In Situ Growth of Nickel–Cobalt Metal Organic Frameworks Guided by a Nickel–Molybdenum Layered Double Hydroxide with Two-Dimensional Nanosheets Forming Flower-Like Struc-Tures for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
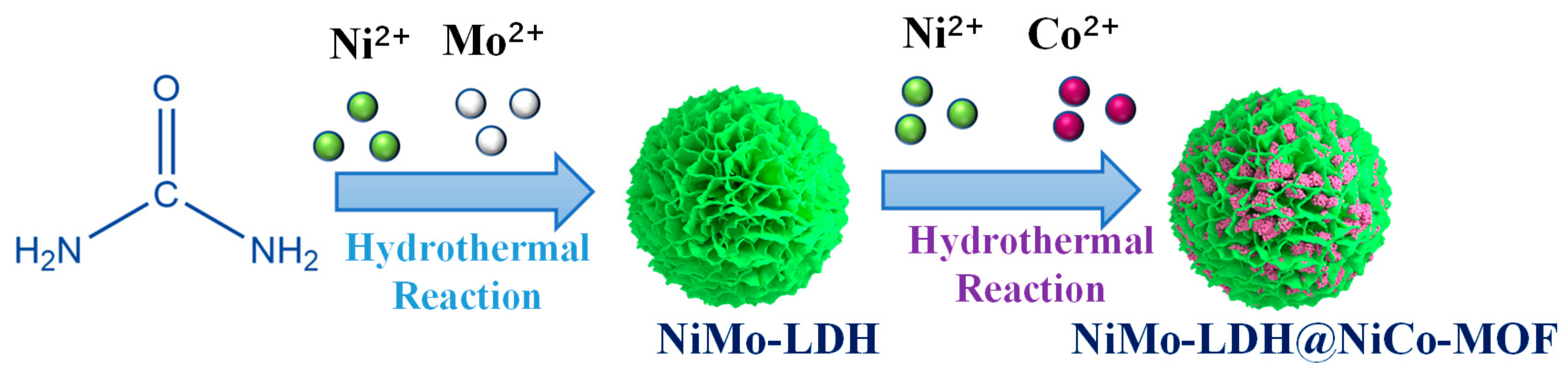
2.1. Synthesis of NiMo-LDH
2.2. Synthesis of NiMo-LDH@NiCo-MOF composites
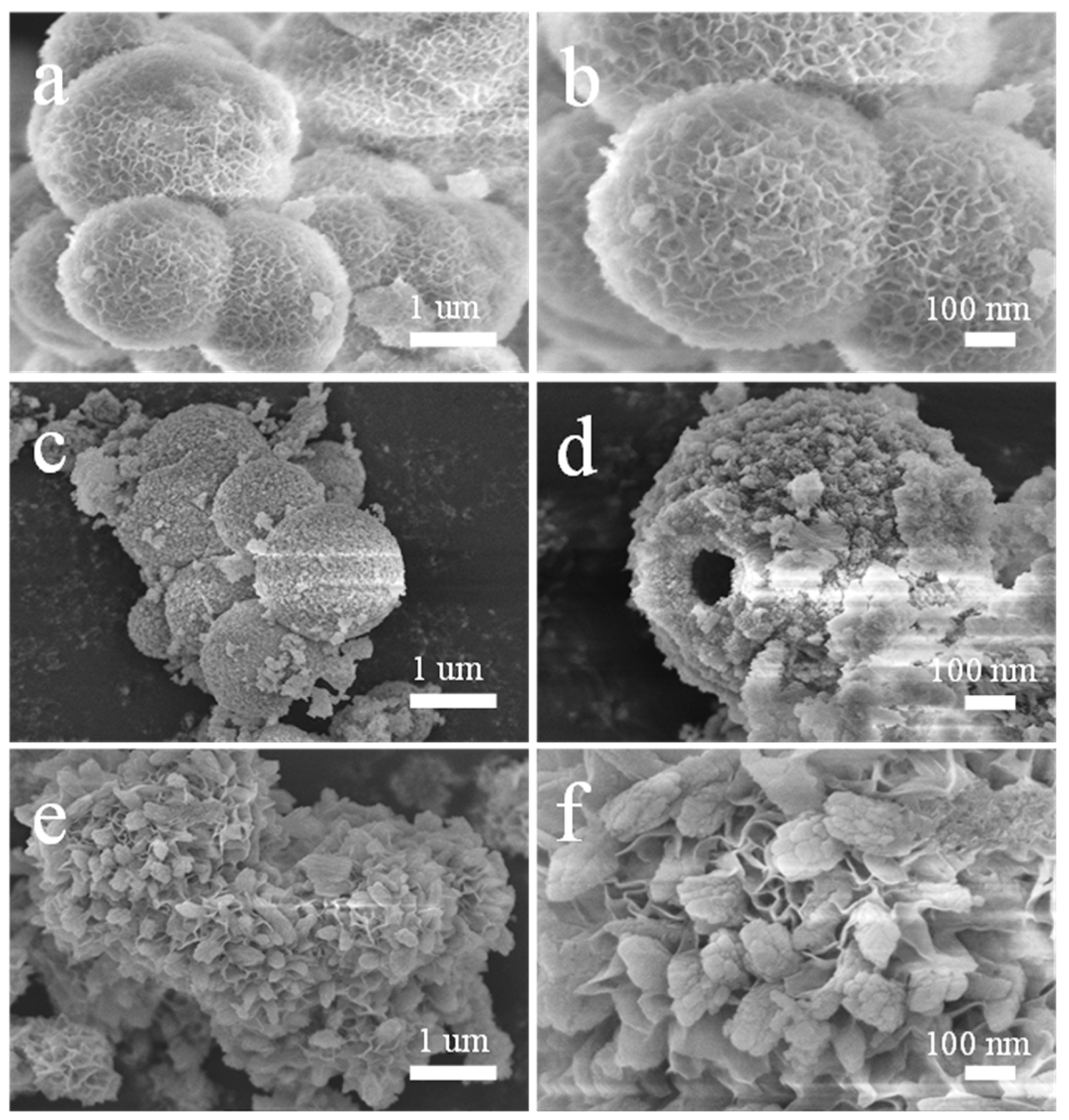
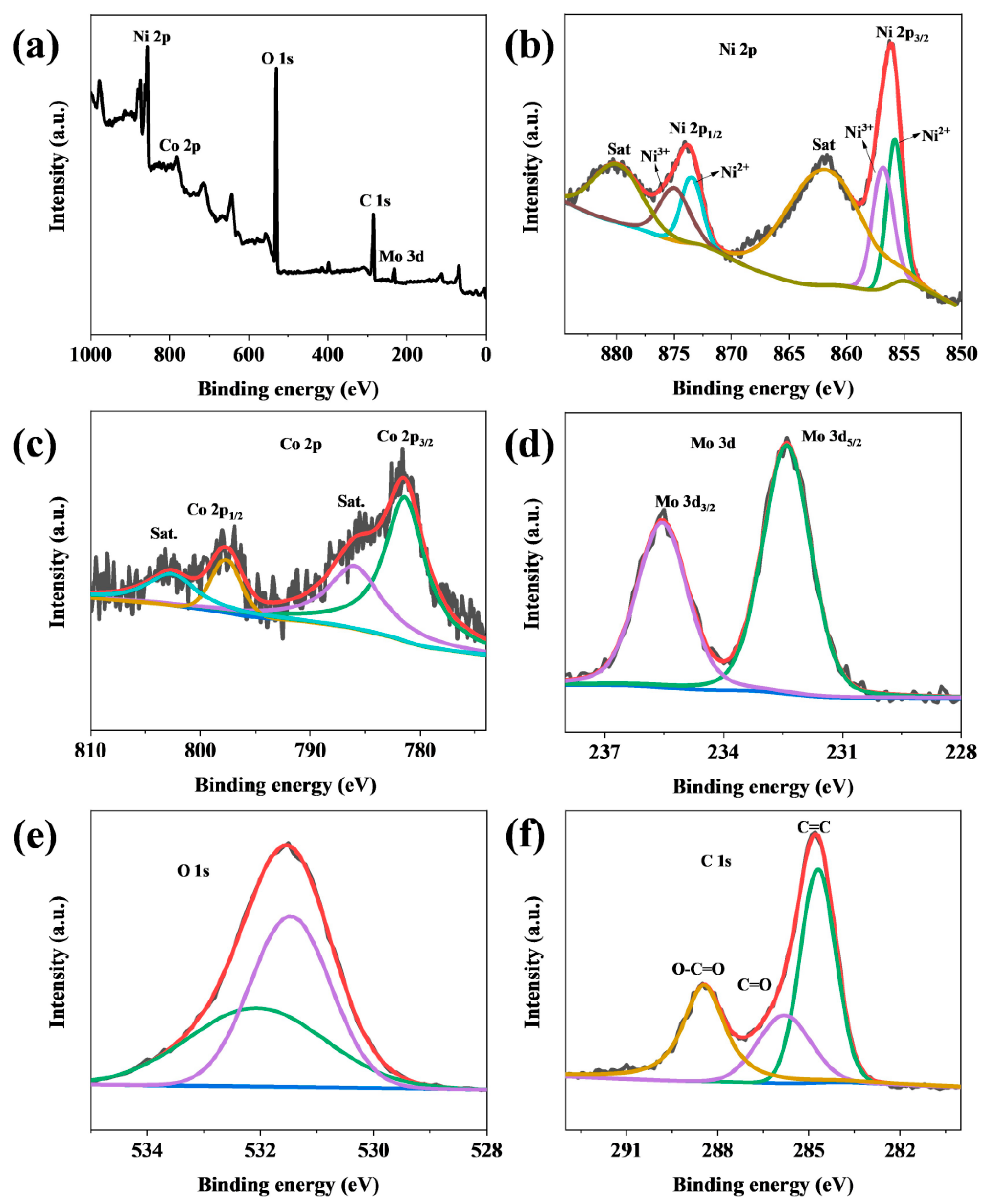
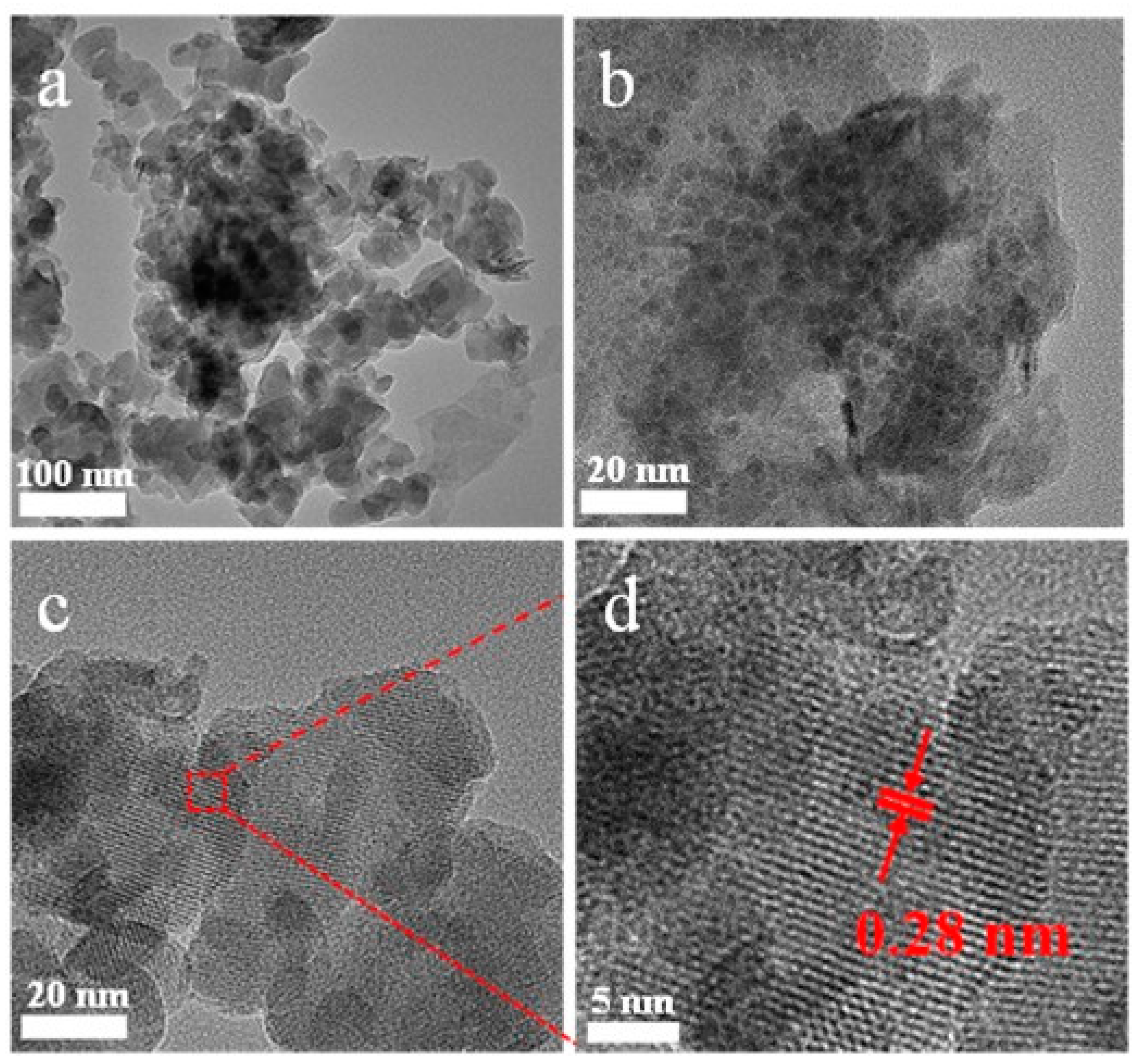
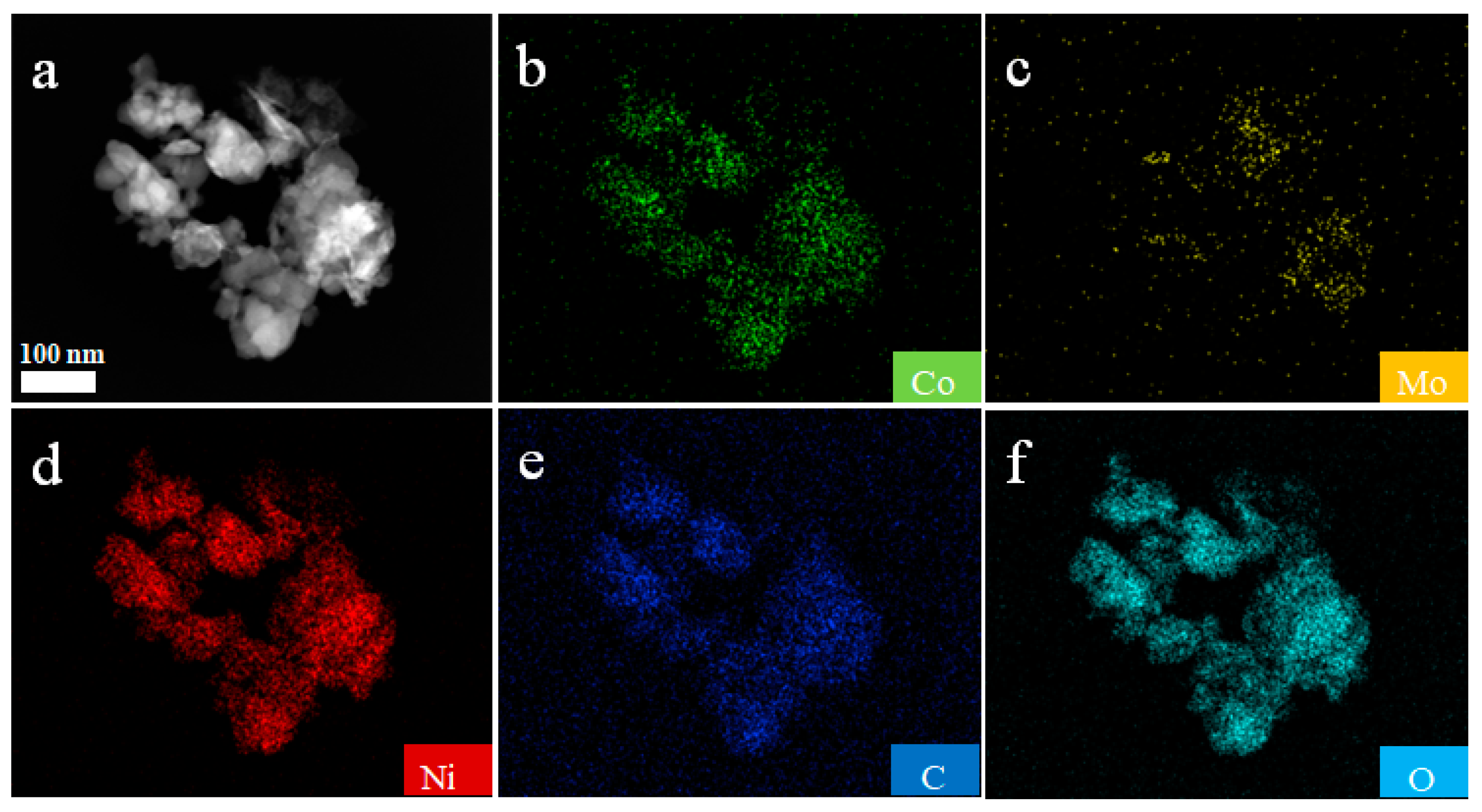
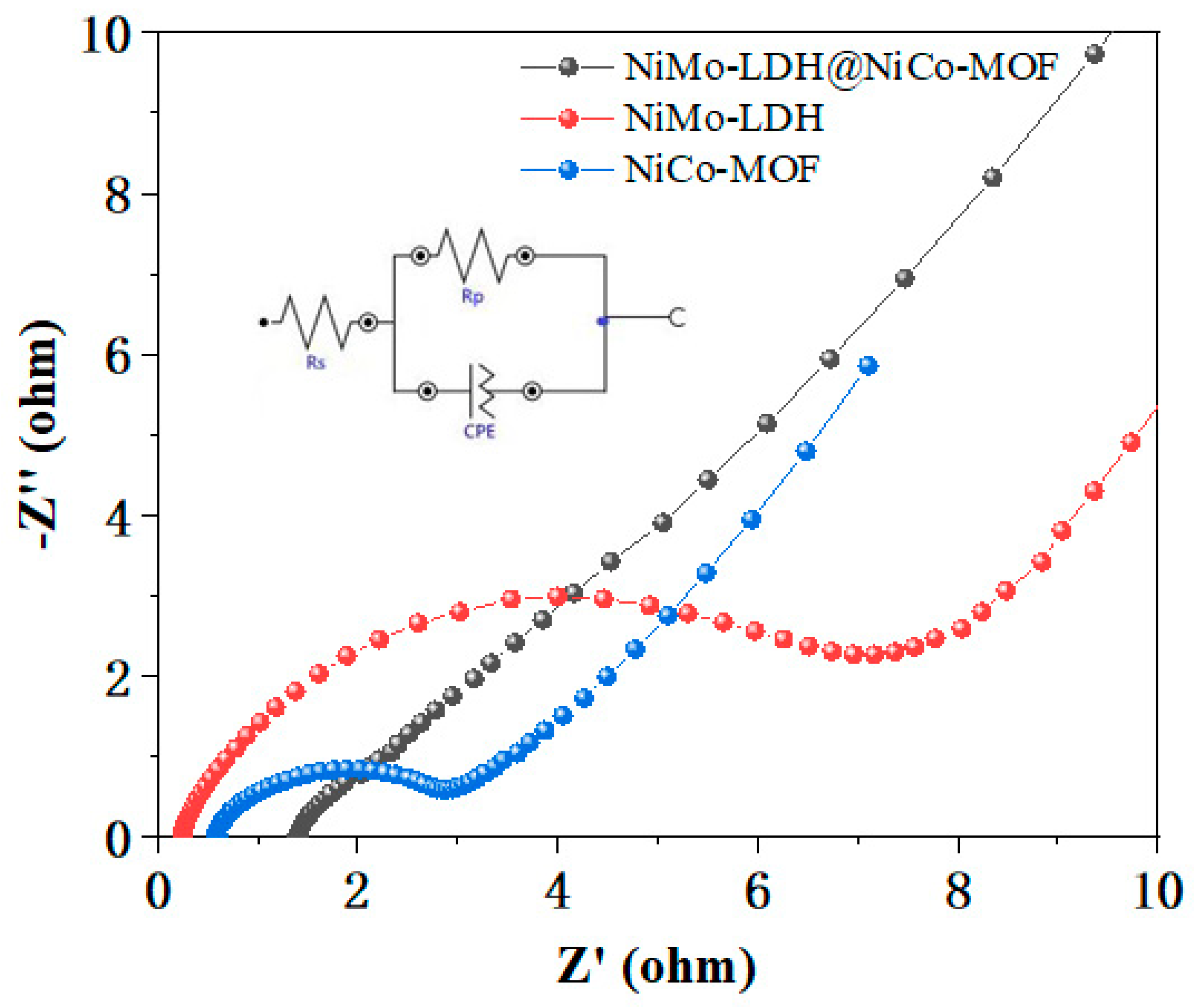
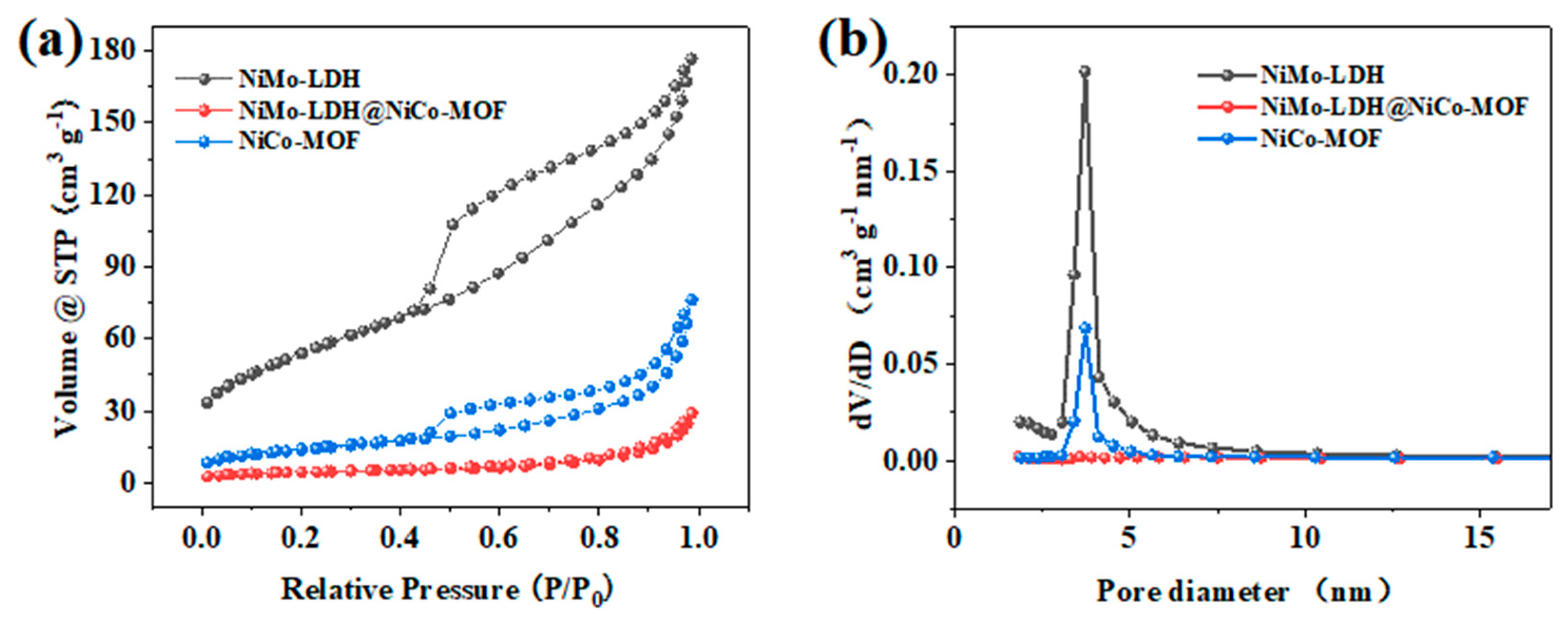
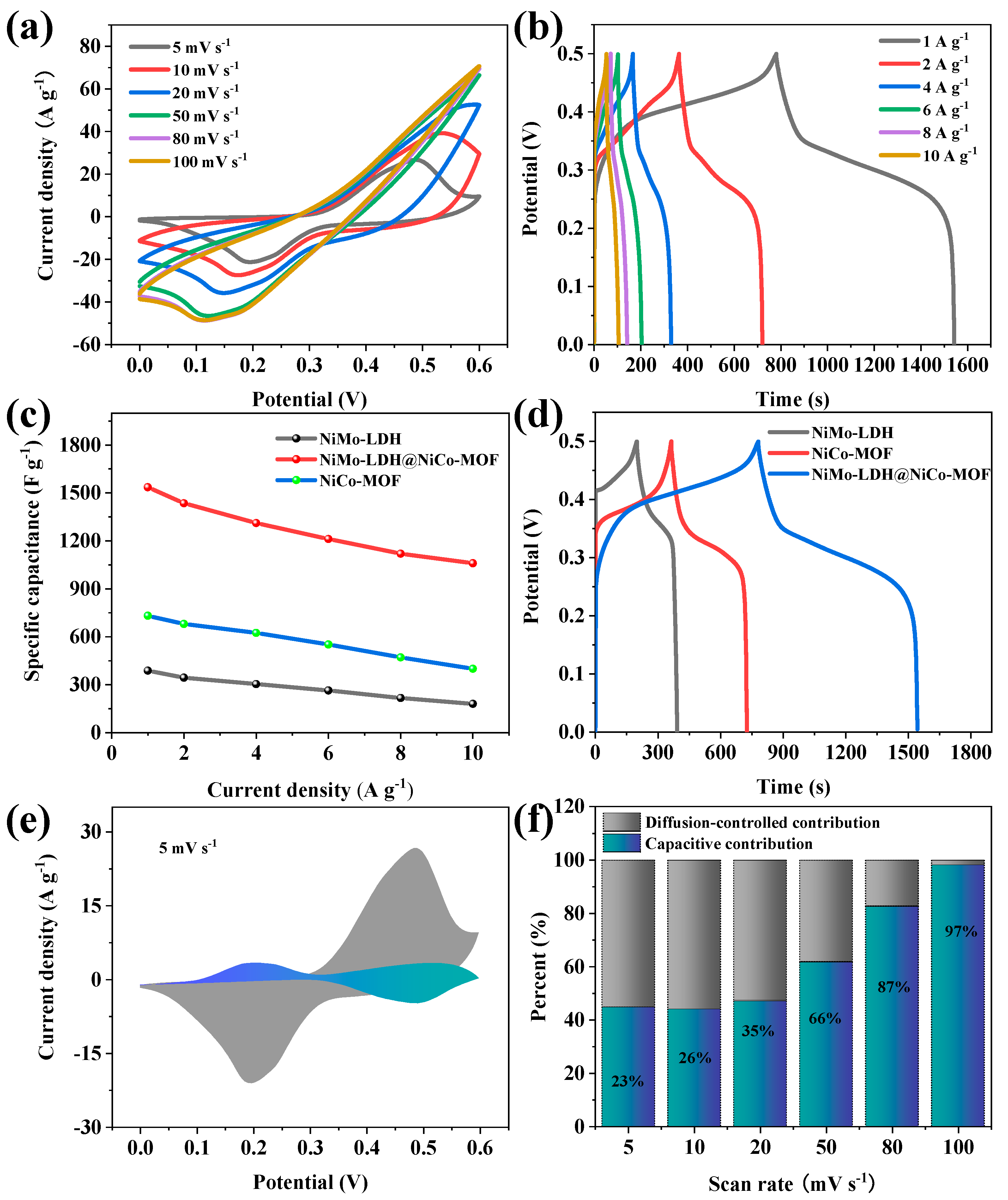
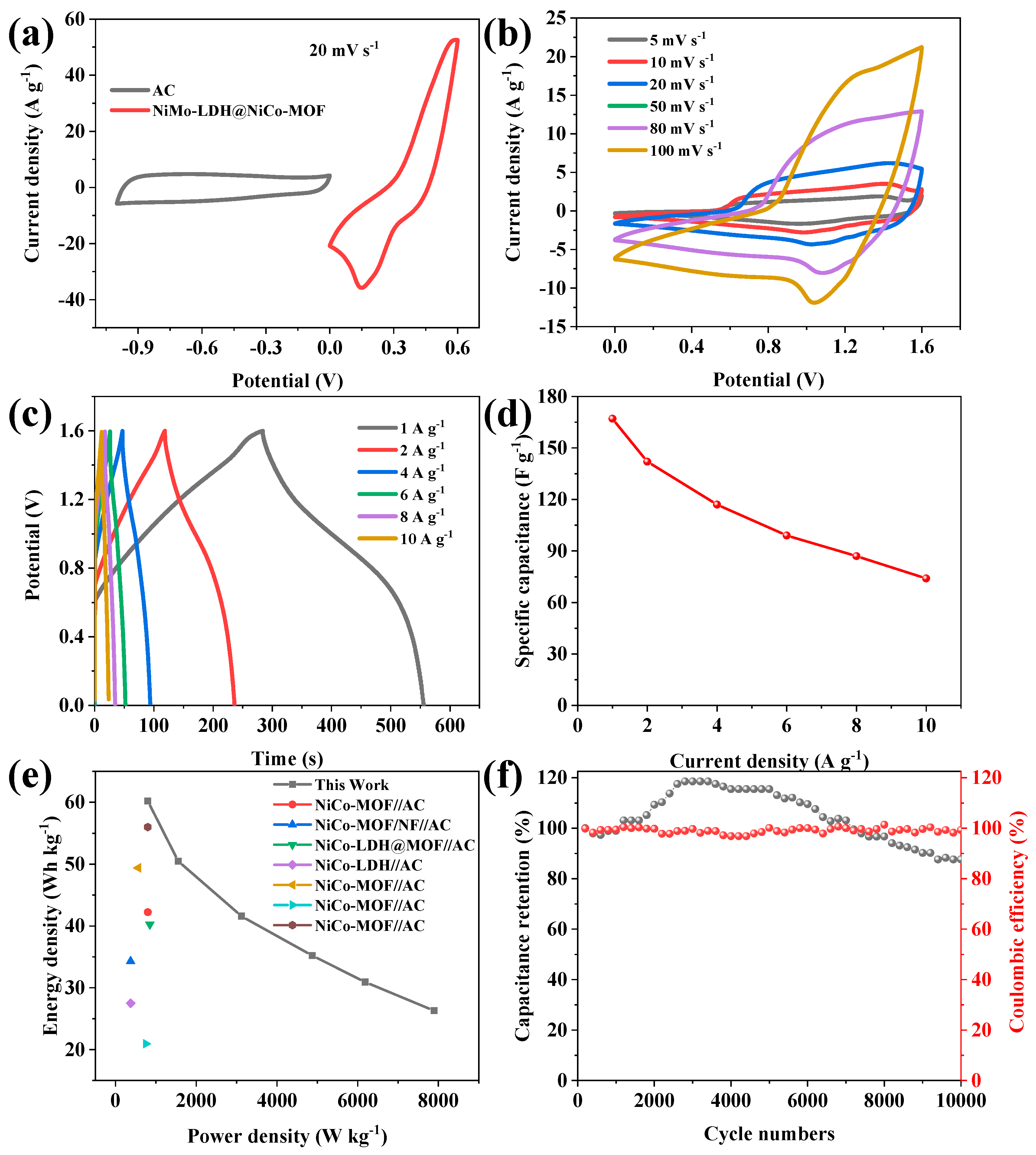
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.-X.; Liu, T. A Review on Nano-/Microstructured Materials Constructed by Electrochemical Technologies for Supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.-E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.; Bai, Y.; Kong, Q.; Pang, H. Framework materials for supercapacitors. Nanotechnol. Rev. 2022, 11, 1005–1046. [Google Scholar] [CrossRef]
- Dedek, I.; Kupka, V.; Jakubec, P.; Sedajova, V.; Jayaramulu, K.; Otyepka, M. Metal-organic framework/conductive polymer hybrid materials for supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Li, P.; Zeng, H. Immobilization of Metal-Organic Framework Nanocrystals for Advanced Design of Supported Nanocatalysts. ACS Appl. Mater. Interfaces 2016, 8, 29551–29564. