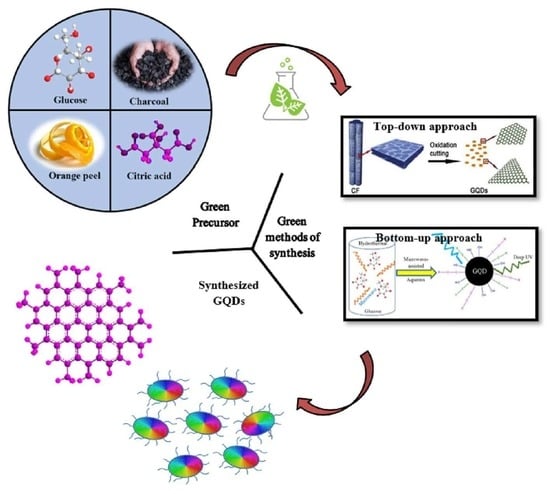
Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs)
Abstract
1. Introduction
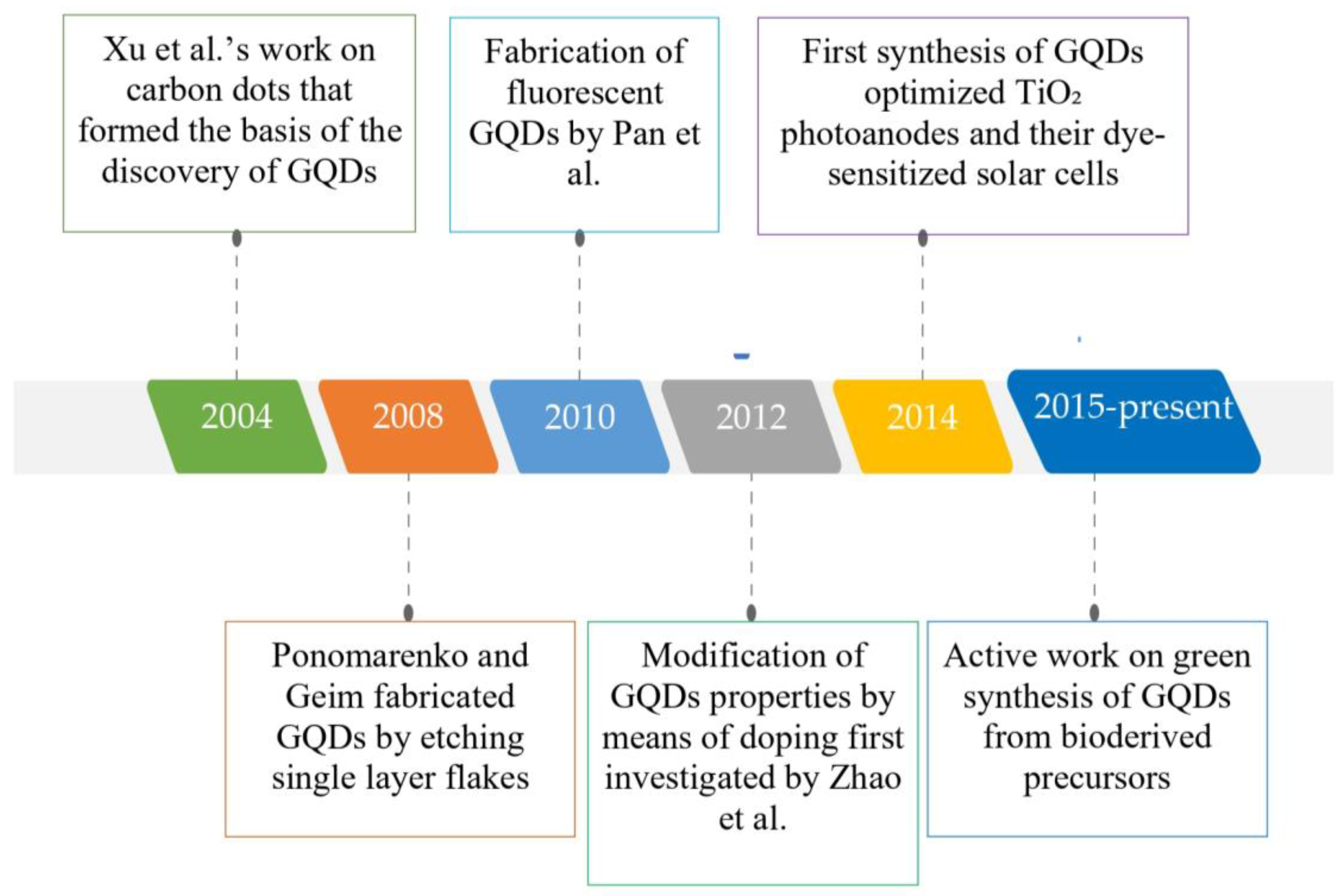
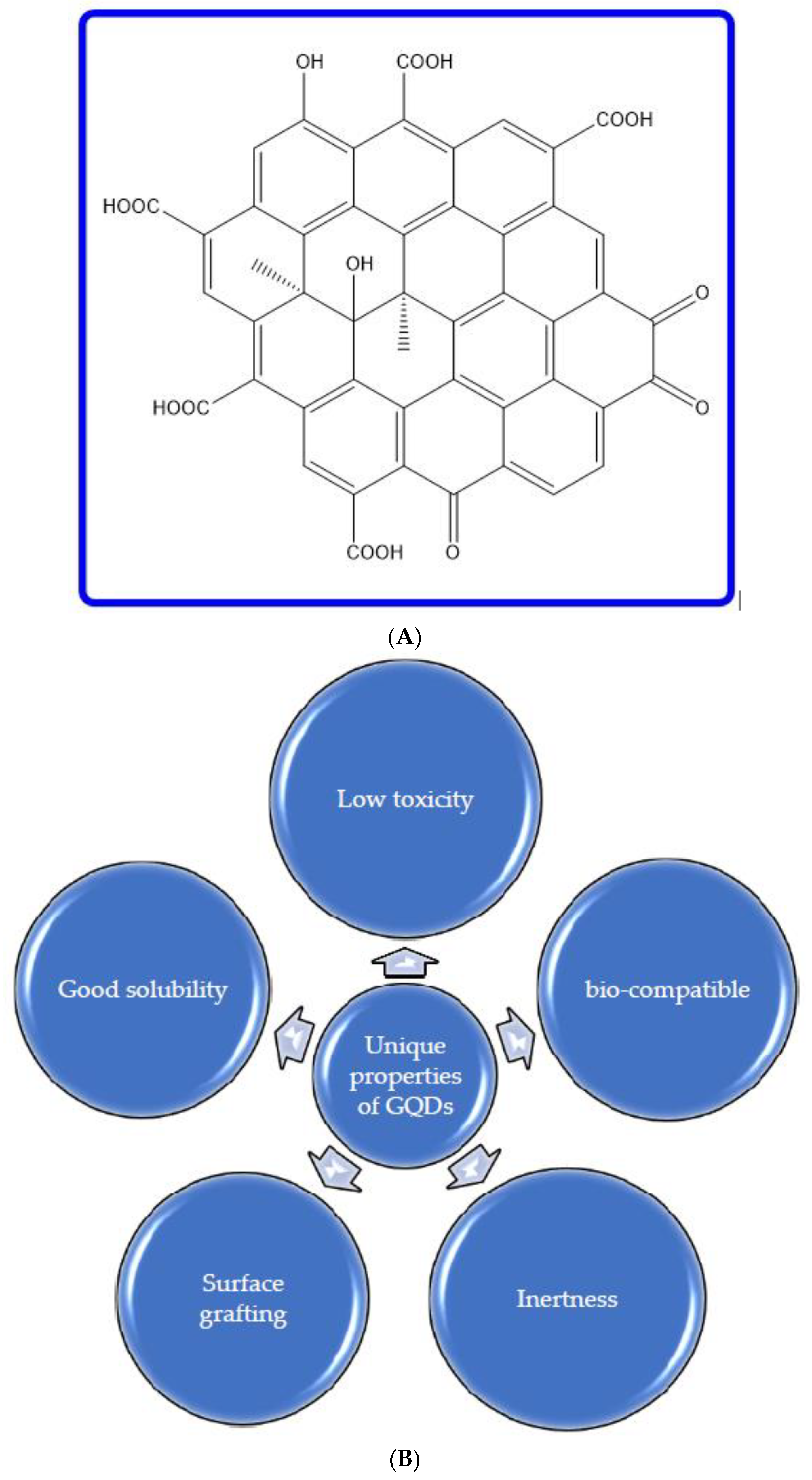
2. Graphene Quantum Dots (GQDs): An Incomparable Nanomaterial
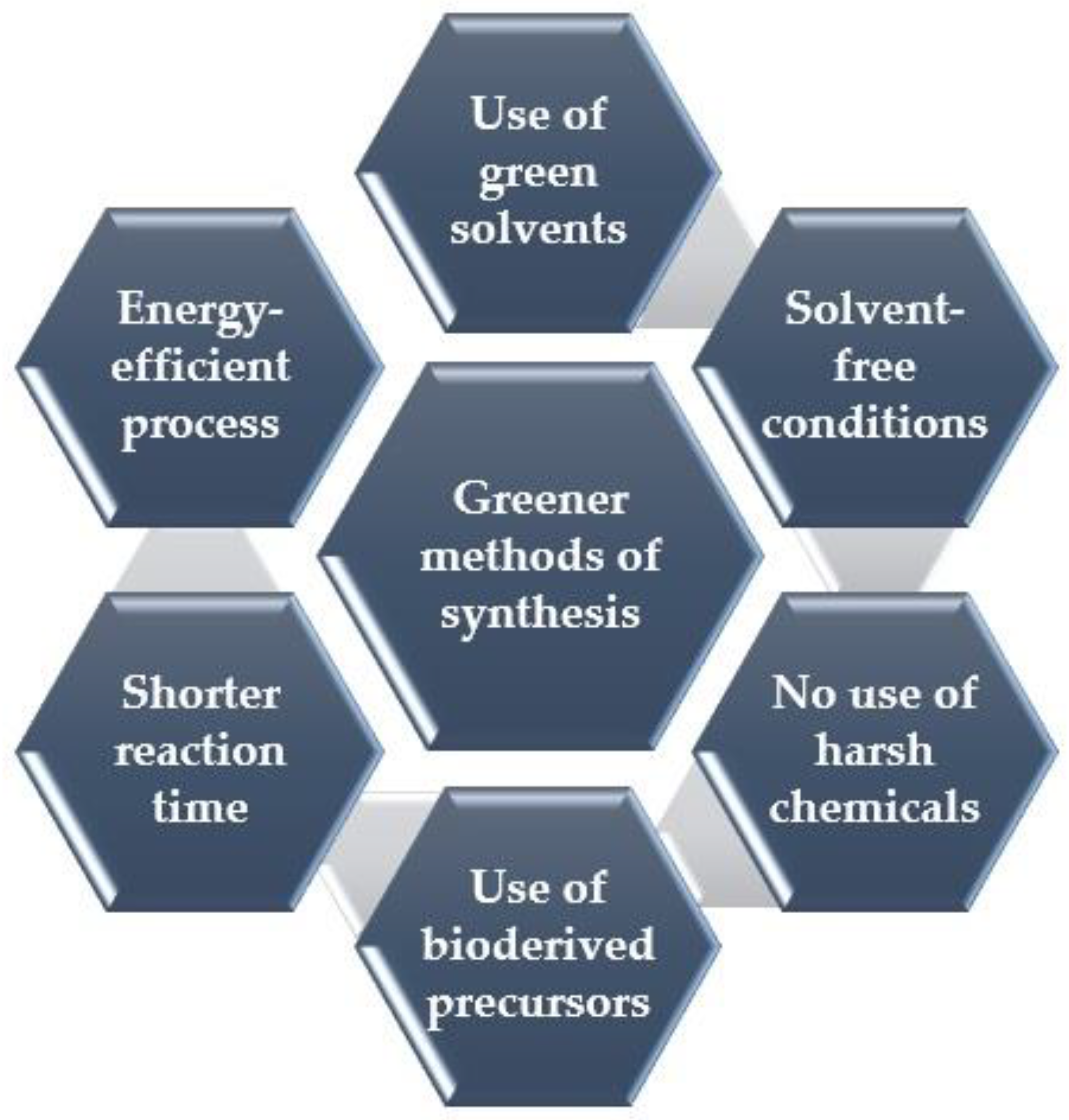
3. Environmentally Friendly Synthesis of GQDs: Greener and Sustainable Approaches
3.1. Strategic Selection of Greener Bioderived Precursors for Eco-Friendly Synthesis of GQDs
3.2. Diverse Routes for the Greener Synthesis of GQDs
