Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.3. General Characterizations
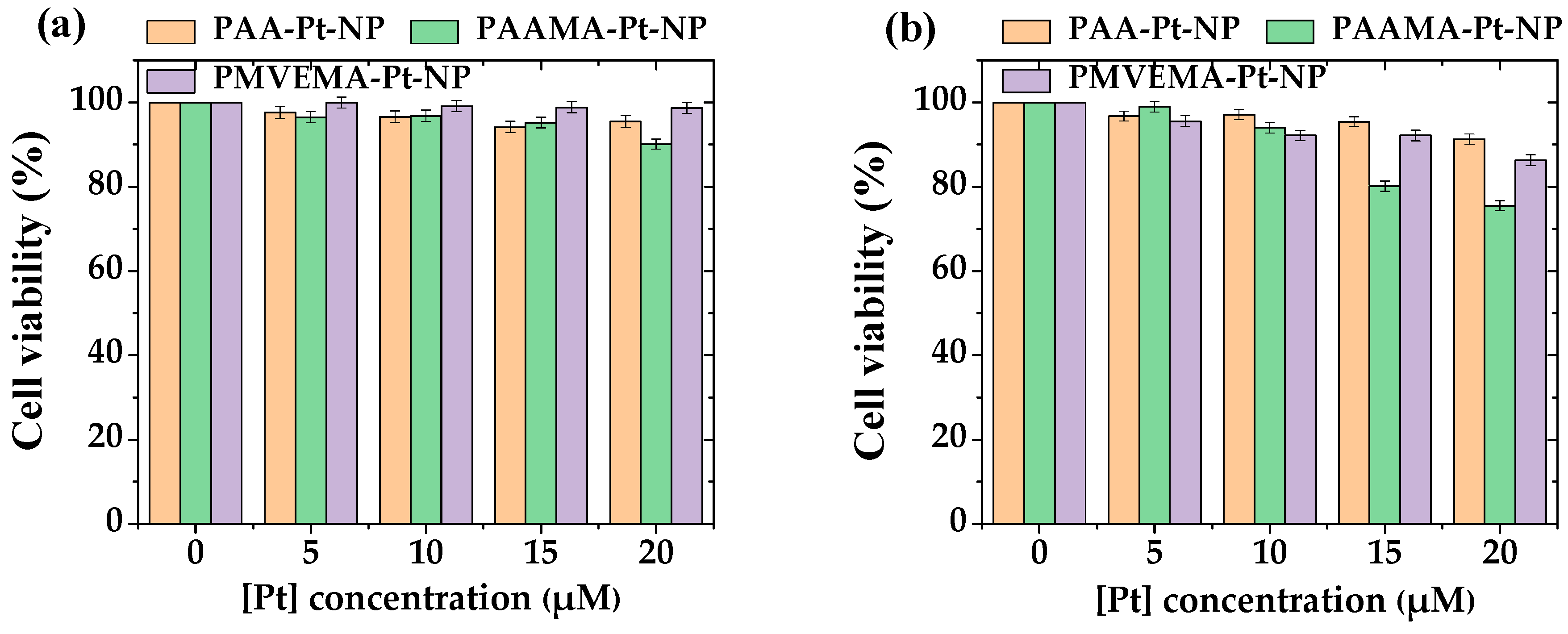
2.4. In Vitro Cell Viability Assay
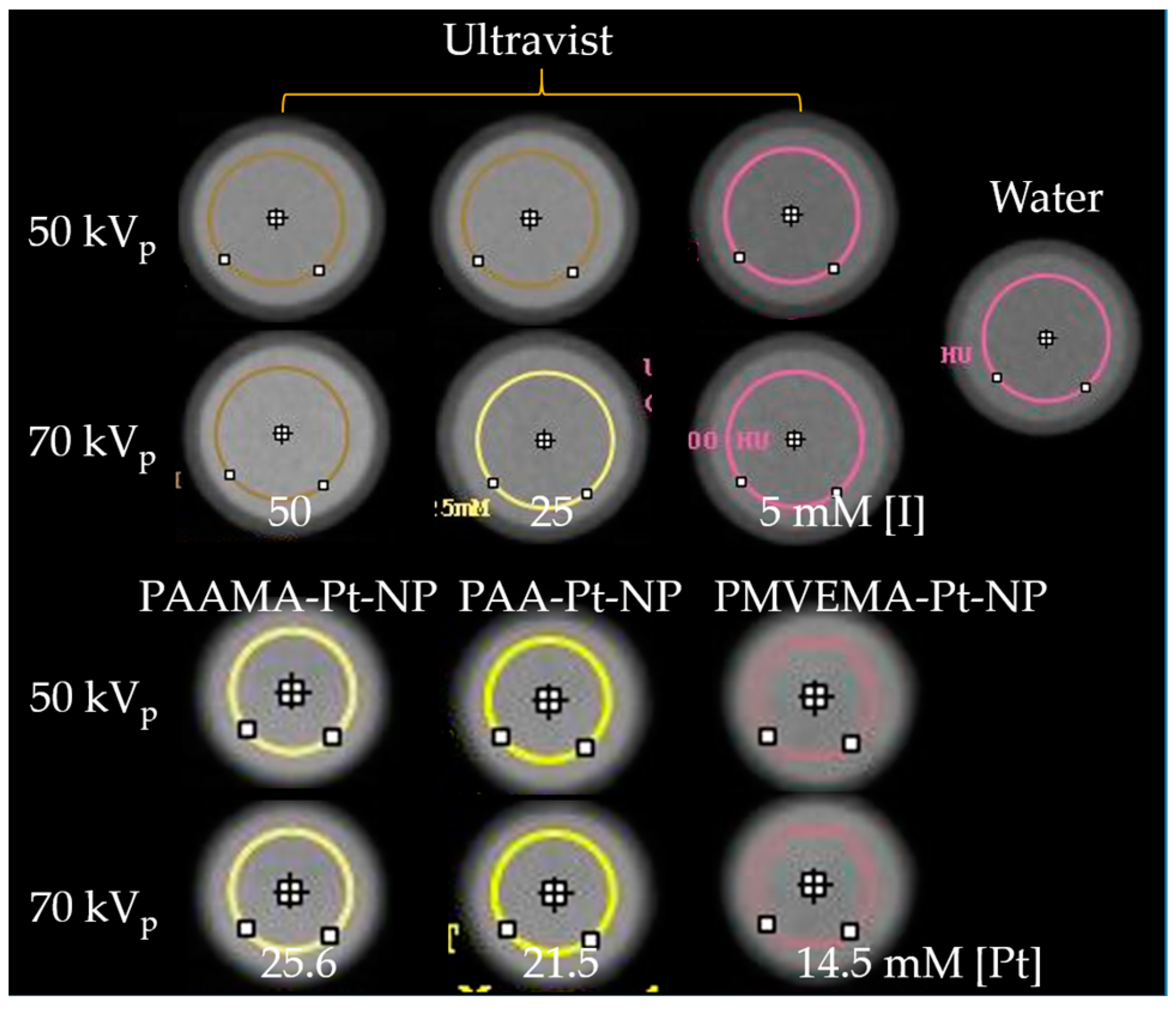
2.5. X-ray Phantom Image Measurements
3. Results
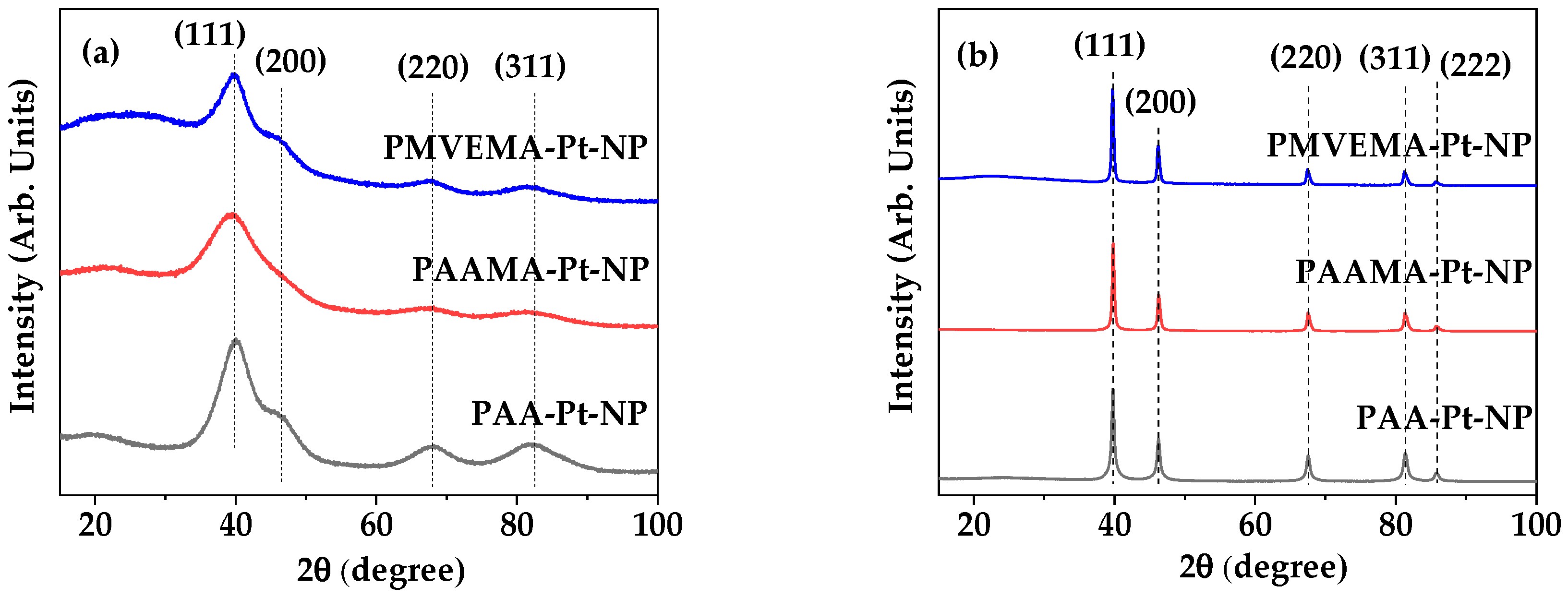
3.1. Physical Characteristics of Polymer-Coated Pt-NPs
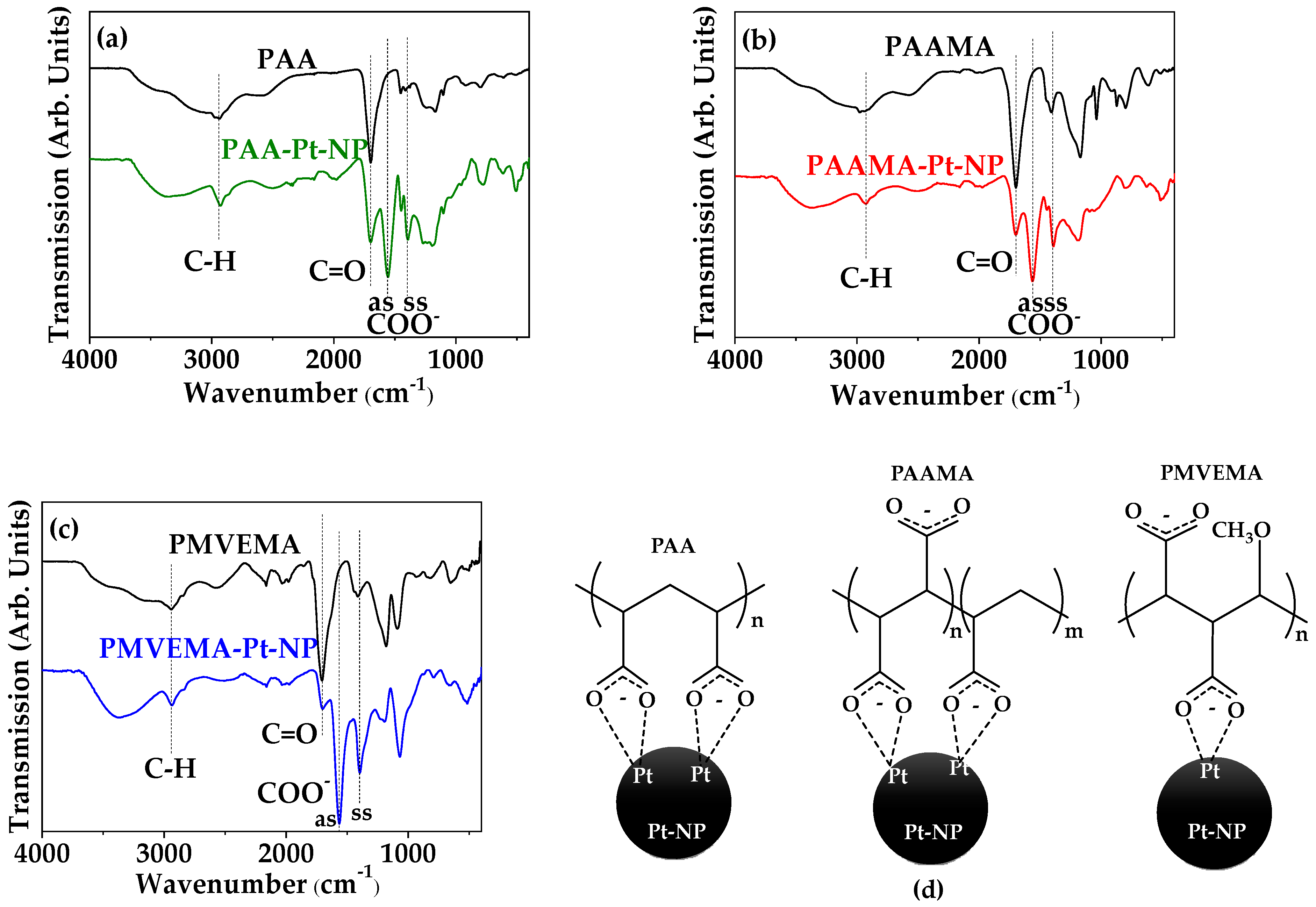
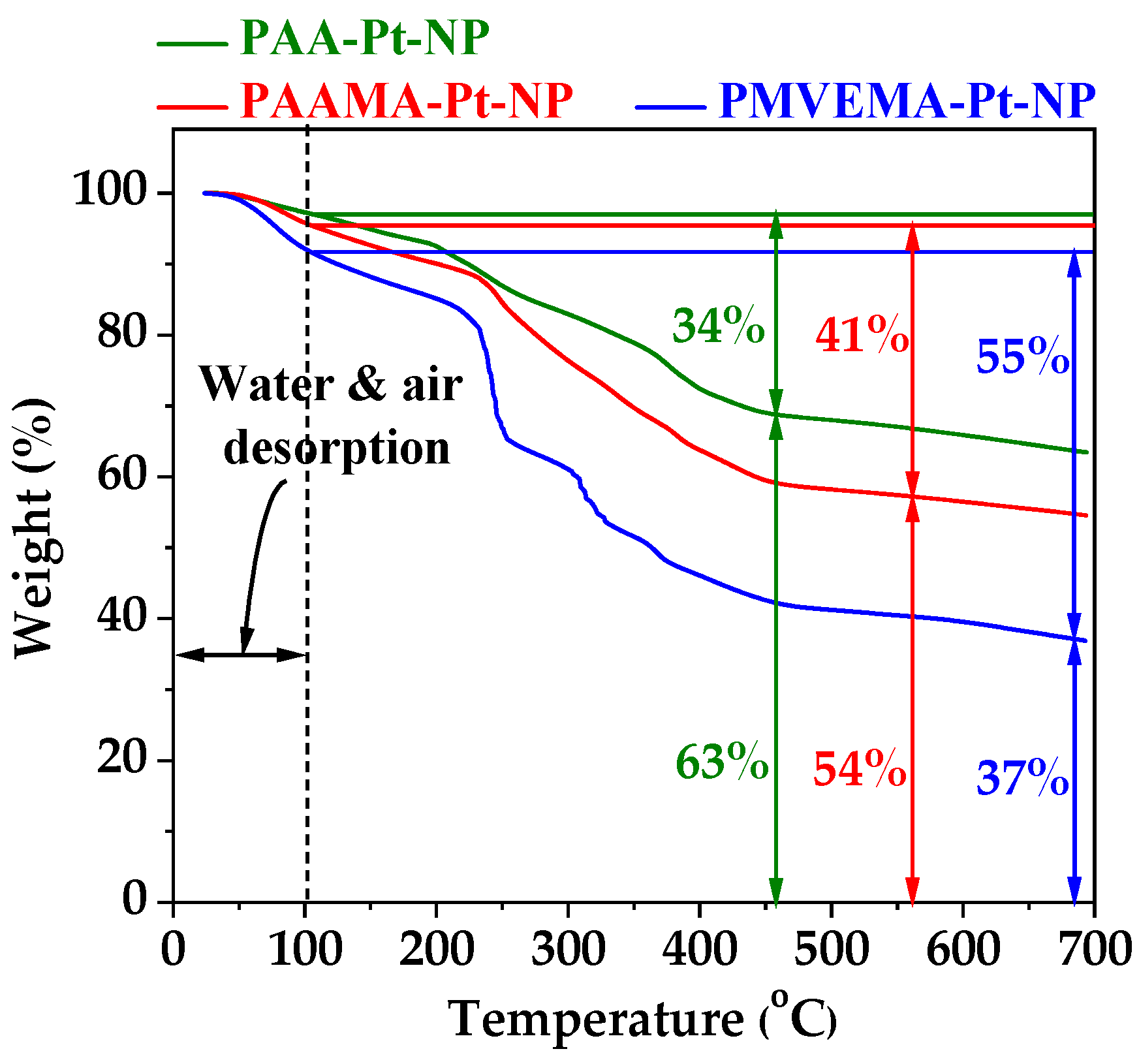
3.2. Polymer-Coating Amount and Structure
3.3. In Vitro Cellular Cytotoxicity Results
3.4. X-ray Phantom Images and X-ray Attenuation Power
4. Discussion
5. Conclusions
- The observed average particle diameter was nearly monodispersed and ultrasmall (i.e., 2.0 nm) for all polymer-coated Pt-NPs;
- Highly negative zeta potentials (<−40 mV) were observed for all polymer-coated Pt-NP solution samples owing to the coating of hydrophilic and biocompatible polymers on the NP surfaces. This led to excellent colloidal stability (no precipitation after synthesis for >1.5 years). Furthermore, all polymer-coated Pt-NP solution samples exhibited low toxicity (>75% cell survival) up to the tested concentration range of 20 μM [Pt], indicating their suitability for biomedical applications;
- The X-ray attenuation power of all polymer-coated Pt-NP solution samples was ~4 times higher than that of the commercial iodine contrast agent Ultravist at the same atomic concentration and ~500 times higher at the same number density, confirming the superiority of the polymer-coated Pt-NPs to iodine contrast agents and thus, their potential as viable high-performance CT contrast agents.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic nanoparticles for biomedical applications: From the soul of the earth to the deep history of ourselves. ACS Appl. Biol. Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Nicola, M.D.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef]
