Primary Amine Functionalized Carbon Dots for Dead and Alive Bacterial Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Apparatus
2.3. Preparation and Modification of the Carbon Dots
2.4. Quantum Yield (QY) Measurement
2.5. Bacterial Culture and Preparation for Imaging
2.6. Stability Study of the Carbon Dots
2.7. Cytotoxicity Test
3. Results and Discussion
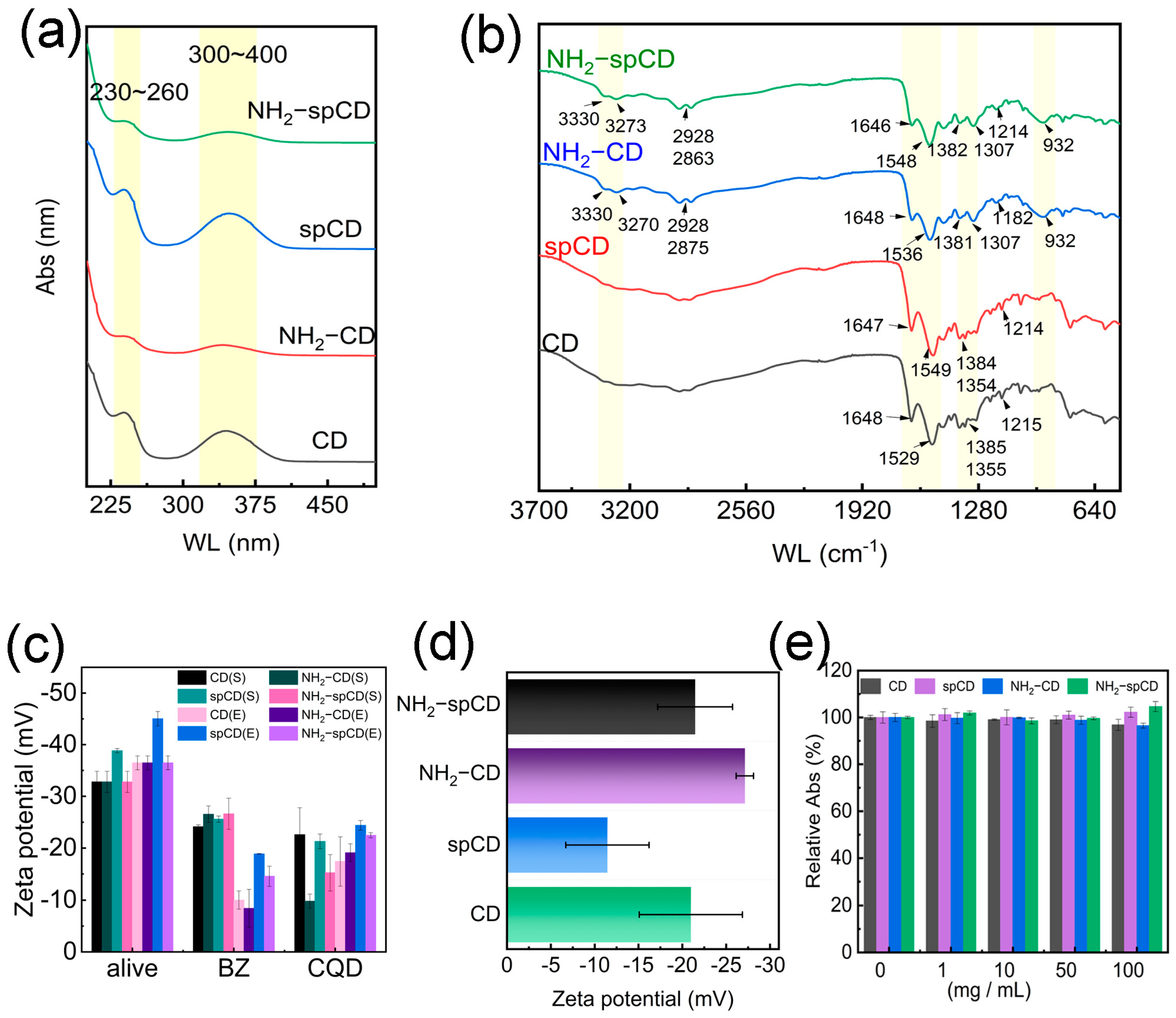
3.1. Fluorescent Characterization of Carbon Dots
3.2. Physical Structural Analysis of Carbon Dots
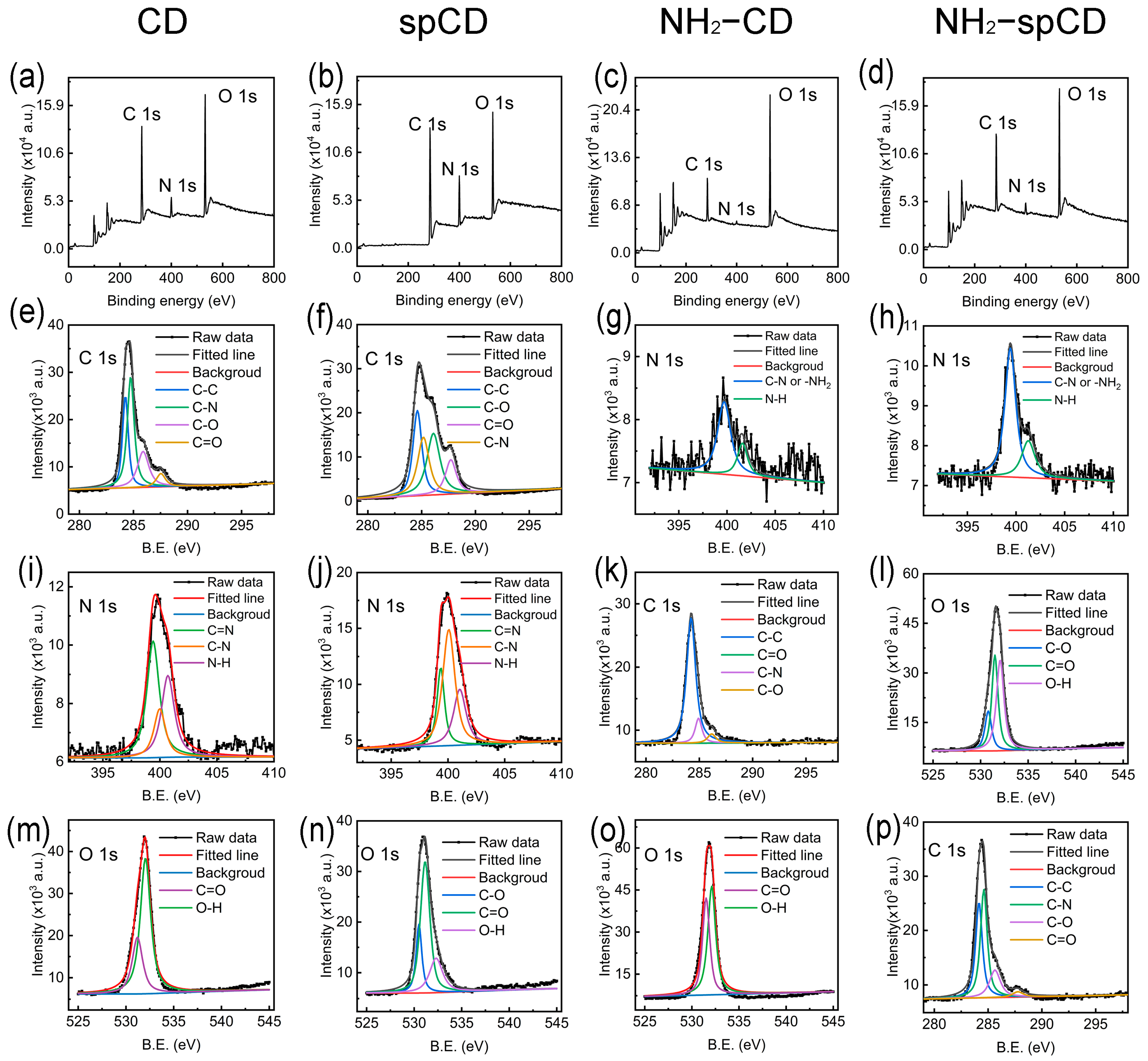
3.3. Chemical Structural Analysis of Carbon Dots
3.4. Bacterial Imaging by Carbon Dots
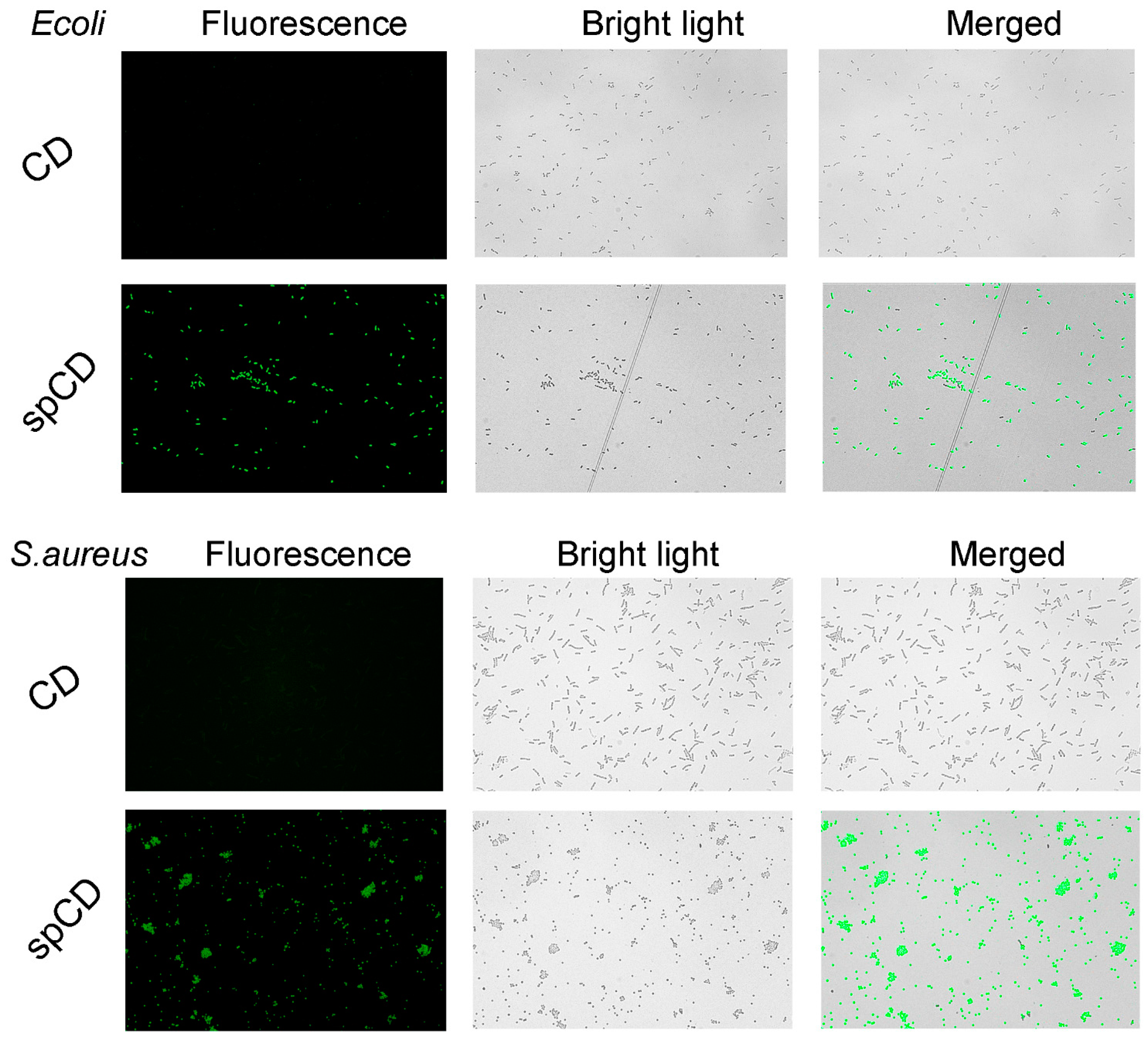
3.4.1. Imaging for Dead Bacteria
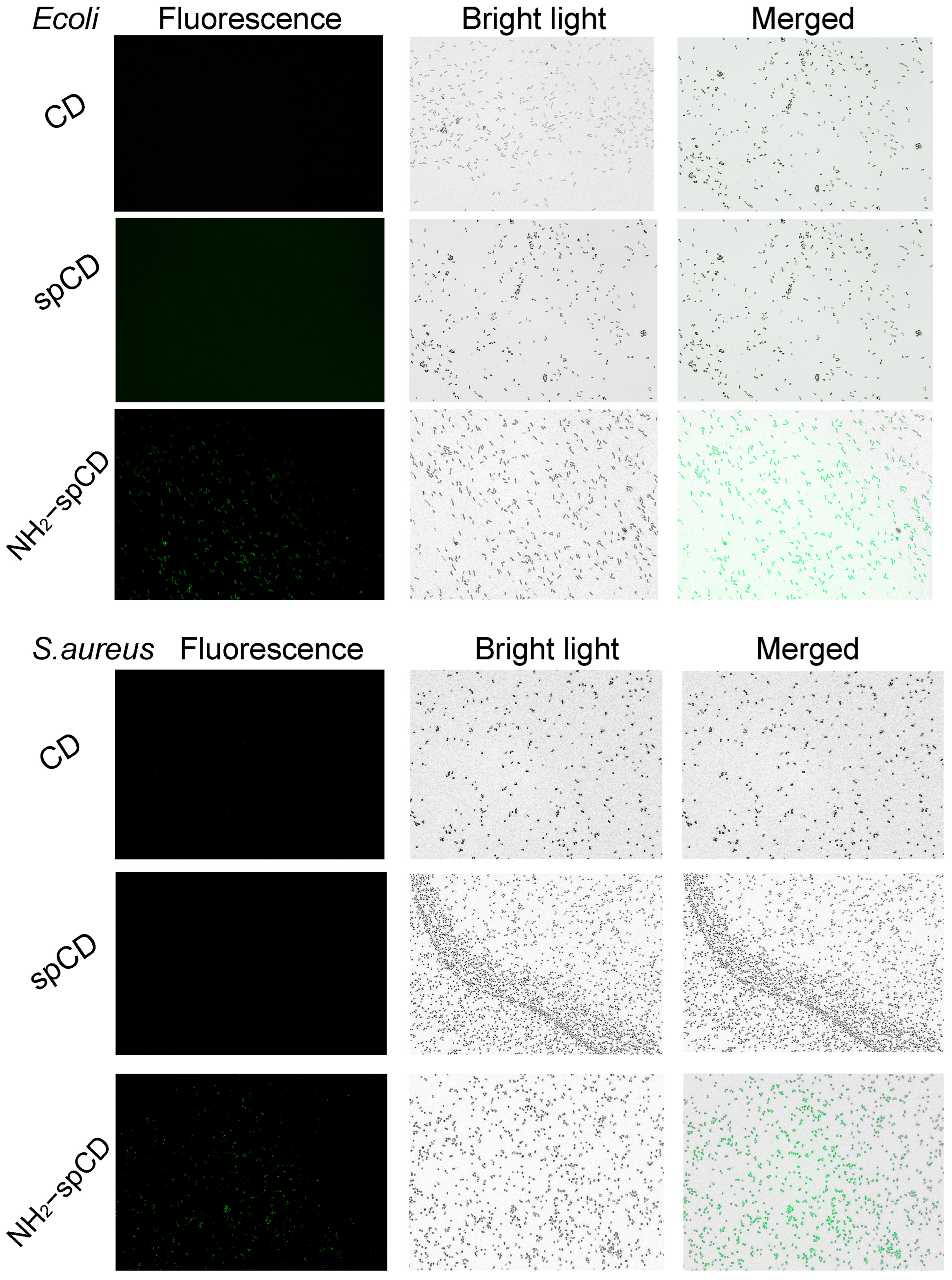
3.4.2. Live Bacterial Imaging
3.4.3. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, D.; Johani, K.; Melo, D.; Lopes, L.; Lima, L.L.; Tipple, A.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Deng, Y.; Zhang, X.; Liu, G.; Huang, Y.; Lin, C.; Li, J.; Yan, H.; Li, X.; Jia, L.; et al. Etiology of acute diarrhea due to enteropathogenic bacteria in Beijing, China. J. Infect. 2012, 65, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of foodborne disease outbreaks caused by clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiong, L.-H.; Zhao, Z.; Wang, Z.; Luo, L.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-based theranostic systems for detection and killing of pathogens. Theranostics 2019, 9, 3223–3248. [Google Scholar] [CrossRef]
- Hickey, M.E.; He, L. SERS imaging analyses of bacteria cells among plant tissues. Talanta 2021, 225, 122008. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Parker, M.F.L.; Flavell, R.R.; Luu, J.M.; Rosenberg, O.S.; Ohliger, M.A.; Wilson, D.M. Small molecule sensors targeting the bacterial cell wall. ACS Infect. Dis. 2020, 6, 1587–1598. [Google Scholar] [CrossRef]
- Vojnovic, I.; Winkelmeier, J.; Endesfelder, U. Visualizing the inner life of microbes: Practices of multi-color single-molecule localization microscopy in microbiology. Biochem. Soc. Trans. 2019, 47, 1041–1065. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, L.; Chen, Y.; Long, Y. Optimization of staining with SYTO 9/propidium iodide: Interplay, kinetics and impact on Brevibacillus brevis. Biotechniques 2020, 69, 88–98. [Google Scholar] [CrossRef]