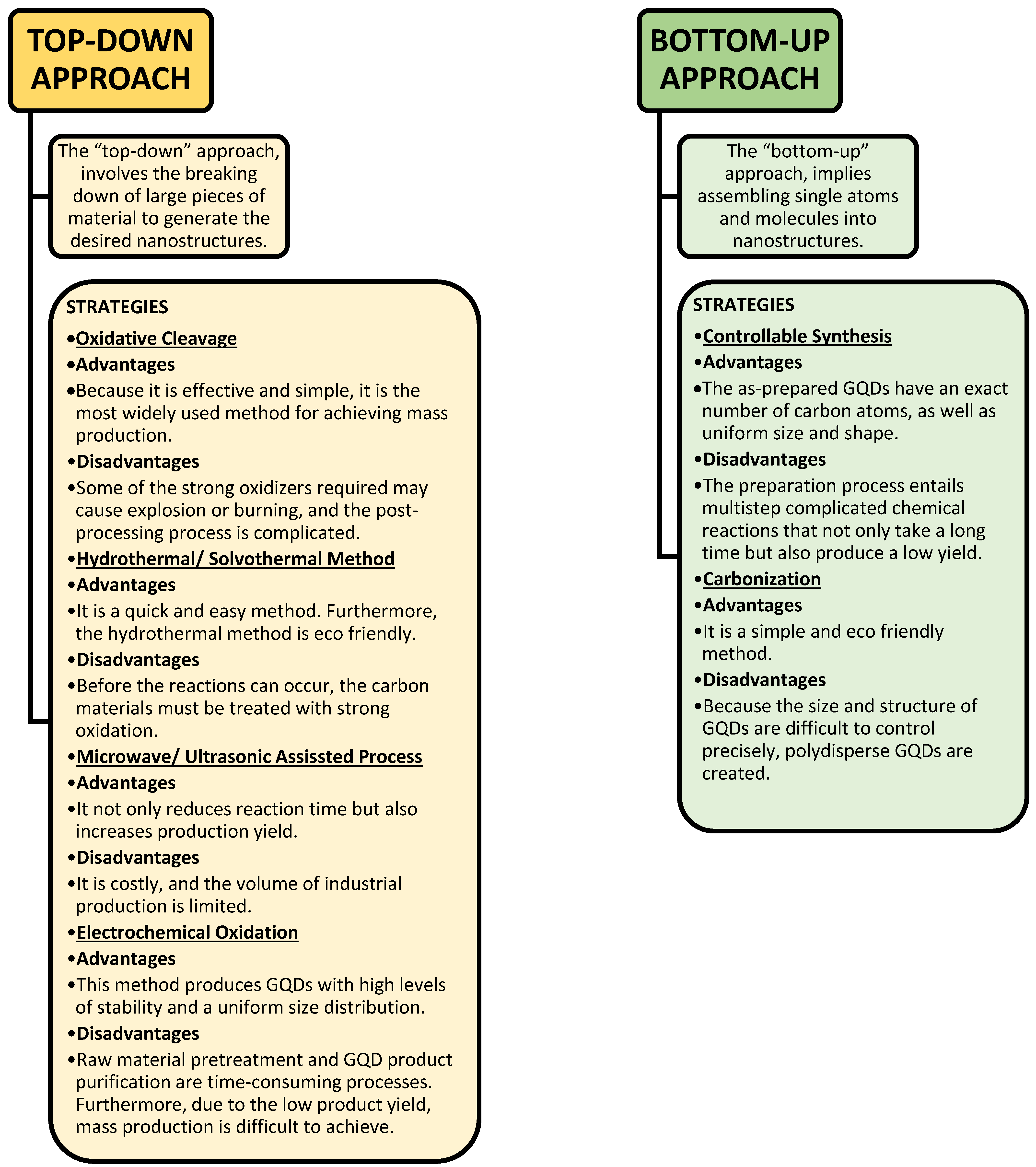
3.2.1. Top-Down Approach for Preparation of GQDs
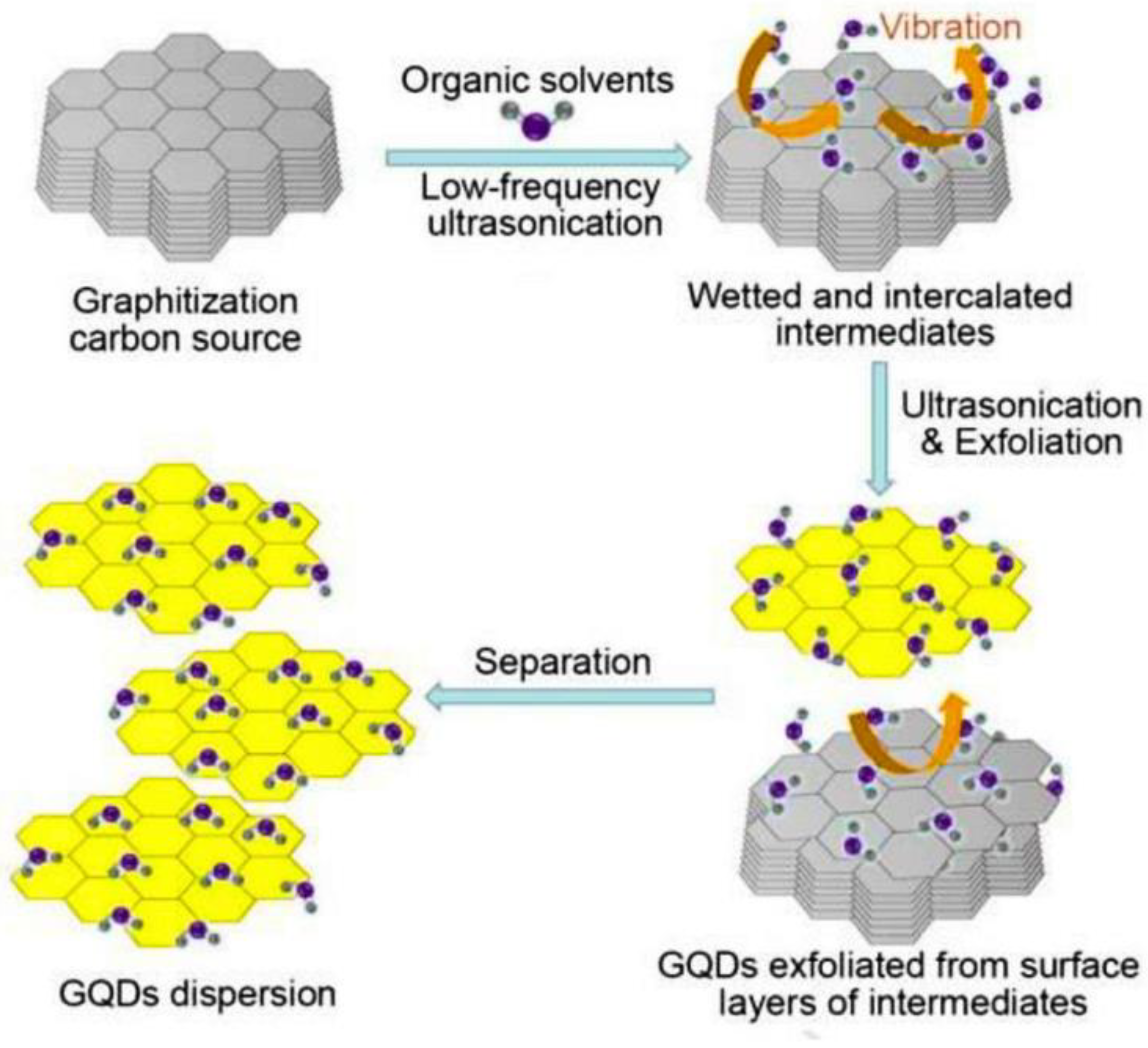
Ultrasound-Assisted Synthesis
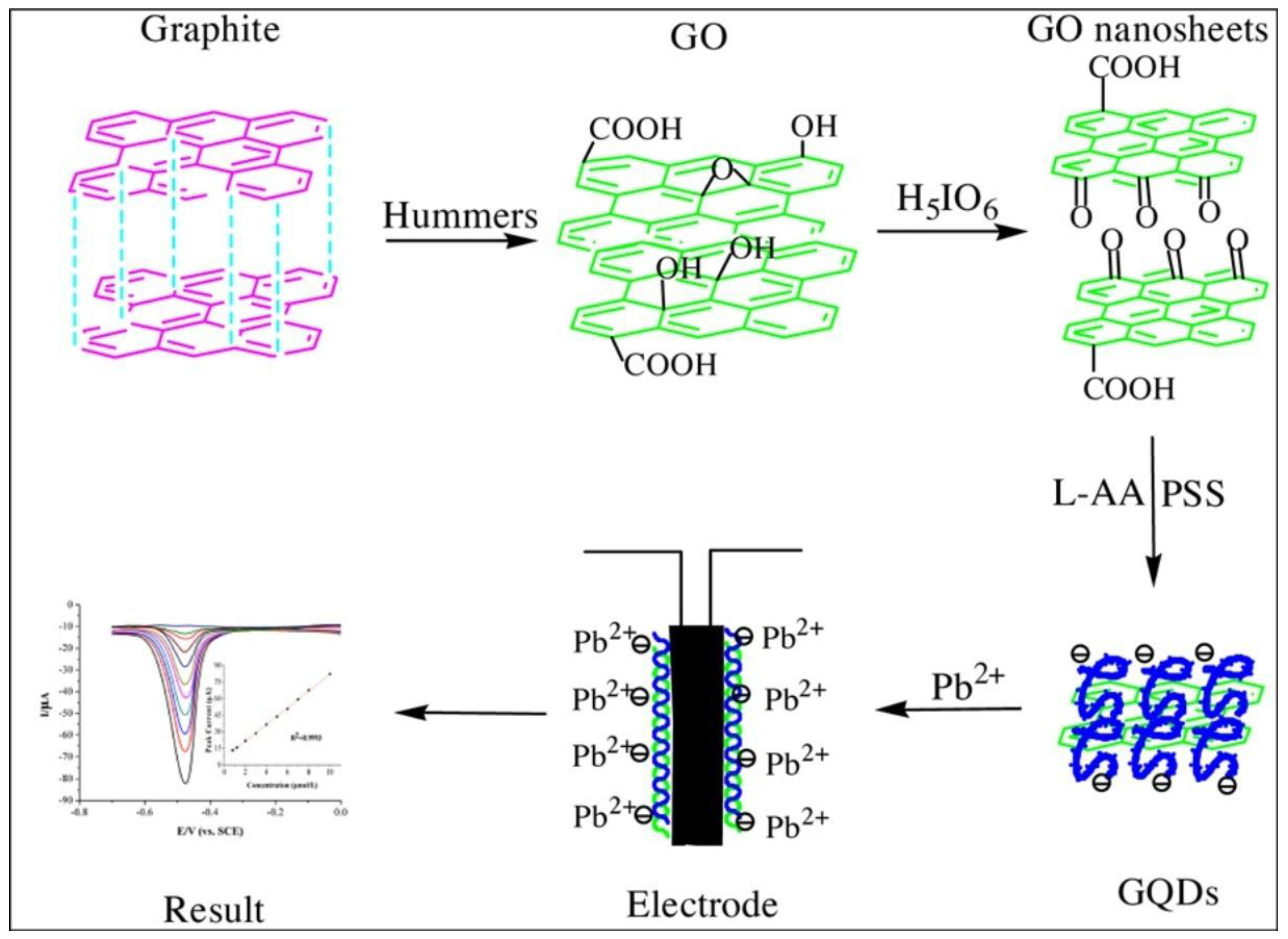
Molecular Oxidation Method for the Synthesis of GQDs
Free-Radical Oxidation for the Synthesis of GQDs
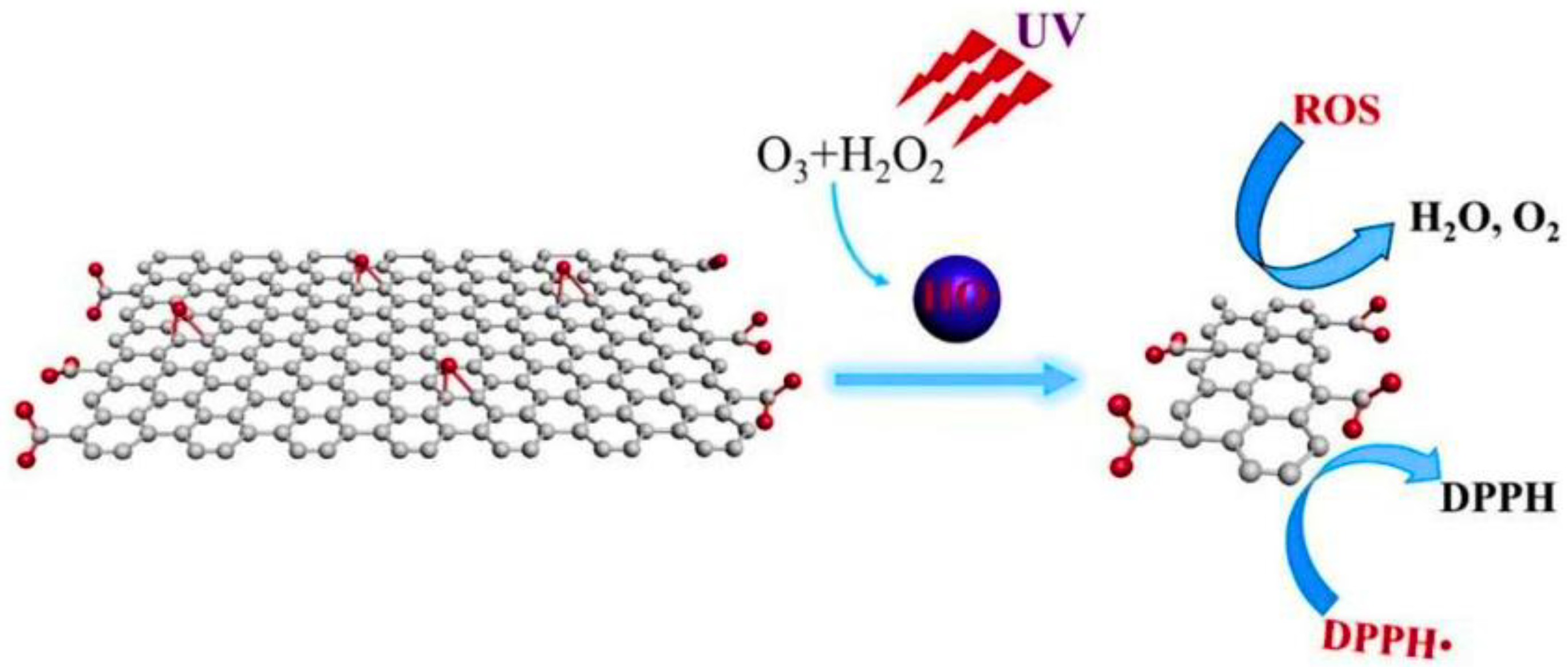
Oxidative Cleavage Method
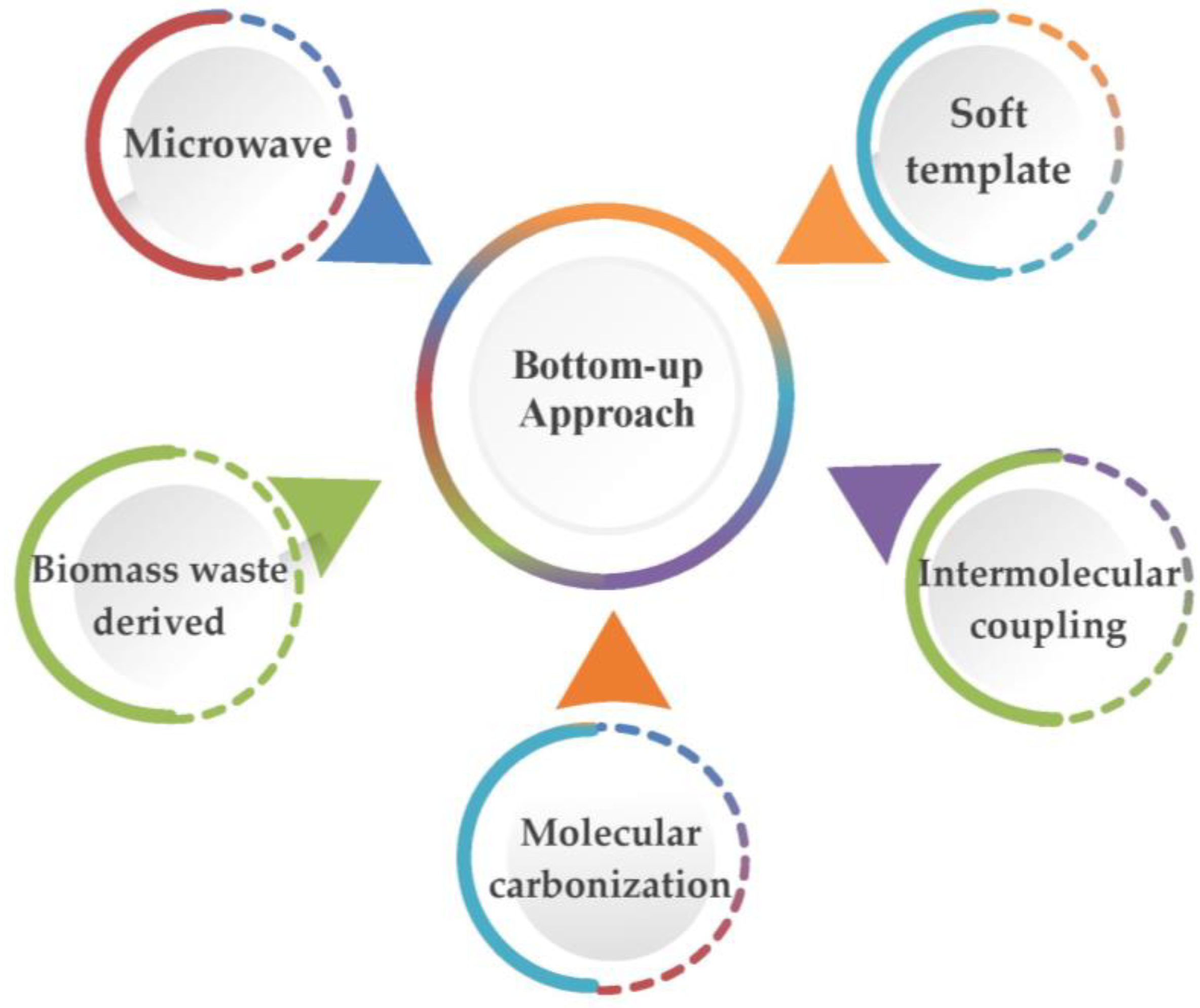
3.2.2. Bottom-Up Approach for the Preparation of GQDs
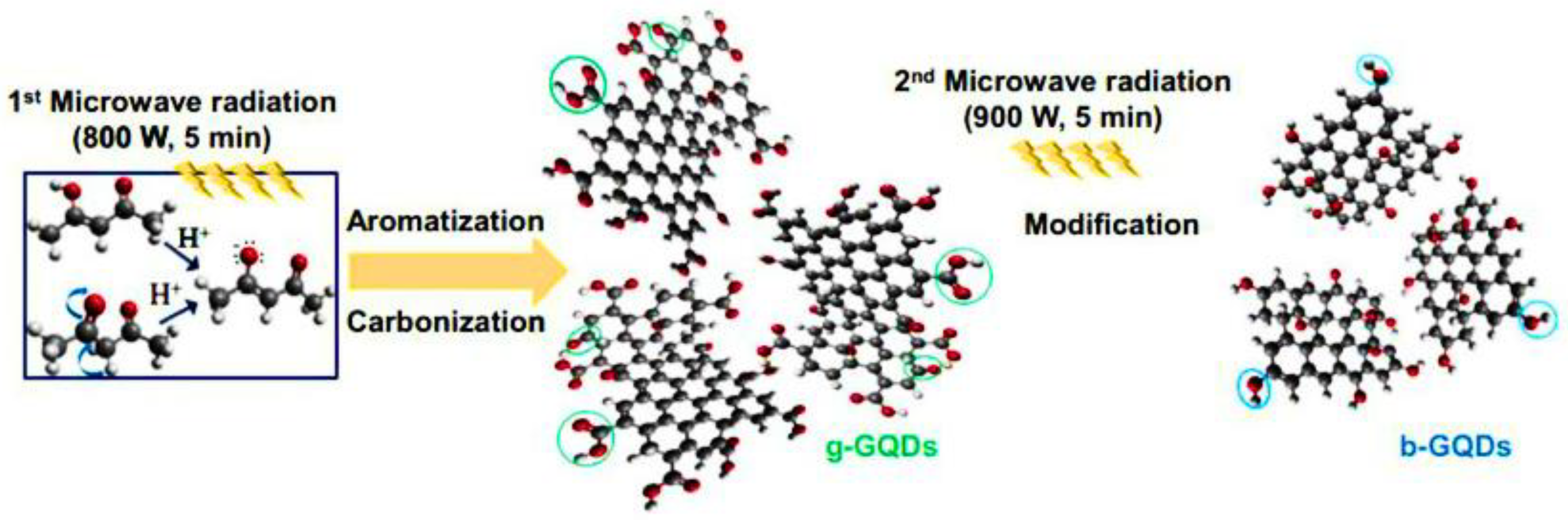
Microwave-Assisted Synthesis of GQDs
