Tunable Raman Gain in Transparent Nanostructured Glass-Ceramic Based on Ba2NaNb5O15 †
Abstract
1. Introduction
2. Materials and Methods
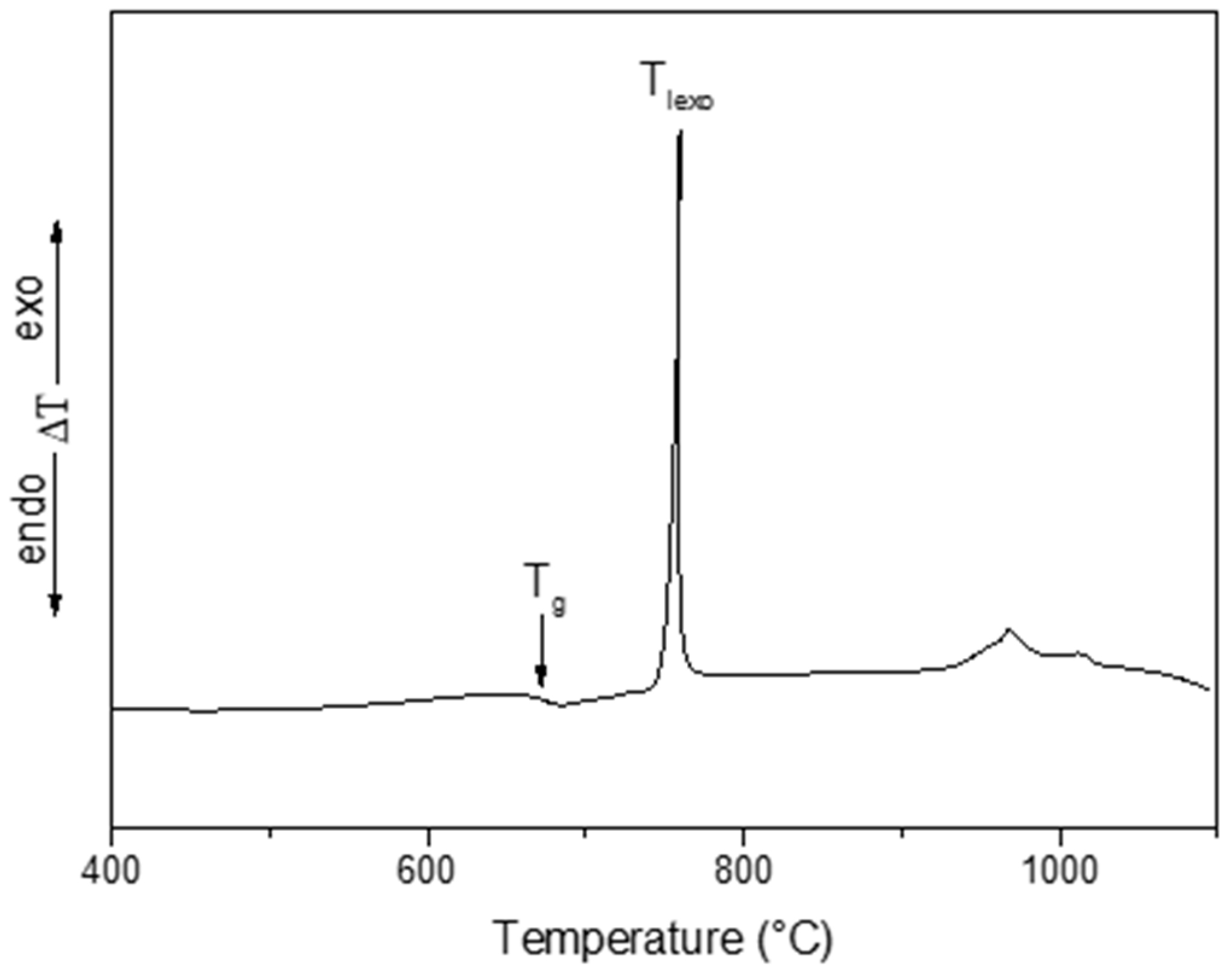
2.1. Preparation of Samples and Thermal Analysis
2.2. Structural Characterization
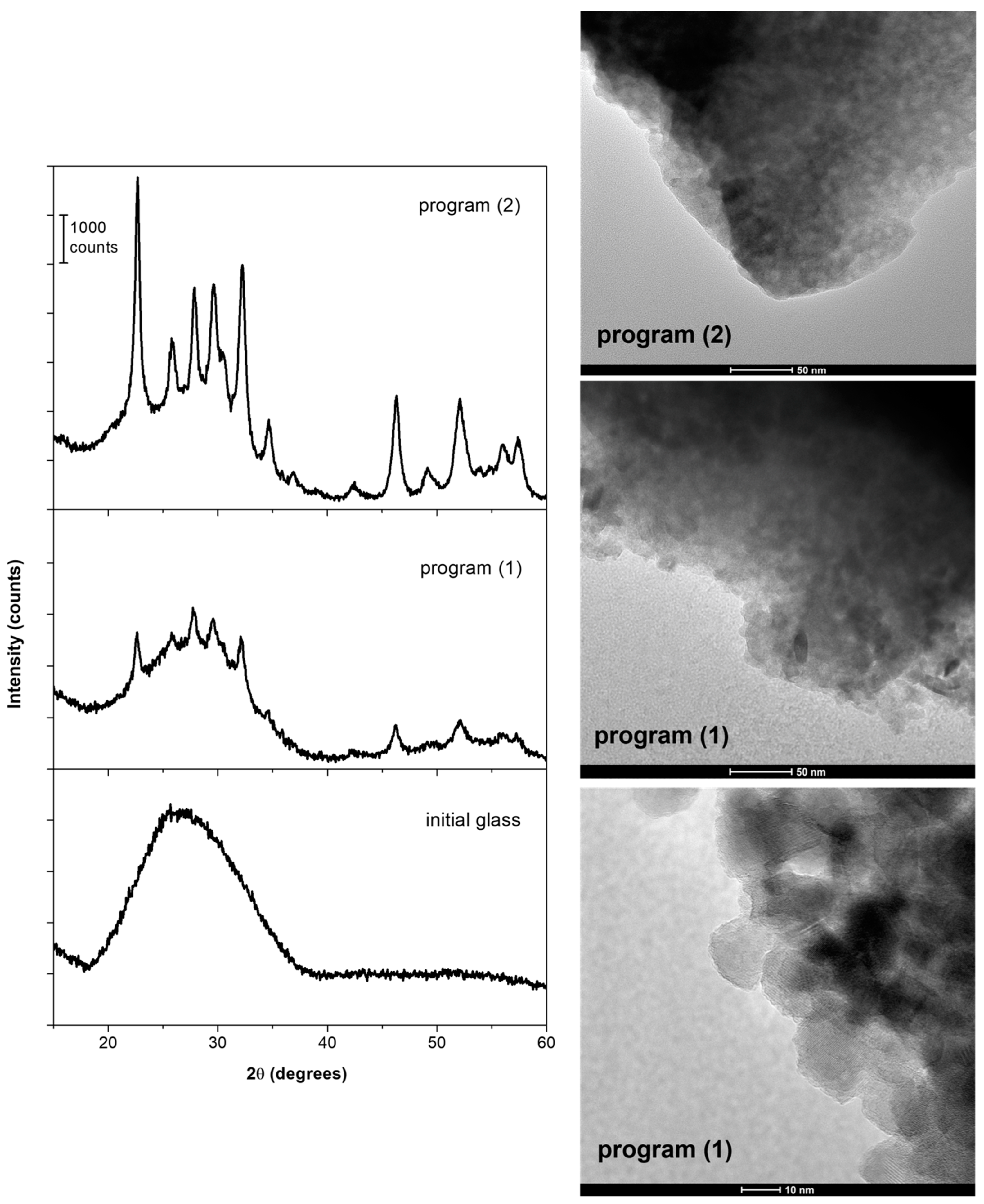
2.2.1. XRD and TEM
2.2.2. Scanning Electron Microscopy (SEM) and EDS
2.2.3. Linear and Nonlinear Optical Characterization
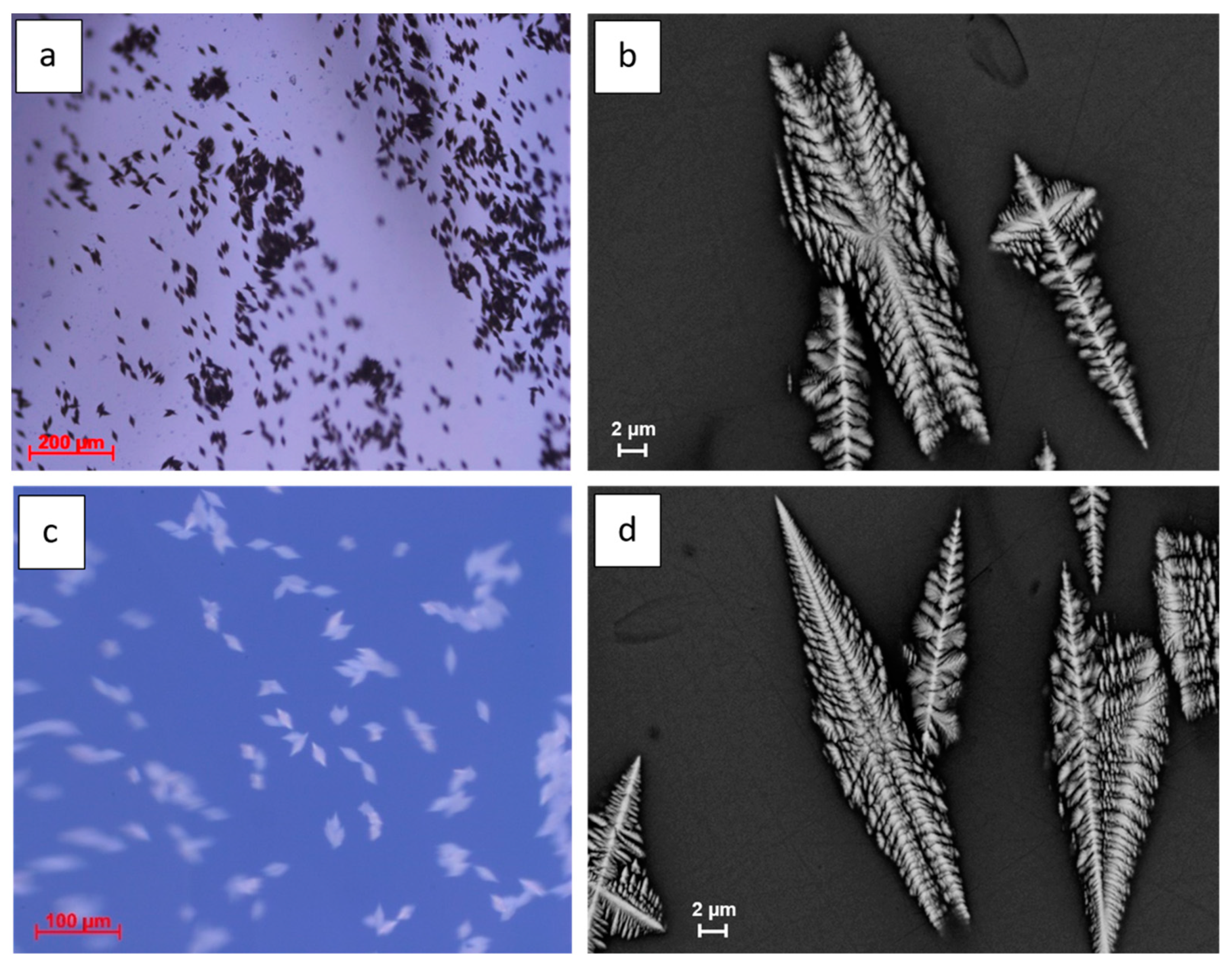
Optical Microscopy
Ellipsometric Characterization
Raman Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, J.A.; Bloembergen, N.; Ducuing, J.; Pershan, P.S. Interactions between Light Waves in a Nonlinear Dielectric. Phys. Rev. 1962, 127, 1918–1939. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Shen, Y.R. The Principles of Nonlinear Optics; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Agrawal, G.P.; Boyd, R.W. Contemporary Nonlinear Optics; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Banfi, G.; Degiorgio, V.; Ricard, D. Nonlinear optical properties of semiconductor nanocrystals. Adv. Phys. 1998, 47, 447–510. [Google Scholar] [CrossRef]
- Prasad, P.N. Nanophotonics; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Haus, J.W. Fundamentals and Applications of Nanophotonics; Woodhead: Sawston, UK, 2016. [Google Scholar]
- Zheludev, N.I. Nonlinear optics on the nano scale. Contemp. Phys. 2010, 43, 365–377. [Google Scholar] [CrossRef]
- Butet, J.; Brevet, P.-F.; Martin, O.J.F. Optical second harmonic generation in plasmonic nanostructures: From fundamental principles to advanced applications. ACS Nano 2015, 9, 10545–10562. [Google Scholar] [CrossRef]
- Bonacina, L.; Brevet, P.-F.; Finazzi, M.; Celebrano, M. Harmonic generation at the nanoscale. J. Appl. Phys. 2020, 127, 230901. [Google Scholar] [CrossRef]
- Hellwarth, R.W. Theory of Stimulated Raman Scattering. Phys. Rev. 1963, 130, 1850–1852. [Google Scholar] [CrossRef]