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Zhao, S.; Chen, H.; Tao, K.; Han, L. Solvent-Controlled Morphology of Amino-Functionalized Bimetal Metal-Organic Frameworks for Asymmetric Supercapacitors. Inorg. Chem. 2020, 59, 11385–11395. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Ji, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Nickel/cobalt bimetallic metal-organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. J. Alloys Compd. 2020, 825, 154069. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, N.; Yang, S.; Lei, D.; Liu, A.; Zhou, Q. Cobalt induced growth of hollow MOF spheres for high performance supercapacitors. Mater. Chem. Front. 2021, 5, 482–491. [Google Scholar] [CrossRef]
- Shi, C.; Du, Y.; Guo, L.; Yang, J.; Wang, Y. Construction of interconnected NiCo layered double hydroxides/metal-organic frameworks hybrid nanosheets for high-performance supercapacitor. J. Energy Storage 2022, 48, 103961. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Guo, Y.; Yuliarto, B.; Jiang, X.; Ide, Y.; Nugraha, N.; Dipojono, H.K.; Yu, A.; Sugahara, Y.; et al. Holey Assembly of Two-Dimensional Iron-Doped Nickel-Cobalt Layered Double Hydroxide Nanosheets for Energy Conversion Application. Chemsuschem 2020, 13, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, D.; Zhang, Y.; Xing, W.; Xue, Q.; Yan, Z. Layered double hydroxides toward high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 15460–15485. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Xu, F.; Xiang, C.; Sui, Q.; Zhang, J.; Sun, L.; Chen, Z. Ultrathin graphene@NiCo2S4@Ni-Mo layered double hydroxide with a 3D hierarchical flowers structure as a high performance positive electrode for hybrid supercapacitor. J. Energy Storage 2022, 52, 105049. [Google Scholar] [CrossRef]
- Jeghan, S.M.N.; Kim, N.; Lee, G. Mo-incorporated three-dimensional hierarchical ternary nickel-cobalt-molybdenum layer double hydroxide for high-efficiency water splitting. Int. J. Hydrogen Energy 2021, 46, 22463–22477. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Zhao, Y.; Lv, H.; Zhou, Z.; Wei, H.; Chen, Z. Well-designed sophisticated structure of sandwich-like CC@NiAl-LDH@GO@NiCo-LDH material with unique advantages for high performance and practicality hybrid quasi-solid-state supercapacitors. J. Colloid Interface Sci. 2022, 609, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, J.; Zhang, W.; Ma, L.; Jiang, Z.; Wang, J.; Huang, Y. Synergistically coupling of 3D FeNi-LDH arrays with Ti(3)C(2)Tx-MXene nanosheets toward superior symmetric supercapacitor. Nano Energy 2022, 91, 106633. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Kang, Y.; Yu, C.C.; Wen, Y.; Hu, M.; Meng, D.; Song, W.; Yang, Y. Interface Engineering of Co-LDH@MOF Heterojunction in Highly Stable and Efficient Oxygen Evolution Reaction. Adv. Sci. 2021, 8, 2002631. [Google Scholar] [CrossRef]
- Tahir, M.U.; Arshad, H.; Xie, W.; Wang, X.; Nawaz, M.; Yang, C.; Su, X. Synthesis of morphology controlled NiCo-LDH microflowers derived from ZIF-67 using binary additives and their excellent asymmetric supercapacitor properties. Appl. Surf. Sci. 2020, 529, 147073. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, Y.; Liu, Y.; Xin, N.; Shi, W. NiCo-layered double-hydroxide and carbon nanosheets microarray derived from MOFs for high performance hybrid supercapacitors. J. Colloid Interface Sci. 2019, 539, 545–552. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Liu, W.; Li, Y.; Zhang, J.; Hou, H.; Yang, J. Ultrathin NiCo-MOF Nanosheets for High-Performance Supercapacitor Electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, Y.; Wang, C.; Guo, L. NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-performance supercapacitors. Appl. Surf. Sci. 2020, 507, 145089. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, N.; Yang, S.; Fan, Q.; Lei, D.; Liu, A.; Chen, X. Shape-controlled synthesis of Ni-based metal-organic frameworks with albizia flower-like spheres@nanosheets structure for high performance supercapacitors. J. Colloid Interface Sci. 2020, 575, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, C.; Chen, Y.; Li, D.; Wu, G.; Wang, C.; Guo, L. 3D flower-like MOF-derived NiCo-LDH integrated with Ti3C2Tx for high-performance pseudosupercapacitors. Electrochim. Acta 2021, 376, 138040. [Google Scholar] [CrossRef]
- Liang, M.; Zhao, M.; Wang, H.; Shen, J.; Song, X. Enhanced cycling stability of hierarchical NiCo2S4@Ni(OH)(2)@PPy core-shell nanotube arrays for aqueous asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 2482–2493. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Morphology-dependent electrochemical properties of cobalt-based metal organic frameworks for supercapacitor electrode materials. Electrochim. Acta 2018, 267, 170–180. [Google Scholar] [CrossRef]
- Lei, X.; Ge, S.; Tan, Y.; Wang, Z.; Li, J.; Li, X.; Hu, G.; Zhu, X.; Huang, M.; Zhu, Y.; et al. High Capacity and Energy Density of Zn-Ni-Co-P Nanowire Arrays as an Advanced Electrode for Aqueous Asymmetric Supercapacitor. ACS Appl. Mater. Inter. 2020, 12, 9158–9168. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Hu, Z.; Wang, W.; Zhang, X.; Qiang, L.; Wang, Q. 3D thin-wall cell structure nickel-cobalt-molybdenum ternary phosphides on carbon cloth as high-performance electrodes for asymmetric supercapacitors. J. Alloys Compd. 