Progress in Research and Application of Metal–Organic Gels: A Review
Abstract
1. Introduction
2. Methods
2.1. Data Acquisition
2.2. Methods
3. Results and Discussion
3.1. Literature Quantity Analysis
3.2. Analysis of Publication Countries
3.3. Cooperation between Scientific Research Authors and Institutions
3.4. Co-Citation Analysis
3.5. The Relationship between Disciplines and Journals
3.6. Analysis of Hotspots in the Research of Metal–Organic Gels
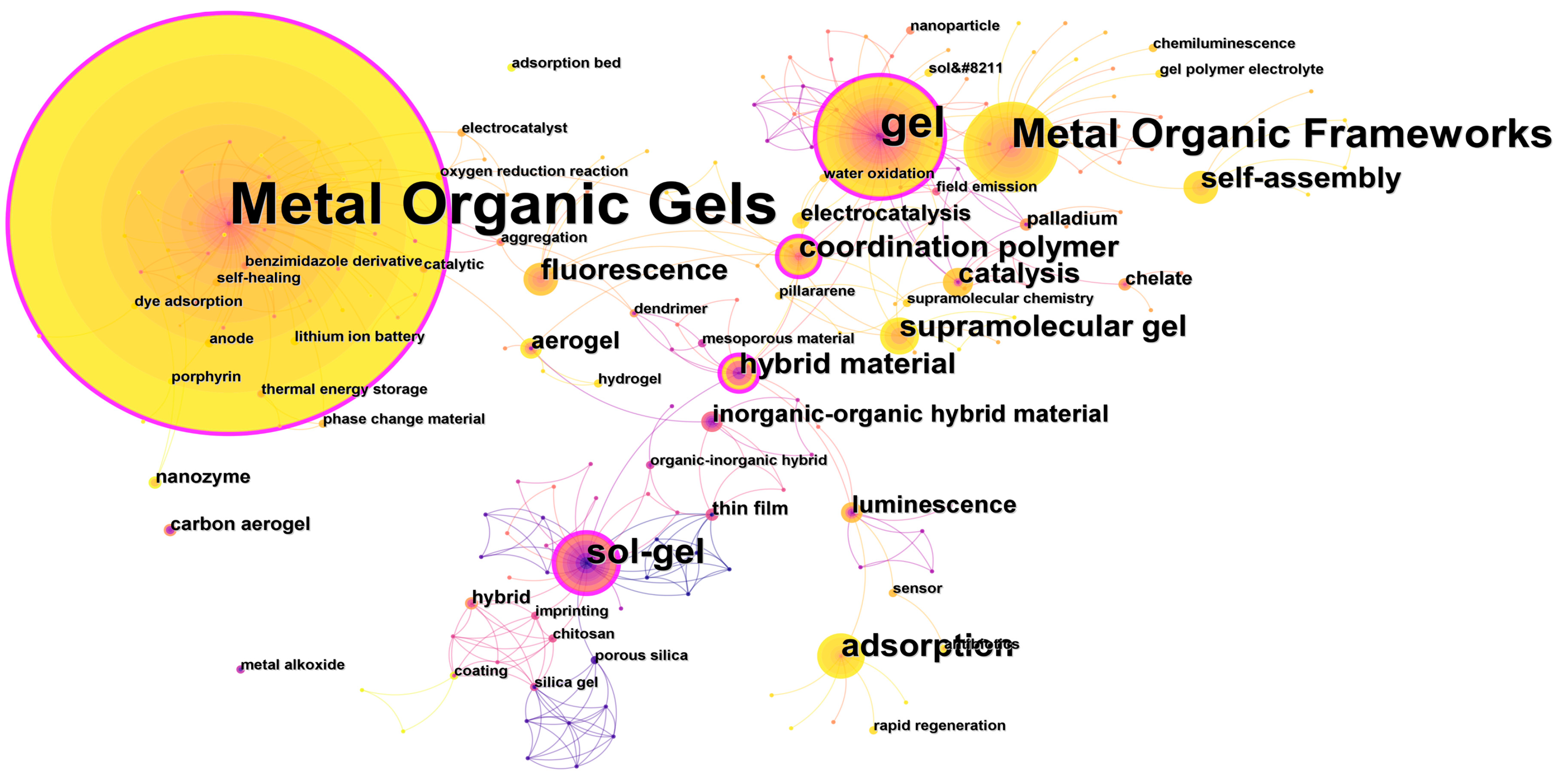
3.6.1. Keyword Co-Occurrence Network Analysis
3.6.2. Keyword Cluster Analysis
3.6.3. Keywords Time Zone Map
3.6.4. Analysis of Burst Keywords
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. Available online: https://www.sciencedirect.com/science/article/pii/S0011916409012193 (accessed on 26 November 2022). [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total. Environ. 2017, 597, 303–320. [Google Scholar] [CrossRef]
- Chen, J.-F.; Liu, X.; Ma, J.-F.; Han, B.-B.; Ding, J.-D.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. A pillar [5]arene-based multiple-stimuli responsive metal–organic gel was constructed for facile removal of mercury ions. Soft Matter 2017, 13, 5214–5218. [Google Scholar] [CrossRef] [PubMed]
- Sutar, P.; Maji, T.K. Coordination polymer gels: Soft metal–organic supramolecular materials and versatile applications. Chem. Commun. 2016, 52, 8055–8074. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Shenglin, X.; Shuqi, C.; Jianyong, Z.; Gangfeng, O.; Liuping, C.; Cheng-Yong, S. A synthetic route to ultralight hierarchically micro/mesoporous Al(III)-carboxylate metal-organic aerogels. Nat. Commun. 2013, 4, 1774. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Z.; Li, Y.; Zou, D. Three-in-one multifunctional luminescent metal-organic gels/sodium alginate beads for high-performance adsorption and detection of chlortetracycline hydrochloride, and high-security anti-counterfeiting. Chem. Eng. J. 2023, 452, 139194. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Chen, L.; Lu, G.; Shi, G.; Xie, X.; Wang, Y.; Sun, J. Enhanced adsorption and visible-light photocatalytic degradation of toluene by CQDs/UiO-66 MOG with hierarchical pores. Chem. Eng. J. 2022, 435, 135033. [Google Scholar] [CrossRef]
- Fang, Y.; Ren, G.; Ma, Y.; Wang, C.; Li, M.; Pang, X.; Pan, Q.; Li, J. Adsorption and reutilization of Pb(II) based on acid-resistant metal-organic gel. Sep. Purif. Technol. 2022, 295, 121253. [Google Scholar] [CrossRef]
- Wang, L.; Ke, F.; Zhu, J. Metal-organic gel templated synthesis of magnetic porous carbon for highly efficient removal of organic dyes. Dalton Trans. 2016, 45, 4541–4547. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26842305 (accessed on 26 November 2022). [CrossRef] [PubMed]
- Flory, P.J. Introductory lecture. Faraday Discuss. Chem. Soc. 1974, 57, 7–18. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.Y.; Yam, V.W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Gebel, G.; Ramasseul, R. Molecular Rods in a Zinc(II) Porphyrin/Cyclohexane Physical Gel: Neutron and X-ray Scattering Characterizations. Langmuir 1996, 12, 4321–4323. [Google Scholar] [CrossRef]
- Kawano, S.-i.; Fujita, N.; Shinkai, S. A Coordination Gelator That Shows a Reversible Chromatic Change and Sol−Gel Phase-Transition Behavior upon Oxidative/Reductive Stimuli. J. Am. Chem. Soc. 2004, 126, 8592–8593. [Google Scholar] [CrossRef]
- Lam, S.-T.; Wang, G.; Yam, V.W.-W. Luminescent Metallogels of Alkynylrhenium(I) Tricarbonyl Diimine Complexes. Organometallics 2008, 27, 4545–4548. [Google Scholar] [CrossRef]
- Ganta, S.; Chand, D.K. Multi-Stimuli-Responsive Metallogel Molded from a Pd(2)L(4)-Type Coordination Cage: Selective Removal of Anionic Dyes. Inorg. Chem. 2018, 57, 3634–3645. [Google Scholar] [CrossRef]
