Abstract
Flame spray pyrolysis (FSP) is an industrially scalable technology that enables the engineering of a wide range of metal-based nanomaterials with tailored properties nanoparticles. In the present review, we discuss the recent state-of-the-art advances in FSP technology with regard to nanostructure engineering as well as the FSP reactor setup designs. The challenges of in situ incorporation of nanoparticles into complex functional arrays are reviewed, underscoring FSP’s transformative potential in next-generation nanodevice fabrication. Key areas of focus include the integration of FSP into the technology readiness level (TRL) for nanomaterials production, the FSP process design, and recent advancements in nanodevice development. With a comprehensive overview of engineering methodologies such as the oxygen-deficient process, double-nozzle configuration, and in situ coatings deposition, this review charts the trajectory of FSP from its foundational roots to its contemporary applications in intricate nanostructure and nanodevice synthesis.
1. Introduction
Flame spray pyrolysis (FSP) is an industrially scalable technique for the synthesis of nanomaterials, which allows to produce many types of metal, or metal-oxide nanoparticles with tailored physicochemical properties [1]. At the heart of the FSP technology lies an intricate process design, involving precursor atomization, combustion, and nanoparticle formation [2]. This enables swift, single-step synthesis, eliminating the need for post-production treatments commonly required in other methodologies. Recent advancements have further elevated the prominence of FSP in the development of nanodevices, i.e., where nanoparticles can be in situ incorporated in complex functional arrays [3]. Thus, FSP not only revolutionizes nanomaterials’ production but, with recent innovations, also paves the way for the next generation of nanodevices [4]. In the present review article, we provide an updated overview of the current state-of-the-art in FSP technology regarding novel reactor and process designs, novel material production, and nanodevice engineering.
In the domain of nanotechnology, nanostructure synthesis represents a critical research area, encompassing a diverse range of methodologies alongside FSP. These alternative techniques, including, but not limited to, chemical vapor deposition, sol-gel processing, and electrospinning, offer unique properties in terms of particle size control, morphology, and chemical composition. The selection of an appropriate synthesis method is contingent upon a set of criteria closely related to the intended application of the nanostructures. Factors such as material versatility, environmental impact, synthesis time, and temperature range play a pivotal role in determining the suitability of a technique for specific applications, which vary from drug delivery systems to photovoltaic devices. In Table 1, we enumerate various methodologies employed in the synthesis of nanomaterials, including FSP, and delineate the specific criteria applicable to their utilization. This careful consideration ensures that the synthesis process aligns with the functional requirements of the end application, thereby maximizing the efficacy and utility of the nanostructures produced. Atomic layer deposition (ALD) [5,6] is an alternative to FSP, particularly for engineering thin films. ALD provides atomic-level precision and high-level conformality, producing highly uniform and defect-free films. The downsides include a slower deposition rate, the need for expensive and controlled-environment equipment, and potentially high costs for precursors [5,6].

Table 1.
The literature summary on various techniques for synthesizing nanostructures and the criteria for their application.
1.1. Integration of Flame Spray Pyrolysis into the Technology Readiness Level (TRL) Scale for Nanomaterial Production
FSP stands out as an innovative and advanced methodology for the synthesis of nanomaterials, which highlights its vital role in producing a wide array of metal oxide nanoparticles with tailored morphologies and compositions [24]. Inherent in FSP, synthesis at elevated temperatures enhances both the crystallinity and physicochemical attributes of the nanoparticles. By adeptly adjusting operational parameters, such as precursor solution concentration, solvent type, flame temperature, oxygen-to-fuel ratio, and particle residence time in the flame zone, researchers can effectively control the nanoparticle size, distribution, and phase composition. Regarding the technology readiness level (TRL) spectrum, FSP for device applications aligns with TRL 6–8 [25]. This placement signifies FSP’s evolution beyond foundational laboratory research [1]. Given the adoption of FSP by certain industries, this positions FSP in the late stages of development and early stages of commercialization, placing it in the TRL 7–8 range (see Figure 1) [25].
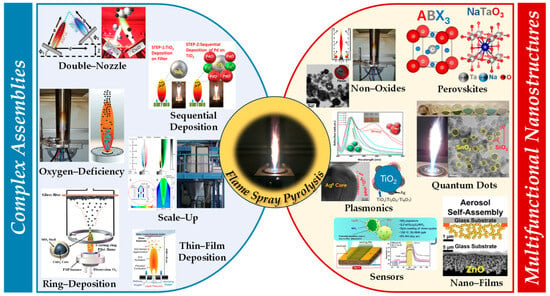
Figure 1.
Figure illustrating the complex assemblies in flame spray pyrolysis (FSP) discussed in this review. These include double-nozzle, sequential deposition, oxygen-deficiency process, ring deposition, sequential/thin-film deposition, and scale-up methods. The resultant advanced nanomaterials/nanodevices encompass perovskites, non-oxides, quantum dots, plasmonics, nanofilms, and sensors.
1.2. Process Design
The process design of FSP [26] begins with the preparation of a metal or metal-organic precursor solution, typically dissolved in an appropriate solvent. This solution undergoes atomization, often facilitated by a high-pressure nozzle, to form a fine spray of droplets [2]. Subsequent ignition of these droplets, often with the aid of an auxiliary flame, leads to the combustion of the solvent and the eventual decomposition of the metal precursors. Within this flame environment, characterized by elevated temperatures, the precursor decomposes, and metal or metal-oxide nanoparticles nucleate and grow [27]. The characteristics of the resultant nanoparticles—size, morphology, crystallinity, and phase composition—can be controlled by diligent choice of the metal precursor, its concentration in the solution, the solvent’s nature, the atomization method, flame temperature, oxygen-to-fuel ratio, and the residence time of particles within the flame. Both inorganic and organic metal salts—including nitrates, acetates, and 2-ethylhexanoates—as well as metalorganic compounds such as acetylacetonates or alkoxides, serve as prevalent precursors [28]. These compounds are soluble in organic solvents, notably xylene (with a standard enthalpy change in combustion [29], = −4550 kJ mol−1), toluene (−3910 kJ mol−1), ethanol (−1376 kJ mol−1), acetonitrile (−1256 kJ mol−1), etc. Another pivotal aspect of the FSP design is its continuous mode of operation that enhances its scalability potential, making it an attractive proposition for industrial applications.
Furthermore, the high-temperature synthesis environment ensures rapid crystallization of particles, obviating the need for post-process annealing. Meierhofer et al. [1] delineated the relationship between temperature and process residence time during each phase of the droplet-to-particle formation, as represented by the red line in Figure 2a and the flame temperature profile in Figure 2b. At the nozzle’s apex, temperature fluctuations range from 500 to 400 °C within the initial 10 μs. Adjacent to the capillary tip, the flame’s core registers the peak temperatures, oscillating between 3500 and 2500 °C (Figure 2). At this juncture, the precursor solution vaporizes, initiating the nucleation of the primary particles. Following initial particle formation, particles fuse cohesively within the temperature range of 1700–600 °C during coagulation and sintering processes. Driven by Brownian motion, these particles collide and coalesce, forming larger entities. As the sintered particles move further through the flame and into cooler regions (<600 °C), they can stick together into agglomerates, forming loose clusters. This clustering is due to physical forces, such as van der Waals interactions. Subsequently, these agglomerates transform into aggregates, binding more firmly through chemical (covalent) bonds in the temperature range of 400–200 °C. The FSP setup typically comprises components like liquid atomizers, combustion chambers, flame torches, and substrate holders for potential direct deposition of nanoparticles.
