Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application
Abstract
1. Introduction
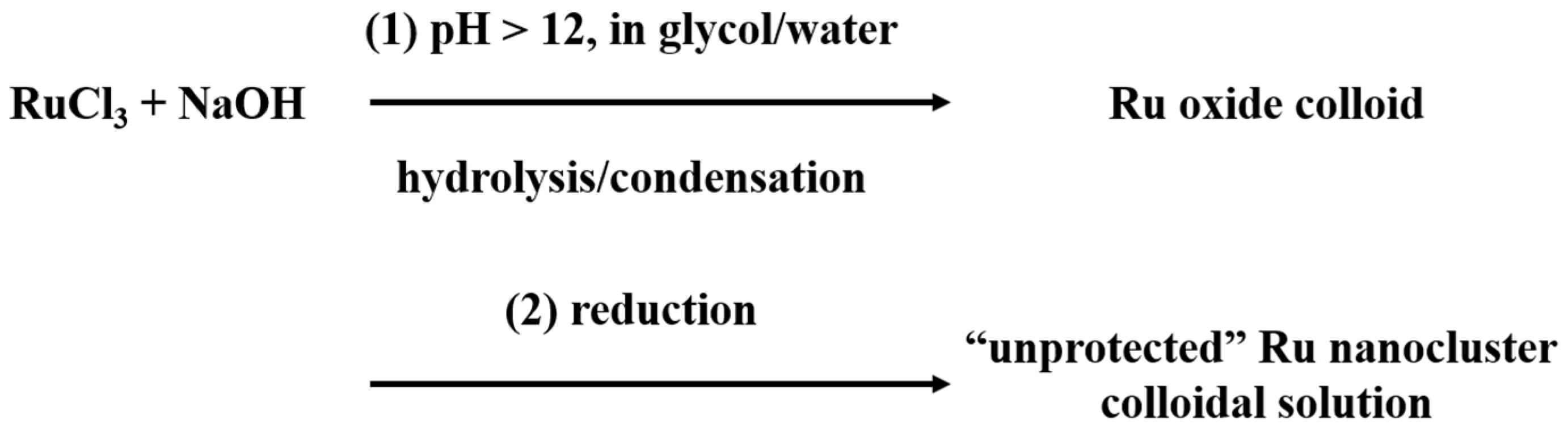
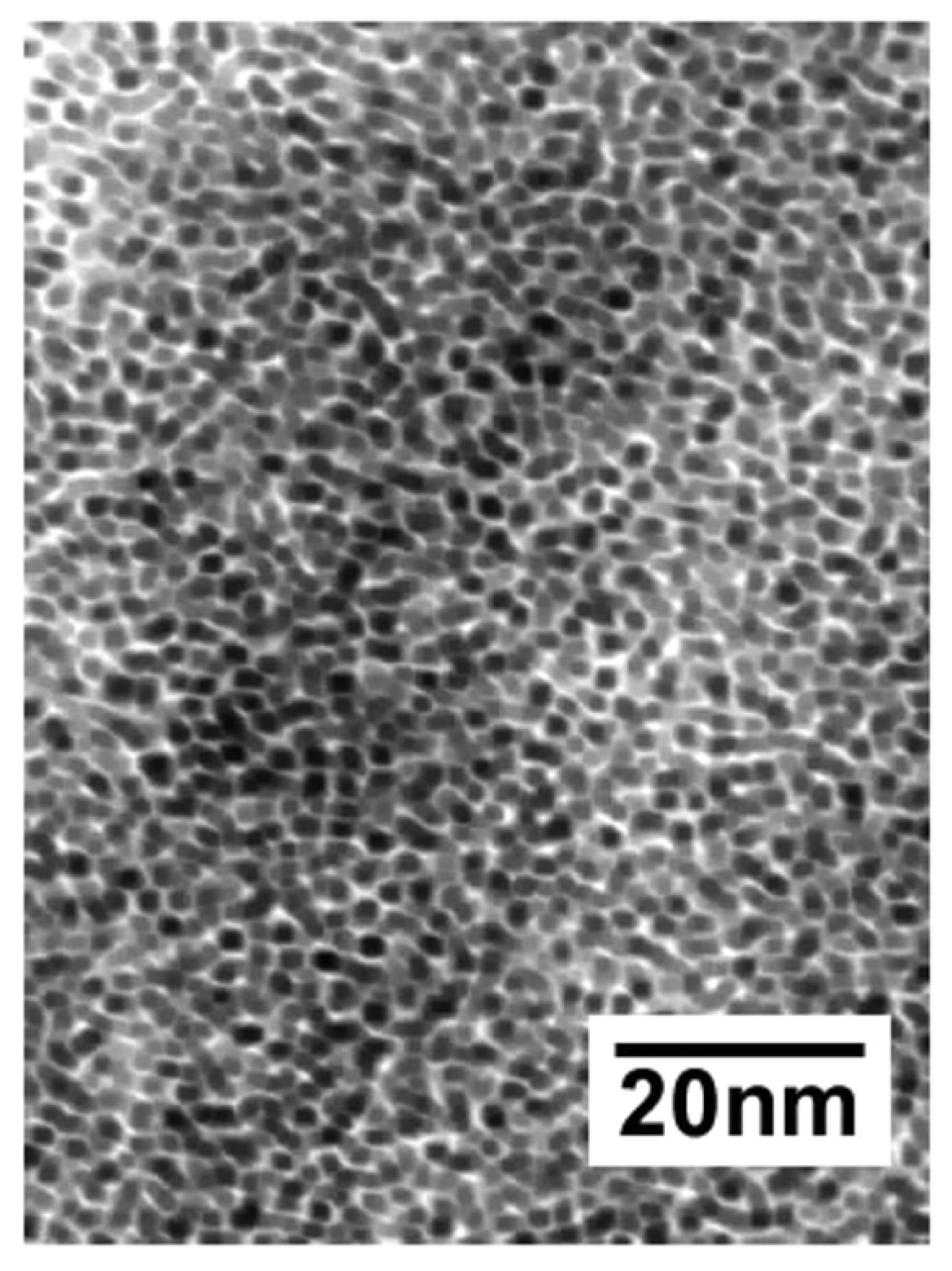
2. Formation Mechanism of Unprotected Nanoclusters
3. Applications of “Unprotected” Metal Nanoclusters
3.1. Application in Catalysis
3.1.1. Exploring the Structure–Function Relationship of Metal Nanocluster Catalysts
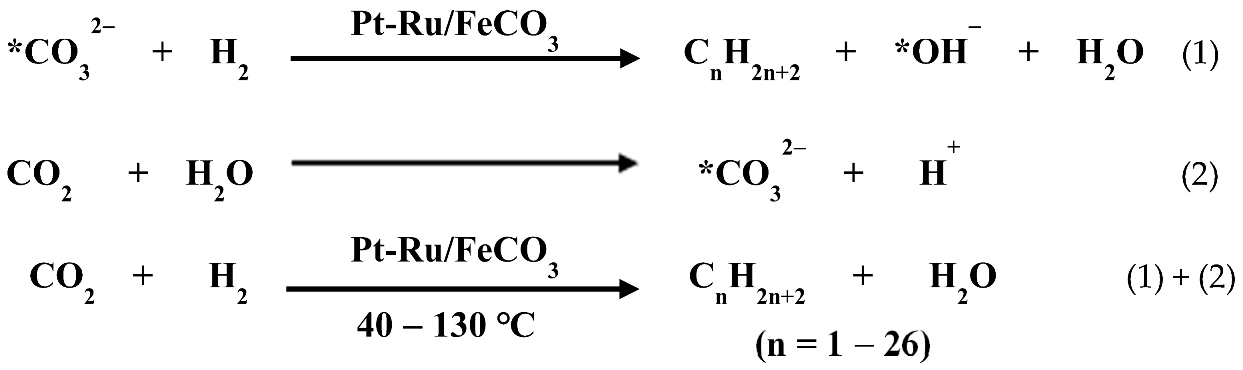
- (1)
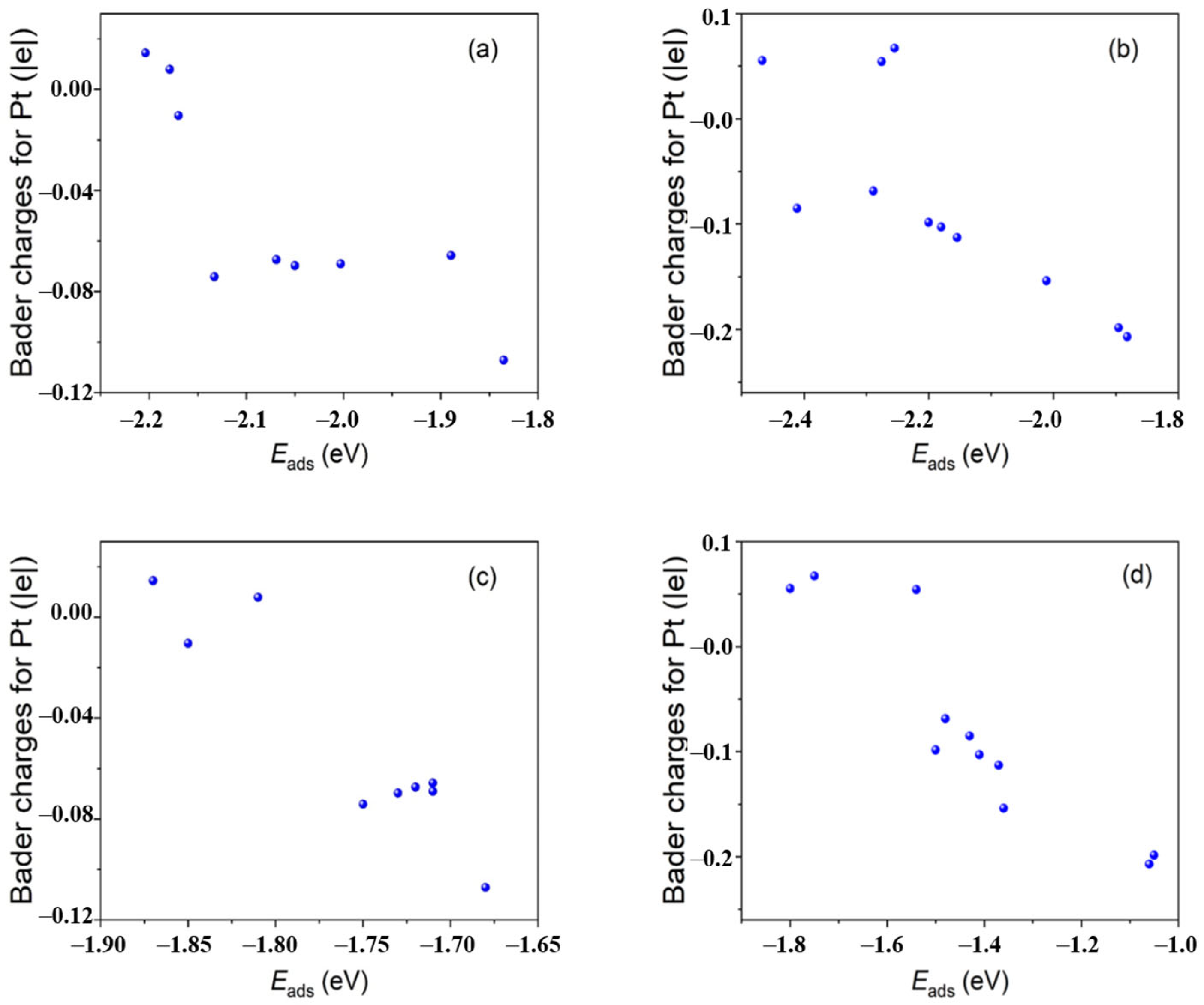
- They change the extent of charge separation between the surface atomic layer and core of the metal nanocluster. The electron donation from the support or ligand will increase the charge separation extent, leaving the surface atomic layer with a more negative charge.
