liu, D.J.; Yang, Y.L.; Zhao, F.Q. Adsorption Effect of Cetyltrimethyl-ammonium Bromide Modified Fly Ash on Methyl Orange Waste Water. Mater. Sci. Eng. 2018, 409, 6–12. [Google Scholar] [CrossRef]
- Seo, K.; Kim, M.; Kim, D.H. Candle-based process for creating a stable superhydrophobic surface. Carbon 2014, 68, 583–596. [Google Scholar] [CrossRef]
- Pang, J.F.; Li, Q.; Wang, B.; Tao, D.J. Preparation and characterization of electroless Ni-Fe-P alloy films on fly ash cenospheres. Powder Technol. 2012, 226, 246–252. [Google Scholar] [CrossRef]
- Munief, W.M.; Heib, F.; Hempel, F.; Lu, X.; Schwartz, M.; Pachauri, V.; Hempelmann, R.; Schmitt, M.; Ingebrandt, S. Silane Deposition via Gas-Phase Evaporation and High-Resolution Surface Characterization of the Ultrathin Siloxane Coatings. Langmuir 2018, 34, 10217–10229. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Weibel, J.A.; Schaber, J.A.; Garimella, S.V. The Wetting State of Water on a Rose Petal. Adv. Mater. Interfaces 2019, 6, 1900652. [Google Scholar] [CrossRef]
- Ghosh, U.U.; Naira, S.; Das, A.; Mukherjee, R.; DasGupta, S. Replicating and resolving wetting and adhesion characteristics of a Rose petal. Colloid Surface A. 2019, 561, 9–17. [Google Scholar] [CrossRef]
- Sun, X.; Xue, B.; Tian, Y.Z.; Xie, L. 3D Porous Poly (L-lactic Acid) Materials with Controllable Multi-scale Microstructures and Their Potential Application in Oil-Water Separation. Appl. Surf. Sci. 2018, 4332, 32256–32261. [Google Scholar] [CrossRef]
- Li, Y.D.; Fu, Q.Q.; Wang, M.; Zeng, J.B. Morphology, crystallization and rheological behavior in poly (butylene succin-ate)/cellulose nanocrystal nanocomposites fabricated by solution coagulation. Carbohydr. Polym. 2017, 164, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Kryszak, B.; Dzienny, P.; Pozniak, B.; Tikhomirov, M.; Hoppe, V.; Ziolkowska, P.; Tylus, W.; Grzymajlo, M.; Gajadhur, A.; et al. Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-ceramisation: A New Way towards Implant Surface Modification. Int. J. Mol. Sci. 2021, 22, 8436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).