Stability and Activity of Rhodium Promoted Nickel-Based Catalysts in Dry Reforming of Methane
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalyst Activity Test
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ocsachoque, M.; Quincoces, C.E.; González, M.G. Effect of Rh Addition on Activity and Stability over NI/γ-AL2O3 Catalysts during Methane Reforming with CO2. Stud. Surf. Sci. Catal. 2007, 167, 397–402. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Wang, X.; Jin, Z.; Zhu, D.; Meng, Q.; Huang, S.; Liu, J.; Fu, Q. Carbon and Hydrogen Isotopes of Methane, Ethane, and Propane: A Review of Genetic Identification of Natural Gas. Earth Sci. Rev. 2019, 190, 247–272. [Google Scholar] [CrossRef]
- Zhou, Y.; Haynes, D.; Baltrus, J.; Roy, A.; Shekhawat, D.; Spivey, J.J. Methane Steam Reforming at Low Steam-to-Carbon Ratio: The Effect of Y Doping in Rh Substituted Lanthanum Zirconates. Appl. Catal. A Gen. 2020, 606, 117802–117814. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zheng, X.; Liu, W.; Cao, Z.; Peng, H. Coke-Resistance over Rh–Ni Bimetallic Catalyst for Low Temperature Dry Reforming of Methane. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Sun, W.; Zhao, G.; Liu, Y.; Lu, Y. Insight into Deactivation of the Carbon-/Sintering-Resistant Ni@Silicalite-1 for Catalytic Partial Oxidation of Methane to Syngas. Fuel 2022, 320, 123892. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically Dispersed Nickel as Coke-Resistant Active Sites for Methane Dry Reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Effect of Ti/Al Ratio on the Performance of Ni/TiO2-Al2O3 Catalyst for Methane Reforming with CO2. Fuel Process. Technol. 2019, 192, 21–35. [Google Scholar] [CrossRef]