- (2)
- They change the charge distribution state of the metal nanocluster surface atomic layer at the atomic (or subatomic) scale and make it more uneven compared with that of the naked metal nanocluster.
- (3)
- They change the distance between some of the metal atoms.
- (4)
- The extent of the change in the charge distribution or atomic spacing is related to the size of the metal nanocluster. Usually, small-sized metal nanoclusters exhibit more significant changes.
- (5)
- Catalytic sites composed of surface metal atoms with different charge states or atomic spacings exhibit different adsorption energies for a reactant and reaction energy barrier, showing different catalytic activities and product selectivities. The species in the support surface (the ion, vacancy or chemical group) or the ligand adjacent to the metal nanocluster can form complex (or synergistic) catalytic sites with metal nanocluster surface atoms, at which the adsorption energies of the reactants and chemical reaction energy barriers are influenced by coordination polarization or hydrogen bonding, resulting in a synergistic catalytic effect.
3.1.2. Application in Fabrication of Smart Catalysts
3.2. Application in Sensor
4. Concluding Remarks and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Guo, S.J.; Wang, E.K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Jin, R.C.; Zeng, C.J.; Zhou, M.; Chen, Y.X. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Pan, M.F.; Yang, J.Y.; Liu, K.X.; Yin, Z.J.; Ma, T.Y.; Liu, S.M.; Xu, L.H.; Wang, S.O. Noble Metal Nanostructured Materials for Chemical and Biosensing Systems. Nanomaterials 2020, 10, 209. [Google Scholar] [CrossRef]
- Lu, L.F.; Zheng, H.; Li, Y.X.; Zhou, Y.H.; Fang, B.Z. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- An, K.; Somorjai, G.A. Size and Shape Control of Metal Nanoparticles for Reaction Selectivity in Catalysis. ChemCatChem 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Schauermann, S.; Nilius, N.; Shaikhutdinov, S.; Freund, H.J. Nanoparticles for Heterogeneous Catalysis: New Mechanistic Insights. Acc. Chem. Res. 2013, 46, 1673–1681. [Google Scholar] [CrossRef]
- Liu, L.C.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.W.; Deng, K.; Gui, L.L.; Tang, Y.Q. Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Zuo, B.J.; Wang, Y.; Wang, Q.L.; Zhang, J.L.; Wu, N.Z.; Peng, L.D.; Gui, L.L.; Wang, X.D.; Wang, R.M.; Yu, D.P. An efficient ruthenium catalyst for selective hydrogenation of ortho-chloronitrobenzene prepared via assembling ruthenium and tin oxide nanoparticles. J. Catal. 2004, 222, 493–498. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Y.; Ji, H.; Wei, Y.G.; Wu, N.Z.; Zuo, B.J.; Wang, Q.L. Magnetic nanocomposite catalysts with high activity and selectivity for selective hydrogenation of ortho-chloronitrobenzene. J. Catal. 2005, 229, 114–118. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.L.; Wang, X.D.; Ren, J.W.; Zuo, B.J.; Tang, Y.Q. Metal nanoclusters stabilized with simple ions and solvents—Promising building blocks for future catalysts. Top. Catal. 2005, 35, 35–41. [Google Scholar] [CrossRef]
- Wang, X.D.; Liang, M.H.; Liu, H.Q.; Wang, Y. Selective hydrogenation of bromonitrobenzenes over Pt/γ-Fe2O3. J. Mol. Catal. A Chem. 2007, 273, 160–168. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.D. Metal Nanoclusters in Catalysis and Materials Science: The Issue of Size Control. In Solvent and Simple Ion-Stabilized Metal Nanoclusters: Chemical Synthesis and Application; Corain, B., Schmid, G., Toshima, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; p. 327. [Google Scholar]
- Liang, M.H.; Wang, X.D.; Liu, H.Q.; Liu, H.C.; Wang, Y. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J. Catal. 2008, 255, 335–342. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, N.; Chao, W.; Liu, H.Q.; Wang, Y. A novel nanocomposite catalytic cathode for direct methanol fuel cells. Electrochim. Acta 2010, 55, 5617–5623. [Google Scholar] [CrossRef]
- Zhang, L.W.; Zheng, N.; Gao, A.; Zhu, C.M.; Wang, Z.Y.; Wang, Y.; Shi, Z.J.; Liu, Y. A robust fuel cell cathode catalyst assembled with nitrogen-doped carbon nanohorn and platinum nanoclusters. J. Power Sources 2012, 220, 449–454. [Google Scholar] [CrossRef]
- Xiao, C.; Liang, M.H.; Gao, A.; Xie, J.L.; Wang, Y.; Liu, H.C. Weak affinity for CO of platinum group metal nanoparticles supported on partially reduced iron oxides. J. Nanopart. Res. 2013, 15, 1822. [Google Scholar] [CrossRef]
- Zhang, L.W.; Gao, A.; Liu, Y.; Wang, Y.; Ma, J.T. PtRu nanoparticles dispersed on nitrogen-doped carbon nanohorns as an efficient electrocatalyst for methanol oxidation reaction. Electrochim. Acta 2014, 132, 416–422. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Cheng, T.; Guo, H.Y.; Sun, B.; Wang, Y. Preparation and application in assembling high-performance fuel cell catalysts of colloidal PtCu alloy nanoclusters. J. Power Sources 2018, 395, 66–76. [Google Scholar] [CrossRef]
- Yu, Y.L.; Huang, J.; Wang, Y. Catalytic Conversion of CO2 to Value-Added Products under Mild Conditions. ChemCatChem 2018, 10, 4863–4867. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Zhao, X.S.; Yu, Y.L.; Liu, Y.; Harada, M.; Ishihara, A.; Wang, Y. Indium oxide supported Pt-In alloy nanocluster catalysts with enhanced catalytic performance toward oxygen reduction reaction. J. Power Sources 2020, 446, 227332. [Google Scholar] [CrossRef]
- Schrader, I.; Warneke, J.; Neumann, S.; Grotheer, S.; Swane, A.A.; Kirkensgaard, J.J.K.; Arenz, M.; Kunz, S. Surface Chemistry of "Unprotected" Nanoparticles: A Spectroscopic Investigation on Colloidal Particles. J. Phys. Chem. C 2015, 119, 17655–17661. [Google Scholar] [CrossRef]
- Huang, B.; Ma, Y.T.; Xiong, Z.L.; Lu, W.D.; Ding, R.; Li, T.T.; Jiang, P.; Liang, M.H. Facile fabrication of Ir/CNT/rGO nanocomposites with enhanced electrocatalytic performance for the hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 3288–3292. [Google Scholar] [CrossRef]
- Ding, J.; Chan, K.Y.; Ren, J.W.; Xiao, F.S. Platinum and platinum-ruthenium nanoparticles supported on ordered mesoporous carbon and their electrocatalytic performance for fuel cell reactions. Electrochim. Acta 2005, 50, 3131–3141. [Google Scholar] [CrossRef]
- Fu, X.Y.; Wang, Y.; Wu, N.Z.; Gui, L.L.; Tang, Y.Q. Surface modification of small platinum nanoclusters with alkylamine and alkylthiol: An XPS study on the influence of organic ligands on the Pt 4f binding energies of small platinum nanoclusters. J. Colloid Interface Sci. 2001, 243, 326–330. [Google Scholar] [CrossRef]