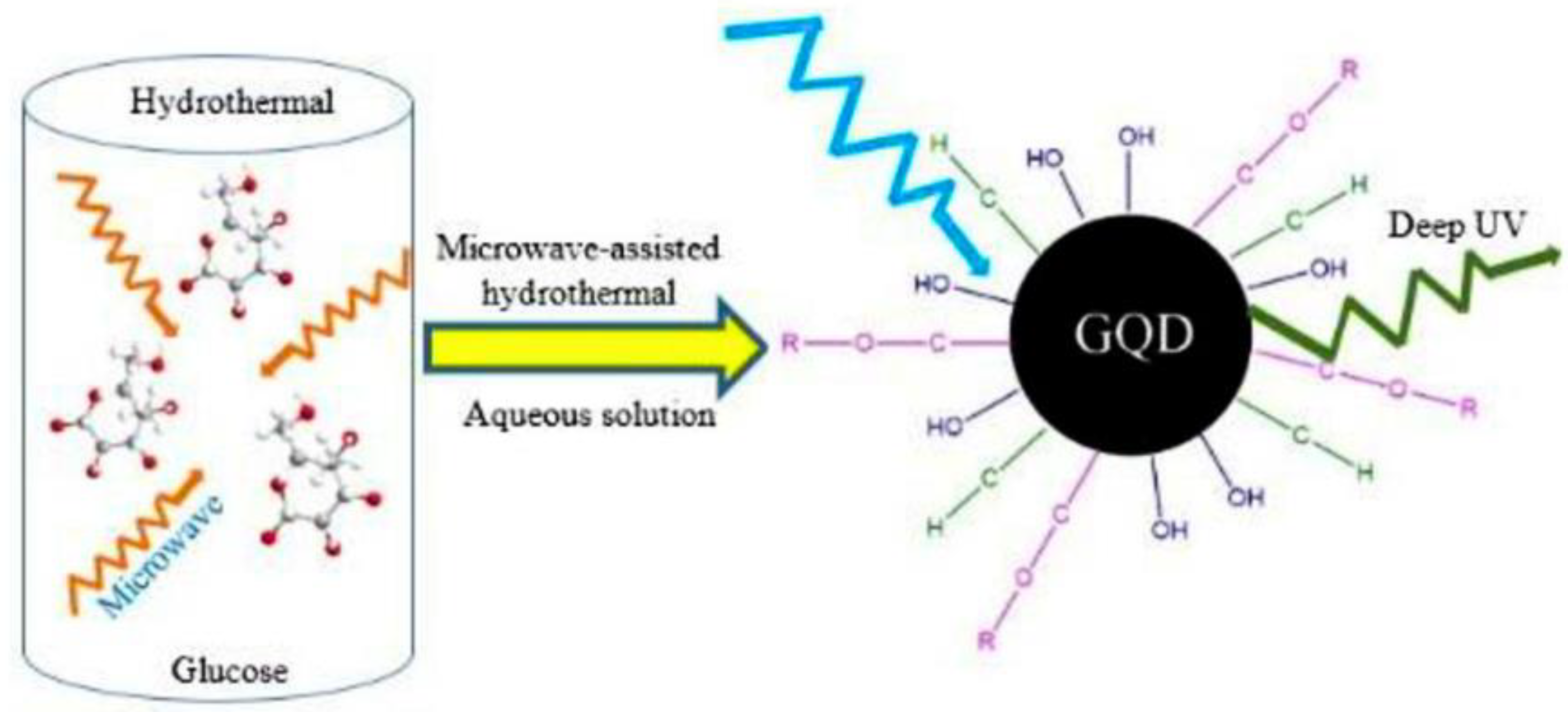
MW-Assisted Hydrothermal Method for the Synthesis of GQDs
Biomass-Waste-Derived Method of the Synthesis of GQDs
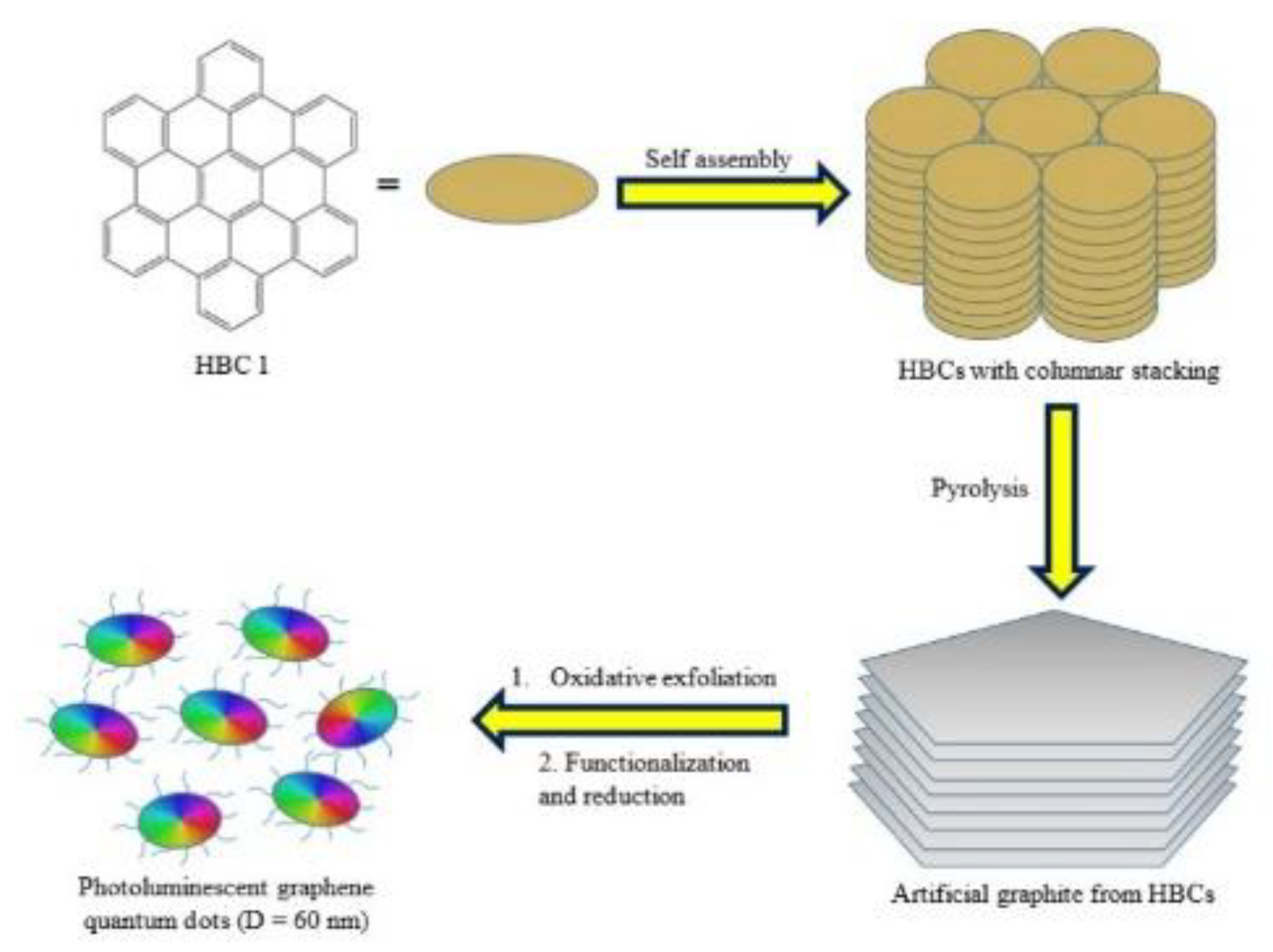
Intermolecular Coupling Method
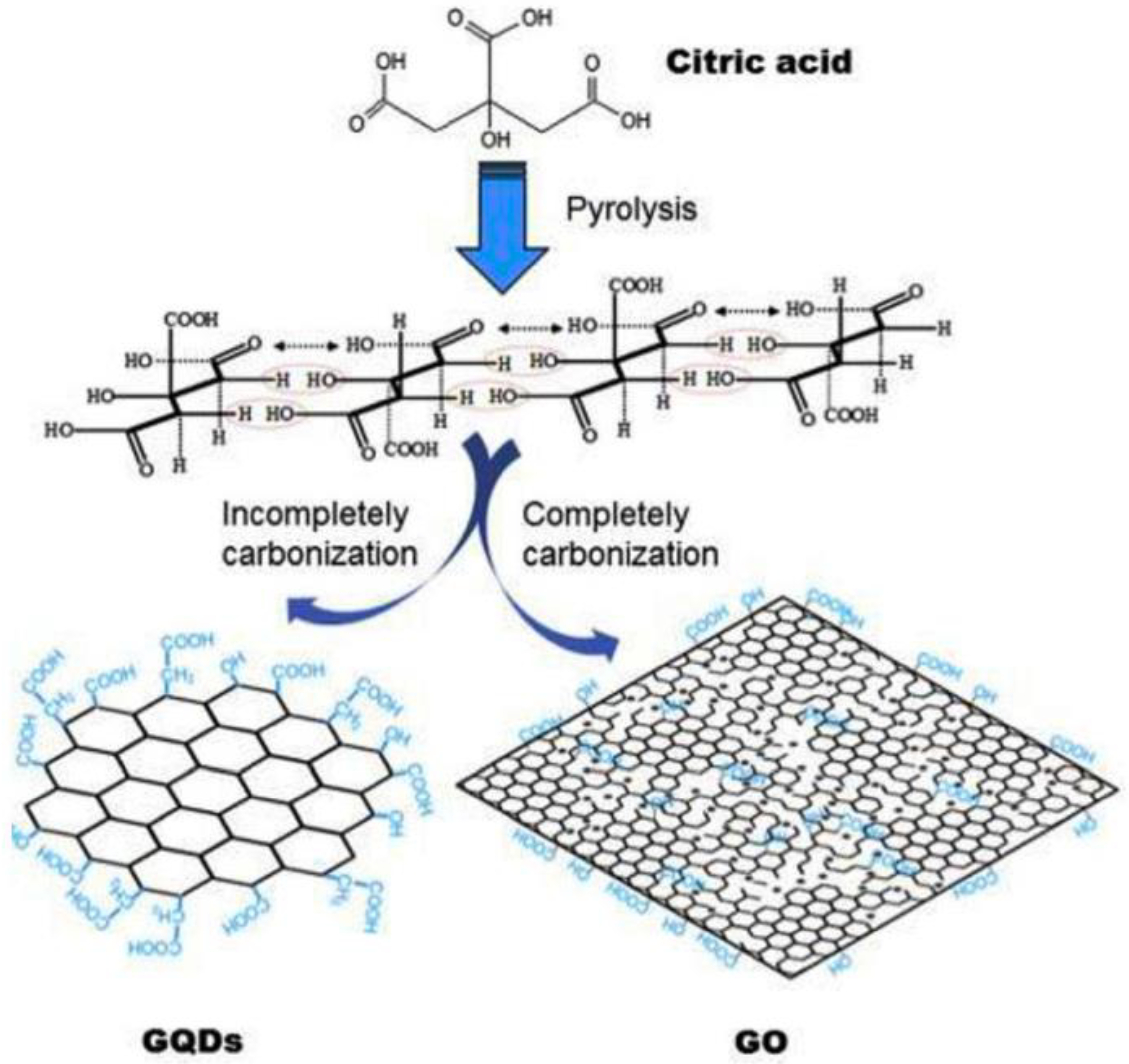
Molecular Carbonization Method
Soft Template Method
3.2.3. Acid-Free Method
4. Comparison between Different Approaches for the Synthesis of GQDs
5. Diverse Approaches for the Modification of GQDs
- Control over the size and shape;
- Surface modification;
- Heteroatom doping.