- Ni, Y.; Li, X.; Hu, J.; Huang, S.; Yu, H. Supramolecular Liquid-Crystalline Polymer Organogel: Fabrication, Multiresponsiveness, and Holographic Switching Properties. Chem. Mater. 2019, 31, 3388–3394. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Dietrich, D.; Licht, C.; Nuhnen, A.; Hofert, S.P.; De Laporte, L.; Janiak, C. Metal-Organic Gels Based on a Bisamide Tetracarboxyl Ligand for Carbon Dioxide, Sulfur Dioxide, and Selective Dye Uptake. ACS Appl. Mater. Interfaces 2019, 11, 19654–19667. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31063354 (accessed on 28 November 2022). [CrossRef] [PubMed]
- Luisi, B.S.; Rowland, K.D.; Moulton, B. Coordination polymer gels: Synthesis, structure and mechanical properties of amorphous coordination polymers. Chem. Commun. 2007, 27, 2802–2804. [Google Scholar] [CrossRef] [PubMed]
- Mollick, S.; Mandal, T.N.; Jana, A.; Fajal, S.; Ghosh, S.K. A hybrid blue perovskite@metal-organic gel (MOG) nanocomposite: Simultaneous improvement of luminescence and stability. Chem. Sci. 2019, 10, 10524–10530. [Google Scholar] [CrossRef]
- Naota, T.; Koori, H. Molecules That Assemble by Sound: An Application to the Instant Gelation of Stable Organic Fluids. J. Am. Chem. Soc. 2005, 127, 9324–9325. [Google Scholar] [CrossRef]
- Shirakawa, M.; Fujita, N.; Tani, T.; Kaneko, K.; Ojima, M.; Fujii, A.; Ozaki, M.; Shinkai, S. Organogels of 8-quinolinol/metal(ii)–chelate derivatives that show electron- and light-emitting properties. Chemistry 2007, 13, 4155–4162. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.200601813 (accessed on 26 November 2022). [CrossRef]
- Wei, S.-C.; Pan, M.; Li, K.; Wang, S.; Zhang, J.; Su, C.-Y. A Multistimuli-Responsive Photochromic Metal-Organic Gel. Adv. Mater. 2014, 26, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Mahendar, C.; Kumar, Y.; Dixit, M.K.; Mukherjee, M.; Kalam, A.; Dubey, M. Conductive Zn(ii)-metallohydrogels: The role of alkali metal cation size in gelation, rheology and conductance. Mol. Syst. Des. Eng. 2021, 6, 654–661. [Google Scholar] [CrossRef]
- Lee, H.; Jung, S.H.; Han, W.S.; Moon, J.H.; Kang, S.; Lee, J.Y.; Jung, J.H.; Shinkai, S. A Chromo-Fluorogenic Tetrazole-Based CoBr2 Coordination Polymer Gel as a Highly Sensitive and Selective Chemosensor for Volatile Gases Containing Chloride. Chem.—Eur. J. 2011, 17, 2823–2827. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201003279 (accessed on 26 November 2022). [CrossRef] [PubMed]
- Zhice, C.; Yuchen, X.; Huwei, W. Visualization analysis of research on climate innovation on CiteSpace. Front. Environ. Sci. 2022, 10, 1–21. [Google Scholar] [CrossRef]
- Ding, X.; Yang, Z. Knowledge mapping of platform research: A visual analysis using VOSviewer and CiteSpace. Electron. Commer. Res. 2020, 22, 787–809. [Google Scholar] [CrossRef]
- Chen, C.; Song, M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS ONE 2019, 14, e0223994. [Google Scholar] [CrossRef]
- Fahimnia, B.; Sarkis, J.; Davarzani, H.J.I.J.o.P.E. Green supply chain management: A review and bibliometric analysis. Int. J. Prod. Econ. 2015, 162, 101–114. [Google Scholar] [CrossRef]
- González-Hernández, I.J.; Granillo-Macías, R.; Rondero-Guerrero, C.; Simón-Marmolejo, I. Marshall-Olkin distributions: A bibliometric study. Scientometrics 2021, 126, 9005–9029. [Google Scholar] [CrossRef]
- Gaviria-Marin, M.; Merigó, J.M.; Baier-Fuentes, H. Knowledge management: A global examination based on bibliometric analysis. Technol. Forecast. Soc. Change 2019, 140, 194–220. Available online: https://www.sciencedirect.com/science/article/pii/S0040162517304055 (accessed on 26 November 2022). [CrossRef]