- Du, X.Y.; Wang, Y.; Mu, Y.Y.; Gui, L.L.; Wang, P.; Tang, Y.Q. A new highly selective H2 sensor based on TiO2/PtO-Pt dual-layer films. Chem. Mater. 2002, 14, 3953–3957. [Google Scholar] [CrossRef]
- Mu, Y.Y.; Du, X.Y.; Wang, Y.; Guo, H.Y.; Gui, L.L. Study on hydrogen sensitivities of TiO2/PtO-Pt and SnO2/PtO-Pt dual-layer films. Acta Chimica Sinica 2003, 61, 8–12. [Google Scholar]
- Mu, Y.Y.; Liang, H.P.; Hu, J.S.; Jiang, L.; Wan, L.J. Controllable Pt nanoparticle deposition on carbon nanotubes as an anode catalyst for direct methanol fuel cells. J. Phys. Chem. B 2005, 109, 22212–22216. [Google Scholar] [CrossRef]
- Neumann, S.; Grotheer, S.; Tielke, J.; Schrader, I.; Quinson, J.; Zana, A.; Oezaslan, M.; Arenz, M.; Kunz, S. Nanoparticles in a box: A concept to isolate, store and re-use colloidal surfactant-free precious metal nanoparticles. J. Mater. Chem. A 2017, 5, 6140–6145. [Google Scholar] [CrossRef]
- Lewis, L.N. Chemical catalysis by colloids and clusters. Chem. Rev. 1993, 93, 2693–2730. [Google Scholar] [CrossRef]
- Schmid, G. Nanoclusters-Building blocks for future nanoelectronic devices? Adv. Eng. Mater. 2001, 3, 737–743. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Tsung, C.-K.; Huang, W.; Somorjai, G.A. Effect of organic capping layers over monodisperse platinum nanoparticles upon activity for ethylene hydrogenation and carbon monoxide oxidation. J. Catal. 2009, 265, 209–215. [Google Scholar] [CrossRef]
- Fenske, D.; Sonstroem, P.; Stoever, J.; Wang, X.D.; Borchert, H.; Al-Shamery, K. Colloidally Prepared Pt Nanoparticles for Heterogeneous Gas-Phase Catalysis: Influence of Ligand Shell and Catalyst Loading on CO Oxidation Activity. ChemCatChem 2010, 2, 198–205. [Google Scholar] [CrossRef]
- Wang, X.D.; Stoever, J.; Zielasek, V.; Altmann, L.; Thiel, K.; Al-Shamery, K.; Baeumer, M.; Borchert, H.; Parisi, J.; Kolny-Olesiak, J. Colloidal Synthesis and Structural Control of PtSn Bimetallic Nanoparticles. Langmuir 2011, 27, 11052–11061. [Google Scholar] [CrossRef]
- Sonstroem, P.; Arndt, D.; Wang, X.D.; Zielasek, V.; Baeumer, M. Ligand Capping of Colloidally Synthesized Nanoparticles-A Way to Tune Metal-Support Interactions in Heterogeneous Gas-Phase Catalysis. Angew. Chem. Int. Ed. 2011, 50, 3888–3891. [Google Scholar] [CrossRef]
- Kunz, S.; Schreiber, P.; Ludwig, M.; Maturi, M.M.; Ackermann, O.; Tschurl, M.; Heiz, U. Rational design, characterization and catalytic application of metal clusters functionalized with hydrophilic, chiral ligands: A proof of principle study. Phys. Chem. Chem. Phys. 2013, 15, 19253–19261. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Xiao, C.X.; Maligal-Ganesh, R.V.; Zhou, L.; Goh, T.W.; Li, X.L.; Tesfagaber, D.; Thiel, A.; Huang, W.Y. Pt Nanoclusters Confined within Metal Organic Framework Cavities for Chemoselective Cinnamaldehyde Hydrogenation. ACS Catal. 2014, 4, 1340–1348. [Google Scholar] [CrossRef]
- Speder, J.; Spanos, I.; Zana, A.; Kirkensgaard, J.J.K.; Mortensen, K.; Altmann, L.; Baeumer, M.; Arenz, M. From single crystal model catalysts to systematic studies of supported nanoparticles. Surf. Sci. 2015, 631, 278–284. [Google Scholar] [CrossRef]
- Ning, X.M.; Yu, H.; Peng, F.; Wang, H.J. Pt nanoparticles interacting with graphitic nitrogen of N-doped carbon nanotubes: Effect of electronic properties on activity for aerobic oxidation of glycerol and electro-oxidation of CO. J. Catal. 2015, 325, 136–144. [Google Scholar] [CrossRef]
- Altmann, L.; Wang, X.; Borchert, H.; Kolny-Olesiak, J.; Zielasek, V.; Parisi, J.; Kunz, S.; Baeumer, M. Influence of Sn content on the hydrogenation of crotonaldehyde catalysed by colloidally prepared PtSn nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 28186–28192. [Google Scholar] [CrossRef]
- Darab, M.; Barnett, A.O.; Lindbergh, G.; Thomassen, M.S.; Sunde, S. The Influence of Catalyst Layer Thickness on the Performance and Degradation of PEM Fuel Cell Cathodes with Constant Catalyst Loading. Electrochim. Acta 2017, 232, 505–516. [Google Scholar] [CrossRef]
- Yan, M.Y.; Wu, T.; Chen, L.F.; Yu, Y.L.; Liu, B.; Wang, Y.; Chen, W.X.; Liu, Y.; Lian, C.; Li, Y.D. Effect of Protective Agents upon the Catalytic Property of Platinum Nanocrystals. ChemCatChem 2018, 10, 2433–2441. [Google Scholar] [CrossRef]
- Ilsemann, J.; Murshed, M.M.; Gesing, T.M.; Kopyscinski, J.; Baeumer, M. On the support dependency of the CO2 methanation—Decoupling size and support effects. Catal. Sci. Technol. 2021, 11, 4098–4114. [Google Scholar] [CrossRef]
- Li, X.H.; You, X.; Ying, P.L.; Xiao, J.L.; Li, C. Some insights into the preparation of Pt/γ-Al2O3 catalysts for the enantioselective hydrogenation of a-ketoesters. Top. Catal. 2003, 25, 63–70. [Google Scholar] [CrossRef]
- Lian, C.; Liu, H.Q.; Xiao, C.; Yang, W.; Zhang, K.; Liu, Y.; Wang, Y. Solvent-free selective hydrogenation of chloronitrobenzene to chloroaniline over a robust Pt/Fe3O4 catalyst. Chem. Commun. 2012, 48, 3124–3126. [Google Scholar] [CrossRef]
- Liu, M.H.; Mo, X.X.; Liu, Y.Y.; Xiao, H.L.; Zhang, Y.; Jing, J.Y.; Colvin, V.L.; Yu, W.W. Selective hydrogenation of o-chloronitrobenzene using supported platinum nanoparticles without solvent. Appl. Catal. A Gen. 2012, 439, 192–196. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Li, X.B.; Yang, Y.; Yang, Q.H.; Li, C. Assembly of ZIF nanostructures around free Pt nanoparticles: Efficient size-selective catalysts for hydrogenation of alkenes under mild conditions. Chem. Commun. 2013, 49, 3330–3332. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Selective conversion of nitroarenes using a carbon nanotube-ruthenium nanohybrid. Chem. Commun. 2015, 51, 1739–1742. [Google Scholar] [CrossRef]
- Schrader, I.; Warneke, J.; Backenkoehler, J.; Kunz, S. Functionalization of Platinum Nanoparticles with L-Proline: Simultaneous Enhancements of Catalytic Activity and Selectivity. J. Am. Chem. Soc. 2015, 137, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Huang, J.; Wang, Y. Catalytic conversion of ferrous carbonate to higher hydrocarbons under mild conditions and its application in transformation of CO2 to liquid fuels. Sustain. Energy Fuels 2020, 4, 96–100. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, W.; Li, X.J.; Jiang, P.; Zhang, N.; Liang, M.H. Copper Single-Atom-Covered Pt Nanoparticles for Selective Hydrogenation of Phenylacetylene. ACS Appl. Nano Mater. 2021, 4, 5292–5300. [Google Scholar] [CrossRef]
- Yu, Y.L.; Cai, Y.C.; Liang, M.H.; Tan, X.; Huang, J.; Jiang, H.; Harada, M.; Wang, Y. Highly selective synthesis of multicarbon compounds by carbon dioxide hydrogenation over Pt nanocrystals anchoring Ru clusters. Catal. Sci. Technol. 2022, 12, 3786–3792. [Google Scholar] [CrossRef]