- Bloembergen, N.; Shen, Y.R. Coupling Between Vibrations and Light Waves in Raman Laser Media. Phys. Rev. Lett. 1964, 12, 504–507. [Google Scholar] [CrossRef]
- Shen, Y.R.; Bloembergen, N. Theory of Stimulated Brillouin and Raman Scattering. Phys. Rev. 1965, 137, A1787–A1805. [Google Scholar] [CrossRef]
- Raymer, M.G.; Walmsley, I.A.; Mostowski, J.; Sobolewska, B. Quantum theory of spatial and temporal coherence properties of stimulated Raman scattering. Phys. Rev. A 1985, 32, 332–344. [Google Scholar] [CrossRef]
- Pask, H. The design and operation of solid-state Raman lasers. Prog. Quantum Electron. 2003, 27, 3–56. [Google Scholar] [CrossRef]
- Cerný, P. Solid state lasers with Raman frequency conversion. Prog. Quantum Electron. 2004, 28, 113–143. [Google Scholar] [CrossRef]
- Piper, J.A.; Pask, H.M. Crystalline Raman Lasers. IEEE J. Sel. Top. Quantum Electron. 2007, 13, 692–704. [Google Scholar] [CrossRef]
- Pask, H.; Dekker, P.; Mildren, R.; Spence, D.; Piper, J. Wavelength-versatile visible and UV sources based on crystalline Raman lasers. Prog. Quantum Electron. 2008, 32, 121–158. [Google Scholar] [CrossRef]
- Boyraz, O.; Jalali, B. Demonstration of a silicon Raman laser. Opt. Express 2004, 12, 5269–5273. [Google Scholar] [CrossRef]
- Rong, H.; Liu, A.; Jones, R.; Cohen, O.; Hak, D.; Nicolaescu, R.; Fang, A.; Paniccia, M. An all-silicon Raman laser. Nature 2005, 433, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Spillane, S.M.; Kippenberg, T.J.; Vahala, K.J. Ultralow-threshold Raman laser using a spherical dielectric microcavity. Nature 2002, 415, 621–623. [Google Scholar] [CrossRef]
- Takahashi, Y.; Inui, Y.; Chihara, M.; Asano, T.; Terawaki, R.; Noda, S. A micrometre-scale Raman silicon laser with a microwatt threshold. Nature 2013, 498, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Latawiec, P.; Venkataraman, V.; Burek, M.J.; Hausmann, B.J.M.; Bulu, I.; Lončar, M. On-chip diamond Raman laser. Optica 2015, 2, 924–928. [Google Scholar] [CrossRef]