- Daniel Leite, M.; Gustavo Nunes, M.; Fabio, A.; Daniel Carvalho de, R. Autonomous Vehicles and People with Disabilities: A Scientometric and Integrative Analysis of the Literature. J. Scientometr. Res. 2022, 11, 332–343. [Google Scholar]
- Bornmann, L.; Haunschild, R. Empirical analysis of recent temporal dynamics of research fields: Annual publications in chemistry and related areas as an example. J. Informetr. 2022, 16, 101253. [Google Scholar] [CrossRef]
- Kleminski, R.; Kazienko, P.; Kajdanowicz, T. Analysis of direct citation, co-citation and bibliographic coupling in scientific topic identification. J. Inf. Sci. 2020, 48, 349–373. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, B.; Chinchilla-Rodríguez, Z.; Sugimoto, C.R.; Huang, Y.; Huang, W.-B. Considering author sequence in all-author co-citation analysis. Inf. Process. Manag. 2020, 57, 102300. Available online: https://www.sciencedirect.com/science/article/pii/S0306457320307950 (accessed on 26 November 2022). [CrossRef]
- Tam, A.Y.-Y.; Wong, K.M.-C.; Wang, G.; Yam, V.W.W. Luminescent metallogels of platinum(ii) terpyridyl complexes: Interplay of metal…Metal, pi-pi and hydrophobic-hydrophobic interactions on gel formation. Chem. Commun. 2007, 20, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 2013, 257, 7–8. [Google Scholar] [CrossRef]
- Ke, Z.; Tam, A.Y.-Y.; Chow, H.-F.; Yam, V.W.-W. A platinum(ii) terpyridine metallogel with an l-valine-modified alkynyl ligand: Interplay of pt pt, π–π and hydrogen-bonding interactions. Chemistry 2013, 19, 15735–15744. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201302702 (accessed on 29 November 2022). [CrossRef]
- Wei, Q.; James, S.L. A metal-organic gel used as a template for a porous organic polymer. Chem. Commun. 2005, 1555–1556. [Google Scholar] [CrossRef]
- Lohe, M.R.; Rose, M.; Kaskel, S. Metal-organic framework (MOF) aerogels with high micro- and macroporosity. Chem. Commun. 2009, 6056–6058. [Google Scholar] [CrossRef]
- Cong, Y.; Liu, S.; Wu, F.; Zhang, H.; Fu, J. Shape memory effect and rapid reversible actuation of nanocomposite hydrogels with electrochemically controlled local metal ion coordination and crosslinking. J. Mater. Chem. B 2020, 8, 9679–9685. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32985643 (accessed on 5 January 2023). [CrossRef]
- Samai, S.; Biradha, K. Chemical and Mechano Responsive Metal–Organic Gels of Bis(benzimidazole)-Based Ligands with Cd(II) and Cu(II) Halide Salts: Self Sustainability and Gas and Dye Sorptions. Chem. Mater. 2012, 24, 1165–1173. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, M.; Tang, D.; Yan, X.; Zhang, Z.; Zhou, Z.; Song, B.; Wang, H.; Li, X.; Yin, S.; et al. Fluorescent metallacage-core supramolecular polymer gel formed by orthogonal metal coordination and host-guest interactions. J. Am. Chem. Soc. 2018, 140, 7674–7680. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29856215 (accessed on 5 January 2023). [CrossRef]
- Yang, Q.; Tan, X.; Wang, S.; Zhang, J.; Chen, L.; Zhang, J.-P.; Su, C.-Y. Porous organic–inorganic hybrid aerogels based on bridging acetylacetonate. Microporous Mesoporous Mater. 2013, 187, 108–113. [Google Scholar] [CrossRef]
- Chen, C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, S.; Pan, H.; Yang, G.; Wang, L.; Tao, C.-A.; Li, H. Synthesis of macroscopic monolithic metal–organic gels for ultra-fast destruction of chemical warfare agents. RSC Adv. 2021, 11, 22125–22130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, Q.; Yin, M.; Ren, X.; Wang, J.; Zhou, Y.-H. A metal–organic gel based on silver salt and 2-amino-5-mercapto-1,3,4-thiadiazole with high antibacterial activity and excellent dye adsorption performance†. New J. Chem. 2016, 40, 9125–9131. [Google Scholar] [CrossRef]