- Pedone, D.; Moglianetti, M.; Luca, E.D.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Zhang, L.; Laug, L.; Munchgesang, W.; Pippel, E.; Gösele, U.; Brandsch, M.; Knez, M. Reducing stress on cells with apoferritin-encapsulated platinum nanoparticles. Nano Lett. 2010, 10, 219–223. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Hinzmann, M.; Wierzbicki, M.; Hotowy, A.; Grodzik, M.; Winnicka, A.; Chwalibog, A. Investigation of platinum nanoparticle properties against U87 glioblastoma multiforme. Arch. Med. Res. 2017, 13, 1322–1334. [Google Scholar] [CrossRef]
- Cho, E.J.; Sun, B.; Doh, K.-O.; Wilson, E.M.; Torregrosa-Allen, S.; Elzey, B.D.; Yeo, Y. Intraperitoneal delivery of platinum with in-situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 2015, 37, 312–319. [Google Scholar] [CrossRef]
- Chen, C.-L.; Kuo, L.-R.; Lee, S.-Y.; Hwu, Y.-K.; Chou, S.-W.; Chen, C.-C.; Chang, F.-H.; Lin, K.-H.; Tsai, D.-H.; Chen, Y.-Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef]
- Bao, Z.; He, M.; Quan, H.; Jiang, D.; Zheng, Y.; Qin, W.; Zhou, Y.; Ren, F.; Guo, M.; Jiang, C. FePt nanoparticles: A novel nanoprobe for enhanced HeLa cells sensitivity to chemoradiotherapy. RSC Adv. 2016, 6, 35124–35134. [Google Scholar] [CrossRef]
- Gopal, J.; Hasan, N.; Manikandan, M.; Wu, H.F. Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci. Rep. 2013, 3, 1260. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Zhou, Q.; Dang, M.; Tang, Y.; Wang, S.; Fu, J.; Teng, Z.; Lu, G. Computed tomography and photoacoustic imaging guided photodynamic therapy against breast cancer based on mesoporous platinum with insitu oxygen generation ability. Acta Pharm. Sin. B. 2020, 10, 1719–1729. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NIST: Gaithersburg, MD, USA, 1995. [Google Scholar] [CrossRef]