- Rukhlenko, I.D.; Kalavally, V. Raman Amplification in Silicon-Nanocrystal Waveguides. J. Light. Technol. 2013, 32, 130–134. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Okano, M.; Shishido, H.; Noda, S.; Takahashi, Y. Implementing a Raman silicon nanocavity laser for integrated optical circuits by using a (100) SOI wafer with a 45-degree-rotated top silicon layer. OSA Contin. 2019, 2, 2098–2112. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Sen, M. An integrable all-silicon slotted photonic crystal Raman laser. J. Appl. Phys. 2019, 126, 233103. [Google Scholar] [CrossRef]
- Cao, L.; Nabet, B.; Spanier, J.E. Enhanced Raman Scattering from Individual Semiconductor Nanocones and Nanowires. Phys. Rev. Lett. 2006, 96, 157402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.P.; Shimazaki, K.; Shiokawa, T.; Suzuki, M.; Ishibashi, K.; Saito, R. Stimulated Raman scattering from individual single-wall carbon nanotubes. Appl. Phys. Lett. 2006, 88, 241101. [Google Scholar] [CrossRef]
- Wu, J.; Gupta, A.K.; Gutierrez, H.R.; Eklund, P.C. Cavity-Enhanced Stimulated Raman Scat-tering from Short GaP Nanowires. Nano Lett. 2009, 9, 3252–3257. [Google Scholar] [CrossRef]
- Cho, C.-H.; Aspetti, C.O.; Park, J.; Agarwal, R. Silicon coupled with plasmon nanocavities generates bright visible hot luminescence. Nat. Photonics 2013, 7, 285–289. [Google Scholar] [CrossRef]
- Agarwal, D.; Ren, M.-L.; Berger, J.S.; Yoo, J.; Pan, A.; Agarwal, R. Nanocavity-Enhanced Giant Stimulated Raman Scattering in Si Nanowires in the Visible Light Region. Nano Lett. 2019, 19, 1204–1209. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Effects of photon density of states on Raman scattering in mesoscopic struc-tures. Phys. Rev. B 2002, 65, 140303. [Google Scholar] [CrossRef]