- Mengting, W.; Siyu, D.; Zhongkui, W.; Xiaodong, W.; Xiaolan, Y.; Baotuo, C. A new type of porous Zn (II) metal-organic gel designed for effective adsorption to methyl orange dye. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127335. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Lal, R.A. Vanadium(v) complex based supramolecular metallogel: Self-assembly and (metallo)gelation triggered by non-covalent and n+h…o hydrogen bonding interactions. Inorg. Chem. Commun. 2020, 111, 107642. [Google Scholar] [CrossRef]
- Wang, Z.; Aoyama, T.; Sánchez-González, E.; Inose, T.; Urayama, K.; Furukawa, S. Control of Extrinsic Porosities in Linked Metal–Organic Polyhedra Gels by Imparting Coordination-Driven Self-Assembly with Electrostatic Repulsion. ACS Appl. Mater. Interfaces 2022, 14, 23660–23668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, H.; Li, C.; Li, S.; Liu, K.; Wang, L. Facile coordination driven synthesis of metal-organic gels toward efficiently electrocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 299, 120641. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, Z.; Li, Y.; Huang, C.; Li, Y. Metal–Organic Gel-Derived Multimetal Oxides as Effective Electrocatalysts for the Oxygen Evolution Reaction. ChemSusChem 2019, 12, 2480–2486. [Google Scholar] [CrossRef]
- 54. Yu, H.-T.; Tang, J.-W.; Feng, Y.-Y.; Feng, W. Structural Design and Application of Azo-based Supramolecular Polymer Systems. Chin. J. Polym. Sci. 2019, 37, 1183–1199. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Chen, J.-H.; Lin, Y.; Chen, H.-T.; Lin, C.-H.; Huang, H.-Y. Nitrogen-doped porous carbon material derived from metal–organic gel for small biomolecular sensing. Chem. Commun. 2017, 53, 5725–5728. [Google Scholar] [CrossRef]
- Sun, S.; Liao, P.; Zeng, L.; He, L.; Zhang, J. UiO-67 metal–organic gel material deposited on photonic crystal matrix for photoelectrocatalytic hydrogen production. RSC Adv. 2020, 10, 14778–14784. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-J.; Zhang, X.; Dong, H.; Yang, D.-D.; Tang, H.-L.; Zhai, Y.-C.; Wei, J.-Z.; Zhang, F.-M. Porous metal–organic gel assisted by l-tartaric acid ligand for efficient and controllable drug delivery†. New J. Chem. 2018, 42, 14789–14795. [Google Scholar] [CrossRef]
- Wang, H.-S.; Wang, X.-Y.; Ding, H.-T.; Hu, X.-Y.; Li, J.; Cheng, C.; Zheng, F. Oral Metal–Organic Gel Protected Whole-Cell Biosensor for in Situ Monitoring Nitrosamines in the Gastrointestinal Tract. Nano Lett. 2022, 22, 8688–8694. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, R.; Maimaituerxun, M. Bibliometric review of carbon neutrality with CiteSpace: Evolution, trends, and framework. Environ. Sci. Pollut. Res. Int. 2022, 29, 76668–76686. Available online: https://www.ncbi.nlm.nih.gov/pubmed/36169840 (accessed on 26 November 2022). [CrossRef]
- Anis Muneerah Shaiful, B.; Siti Zubaidah, O.; Mohammad Faizulizwan Mohamad, F.; Mohd Zul Amzar, Z.; Saidatul Akmal, B.; Mohammad Aminul, I.; Zarina, A.; Nowshad, A.; Halina, M. Facile synthesis of Zr-based metal-organic gel (Zr-MOG) using “green” sol-gel approach. Surf. Interfaces 2021, 27, 101469. [Google Scholar] [CrossRef]
- Gu, D.; Yang, W.; Lin, D.; Qin, X.; Yang, Y.; Wang, F.; Pan, Q.; Su, Z. Water-stable lanthanide-based metal–organic gel for the detection of organic amines and white-light emission. J. Mater. Chem. C 2020, 8, 13648–13654. [Google Scholar] [CrossRef]
- Li, L.; Cong, Y.; He, L.; Wang, Y.; Wang, J.; Zhang, F.-M.; Bu, W. Multiple stimuli-responsive supramolecular gels constructed from metal–organic cycles†. Polym. Chem. 2016, 7, 6288–6292. [Google Scholar] [CrossRef]