- Kongkanand, A.; Vinodgopal, K.; Kuwabata, S.; Kamat, P.V. Highly dispersed Pt catalysts on single-walled carbon nanotubes and their role in methanol oxidation. J. Phys. Chem. B 2006, 110, 16185–16188. [Google Scholar] [CrossRef]
- Joo, J.B.; Kim, P.; Kim, W.; Yi, J. Preparation of Pt supported on mesoporous carbons for the reduction of oxygen in polymer electrolyte membrane fuel cell (PEMFC). J. Electroceramics 2006, 17, 713–718. [Google Scholar] [CrossRef]
- Baturina, O.A.; Garsany, Y.; Zega, T.J.; Stroud, R.M.; Schull, T.; Swider-Lyons, K.E. Oxygen Reduction Reaction on Platinum/Tantalum Oxide Electrocatalysts for PEM Fuel Cells. J. Electrochem. Soc. 2008, 155, B1314–B1321. [Google Scholar] [CrossRef]
- Garsany, Y.; Epshteyn, A.; Purdy, A.P.; More, K.L.; Swider-Lyons, K.E. High-Activity, Durable Oxygen Reduction Electrocatalyst: Nanoscale Composite of Platinum-Tantalum Oxyphosphate on Vulcan Carbon. J. Phys. Chem. Lett. 2010, 1, 1977–1981. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.X.; Yang, L.P.; Wang, F.; Yin, J. Unprotected Pt nanoclusters anchored on ordered mesoporous carbon as an efficient and stable catalyst for oxygen reduction reaction. Electrochim. Acta 2019, 297, 539–544. [Google Scholar] [CrossRef]
- Brauns, E.; Morsbach, E.; Kunz, S.; Baeumer, M.; Lang, W. A fast and sensitive catalytic gas sensors for hydrogen detection based on stabilized nanoparticles as catalytic layer. Sens. Actuators B Chem. 2014, 193, 895–903. [Google Scholar] [CrossRef]
- Wu, X.; Li, W.; Zhang, G.; Zhang, Q.; Cheng, Y. Highly sensitive glucose biosensor preliminary applied for detecting the glucose levels of cerebrospinal fluid in patients with traumatic brain injury. Anal. Methods 2014, 6, 9698–9704. [Google Scholar] [CrossRef]
- Morsbach, E.; Kunz, S.; Baeumer, M. Novel nanoparticle catalysts for catalytic gas sensing. Catal. Sci. Technol. 2016, 6, 339–348. [Google Scholar] [CrossRef]
- Dave, P.; Agrawal, B.; Thakarda, J.; Bhowmik, S.; Maity, P. An organometallic ruthenium nanocluster with conjugated aromatic ligand skeleton for explosive sensing. J. Chem. Sci. 2019, 131, 14. [Google Scholar] [CrossRef]
- Altmann, L.; Sturm, H.; Brauns, E.; Lang, W.; Baeumer, M. Novel catalytic gas sensors based on functionalized nanoparticle layers. Sens. Actuators B Chem. 2012, 174, 145–152. [Google Scholar] [CrossRef]
- Gebauer, C.; Jusys, Z.; Wassner, M.; Huesing, N.; Behm, R.J. Membrane Fuel Cell Cathode Catalysts Based on Titanium Oxide Supported Platinum Nanoparticles. ChemPhysChem 2014, 15, 2094–2107. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Chen, Z.Y.; Mao, J.X.; Zhou, R.X. The size effect and high activity of nanosized platinum supported catalysts for low temperature oxidation of volatile organic compounds. Chin. J. Chem. Eng. 2021, 39, 135–143. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Zhou, W.J.; Qiu, J.S.; Li, H.Q.; Sun, G.Q.; Xin, Q. Homogeneous and controllable Pt particles deposited on multi-wall carbon nanotubes as cathode catalyst for direct methanol fuel cells. Carbon 2004, 42, 436–439. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Xin, Q. Application of novel carbon nanomaterials in low-temperature fuel cell catalysts. Chinese J. Catal. 2004, 25, 839–843. [Google Scholar]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-surface-area catalyst design: Synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef]
- Yang, G.W.; Gao, G.Y.; Zhao, G.Y.; Li, H.L. Effective adhesion of Pt nanoparticles on thiolated multi-walled carbon nanotubes and their use for fabricating electrocatalysts. Carbon 2007, 45, 3036–3041. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Yeh, C.-T. Poly(vinylpyrrolidone)-modified graphite carbon nanofibers as promising supports for PtRu catalysts in direct methanol fuel cells. J. Am. Chem. Soc. 2007, 129, 9999–10010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.F.; Hu, J.S.; Zhong, L.S.; Wan, L.J.; Song, W.G. In situ one-step method for preparing carbon nanotubes and pt composite catalysts and their performance for methanol oxidation. J. Phys. Chem. C 2007, 111, 11174–11179. [Google Scholar] [CrossRef]
- Qi, J.; Jiang, L.H.; Jing, M.Y.; Tang, Q.W.; Sun, G.Q. Preparation of Pt/C via a polyol process—Investigation on carbon support adding sequence. Int. J. Hydrogen Energy 2011, 36, 10490–10501. [Google Scholar] [CrossRef]
- Muthuswamy, N.; de la Fuente, J.L.G.; Ochal, P.; Giri, R.; Raaen, S.; Sunde, S.; Ronning, M.; Chen, D. Towards a highly-efficient fuel-cell catalyst: Optimization of Pt particle size, supports and surface-oxygen group concentration. Phys. Chem. Chem. Phys. 2013, 15, 3803–3813. [Google Scholar] [CrossRef]
- Zhu, C.M.; Gao, A.; Wang, Y.; Liu, Y. Pt-Cu bimetallic electrocatalysts with enhanced catalytic properties for oxygen reduction. Chem. Commun. 2014, 50, 13889–13892. [Google Scholar] [CrossRef]
- Eckardt, M.; Gebauer, C.; Jusys, Z.; Wassner, M.; Huesing, N.; Behm, R.J. Oxygen reduction reaction activity and long-term stability of platinum nanoparticles supported on titania and titania-carbon nanotube composites. J. Power Sources 2018, 400, 580–591. [Google Scholar] [CrossRef]
- Mao, S.S.; Mao, G. Supported Nanoparticle Catalyst. U.S. Patent 6686308 B2 3 February 2004. [Google Scholar]
- Ledendecker, M.; Paciok, P.; Osowiecki, W.T.; Pander, M.; Heggen, M.; Gohl, D.; Kamat, G.A.; Erbe, A.; Mayrhofer, K.J.J.; Alivisatos, A.P. Engineering gold-platinum core-shell nanoparticles by self-limitation in solution. Commun. Chem. 2022, 5, 71. [Google Scholar] [CrossRef]
- Loof, D.; Thueringer, O.; Schowalter, M.; Mahr, C.; Pranti, A.S.; Lang, W.; Rosenauer, A.; Zielasek, V.; Kunz, S.; Baeumer, M. Synthesis and Characterization of Ligand-Linked Pt Nanoparticles: Tunable, Three-Dimensional, Porous Networks for Catalytic Hydrogen Sensing. Chemistryopen 2021, 10, 697–712. [Google Scholar] [CrossRef]
- Lang, R.; Xi, W.; Liu, J.C.; Wang, X.D.; Luo, J.; Qiao, B.T.; Li, J.; Zhang, T. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 2019, 10, 234. [Google Scholar] [CrossRef]
- Bock, C.; Paquet, C.; Couillard, M.; Botton, G.A.; MacDougall, B.R. Size-selected synthesis of PtRu nano-catalysts: Reaction and size control mechanism. J. Am. Chem. Soc. 2004, 126, 8028–8037. [Google Scholar] [CrossRef]
- Harpeness, R.; Peng, Z.; Liu, X.S.; Pol, V.G.; Koltypin, Y.; Gedanken, A. Controlling the agglomeration of anisotropic Ru nanoparticles by the microwave-polyol process. J. Colloid Interface Sci. 2005, 287, 678–684. [Google Scholar] [CrossRef]