- Marshall, A.P.; Shirley, J.D.; Carlson, E.E. Carlson Enzyme-targeted fluorescent small-molecule probes for bacterial imaging. Curr. Opin. Chem. Biol. 2020, 57, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, W.; Chung, J.; Yin, J.; Yoon, J. Recent progress in fluorescent probes for bacteria. Chem. Soc. Rev. 2021, 50, 7725–7744. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Pv, S.; Stanley, J.; Babu, T.G.S. Multicolor emitting N/S-doped carbon dots as a fluorescent probe for imaging pathogenic bacteria and human buccal epithelial cells. Microchim. Acta 2019, 186, 157. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Wang, Z.; Huang, X.; Li, J.; Yao, C.; Zhou, N.; Shen, J. Insight into the effect of particle size distribution differences on the antibacterial activity of carbon dots. J. Colloid Interface Sci. 2021, 584, 505–519. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, Y.-H.; Gao, G.; Chen, X.; Jia, H.-R.; Li, Y.-H.; Chen, Z.; Wu, F.-G. Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Interfaces 2016, 8, 32170–32181. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Zhang, R.; Huang, X.; Xiao, X. Facile one-pot synthesis of multifunctional protamine sulfate-derived carbon dots for antibacterial applications and fluorescence imaging of bacteria. N. J. Chem. 2021, 45, 1010–1019. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent development of carbon quantum dots: Biological toxicity, antibacterial properties and application in foods. Food Rev. Int. 2020, 38, 1513–1532. [Google Scholar] [CrossRef]
- AValizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Dong, H.; Sarkes, D.A.; Stratis-Cullum, D.N.; Hurley, M.M. Direct conjugation of fluorescent quantum dots with E. coli via surface-displayed histidine-containing peptides. Colloids Surf. B-Biointerfaces 2021, 203, 259–265. [Google Scholar] [CrossRef]