- Moglianetti, M.; Luca, E.D.; Pedone, D.; Marotta, R.; Catelani, T.; Sartori, B.; Amenitsch, H.; Retta, S.F.; Pompa, P.P. Platinum nanozymes recover cellular ROS homeostasis in an oxidative stress-mediated disease model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 2009, 22, 340–349. [Google Scholar] [CrossRef]
- Horie, M.; Kato, H.; Endoh, S.; Fujita, K.; Nishio, K.; Komaba, L.K.; Fukui, H.; Nakamura, A.; Miyauchi, A.; Nakazato, T.; et al. Evaluation of cellular influences of platinum nanoparticles by stable medium dispersion. Metallomics 2011, 3, 1244–1252. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef]
- Rabin, O.; Perez, J.M.; Grimm, J.; Wojtkiewicz, G.; Weissleder, R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater. 2006, 5, 118–122. [Google Scholar] [CrossRef]
- Bonitatibus, P.J.; Torres, A.S.; Goddard, G.D.; FitzGerald, P.F.; Kulkarni, A.M. Synthesis, characterization, and computed tomography imaging of a tantalum oxide nanoparticle imaging agent. Chem. Commun. 2010, 46, 8956–8958. [Google Scholar] [CrossRef]
- Oh, M.H.; Lee, N.; Kim, H.; Park, S.P.; Piao, Y.; Lee, J.; Jun, S.W.; Moon, W.K.; Choi, S.H.; Hyeon, T. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J. Am. Chem. Soc. 2011, 133, 5508–5515. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Nanoparticulate X-ray computed tomography contrast agents: From design validation to in vivo applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Yuan, Q.; He, Y.; Lu, L. A high-performance ytterbium-based nanoparticulate contrast agent for in vivo X-ray computed tomography imaging. Angew. Chem. Int. Ed. 2012, 24, 1466–1471. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Xu, W.; Kim, S.J.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Park, J.A.; Kim, T.J.; Lee, G.H. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci. Rep. 2015, 5, 8549. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Liu, J.; Gu, S.; Yuan, Q.; Ren, J.; Qu, X. Long-circulating Er3+-doped Yb2O3 up-conversion nanoparticle as an in vivo X-ray CT imaging contrast agent. Biomaterials 2012, 33, 6748–6757. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Huang, C.; Huang, Y.; Jia, N. Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J. Mater. Chem. B 2017, 5, 3498–3510. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A.; Mehrdel, B.; Khaniabadi, P.M.; Khaniabadi, B.M. Green sonochemical synthesis platinum nanoparticles as a novel contrast agent for computed tomography. Mater. Today Commun. 2021, 27, 102480. [Google Scholar] [CrossRef]
- Fu, B.; Dang, M.; Tao, J.; Li, Y.; Tang, Y. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J. Colloid Interface Sci. 2020, 570, 197–204. [Google Scholar] [CrossRef]
- Chu, C.-H.; Cheng, S.-H.; Chen, N.-T.; Liao, W.-N.; Lo, L.-W. Microwave-synthesized platinum-embedded mesoporous silica nanoparticles as dual-modality contrast agents: Computed tomography and optical imaging. Int. J. Mol. Sci. 2019, 20, 1560. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, L.; Gong, F.; Dong, Z.; Liang, C.; Wang, M.; Feng, L.; Li, Y.; Liu, Z.; Li, C.; et al. Platinum nanoworms for imaging-guided combined cancer therapy in the second near-infrared window. J. Mater. Chem. B 2018, 6, 5069–5079. [Google Scholar] [CrossRef]
- Yu, S.-B.; Watson, A.D. Metal-based X-ray contrast media. Chem. Rev. 1999, 99, 2353–2377. [Google Scholar] [CrossRef]
- Yim, E.S.; Zhao, B.; Myung, D.; Kourtis, L.C.; Frank, C.W.; Carter, D.; Smith, R.L.; Goodman, S.B. Biocompatibility of poly(ethylene glycol)/poly(acrylic acid) interpenetrating polymer network hydrogel particles in RAW 264.7 macrophage and MG-63 osteoblast cell lines. J. Biomed. Mater. Res. A 2009, 91, 894–902. [Google Scholar] [CrossRef]