- He, B.L.; Chen, Y.X.; Liu, H.F.; Liu, Y. Synthesis of solvent-stabilized colloidal nanoparticles of platinum, rhodium, and ruthenium by microwave-polyol process. J. Nanosci. Nanotechnol. 2005, 5, 266–270. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, G.Q.; Li, N.; Sun, S.G.; Su, D.S.; Xin, Q. Design and preparation of highly active Pt-Pd/C catalyst for the oxygen reduction reaction. J. Phys. Chem. C 2007, 111, 5605–5617. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, G.Q.; Gaot, Y.; Jiang, Q.; Jia, Z.Q.; Xin, Q. Effect of reaction atmosphere on the electrocatalytic activities of Pt/C and PtRu/C obtained in a polyol process. J. Phys. Chem. C 2007, 111, 15192–15200. [Google Scholar] [CrossRef]
- Navin, J.K.; Grass, M.E.; Somorjai, G.A.; Marsh, A.L. Characterization of Colloidal Platinum Nanoparticles by MALDI-TOF Mass Spectrometry. Anal. Chem. 2009, 81, 6295–6299. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Wang, Z.B.; Gu, D.M.; Yin, G.P. Performance of Pt/C catalysts prepared by microwave-assisted polyol process for methanol electrooxidation. J. Power Sources 2010, 195, 1799–1804. [Google Scholar] [CrossRef]
- Sevjidsuren, G.; Zils, S.; Kaserer, S.; Wolz, A.; Ettingshausen, F.; Dixon, D.; Schoekel, A.; Roth, C.; Altantsog, P.; Sangaa, D.; et al. Effect of Different Support Morphologies and Pt Particle Sizes in Electrocatalysts for Fuel Cell Applications. J. Nanomater. 2010, 2010, 852786. [Google Scholar] [CrossRef]
- Nguyen Viet, L.; Nguyen Duc, C.; Hayakawa, T.; Hirata, H.; Lakshminarayana, G.; Nogami, M. The synthesis and characterization of platinum nanoparticles: A method of controlling the size and morphology. Nanotechnology 2010, 21, 035605. [Google Scholar]
- Nguyen Viet, L.; Nguyen Duc, C.; Tomokatsu, H.; Matsubara, T.; Ohtaki, M.; Nogami, M. Sharp cubic and octahedral morphologies of poly(vinylpyrrolidone)-stabilised platinum nanoparticles by polyol method in ethylene glycol: Their nucleation, growth and formation mechanisms. J. Exp. Nanosci. 2012, 7, 133–149. [Google Scholar]
- Steinfeldt, N. In Situ Monitoring of Pt Nanoparticle Formation in Ethylene Glycol Solution by SAXS-Influence of the NaOH to Pt Ratio. Langmuir 2012, 28, 13072–13079. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Arellano-Jimenez, M.J.; Carter, C.B.; Agrios, A.G. Preparation of functionalized platinum nanoparticles: A comparison of different methods and reagents. J. Nanopart. Res. 2013, 15, 1744. [Google Scholar] [CrossRef]
- Proch, S.; Kodama, K.; Inaba, M.; Oishi, K.; Takahashi, N.; Morimoto, Y. The “Particle Proximity Effect” in Three Dimensions: A Case Study on Vulcan XC 72R. Electrocatalysis 2016, 7, 249–261. [Google Scholar] [CrossRef]
- Wand, P.; Bartl, J.D.; Heiz, U.; Tschurl, M.; Cokoja, M. Functionalization of small platinum nanoparticles with amines and phosphines: Ligand binding modes and particle stability. J. Colloid Interface Sci. 2016, 478, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Neumann, S.; Kacenauskaite, L.; Bucher, J.; Kirkensgaard, J.J.K.; Simonsen, S.B.; Kuhn, L.T.; Zana, A.; Vosch, T.; Oezaslan, M.; et al. Solvent-Dependent Growth and Stabilization Mechanisms of Surfactant-Free Colloidal Pt Nanoparticles. Chem. Eur. J. 2020, 26, 9012–9023. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, S.; Li, D.S.; Newalkar, B.; Katsuki, H.; Bhalla, A.S. Microwave-polyol process for Pt and Ag nanoparticles. Langmuir 2002, 18, 5959–5962. [Google Scholar] [CrossRef]
- Yang, J.; Deivaraj, T.C.; Too, H.P.; Lee, J.Y. Acetate stabilization of metal nanoparticles and its role in the preparation of metal nanoparticles in ethylene glycol. Langmuir 2004, 20, 4241–4245. [Google Scholar] [CrossRef]
- Schroder, J.; Neumann, S.; Kunz, S. Visible-Light-Induced Synthesis of ”Surfactant-Free” Pt Nanoparticles in Ethylene Glycol as a Synthetic Approach for Mechanistic Studies on Nanoparticle Formation. J. Phys. Chem. C 2020, 124, 21798–21809. [Google Scholar] [CrossRef]
- Quinson, J.; Jensen, K.M.O. From platinum atoms in molecules to colloidal nanoparticles: A review on reduction, nucleation and growth mechanisms. Adv. Colloid Interface Sci. 2020, 286, 102300. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Zhou, W.J.; Qiu, J.S.; Zhou, Z.H.; Sun, G.Q.; Xin, Q. Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Chen, L.F.; Yu, Y.L.; Kuwa, M.; Cheng, T.; Liu, Y.; Murakami, H.; Harada, M.; Wang, Y. Insight into the Formation Mechanism of "Unprotected" Metal Nanoclusters. Acta Phys. Chim. Sin. 2020, 36, 1907008. [Google Scholar] [CrossRef]
- Quinson, J.; Kunz, S.; Arenz, M. Beyond Active Site Design: A Surfactant-Free Toolbox Approach for Optimized Supported Nanoparticle Catalysts. ChemCatChem 2021, 13, 1692–1705. [Google Scholar] [CrossRef]
- Cardenastrivino, G.; Klabunde, K.J.; Dale, E.B. Living colloidal palladium in nonaqueous solvents. formation, stability, and film-forming properties. clustering of metal atoms in organic media. Langmuir 1987, 3, 986–992. [Google Scholar] [CrossRef]
- Esumi, K.; Tano, T.; Meguro, K. Preparation of organo palladium particles from thermal decomposition of its organic complex in organic solvents. Langmuir 1989, 5, 268–270. [Google Scholar] [CrossRef]
- Tano, T.; Esumi, K.; Meguro, K. Preparation of organopalladium sols by thermal decomposition of palladium acetate. J. Colloid Interface Sci. 1989, 133, 530–533. [Google Scholar] [CrossRef]
- Curtis, A.C.; Duff, D.G.; Edwards, P.P.; Jefferson, D.A.; Johnson, B.F.G.; Kirkland, A.I.; Wallace, A.S. Preparation and structural characterization of an unprotected copper sol. J. Phys. Chem. 1988, 92, 2270–2275. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, N.N.; Fang, B.Z.; Li, H.; Bi, X.T.T.; Wang, H.J. Carbon-Supported Pt-Based Alloy Electrocatalysts for the Oxygen Reduction Reaction in Polymer Electrolyte Membrane Fuel Cells: Particle Size, Shape, and Composition Manipulation and Their Impact to Activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, G.Q.; Cao, L.; Jiang, L.H.; Xin, Q. Comparison of different promotion effect of PtRu/C and PtSn/C electrocatalysts for ethanol electro-oxidation. Electrochim. Acta 2007, 52, 6622–6629. [Google Scholar] [CrossRef]
- Speder, J.; Altmann, L.; Roefzaad, M.; Baeumer, M.; Kirkensgaard, J.J.K.; Mortensen, K.; Arenz, M. Pt based PEMFC catalysts prepared from colloidal particle suspensions—A toolbox for model studies. Phys. Chem. Chem. Phys. 2013, 15, 3602–3608. [Google Scholar] [CrossRef]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Kunz, S.; Arenz, M. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Martinez, E.Y.; Li, C.W. Surface functionalization of Pt nanoparticles with metal chlorides for bifunctional CO oxidation. Polyhedron 2019, 170, 239–244. [Google Scholar] [CrossRef]