5.1. Size and Shape Control of GQDs
5.2. Surface Modification of GQDs
5.3. Heteroatom Doping of GQDs
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| N-GQDs | Glucose | 5.3 nm, spherical | Photo electronics and fluorescent probing | [102] |
| GQDs | Clitoria ternatea | 10 nm, spherical | Treatment of Alzheimer’s disease | [103] |
| GQDs | Glucose | Less than 5 nm, | Detection of Al3+ ions | [199] |
| GQDs | Citric acid | Around 15 nm | For the synthesis of xanthenes | [200] |
| Graphene-TiO2 QDs | Graphene oxide | About 3 nm, spherical | In a photoelectrochemical cell | [201] |
| GQDs | Organic precursors | Spherical | Removal of triazine | [105] |
| N-GQDs | Citric acid and urea | Circular or elliptical | In optical, electronic, and biomedical devices | [106] |
| B-GQDs | Graphene oxide and borax | 4 nm | Cell imaging | [107] |
| GQDs | Mangifera indica (mango) | 2–8 nm | Near-infrared bioimaging and intracellular nano-thermometry | [108] |
| GQDs | Graphene oxide | - | ECL biosensing and imaging | [104] |
| B-GQDs | Bulk boron carbide (B4C) crystals | Around 6 nm | Dye-sensitized solar cell | [109] |
| His-GQDs | Citric acid and histidine | - | Supercapacitor | [202] |
| N-GQDs | Sodium citrate and triethanolamine | Around 5.6 nm | As fluorescent ink and in the detection of ferric ions | [112] |
| GQDs | Grape seed extract | 50–60 nm | Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing | [113] |
| GQDs | Graphite | 2–5 nm | - | [114] |
| GQDs | 1,3,6-trinitropyrene | 4.12 nm | Live-cell staining and white LEDs | [115] |
| GQDs | acetylacetone | 5 nm, 2.3 nm | In enzyme-free biosensors, bioimaging and optoelectronic devices | [116] |
| Amine-functionalized GQDs | Glucose, H2O2, and NH3 | 3.78 nm, quasi-spherical | In visible-light photocatalytic systems | [203] |
| GQDs | aspartic acid and NH4HCO3 mixture | 2.1 nm | Fluorescent probes for detection of iron ions and pH value | [204] |
| GQDS | De-oiled asphalt | 2.65 nm, | As surfactant for asphalt emulsion | [205] |
| GQDs | Citric acid | - | Live-cell imaging | [117] |
| N-GQDs | Citric acid and semi-carbazide hydrochloride | 4–8 nm, | Sensing and imaging | [206] |
| GQDs | Opuntia sp. extract | 2.6 ± 0.63 nm, spherical | Phosphate detection | [207] |
| GQDs | Lemon juice | 5–10 nm, spherical | Biomedical and chemical sensing | [208] |
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| GQDs | Citric acid | Around 15 nm | - | [46] |
| GQDs | DI water and glucose | 8 nm | - | [152] |
| GQDs | Citric acid | 1.5 nm at pH 9, 1 nm at pH 10, below 2 nm at pH 12, spherical | - | [153] |
| GQDs | Corn powder | 20–40 nm | Reduce charge recombination and increase free charge carriers | [161] |
| GQDs | Corn powder | 20–40 nm | In dye-sensitized solar cells | [162] |
| GQDs | Polycyclic aromatic hydrocarbon | 5–10 nm | Bioimaging and sensing of Fe3+ ions and hydrogen peroxide | [209] |
| N-GQDs | Citric acid | 3.5 nm | Fe3+ ions detection | [163] |
| N-GQDs | Citric acid and urea | 6 nm | Electrochemical sensing of hydroxyurea | [210] |
| Amino-functionalized GQDs | Asphalt | Less than 4 nm | Fe3+ detection | [168] |
| Nitrogen and phosphorus co-doped GQDs | Adenosine triphosphate | - | Cellular imaging | [164] |
| N-GQDs | Citric acid | 5–10 nm, spherical | Cell labeling, bioimaging | [165] |
| GQDs | Citric acid and urea | 5–10 nm, quasi-spherical | Optical, sensing, energy, and biological applications | [211] |
| GQDs | Glucose | Around 20 nm, spherical | Determination of free chlorine | [212] |
| N-GQDs | Orange juice | - | For turn-off sensing of TNP in an aqueous medium | [166] |
| N-GQDs | 4-nitrophenol | 5.2 nm | As metal-free photocatalysts for reduction of 4-nitrophenol | [167] |
| GQDs | En, Gl, Eg, Me, Dmf, Ac, Et, To, Ct | Less than 3 nm | - | [169] |
| GQDs | EDTA | Around 8.2 nm | Bioimaging and optoelectronic applications | [213] |
| GQDs | Honey | 2.4 nm | As stable security ink and white-light emission | [150] |
| GQDs | Glucose | 7–10 nm | As optical sensor for glucose | [214] |
| GQDs | L-cysteine | 4–8 nm, elliptical | Selective sensing of curcumin | [215] |
| Amino-functionalized GQDs | Glucose | 3–4 nm | Detection of copper ions | [216] |
| N-GQDs | Ammonium citrate | 1–5 nm | Fabrication of BiOBr nanohybrids | [217] |
| N-GQDs | Ethanolamine | 1–5 nm | Biological imaging, drug delivery | [218] |
| N-GQDs | Polyethylimine | 7–12 nm | Heavy metal ions recognition and bio-labeling | [219] |
| GQDs | Citric acid | 12.7 nm | Biomedical applications | [220] |
| GQDs | Glucose and citric acid | 22 nm, 8 nm | As fluorescence probe for the detection of Au(III) ion | [221] |
| N-GQDs | Blue citric acid and glycine | 3–8 nm | Nanomedicine | [222] |
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| S-GQDs | Paraffin oil and carbon disulfide | 2.46 nm, spherical | High-performance optoelectronic devices | [174] |
| N-GQDs | Citric acid | - | Phosphor-based light-emitting diodes | [175] |
| Histidine-functionalized GQDs | Citric acid and histidine | 3.2 nm | For the synthesis of polystyrene microspheres for adsorption of Cu2+ | [176] |
| Histidine-functionalized GQDs | Citric acid and histidine | - | Volumetric sensing of dopamine | [177] |
| GQDs | HBC | 60 nm, disk-like | - | [173] |
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| GQDs | Coffee grounds | - | Sensing of heavy metals | [84] |
| GQDs | H2O2 | 35 nm | Cellular imaging, drug delivery | [85] |
| GQDs | Graphene sheets | 10 nm (diameter) | Biological labeling | [16] |
| GQDs | Glucose | 1.65 nm, spherical | DUV photonic devices | [120] |
| GQDs | Glucose | <5 nm ± 0.55, spherical | Optical applications | [121] |
| p-GQDs | Graphene Oxide | 2–6 nm | Biological optoelectronic | [122] |
| GQDs-F, GQDs-P | Fluorinated graphene oxide | GQDs-F- 5.6 nm GQDs-P- 6.0 nm | Solar cells, biosensors, and bioimaging | [123] |
| GQDs | Citric acid | 10 nm | Adsorbent for toxic carbamate pesticide oxamyl | [124] |
| N-GQDs | Urea | 3 nm, crystalline structure | Biolabeling and bioimaging | [125] |
| N-GQDs | Glucose | 3–4 nm, spherical | Dopamine sensors | [126] |
| WGQDs | Graphite | Lateral size 1.25–2.75 nm | WLEDs | [127] |
| FGQDs | Glucose | <3 nm | Biomedical applications, treatment of amyloidosis | [223] |
| GODQs | Graphene oxide | 7–12 nm, zigzag structure | biosensing, imaging, and labeling | [224] |
| GQDs | Glucose | 1.1 nm, spherical | Supercapacitors | [225] |
| RGQDs and NGQDs | Graphene oxide and glucosamine precursors | 3–6 nm size | Imaging-based Temperature sensors | [226] |
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| a-GQDs, b-GQDs, c-GQDs | Anthracite, bituminous, coke | b-GQDs- 2.96 ± 0.96 nm, crystalline hexagonal c-GQDs- 5.8 ± 1.7 nm a-GQDs- 29 ± 11 nm | Bioimaging, biomedicine, photovoltaics, and optoelectronics | [59] |
| GQDs | low-cost coal | 20 ± 10.25 nm, | Bioimaging applications | [133] |
| Neem-derived GQDs, fenugreek-derived GQDs | Neem and Fenugreek leaves | Neem- 2–8 nm, spherical Fenugreek- 3–10 nm, spherical | White LEDs | [31] |
| Am-GQDs | Neem leaves | 5–6 nm, crystalline | Soil and water diagnosis, food safety surveillance | [33] |
| N-GQDs | Marigold | 3.2 nm, uniform crystal lattice | Detection of Fe3+ in water, bioimaging applications | [134] |
| GODQs | Paper waste | 1.2 nm, spherical | Optical | [136] |
| GQDs | CTP | 1.7 ± 0.4 nm, hexagonal lattice | Applications in aqueous systems | [137] |
| GQDs | Graphitic waste | 10 ± 0.5 nm, spherical | Humidity sensors | [139] |
| GQDs | Sugarcane bagasse | 2–6 nm, hexagonal | Nanoprobes for multicolor bioimaging | [140] |
| GQDs | Cellulose polymer | 1–5 nm | Bioimaging and biolabeling | [69] |
| GQDs | Spent tea | 1.6 ± 0.55 nm, uneven shape (GQDs-500) | Biomolecules and metal ion sensors | [227] |
| RH-GQDs | Rice husk | 3–6 nm, crystalline lattice | Fluorescent bio-probes | [32] |
| LGQDs | Alkali lignin | - | Ultrasensitive biosensors | [228] |
| N-S co-doped GQDs | Graphite waste (powdered) | 10 ± 0.5 nm, spherical | Humidity sensing | [138] |
| Reduced-GQDs (rGQDs) | Glucose | nearly spherical, 4–14 nm | Electrochemical applications | [229] |
| NIR-GQDs (Near infrared GQDs) | cis-cyclobutane-1,2-dicarboxylic acid (CBDA-2) | 6.5 ± 3.5 nm | Cell imaging and metal ion detection | [230] |
| N-GQDs | Arjuna bark, waste melamine sponge | 2–3 nm, spherical | Bioimaging, H2O2 sensing | [141] |
| N-GQDs | Pineapple leaf fiber | 3.7 nm, spherical | Hg2+ detection | [231] |
| GQDs | Marigold flower (Tagetes erecta) | ~5.7 nm | Supercapacitors | [135] |
| GQDs | Corn straw | 2.67 nm | PO43− detection | [232] |
| Type of GQDs | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| GQDs | - | 3–5 nm | Biosensors, bioimaging, laser, and light-emitting diodes | [70] |
| GQDs | Graphene oxide | - | Sensing of alkaline phosphatase | [71] |
| GQDs | Ethyl acetoacetate, NaOH | 2–10 nm, spherical | Biophotonic | [72] |
| GQDs | Graphene oxide | 4–10 nm | Solar cells, fluorescent detection, cellular imaging | [73] |
| GQDs | Leather | 5 nm, spherical | High-contrast bioimaging, biosensing, photovoltaics, and drug delivery | [74] |
| GQDs | Graphene flakes | 2–4 nm | Thin-film electronic and optoelectronic devices | [75] |
| PGQDs, EGQDs, GOGQDs | Natural graphite, expanded graphite, and oxide graphite | 2~4 nm | - | [76] |
| LD-GQDs, HD-GQDs | Graphitic carbon | HD-GQDs- 2–6 nm, curved shape and zigzag edges LD-GQDs- 2–9 nm, rectangle, and hexagon | Biology, electronic, energy, and engineering | [77] |
| GQDs | Graphene oxide | 5–7 nm | Optoelectronic applications | [78] |
| C-GQDs | Coal | 3.2 ± 1.0 nm, | Sensor applications | [79] |
| GQDs | Miscanthus | - | - | [233] |
| Type of GQD | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| GQDs | MWCNT | Less than 5 nm, zigzag structure | - | [99] |
| GQDs | Carbon fibers | 1–4 nm | Bioimaging and biosensing | [18] |
| GQDs | Graphene oxide | - | Bioscience and energy applications | [193] |
| GQDs | Graphene nanoribbons | 100–200 nm | Humidity/pressure sensing applications | [234] |
| GQDs | Carbon black | Less than 10 nm | DSSCs | [100] |
| GQDs | Graphene oxide | 1–3 nm | - | [94] |
| GQDs | GO | 15 nm, Spherical | Sensing | [235] |
| N-GQDs | Low-cost graphite | 5 nm | Bioimaging | [86] |
| GQDs | GO | 20–30 nm, quasi-hexagonal | Drug-delivery applications | [236] |
| Coal-like precursors: carbon fiber, Ethiopia, Mandheling, and Kenya AA |
| Biomedicine | [237] |
| Type of GQD | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| Porous graphene (PGN) | Graphene oxide | 5–150 nm | Detectable molecular separation | [65] |
| GQDs | Graphene oxide, H2O2 | 1–6 nm | - | [66] |
| Graphene nanosheets | Carbon nanotubes | 1–3 nm | Supercapacitors, solar cells, etc. | [81] |
| GQDs | Vulcan XC-72 carbon black | - | - | [83] |
| GQDs | C60 cage | - | Effective peroxidase mimic | [82] |
| CQDs | Chinese ink | 1–6 nm | Electrochemical and photoluminescent applications | [238] |
| Type of GQD | Precursor | Size and Shape | Application | Reference |
|---|---|---|---|---|
| GQDs | Graphene oxide | 4–5 nm, crystalline | - | [178] |
| GQDs | Graphene oxide | 1–5 nm, hexagonal lattice | Cancer detection or bioimaging | [179] |
| GQDs | Graphite (G), MWCNTs, carbon fiber (CF), and charcoal (C) | 2–8 nm, crystalline lattice | Industrial manufacturing with carbon precursors | [180] |
| Graphene oxide quantum dots | Black carbon | 3–4.5 nm | Bioimaging and biological applications | [181] |
6. Characterization Techniques and Structural Analysis of GQDs
6.1. Fourier-Transform Infrared Spectroscopy (FTIR)
6.2. Transmission Electron Microscopy (TEM)
6.3. UV Spectroscopy
6.4. Other Spectroscopy Studies
7. Concluding Remarks and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GQDs | graphene quantum dots |
| NPs | nanoparticles |
| CNTs | carbon nanotubes |
| GO | graphene oxide |
| N-GQDs | nitrogen-doped graphene quantum dots |
| PGQDs | pristine graphene quantum dots |
| EGQDs | expanded graphene quantum dots |
| GOGQDs | graphene oxide graphene quantum dots |
| LD-GQDs | low-defects graphene quantum dots |
| HD-GQDs | high-defects graphene quantum dots |
| C-GQDs | coal-derived graphene quantum dots |
| F-GQDs | fenugreek-derived graphene quantum dots |
| CQDs | carbon quantum dots |
| PL | photoluminescence |
| TATB | 1,3,5-triamino-2,4,6-trinitrobenzene |
| HBC | hexa-peri-hexabenzocoronene |
| CA | citric acid |
| His-GQD-GMA | histidine–graphene quantum dot–graphene micro-aerogel |
| ATP | adenosine triphosphate |
| N and P co-doped GQDs | graphene quantum dots co-doped with nitrogen and phosphorus |
| QY | quantum yield |
| FL | fluorescence |
| TNP | 2,4,6-trinitrophenol |
| En | ethylenediamine |
| Gl | glycerol |
| Eg | ethylene glycol |
| Me | methanol |
| Dmf | dimethyl formative |
| Ac | acetone |
| Et | ethanol |
| To | toluene |
| Ct | carbon tetrachloride |
| PEI | polyethyleneimine |
| N-GO | nitrogen-doped graphene oxide |
| MWCNT | multiwalled carbon nanotube |
| G-GQDs | graphite-derived graphene quantum dots |
| M-GQDs | MWCNT-derived graphene quantum dots |
| CF-GQDs | carbon fiber-derived graphene quantum dots |
| C-GQDs | charcoal-derived graphene quantum dots |
| a-GQDs | anthracite-derived GQDs |