- Ross, J.R.H. Natural Gas Reforming and CO2 Mitigation. Catal. Today 2005, 100, 151–158. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Fabiano, C.; Pino, L.; Laganà, M.; Recupero, V. Hydrogen-Rich Gas Production by Steam Reforming of n-Dodecane: Part I: Catalytic Activity of Pt/CeO2 Catalysts in Optimized Bed Configuration. Appl. Catal. B Environ. 2016, 199, 350–360. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Rogozhnikov, V.N.; Simonov, P.A.; Snytnikov, P.V.; Salanov, A.N.; Kulikov, A.V.; Gerasimov, E.Y.; Belyaev, V.D.; Potemkin, D.I.; Sobyanin, V.A. Highly Dispersed Rh/Ce0.75Zr0.25O2-δ-ƞ-Al2O3/FeCrAl Wire Mesh Catalyst for Autothermal n-Hexadecane Reforming. Mater. Lett. 2018, 214, 290–292. [Google Scholar] [CrossRef]
- Amjad, U.E.S.; Quintero, C.W.M.; Ercolino, G.; Italiano, C.; Vita, A.; Specchia, S. Methane Steam Reforming on the Pt/CeO2 Catalyst: Effect of Daily Start-Up and Shut-Down on Long-Term Stability of the Catalyst. Ind. Eng. Chem. Res. 2019, 58, 16395–16406. [Google Scholar] [CrossRef]
- Chung, W.C.; Chang, M.B. Review of Catalysis and Plasma Performance on Dry Reforming of CH4 and Possible Synergistic Effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Myint, M.N.Z.; Yan, B.; Wan, J.; Zhao, S.; Chen, J.G. Reforming and Oxidative Dehydrogenation of Ethane with CO2 as a Soft Oxidant over Bimetallic Catalysts. J. Catal. 2016, 343, 168–177. [Google Scholar] [CrossRef]
- Meshkani, F.; Rezaei, M.; Andache, M. Investigation of the Catalytic Performance of Ni/MgO Catalysts in Partial Oxidation, Dry Reforming and Combined Reforming of Methane. J. Ind. Eng. Chem. 2014, 20, 1251–1260. [Google Scholar] [CrossRef]
- Tang, Y.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Aminonitro Groups Surrounding a Fused Pyrazolotriazine Ring: A Superior Thermally Stable and Insensitive Energetic Material. ACS Appl. Energy Mater. 2019, 2, 2263–2267. [Google Scholar] [CrossRef]