- Cheng, T.; Tan, X.; Chen, L.F.; Zhao, X.S.; Kotegawa, F.; Huang, J.; Liu, Y.; Jiang, H.; Harada, M.; Wang, Y. A Robust Electrocatalyst for Oxygen Reduction Reaction Assembled with Pt Nanoclusters and a Melem-Modified Carbon Support. Energy Technol. 2022, 10, 2200680. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, P.J.; Hu, J.T.; Tu, Y.C.; Gong, Z.M.; Cui, Y.; Deng, D.H. Electron penetration triggering interface activity of Pt-graphene for CO oxidation at room temperature. Nat. Commun. 2021, 12, 5814. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F. Study on the Structure and Properties of Novel Nanocomposite Catalysts for Fuel Cell. Ph.D. Thesis, Peking University, Beijing, China, 2019. [Google Scholar]
- Morsbach, E.; Nesselberger, M.; Warneke, J.; Harz, P.; Arenz, M.; Baeumer, M.; Kunz, S. 1-Naphthylamine functionalized Pt nanoparticles: Electrochemical activity and redox chemistry occurring on one surface. New J. Chem. 2015, 39, 2557–2564. [Google Scholar] [CrossRef]
- Song, H.; Rioux, R.M.; Hoefelmeyer, J.D.; Komor, R.; Niesz, K.; Grass, M.; Yang, P.D.; Somorjai, G.A. Hydrothermal growth of mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: Synthesis, characterization, and catalytic properties. J. Am. Chem. Soc. 2006, 128, 3027–3037. [Google Scholar] [CrossRef]
- Yuan, X.; Yan, N.; Xiao, C.X.; Li, C.N.; Fei, Z.F.; Cai, Z.P.; Kou, Y.; Dyson, P.J. Highly selective hydrogenation of aromatic chloronitro compounds to aromatic chloroamines with ionic-liquid-like copolymer stabilized platinum nanocatalysts in ionic liquids. Green Chem. 2010, 12, 228–233. [Google Scholar] [CrossRef]
- Wand, P.; Kratzer, E.; Heiz, U.; Cokoja, M.; Tschurl, M. High stability of thiol-protected colloidal platinum nanoparticles with reduced ligand coverages in the hydrogenation of 3-hexyne. Catal. Commun. 2017, 100, 85–88. [Google Scholar] [CrossRef]
- Sulce, A.; Mitschke, N.; Azov, V.; Kunz, S. Molecular Insights into the Ligand-Reactant Interactions of Pt Nanoparticles Functionalized with alpha-Amino Acids as Asymmetric Catalysts for beta-Keto Esters. ChemCatChem 2019, 11, 2732–2742. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.L. Influence of surface capping on oxygen reduction catalysis: A case study of 1.7 nm Pt nanoparticles. Surf. Sci. 2016, 648, 120–125. [Google Scholar] [CrossRef]
- Schrader, I.; Neumann, S.; Sulce, A.; Schmidt, F.; Azov, V.; Kunz, S. Asymmetric Heterogeneous Catalysis: Transfer of Molecular Principles to Nanoparticles by Ligand Functionalization. ACS Catal. 2017, 7, 3979–3987. [Google Scholar] [CrossRef]
- Sulce, A.; Backenkohler, J.; Schrader, I.; Delle Piane, M.; Azov, V.; Kunz, S. Ligand-functionalized Pt nanoparticles as asymmetric heterogeneous catalysts: Molecular reaction control by ligand-reactant interactions. Catal. Sci. Technol. 2018, 8, 6062–6075. [Google Scholar] [CrossRef]
- Nguyen Viet, L.; Ohtaki, M.; Nogami, M.; Tong Duy, H. Effects of heat treatment and poly(vinylpyrrolidone) (PVP) polymer on electrocatalytic activity of polyhedral Pt nanoparticles towards their methanol oxidation. Colloid. Polym. Sci. 2011, 289, 1373–1386. [Google Scholar]
- Wang, X.; Sonstroem, P.; Arndt, D.; Stoever, J.; Zielasek, V.; Borchert, H.; Thiel, K.; Al-Shamery, K.; Baeumer, M. Heterogeneous catalysis with supported platinum colloids: A systematic study of the interplay between support and functional ligands. J. Catal. 2011, 278, 143–152. [Google Scholar] [CrossRef]
- Morsbach, E.; Brauns, E.; Kowalik, T.; Lang, W.; Kunz, S.; Baeumer, M. Ligand-stabilized Pt nanoparticles (NPs) as novel materials for catalytic gas sensing: Influence of the ligand on important catalytic properties. Phys. Chem. Chem. Phys. 2014, 16, 21243–21251. [Google Scholar] [CrossRef]
- Pietron, J.J.; Garsany, Y.; Baturina, O.; Swider-Lyons, K.E.; Stroud, R.M.; Ramaker, D.E.; Schull, T.L. Electrochemical observation of ligand effects on oxygen reduction at ligand-stabilized Pt nanoparticle electrocatalysts. Electrochem. Solid State Lett. 2008, 11, B161–B165. [Google Scholar] [CrossRef]
- An, N.; Li, S.; Duchesne, P.N.; Wu, P.; Zhang, W.L.; Jia, M.J.; Zhang, W.X. Size Effects of Platinum Colloid Particles on the Structure and CO Oxidation Properties of Supported Pt/Fe2O3 Catalysts. J. Phys. Chem. C 2013, 117, 21254–21262. [Google Scholar] [CrossRef]
- Zheng, N.; Zhu, C.M.; Sun, B.; Shi, Z.J.; Liu, Y.; Wang, Y. Nanocomposite Cathode Catalyst with High Methanol Tolerance and Durability. Acta Phys.-Chim. Sin. 2012, 28, 2263–2268. [Google Scholar]
- Yu, W.J.; Lou, L.-L.; Yu, K.; Li, S.S.; Shi, Y.; Liu, S.X. Pt nanoparticles stabilized by thermosensitive polymer as effective and recyclable catalysts for the asymmetric hydrogenation of ethyl pyruvate. RSC Adv. 2016, 6, 52500–52508. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; Feng, B.; Li, F.; Wang, F. One-pot synthesis of unprotected PtPd nanoclusters with enhanced catalytic activity, durability, and methanol-tolerance for oxygen reduction reaction. Appl. Surf. Sci. 2019, 473, 318–325. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Gao, Z.R.; Qin, X.T.; Xu, Y.; Wang, Z.H.; Zhou, W.; Ma, D. Importance of Species Heterogeneity in Supported Metal Catalysts. J. Am. Chem. Soc. 2022, 144, 5108–5115. [Google Scholar] [CrossRef] [PubMed]
- Jawale, D.V.; Kouatchou, J.A.T.; Fossard, F.; Miserque, F.; Geertsen, V.; Gravel, E.; Doris, E. Catalytic hydrothiolation of alkenes and alkynes using bimetallic RuRh nanoparticles on carbon nanotubes. Green Chem. 2022, 24, 1231–1237. [Google Scholar] [CrossRef]
- Bizzotto, F.; Arenz, M.; Quinson, J. Surfactant-free Ir nanoparticles synthesized in ethanol: Catalysts for the oxygen evolution reaction. Materials Letters 2022, 308, 131209. [Google Scholar] [CrossRef]
- Kawawaki, T.; Shimizu, N.; Funai, K.; Mitomi, Y.; Hossain, S.; Kikkawa, S.; Osborn, D.J.; Yamazoe, S.; Metha, G.F.; Negishi, Y. Simple and high-yield preparation of carbon-black-supported similar to 1 nm platinum nanoclusters and their oxygen reduction reactivity. Nanoscale 2021, 13, 14679–14687. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, Z.J.; Li, C.R.; Xiao, M.Q.; Wu, Z.Y.; Zhang, D.K.; Zhang, C.C.; Xiao, Y.; Chu, M.Y.; Genest, A.; et al. Ru-Catalyzed Reverse Water Gas Shift Reaction with Near-Unity Selectivity and Superior Stability. ACS Mater. Lett. 2021, 3, 1652–1659. [Google Scholar] [CrossRef]