- Peng, F. Laser-induced phonon amplification in a single-walled nano tube. Europhys. Lett. 2006, 73, 116–120. [Google Scholar] [CrossRef]
- Prince, R.C.; Frontiera, R.R.; Potma, E.O. Stimulated Raman Scattering: From Bulk to Nano. Chem. Rev. 2017, 117, 5070–5094. [Google Scholar] [CrossRef]
- Sirleto, L.; Vergara, A.; Ferrara, M.A. Advances in Stimulated Raman Scattering in Nanostructures. Adv. Opt. Photonics 2017, 9, 169–217. [Google Scholar] [CrossRef]
- Ruan, Z.; Fan, S. Superscattering of Light from Subwavelength Nanostructures. Phys. Rev. Lett. 2010, 105, 013901. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, R.; Taubner, T.; Keilmann, F. Phonon-enhanced light–matter interaction at the na-nometer scale. Nature 2002, 418, 159–162. [Google Scholar] [CrossRef]
- Tribelsky, M.I.; Luk’Yanchuk, B.S. Anomalous Light Scattering by Small Particles. Phys. Rev. Lett. 2006, 97, 263902. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Arivuoli, D. Nanomaterials for nonlinear optical applications: A review. Rev. Adv. Mater. Sci. 2012, 30, 243–253. [Google Scholar]
- Tanaka, K. Optical Nonlinearity in Photonic Glasses. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 1. ISBN 978-3-319-48931-5. [Google Scholar]
- Wu, K.; Pan, S. A review on structure-performance relationship toward the optimal design of infrared nonlinear optical materials with balanced performances. Co-Ord. Chem. Rev. 2018, 377, 191–208. [Google Scholar] [CrossRef]
- Dymshits, O.; Shepilov, M.; Zhilin, A. Transparent glass-ceramics for optical applications. MRS Bull. 2017, 42, 200–205. [Google Scholar] [CrossRef]
- Mazurin, O.V.; Porai-Koshits, E.A. Phase Separation in Glass; Elsevier Science: Burlington, VT, USA, 1984; ISBN 978-0-08-098365-3. [Google Scholar]
- Feng, X.; Lun, Y.; Jiang, X.; Qiu, J.; Yu, H.; Zhou, S. Manipulating Nonlinear Optical Response via Domain Control in Nanocrystal-in-Glass Composites. Adv. Mater. 2021, 33, e2006482. [Google Scholar] [CrossRef]