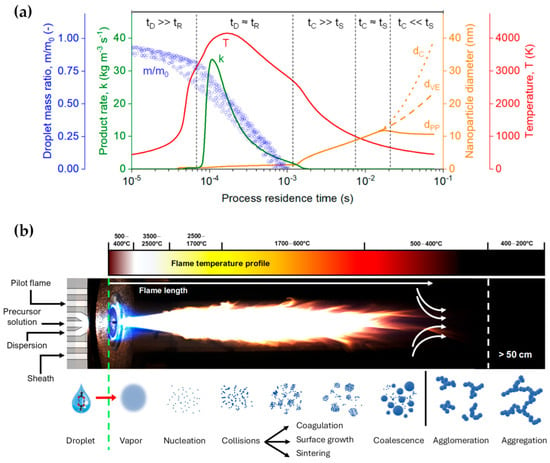
Figure 2.
(a) Temporal scales in the fabrication of ZrO2 nanoparticles via FSP. A time-evolving analysis encompasses the dynamics of the droplet mass ratio, the rate of product formation, nanoparticle diameter, and gas temperature, serving to demarcate distinct phases within the manufacturing process. Reprinted (adapted) with permission from [1]. Copyright 2021 American Chemical Society. (b) Visualization of actual FSP flame, depicting the synthesis parameters (pilot flame, precursor solution, dispersion, sheath gas). Concurrently, a graphical representation of the flame’s temperature distribution, congruent with that depicted in (a), is presented. Below the flame, a comprehensive elucidation of the droplet-to-particle transformation process in the production of nanoparticles is provided.
1.3. Recent Advancements in Product/Nanodevice Development
Figure 3 provides a chronological tracing of the literature articles related to FSP, highlighted by pertinent reviews. Introduced in the 1970s by Sokolowski et al. [30], FSP was utilized for synthesizing Al2O3 nanoparticles from an aluminum acetylacetonate precursor in a benzene-ethanol solution via an ultrasound nozzle. Despite the initial decline in interest, the technique was refined in the 1990s by Laine and colleagues at the University of Michigan [7,31]. In a pilot-scale FSP reactor, a double-alkoxide (Mg-Al) precursor in an alcoholic solution was employed to yield spinel MgAl2O4 nanoparticles at rates between 50 and 100 g/h. Concurrently, scientists at Tampere University of Technology employed the FSP method for various metal oxide syntheses and conducted detailed optical diagnostics on the produced aerosols [32]. By the commencement of the 21st century, Pratsinis’s team at the Swiss Federal Institute of Technology in Zürich further adapted FSP, highlighting its potential in catalytic material development [33].
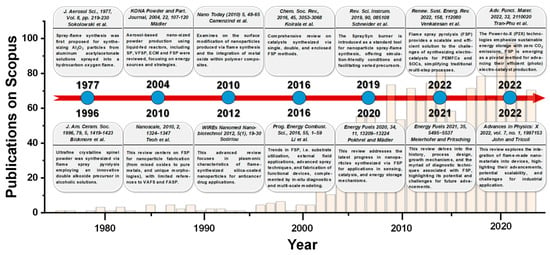
Figure 3.
Timeline of the flame spray pyrolysis (FSP) technology, and some pertinent review articles. The bar graph depicts the annual publication frequency (1365 documents in total) from 1977 to 2023, sourced from Scopus using the keyword ‘Flame Spray Pyrolysis’ [1,2,30,31,34,35,36,37,38,39,40,41,42,43].
Mädler’s review in 2004 [34] emphasized the increasing use of liquid-fed reactors for the aerosol-based synthesis of nano-sized powders. The rising interest in aerosol processes and the growing demand for various functional metal oxides have accelerated the R&D of these reactors. He examined four primary spray techniques: spray pyrolysis in a tubular reactor (SP), vapor flame reactor spray pyrolysis (VFSP), emulsion combustion method (ECM), and flame spray pyrolysis (FSP), comparing their energy sources and reaction mechanisms. He also outlined methods to produce consistent products and their specific applications [34]. In 2010, Teoh and colleagues [2] presented an exhaustive review focusing on FSP as a method for nanoparticle synthesis, spanning from mixed oxides to pure metals and encompassing specialized morphologies, such as core-shell structures, with minimal references to VAFS and FASP. Conversely, Camenzind and associates [35] delve into the surface functionalization of nanoparticles generated through flame synthesis and the incorporation of metal oxide within polymer composites. Moreover, in 2013, Sotiriou [36] provided an in-depth review emphasizing the plasmonic properties of flame-synthesized silica-coated nanoparticles and their potential applications in anticancer drug delivery.
Koirala et al. in 2016 [37] conducted a thorough examination of catalysts produced through single, double, and enclosed FSP techniques. In the same year, Li and his colleagues [38] detailed advancements in FSP, encompassing substrate usage, applications of external fields, innovative spray methodologies, and the construction of functional apparatus, supplemented by in situ diagnostics and multi-scale simulations. In 2019, Schneider et al. [39] presented the SpraySyn burner as a benchmark instrument for the spray-flame synthesis of nanoparticles. This apparatus offers conditions amenable to simulation and accommodates a variety of precursors. One year later, Pokhrel and Mädler’s review [40] outlined recent advancements in nanoparticles produced through FSP for sensing, catalysis, and energy storage applications, while Meierhofer and Fritsching in 2021 [1] provided a detailed analysis of FSP’s historical context, design, growth mechanisms, and diagnostic methodologies, emphasizing its prospective opportunities and challenges.
Meanwhile, Venkatesan et al. [41] highlighted that FSP offers a scalable and proficient approach to address the complexities of electrocatalyst synthesis for polymer electrolyte membrane fuel cells (PEMFCs) and solid oxide cells (SOCs), streamlining conventional multistage procedures. In 2022, Tran-Phu et al. [42] presented a review on Power-to-X (P2X) technologies, underscoring the significance of sustainable energy storage with zero CO2 emissions. Within this context, FSP is identified as a crucial technique for enhancing the production of efficient (photo)electrocatalysts. Ultimately, within that year, John and Tricoli’s review [43] probed the particle formation mechanism, drawing insights from micro-explosions in single droplet experiments across diverse precursor–solvent pairs. The discussion emphasizes the importance of layer fabrication for industrial applications, including gas sensors, catalysis, and energy storage.