- Lu, S.-M.; Huang, J.-C.; Liu, G.-T.; Lin, Z.-W.; Li, Y.-T.; Huang, X.-H.; Huang, C.-C.; Wu, S.-T. Ammonia-modulated reversible gel–solution phase transition and fluorescence switch for a salicylhydrazide-based metal–organic gel†. RSC Adv. 2017, 7, 30979–30983. [Google Scholar] [CrossRef]
- Tan, S.Y.; Ang, C.Y.; Mahmood, A.; Qu, Q.; Li, P.; Zou, R.; Zhao, Y. Doxorubicin-Loaded Metal–Organic Gels for pH and Glutathione Dual-Responsive Release. ChemNanoMat 2016, 2, 504. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, J. Metal-Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2019, 59, 9922. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, T.X. Postsynthetic Modification of Mixed-Ligand Metal-Organic Gels for Adsorbing Nonpolar Organic Solvents. ChemistrySelect 2021, 6, 12351. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Z.; Jia, A.; Wang, Y.; Li, N.; Liu, Z.; Li, Y.; Zhang, H. New insight into adsorption and co-adsorption of chlortetracycline hydrochloride and ciprofloxacin hydrochloride by Ga-based metal-organic gel/sodium alginate composite beads. Sep. Purif. Technol. 2023, 312, 123408. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Pathway Dependence in Redox-Driven Metal-Organic Gels. Chem.—Eur. J. 2020, 26, 6130–6135. [Google Scholar] [CrossRef]
- Sun, S.; Wei, C.; Xiao, Y.; Li, G.; Zhang, J. Zirconium-based metal–organic framework gels for selective luminescence sensing. RSC Adv. 2020, 10, 44912–44919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, B.-H.; Liu, D.-J. Metal–Organic Frameworks and Metal–Organic Gels for Oxygen Electrocatalysis: Structural and Compositional Considerations. Adv. Mater. 2021, 33, 2008023. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Zheng, J.; He, C.; Zhang, J.; Xie, Y.; Li, Y.; Tang, B.; Rui, Y.; Liu, F. Self-healable metal-organic gel membranes as anodes with high lithium storage. Electrochim. Acta 2021, 386, 138334. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Li, H.L.; Zhang, X.H.; Li, Q.P.; Luo, S.W.; Liu, F.Q. Antibiofouling Performance of a Metal-Organic Gel Based on 2-Amino-5-Mercapto-1,3,4-Thiadiazole and CuI. Adv. Mater. Interfaces 2022, 9, 2201688. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Lee, K.S. Moving bed adsorption process based on a PEI-silica sorbent for CO2 capture. Int. J. Greenh. Gas. Control. 2017, 67, 10–19. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- İsmail, O.; Gökçe Kocabay, Ö. Absorption and adsorption studies of polyacrylamide/sodium alginate hydrogels. Colloid. Polym. Sci. 2021, 299, 783–796. [Google Scholar] [CrossRef]
- Qing, Z.; Wang, L.; Liu, X.; Song, Z.; Qian, F.; Song, Y. Simply synthesized sodium alginate/zirconium hydrogel as adsorbent for phosphate adsorption from aqueous solution: Performance and mechanisms. Chemosphere 2021, 291, 133103. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Xu, J.-Y.; Yang, X.-L. MXene/Sodium Alginate Gel Beads for Adsorption of Methylene Blue. Mater. Chem. Phys. 2021, 260, 124123. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, X.; Feng, S. Preparation of microgel/sodium alginate composite granular hydrogels and their Cu2+ adsorption properties. RSC Adv. 2016, 6, 100511–100518. [Google Scholar] [CrossRef]
- Panja, A.; Ghosh, K. Triazole-amide isosteric pyridine-based supramolecular gelators in metal ion and biothiol sensing with excellent performance in adsorption of heavy metal ions and picric acid from water†. New. J. Chem. 2018, 43, 934–945. [Google Scholar] [CrossRef]