- Shoynkhorova, T.B.; Simonov, P.A.; Potemkin, D.I.; Snytnikov, P.V.; Belyaev, V.D.; Ishchenko, A.V.; Svintsitskiy, D.A.; Sobyanin, V.A. Highly Dispersed Rh-, Pt-, Ru/Ce0.75Zr0.25O2–δ Catalysts Prepared by Sorption-Hydrolytic Deposition for Diesel Fuel Reforming to Syngas. Appl. Catal. B Environ. 2018, 237, 237–244. [Google Scholar] [CrossRef]
- Lee, S.; Bae, M.; Bae, J.; Katikaneni, S.P. Ni–Me/Ce0.9Gd0.1O2−x (Me: Rh, Pt and Ru) Catalysts for Diesel Pre-Reforming. Int. J. Hydrogen Energy 2015, 40, 3207–3216. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, X.; Hou, F.; Wu, C.; Wang, L.; Li, G. Tuning Metal-Support Interaction and Oxygen Vacancies of Ceria Supported Nickel Catalysts by Tb Doping for n-Dodecane Steam Reforming. Appl. Surf. Sci. 2020, 503, 144319. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, J.S.; Lee, Y.J.; Park, N.K.; Kim, Y.C.; Hong, S.I.; Moon, D.J. Steam Reforming of N-Hexadecane over Noble Metal-Modified Ni-Based Catalysts. Catal. Today 2008, 136, 228–234. [Google Scholar] [CrossRef]
- Neuberg, S.; Pennemann, H.; Shanmugam, V.; Zapf, R.; Kolb, G. Promoting Effect of Rh on the Activity and Stability of Pt-Based Methane Combustion Catalyst in Microreactors. Catal. Commun. 2021, 149, 106202. [Google Scholar] [CrossRef]
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G. Activity and Stability Enhancement of Ni-MCM-41 Catalysts by Rh Incorporation for Hydrogen from Dry Reforming of Methane. Int. J. Hydrogen Energy 2010, 35, 2296–2304. [Google Scholar] [CrossRef]
- Khalighi, R.; Bahadoran, F.; Panjeshahi, M.H.; Zamaniyan, A.; Tahouni, N. High Catalytic Activity and Stability of X/CoAl2O4 (X = Ni, Co, Rh, Ru) Catalysts with No Observable Coke Formation Applied in the Autothermal Dry Reforming of Methane Lined on Cordierite Monolith Reactors. Microporous Mesoporous Mater. 2020, 305, 110371. [Google Scholar] [CrossRef]