- Dong, Z.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Wang, C. Biocompatible AIE material from natural resources: Chitosan and its multifunctional applications. Carbohydr. Polym. 2020, 227, 115338. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.-W.; Bao, Y.-W.; Wang, H.-Y.; Chen, Z.; Wu, F.-G. Bacteria-derived fluorescent carbon dots for microbial live/dead differentiation. Nanoscale 2017, 9, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Wiraja, C.; Palanisamy, K.; Marsili, E.; Xu, C. Heat shock mediated labelling of Pseudomonas aeruginosa with quantum dots. Colloids Surf. B-Biointerfaces 2016, 142, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Song, Y.; Huang, H.; Liu, Y.; Fu, Y.; Huang, J.; Li, H.; Qu, H.; Kang, Z. Fluorescent carbon dots with tunable negative charges for bio-imaging in bacterial viability assessment. Carbon 2017, 120, 95–102. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Lu, F.; Wang, H.; Zhang, M.; Yang, J.; Huang, J.; Huang, H.; Liu, Y.; Kang, Z. Fluorescent carbon dots with highly negative charges as a sensitive probe for real-time monitoring of bacterial viability. J. Mater. Chem. B 2017, 5, 6008–6015. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Hou, T.; Guan, P.; Qiao, Y.; Guo, L.; Teng, Y.; Hu, X.; Wu, H. Lasting tracking and rapid discrimination of live gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl. Mater. Interfaces 2021, 13, 1277–1287. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Dong, L.; Fu, D.; Chen, Z. Imaging biofilm-encased microorganisms using carbon dots derived from L.plantarum. Nanoscale 2017, 9, 9056–9064. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Q.; Zhu, S.; Bu, W.; Yang, M.; Liu, X.; Meng, Y.; Yu, W.; Sun, H.; Yang, B. Controllable acidophilic dual-emission fluorescent carbonized polymer dots for selective imaging of bacteria. Nanoscale 2019, 11, 9526–9532. [Google Scholar] [CrossRef]
- Fu, C.-C.; Wu, C.-Y.; Chien, C.-C.; Hsu, T.-H.; Ou, S.-F.; Chen, S.-T.; Wu, C.-H.; Hsieh, C.-T.; Juang, R.-S.; Hsueh, Y.-H. Polyethylene glycol(6000)/carbon nanodots as fluorescent bioimaging agents. Nanomaterials 2020, 10, 677. [Google Scholar] [CrossRef]