- Marotta, A.; Pernice, P.; Aronne, A.; Buri, A. Glass transition temperature and devitrification behaviour of glasses in the Li2SiO3 Na2SiO3 composition range. J. Non-Cryst. Solids 1991, 127, 159–164. [Google Scholar] [CrossRef]
- Fanelli, E.; Giannetti, C.; Aronne, A.; Pagliara, S.; Esposito, S.; Ferrini, G. Influence of the Devitrification Mechanism on Second Harmonic Generation Efficiency and Transparency in Ba2NaNb5O15 Nanostructures. J. Phys. Chem. C 2012, 116, 26874–26880. [Google Scholar] [CrossRef]
- Vigouroux, H.; Fargin, E.; Fargues, A.; Le Garrec, B.; Dussauze, M.; Rodriguez, V.; Adamietz, F.; Mountrichas, G.; Kamitsos, E.; Lotarev, S.; et al. Crystallization and Second Harmonic Generation of Lithium Niobium Silicate Glass Ceramics. J. Am. Ceram. Soc. 2011, 94, 2080–2086. [Google Scholar] [CrossRef]
- Guo, Y.; Kao, C.K.; Li, H.E.; Chiang, K.S. Nonlinear Photonics: Nonlinearities in Optics, Opto-Electronics and Fiber Communications; Springer: Heidelberg, Germany, 2002. [Google Scholar]
- Stolen, R.H. Fundamentals of Raman Amplification in Fibers. In Springer Series in Optical Sciences; Springer Science and Business Media LLC: Berlin, Germany, 2007; Volume 90, pp. 35–39. [Google Scholar]
- Manikandan, N.; Ryasnyanskiy, A.; Toulouse, J. Thermal and optical properties of TeO2–ZnO–BaO glasses. J. Non-Cryst. Solids 2013, 358, 947. [Google Scholar] [CrossRef]
- Lin, A.; Ryasnyanskiy, A.; Toulouse, J. Fabrication and characterization of a water-free mid-infrared fluorotellurite glass. Opt. Lett. 2011, 36, 740. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Choi, D.-Y.; Madden, S.; Luther-Davies, B. Interplay between Raman scattering and four-wave mixing in As2S3 chalcogenide glass waveguides. J. Opt. Soc. Am. B Opt. Phys. 2011, 28, 2777. [Google Scholar] [CrossRef]