- Tarifa, P.; Schiaroli, N.; Ho, P.H.; Cañaza, F.; Ospitali, F.; de Luna, G.S.; Lucarelli, C.; Fornasari, G.; Vaccari, A.; Monzon, A.; et al. Steam Reforming of Clean Biogas over Rh and Ru Open-Cell Metallic Foam Structured Catalysts. Catal. Today 2022, 383, 74–83. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, X.; Wang, L.; Li, G. Optimizing the Preparation of Ni-Ce-Pr Catalysts for Efficient Hydrogen Production by n-Dodecane Steam Reforming. Int. J. Energy Res. 2020, 44, 1828–1842. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Keul, C.; Eichberger, M.; Figge, D.; Edelhoff, D.; Lümkemann, N. Three Generations of Zirconia: From Veneered to Monolithic. Part I. Quintessence Int. 2017, 48, 369–380. [Google Scholar]
- Zhang, X.; Peng, L.; Fang, X.; Cheng, Q.; Liu, W.; Peng, H.; Gao, Z.; Zhou, W.; Wang, X. Ni/Y2B2O7 (B[Dbnd]Ti, Sn, Zr and Ce) Catalysts for Methane Steam Reforming: On the Effects of B Site Replacement. Int. J. Hydrogen Energy 2018, 43, 8298–8312. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 Catalyst with Ultra-High Sintering and Coking Resistance for Dry Reforming of Methane to Prepare Syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Fedorov, A.; Chen, P.; Müller, C.R.; Copéret, C. Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar] [CrossRef]
- Pedrero, C.M.; Carrazán, S.G.; Ruiz, P. Preliminary Results on the Role of the Deposition of Small Amounts of ZrO2 on Al2O3 Support on the Partial Oxidation of Methane and Ethane over Rh and Ni Supported Catalysts. Catal. Today 2021, 363, 111–121. [Google Scholar] [CrossRef]
- Lv, J.; Wang, D.; Wei, B.; Jin, L.; Li, Y.; Hu, H. Integrated Process of Coal Pyrolysis with Dry Reforming of Low Carbon Alkane over Ni/La2O3-ZrO2 with Different La/Zr Ratio. Fuel 2021, 292, 120412. [Google Scholar] [CrossRef]
- Achouri, I.E.; Abatzoglou, N.; Fauteux-Lefebvre, C.; Braidy, N. Diesel Steam Reforming: Comparison of Two Nickel Aluminate Catalysts Prepared by Wet-Impregnation and Co-Precipitation. Catal. Today 2013, 207, 13–20. [Google Scholar] [CrossRef]