Herein in this review, we focus on recent advancements in product and nanodevice development by FSP. In Section 2, we review key aspects of engineering of complex assemblies via FSP, as well as mechanisms and process designs such as the oxygen-deficient process, double-nozzle configuration, in situ coatings, FSP deposition, sequential/nano-film deposition, and scale-up industrial production that enable the creation of intricate nanostructures. In Section 3, we discuss examples of complex functional nanostructures and nanodevices synthesized via FSP, such as perovskites, non-oxides, quantum dots, plasmonics, nanofilms, and sensors unraveling their potential applications and the scientific underpinnings that govern their multifaceted functionality.
2. Engineering of Complex Nanoassemblies by Flame Spray Pyrolysis
Complex configurations via FSP encompass a wide range of materials—see details in Section 3. In this section, we review the FSP process principles that can be used to achieve engineering of the advanced nanostructures. In brief, for the sake of the presentation, we can categorize the FSP methodologies as
- -
- Oxygen-deficient FSP process,
- -
- Double-nozzle FSP configuration,
- -
- In situ coatings FSP deposition,
- -
- Sequential/nanofilm FSP deposition.
Finally, we discuss some aspects of scale-up FSP. By this, we refer to the pilot FSP reactors that have been reported so far by various research laboratories and their application in novel nanomaterials engineering.
2.1. Oxygen-Deficient FSP Process
The concept of oxygen-deficient synthesis can pertain to anoxic or reduced metal oxides. In the literature, these are referenced as MKOL−x where K and L are the stoichiometry coefficients that determine the stable crystal phase MKOL. In this terminology, x signifies the O-deficiency coefficient.
Here, for the sake of the discussion, we classify these materials in three cases:
- [i]
- O-vacancies generation with no change in the crystal phase: lack of O atoms from the lattice, compared to the formal stoichiometry of the nominal crystal phase, with no modification of the crystal phase.
- [ii]
- Generation or reduced metal atoms with no change in the crystal phase: lack of O atoms from the lattice can stabilize lower-oxidation states of the metal atoms.
Often, cases [i] and [ii] are interlinked since the reduction in individual metal atoms in the lattice can be triggered thermodynamically from the generation of one or more O-vacancies in its immediate vicinity.
- [iii]
- Stabilization of a reduced crystal phase via lack of O atoms: certain O-deficient metal oxides can stabilize reduced phases. This occurs when a significant fraction part of the metal atoms is reduced. For example, magnetite Fe3O4, which contains one Fe2+ and two Fe3+, can be formed from Fe2O3 (two Fe3+) when 1/3 of the Fe3+-atoms is reduced to Fe2+. Further reduction in all Fe atoms to Fe2+ forms the FeO phase, while further reduction to Fe0-atoms forms the metallic, zero-valent-iron material. Similarly, Cu2O (SnO) is formed when all Cu2+ (Sn4+) atoms in CuO (SnO2) are reduced to the Cu1+ (Sn2+) state.
Table 2 presents a list of literature data on the use of anoxic FSP to engineer nanostructures. The concept of using an oxygen-lean FSP was pioneered by Grass et al. to produce oxygen-deficient metal-oxide particles [44] by placing the FSP nozzle inside a glove box filled with inert nitrogen and regulating the intake of oxidizing gas as illustrated in Figure 4b,c. The dispersion gas mixture in the flame can shift from a CO2/H2O composition (representing traditional, oxidizing flames, see Figure 4a) to a CO/H2/H2O mixture (under reducing conditions) [44]. Noble metal nanoparticles, including Pt, Au, Ag, and their alloys, can typically be produced even in oxygen-rich FSP, i.e., due to the thermodynamic preference of the metal state vs. the oxide state by the noble metal atoms. However, creating non-noble metals necessitates a reductive environment. When cobalt or bismuth organic precursors [45], such as cobalt(II)- and bismuth(III)-2-ethylhexanoate (more information in Table 2), are burned in a controlled atmosphere (with O2 levels less than 100 ppm) and with a high fuel-to-oxygen ratio (see Figure 4b), it enables the swift production of pure Co and Bi metal nanoparticles, enhancing the conventional flame process. With this experimental setup, Stark et al. have explored the creation of metallic bismuth nanoparticles ensuring no soot formation [46]. While the reducing environment might be beneficial for producing metallic particles on a large scale [45,47], it comes with the risks of incomplete combustion [1]. In the case where the oxygen supply is further constrained, a fine carbonaceous layer tends to form on these metal nanoparticles [47,48]. Using this experimental setup, NiMo nanoalloys [49] and ZnS nanocompounds [50] have been reported.
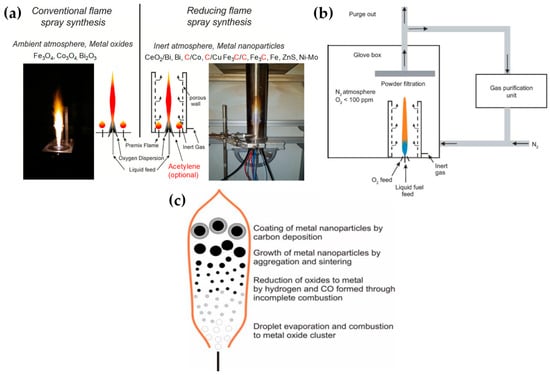
Figure 4.
(a) Conventional FSP (left) and our reducing FSP (right), where the anoxic flame is produced by in situ introduction of reducing dispersion gas, e.g., CH4. (b) An anoxic FSP reactor, used by Stark, with the whole reactor enclosed in a glove box filled with an inert atmosphere. By adjusting the gas flow rates, it is possible to achieve highly reduced conditions (O2 < 100 ppm). Used with permission of Royal Society of Chemistry from [45]; permission conveyed through Copyright Clearance Center, Inc. (c) Schematic depiction of the step-by-step transformation from precursor to oxide, metal, and carbon-coated metal nanoparticles during the reducing flame synthesis process: Initially, the precursor undergoes evaporation and combustion, resulting in oxide nanoparticles. These particles can then be further reduced to their metallic form by H2 and CO. Throughout this procedure, the nanoparticles increase in size due to aggregation and sintering. By introducing acetylene, these metal nanoparticles can acquire a carbon coating layer. Reproduced with permission from ref. [47,48]. Copyright 2007 Wiley-VCH.
Strobel and Pratsinis used an oxygen-deficiency FSP process [51] in order to synthesize Fe2O3, Fe3O4, and FeO nanoparticles. Their setup featured an FSP nozzle with a metal tube (4 cm in diameter and 40 cm in length) positioned directly above it (as shown in Figure 4b). Situated 20 cm above the FSP nozzle and angled at 45°, an internal mix spray nozzle was directed downward. This nozzle delivered deionized water at a rate of 10 mL/min, dispersed using 5 L/min of N2. A different oxygen-deficiency FSP setup for the production of Fe3O4 nanoparticles may be the utilization of a laminar, inverse diffusion flame [52]. This method takes advantage of the properties of the inverse flame, created when an oxidizer is injected into a flow of surrounding fuel [53]. Contrary to conventional flame approaches, this setup ensures that the iron particle formation occurs in a predominantly reducing atmosphere. As illustrated in Figure 5a, the burner features two concentric brass tubes with specific outer diameters, enclosed within an 11.4 cm diameter acrylic chamber. This chamber is crucial for protecting the flame from ambient air, preventing additional particle oxidation and potential secondary diffusion flame formation due to excess fuel reacting with room air. The oxidizer, either pure O2 or an O2-Ar mixture, is released from the innermost tube and is encircled by a blend of fuel (methane or ethylene), argon, and iron precursor vapor. A N2 flow enveloped the resulting inverse flame.