| b-GQDs | bituminous coal-derived GQDs |
| c-GQDs | coke-derived GQDs |
| PLAL | pulsed laser ablation in liquid |
| GOQDs | graphene oxide quantum dots |
| mGQDs | Mangifera indica derived GQDs |
| RH-GQDs | rice-husk-derived GQDs |
| LGQDs | lignin-derived GQDs |
| MAH | microwave-assisted hydrothermal |
| WGQDs | white fluorescent GQDs |
| WLEDs | white-light-emitting diodes |
| CL | chemiluminescence |
| DSSC | dye-sensitized solar cells |
| XRD | X-ray diffraction |
| TEM | transmission electron microscope |
| FITR | Fourier-transform infrared spectroscopy |
| ETL | electron transport layer |
References
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Varma, R.S. Greener Approach to Nanomaterials and Their Sustainable Applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Varma, R.S. Greener and Sustainable Chemistry. Appl. Sci. 2014, 4, 493–497. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Biofactories: Engineered Nanoparticles: Via Genetically Engineered Organisms. Green Chem. 2019, 21, 4583–4603. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Shokrian Hajibehzad, S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A.; et al. Plant Molecular Farming: Production of Metallic Nanoparticles and Therapeutic Proteins Using Green Factories. Green Chem. 2019, 21, 1845–1865. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2015, 18, 20–52. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and MiRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]
- Zahir, N.; Magri, P.; Luo, W.; Gaumet, J.J.; Pierrat, P. Recent Advances on Graphene Quantum Dots for Electrochemical Energy Storage Devices. Energy Environ. Mater. 2021, 5, 201–214. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Schneider, R.; Mercante, L.A.; Correa, D.S. A Review on Graphene Quantum Dots and Their Nanocomposites: From Laboratory Synthesis towards Agricultural and Environmental Applications. Environ. Sci. Nano 2020, 7, 3710–3734. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Gao, K.; Chen, N.; Sun, X.; Ti, D.; Bai, C.; Cui, R.; Qu, L. Graphene Quantum Dots for Energy Storage and Conversion: From Fabrication to Applications. Mater. Chem. Front. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene Quantum Dots and Their Possible Energy Applications: A Review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Matsui, T.; Sai, H.; Bidiville, A.; Hsu, H.J.; Matsubara, K. Progress and Limitations of Thin-Film Silicon Solar Cells. Sol. Energy 2018, 170, 486–498. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, K. Minimal Realization of Linear System Based on New Smith-Mcmillan Normal Form of Transfer Function Matrix. Adv. Syst. Sci. Appl. 2010, 10, 531–537. [Google Scholar]
- Mhatre, V.H.; Kcm, J.-A.L. Genetic Changes NIH Public Access. Bone 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Shaker, M.; Riahifar, R.; Li, Y. A Review on the Superb Contribution of Carbon and Graphene Quantum Dots to Electrochemical Capacitors’ Performance: Synthesis and Application. FlatChem 2020, 22, 100171. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Bressi, V.; Ferlazzo, A.; Iannazzo, D.; Espro, C. Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives. Nanomaterials 2021, 11, 1120. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of Gold Nanoparticles Using Tea: A Green Chemistry Experiment. J. Chem. Educ. 2012, 89, 1316–1318. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Sachdeva, S. One Pot and Solvent-Free Synthesis of 2,9,16,23-Tetrachlorometal(II) Phthalocyanines. Green Chem. Lett. Rev. 2012, 5, 83–87. [Google Scholar] [CrossRef]
- Pant, P.; Bansal, R.; Gulati, S.; Kumar, S.; Kodwani, R. Porous and Chelated Nanostructured Multifunctional Materials: Recoverable and Reusable Sorbents for Extraction of Metal Ions and Catalysts for Diverse Organic Reactions. J. Nanostruct. Chem. 2016, 6, 145–157. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, S.; Gulati, S.; Pandey, A. Fabrication of a Novel Nano-Composite Carbon Paste Sensor Based on Silica-Nanospheres Functionalized with Isatin Thiosemicarbazone for Potentiometric Monitoring of Cu2+ Ions in Real Samples. Anal. Methods 2013, 5, 1414–1426. [Google Scholar] [CrossRef]
- Kumar, S.; Diwan, A.; Singh, P.; Gulati, S.; Choudhary, D.; Mongia, A.; Shukla, S.; Gupta, A. Functionalized Gold Nanostructures: Promising Gene Delivery Vehicles in Cancer Treatment. RSC Adv. 2019, 9, 23894–23907. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Green Synthesis, Biomedical and Biotechnological Applications of Carbon and Graphene Quantum Dots. A Review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Shaik, S.A.; Sengupta, S.; Varma, R.S.; Gawande, M.B.; Goswami, A. Syntheses of N-Doped Carbon Quantum Dots (NCQDs) from Bioderived Precursors: A Timely Update. ACS Sustain. Chem. Eng. 2021, 9, 3–49. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Mousavi Khadem, S.S.; Habibzadeh, S.; Esmaeili, A.; Abida, O.; Vatanpour, V.; Rabiee, N.; Bagherzadeh, M.; Iravani, S.; Reza Saeb, M.; et al. Quantum Dots for Photocatalysis: Synthesis and Environmental Applications. Green Chem. 2021, 23, 4931–4954. [Google Scholar] [CrossRef]
- Gawande, M.B.; Moores, A.; Varma, R.S. ACS Sustainable Chemistry & Engineering Virtual Special Issue on N-Doped Carbon Materials: Synthesis and Sustainable Applications. ACS Sustain. Chem. Eng. 2021, 9, 3975–3976. [Google Scholar] [CrossRef]
- Sharma, K.; Raizada, P.; Hasija, V.; Singh, P.; Bajpai, A.; Nguyen, V.H.; Rangabhashiyam, S.; Kumar, P.; Nadda, A.K.; Kim, S.Y.; et al. ZnS-Based Quantum Dots as Photocatalysts for Water Purification. J. Water Process Eng. 2021, 43, 102217. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Chuang, C.; Liou, Y.R.; Chen, Y.F.; Joly, J.; Liang, C.T.; Chang, H.T. Plant Leaf-Derived Graphene Quantum Dots and Applications for White LEDs. New J. Chem. 2014, 38, 4946–4951. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-Scale and Controllable Synthesis of Graphene Quantum Dots from Rice Husk Biomass: A Comprehensive Utilization Strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, A.; Biswal, M.; Mhamane, D.; Gokhale, R.; Patil, S.; Guin, D.; Ogale, S. Large Scale Synthesis of Graphene Quantum Dots (GQDs) from Waste Biomass and Their Use as an Efficient and Selective Photoluminescence on-off-on Probe for Ag+ Ions. Nanoscale 2014, 6, 11664–11670. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lee, S.-T. Carbon Dots: Advances in Nanocarbon Applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dot Biopolymer-Based Flexible Functional Films for Antioxidant and Food Monitoring Applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Ma, Y.; Wang, X.; Liu, Y.; Huang, H.; Kang, Z. Size-Dependent Antibacterial of Carbon Dots by Selective Absorption and Differential Oxidative Stress of Bacteria. J. Colloid Interface Sci. 2023, 634, 44–53. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L.S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhao, X.; Xiao, L.; Zeng, H.; Sun, H. Nonlinear Absorption and Low-Threshold Multiphoton Pumped Stimulated Emission from All-Inorganic Perovskite Nanocrystals. Nano Lett. 2016, 16, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Geys, J.; Nemmar, A.; Verbeken, E.; Smolders, E.; Ratoi, M.; Hoylaerts, M.F.; Nemery, B.; Hoet, P.H.M. Acute Toxicity and Prothrombotic Effects of Quantum Dots: Impact of Surface Charge. Environ. Health Perspect. 2008, 116, 1607–1613. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Li, J.; Pan, M.; Bai, L.; Luoshan, M.; Zhao, X. Graphene Quantum Dots Optimization of Dye-Sensitized Solar Cells. Electrochim. Acta 2014, 137, 634–638. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2021, 33, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene Quantum Dots Synthesis and Energy Application: A Review. Carbon Lett. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumar, P. Current and Future Perspectives of Carbon and Graphene Quantum Dots: From Synthesis to Strategy for Building Optoelectronic and Energy Devices. Renew. Sustain. Energy Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on Greener Pathways: From the Use of Alternate Energy Inputs and Benign Reaction Media to Sustainable Applications of Nano-Catalysts in Synthesis and Environmental Remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Puri, A. Green Chemistry Solutions to Water Pollution; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124165762. [Google Scholar]
- Cue, B.W.; Zhang, J. Green Process Chemistry in the Pharmaceutical Industry. Green Chem. Lett. Rev. 2009, 2, 193–211. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanoparticles 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D. New Paradigms for the Synthesis of Graphene Quantum Dots from Sustainable Bioresources. New J. Chem. 2017, 41, 8706–8710. [Google Scholar] [CrossRef]
- Tade, R.S.; Nangare, S.N.; Patil, A.G.; Pandey, A.; Deshmukh, P.K.; Patil, D.R.; Agrawal, T.N.; Mutalik, S.; Patil, A.M.; More, M.P.; et al. Recent Advancement in Bio-Precursor Derived Graphene Quantum Dots: Synthesis, Characterization and Toxicological Perspective. Nanotechnology 2020, 31, 292001. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.Q.; Li, N.; Zhu, J.L.; Zhang, T.; Zhang, W.; Liu, B. Preparation of Graphene Quantum Dots and Their Application in Cell Imaging. J. Nanomater. 2016, 2016, 9245865. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence Mechanism in Graphene Quantum Dots: Quantum Confinement Effect and Surface/Edge State. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Ye, R.; Xiang, C.; Lin, J.; Peng, Z.; Huang, K.; Yan, Z.; Cook, N.P.; Samuel, E.L.G.; Hwang, C.C.; Ruan, G.; et al. Coal as an Abundant Source of Graphene Quantum Dots. Nat. Commun. 2013, 4, 2943. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface Modification and Characterisation of a Coal-Based Activated Carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Kawano, T.; Kubota, M.; Onyango, M.S.; Watanabe, F.; Matsuda, H. Preparation of Activated Carbon from Petroleum Coke by KOH Chemical Activation for Adsorption Heat Pump. Appl. Therm. Eng. 2008, 28, 865–871. [Google Scholar] [CrossRef]
- Xie, X.; Goodell, B. Thermal Degradation and Conversion of Plant Biomass into High Value Carbon Products. ACS Symp. Ser. 2014, 1158, 147–158. [Google Scholar] [CrossRef]
- Schmidt, L.D.; Dauenhauer, P.J. Chemical Engineering: Hybrid Routes to Biofuels. Nature 2007, 447, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-Negative Biofuels from Low-Input High-Diversity Grassland Biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; He, P.; Wang, G.; Ding, G.; Xie, X. Insights into the Oxidation Mechanism of Sp2-Sp3 Hybrid Carbon Materials: Preparation of a Water-Soluble 2D Porous Conductive Network and Detectable Molecule Separation. Langmuir 2017, 33, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, S.; Wang, G.; Mo, R.; He, P.; Sun, J.; Di, Z.; Kang, Z.; Yuan, N.; Ding, J.; et al. A New Mild, Clean and Highly Efficient Method for the Preparation of Graphene Quantum Dots without by-Products. J. Mater. Chem. B 2015, 3, 6871–6876. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wang, C.; Wu, X.; Yang, Y.; Zheng, B.; Wu, H.; Guo, S.; Zhang, J. Photo-Fenton Reaction of Graphene Oxide: A New Strategy to Prepare Graphene Quantum Dots for DNA Cleavage. ACS Nano 2012, 6, 6592–6599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Tang, J.; Lee, K.J.; Pol, V.G.; Gedanken, A. In Situ Sonochemical Synthesis of Luminescent Sn@C-Dots and a Hybrid Sn@C-Dots@Sn Anode for Lithium-Ion Batteries. RSC Adv. 2016, 6, 66256–66265. [Google Scholar] [CrossRef]
- Chen, W.; Shen, J.; Lv, G.; Li, D.; Hu, Y.; Zhou, C.; Liu, X.; Dai, Z. Green Synthesis of Graphene Quantum Dots from Cotton Cellulose. ChemistrySelect 2019, 4, 2898–2902. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, G.; Jiang, H.; Chen, L.; Zhang, X. One-Step Ultrasonic Synthesis of Graphene Quantum Dots with High Quantum Yield and Their Application in Sensing Alkaline Phosphatase. Chem. Commun. 2015, 51, 948–951. [Google Scholar] [CrossRef]
- Sarkar, S.; Gandla, D.; Venkatesh, Y.; Bangal, P.R.; Ghosh, S.; Yang, Y.; Misra, S. Graphene Quantum Dots from Graphite by Liquid Exfoliation Showing Excitation-Independent Emission, Fluorescence Upconversion and Delayed Fluorescence. Phys. Chem. Chem. Phys. 2016, 18, 21278–21287. [Google Scholar] [CrossRef]
- Wen, J.; Li, M.; Xiao, J.; Liu, C.; Li, Z.; Xie, Y.; Ning, P.; Cao, H.; Zhang, Y. Novel Oxidative Cutting Graphene Oxide to Graphene Quantum Dots for Electrochemical Sensing Application. Mater. Today Commun. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Kanwal, S.; Jahan, S.; Mansoor, F. An Ultrasonic-Assisted Synthesis of Leather-Derived Luminescent Graphene Quantum Dots: Catalytic Reduction and Switch on-off Probe for Nitro-Explosives. RSC Adv. 2020, 10, 22959–22965. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Siddiqui, G.U.D.; Yang, Y.J.; Lee, K.T.; Um, K.; Choi, K.H. Direct Synthesis of Graphene Quantum Dots from Multilayer Graphene Flakes through Grinding Assisted Co-Solvent Ultrasonication for All-Printed Resistive Switching Arrays. RSC Adv. 2016, 6, 5068–5078. [Google Scholar] [CrossRef]