- INGEL, R.P.; III, D.L. Lattice Parameters and Density for Y2O3-Stabilized ZrO2. J. Am. Ceram. Soc. 1986, 69, 325–332. [Google Scholar] [CrossRef]
- Darby, R.J.; Kumar, R.V. Activation Enthalpies for Oxygen Ion Motion in Cubic Yttria-Stabilized Zirconia. J. Mater. Sci. 2008, 43, 6567–6570. [Google Scholar] [CrossRef]
- Patel, R.; Fakeeha, A.H.; Kasim, S.O.; Sofiu, M.L.; Ibrahim, A.A.; Abasaeed, A.E.; Kumar, R.; Al-Fatesh, A.S. Optimizing Yttria-Zirconia Proportions in Ni Supported Catalyst System for H2 Production through Dry Reforming of Methane. Mol. Catal. 2021, 510, 111676. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, X.; Chen, X.; Jiang, L.; Zheng, Y. Synthesis of Mg-Doped Ordered Mesoporous Pd-Al2O3 with Different Basicity for CO, NO, and HC Elimination. Ind. Eng. Chem. Res. 2017, 56, 1687–1695. [Google Scholar] [CrossRef]
- Oliveira, D.; Andrada, A.S. Synthesis of Ordered Mesoporous Silica MCM-41 with Controlled Morphology for Potential Application in Controlled Drug Delivery Systems. Ceramica 2019, 65, 170–179. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Alkaline Earth Promoters (MgO, CaO, and BaO) on the Activity and Coke Formation of Ni Catalysts Supported on Nanocrystalline Al2O2 in Dry Reforming of Methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry Reforming of Methane over Ni/MgO-Al2O2 Catalysts Prepared by Two-Step Hydrothermal Method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-Oxide-Modified Nickel as a Non-Noble Metal Catalyst for Selective Decomposition of Hydrous Hydrazine to Hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas Reforming of Carbon Dioxide to Syngas Production over Ni-Mg-Al Catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In Situ Auto-Gasification of Coke Deposits over a Novel Ni-Ce/W-Zr Catalyst by Sequential Generation of Oxygen Vacancies for Remarkably Stable Syngas Production via CO2-Reforming of Methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Mi, Y.; Yang, S.; Wang, Z.; Liu, W.; Wu, D.; Peng, H. Trifunctional Strategy for the Design and Synthesis of a Ni-CeO2@SiO2 Catalyst with Remarkable Low-Temperature Sintering and Coking Resistance for Methane Dry Reforming. Chin. J. Catal. 2021, 42, 1808–1820. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-Reforming of Methane to Produce Syngas over Co-Ni/SBA-15 Catalyst: Effect of Support Modifiers (Mg, La and Sc) on Catalytic Stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Wang, C.; Jie, X.; Qiu, Y.; Zhao, Y.; Al-Megren, H.A.; Alshihri, S.; Edwards, P.P.; Xiao, T. The Importance of Inner Cavity Space within Ni@SiO2 Nanocapsule Catalysts for Excellent Coking Resistance in the High-Space-Velocity Dry Reforming of Methane. Appl. Catal. B Environ. 2019, 259, 118019. [Google Scholar] [CrossRef]
- Li, R.; Xu, W.; Deng, J.; Zhou, J. Coke-Resistant Ni-Co/ZrO2-CaO-Based Microwave Catalyst for Highly Effective Dry Reforming of Methane by Microwave Catalysis. Ind. Eng. Chem. Res. 2021, 60, 17458–17468. [Google Scholar] [CrossRef]
- Fidalgo, B.; Arenillas, A.; Menéndez, J.A. Mixtures of Carbon and Ni/Al2O2 as Catalysts for the Microwave-Assisted CO2 Reforming of CH4. Fuel Process. Technol. 2011, 92, 1531–1536. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Tkachenko, O.P.; Kapustin, G.I. Nickel-Alumina Catalysts in the Reaction of Carbon Dioxide Re-Forming of Methane under Thermal and Microwave Heating. Ind. Eng. Chem. Res. 2017, 56, 13034–13039. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Wang, H.; Wang, J.; Song, Z.; Zhao, X.; Ma, C. Methane Dry and Mixed Reforming on the Mixture of Bio-Char and Nickel-Based Catalyst with Microwave Assistance. J. Anal. Appl. Pyrolysis 2017, 125, 318–327. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent Developments in Methane Decomposition over Heterogeneous Catalysts: An Overview. Mater. Renew. Sustain. Energy 2020, 9, 8. [Google Scholar] [CrossRef]