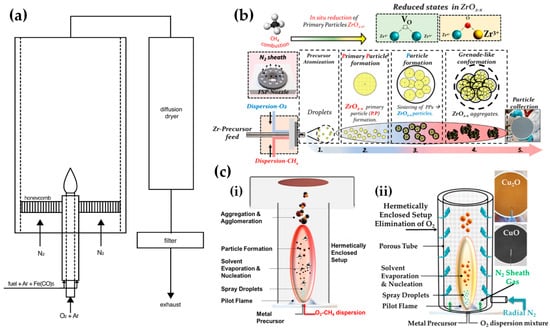
Figure 5.
(a) Experimental setup of laminar, inverse diffusion flame stabilized on a burner for the synthesis of magnetic iron oxide nanoparticles with reduced oxidation state. Reprinted from [52], with permission from Elsevier. (b) The concept of the novel anoxic FSP, as developed by our lab, for ZrO2−x production. Reprinted from [54]. (c) (i) Schematic representation of anoxic FSP reactor used for the synthesis of C@Cu2O/Cu0 nanoparticles. Reprinted from [55]. (ii) Anoxic FSP reactor configuration utilized for creating CuO and Cu2O nanomaterials. Reprinted from [55,56].
Recently, we have exemplified a novel anoxic FSP process, to engineer ZrO2–x (see Figure 5b) [54] and C@Cu2O/Cu0 (see Figure 5c) [55] nanoparticles. Our anoxic FSP concept relies on the combustion of CH4 in the dispersion gas. This introduces reducing agents that can modify the primary Zr particle by creating oxygen vacancies (VO). XPS and EPR confirm that the increased dispersion of the CH4 promotes the formation of oxygen vacancies [54]. A more complicated oxygen-deficiency FSP setup, which includes a dispersion feed consisting of {oxygen (O2)–methane (CH4)} mixture, in tandem with enclosed FSP flame with radial N2, is necessary for the synthesis of non-graphitized carbon/Cu2O/Cu0 heterojunction (see Figure 5c) [55]. The modification in the dispersion gas mixture leads to increased temperatures and generates reducing agents for the controlled phase transformation from CuO to Cu2O and Cu0 (see Figure 5c).

Table 2.
The literature summary of FSP characteristics/conditions for the production of oxygen-deficient nanostructures.
Table 2.
The literature summary of FSP characteristics/conditions for the production of oxygen-deficient nanostructures.
| Nano- Structure | FSP Configuration | Precursor(s) | Solvent | Molarity (mol L−1) | Precursor Flow (mL min−1) | Pilot Flame O2/CH4 (L min−1) | N2 Flow (L min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| C-Co | The flame is encased in a porous tube enabling the addition of inert cooling gases and acetylene. The flame is operated in a glove box in an N2 atmosphere at an O2 < 100 ppm. | Cobalt(II) 2-ethylhexanoate | Tetrahydrofuran | 6 | 2.2/1.2 | 45 | [48,57] | |
| Bi | The spray nozzle was placed in a Glove box fed with N2. A sinter metal tube (inner diameter 25 mm) surrounding the flame allowed for N2 radial flow. | Bismuth(III) 2-ethylhexanoate | Tetrahydrofuran | 6 | 2.2/1.2 | 25 | [46] | |
| CeO2/Bi | Bismuth(III) 2-ethylhexanoate, Cerium(III) octoate | Tetrahydrofuran | 6 | 2.2/1.2 | 45 | [45] | ||
| C-Cu | The flame is encased in a porous tube enabling the addition of inert cooling gases. The flame is operated in a glove box in an N2 atmosphere at an O2 < 100 ppm. | Cu(II)-2-ethylhexanoate | Tetrahydrofuran | 4.5 | 2.2/1.2 | 45 | [47] | |
| Ni-Mo | Ni(II)-ethylhexanoate, Mo(II)-ethylhexanoate | Tetrahydrofuran | 6 | 2.2/1.2 | 45 | [49] | ||
| FexOy | FSP nozzle with an Inconel metal tube (ID = 4 cm, length = 40 cm). Water was fed into this nozzle and dispersed by N2 gas. | Fe(III) nitrate nonahydrate, Fe(II) naphthenate | 2-ethylhexanoic acid/THF/ethanol (2/2/1) | 0.9 | 5 | 2.5/1 | 40 | [51] |
| α-Fe/Fe3O4 | Laminar, inverse diffusion flame stabilized on a burner. | Iron pentacarbonyl | [52] | |||||
| ZrO2–x | A single-nozzle FSP reactor featuring an enclosed flame utilizing a mixture of dispersion gases, O2 and CH4, to establish a reductive reaction environment. | Zirconium(IV) Propoxide | Xylene/acetonitrile (2.2/1.0) | 0.25 | 3 | 4/2 | 15 | [54] |
| C@Cu2O/ CuO/Cu0, Cu2O/CuO | A single-nozzle FSP reactor featuring enclosed flame utilizing a mixture of dispersion gases, O2 and CH4, to establish a reductive reaction environment. A perforated tube permits the introduction of radial N2 gas. | Copper(II) nitrate trihydrate | Acetonitrile/ ethylenglycol (1/1) | 0.25 | 3 | 2/1.2 | 10 | [55,56] |
2.2. Double-Nozzle FSP Configuration
In the case of mixed structures, e.g., heterojunctions, core-shell compositions, etc., the application of two FSP nozzles that operate in tandem offers advantages. Typical examples include the cases where a nanomaterial (NP1) and a cocatalytic nanomaterial (NP2) are combined. In the conventional single-nozzle FSP, a single precursor contains both the elements of nanomaterial (NP1) and nanomaterial (NP2) and produces the combined material in a single flame (see Figure 6a).
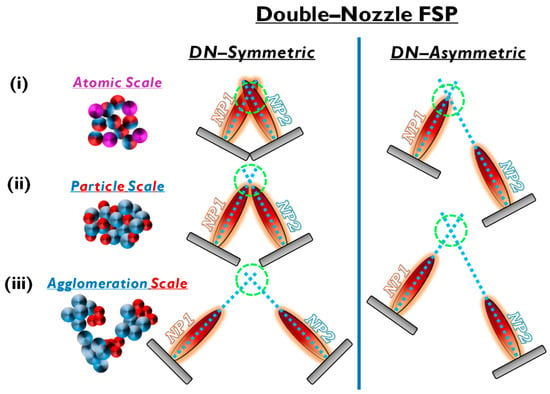
Figure 6.