- Gao, H.; Xue, C.; Hu, G.; Zhu, K. Production of Graphene Quantum Dots by Ultrasound-Assisted Exfoliation in Supercritical CO2/H2O Medium. Ultrason. Sonochem. 2017, 37, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhu, Y.; Shi, C.; Pei, Y.T. Large-Scale Synthesis of Defect-Selective Graphene Quantum Dots by Ultrasonic-Assisted Liquid-Phase Exfoliation. Carbon 2016, 109, 373–383. [Google Scholar] [CrossRef]
- Sandeep Kumar, G.; Roy, R.; Sen, D.; Ghorai, U.K.; Thapa, R.; Mazumder, N.; Saha, S.; Chattopadhyay, K.K. Amino-Functionalized Graphene Quantum Dots: Origin of Tunable Heterogeneous Photoluminescence. Nanoscale 2014, 6, 3384–3391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Ren, S.; Dang, Y.; Liu, G.; Zhang, R.; Zhang, K.; Long, X.; Jia, K. Coal-Derived Graphene Quantum Dots Produced by Ultrasonic Physical Tailoring and Their Capacity for Cu(II) Detection. ACS Sustain. Chem. Eng. 2019, 7, 9793–9799. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, F.; Zhao, C.; Lv, Y.; Ma, G.; Wei, W.; Tian, Z. Beyond a Carrier: Graphene Quantum Dots as a Probe for Programmatically Monitoring Anti-Cancer Drug Delivery, Release, and Response. ACS Appl. Mater. Interfaces 2017, 9, 27396–27401. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Chiang, Y.M.; Tzou, D.Y.; Chen, Y.F.; Gandomi, Y.A. Optimization of Graphene Quantum Dots by Chemical Exfoliation from Graphite Powders and Carbon Nanotubes. Mater. Chem. Phys. 2018, 215, 104–111. [Google Scholar] [CrossRef]
- Wang, N.; Li, L.; Zhou, N.; Chen, S. Cage Breaking of C60 Into Photoluminescent Graphene Oxide Quantum Dots: An Efficient Peroxidase Mimic. Phys. Status Solidi Basic Res. 2018, 255, 2–5. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Zheng, Y.; Tang, N.; Li, M.; Zhong, W.; Du, Y. Gram-Scale Synthesis of High-Purity Graphene Quantum Dots with Multicolor Photoluminescence. RSC Adv. 2015, 5, 103428–103432. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile Synthesis of Fluorescent Graphene Quantum Dots from Coffee Grounds for Bioimaging and Sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Tian, R.; Zhong, S.; Wu, J.; Jiang, W.; Shen, Y.; Jiang, W.; Wang, T. Solvothermal Method to Prepare Graphene Quantum Dots by Hydrogen Peroxide. Opt. Mater. 2016, 60, 204–208. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Xie, W.; Wang, Y.; Jia, X.; Nawaz, A.; Song, M.; Gong, J.R. Preparation of Blue- and Green-Emissive Nitrogen-Doped Graphene Quantum Dots from Graphite and Their Application in Bioimaging. Mater. Sci. Eng. C 2021, 119, 111642. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Li, Q.; Yang, Y.; Wang, G.; You, X.; Mao, B.; Wang, H.; Ma, Y.; He, P.; et al. Electrochemical cutting in weak aqueous electrolytes: The strategy for efficient and controllable preparation of graphene quantum dots. Langmuir 2018, 34, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.X.; Wang, J.; Lim, A.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V. One Step Electro-Oxidative Preparation of Graphene Quantum Dots from Wood Charcoal as a Peroxidase Mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Dai, L.; Li, L.S. Nitrogen-Doped Colloidal Graphene Quantum Dots and Their Size-Dependent Electrocatalytic Activity for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 18932–18935. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.H.; Lim, K.H.; Li, J.; Chen, P. Facile Synthesis of Graphene Quantum Dots from 3D Graphene and Their Application for Fe3+ Sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Wang, Z.; Shen, H.; Xu, T.; Sun, L.; Li, H.; Chen, W.; Jiang, X.; Ding, G.; et al. Ultra-High Quantum Yield of Graphene Quantum Dots: Aromatic-Nitrogen Doping and Photoluminescence Mechanism. Part. Part. Syst. Charact. 2015, 32, 434–440. [Google Scholar] [CrossRef]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A.; Farzaneh, F. Tryptophan-Functionalized Graphene Quantum Dots with Enhanced Curcumin Loading Capacity and PH-Sensitive Release. J. Drug Deliv. Sci. Technol. 2021, 61, 102137. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Liu, X.Q.; Li, S.; Wang, L.; Ma, N.; Qiu, D. Free-Radical-Assisted Rapid Synthesis of Graphene Quantum Dots and Their Oxidizability Studies. Langmuir 2016, 32, 8641–8649. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.Y.; Tian, J.; Huang, Z. Rapid and Facile Synthesis of Graphene Quantum Dots with High Antioxidant Activity. Inorg. Chem. Commun. 2020, 122, 108288. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Via, B.K. Facile Synthesis of Soluble Graphene Quantum Dots and Its Improved Property in Detecting Heavy Metal Ions. Colloids Surf. B Biointerfaces 2014, 118, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, F.; Wu, C.; Guo, T. Optical Properties of Fluorescent Zigzag Graphene Quantum Dots Derived from Multi-Walled Carbon Nanotubes. Appl. Phys. Lett. 2014, 104, 063109. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, S.Y. Carbon Black-Derived Graphene Quantum Dots Composited with Carbon Aerogel as a Highly Efficient and Stable Reduction Catalyst for the Iodide/Tri-Iodide Couple. Nanoscale 2015, 7, 1209–1215. [Google Scholar] [CrossRef]
- Piyasena, P.; Dussault, C.; Koutchma, T.; Ramaswamy, H.S.; Awuah, G.B. Radio Frequency Heating of Foods: Principles, Applications and Related Properties—A Review. Crit. Rev. Food Sci. Nutr. 2003, 43, 587–606. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, Y.; Li, P.; Wang, Z.; Cao, B.; Qi, F.; Liu, J.; Qiu, Z.; Zhang, W. Ultrafast Ammonia-Driven, Microwave-Assisted Synthesis of Nitrogen-Doped Graphene Quantum Dots and Their Optical Properties. Nanophotonics 2017, 6, 259–267. [Google Scholar] [CrossRef]
- Tak, K.; Sharma, R.; Dave, V.; Jain, S.; Sharma, S. Clitoria Ternatea Mediated Synthesis of Graphene Quantum Dots for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2020, 11, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Ji, J.; Fei, R.; Wang, C.Z.; Lu, Q.; Zhang, J.R.; Jiang, L.P.; Zhu, J.J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Soriano, M.L.; Sciortino, A.; Cannas, M.; Messina, F.; Cardenas, S. One-Pot Synthesis of Graphene Quantum Dots and Simultaneous Nanostructured Self-Assembly: Via a Novel Microwave-Assisted Method: Impact on Triazine Removal and Efficiency Monitoring. RSC Adv. 2018, 8, 29939–29946. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Ashraf Gandomi, Y.; Chang, J.K.; Li, J.; Li, J.; Zhang, H.; Guo, Q.; Lau, K.C.; Pandey, R. Microwave Growth and Tunable Photoluminescence of Nitrogen-Doped Graphene and Carbon Nitride Quantum Dots. J. Mater. Chem. C 2019, 7, 5468–5476. [Google Scholar] [CrossRef]
- Hai, X.; Mao, Q.X.; Wang, W.J.; Wang, X.F.; Chen, X.W.; Wang, J.H. An Acid-Free Microwave Approach to Prepare Highly Luminescent Boron-Doped Graphene Quantum Dots for Cell Imaging. J. Mater. Chem. B 2015, 3, 9109–9114. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Srivastava, R.; Thakur, M.; Gurung, R.B. Graphene Quantum Dots from Mangifera Indica: Application in near-Infrared Bioimaging and Intracellular Nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Vijaya, P.M.; Kumar, M.P.; Takahashi, C.; Kundu, S.; Narayanan, T.N.; Pattanayak, D.K. Boron-Doped Graphene Quantum Dots: An Efficient Photoanode for a Dye Sensitized Solar Cell. New J. Chem. 2019, 43, 14313–14319. [Google Scholar] [CrossRef]
- Nguyen, H.Y.; Le, X.H.; Dao, N.T.; Pham, N.T.; Vu, T.H.H.; Nguyen, N.H.; Pham, T.N. Microwave-Assisted Synthesis of Graphene Quantum Dots and Nitrogen-Doped Graphene Quantum Dots: Raman Characterization and Their Optical Properties. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025005. [Google Scholar] [CrossRef]
- Qu, D.; Zheng, M.; Zhang, L.; Zhao, H.; Xie, Z.; Jing, X.; Haddad, R.E.; Fan, H.; Sun, Z. Formation Mechanism and Optimization of Highly Luminescent N-Doped Graphene Quantum Dots. Sci. Rep. 2014, 4, 5294. [Google Scholar] [CrossRef]
- Ren, Q.; Ga, L.; Ai, J. Rapid Synthesis of Highly Fluorescent Nitrogen-Doped Graphene Quantum Dots for Effective Detection of Ferric Ions and as Fluorescent Ink. ACS Omega 2019, 4, 15842–15848. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Yang, J.; Park, J.; Lee, K.; Kim, S.; Park, Y.; Lee, H. Mass Production of Graphene Quantum Dots by One-Pot Synthesis Directly from Graphite in High Yield. Small 2014, 10, 866–870. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Liu, Y.; Pan, D.; Li, Z.; Wang, L.; Wu, M. Three Minute Ultrarapid Microwave-Assisted Synthesis of Bright Fluorescent Graphene Quantum Dots for Live Cell Staining and White LEDs. ACS Appl. Nano Mater. 2018, 1, 1623–1630. [Google Scholar] [CrossRef]
- Umrao, S.; Jang, M.H.; Oh, J.H.; Kim, G.; Sahoo, S.; Cho, Y.H.; Srivastva, A.; Oh, I.K. Microwave Bottom-up Route for Size-Tunable and Switchable Photoluminescent Graphene Quantum Dots Using Acetylacetone: New Platform for Enzyme-Free Detection of Hydrogen Peroxide. Carbon 2015, 81, 514–524. [Google Scholar] [CrossRef]
- Zhuang, Q.; Wang, Y.; Ni, Y. Solid-Phase Synthesis of Graphene Quantum Dots from the Food Additive Citric Acid under Microwave Irradiation and Their Use in Live-Cell Imaging. Luminescence 2016, 31, 746–753. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, S.; Tan, T.T.Y.; Xiao, F.X. Graphene Quantum Dots (GQDs) and Its Derivatives for Multifarious Photocatalysis and Photoelectrocatalysis. Catal. Today 2018, 315, 171–183. [Google Scholar] [CrossRef]
- Ozhukil Valappil, M.; Pillai, V.K.; Alwarappan, S. Spotlighting Graphene Quantum Dots and beyond: Synthesis, Properties and Sensing Applications. Appl. Mater. Today 2017, 9, 350–371. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-Dependent Structural and Optical Characteristics of Glucose-Derived Graphene Quantum Dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Chen, S.; Hai, X.; Xia, C.; Chen, X.W.; Wang, J.H. Preparation of Excitation-Independent Photoluminescent Graphene Quantum Dots with Visible-Light Excitation/Emission for Cell Imaging. Chem. Eur. J. 2013, 19, 15918–15923. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ji, H.; Ju, E.; Guan, Y.; Ren, J.; Qu, X. Synthesis of Fluorinated and Nonfluorinated Graphene Quantum Dots through a New Top-down Strategy for Long-Time Cellular Imaging. Chem. Eur. J. 2015, 21, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sadeghi, N.; Tyagi, I.; Gupta, V.K.; Fakhri, A. Adsorption of Toxic Carbamate Pesticide Oxamyl from Liquid Phase by Newly Synthesized and Characterized Graphene Quantum Dots Nanomaterials. J. Colloid Interface Sci. 2016, 478, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, Y.; Zhao, C. Microwave-Assisted Synthesis of Nitrogen-Doped Multi-Layer Graphene Quantum Dots with Oxygen-Rich Functional Groups. Aust. J. Chem. 2016, 69, 357–360. [Google Scholar] [CrossRef]
- Ben Aoun, S. Nanostructured Carbon Electrode Modified with N-Doped Graphene Quantum Dots—Chitosan Nanocomposite: A Sensitive Electrochemical Dopamine Sensor. R. Soc. Open Sci. 2017, 4, 171199. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Qi, G.; Chen, K.; Zou, M.; Yuwen, L.; Zhang, X.; Huang, W.; Wang, L. Microwave-Assisted Preparation of White Fluorescent Graphene Quantum Dots as a Novel Phosphor for Enhanced White-Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2739–2744. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, D.; Yang, C.; Wu, X.; Hu, Y.; Zhang, Y.; Yuwen, L.; Yeow, E.K.L.; Weng, L.; Huang, W.; et al. Graphene Quantum Dots Modified with Adenine for Efficient Two-Photon Bioimaging and White Light-Activated Antibacteria. Appl. Surf. Sci. 2018, 434, 155–162. [Google Scholar] [CrossRef]
- Jénnifer Gómez, I.; Vázquez Sulleiro, M.; Dolečková, A.; Pizúrová, N.; Medalová, J.; Bednařík, A.; Preisler, J.; Nečas, D.; Zajíčková, L. Structure Elucidation of Multicolor Emissive Graphene Quantum Dots towards Cell Guidance. Mater. Chem. Front. 2022, 6, 145–154. [Google Scholar] [CrossRef]
- Chaudhary, P.; Verma, A.; Mishra, A.; Yadav, D.; Pal, K.; Yadav, B.C.; Ranjith Kumar, E.; Thapa, K.B.; Mishra, S.; Dwivedi, D.K. Preparation of Carbon Quantum Dots Using Bike Pollutant Soot: Evaluation of Structural, Optical and Moisture Sensing Properties. Phys. E Low Dimens. Syst. Nanostruct. 2022, 139, 115174. [Google Scholar] [CrossRef]
- Rajamanikandan, S.; Biruntha, M.; Ramalingam, G. Blue Emissive Carbon Quantum Dots (CQDs) from Bio-Waste Peels and Its Antioxidant Activity. J. Clust. Sci. 2022, 33, 1045–1053. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green Synthesis of Carbon Quantum Dots from Lemon Peel Waste: Applications in Sensing and Photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Kang, S.; Kim, K.M.; Jung, K.; Son, Y.; Mhin, S.; Ryu, J.H.; Shim, K.B.; Lee, B.; Han, H.S.; Song, T. Graphene Oxide Quantum Dots Derived from Coal for Bioimaging: Facile and Green Approach. Sci. Rep. 2019, 9, 4101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Ma, J.M.; Yang, Y.S.; Ru, J.X.; Liu, X.Y.; Ma, Y.; Guo, H.C. Synthesis of Nitrogen-Doped Graphene Quantum Dots (N-GQDs) from Marigold for Detection of Fe3+ Ion and Bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Sagar, P.; Srivastava, M.; Singh, A.K.; Singh, J.; Srivastava, S.K.; Srivastava, A. Excellent Supercapacitive Performance of Graphene Quantum Dots Derived from a Bio-Waste Marigold Flower (Tagetes Erecta). Int. J. Hydrogen Energy 2021, 46, 38416–38424. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Hassanzadeh, S.; Hakkarainen, M. Valorization of Cellulose and Waste Paper to Graphene Oxide Quantum Dots. RSC Adv. 2015, 5, 26550–26558. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; He, H.; Huang, G.; Xing, B.; Jia, J.; Zhang, C. Green Preparation of High Yield Fluorescent Graphene Quantum Dots from Coal-Tar-Pitch by Mild Oxidation. Nanomaterials 2018, 8, 844. [Google Scholar] [CrossRef]