- Miao, X.; Xu, W.; Cha, H.; Chang, Y.; Oh, I.T.; Chae, K.S.; Tegafaw, T.; Ho, S.L.; Kim, S.J.; Lee, G.H. Ultrasmall Gd2O3 nanoparticles surface-coated by polyacrylic acid (PAA) and their PAA-size dependent relaxometric properties. Appl. Surf. Sci. 2019, 477, 111–115. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Liu, S.; Yue, H.; Park, J.A.; Cha, H.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Ahmad, M.Y.; Miao, X.; et al. Hydrophilic biocompatible poly(acrylic acid-co-maleic acid) polymer as a surface-coating ligand of ultrasmall Gd2O3 nanoparticles to obtain a high r1 value and T1 MR images. Diagnostics 2020, 11, 2. [Google Scholar] [CrossRef]
- Rivas, B.L.; Seguel, G.V. Poly(acrylic acid-co-maleic acid)–metal complexes with copper(II), cobalt(II), and nickel(II): Synthesis, characterization and structure of its metal chelates. Polyhedron 1999, 18, 2511–2518. [Google Scholar] [CrossRef]
- Mlih, R.; Suazo-Hernández, J.; Liang, Y.; Tombácz, E.; Bol, R.; Klumpp, E. Polyacrylic-co-maleic-acid-coated magnetite nanoparticles for enhanced removal of heavy metals from aqueous solutions. Colloids Interfaces 2023, 7, 5. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Almeida, P.V.; Mäkilä, E.; Correia, A.; Ferreira, M.P.A.; Kaasalainen, M.; Salonen, J.; Hirvonen, J.; Santos, H.A. Poly(methyl vinyl ether-alt-maleic acid)-functionalized porous silicon nanoparticles for enhanced stability and cellular internalization. Macromol. Rapid Commun. 2014, 35, 624–629. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Ahmad, M.W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In vivo positive magnetic resonance imaging applications of poly(methyl vinyl ether-alt-maleic acid)-coated ultra-small paramagnetic gadolinium oxide nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Lee, J.J.; Jin, S.; Baeck, J.S.; et al. A facile synthesis, in vitro and in vivo MR studies of D-glucuronic acid-coated ultrasmall Ln2O3 (Ln = Eu, Gd, Dy, Ho, and Er) nanoparticles as a new potential MRI contrast agent. ACS Appl. Mater. Interfaces 2011, 3, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Davey, W.P. Precision measurements of the lattice constants of twelve common metals. Phys. Rev. 1925, 25, 753–761. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; p. 1148. [Google Scholar]
- Duckworth, O.W.; Martin, S.T. Surface complexation and dissolution of hematite by C1-C6 dicarboxylic acids at pH = 5.0. Geochim. Cosmochim. Acta 2001, 65, 4289–4301. [Google Scholar] [CrossRef]
- Hug, S.J.; Bahnemann, D. Infrared spectra of oxalate, malonate and succinate adsorbed on the aqueous surface of rutile, anatase and lepidocrocite measured with in situ ATR-FTIR. J. Electron Spectrosc. Relat. Phenom. 2006, 150, 208–219. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Benoit, D.N.; Zhu, H.; Lilierose, M.H.; Verm, R.A.; Ali, N.; Morrison, A.N.; Fortner, J.D.; Avendano, C.; Colvin, V.L. Measuring the grafting density of nanoparticles in solution by analytical ultracentrifugation and total organic carbon analysis. Anal. Chem. 2012, 84, 9238–9245. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Lennox, R.B. Polymer-stabilized gold nanoparticles with high grafting densities. Langmuir 2004, 20, 2867–2873. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; pp. 4–75. [Google Scholar]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]

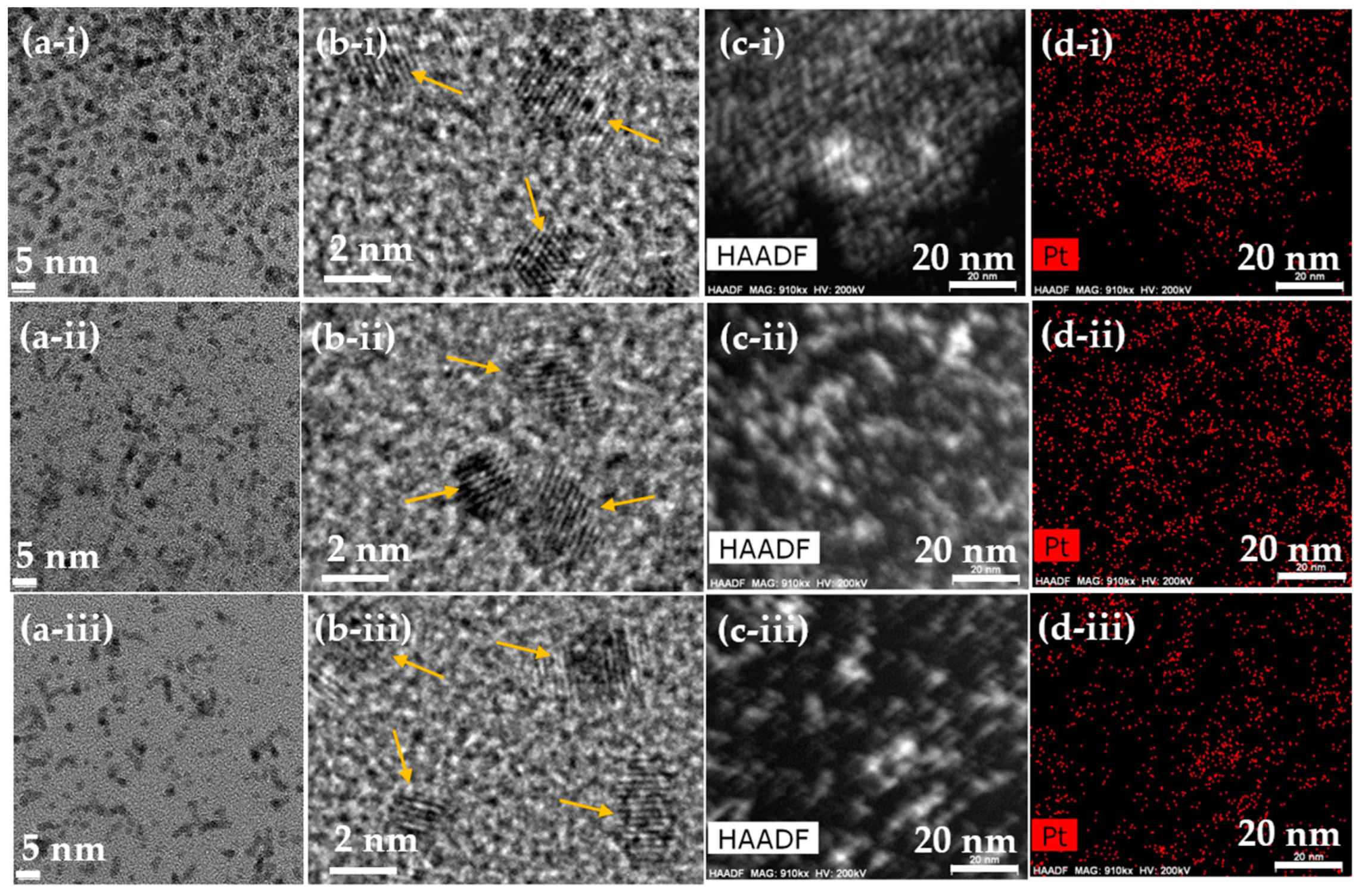
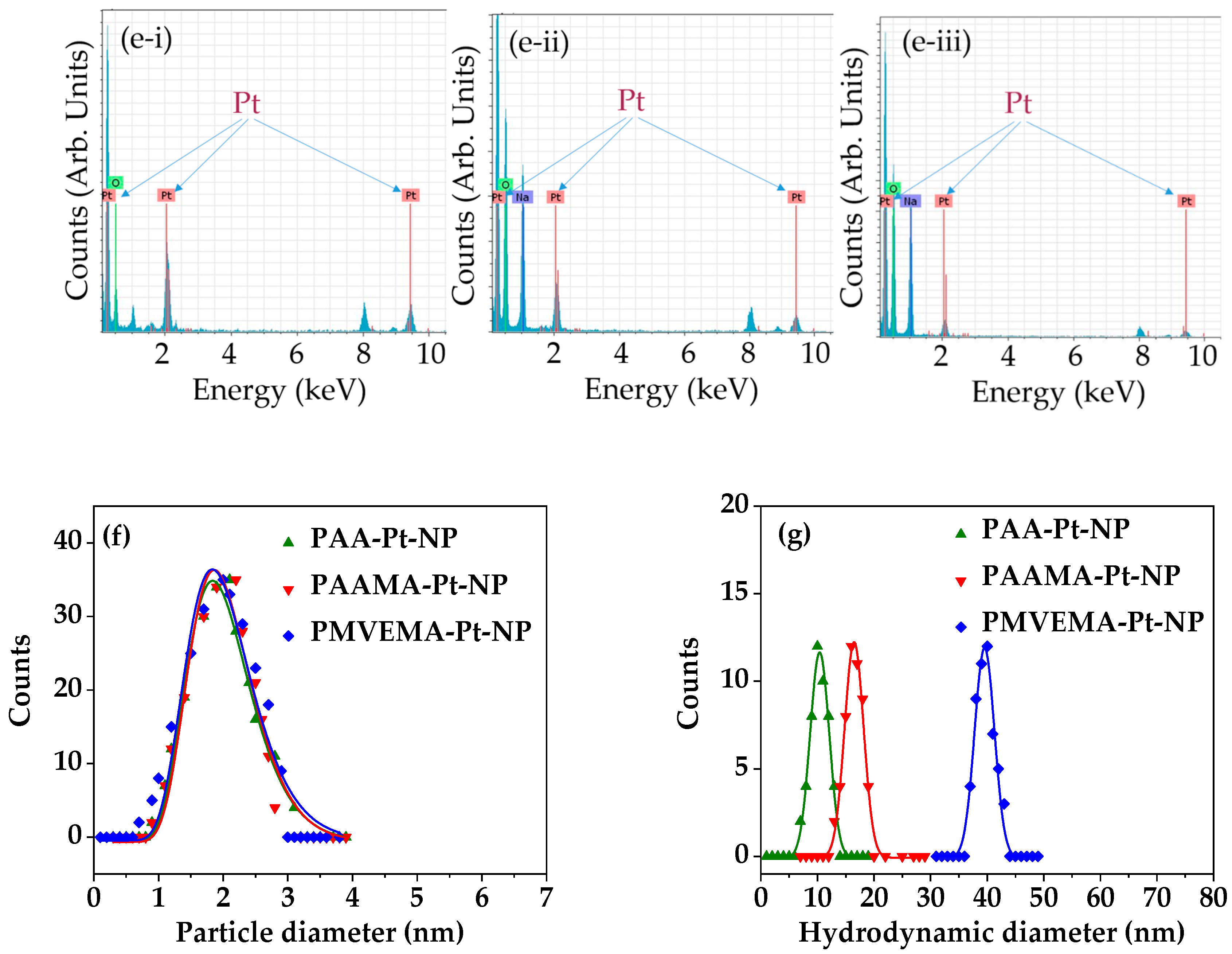
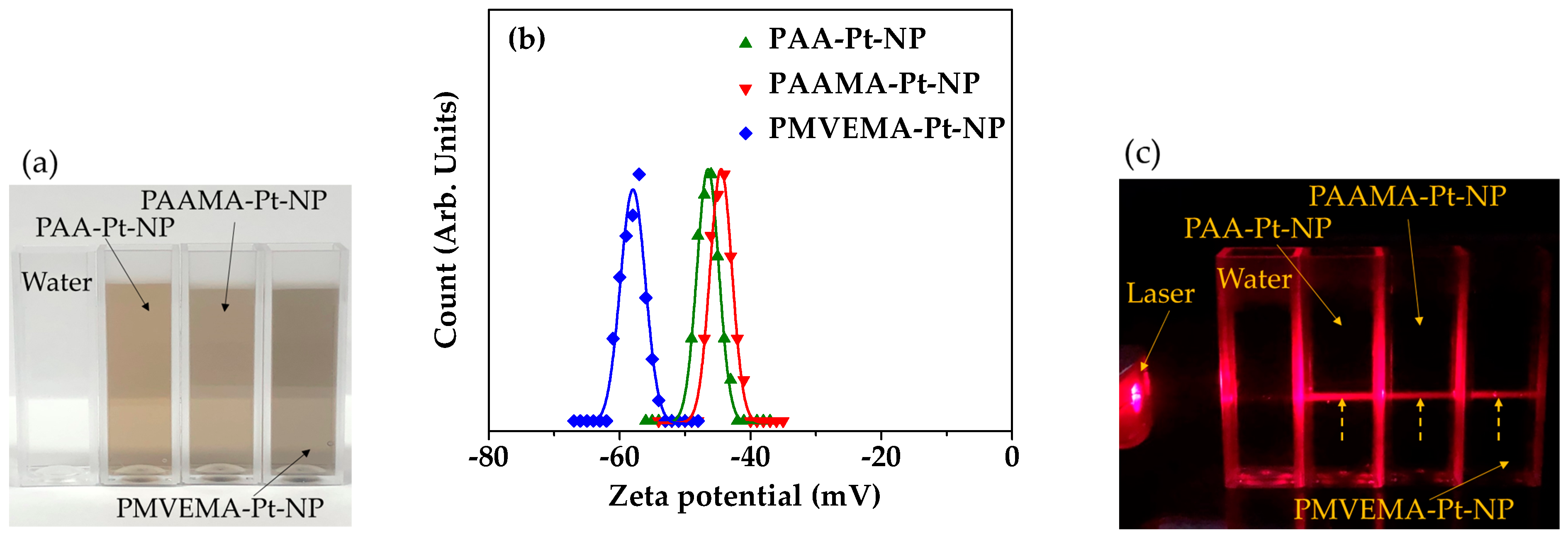

| Coating Polymer | davg (nm) | aavg (nm) | ξavg (mV) |
|---|---|---|---|