Symmetric and asymmetric DN-FSP configuration for two particle formation regarding the (i) atomic, (ii) particle, or (iii) agglomeration scale.
Double-nozzle FSP entails two independent spray flames, with the precursor of NP1 inserted in a different flame than NP2 (see Figure 6). This method unlocks several options for independent size control, mixing, and specific deposition for the two nanomaterials by altering the primary geometrical parameters of distance and intersection of the flames. As shown in Figure 6: (i) At a small flame-intersection distance, where the centers of the flames are in contact, the atoms are in the preliminary stages of crystallization, producing well-mixed particles, tending to be similar to the single-nozzle FSP. In this case, the second flame substantially increases the synthesis overall temperature. (ii) When the intersection occurs after the endpoints of the flames, the materials are well crystallized, resulting in well-mixed primary particles of NP1 and NP2. (iii) At increased intersection distance, the two materials mix at their sintering stage or bigger distances at the agglomeration stage.
Thus, by changing the geometrical disposition of the two flames via the parameters a, b, d, Φ1, Φ2, and Z (see Figure 7b), the symmetrical/asymmetrical DN-FSP configuration offers a versatile technology that allows for the control of composite configurations at different synthesis stages, i.e., at the atomic scale, at the particle scale, or the aggregate’s scale (see Figure 6). Table 3 presents a list of the literature data on the use of DN-FSP to engineer nano-heterostructures.
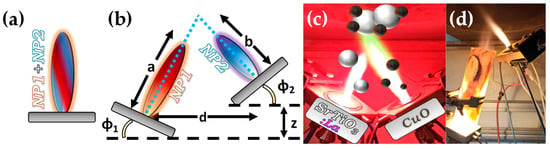
Figure 7.
(a) Schematic example of SN-FSP where two precursors are mixed before being fed to the flame. (b) Geometry parameters of DN-FSP. (c) Example of a symmetrical DN-FSP, used for engineering of La-doped SrTiO3, with surface deposition of CuO. Reprinted from [58]. (d) Example of asymmetrical DN-FSP.
Al2O3: DN-FSP was first implemented by Strobel et al. [59], producing in one nozzle Al2O3 and in the second nozzle Pt/BaCO3, thus forming individual Al2O3 and monoclinic BaCO3 nanoparticles. Increasing the internozzle distance delayed flame product mixing, increasing the crystallinity of BaCO3. In contrast, the single-nozzle process yielded Al2O3 particles with amorphous Ba species. The two-nozzle process enhanced NOx storage behavior, while the single-nozzle approach showed negligible NOx retention [59]. Following this successful novelty method, a series of Al2O3-based articles were published, herein chronologically presented: Minnermann et al. [60] produced in one nozzle Al2O3 and in the other pure oxide or mixed CoOx. Single flame synthesis is inadequate for producing an effective Al2O3/Co FT catalyst due to inadequate reducible cobalt oxide support particle size. The DN-FSP geometry significantly influences the resulting catalyst, yielding smaller alumina particles as the intersection distance increases, resulting in good adhesion of the two oxides and good stabilization. Høj et al. [61] produced Al2O3/CoMo by DN-FSP, and varying flame mixing distances (81–175 mm) minimized the formation of CoAl2O4, detectable only at short flame distances. Notably, employing DN-FSP synthesis achieved superior promotion of the active molybdenum sulfide phase, potentially attributed to reduced CoAl2O4 formation, consequently enhancing Co availability for promotion. Schubert et al. [62], through DN-FSP, produced Al2O3/Co enhanced with Pt (0.03, 0.43 wt%) deposition in the first nozzle and other materials in the second nozzle. Noble metals enhance catalyst reducibility, yielding abundant metallic Co sites. Due to their high cost, optimizing synthetic strategies for low concentrations is essential. Regardless of the preparation approach, adding 0.03 wt% Pt significantly improves catalytic activity in CO2 methanation, and 0.43 wt% Pt marginally increases the catalyst reduction. Using DN-FSP, Horlyck et al. [63] produced Al2O3/Co with Lanthanum doping (0–15 wt%). Increased La content and wider nozzle distance suppressed undesirable CoAl2O4 spinel phase, promoting easily reducible Co species. La addition enhanced carbon resistance, ensuring maximum methane conversions at 15 wt% La without catalyst deactivation or carbon formation. Stahl et al. [64] used DN-FSP to produce Co/Al2O3; in the nozzle of Al2O3, one additional particle—SmOx, ZrOx, or Pt—was formed contributing different cocatalytic effects, enhancing surface hydrogen or carbon oxide concentrations (see Figure 8a,b). All catalysts had consistent morphology with interconnected 12 nm alumina oxides and 8 nm cobalt oxides. For CO2 methanation, Pt and zirconia proved optimal, aligning with Pt-enhanced H2 adsorption and zirconia’s higher CO2 adsorption due to oxide sites with medium basicity.
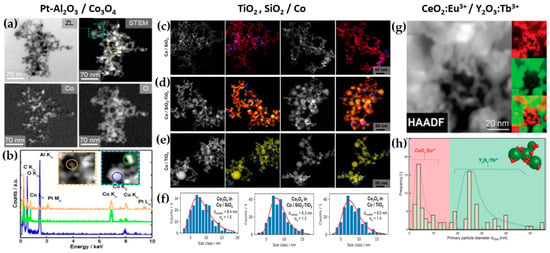
Figure 8.
(a) TEM images revealing the local distribution of cobalt and oxygen for Pt-Al2O3/Co3O4, (b) EDX measurements for chemical composition. Reprinted from [64]. DΝ-FSP-prepared (c) SiO2/Co, (d) SiO2-TiO2/Co, (e) and TiO2/Co; left images show STEM-HAADF and right images show EDX mappings of the elements Co (blue), Si (red) and Ti (yellow). (f) Particle size distributions of Co3O4 for the materials SiO2, SiO2-TiO2, and TiO2. Reproduced with permission from ref. [65]. Copyright 2022 Wiley-VCH. (g) STEM-HAADF of the nano-mixed CeO2:Eu3+/Y2O3:Tb3+ and its elemental mapping for Ce in red and Y in green, (h) dTEM distribution of CeO2:Eu3+ and Y2O3:Tb3+. Reprinted from [66], with permission from Elsevier.