- Jlassi, K.; Mallick, S.; Eribi, A.; Chehimi, M.M.; Ahmad, Z.; Touati, F.; Krupa, I. Facile Preparation of N-S Co-Doped Graphene Quantum Dots (GQDs) from Graphite Waste for Efficient Humidity Sensing. Sens. Actuators B Chem. 2021, 328, 129058. [Google Scholar] [CrossRef]
- Mohan, A.N.; Manoj, B. Biowaste Derived Graphene Quantum Dots Interlaced with SnO2 Nanoparticles-a Dynamic Disinfection Agent against: Pseudomonas Aeruginosa. New J. Chem. 2019, 43, 13681–13689. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-Scale Synthesis of Single-Crystalline Graphene Quantum Dots Derived from Lignin Biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Khose, R.V.; Bangde, P.; Bondarde, M.P.; Dhumal, P.S.; Bhakare, M.A.; Chakraborty, G.; Ray, A.K.; Dandekar, P.; Some, S. Waste Derived Approach towards Wealthy Fluorescent N-Doped Graphene Quantum Dots for Cell Imaging and H2O2 Sensing Applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120453. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Chen, L.; Huang, X. Monodispersed Hard Carbon Spherules with Uniform Nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Ortiz Balbuena, J.; Tutor De Ureta, P.; Rivera Ruiz, E.; Mellor Pita, S. Enfermedad de Vogt-Koyanagi-Harada. Med. Clin. 2016, 146, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.W.; Hsiao, Y.H.; Peng, Y.K.; Chou, P.T. Facile Synthesis of Highly Emissive Carbon Dots from Pyrolysis of Glycerol; Gram Scale Production of Carbon Dots/MSiO2 for Cell Imaging and Drug Release. J. Mater. Chem. 2012, 22, 14403–14409. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Karakassides, M.; Giannelis, E.P. Surface Functionalized Carbogenic Quantum Dots. Small 2008, 4, 455–458. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, D.; Chen, X.; Wang, F.; Song, H.; Shen, D. Long Lifetime Pure Organic Phosphorescence Based on Water Soluble Carbon Dots. Chem. Commun. 2013, 49, 5751–5753. [Google Scholar] [CrossRef] [PubMed]
- Click, M. Manuscript Click Here to View Linked References. Brain 2010, 2, 617–638. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. One-Pot Green Synthesis of Optically PH-Sensitive Carbon Dots with Upconversion Luminescence. Nanoscale 2012, 4, 5572–5575. [Google Scholar] [CrossRef]
- Kalita, H.; Mohapatra, J.; Pradhan, L.; Mitra, A.; Bahadur, D.; Aslam, M. Efficient Synthesis of Rice Based Graphene Quantum Dots and Their Fluorescent Properties. RSC Adv. 2016, 6, 23518–23524. [Google Scholar] [CrossRef]
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D.; Joseph, K. Simple and Cost-Effective Synthesis of Fluorescent Graphene Quantum Dots from Honey: Application as Stable Security Ink and White-Light Emission. Part. Part. Syst. Charact. 2016, 33, 70–74. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to up-Conversion Bioimaging Applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Synthesis of Green-Photoluminescent Single Layer Graphene Quantum Dots: Determination of HOMO and LUMO Energy States. J. Lumin. 2017, 192, 180–183. [Google Scholar] [CrossRef]
- Naik, J.P.; Sutradhar, P.; Saha, M. Molecular Scale Rapid Synthesis of Graphene Quantum Dots (GQDs). J. Nanostruct. Chem. 2017, 7, 85–89. [Google Scholar] [CrossRef]
- Li, X.; Lau, S.P.; Tang, L.; Ji, R.; Yang, P. Sulphur Doping: A Facile Approach to Tune the Electronic Structure and Optical Properties of Graphene Quantum Dots. Nanoscale 2014, 6, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Shokri, M. A New Humidity Sensor Based upon Graphene Quantum Dots Prepared via Carbonization of Citric Acid. Sens. Actuators B Chem. 2016, 222, 728–734. [Google Scholar] [CrossRef]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T. Chemiluminescence of Graphene Quantum Dots and Its Application to the Determination of Uric Acid. J. Lumin. 2014, 153, 73–78. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Magnetic Nanoparticles Embedded with Graphene Quantum Dots and Multiwalled Carbon Nanotubes as a Sensing Platform for Electrochemical Detection of Progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Diao, J.; Wang, T.; Li, L. Graphene Quantum Dots as Nanoprobes for Fluorescent Detection of Propofol in Emulsions. R. Soc. Open Sci. 2019, 6, 181753. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.M.; Guo, M.M.; Song, X.L.; Dai, H.Y.; Liang, Z.H. Graphene Quantum Dots Modified Glassy Carbon Electrode via Electrostatic Self-Assembly Strategy and Its Application. Electrochim. Acta 2016, 190, 455–462. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Dehbozorgi, A. Determination of Dopamine in the Presence of Ascorbic and Uric Acids by Fluorometric Method Using Graphene Quantum Dots. Spectrosc. Lett. 2016, 49, 319–325. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Safardoust-Hojaghan, H. Synthesis of Graphene Quantum Dots from Corn Powder and Their Application in Reduce Charge Recombination and Increase Free Charge Carriers. J. Mol. Liq. 2017, 242, 447–455. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Farangi, M. Facile Synthesis of Graphene Quantum Dots from Corn Powder and Their Application as down Conversion Effect in Quantum Dot-Dye-Sensitized Solar Cell. J. Mol. Liq. 2018, 251, 267–272. [Google Scholar] [CrossRef]
- Van Tam, T.; Trung, N.B.; Kim, H.R.; Chung, J.S.; Choi, W.M. One-Pot Synthesis of N-Doped Graphene Quantum Dots as a Fluorescent Sensing Platform for Fe3+ Ions Detection. Sens. Actuators B Chem. 2014, 202, 568–573. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, Y.; Routh, P.; Sk, M.A.; Than, A.; Lin, M.; Zhang, J.; Chen, J.; Sun, H.; Chen, P. Nitrogen and Phosphorus Co-Doped Graphene Quantum Dots: Synthesis from Adenosine Triphosphate, Optical Properties, and Cellular Imaging. Nanoscale 2015, 7, 8159–8165. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Pang, A.; Yang, J. Facile Synthesis and Photoluminescence Characteristics of Blue-Emitting Nitrogen-Doped Graphene Quantum Dots. Nanotechnology 2016, 27, 165704. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. A Fluorescent Probe Based on Nitrogen Doped Graphene Quantum Dots for Turn off Sensing of Explosive and Detrimental Water Pollutant, TNP in Aqueous Medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 37–43. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Qian, Y.; Zhuang, J.; Hu, L.; Chen, Q.; Zhou, S. Nitrogen-Doped Graphene Quantum Dots as Metal-Free Photocatalysts for Near-Infrared Enhanced Reduction of 4-Nitrophenol. ACS Appl. Nano Mater. 2019, 2, 7043–7050. [Google Scholar] [CrossRef]
- Wang, R.; Fan, H.; Jiang, W.; Ni, G.; Qu, S. Amino-Functionalized Graphene Quantum Dots Prepared Using High-Softening Point Asphalt and Their Application in Fe3+ Detection. Appl. Surf. Sci. 2019, 467–468, 446–455. [Google Scholar] [CrossRef]
- Liu, H.; Lv, X.; Li, C.; Qian, Y.; Wang, X.; Hu, L.; Wang, Y.; Lin, W.; Wang, H. Direct Carbonization of Organic Solvents toward Graphene Quantum Dots. Nanoscale 2020, 12, 10956–10963. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Kwon, W.; Lee, G.; Do, S.; Joo, T.; Rhee, S.W. Size-Controlled Soft-Template Synthesis of Carbon Nanodots toward Versatile Photoactive Materials. Small 2014, 10, 506–513. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Li, Z.; Shen, J.; Yang, Y.; Cui, X.; Yang, G. Bottom-Up Fabrication of Single-Layered Nitrogen-Doped Graphene Quantum Dots through Intermolecular Carbonization Arrayed in a 2D Plane. Chem. Eur. J. 2016, 22, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, D.; Feng, X.; Müllen, K. Bottom-up Fabrication of Photoluminescent Graphene Quantum Dots with Uniform Morphology. J. Am. Chem. Soc. 2011, 133, 15221–15223. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tang, L.; Xiang, J.; Ji, R.; Lai, S.K.; Yuan, S.; Lau, S.P. Facile Preparation of Sulphur-Doped Graphene Quantum Dots for Ultra-High Performance Ultraviolet Photodetectors. New J. Chem. 2017, 41, 10447–10451. [Google Scholar] [CrossRef]
- Do, S.; Kwon, W.; Rhee, S.W. Soft-Template Synthesis of Nitrogen-Doped Carbon Nanodots: Tunable Visible-Light Photoluminescence and Phosphor-Based Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 4221–4226. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Zhou, X.; Li, Z.; Liu, J. Fabrication of Zinc-Histidine-Functionalized Graphene Quantum Dot Framework Amphiphilic Nanoparticles and Application in the Synthesis of Polystyrene Microspheres for Adsorption of Cu2+ by Pickering Emulsion Polymerization. RSC Adv. 2016, 6, 102534–102541. [Google Scholar] [CrossRef]
- Ruiyi, L.; Sili, Q.; Zhangyi, L.; Ling, L.; Zaijun, L. Histidine-Functionalized Graphene Quantum Dot-Graphene Micro-Aerogel Based Voltammetric Sensing of Dopamine. Sens. Actuators B Chem. 2017, 250, 372–382. [Google Scholar] [CrossRef]
- Shin, Y.; Park, J.; Hyun, D.; Yang, J.; Lee, H. Generation of Graphene Quantum Dots by the Oxidative Cleavage of Graphene Oxide Using the Oxone Oxidant. New J. Chem. 2015, 39, 2425–2428. [Google Scholar] [CrossRef]
- Nair, R.V.; Thomas, R.T.; Sankar, V.; Muhammad, H.; Dong, M.; Pillai, S. Rapid, Acid-Free Synthesis of High-Quality Graphene Quantum Dots for Aggregation Induced Sensing of Metal Ions and Bioimaging. ACS Omega 2017, 2, 8051–8061. [Google Scholar] [CrossRef]
- Shin, Y.; Park, J.; Hyun, D.; Yang, J.; Lee, J.H.; Kim, J.H.; Lee, H. Acid-Free and Oxone Oxidant-Assisted Solvothermal Synthesis of Graphene Quantum Dots Using Various Natural Carbon Materials as Resources. Nanoscale 2015, 7, 5633–5637. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, C.; Liu, D.; Wang, H.; Su, W.; Li, H.; Zhang, Y.; Yao, S. A Facile and Simple Method for Synthesis of Graphene Oxide Quantum Dots from Black Carbon. Green Chem. 2017, 19, 900–904. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and Applications of Graphene Quantum Dots: A Review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the Tunable Photoluminescence Properties of Graphene Quantum Dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Mohanty, N.; Moore, D.; Xu, Z.; Sreeprasad, T.S.; Nagaraja, A.; Rodriguez, A.A.; Berry, V. Nanotomy-Based Production of Transferable and Dispersible Graphene Nanostructures of Controlled Shape and Size. Nat. Commun. 2012, 3, 844. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, X.; Li, L.S. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable Size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 Molecules into Graphene Quantum Dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Li, Y. Antioxidant Activity of Graphene Quantum Dots Prepared in Different Electrolyte Environments. Nanomaterials 2019, 9, 1708. [Google Scholar] [CrossRef]
- Zheng, A.X.; Cong, Z.X.; Wang, J.R.; Li, J.; Yang, H.H.; Chen, G.N. Highly-Efficient Peroxidase-like Catalytic Activity of Graphene Dots for Biosensing. Biosens. Bioelectron. 2013, 49, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, R.; Uemura, Y.; Naito, H.; Naka, K.; Haino, T. Chemical Functionalisation and Photoluminescence of Graphene Quantum Dots. Chem. Eur. J. 2016, 22, 8198–8206. [Google Scholar] [CrossRef]
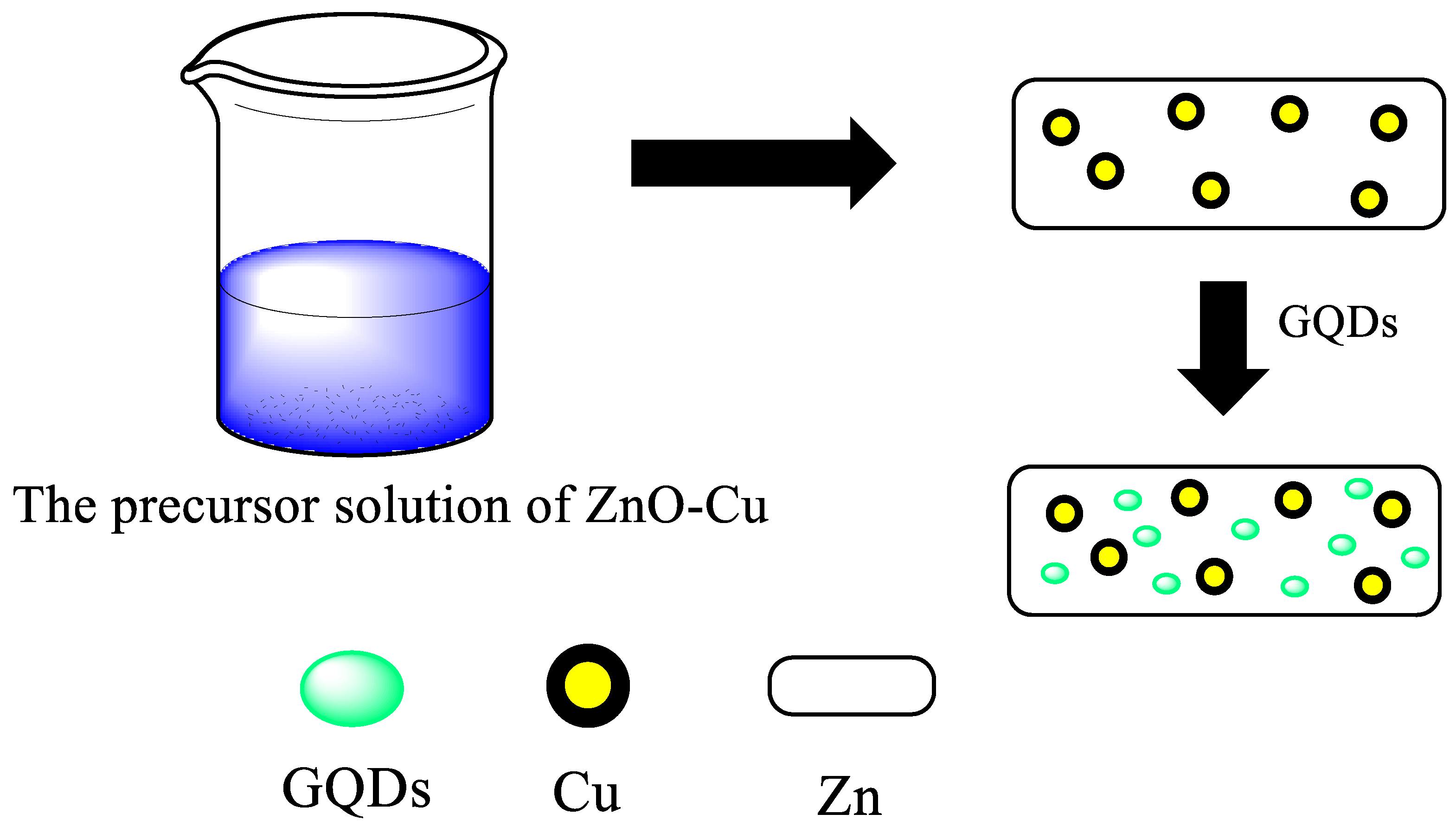
- Li, Y.; Wang, L.; Ge, J.; Wang, J.; Li, Q.; Wan, W.; Zhang, B.; Liu, X.; Xue, W. Graphene Quantum Dots Modified ZnO + Cu Heterostructure Photocatalysts with Enhanced Photocatalytic Performance. RSC Adv. 2016, 6, 106508–106515. [Google Scholar] [CrossRef]
- Hu, S.; Tian, R.; Wu, L.; Zhao, Q.; Yang, J.; Liu, J.; Cao, S. Chemical Regulation of Carbon Quantum Dots from Synthesis to Photocatalytic Activity. Chem. Asian J. 2013, 8, 1035–1041. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Gao, N.; Ren, J.; Qu, X. Improvement of Photoluminescence of Graphene Quantum Dots with a Biocompatible Photochemical Reduction Pathway and Its Bioimaging Application. ACS Appl. Mater. Interfaces 2013, 5, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile Preparation and Upconversion Luminescence of Graphene Quantum Dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-Doped Graphene Materials: Syntheses, Properties and Applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S. A Facile Ultrasonic-Assisted Fabrication of Nitrogen-Doped Carbon Dots/BiOBr up-Conversion Nanocomposites for Visible Light Photocatalytic Enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef]