- Pei, W.B.; Dai, L.Y.; Liu, Y.X.; Deng, J.G.; Jing, L.; Zhang, K.F.; Hou, Z.Q.; Han, Z.; Rastegarpanah, A.; Dai, H.X. PtRu nanoparticles partially embedded in the 3DOM Ce0.7Zr0.3O2 skeleton: Active and stable catalysts for toluene combustion. J. Catal. 2020, 385, 274–288. [Google Scholar] [CrossRef]
- Li, X.B.; Liu, X.; Yang, Y.; Zhao, J.; Li, C.; Yang, Q.H. Entrapment of metal nanoparticles within nanocages of mesoporous silicas aided by co-surfactants. J. Mater. Chem. 2012, 22, 21045–21050. [Google Scholar] [CrossRef]
- Zhao, K.; Nie, X.W.; Wang, H.Z.; Chen, S.; Quan, X.; Yu, H.T.; Choi, W.Y.; Zhang, G.H.; Kim, B.; Chen, J.G.G. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon. Nat. Commun. 2020, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Li, X.Y.; Gao, J.H.; Wang, J.; Ma, G.Y.; Wen, X.D.; Yang, Y.; Li, Y.W.; Ding, M.Y. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Wang, J.J.; Qu, Y.Z.; Liu, H.L.; Tang, C.Z.; Miao, S.; Feng, Z.C.; An, H.Y.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.J.; Yao, R.W.; Wen, Z.Y.; Fang, C.Y.; Guo, L.S.; Xu, H.Y.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, S.G.; Bu, X.N.; Dang, S.S.; Liu, Z.Y.; Wang, H.; Zhong, L.S.; Qiu, M.H.; Yang, C.G.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, J.Q.; Zhou, W.; Cheng, K.; Zhang, Q.H.; Kang, J.C.; Wang, Y. Highly Active ZnO-ZrO2 Aerogels Integrated with H-ZSM-5 for Aromatics Synthesis from Carbon Dioxide. ACS Catal. 2020, 10, 302–310. [Google Scholar] [CrossRef]
- Boreriboon, N.; Jiang, X.; Song, C.S.; Prasassarakich, P. Fe-based bimetallic catalysts supported on TiO2 for selective CO2 hydrogenation to hydrocarbons. J. CO2 Util 2018, 25, 330–337. [Google Scholar] [CrossRef]
- Ni, Y.M.; Chen, Z.Y.; Fu, Y.; Liu, Y.; Zhu, W.L.; Liu, Z.M. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.H.; Zhang, P.P.; Fang, Y.; Yoneyama, Y.; Yang, G.H.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy to Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2019, 9, 895–901. [Google Scholar] [CrossRef]
- Huang, J.; Cai, Y.C.; Yu, Y.L.; Wang, Y.A. Conversion of CO2 to Multi-carbon Compounds over a CoCO3 Supported Ru-Pt Catalyst Under Mild Conditions. Chem. Res. Chin. Univ. 2022, 38, 223–228. [Google Scholar] [CrossRef]
- Yang, W.H.; Wang, H.H.; Chen, D.H.; Zhou, Z.Y.; Sun, S.G. Facile synthesis of a platinum-lead oxide nanocomposite catalyst with high activity and durability for ethanol electrooxidation. Phys. Chem. Chem. Phys. 2012, 14, 16424–16432. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, F.; Zhang, L.; Pan, S.X.; Bian, C.Q.; Zheng, X.M.; Meng, X.J.; Xiao, F.S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. 2015, 51, 5936–5938. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Mao, J.X.; Zhou, R.X. Preparation of size-controlled Pt supported on Al2O3 nanocatalysts for deep catalytic oxidation of benzene at lower temperature. Appl. Surf. Sci. 2019, 465, 15–22. [Google Scholar] [CrossRef]
- Sonstroem, P.; Adam, M.; Wang, X.; Wilhelm, M.; Grathwohl, G.; Baeumer, M. Colloidal Nanoparticles Embedded in Ceramers: Toward Structurally Designed Catalysts. J. Phys. Chem. C 2010, 114, 14224–14232. [Google Scholar] [CrossRef]
- Klimavicius, V.; Neumann, S.; Kunz, S.; Gutmann, T.; Buntkowsky, G. Room temperature CO oxidation catalysed by supported Pt nanoparticles revealed by solid-state NMR and DNP spectroscopy. Catal. Sci. Technol. 2019, 9, 3743–3752. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Li, L.; Pan, X.L.; Wang, X.D.; Zhang, T. Local structure of Pt species dictates remarkable performance on Pt/Al2O3 for preferential oxidation of CO in H2. Appl. Catal. B Environ. 2021, 282, 119588. [Google Scholar] [CrossRef]
- Neumann, S.; Gutmann, T.; Buntkowsky, G.; Paul, S.; Thiele, G.; Kunz, S. Insights into the reaction mechanism and particle size effects of CO oxidation over supported Pt nanoparticle catalysts. J. Catal. 2019, 377, 662–672. [Google Scholar] [CrossRef]
- Neumann, S.; Doebler, H.H.; Keil, S.; Erdt, A.J.; Gutsche, C.; Kunz, S. Effects of Particle Size on Strong Metal-Support Interactions Using Colloidal "Surfactant-Free" Pt Nanoparticles Supported on Fe3O4. ACS Catal. 2020, 10, 4136–4150. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, G.; Lian, H.L.; Jia, M.J.; Zhao, G.M.; Jiang, D.Z.; Zhang, W.X. Low-temperature CO oxidation over supported Pt catalysts prepared by colloid-deposition method. Catal. Commun. 2008, 9, 1045–1049. [Google Scholar] [CrossRef]
- An, N.; Yuan, X.L.; Pan, B.; Li, Q.L.; Li, S.Y.; Zhang, W.X. Design of a highly active Pt/Al2O3 catalyst for low-temperature CO oxidation. RSC Adv. 2014, 4, 38250–38257. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Geng, L.L.; Cui, J.Y.; Wu, S.J.; Zhang, W.X. Role of the FeOx support in constructing high-performance Pt/FeOx catalysts for low-temperature CO oxidation. Catal. Sci. Technol. 2016, 6, 1546–1554. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.X.; Li, X.Y.; Lin, J.; Lin, S.; Wang, X.D.; Zhang, T. Identification of Active Sites on High-Performance Pt/Al2O3 Catalyst for Cryogenic CO Oxidation. ACS Catal. 2020, 10, 8815–8824. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.Q.; Qiao, B.T.; Li, J.; Liu, J.Y.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M.; Gates, B.C. Atomically Dispersed Supported Metal Catalysts. Annu. Rew. Chem. Biomol. Eng. 2012, 3, 545–574. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Li, L.; Qjao, B.T.; Liu, J.Y.; Su, Y.; Wang, X.D. Identifying Size Effects of Pt as Single Atoms and Nanoparticles Supported on FeOx for the Water-Gas Shift Reaction. ACS Catal. 2018, 8, 859–868. [Google Scholar] [CrossRef]
- Sun, L.; Cao, L.R.; Su, Y.; Wang, C.J.; Lin, J.; Wang, X.D. Ru-1/FeOx single-atom catalyst with dual active sites for water gas shift reaction without methanation. Appl. Catal. B Environ. 2022, 318, 121841. [Google Scholar] [CrossRef]
- Lin, J.; Qiao, B.T.; Li, N.; Li, L.; Sun, X.C.; Liu, J.Y.; Wang, X.D.; Zhang, T. Little do more: A highly effective Pt1/FeOx single-atom catalyst for the reduction of NO by H2. Chem. Commun. 2015, 51, 7911–7914. [Google Scholar] [CrossRef]
- Ding, X.M.; Liang, Y.L.; Zhang, H.L.; Zhao, M.; Wang, J.L.; Chen, Y.Q. Preparation of Reduced Pt-Based Catalysts with High Dispersion and Their Catalytic Performances for NO Oxidation. Acta Phys. Chim. Sin. 2022, 38, 2005009. [Google Scholar] [CrossRef]
- Arminio-Ravelo, J.A.; Quinson, J.; Pedersen, M.A.; Kirkensgaard, J.J.K.; Arenz, M.; Escudero-Escribano, M. Synthesis of Iridium Nanocatalysts for Water Oxidation in Acid: Effect of the Surfactant. ChemCatChem 2020, 12, 1282–1287. [Google Scholar] [CrossRef]