TiO2: Grossmann et al., through the utilization of DN-FSP, produced TiO2 with deposited Pt particles [67]. Geometric configurations in DN-FSP strongly influenced Pt particle size and distribution on TiO2. Larger intersection distances and smaller angles result in nonuniform large and broadly distributed Pt clusters on TiO2. Conversely, smaller distances and larger angles enhance Pt dispersion and a uniform mixing, akin to single flame; however, DN-FSP allows for individual tuning of compound particle sizes. Solakidou et al. produced {TiO2-Noble metal} nanohybrids, with deposition of Pt0, Pd0, Au0, or Ag0 [68]. As shown, DN-FSP is superior vs. single-nozzle-FSP for finely dispersing noble metals on TiO2 support, achieving a narrower size distribution [50]. DN-FSP promoted intraband states in TiO2/noble metal, reducing the band gap. Efficient H2 generation presented the following trend: Pt0 > Pd0 > Au0 > Ag0, in line with a higher Schottky barrier upon TiO2 contact [50]. Gäßler et al. produced SiO2, TiO2, and SiO2-TiO2 mixture with DN-FSP deposition of Co3O4 (see Figure 8c–f) [65]: titania, comprising anatase and rutile phases, the SiO2-TiO2 mixed support, with separate anatase and silica phases. H2O adsorption varies significantly based on the support: SiO2 < SiO2-TiO2 < TiO2. CH4 formation rate increased with higher TiO2 fractions, while CO formation rate peaked in the mixed support. Psathas et al. used DN-FSP to engineer heterojunctions of perovskite SrTiO3 with deposited CuO nanoparticles (0.5 to 2 wt%) [58]. Higher CuO deposition led to larger SrTiO3 particle sizes due to increased enthalpy from the second flame [40]. Scanning TEM depicted small CuO particles (<2 nm), mainly found on the surface of SrTiO3. The dopant concentration significantly controlled the selective production of H2 or CH4 from H2O/CH3OH. CuO incorporation drastically shifted production to CH4, achieving a rate of 1.5 mmol g−1 h−1 for the La:SrTiO3/CuO catalyst (0.5 wt%) [58].

Table 3.
The literature summary of characteristics/conditions for nanostructures synthesized by symmetric and asymmetric DN-FSP methods.
Table 3.
The literature summary of characteristics/conditions for nanostructures synthesized by symmetric and asymmetric DN-FSP methods.
| Nanomaterial | Geometric Parameters (cm) | Precursor(s) * | Molarity * (mol L−1) | Precursor * (mL min−1) | Oxygen * (L min−1) | Size (nm) | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Symmetric DN–FSP | ||||||||
| Al2O3/Pt/Ba | φ = 30°, d = 3–7 | Al(III) tri-sec-butoxide/ Ba(II) 2-ethylhexanoate, Pt(II) acetylacetonate | 0.5/ 15.4 wt% Ba, <1 wt% Pt | 5/ 3 | 5/ 5 | 10–20 | 120–160 | [59] |
| Al2O3/Co | φ = 20°, d = 11, a = 16 | Al-sec-butoxide/Co naphthenate | 0.5/ 10 wt% Co | 5/ 5 | 5/ 5 | 15–30 | 111–122 | [63] |
| TiO2/Pt0, Pd0, Au0, Ag0 | φ = 30°, d = 11 | Ti(IV) isopropoxide/ Pt(II), Pd(II), Au(III), Ag(I) acetylacetonate | 0.64/ 0–5 wt% | 5/ 3–7 | 5/ 3–7 | 10–20 | 72–200 | [68] |
| TiO2-SiO2/ Co | φ = 20° d = 15, a = 22 | Ti(IV) isopropoxide, TEOS/ Co naphthenate | 0.9/ 9 wt% Co | 5/ 5 | 5/ 5 | 7–16 | 86–284 | [65] |
| SrTiO3/CuO | φ = 20°, d = 8, a = 10 | Sr acetate, Ti(VI) isopropoxide/ Cu(II) nitrate trihydrate | 0.4/ 2–0.5 wt% Cu | 5/ 5 | 5/ 5 | 45–55 | 32–57 | [58] |
| ZrO2/CuO | φ = 10° | Zr 2-ethylhexanoate/Cu(II) 2-ethylhexanoate | 0.5/ 11 wt% Cu | 5/ 5 | 5/ 5 | 10–20 | 106–114 | [69] |
| LiMn2O4/AlPO4 | φ = 20°, d = 17 | Li and Mn(III) acetylacetonate/Al-tri-sec-butoxide, triethyl phosphate | –/ 0–5 wt% AlPO4 | 3–7/ 5 | 3–7/ 5 | 7–22 | 64–195 | [70] |
| CeO2:Eu3+/ Y2O3:Tb3+ | φ = 30° | Ce 2-ethylhexanoate/Y nitrate hexahydrate | 0.3/ 0.4 | 3–12/ 3–12 | 3–8/ 3–8 | 5 | 185 | [66] |
| Asymmetric DN–FSP | ||||||||
| SiO2/Ce0.7 Zr0.3O2 | φ1 = 20°, φ2 = 35°, a = 23, b = 5 d = 11, z = 10 | TEOS/Ce 2-ethylhexanoate, Zr(IV) n-propoxide | 0.5/ 0.19 | 3–7/ 5 | 5/ 5 | 18.5–28.5 | 217–363 | [71] |
| NaTaO3/NiO–Pt0 | φ1 = 30°, φ2 = 15°, a = 20, b = 24, d = 11, z = 3 | Na 2-ethylhexanoate, Ta(V) chloride/Ni(II) 2-ethylhexanoate, Pt(II) acetylacetonate | 0.1–0.6/ 0.5 wt% Ni, 0.5 wt% Pt | 3–9/ 5 | 3–9/ 5 | 12–34 | 19–84 | [72] |
* In the setup, because there are two distinct nozzles, parameters related to them are differentiated and denoted using a slash: nozzle 1/nozzle 2.
Other particles: Tada et al., using DN-FSP, produced a ZrO2/CuO heterostructure [69]. Changing the geometrical parameters of DN-FSP altered the proportion of interfacial sites vs. copper surface sites. As active sites are primarily at the metal–oxide interface, ZrO2/CuO with smaller CuO clusters exhibited higher activity in methanol synthesis via CO2 hydrogenation. Gockeln et al., by a combination of DN-FSP and a lamination technique [73], synthesized in situ carbon-coated nano-Li4Ti5O12 Li-ion battery electrodes. Li et al. synthesized LiMn2O4 spinel as a cathode material for Li-ion batteries via screening 16 different precursor–solvent combinations [70]. To overcome the drawback of capacity fading, the deposition of AlPO4 (1–5%) via DN-FSP was homogeneously mixed with LiMn2O4. The optimal 1% AlPO4 with LiMn2O4 demonstrated an energy density of 116.1 mA h g−1 at 1 C (one-hour discharge). Henning et al. used DN-FSP to engineer luminescent biosensors CeO2:Eu3+/Y2O3:Tb3+ [66]. CeO2:Eu3+ nanoparticles (6 nm, 22 wt%) and Y2O3:Tb3+ nanoparticles (32.5 nm, 78 wt%) were shown to function as robust optical-based ratiometric H2O2 biosensors (see Figure 8g,h). Based on the collective effect, H2O2 caused significant luminescence quenching in CeO2:Eu3+ nanocrystals, but Y2O3:Tb3+ nanoparticles were unaffected [48].