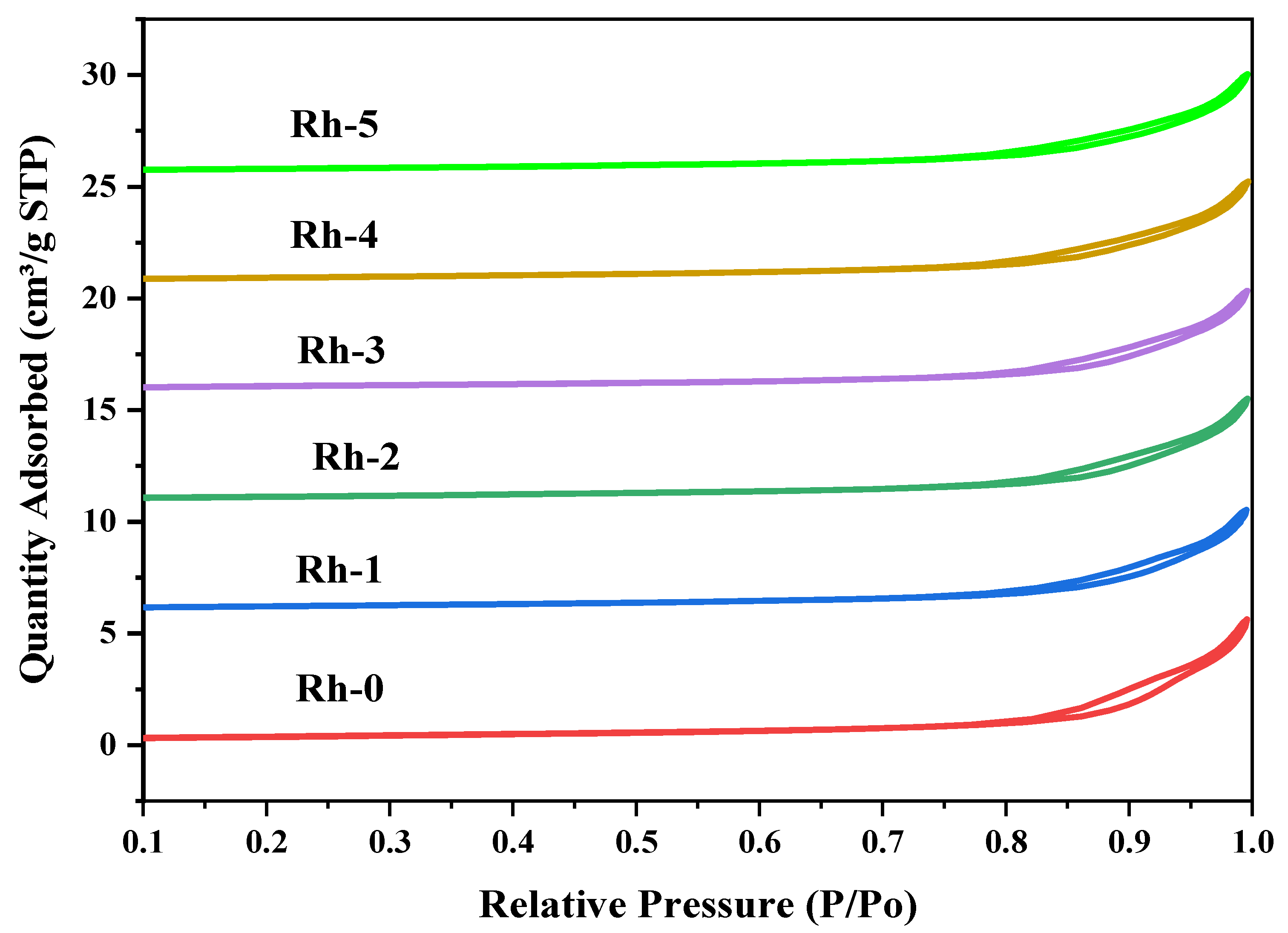
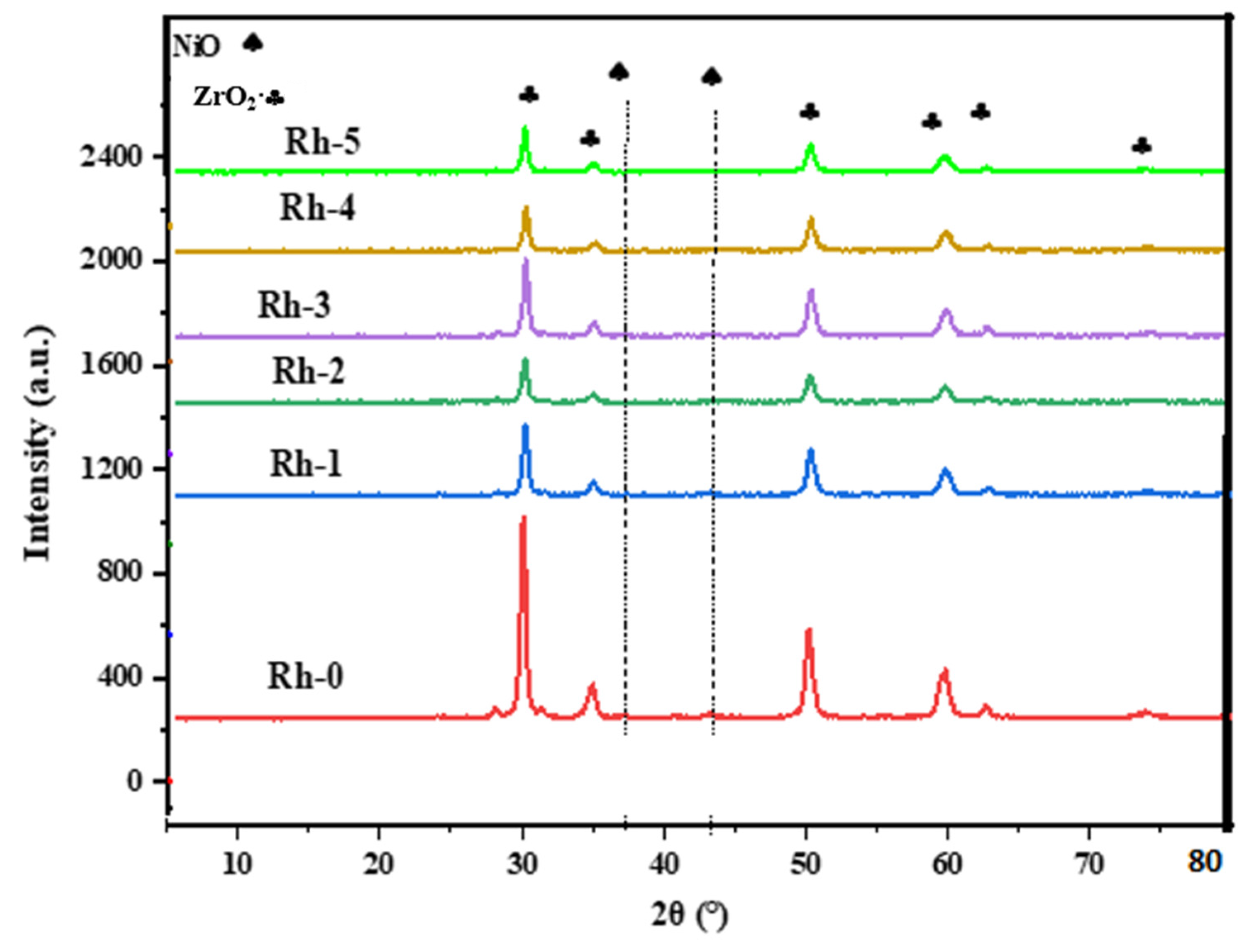

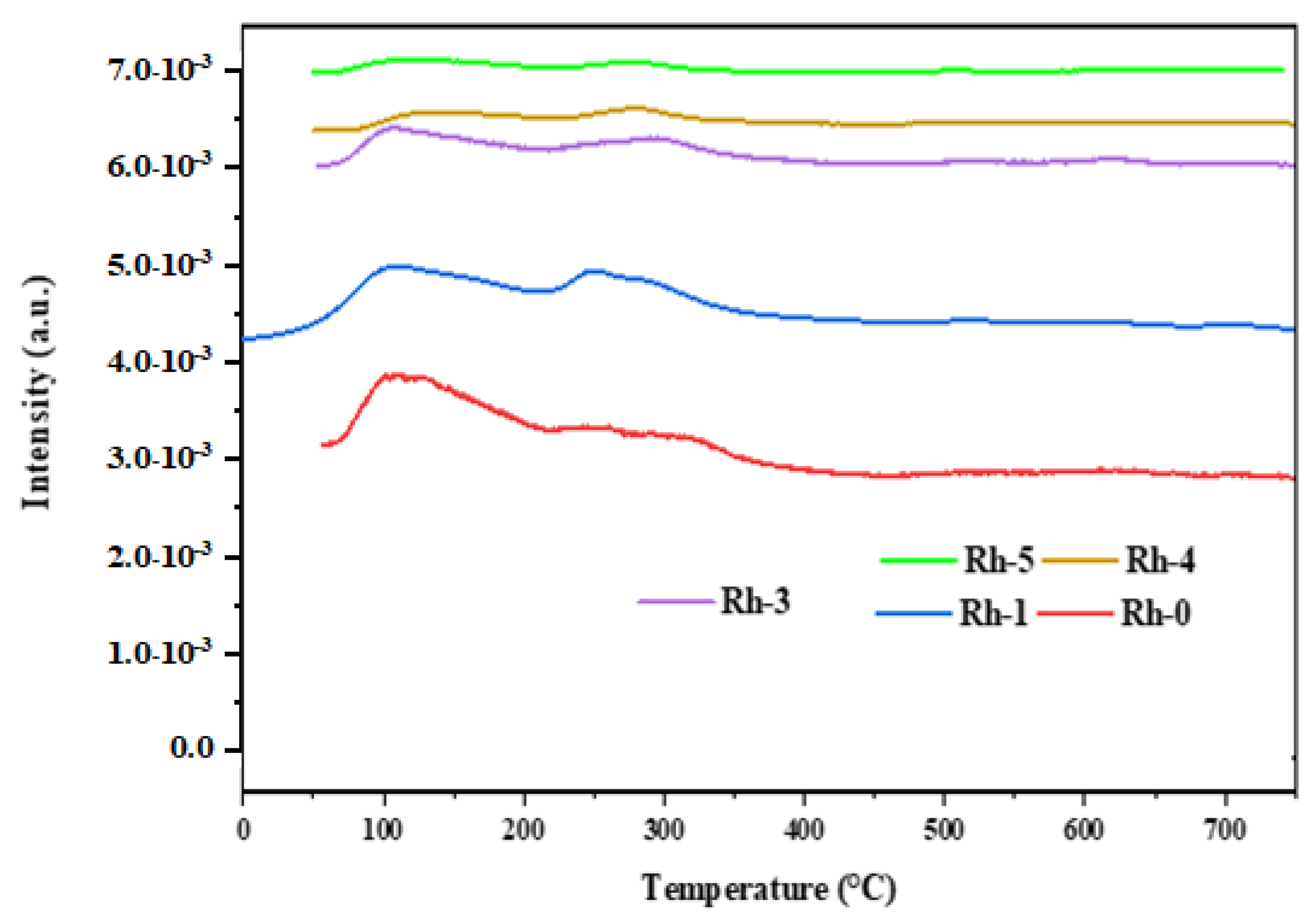
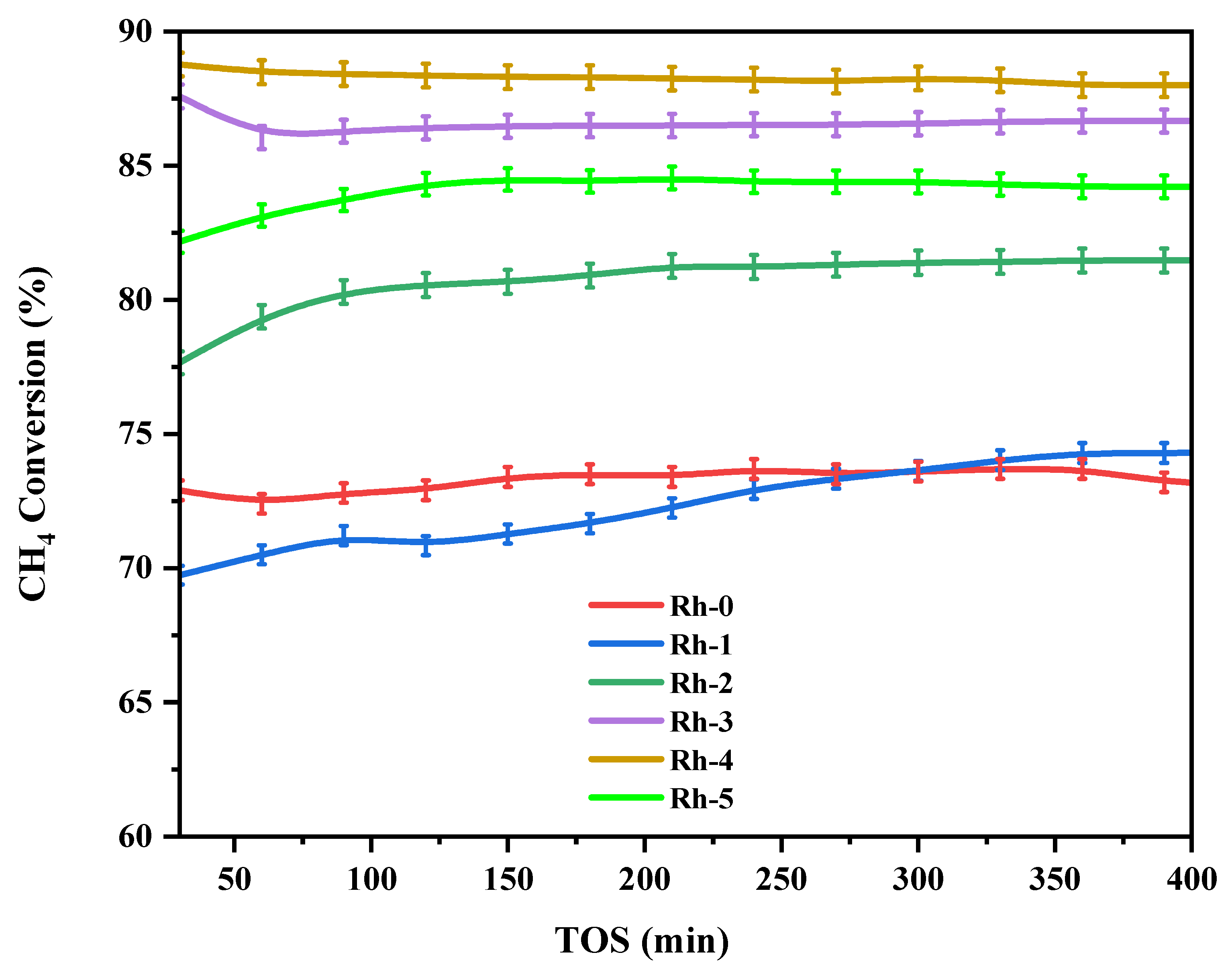
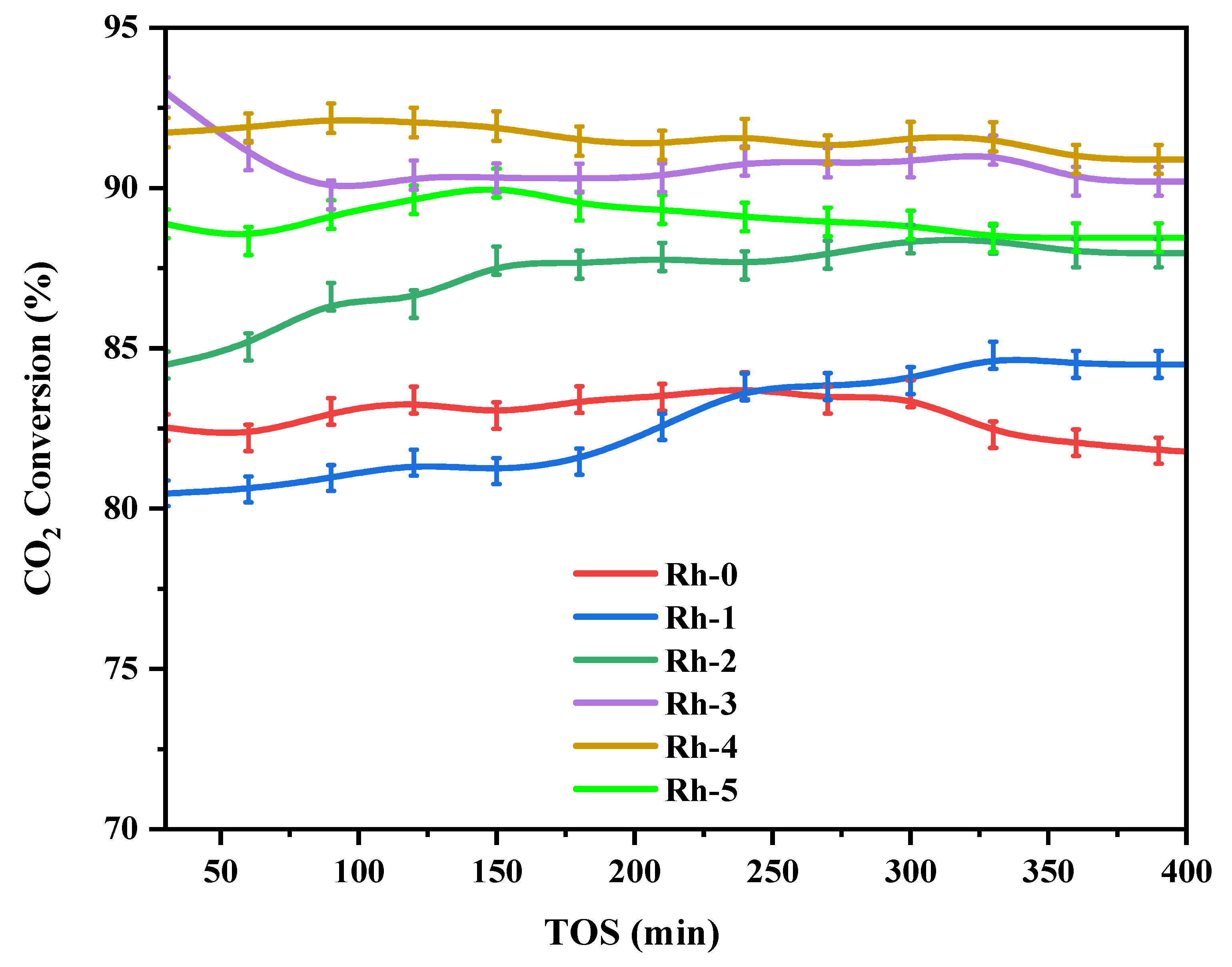
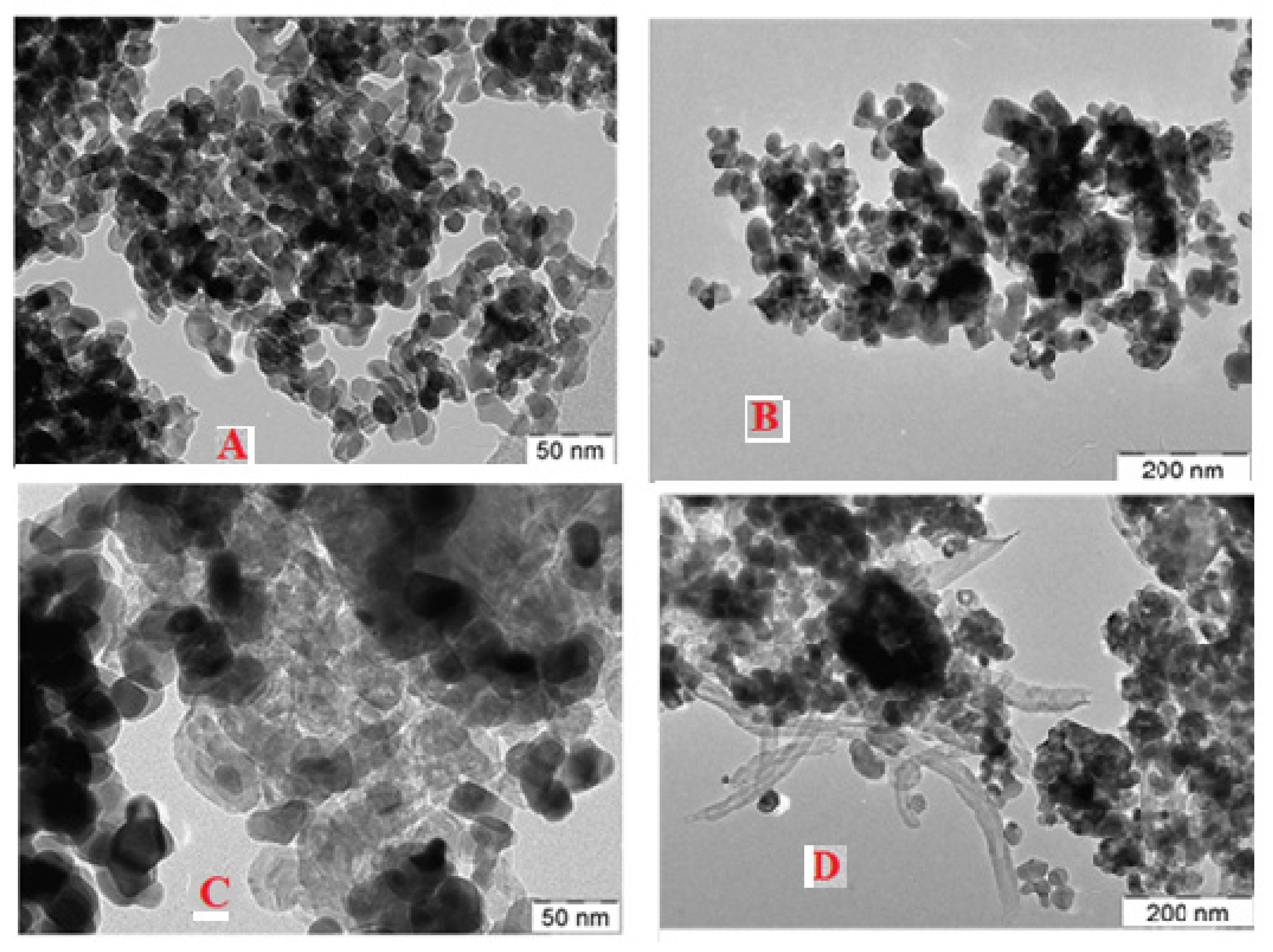
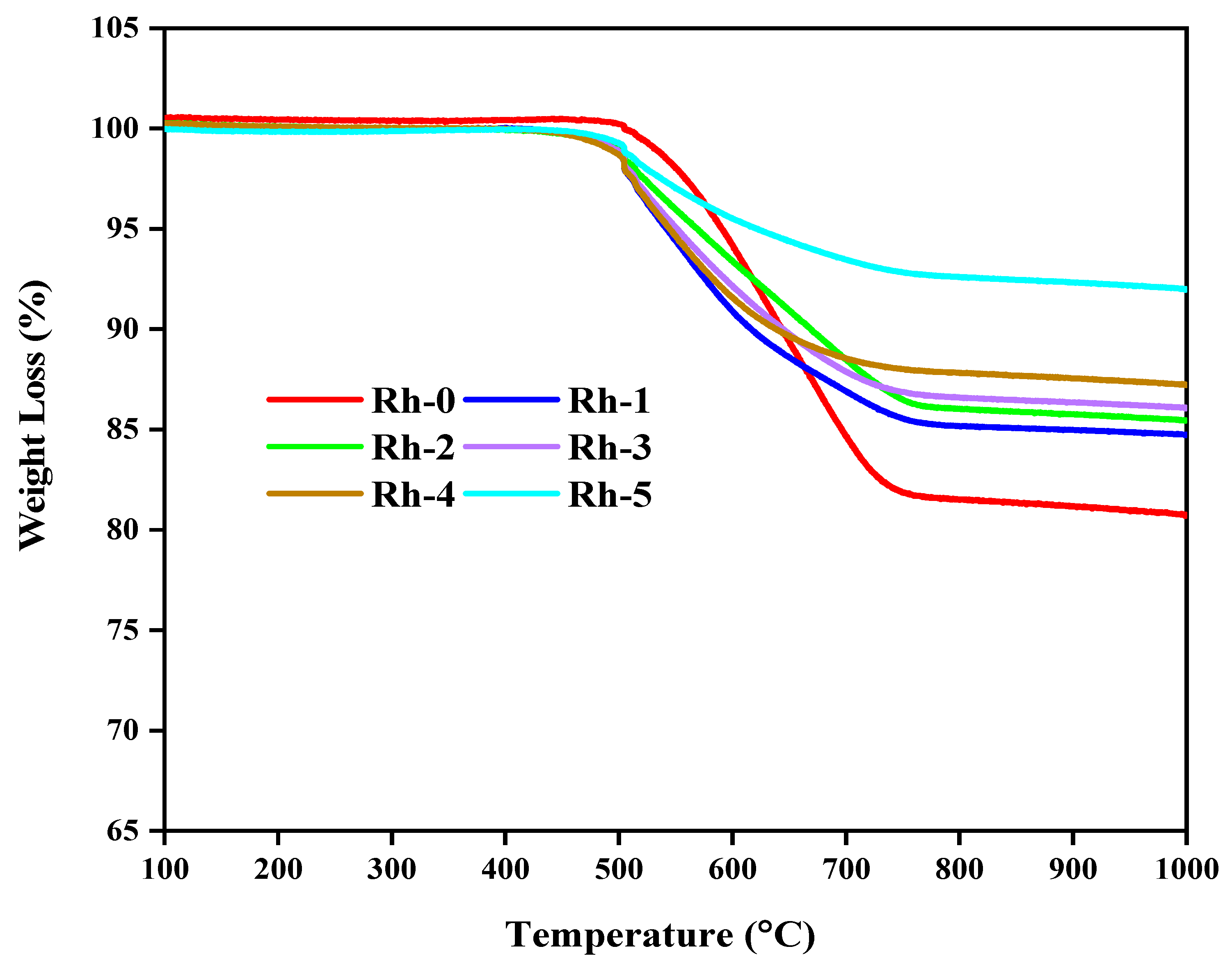
| Sample-Type | BET Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Rh-0 | 31 | 0.19 | 25.00 |
| Rh-1 | 27 | 0.15 | 23.00 |
| Rh-2 | 27 | 0.16 | 23.00 |
| Rh-3 | 27 | 0.15 | 24.00 |
| Rh-4 | 28 | 0.15 | 22.00 |
| Rh-5 | 27 | 0.15 | 22.00 |
| Sample | Description | H2 Consumption (mol/gcat) |
|---|---|---|
| Rh-0 | 5% Ni /8%Y2O3 + 92% ZrO2 | 0.68 |
| Rh-1 | 5% Ni+ 1% Rh/8% Y2O3 + 92% ZrO2 | 0.80 |
| Rh-2 | 5% Ni+ 2% Rh/8% Y2O3 + 92% ZrO2 | 0.90 |
| Rh-3 | 5% Ni+ 3% Rh/8% Y2O3 + 92% ZrO2 | 1.00 |
| Rh-4 | 5% Ni+ 4% Rh/8% Y2O3 + 92% ZrO2 | 1.00 |
| Rh-5 | 5% Ni+ 5% Rh/8% Y2O3 + 92% ZrO2 | 1.00 |
| Sample | Description | CO2 Consumption (μmol/gcat) |
|---|---|---|
| Rh-0 | 5% Ni /8%Y2O3 + 92% ZrO2 | 0.72 |
| Rh-1 | 5% Ni+ 1% Rh/8% Y2O3 + 92% ZrO2 | 0.8 |
| Rh-2 | 5% Ni+ 2% Rh/8% Y2O3 + 92% ZrO2 | 0.9 |
| Rh-3 | 5% Ni+ 3% Rh/8% Y2O3 + 92% ZrO2 | 1.0 |
| Rh-4 | 5% Ni+ 4% Rh/8% Y2O3 + 92% ZrO2 | 1.0 |
| Rh-5 | 5% Ni+ 5% Rh/8% Y2O3 + 92% ZrO2 | 1.0 |
| Catalyst | T (°C) | CH4/CO2 | CH4 Conversion (%) | Ref. |
|---|---|---|---|---|
| Ni-2.5%Ce/W-Zr | 700 | 1:1 | 78 | [44] |
| Ni-CeO2@SiO2 | 800 | 1:1 | 80 | [45] |
| Co-Ni/Sc-SBA-15 | 700 | 1:1 | 66 | [46] |
| Ni@SiO2 | 700 | 1:1 | 71 | [47] |
| Ni-Co/ZrO2-CaO + SiC | 800 | 1:1 | 97 | [48] |
| 5.6 wt.% Ni/Al2O3 + FY5 | 800 | 1:1 | 90 | [49] |
| 10%Ni/ Al2O3 − F | 700 | 1:1 | 72 | [50] |
| 20%Ni/ Al2O3 (mixed with biochar) | 800 | 1:1 | 79 | [51] |
| 5% Ni+ 4% Rh/8% Y2O3 + 92% ZrO2 | 800 | 1:1 | 89 | The present work |
| Catalyst | Thermodynamics Parameter | ||