- Apaydın, S.; Török, M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042–2050. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. Beta-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Jovanović, S.P.; Mašković, P.Z.; Danko, M.; Mičušík, M.; Pavlović, V.B.; Milivojević, D.D.; Kleinová, A.; Špitalský, Z.; Marković, B.M.T. Photo-induced antibacterial activity of four graphene based nanomaterials on a wide range of bacteria. RSC Adv. 2018, 8, 31337–31347. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.G.; Robby, A.I.; Phuong, P.T.M.; In, I.; Park, S.Y. Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 97, 613–623. [Google Scholar] [CrossRef] [PubMed]
- ravlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S- and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Yu, X.-W.; Liu, X.; Jiang, Y.-W.; Li, Y.-H.; Gao, G.; Zhu, Y.-X.; Lin, F.; Wu, F.-G. Rose bengal-derived ultrabright sulfur-doped carbon dots for fast discrimination between live and dead cells. Anal. Chem. 2022, 94, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Gagic, M.; Kociova, S.; Smerkova, K.; Michalkova, H.; Setka, M.; Svec, P.; Pribyl, J.; Masilko, J.; Balkova, R.; Heger, Z.; et al. One-pot synthesis of natural amine-modified biocompatible carbon quantum dots with antibacterial activity. J. Colloid Interface Sci. 2020, 580, 30–48. [Google Scholar] [CrossRef]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- HJian, H.-J.; Yu, J.; Li, Y.-J.; Unnikrishnan, B.; Huang, Y.-F.; Luo, L.-J.; Ma, D.H.-K.; Harroun, S.G.; Chang, H.-T.; Lin, H.-J.; et al. Highly adhesive carbon quantum dots from biogenic amines for prevention of biofilm formation. Chem. Eng. J. 2020, 386, 123913. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Lu, C.-D. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob. Agents Chemother. 2007, 51, 2070–2077. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Lin, F.; Guo, Y.; Wu, F. One-step synthesis of epoxy group-terminated organosilica nanodots: A versatile nanoplatform for imaging and eliminating multidrug-resistant bacteria and their biofilms. Small 2019, 15, 1901647. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.-H.; Chen, X.; Wu, F.-G. One-step synthesis of carbon dots with bacterial contact-enhanced fluorescence emission: Fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon 2019, 146, 827–839. [Google Scholar] [CrossRef]