- Prasad, K.S.; Pallela, R.; Kim, D.M.; Shim, Y.B. Microwave-Assisted One-Pot Synthesis of Metal-Free Nitrogen and Phosphorus Dual-Doped Nanocarbon for Electrocatalysis and Cell Imaging. Part. Part. Syst. Charact. 2013, 30, 557–564. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Chen, J.; Feng, H. Si doped CQDs: A facile and general preparation strategy, bioimaging application, and multifunctional sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.Y.; Liang, R.P.; Li, Y.H.; Qiu, J.D. Boron-Doped Graphene Quantum Dots for Selective Glucose Sensing Based on the “Abnormal” Aggregation-Induced Photoluminescence Enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef]
- Yao, M.; Huang, J.; Deng, Z.; Jin, W.; Yuan, Y.; Nie, J.; Wang, H.; Du, F.; Zhang, Y. Transforming Glucose into Fluorescent Graphene Quantum Dots: Via Microwave Radiation for Sensitive Detection of Al3+ ions Based on Aggregation-Induced Enhanced Emission. Analyst 2020, 145, 6981–6986. [Google Scholar] [CrossRef]
- Moghanlo, S.P.; Valizadeh, H. Microwave-Assisted Preparation of Graphene Quantum Dots Immobilized Nanosilica as an Efficient Heterogeneous Nanocatalyst for the Synthesis of Xanthenes. Org. Commun. 2019, 12, 14–25. [Google Scholar] [CrossRef]
- Alves, A.K.; Frantz, A.C.S.; Berutti, F.A. Microwave-Assisted Oleothermal Synthesis of Graphene-TiO2 Quantum Dots for Photoelectrochemical Oxygen Evolution Reaction. FlatChem 2018, 12, 26–34. [Google Scholar] [CrossRef]
- Qiu, H.; Sun, X.; An, S.; Lan, D.; Cui, J.; Zhang, Y.; He, W. Microwave Synthesis of Histidine-Functionalized Graphene Quantum Dots/Ni-Co LDH with Flower Ball Structure for Supercapacitor. J. Colloid Interface Sci. 2020, 567, 264–273. [Google Scholar] [CrossRef]
- Van Tam, T.; Altahtamouni, T.M.; Le Minh, V.; Ha, H.K.P.; Chung, N.T.K.; Van Thuan, D. One-Pot Microwave-Assisted Green Synthesis of Amine-Functionalized Graphene Quantum Dots for High Visible Light Photocatalytic Application. Comptes Rendus Chim. 2019, 22, 822–828. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Song, L.; Liu, X.; Hu, Z. Microwave Assisted One-Pot Synthesis of Graphene Quantum Dots as Highly Sensitive Fluorescent Probes for Detection of Iron Ions and PH Value. Talanta 2016, 150, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, C.; Yang, M. Microwave-Assisted One-Pot Conversion from Deoiled Asphalt to Green Fluorescent Graphene Quantum Dots and Their Interfacial Properties. J. Dispers. Sci. Technol. 2017, 38, 769–774. [Google Scholar] [CrossRef]
- Wu, D.; Qu, C.; Wang, J.; Yang, R.; Qu, L. Highly Sensitive and Selective Fluorescence Sensing and Imaging of Fe3+ Based on a Novel Nitrogen-Doped Graphene Quantum Dots. Luminescence 2021, 36, 1592–1599. [Google Scholar] [CrossRef]
- Centeno, L.; Romero-García, J.; Alvarado-Canché, C.; Gallardo-Vega, C.; Télles-Padilla, G.; Díaz Barriga-Castro, E.; Cabrera-Álvarez, E.N.; Ledezma-Pérez, A.; de León, A. Green Synthesis of Graphene Quantum Dots from Opuntia sp. Extract and Their Application in Phytic Acid Detection. Sens. Bio Sens. Res. 2021, 32, 100412. [Google Scholar] [CrossRef]
- Thi, T.; Quyen, B.; Nhon, N.H.; Nguyen, T.; Duong, T.; Nguyen, N.; My, T.; Thien, D.V.H.; Huynh, L.; Thanh, V. Rapid and Simple Synthesis of Graphene Quantum Dots/Ag Nanocomposites and Its Application for Glucose Detection by Photoluminescence Spectroscopy. Int. J. Sci. Eng. Sci. 2021, 5, 1–5. [Google Scholar]
- Zhou, L.; Geng, J.; Liu, B. Graphene Quantum Dots from Polycyclic Aromatic Hydrocarbon for Bioimaging and Sensing of Fe3+ and Hydrogen Peroxide. Part. Part. Syst. Charact. 2013, 30, 1086–1092. [Google Scholar] [CrossRef]
- Pathak, P.K.; Kumar, A.; Prasad, B.B. Functionalized Nitrogen Doped Graphene Quantum Dots and Bimetallic Au/Ag Core-Shell Decorated Imprinted Polymer for Electrochemical Sensing of Anticancerous Hydroxyurea. Biosens. Bioelectron. 2019, 127, 10–18. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Yuan, C.Y.; Gandomi, Y.A.; Chang, J.K.; Fu, C.C.; Yang, J.W.; Juang, R.S. Fluorescence of Functionalized Graphene Quantum Dots Prepared from Infrared-Assisted Pyrolysis of Citric Acid and Urea. J. Lumin. 2020, 217, 116774. [Google Scholar] [CrossRef]
- Hallaj, T.; Amjadi, M.; Manzoori, J.L.; Shokri, R. Chemiluminescence Reaction of Glucose-Derived Graphene Quantum Dots with Hypochlorite, and Its Application to the Determination of Free Chlorine. Microchim. Acta 2014, 182, 789–796. [Google Scholar] [CrossRef]
- Ma, C.B.; Zhu, Z.T.; Wang, H.X.; Huang, X.; Zhang, X.; Qi, X.; Zhang, H.L.; Zhu, Y.; Deng, X.; Peng, Y.; et al. A General Solid-State Synthesis of Chemically-Doped Fluorescent Graphene Quantum Dots for Bioimaging and Optoelectronic Applications. Nanoscale 2015, 7, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Ebrahim, S.; Soliman, M. Graphene Quantum Dots Prepared from Glucose as Optical Sensor for Glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Sudarsanakumar, C.; Thomas, S.; Mathew, S.; Arundhathi, S.; Raj, D.R.; Prasanth, S.; Thomas, R.K. Selective Sensing of Curcumin Using L-Cysteine Derived Blue Luminescent Graphene Quantum Dots. Mater. Res. Bull. 2019, 110, 32–38. [Google Scholar] [CrossRef]
- Van Tam, T.; Choi, W.M. One-Pot Synthesis of Highly Fluorescent Amino-Functionalized Graphene Quantum Dots for Effective Detection of Copper Ions. Curr. Appl. Phys. 2018, 18, 1255–1260. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Jiang, D.; Du, X.; Qian, J.; Mao, H.; Wang, K. Atmospheric Pressure Synthesis of Nitrogen Doped Graphene Quantum Dots for Fabrication of BiOBr Nanohybrids with Enhanced Visible-Light Photoactivity and Photostability. Carbon 2016, 96, 1157–1165. [Google Scholar] [CrossRef]
- Zhu, W.; Song, H.; Zhang, L.; Weng, Y.; Su, Y.; Lv, Y. Fabrication of Fluorescent Nitrogen-Rich Graphene Quantum Dots by Tin(IV) Catalytic Carbonization of Ethanolamine. RSC Adv. 2015, 5, 60085–60089. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, Y.; Xu, J.; Wang, A.; Wu, S.; Shen, J. The Carbonization of Polyethyleneimine: Facile Fabrication of N-Doped Graphene Oxide and Graphene Quantum Dots. RSC Adv. 2015, 5, 105855–105861. [Google Scholar] [CrossRef]
- More, M.P.; Lohar, P.H.; Patil, A.G.; Patil, P.O.; Deshmukh, P.K. Controlled Synthesis of Blue Luminescent Graphene Quantum Dots from Carbonized Citric Acid: Assessment of Methodology, Stability, and Fluorescence in an Aqueous Environment. Mater. Chem. Phys. 2018, 220, 11–22. [Google Scholar] [CrossRef]
- Amjadi, M.; Shokri, R.; Hallaj, T. A New Turn-off Fluorescence Probe Based on Graphene Quantum Dots for Detection of Au(III) Ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 619–624. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, Y.C.; Ren, W.W.; Bai, A.M.; Li, X.Y.; Hu, Y.J. Preparation of Graphene Quantum Dots with Glycine as Nitrogen Source and Its Interaction with Human Serum Albumin. Luminescence 2021, 36, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Huang, H.; Li, P.; Wang, C.; Yang, Y. Fluorine Functionalized Graphene Quantum Dots as Inhibitor against HIAPP Amyloid Aggregation. ACS Chem. Neurosci. 2017, 8, 1368–1377. [Google Scholar] [CrossRef]
- Hu, C.; Su, T.R.; Lin, T.J.; Chang, C.W.; Tung, K.L. Yellowish and Blue Luminescent Graphene Oxide Quantum Dots Prepared: Via a Microwave-Assisted Hydrothermal Route Using H2O2 and KMnO4 as Oxidizing Agents. New J. Chem. 2018, 42, 3999–4007. [Google Scholar] [CrossRef]
- Vandana, M.; Ashokkumar, S.P.; Vijeth, H.; Yesappa, L.; Devendrappa, H. Synthesis and Characterization of Polypyrrole—Graphene Quantum Dots Nanocomposites for Supercapacitor Application. AIP Conf. Proc. 2019, 2115, 030535. [Google Scholar] [CrossRef]
- Lee, B.H.; McKinney, R.L.; Hasan, M.T.; Naumov, A.V. Graphene Quantum Dots as Intracellular Imaging-Based Temperature Sensors. Materials 2021, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Tabish, T.A.; Bull, S.J.; Lim, T.M.; Phan, A.N. High Yield Synthesis of Graphene Quantum Dots from Biomass Waste as a Highly Selective Probe for Fe3+ Sensing. Sci. Rep. 2020, 10, 21262. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xia, G.; Zhong, W.; Chen, L.; Chen, L.; Wang, Y.; Min, Y.; Li, K. Direct Transformation of Lignin into Fluorescence-Switchable Graphene Quantum Dots and Their Application in Ultrasensitive Profiling of a Physiological Oxidant. Green Chem. 2019, 21, 3343–3352. [Google Scholar] [CrossRef]
- Veeresh, S.; Ganesh, H.; Nagaraju, Y.S.; Vandana, M.; Ashokkumar, S.P.; Vijeth, H.; Prasad, M.V.N.A.; Devendrappa, H. UV-Irradiated Hydrothermal Synthesis of Reduced Graphene Quantum Dots for Electrochemical Applications. Diam. Relat. Mater. 2021, 114, 108289. [Google Scholar] [CrossRef]
- Reagen, S.; Wu, Y.; Liu, X.; Shahni, R.; Bogenschuetz, J.; Wu, X.; Chu, Q.R.; Oncel, N.; Zhang, J.; Hou, X.; et al. Synthesis of Highly Near-Infrared Fluorescent Graphene Quantum Dots Using Biomass-Derived Materials for In Vitro Cell Imaging and Metal Ion Detection. ACS Appl. Mater. Interfaces 2021, 13, 43952–43962. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zou, Y.; Cao, X.; Lin, Z. Color-Tunable Fluorescent Nitrogen-Doped Graphene Quantum Dots Derived from Pineapple Leaf Fiber Biomass to Detect Hg2+. Chin. J. Anal. Chem. 2021, 50, 69–76. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Zhao, X.; Yuan, J.; Zhao, H.; Wang, G.; Li, M. Synthesis of Corn Straw-Based Graphene Quantum Dots (GQDs) and Their Application in PO43- Detection. J. Environ. Chem. Eng. 2022, 10, 107150. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene Oxide and Graphene Quantum Dots from Miscanthus via Ultrasound-Assisted Mechano-Chemical Cracking Method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef] [PubMed]
- Sreeprasad, T.S.; Rodriguez, A.A.; Colston, J.; Graham, A.; Shishkin, E.; Pallem, V.; Berry, V. Electron-Tunneling Modulation in Percolating Network of Graphene Quantum Dots: Fabrication, Phenomenological Understanding, and Humidity/Pressure Sensing Applications. Nano Lett. 2013, 13, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Mehata, M.S.; Biswas, S. Synthesis of Fluorescent Graphene Quantum Dots from Graphene Oxide and Their Application in Fabrication of GQDs@AgNPs Nanohybrids and Sensing of H2O2. Ceram. Int. 2021, 47, 19063–19072. [Google Scholar] [CrossRef]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-Conjugated Graphene Quantum Dots as Versatile and PH-Sensitive Nanocarriers for Enhanced Delivery of Curcumin Targeting to Breast Cancer. Mater. Sci. Eng. C 2021, 121, 111809. [Google Scholar] [CrossRef]
- Kim, D.J.; Yoo, J.M.; Suh, Y.; Kim, D.; Kang, I.; Moon, J.; Park, M.; Kim, J.; Kang, K.S.; Hong, B.H. Graphene Quantum Dots from Carbonized Coffee Bean Wastes for Biomedical Applications. Nanomaterials 2021, 11, 1423. [Google Scholar] [CrossRef]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-Scale Fabrication of Heavy Doped Carbon Quantum Dots with Tunable-Photoluminescence and Sensitive Fluorescence Detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar] [CrossRef]
- Khodadadei, F.; Safarian, S.; Ghanbari, N. Methotrexate-Loaded Nitrogen-Doped Graphene Quantum Dots Nanocarriers as an Efficient Anticancer Drug Delivery System. Mater. Sci. Eng. C 2017, 79, 280–285. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene Quantum Dots Based Systems as HIV Inhibitors. Bioconjug. Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H. Graphene and Carbon Quantum Dots: A review on syntheses, biological and sensing applications for neurotransmitter determination. Synth. Charact. Biol. Sens. 2020, 15406–15429. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Sun, R.C.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Rajender, G.; Goswami, U.; Giri, P.K. Solvent Dependent Synthesis of Edge-Controlled Graphene Quantum Dots with High Photoluminescence Quantum Yield and Their Application in Confocal Imaging of Cancer Cells. J. Colloid Interface Sci. 2019, 541, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M.; Hill, C.; Carolina, N. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A Review on Syntheses, Properties, Characterization and Bioanalytical Applications of Fluorescent Carbon Dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Green Synthesis of Highly Fluorescent Carbon Quantum Dots from Sugarcane Bagasse Pulp. Appl. Surf. Sci. 2016, 390, 435–443. [Google Scholar] [CrossRef]
- Wu, J.; Lin, M.; Cong, X.; Liu, H.; Tan, P. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally Derived Carbon Dots In Situ Confined Self-Healing and Breathable Hydrogel Monolith for Anomalous Diffusion-Driven Phytomedicine Release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulati, S.; Baul, A.; Amar, A.; Wadhwa, R.; Kumar, S.; Varma, R.S. Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs). Nanomaterials 2023, 13, 554. https://doi.org/10.3390/nano13030554
Gulati S, Baul A, Amar A, Wadhwa R, Kumar S, Varma RS. Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs). Nanomaterials. 2023; 13(3):554. https://doi.org/10.3390/nano13030554
Chicago/Turabian StyleGulati, Shikha, Arikta Baul, Anoushka Amar, Rachit Wadhwa, Sanjay Kumar, and Rajender S. Varma. 2023. "Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs)" Nanomaterials 13, no. 3: 554. https://doi.org/10.3390/nano13030554
APA StyleGulati, S., Baul, A., Amar, A., Wadhwa, R., Kumar, S., & Varma, R. S. (2023). Eco-Friendly and Sustainable Pathways to Photoluminescent Carbon Quantum Dots (CQDs). Nanomaterials, 13(3), 554. https://doi.org/10.3390/nano13030554