|---|---|---|---|
| ΔH800, kJ/kmol | ΔS800, kJ/(kmol.K) | ΔG800, kJ/kmol | |
| Rh-0 | +136,845.2 | +54.4208 | +78,444 |
| Rh-1 | +137,654.4 | +54.5777 | +79,085 |
| Rh-2 | +145,049.0 | +56.2075 | +84,730 |
| Rh-3 | +150,385.0 | +57.2514 | +88,946 |
| Rh-4 | +151,860.1 | +57.5033 | +90,151 |
| Rh-5 | +148,103.0 | +56.8009 | +87,147 |
| Reaction | Keq (800 °C) | ||
| DRM: CH4(g) + CO2(g) = 2H2(g) + 2CO(g) | 151.348 | ||
| RWGS: CO2(g) + H2(g) = CO(g) + H2O(g) | 0.912605 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, J.; Al-Fatesh, A.S.; Ibrahim, A.A.; Frusteri, F.; Abasaeed, A.E.; Fakeeha, A.H.; Albaqi, F.; Anojaidi, K.; Alreshaidan, S.B.; Albinali, I.; et al. Stability and Activity of Rhodium Promoted Nickel-Based Catalysts in Dry Reforming of Methane. Nanomaterials 2023, 13, 547. https://doi.org/10.3390/nano13030547
Saleh J, Al-Fatesh AS, Ibrahim AA, Frusteri F, Abasaeed AE, Fakeeha AH, Albaqi F, Anojaidi K, Alreshaidan SB, Albinali I, et al. Stability and Activity of Rhodium Promoted Nickel-Based Catalysts in Dry Reforming of Methane. Nanomaterials. 2023; 13(3):547. https://doi.org/10.3390/nano13030547
Chicago/Turabian StyleSaleh, Jehad, Ahmed Sadeq Al-Fatesh, Ahmed Aidid Ibrahim, Francesco Frusteri, Ahmed Elhag Abasaeed, Anis Hamza Fakeeha, Fahad Albaqi, Khalid Anojaidi, Salwa B. Alreshaidan, Ibrahim Albinali, and et al. 2023. "Stability and Activity of Rhodium Promoted Nickel-Based Catalysts in Dry Reforming of Methane" Nanomaterials 13, no. 3: 547. https://doi.org/10.3390/nano13030547
APA StyleSaleh, J., Al-Fatesh, A. S., Ibrahim, A. A., Frusteri, F., Abasaeed, A. E., Fakeeha, A. H., Albaqi, F., Anojaidi, K., Alreshaidan, S. B., Albinali, I., Al-Rabiah, A. A., & Bagabas, A. (2023). Stability and Activity of Rhodium Promoted Nickel-Based Catalysts in Dry Reforming of Methane. Nanomaterials, 13(3), 547. https://doi.org/10.3390/nano13030547