- Li, D.; Sun, P.-P.; Li, J.-G.; Yan, S.-S.; Wang, D.; Tang, B.T. Recent advances of polymers with aggregation-induced emission characteristics in phototheranostics. Acta Polym. Sin. 2022, 53, 856–872. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Tan, J.; Chang, Z.; Liu, X.; Ma, W.; Xu, Y. Near-infrared regulated nanozymatic/photothermal/photodynamic triple-therapy for combating multidrug-resistant bacterial infections via oxygen-vacancy molybdenum trioxide nanodots. Small 2021, 17, e2005739. [Google Scholar] [CrossRef] [PubMed]
- Mandani, S.; Rezaei, B.; Ensafi, A.A.; Sabzalian, M.R. Development of a new simple spectroscopic determination coupled acid-motivated delivery system based on fluorescence turn-off MSNs@MPA-ZnS QDs for infection. Microporous Mesoporous Mater. 2021, 317, 110971. [Google Scholar] [CrossRef]
- Niu, F.; Xu, Y.; Liu, J.; Song, Z.; Liu, M.; Liu, J. Controllable electrochemical/electroanalytical approach to generate nitrogen-doped carbon quantum dots from varied amino acids: Pinpointing the utmost quantum yield and the versatile photoluminescent and electrochemiluminescent applications. Electrochim. Acta 2017, 236, 239–251. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Zhang, G.; Yang, X.; Cao, W.; Gong, X.-D.; Xing, X. Design of pH-responsive dissociable nanosystem based on carbon dots with enhanced anti-biofilm property and excellent biocompatibility. ACS Appl. Bio Mater. 2020, 3, 1105–1115. [Google Scholar] [CrossRef]
- Hirsch, J.G. The antimycobacterial activity of various amines related to spermine in chemical structure. J. Exp. Med. 1953, 97, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Lotha, R.; Shamprasad, B.R.; Sundaramoorthy, N.S.; Nagarajan, S.; Sivasubramanian, A. Biogenic phytochemicals (cassinopin and isoquercetin) capped copper nanoparticles (ISQ/CAS@CuNPs) inhibits MRSA biofilms. Microb. Pathog. 2019, 132, 178–187. [Google Scholar] [CrossRef]