- Shakeri, S.; Hatami, M. Self-tunable chalcogenide Raman laser. J. Opt. Soc. Am. B Opt. Phys. 2010, 27, 679. [Google Scholar] [CrossRef]
- Pernice, P.; Sirleto, L.; Vergara, A.; Aronne, A.; Gagliardi, M.; Fanelli, E.; Righini, G.C. Large Raman Gain in a Stable Nanocomposite Based on Niobiosilicate Glass. J. Phys. Chem. C 2011, 115, 17314–17319. [Google Scholar] [CrossRef]
- Sirleto, L.; Aronne, A.; Gioffrè, M.; Fanelli, E.; Righini, G.C.; Pernice, P.; Vergara, A. Compositional and thermal treatment effects on Raman gain and bandwidth in nanostructured silica based glasses. Opt. Mater. 2013, 36, 408–413. [Google Scholar] [CrossRef]
- Ramesh, P.; Hegde, V.; Pramod, A.; Eraiah, B.; Rao, S.V.; Shisina, S.; Das, S.; Agarkov, D.; Eliseeva, G.; Jagannath, G.; et al. Effect of Eu3+ in tuning the ultrafast third-order optical nonlinearity in heavy metal borate glasses. Opt. Mater. 2020, 108, 110051. [Google Scholar] [CrossRef]
- Chukhchin, D.G.; Malkov, A.; Tyshkunova, I.V.; Mayer, L.V.; Novozhilov, E.V. Diffractometric method for determining the degree of crystallinity of materials. Crystallogr. Rep. 2016, 61, 371–375. [Google Scholar] [CrossRef]
- Pouchou, J.-L.; Pichoir, F. Quantitative Analysis of Homogeneous or Stratified Microvolumes Applying the Model “PAP”. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Springer US: Boston, MA, USA, 1991; pp. 31–75. ISBN 978-1-4899-2617-3. [Google Scholar]
- Müller, H.; Strubel, C.; Bange, K. Characterization and identification of local defects in glass. Scanning 2006, 23, 14–23. [Google Scholar] [CrossRef]
- Singh, S.; Draegert, D.A.; Geusic, J.E. Optical and Ferroelectric Properties of Barium Sodium Niobate. Phys. Rev. B 1970, 2, 2709–2724. [Google Scholar] [CrossRef]