- Shijina, K.; Illathvalappil, R.; Sumitha, N.S.; Sailaja, G.S.; Kurungot, S.; Nair, B.N.; Peer Mohamed, A.; Anilkumar, G.M.; Yamaguchi, T.; Hareesh, U.S. Melamine formaldehyde–metal organic gel interpenetrating polymer network derived intrinsic Fe–N-doped porous graphitic carbon electrocatalysts for oxygen reduction reaction†. New. J. Chem. 2018, 42, 18690–18701. [Google Scholar] [CrossRef]
- He, L.; Li, Y.; Wu, Q.; Wang, D.M.; Li, C.M.; Huang, C.Z.; Li, Y.F. Ru(III)-Based Metal–Organic Gels: Intrinsic Horseradish and NADH Peroxidase-Mimicking Nanozyme. ACS Appl. Mater. Interfaces 2019, 11, 29158–29166. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Z.; Ma, T.; Liu, Z.; Li, Y.; Zou, D. Luminescent cellulose-based porous binary metal-organic gels in an adsorption bed for effective adsorption and sensitive detection of chlortetracycline hydrochloride. J. Hazard. Mater. 2021, 414, 125473. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33652220 (accessed on 20 November 2022). [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Xia, Y.; Li, D.; Yin, B.; Hou, R. A novel tetrathiafulvalene based liquid crystalline organogelator: Synthesis, self-assembly properties and potential utilization. New J. Chem. 2022, 46, 22663–22671. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, H.; Rehman, S.; Zhang, P. Energy-efficient capture of volatile organic compounds from humid air by granular metal organic gel. J. Hazard. Mater. 2021, 411, 125057. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33465540 (accessed on 21 November 2022). [CrossRef] [PubMed]
- Hong, Y.; Gao, Z.; Chen, M.; Hao, J.; Dong, S. Metal-Organic Gels of Catechol-Based Ligands with Ni(II) Acetate for Dye Adsorption. Langmuir 2018, 34, 9435–9441. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-j.; Ji, Y.-X.; Cao, J.-F.; Xin, Z.-F. Polyoxometalate encapsulated in metal-organic gel as an efficient catalyst for visible-light-driven dye degradation applications. Appl. Organomet. Chem. 2018, 32, e4206. [Google Scholar] [CrossRef]
- Zhang, W.; Dynes, J.J.; Hu, Y.; Jiang, P.; Ma, S. Porous metal-metalloporphyrin gel as catalytic binding pocket for highly efficient synergistic catalysis. Nat. Commun. 2019, 10, 1913. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31015441 (accessed on 26 November 2022). [CrossRef]
- Li, B.; Zhou, X.; Yan, B.; Li, Y.; Ye, H.; Huang, G.; Zhou, Q. In2S3-Polymer Hybrid Gels Derived from In(III) Metal–Organic Gels for Dye Adsorption, Photodegradation, and Bacteria Removal. Macromol. Mater. Eng. 2021, 306, 2100290. [Google Scholar] [CrossRef]
- Fu, H.G.; You, J. Novel Porous Rhodium Metal-Organic Aerogel for Efficient Removal of Organic Dyes and Catalysis of Si-H Insertion Reactions. ACS Omega 2021, 6, 26766–26772. [Google Scholar] [CrossRef]
- Sun, Y.; Dramou, P.; Song, Z.; Zheng, L.; Zhang, X.; Ni, X.; He, H.J.M.J. Lanthanide Metal Doped Organic Gel as Ratiometric Fluorescence Probe for Selective Monitoring of Ciprofloxacin. Microchem. J. 2022, 179, 107476. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Liu, P.; Zhang, G.; Song, L.; Zou, X.; Fu, Y. A Superstable Luminescent Lanthanide Metal Organic Gel Utilized in an Electrochemiluminescence Sensor for Epinephrine Detection with a Narrow Potential Sweep Range. ACS Sens. 2021, 6, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, M.; Fang, X.; Zhang, L.; Ji, L.; Liu, Y.; Xu, K.; Yu, H. Dual channel detecting of CO in a metal complex gel system: Phase transition and fluorescence enhancement. Colloid. Interface Sci. Commun. 2022, 48, 100613. Available online: https://www.sciencedirect.com/science/article/pii/S2215038222000309 (accessed on 23 November 2022). [CrossRef]
- Zhao, T.T.; Jiang, Z.W.; Zhen, S.J.; Huang, C.Z.; Li, Y.F. A copper(II)/cobalt(II) organic gel with enhanced peroxidase-like activity for fluorometric determination of hydrogen peroxide and glucose. Microchim. Acta 2019, 186, 168. [Google Scholar] [CrossRef]