Asymmetric Double Flame: Lovell et al. utilized asymmetric-DN-FSP geometry to control the SiO2 interaction with Ce0.7Zr0.3O2 nanoparticles [71]. Tuning the intersection distance during DN-FSP (18.5 to 28.5 cm) prevented silica coating. Short intersection distances led to high surface-area silica encapsulating ceria-zirconia, while longer distances suppressed this encapsulation. The material at longer intersection distances, used as Ni support for dry methane reforming, showed enhanced oxygen storage capacity and basicity, yielding a highly selective catalyst. Psathas et al. used asymmetrical-DN-FSP-deposited NiO or Pt0 nanomaterials on the surface of Ta2O5 or the perovskite NaTaO3 [72]. Single-step synthesis of the smallest produced NaTaO3 (<15 nm), with finely dispersed NiO or Pt0 (<3 nm). NaTaO3/NiO produced from FSP had half the photocatalytic hydrogen production than those from DN-FSP. Also, DN-FSP had a ten times higher yield than the conventional deposition of wet-impregnated NiO. Similar results were found for the photocatalytic efficiency of NaTaO3/Pt0, which was 30% more photocatalytically active than the conventional liquid-Pt photo-deposition method [54].
2.3. In Situ Coatings by FSP
Apart from heterojunction engineering, FSP allows for in situ engineering of core-shell structures, i.e., where a hermetic layer can be deposited on the core particle. Hansen et al. were the first to present this concept, using a spraying-ring apparatus, which facilitated the synthesis of ZnO particles (see Figure 9a) [74]. This research revolved around the exploration of how introducing cold air to cool a flame could influence the formation of ZnO particles. Their findings indicated that a swift drop in temperature downstream from the peak greatly benefitted the creation of particles with significant specific surface area [74]. Later on, this ring-spraying setup was predominantly employed to produce core-shell particles. Teleki et al. demonstrated that a hermetic SiO2-layer can be formed around TiO2 nanoparticle FSP reactor via injection of hexamethyldisiloxane (HMDSO) vapor—a precursor for SiO2—on the TiO2-forming stream (see Figure 9b–d) [75]. Addressing the problem of distinct Si and Ti domains found in earlier research [76], they incorporated a toroidal ring in an encapsulated FSP reactor. This facilitated the separate introduction of the gaseous Si precursor, HMDSO. The study emphasized that under specific conditions, it is possible to achieve the desired coatings in a single-phase system, avoiding the complexities of multiple phases [76]. In subsequent research, the same team (see Figure 9e–g) [77] utilized experimental and computational fluid dynamics (CFD) techniques to investigate the integrity of resultant coatings. Flame-made nanoparticles have been effectively coated in a single step, achieving notable production rates of 30 g/h. Predominantly, rutile TiO2 nanoparticles (enriched with Al) with an approximate diameter of 40 nm were synthesized with an in situ coating of 20 wt% SiO2. CFD further clarified the effects of merging the TiO2 aerosol with the HMDSO vapor stream jets (see Figure 9g).
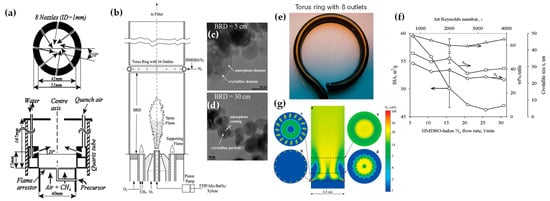
Figure 9.
(a) Overhead perspective of the stainless steel segment in the quench ring according to the research work of Hansen et al. [74]; every nozzle is angled at 10° compared with a hypothetical line passing through the central axis. Reproduced with permission from ref. [74]. Copyright 2001 Wiley-VCH. (b) Experimental configuration for in situ SiO2 coating of TiO2 nanoparticles produced by Teleki et al. [75], using a toroidal pipe ring with 16 gas exits to inject HMDSO-laden N2. At burner ring distances (BRD) of (c) 5 cm and (d) 30 cm, this leads to distinct SiO2/Al2O3/TiO2 or SiO2-layered Al/TiO2 particles, each containing 4 wt% Al2O3 and 20 wt% SiO2, respectively. Reprinted (adapted) with permission from [75]. Copyright 2008 American Chemical Society. (e) Toroidal pipe ring equipped with 8 outlets for injection of the HMDSO-laden N2. (f) Impact of the ring N2 flow rate (along with the associated jet Reynolds number at 300 K) under standard coating conditions on SSA (depicted by circles), rutile weight percentage (represented by triangles), anatase (shown as squares), and rutile (illustrated by diamonds) crystallite sizes of 20Si-coated Al/TiO2. (g) Graphical representations of the (a) N2 volume percentage for 16 jets with a combined volume flow of 15.8 L/min N2 (v0 = 58 m/s). The related cross-sectional views are displayed at heights of 0 (b), 0.2 (c), 1 (d), and 3 cm (e) above the outlet level. The logarithmic color gradient extends from <1 (in blue) to 100 (in red) % v/v of N2. Reprinted (adapted) with permission from [77]. Copyright 2009 American Chemical Society.
Building upon similar principles, Sotiriou et al. demonstrated the one-step production of Ag/SiO2 core-shell nanoparticles [78]. The innovative design of their apparatus, featuring a torus ring with multiple jets, ensured the accurate in-flight coating of particles (shown in Figure 10a). A crucial aspect of their work was to understand how the SiO2 content in resultant particles can be manipulated through process adjustments, influencing the size of the nanosilver particles and preventing their agglomeration. The particles were covered in-flight by injecting HMDSO through a torus ring equipped with multiple evenly spaced and uniform jets, each having a diameter of 0.6 mm. This injection occurred at a specific height referred to as the burner ring distance (BRD). Regardless of the BRD values, the length of the tube above the torus ring was maintained at 40 cm. HMDSO vapor-laden N2 gas at a flow rate of 0.8 L/min was obtained by bubbling nitrogen gas through liquid HMDSO at varying temperatures, through the jet openings. The amount of HMDSO injected was adjusted to achieve a targeted theoretical coating thickness (CT) of either 3 or 6 nm [79]. The SiO2 content in the resulting particles was determined under complete saturation conditions. Our research group extended this knowledge and developed Ag@SiO2 particles with a slightly modified experimental setup [80,81] illustrated in Figure 10b. The HMDSO vapor was generated by bubbling N2 gas through 300 cm3 of HMDSO contained in a glass flask, which was maintained at a temperature of 10 °C. Under saturation conditions, this configuration resulted in a theoretical SiO2 production rate of 5.9 g/h, equal to 20 wt% SiO2 in the product powder [80]. More recently, we have demonstrated that ring-coating FSP allows engineering safe-by-design core-shell SiO2 materials with diminished reactive oxygen species (ROS) generation [82]. As shown in [64], this stems from the flexibility of ring-coating FSP toward modulating the thermal profile during nano-SiO2 synthesis. A cooler SiO2-formation process allowed for the surface passivation of the nanosilica, consequently decreasing its ROS generation potential [64]. In this process, the N2 gas, which served as a carrier of the atom to be dispersed, played a pivotal role in the cooling dynamics [83]. More recent research works include the use of ring-spraying FSP for the production of stable core-shell ZnO@SiO2 [84], CuOx@SiO2 (see Figure 10d) [85], SiO2@YAlO3:Nd3+ [86], and SiO2-coated Y2O3:Tb3+ [87] along with their FSP characteristics listed in Table 4.