- Lin, K.; Gong, P.; Chu, S.; Li, Q.; Lin, Z.; Wu, H.; Wang, Q.; Wang, J.; Kim, M.J.; Kato, K.; et al. Strong Second Harmonic Generation in a Tungsten Bronze Oxide by Enhancing Local Structural Distortion. J. Am. Chem. Soc. 2020, 142, 7480–7486. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fu, M.; Stennett, M.; Vilarinho, P.; Levin, I.; Randall, C.A.; Gardner, J.; Morrison, F.; Reaney, I. A Crystal-Chemical Framework for Relaxor versus Normal Ferroelectric Behavior in Tetragonal Tungsten Bronzes. Chem. Mater. 2015, 27, 3250–3261. [Google Scholar] [CrossRef]

| Refractive Index | Peak Position (cm−1) | FWHM (cm−1) | Sigma/SigmaSiO2 | I/ISiO2 | g/gSiO2 | |
|---|---|---|---|---|---|---|
| SiO2 glass | 1.461 | 450 | 190 | 1 | 1 | 1 |
| Initial BaNaNS glass | 1.983 | A = 685 B = 800 C = 886 | 110 60 70 | 71 32 41 | 52 43 46 | 7.5 |
| Nucleated BaNaNS glass subjected to thermal program (1) | 2.014 | A = 640 B = 810 C = 915 | 80 105 100 | 292 62 19 | 292 47 15 | 40 |
| Nucleated BaNaNS glass subjected to thermal program (2) | 2.025 | A = 640 B = 810 C = 915 | 80 105 100 | 292 62 19 | 292 47 15 | 40 |
| Nucleated BaNaNS glass subjected to thermal program (3) | 1.989 | A = 640 B = 808 | 70 160 | 91 119 | 104 60 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernice, P.; Sirleto, L.; Rossi, M.; Iodice, M.; Vergara, A.; Di Girolamo, R.; Luciani, G.; Imparato, C.; Aronne, A.
Tunable Raman Gain in Transparent Nanostructured Glass-Ceramic Based on Ba2NaNb5O15
Pernice P, Sirleto L, Rossi M, Iodice M, Vergara A, Di Girolamo R, Luciani G, Imparato C, Aronne A.
Tunable Raman Gain in Transparent Nanostructured Glass-Ceramic Based on Ba2NaNb5O15
Pernice, Pasquale, Luigi Sirleto, Manuela Rossi, Mario Iodice, Alessandro Vergara, Rocco Di Girolamo, Giuseppina Luciani, Claudio Imparato, and Antonio Aronne.
2023. "Tunable Raman Gain in Transparent Nanostructured Glass-Ceramic Based on Ba2NaNb5O15
Pernice, P., Sirleto, L., Rossi, M., Iodice, M., Vergara, A., Di Girolamo, R., Luciani, G., Imparato, C., & Aronne, A.
(2023). Tunable Raman Gain in Transparent Nanostructured Glass-Ceramic Based on Ba2NaNb5O15