| PAA | 2.0 ± 0.2 | 10.4 ± 1.0 | −46.5 ± 1.0 |
| PAAMA | 2.0 ± 0.2 | 20.5 ± 1.0 | −44.2 ± 1.0 |
| PMVEMA | 2.0 ± 0.2 | 37.5 ± 1.0 | −57.3 ± 1.0 |
| PAA | PAAMA | PMVEMA | PAA-Pt-NPs | PAAMA-Pt-NPs | PMVEMA-Pt-NPs | |
|---|---|---|---|---|---|---|
| C–H stretch | 2940 ± 5 | 2935 ± 5 | 2942 ± 5 | 2926 ± 5 | 2922 ± 5 | 2935 ± 5 |
| C=O stretch | 1697 ± 5 | 1693 ± 5 | 1699 ± 5 | 1697 ± 5 | 1693 ± 5 | 1699 ± 5 |
| COO− antisymmetric stretch | - | - | - | 1553 ± 5 | 1556 ± 5 | 1560 ± 5 |
| COO− symmetric stretch | - | - | - | 1398 ± 5 | 1385 ± 5 | 1390 ± 5 |
| Coating Polymer | Surface-Coating Amount | ||
|---|---|---|---|
| P 1 (wt. %) | σ 2 (nm−2) | Npolymer 3 | |
| PAA | 34 ± 1 | 1.2 ± 0.1 | 12.4 ± 0.1 |
| PAAMA | 41 ± 1 | 1.0 ± 0.1 | 11.0 ± 0.1 |
| PMVEMA | 55 ± 1 | 0.10 ± 0.05 | 1.1 ± 0.1 |
| Chemical | Natom | Concentration (mM [Pt] or [I]) | Number Density (1/L) | X-ray Attenuation Power (HU) | X-ray Attenuation Efficiency (η) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 50 kVp | 70 kVp | (HU/mM) | [HU/(1/L)] × 10−19 | ||||||
| 50 kVp | 70 kVp | 50 kVp | 70 kVp | ||||||
| PAA-Pt-NP | 378 ± 5 | 21.5 ± 0.5 | 3.4 ± 0.1 × 1019 | 371 ± 30 | 423 ± 45 | 16.4 ± 0.1 | 18.4 ± 0.1 | 102.7 ± 0.5 | 115.1 ± 0.5 |
| PAAMA-Pt-NP | 378 ± 5 | 25.6 ± 0.5 | 4.1 ± 0.1 × 1019 | 413 ± 30 | 459 ± 32 | ||||
| PMVEMA-Pt-NP | 378 ± 5 | 14.5 ± 0.5 | 2.3 ± 0.1 × 1019 | 288 ± 45 | 325 ± 46 | ||||
| Ultravist | 3 | 100.0 ± 0.5 | 20.0 ± 0.1 × 1021 | 398 ± 39 | 487 ± 38 | 4.0 ± 0.1 | 5.0 ± 0.1 | 0.20 ± 0.01 | 0.25 ± 0.01 |
| 3 | 50.0 ± 0.5 | 10.0 ± 0.1 × 1021 | 207 ± 34 | 273 ± 37 | |||||
| 3 | 25.0 ± 0.5 | 5.0 ± 0.1 × 1021 | 75 ± 32 | 82 ± 46 | |||||
| 3 | 5.0 ± 0.5 | 1.0 ± 0.1 × 1021 | 24 ± 48 | 14 ± 33 | |||||
| Water | - | - | - | 0 ± 32 | 0 ± 42 | - | - | - | - |
| Pt-NP Type | Coating Ligand | davg (nm) | η (Hu/mM) | Ref. |