- Qin, Z.-S.; Dong, W.-W.; Zhao, J.; Wu, Y.-P.; Zhang, Q.; Li, D.-S. A water-stable Tb(iii)-based metal–organic gel (MOG) for detection of antibiotics and explosives. Inorg. Chem. Front. 2018, 5, 120–126. [Google Scholar] [CrossRef]
- Karan, C.K.; Mallick, S.; Raj, C.R.; Bhattacharjee, M. A Self-Healing Metal–Organic Hydrogel for an All-Solid Flexible Supercapacitor. Chemistry 2019, 25, 14775–14779. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201903561 (accessed on 26 November 2022). [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, L.; Rao, H.-S.; Yang, Q.-L.; Zhang, J.; Chen, H.-Y.; Chen, L.; Kuang, D.-B.; Su, C.-Y. A novel metal–organic gel based electrolyte for efficient quasi-solid-state dye-sensitized solar cells†. J. Mater. Chem. A 2014, 2, 15406–15413. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Rao, H.-S.; Cao, Y.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. In situ gelation of Al(III)-4-tert-butylpyridine based metal-organic gel electrolyte for efficient quasi-solid-state dye-sensitized solar cells. J. Power Sources 2017, 343, 148–155. [Google Scholar] [CrossRef]





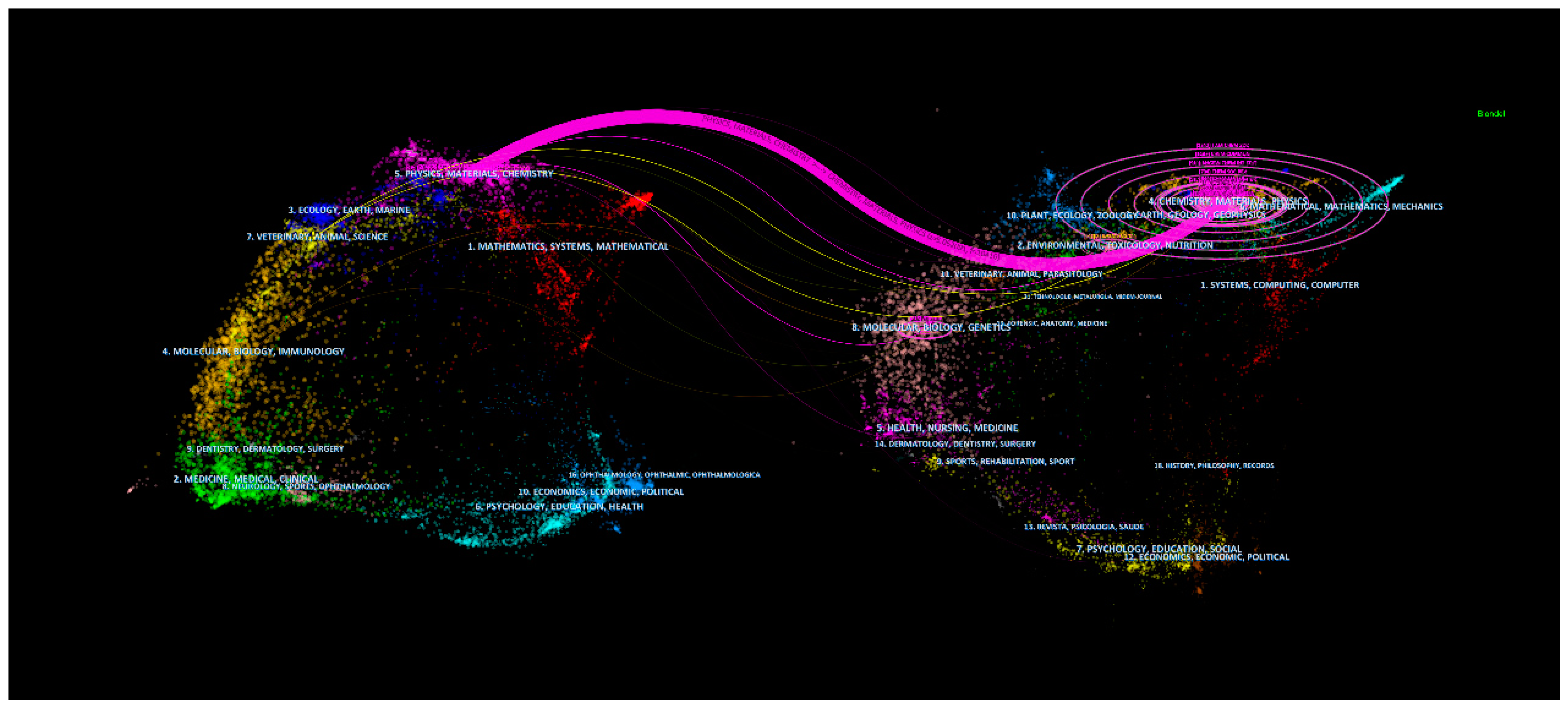


| Rank | Countries | Publication Number | Percentage (%) | First Published Year | Centrality |
|---|---|---|---|---|---|
| 1 | China | 224 | 41.71 | 1998 | 0.31 |
| 2 | Japan | 54 | 10.06 | 2001 | 0.23 |
| 3 | India | 45 | 8.38 | 1994 | 0.06 |
| 4 | USA | 28 | 5.21 | 1993 | 0.02 |
| 5 | France | 20 | 3.72 | 1995 | 0.18 |
| 6 | Germany | 20 | 3.72 | 2000 | 0.19 |
| 7 | Spain | 17 | 3.17 | 1999 | 0.13 |
| 8 | South Korea | 13 | 2.42 | 2000 | 0.13 |
| 9 | Russia | 12 | 2.23 | 1999 | 0.07 |
| 10 | Austria | 11 | 2.05 | 2000 | 0 |
| Rank | Frequency | Author | Year | Half-Value Period |
|---|---|---|---|---|
| 1 | 26 | Zhang, Jianyong | 2009 | 6.5 |
| 2 | 24 | Li, Yuan Fang | 2017 | 1.5 |
| 3 | 16 | Huang, Cheng Zhi | 2017 | 1.5 |
| 4 | 10 | Su, Cheng-Yong | 2009 | 4.5 |
| 5 | 9 | Chen, Liuping | 2009 | 4.5 |
| 6 | 8 | Jiang, Zhong Wei | 2017 | 1.5 |
| 7 | 7 | Li, Yang | 2018 | 0.5 |
| 8 | 7 | He, Li | 2017 | 1.5 |
| 9 | 6 | Biradha, Kumar | 2012 | 4.5 |
| 10 | 6 | Schubert, U | 1994 | 5.5 |
| Rank | Frequency | Institution | Year | Half-Value Period |
|---|---|---|---|---|
| 1 | 26 | Sun Yat-sen University | 2009 | 7.5 |
| 2 | 24 | Southwest University | 2015 | 3.5 |
| 3 | 16 | Chinese Acad Sci | 2007 | 7.5 |
| 4 | 10 | Indian Inst Technol | 2012 | 4.5 |
| 5 | 9 | Vienna Univ Technol | 2000 | 3.5 |
| 6 | 8 | Jilin University | 2016 | 5.5 |
| 7 | 7 | Qingdao Univ Sci & Technol | 2006 | 13.5 |
| 8 | 7 | Indian Assoc Cultivat Sci | 2013 | 0.5 |
| 9 | 6 | Tokyo Inst Technol | 2000 | 9.5 |
| 10 | 6 | Kyoto Univ | 1998 | 22.5 |
| Rank | Citations | Author | Year | Journal | Title | Reference |
|---|---|---|---|---|---|---|
| 1 | 72 | Piepenbrock | 2010 | CHEM REV | Metal- and Anion-Binding Supramolecular Gels | [11] |
| 2 | 60 | Li L | 2013 | NAT COMMUN | A synthetic route to ultralight hierarchically micro/mesoporous Al(III)-carboxylate metal-organic aerogels | [37] |
| 3 | 54 | Zhang JY | 2013 | COORDIN CHEM REV | Metal-organic gels: From discrete metallogelators to coordination polymers | [38] |
| 4 | 53 | Tam AYY | 2013 | CHEM SOC REV | Recent advances in metallogels | [39] |
| 5 | 37 | Wei Q | 2005 | CHEM COMMUN | A metal–organic gel used as a template for a porous organic polymer | [40] |
| 6 | 33 | Lohe MR | 2009 | CHEM COMMUN | Metal–organic framework (MOF) aerogels with high micro- and macroporosity | [41] |
| 7 | 32 | Sutar P | 2016 | CHEM COMMUN | Coordination polymer gels: soft metal–organic supramolecular materials and versatile applications | [42] |