2019, 772, 683–692. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Zeng, Y.; Cheng, D.; Xiong, Y.; Bu, Y. Rational construction of triangle-like nickel-cobalt bimetallic metal-organic framework nanosheets arrays as battery-type electrodes for hybrid supercapacitors. J. Colloid Interface Sci. 2019, 555, 42–52. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Huang, F.; Yu, X.; Liu, M.; Zhang, H. Interface modification of hierarchical Co9S8@NiCo layered dihydroxide nanotube arrays using polypyrrole as charge transfer layer in flexible all -solid asymmetric supercapacitors, J. Power Sources 2019, 439, 227103. [Google Scholar] [CrossRef]
- Hang, X.; Zhao, J.; Xue, Y.; Yang, R.; Pang, H. Synergistic effect of Co/Ni bimetallic metal-organic nanostructures for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 628, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, L.; Du, M.; Song, Y.; Wu, Z.; Zheng, Q. Hierarchical NiCo-layered double hydroxide nanoscroll@PANI nanocomposite for high performance battery-type supercapacitor. Electrochim. Acta 2020, 338, 135869. [Google Scholar] [CrossRef]
- Ramachandran, R.; Lan, Y.; Xu, Z.-X.; Wang, F. Construction of NiCo-Layered Double Hydroxide Microspheres from Ni-MOFs for High-Performance Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 6633–6643. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. Wire spherical-shaped Co-MOF electrode materials for high-performance all-solid-state flexible asymmetric supercapacitor device. J. Alloys Compd. 2021, 879, 160423. [Google Scholar] [CrossRef]
- Amin, K.M.; Krois, K.; Muench, F.; Etzold, B.J.M.; Ensinger, W. Hierarchical pipe cactus-like Ni/NiCo-LDH core-shell nanotube networks as a self-supported battery-type electrode for supercapacitors with high volumetric energy density. J. Mater. Chem. A 2022, 10, 12473–12488. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, N. Construction of sulfide nanoparticles on hydrangea-like nickel-cobalt hydroxide for enhanced pseudocapacitance. J. Energy Storage 2022, 53, 105097. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Li, S.; Liao, X.; Sun, S. Water-promoted zeolitic imidazolate framework-67 transformation to Ni-Co layered double hydroxide hollow microsphere for supercapacitor electrode material. J. Mater. Sci.—Mater. Electron. 2017, 28, 9221–9227. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Dai, L.; Guo, F.; Mi, H.; Ji, C.; Sun, L. Kinetics-Favorable Ultrathin NiCo-MOF Nanosheets with Boosted Pseudocapacitive Charge Storage for Quasi-Solid-State Hybrid Supercapacitors. Inorg. Chem. 2022, 61, 3866–3874. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zou, Y.; Xu, F.; Xiang, C.; Sun, L. In Situ Growth of Nickel–Cobalt Metal Organic Frameworks Guided by a Nickel–Molybdenum Layered Double Hydroxide with Two-Dimensional Nanosheets Forming Flower-Like Struc-Tures for High-Performance Supercapacitors. Nanomaterials 2023, 13, 581. https://doi.org/10.3390/nano13030581
Cheng C, Zou Y, Xu F, Xiang C, Sun L. In Situ Growth of Nickel–Cobalt Metal Organic Frameworks Guided by a Nickel–Molybdenum Layered Double Hydroxide with Two-Dimensional Nanosheets Forming Flower-Like Struc-Tures for High-Performance Supercapacitors. Nanomaterials. 2023; 13(3):581. https://doi.org/10.3390/nano13030581
Chicago/Turabian StyleCheng, Cheng, Yongjin Zou, Fen Xu, Cuili Xiang, and Lixian Sun. 2023. "In Situ Growth of Nickel–Cobalt Metal Organic Frameworks Guided by a Nickel–Molybdenum Layered Double Hydroxide with Two-Dimensional Nanosheets Forming Flower-Like Struc-Tures for High-Performance Supercapacitors" Nanomaterials 13, no. 3: 581. https://doi.org/10.3390/nano13030581
APA StyleCheng, C., Zou, Y., Xu, F., Xiang, C., & Sun, L. (2023). In Situ Growth of Nickel–Cobalt Metal Organic Frameworks Guided by a Nickel–Molybdenum Layered Double Hydroxide with Two-Dimensional Nanosheets Forming Flower-Like Struc-Tures for High-Performance Supercapacitors. Nanomaterials, 13(3), 581. https://doi.org/10.3390/nano13030581