|---|---|---|---|---|
| Mesoporous Pt-NP | Ascorbic acid | 70 | 3.0 at 120 kVp | [16] |
| Spherical Pt-NP | Bovine serum albumin | 2.1 | 16.8 at 120 kVp | [32] |
| Spherical Pt-NP | Extract from Prosopis farcta fruits | 3.8 | 6.9 at 80 kVp | [33] |
| Mesoporous Pt-NP | Polyethylene glycol | 94 | 5.5 at 120 kVp | [34] |
| Spherical Pt-NP embedded in ~50 nm mesoporous silica NP | Polyethylene glycol | 3 | 3.0 at 70 kVp | [35] |
| Pt nanoworm | Polyethylene glycol | ~3 × ~10 | 4.7 | [36] |
| Spherical Pt-NP | PAA, PAAMA, PMVEMA | 2.0 | 16.4 at 50 kVp, 18.4 at 70 kVp | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, A.K.A.A.; Ghazanfari, A.; Liu, S.; Tegafaw, T.; Ahmad, M.Y.; Zhao, D.; Liu, Y.; Yang, S.H.; Hwang, D.W.; Yang, J.-u.; et al. Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials 2023, 13, 806. https://doi.org/10.3390/nano13050806
Saidi AKAA, Ghazanfari A, Liu S, Tegafaw T, Ahmad MY, Zhao D, Liu Y, Yang SH, Hwang DW, Yang J-u, et al. Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials. 2023; 13(5):806. https://doi.org/10.3390/nano13050806
Chicago/Turabian StyleSaidi, Abdullah Khamis Ali Al, Adibehalsadat Ghazanfari, Shuwen Liu, Tirusew Tegafaw, Mohammad Yaseen Ahmad, Dejun Zhao, Ying Liu, So Hyeon Yang, Dong Wook Hwang, Ji-ung Yang, and et al. 2023. "Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers" Nanomaterials 13, no. 5: 806. https://doi.org/10.3390/nano13050806
APA StyleSaidi, A. K. A. A., Ghazanfari, A., Liu, S., Tegafaw, T., Ahmad, M. Y., Zhao, D., Liu, Y., Yang, S. H., Hwang, D. W., Yang, J.-u., Park, J. A., Jung, J. C., Nam, S.-W., Chang, Y., & Lee, G. H. (2023). Facile Synthesis and X-ray Attenuation Properties of Ultrasmall Platinum Nanoparticles Grafted with Three Types of Hydrophilic Polymers. Nanomaterials, 13(5), 806. https://doi.org/10.3390/nano13050806