| 8 | 32 | Samai S | 2012 | CHEM MATER | Chemical and Mechano Responsive Metal–Organic Gels of Bis(benzimidazole)-Based Ligands with Cd(II) and Cu(II) Halide Salts: Self Sustainability and Gas and Dye Sorptions | [43] |
| 9 | 32 | Sangeetha | 2005 | CHEM SOC REV | Supramolecular gels: Functions and uses | [44] |
| 10 | 30 | Xiang SL | 2012 | J MATER CHEM | Porous organic–inorganic hybrid aerogels based on Cr3+/Fe3+ and rigid bridging carboxylates | [45] |
| Rank | Frequency | Keywords | Centrality |
|---|---|---|---|
| 1 | 101 | Metal Organic Gels | 0.35 |
| 2 | 29 | gel | 0.28 |
| 3 | 22 | Metal Organic Frameworks | 0.09 |
| 4 | 14 | sol-gel | 0.22 |
| 5 | 11 | adsorption | 0.03 |
| 6 | 9 | supramolecular gel | 0.03 |
| 7 | 9 | coordination polymer | 0.31 |
| 8 | 8 | hybrid material | 0.29 |
| 9 | 8 | self-assembly | 0.03 |
| 10 | 8 | fluorescence | 0.03 |
| 11 | 7 | catalysis | 0.03 |
| 12 | 5 | inorganic-organic hybrid material | 0.03 |
| ID | Number of Nodes | Silhouette | Average Years | Main Content |
|---|---|---|---|---|
| 0 | 52 | 0.973 | 2017 | metal–organic gel (17.65, 0.0001); metallogel (10.32, 0.005); metal–organic gels (7.84, 0.01); thermal energy storage (6.86, 0.01); catalytic (6.86, 0.01) |
| 1 | 42 | 0.911 | 2012 | gels (37.64, 0.0001); chelates (14.12, 0.001); pillararenes (9.38, 0.005); coordination polymers (9.38, 0.005); nanoparticles (9.38, 0.005) |
| 2 | 30 | 0.995 | 1999 | thin film (19.69, 0.0001); sol–gel (17.06, 0.0001); pzt films (6.49, 0.05); moisture (6.49, 0.05); surface (6.49, 0.05) |
| 3 | 18 | 0.924 | 2017 | catalysis (9.46, 0.005); self-assembly (9.46, 0.005); bifunctional oxygen electrocatalyst (6.56, 0.05); 4-nitrophenol (6.56, 0.05); organophosphorus pesticides (6.56, 0.05) |
| 4 | 18 | 0.994 | 2002 | silica gel (11.75, 0.001); transition metal salts (7.69, 0.01); polyoxazoline (7.69, 0.01); pyrolysis (7.69, 0.01); organic–inorganic (7.69, 0.01) |
| 5 | 18 | 0.993 | 2004 | sol–gel hybrid coatings (7.56, 0.01); functional groups (7.56, 0.01); metal alkoxide (7.56, 0.01); organoalkoxysilanes (7.56, 0.01); abrasion resistance (7.56, 0.01) |
| 6 | 17 | 0.967 | 2015 | metal–organic frameworks (14.61, 0.001); metal–organic framework (12.61, 0.001); heavy metal ion (6.27, 0.05); drug loading (6.27, 0.05); assembly (6.27, 0.05) |
| 7 | 14 | 0.950 | 2017 | supramolecular gels (10.75, 0.005); glucosamine (7.21, 0.01); carbohydrate (7.21, 0.01); Ag+ sensing (7.21, 0.01); ionic conductivity (7.21, 0.01) |
| 8 | 13 | 0.941 | 2011 | aggregation (15.25, 0.0001); sol–gel processes (11.48, 0.001); fluorescence (9.79, 0.005); ionic liquids (7.56, 0.01); benzimidazolylidene complexes (7.56, 0.01) |
| 9 | 13 | 0.985 | 2010 | adsorption (13.9, 0.001); phosphor (13.9, 0.001); luminescence (13.9, 0.001); in situ synthesis of metal–organic frameworks (6.9, 0.01); alginate gel (6.9, 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Li, S.; Shi, C.; Huo, M.; Lin, Y. Progress in Research and Application of Metal–Organic Gels: A Review. Nanomaterials 2023, 13, 1178. https://doi.org/10.3390/nano13071178
Liu G, Li S, Shi C, Huo M, Lin Y. Progress in Research and Application of Metal–Organic Gels: A Review. Nanomaterials. 2023; 13(7):1178. https://doi.org/10.3390/nano13071178
Chicago/Turabian StyleLiu, Gen, Siwen Li, Chunyan Shi, Mingxin Huo, and Yingzi Lin. 2023. "Progress in Research and Application of Metal–Organic Gels: A Review" Nanomaterials 13, no. 7: 1178. https://doi.org/10.3390/nano13071178
APA StyleLiu, G., Li, S., Shi, C., Huo, M., & Lin, Y. (2023). Progress in Research and Application of Metal–Organic Gels: A Review. Nanomaterials, 13(7), 1178. https://doi.org/10.3390/nano13071178