- DShan, D.; Hsieh, J.-T.; Bai, X.; Yang, J. Citrate-based fluorescent biomaterials. Adv. Healthc. Mater. 2018, 7, 1800532. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Li, B.; Fan, X.; Wang, W.; Liu, P.; Xu, S.; Luo, X. Nitrogen doped carbon dots: Mechanism investigation and their application for label free CA125 analysis. J. Mater. Chem. B 2019, 7, 3053–3058. [Google Scholar] [CrossRef]
- Xu, Q.; Li, B.; Ye, Y.; Cai, W.; Li, W.; Yang, C.; Chen, Y.; Xu, M.; Li, N.; Zheng, X.; et al. Synthesis, mechanical investigation, and application of nitrogen and phosphorus co-doped carbon dots with a high photoluminescent quantum yield. Nano Res. 2018, 11, 3691–3701. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.-C. Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Bose, M.; Ganguly, S.; Mondal, S.; Das, A.K.; Banerjee, S.; Das, N.C. Green approach to photoluminescent carbon dots for imaging of gram-negative bacteria Escherichia coli. Nanotechnology 2017, 28, 195501. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 213, 434–443. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chen, H.-H.; Chen, S.-Y.; Chang, C.-Y.; Chen, P.-C.; Hou, Y.-I.; Shao, Y.-T.; Kao, H.-F.; Hsu, C.-L.L.; Chen, Y.-C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Lin, F.; Li, C.; Chen, Z. Bacteria-derived carbon dots inhibit biofilm formation of Escherichia coli without affecting cell growth. Front. Microbiol. 2018, 9, 259. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, F.; Song, J.; Luo, Q.; Yang, Y.; Mei, C.; Xu, M.; Liao, B. Function-oriented graphene quantum dots probe for single cell in situ sorting of active microorganisms in environmental samples. Front. Microbiol. 2021, 12, 659111. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Yeh, T.-S.; Chang, C.-Y.; Liu, J.-C.; Chen, C.-H.; So, E.C.; Wu, P.-C. Amino-functionalized nitrogen-doped graphene quantum dots for efficient enhancement of two-photon-excitation photodynamic therapy: Functionalized nitrogen as a bactericidal and contrast agent. Int. J. Nanomed. 2020, 15, 6961–6973. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Wang, Y.; Xiong, Z.; Zhao, X.; Xia, Y. Chitosan-derived N-doped carbon dots for fluorescent determination of nitrite and bacteria imaging. Spectrochim. Acta A 2021, 251, 119468. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, D.; Wan, Y.; Yang, Y. One-step synthesis of carbon dots for selective bacterial inactivation and bacterial differentiation. Anal. Bioanal. Chem. 2020, 412, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.-H.; Cheng, X.; Bao, Y.-W.; Hua, X.-W.; Gao, G.; Zhang, X.; Jiang, Y.-W.; Zhu, Y.-X.; Wu, F.-G. Multifunctional quaternized carbon dots with enhanced biofilm penetration and eradication efficiencies. J. Mater. Chem. B 2019, 7, 5104–5114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, R.; Liu, X.; Huang, X.; Xiao, X.; Yuan, L. One-step synthesis of blue-green luminescent carbon dots by a low-temperature rapid method and their high-performance antibacterial effect and bacterial imaging. Nanotechnology 2021, 32, 155101. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Gao, X.; Shi, Y.; Cheng, C.; Shi, Z.; Jiao, M.; Cao, F.; Xu, Z.; Li, X.-M.; Zhang, J. Augmented graphene quantum dot-light irradiation therapy for bacteria-infected wounds. ACS Appl. Mater. Interfaces 2020, 12, 40153–40162. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Han, F.; Cao, W.; Zhang, G.; Li, J.; Zhou, J.; Gong, X.; Turnbull, G.; Shu, W.; Xia, L.; et al. Carbon quantum dots derived from lysine and arginine simultaneously scavenge bacteria and promote tissue repair. Appl. Mater. Today 2020, 19, 100601. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Wen, Z.; Zhou, J.; Wang, X.; Wu, Q.; Wang, C. Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron(III) detection and multicolor bioimaging. J. Colloid Interface Sci. 2018, 521, 33–41. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, H.; Yang, Y.; Zhu, Y.; Chen, X.; Sun, J.; Yu, K.; Fan, H.; Zhang, X. Investigation of luminescent mechanism: N-rich carbon dots as luminescence centers in fluorescent hydroxyapatite prepared using a typical hydrothermal process. J. Mater. Chem. B 2017, 5, 3749–3757. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Bouckaert, J.; Dumitrascu, N.; Szunerits, S.; Boukherroub, R. Enhanced antibacterial activity of carbon dots functionalized with ampicillin combined with visible light triggered photodynamic effects. Colloids Surf. B-Biointerfaces 2018, 170, 347–354. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Rodríguez, C.; Chen, A.; Rivera, J.A.; Mohr, M.A.; Liang, Y.; Natan, R.G.; Sun, W.; Milkie, D.E.; Bifano, T.G.; Chen, X.; et al. An adaptive optics module for deep tissue multiphoton imaging in vivo. Nat. Methods 2021, 18, 1259–1264. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Winckler, P.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Shedding Light on the Formation and Structure of Kombucha Biofilm Using Two-Photon Fluorescence Microscopy. Front. Microbiol. 2021, 12, 725379. [Google Scholar] [CrossRef] [PubMed]



| Types | Dead 1 | Dead 2 | Alive | QY/% | Average Size/nm | Average Zeta Potential/mV | Functional Groups/Percursors |
|---|---|---|---|---|---|---|---|
| CD | ✓ | —— 3 | —— | 48.97% | 2.01 | −21.0 | |
| spCD | ✓ | ✓ | —— | 66.46% | 1.38 | −11.5 | Spermine |
| NH2–CD | ✓ | —— | —— | 40.42% | 1.99 | −27.1 | Primary amine |
| NH2–spCD | ✓ | ✓ | ✓ | 53.47% | 2.36 | −21.5 | Spermine/ primary amine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhong, D.; Yu, L.; Shi, Y.; Xu, Y. Primary Amine Functionalized Carbon Dots for Dead and Alive Bacterial Imaging. Nanomaterials 2023, 13, 437. https://doi.org/10.3390/nano13030437
Liu Y, Zhong D, Yu L, Shi Y, Xu Y. Primary Amine Functionalized Carbon Dots for Dead and Alive Bacterial Imaging. Nanomaterials. 2023; 13(3):437. https://doi.org/10.3390/nano13030437
Chicago/Turabian StyleLiu, Yuting, Di Zhong, Lei Yu, Yanfeng Shi, and Yuanhong Xu. 2023. "Primary Amine Functionalized Carbon Dots for Dead and Alive Bacterial Imaging" Nanomaterials 13, no. 3: 437. https://doi.org/10.3390/nano13030437
APA StyleLiu, Y., Zhong, D., Yu, L., Shi, Y., & Xu, Y. (2023). Primary Amine Functionalized Carbon Dots for Dead and Alive Bacterial Imaging. Nanomaterials, 13(3), 437. https://doi.org/10.3390/nano13030437