- Gebauer, C.; Jusys, Z.; Behm, R.J. On the Role of the Support in Pt Anode Catalyst Degradation under Simulated H2 Fuel Starvation Conditions. J. Electrochem. Soc. 2018, 165, J3342–J3349. [Google Scholar] [CrossRef]
- Sun, X.C.; Lin, J.; Chen, Y.; Wang, Y.H.; Li, L.; Miao, S.; Pan, X.L.; Wang, X.D. Unravelling platinum nanoclusters as active sites to lower the catalyst loading for formaldehyde oxidation. Commun. Chem. 2019, 2, 27. [Google Scholar] [CrossRef]
- An, N.H.; Yu, Q.S.; Liu, G.; Li, S.Y.; Jia, M.J.; Zhang, W.X. Complete oxidation of formaldehyde at ambient temperature over supported Pt/Fe2O3 catalysts prepared by colloid-deposition method. J. Hazard. Mater. 2011, 186, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.; Kondratenko, V.A.; Steinfeldt, N.; Sebek, M.; Kondratenko, E.V. Highly selective ammonia oxidation to nitric oxide over supported Pt nanoparticles. J. Catal. 2013, 301, 210–216. [Google Scholar] [CrossRef]
- Allagui, A.; Oudah, M.; Tuaev, X.; Ntais, S.; Almomani, F.; Baranova, E.A. Ammonia electro-oxidation on alloyed PtIr nanoparticles of well-defined size. Int. J. Hydrogen Energy 2013, 38, 2455–2463. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Shah, N.; Dauvois, V.; Li, H.Y.; Namboothiri, I.N.N.; Doris, E. Cooperative Dehydrogenation of N-Heterocycles Using a Carbon Nanotube-Rhodium Nanohybrid. Chem. Eur. J. 2015, 21, 7039–7042. [Google Scholar] [CrossRef]
- Escorcia, N.J.; LiBretto, N.J.; Miller, J.T.; Li, C.W. Colloidal Synthesis of Well-Defined Bimetallic Nanoparticles for Nonoxidative Alkane Dehydrogenation. ACS Catal. 2020, 10, 9813–9823. [Google Scholar] [CrossRef]
- Wang, X.D.; Altmann, L.; Stöever, J.; Zielasek, V.; Bäeumer, M.; Al-Shamery, K.; Borchert, H.; Parisi, J.; Kolny-Olesiak, J. Pt/Sn Intermetallic, Core/Shell and Alloy Nanoparticles: Colloidal Synthesis and Structural Control. Chem. Mater. 2013, 25, 1400–1407. [Google Scholar] [CrossRef]
- Zhang, K.F.; Liu, Y.X.; Deng, J.G.; Jing, L.; Pei, W.B.; Han, Z.; Zhang, X.; Dai, H.X. Ru Nanoparticles Supported on Oxygen-Deficient 3DOM BiVO4: High-Performance Catalysts for the Visible-Light-Driven Selective Oxidation of Benzyl Alcohol. ChemCatChem 2019, 11, 6398–6407. [Google Scholar] [CrossRef]
- Bai, Y.; Li, W.; Liu, C.; Yang, Z.H.; Feng, X.; Lu, X.H.; Chan, K.-Y. Stability of Pt nanoparticles and enhanced photocatalytic performance in mesoporous Pt-(anatase/TiO2(B)) nanoarchitecture. J. Mater. Chem. 2009, 19, 7055–7061. [Google Scholar] [CrossRef]
- Ding, Y.L.; Bai, Y.; Li, W.; Chen, S.S.; Zhu, Y.D.; Zhu, Y.H.; Yang, Z.H.; Lu, X.H. Highly Crystalline TiO2 Whisker Modified with Pt and Its Photocatalytic Performance. Chinese J. Catal. 2010, 31, 1271–1276. [Google Scholar]
- Li, F.J.; Tang, D.-M.; Chen, Y.; Golberg, D.; Kitaura, H.; Zhang, T.; Yamada, A.; Zhou, H.S. Ru/ITO: A Carbon-Free Cathode for Nonaqueous Li-O2 Battery. Nano Lett. 2013, 13, 4702–4707. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Chen, Y.; Tang, D.M.; Jian, Z.L.; Liu, C.; Golberg, D.; Yamada, A.; Zhou, H.S. Performance-improved Li-O2 battery with Ru nanoparticles supported on binder-free multi-walled carbon nanotube paper as cathode. Energy Environ. Sci. 2014, 7, 1648–1652. [Google Scholar] [CrossRef]
- Sun, K.; Fan, B.; Ouyang, J. Nanostructured Platinum Films Deposited by Polyol Reduction of a Platinum Precursor and Their Application as Counter Electrode of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 4237–4244. [Google Scholar] [CrossRef]
- Yin, X.; Xue, Z.S.; Liu, B. Electrophoretic deposition of Pt nanoparticles on plastic substrates as counter electrode for flexible dye-sensitized solar cells. J. Power Sources 2011, 196, 2422–2426. [Google Scholar] [CrossRef]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuators B Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef]
- Yang, H.P.; Zhu, Y.F. Glucose biosensor based on nano-SiO2 and “unprotected” Pt nanoclusters. Biosens. Bioelectron. 2007, 22, 2989–2993. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, S.H.; Xiang, Y.; Li, W.J.; Zhong, X.; Che, X.; Li, J.J. Glucose biosensor based on the highly efficient immobilization of glucose oxidase on Prussian blue-gold nanocomposite films. J. Mol. Catal. B: Enzym. 2011, 69, 1–7. [Google Scholar] [CrossRef]
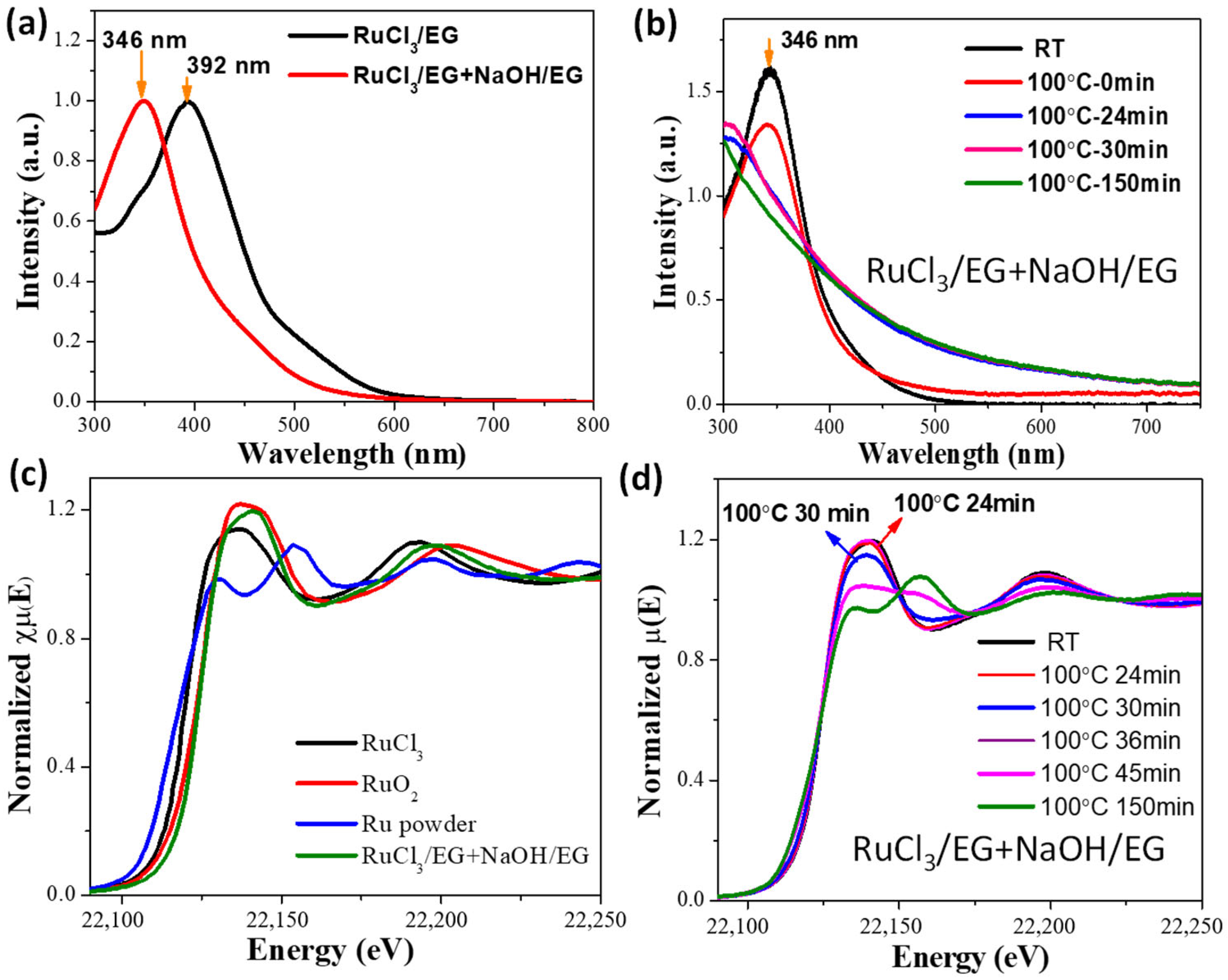
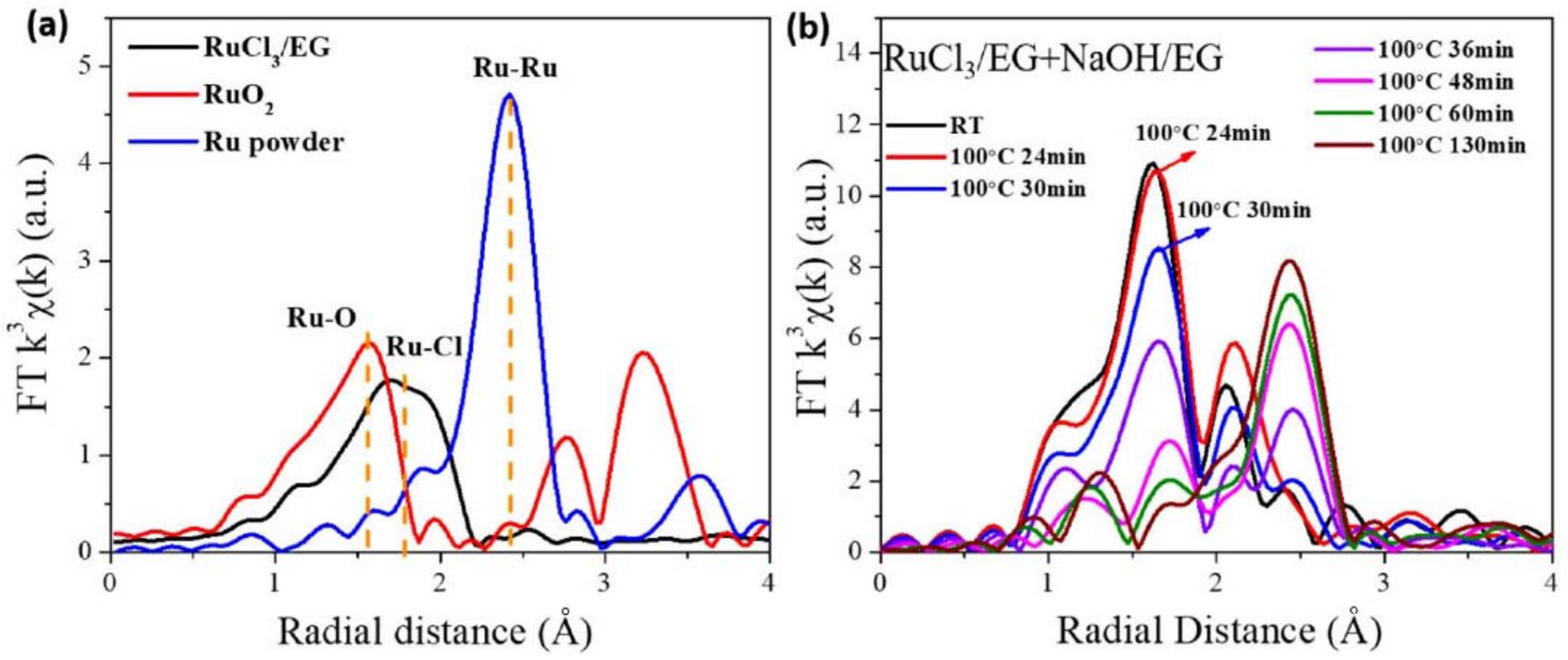
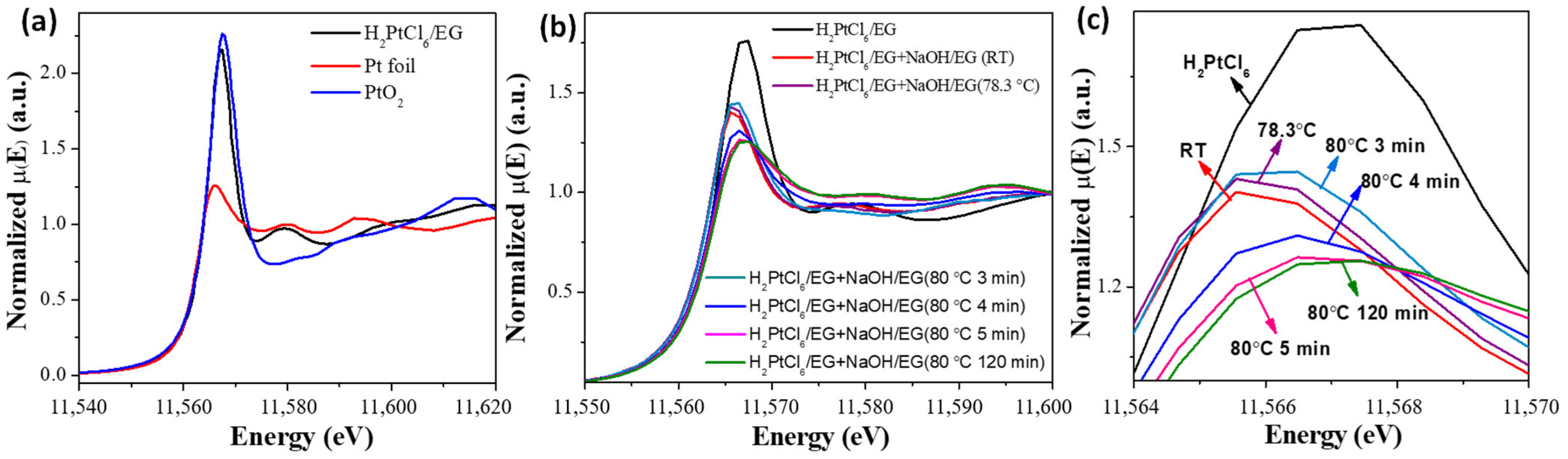
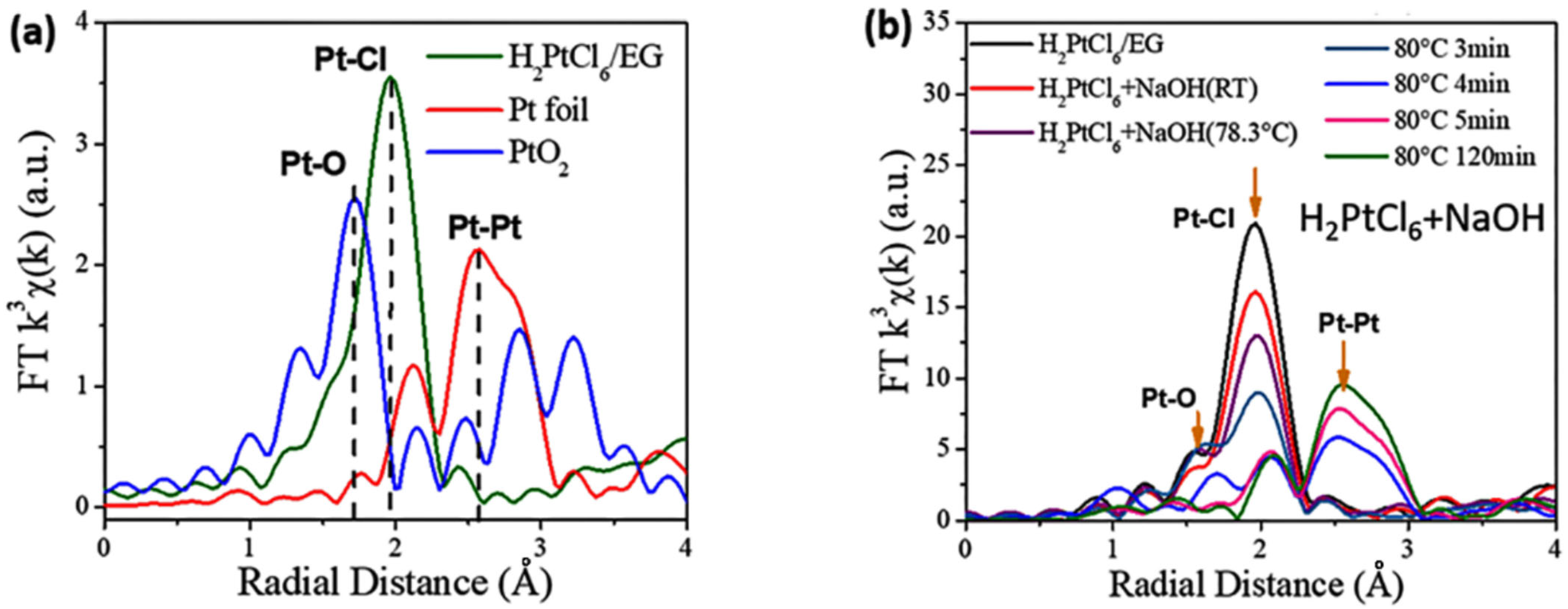


| Time/min | T/°C | CN(Ru–Cl) | CN(Ru–O) | CN(Ru–Ru) | R(Ru–Cl)/Å | R(Ru–O)/Å | R(Ru–Ru)/Å |
|---|---|---|---|---|---|---|---|
| 0 | 27 | 0.56 | 6.27 | 2.40 | 2.05 | ||
| 2 | 48.9 | 5.65 | 2.05 | ||||
| 36 | 100 | 4.45 | 2.06 | ||||
| 39 | 100 | 3.56 | 0.21 | 2.07 | 2.68 | ||
| 61 | 100 | 1.57 | 2.87 | 2.12 | 2.71 | ||
| 62 | 100 | 3.58 | 2.71 | ||||
| 120 | 100 | 3.83 | 2.71 |
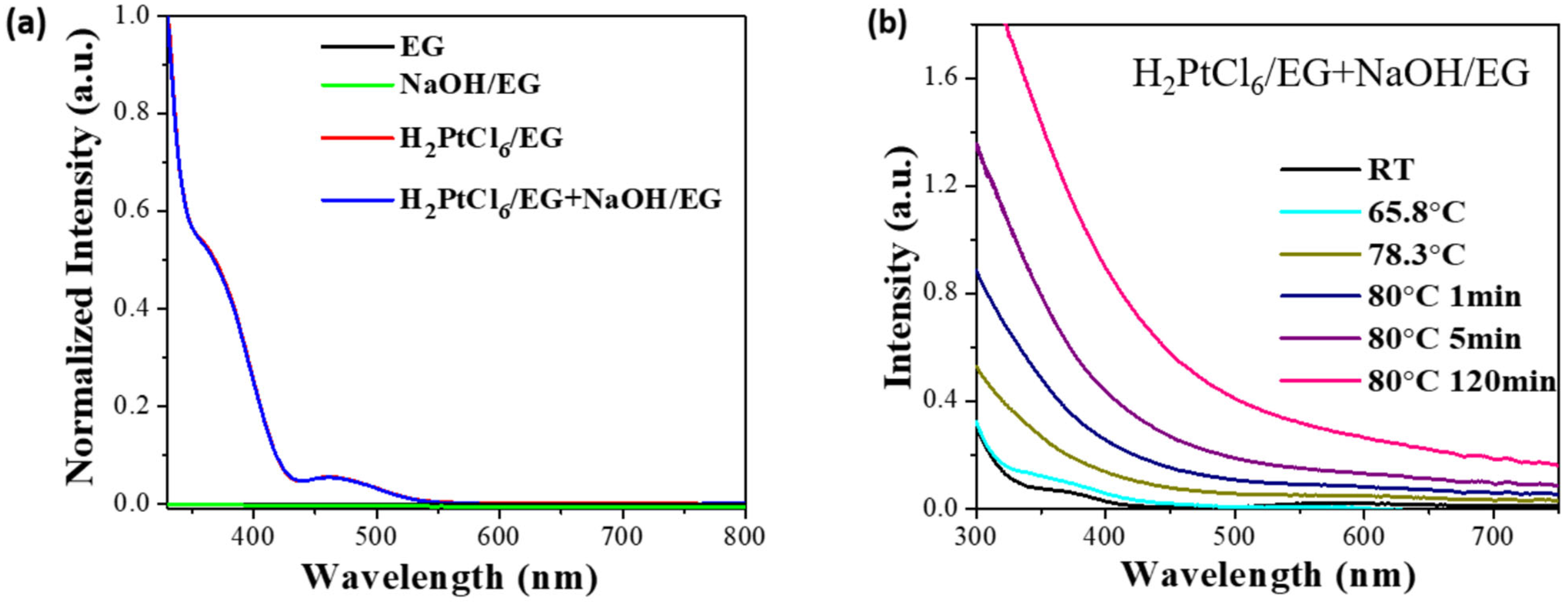
| Time/min | T/℃ | Bond | CN | r/Å | ΔE/eV | σ2/Å2 | R/% |
|---|---|---|---|---|---|---|---|
| 0 | RT (24.9) | Pt–Cl | 4.86 | 2.31 | 12.24 | 0.0027 | 0.24 |
| 7 | 78.3 | Pt–Cl | 3.05 | 2.32 | 12.22 | 0.0030 | 0.71 |
| Pt–O | 1.62 | 2.01 | 10.54 | 0.0009 | |||
| 9 | 80.0 | Pt–Cl | 2.09 | 2.33 | 14.97 | 0.0026 | 0.61 |
| Pt–O | 2.98 | 2.05 | 11.83 | 0.0051 | |||
| 10 | 80.0 | Pt–O | 1.97 | 2.13 | 7.75 | 0.0037 | 0.95 |
| Pt–Pt | 3.20 | 2.74 | 5.53 | 0.0046 | |||
| 11 | 80.0 | Pt–Pt | 5.34 | 2.74 | 5.53 | 0.0074 | 0.45 |
| 90 | 80.0 | Pt–Pt | 9.09 | 2.76 | 8.82 | 0.0076 | 1.77 |
| Reaction Condition | dav (TEM)/nm | dav (DLS)/nm |
|---|---|---|
| – | 3.7 | 7.1 |
| 80 ℃–30 min | 2.4 | 6.3 |
| 80 ℃–60 min | 1.6 | 5.9 |
| 80 ℃–90 min | 1.4 | 3.7 |
| Catalyst | Reaction Energy Barriers (eV) | ||
|---|---|---|---|
| CH2 + CH2 Coupling | CH2 Hydrogenation | CH3 Hydrogenation | |
| Pt42-Ru8 | 0.31 | 0.55 | 0.53 |
| Ru50 | 1.44 | 0.85 | 0.77 |
| Pt50 | 0.78 | 0.92 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hao, M. Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application. Nanomaterials 2023, 13, 565. https://doi.org/10.3390/nano13030565
Wang Y, Hao M. Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application. Nanomaterials. 2023; 13(3):565. https://doi.org/10.3390/nano13030565
Chicago/Turabian StyleWang, Yuan, and Menggeng Hao. 2023. "Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application" Nanomaterials 13, no. 3: 565. https://doi.org/10.3390/nano13030565
APA StyleWang, Y., & Hao, M. (2023). Metal Nanoclusters Synthesized in Alkaline Ethylene Glycol: Mechanism and Application. Nanomaterials, 13(3), 565. https://doi.org/10.3390/nano13030565