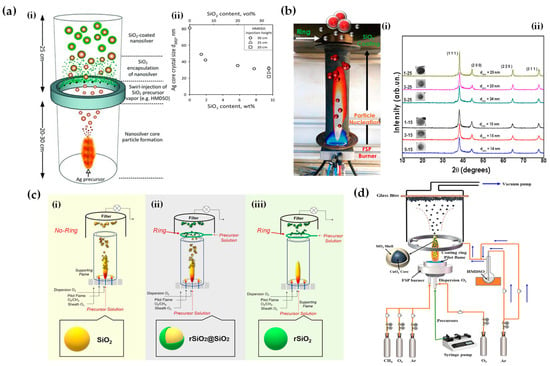
Figure 10.
(a) (i) Illustration of the enclosed FSP setup for producing Ag@SiO2 nanoparticles, originally developed by Sotiriou et al. [78]. The ring situated between the two tubes aids in swirling the SiO2 precursor vapor, ensuring the precise control of the SiO2 core. (ii) Adjustment of the crystallite size of nanosilver can be achieved by varying the SiO2 content in the final nanosilver particles and by altering the injection height of the SiO2 precursor vapor to 30 (circles), 25 (triangles), or 20 cm (squares) above the flame spray burner. Enhancing the SiO2 content helps to prevent the agglomeration and crystal growth of Ag nanoparticles. Additionally, introducing HMDSO (the SiO2 precursor) at reduced heights cools the flame aerosol, further inhibiting Ag crystal growth. Reproduced with permission from ref. [78]. Copyright 2010 Wiley-VCH. (b) (i) Schematic depiction of the enclosed FSP reactor where the one-step Ag coating SiO2 particles occur in-flight [80]. Contrary to Sotiriou et al.’s findings [78], the metal ring was at the top of the FSP-enclosed flame. (ii) XRD patterns of SiO2@Ag0 nanoparticles with variations in size and shell thickness. Reprinted (adapted) with permission from [80]. Copyright 2019 American Chemical Society. (c) Schematic illustration of FSP reactor configurations: (i) designed for high-temperature nano-SiO2 production, (ii) tailored for the hybrid rSiO2@SiO2 nanosilica, and (iii) set up for low-temperature nano-rSiO2 synthesis. Reprinted (adapted) with permission from [82]. Copyright 2022 American Chemical Society. (d) FSP apparatus for creating core-shell CuOx@SiO2 nanoparticles. Reprinted from [85], with permission from Elsevier.

Table 4.
The literature summary of FSP characteristics/conditions for the production of core-shell nanostructures.
2.4. Sequential FSP Deposition
The concept of sequential deposition was originally used for the fabrication of multilayer films, sensors [89], and fuel-cell applications [41]. Mädler and his colleagues fabricated multilayer films for gas sensing where two different sensing layers were deposited on ceramic substrates sequentially: pure SnO2 onto Pd/SnO2 [90] or Pd/Al2O3 layer on top of a Pd/SnO2 layer (refer to Figure 11a) [89]. Recently, we have developed a sequential-deposition flame spray pyrolysis (SD-FSP) technique for the controlled synthesis of PdO/Pd0/TiO2 nano-heterostructures [91]. SD-FSP is a two-phase process in which a nanometric TiO2 particle layer is deposited on a glass-fiber filter in the first FSP step. Then, in a second FSP phase, Pd particles are produced in an FSP flame under conditions that permit control over the Pd NP size and PdO/Pd0 ratio (as shown in Figure 11b). In this SD-FSP process, combustion of the Pd precursor under open-flame conditions permits ambient O2 entrainment without O2 consumption by TiO2 formation during the combustion [91].
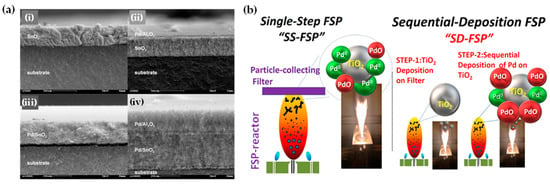
Figure 11.
(a) The concept of sequential deposition technique, as originally exemplified by Sahm et al. [89], for the engineering of multilayer films. Cross-sectional SEM images of a SnO2 layer are shown (i); a Pd/Al2O3 layer over a SnO2 layer (ii); a Pd/SnO2 layer (iii); and a Pd/Al2O3 layer on the top of a Pd/SnO2 layer (iv)—all of which were deposited on ceramic bases. Reprinted from [89], with permission from Elsevier. (b) A schematic depiction of the FSP process employed for the deposition of Pd onto the TiO2 surface in two stages (sequential deposition, SD-FSP). Reprinted (adapted) with permission from [91]. Copyright 2020 American Chemical Society.
FSP has been adeptly employed for the synthesis of films characterized by a porous network comprising nanoparticles whose porosity can be modulated. Such films have exhibited superior efficacy in applications like chemical sensors [92], photodetectors [93], and solar cells [94]. Homogeneity in films still remains a challenge, especially since cracks can easily form when drying films, i.e., created through traditional wet-phase coating methods. However, films formed in the gas phase [2] do not require drying, leading to more consistent layers, e.g., as in chemical vapor deposition (CVD) methods [95]. The film’s structure resulting from FSP deposition largely relies on the substrate temperature and the stage of particle formation when reaching the substrate. Characteristically, Figure 12b illustrates a schematic of a nanofilm deposition procedure. The nature of the deposit—whether it be precursor droplets, a mix of precursor and product vapors, or product particles exhibiting various agglomeration levels—depends on the particle’s formation phase when it reaches the substrate. This characteristic can be controlled by adjusting the substrate’s position or by modulating the precursor and gas flow rates. When uncovered precursor comes into contact with the substrate, it produces denser films [28] whereas airborne product particles result in highly porous particle films, as depicted in Figure 13a. The porosity of such films can either remain intact or transform into denser formations through a sintering process, contingent on the substrate’s temperature. The nanofilm deposition procedure can be clarified in the research work of Kavitha et al. where they produced TiO2, ZnO [96], and Al2O3, ZnO, ZnO-20 mol% MgO, and ZrO2-Y2O3 [97] films. Films of the aforementioned oxides were applied onto amorphous silica bases measuring 10 mm 10 mm. The setup for deposition, as depicted in Figure 13b, comprises a liquid sprayer, a division chamber, a flame, and a holder for the substrate. In [38], a combination of precursor solution and pressurized air (at 16 lpm) was directed into the atomizer, which generated atomized droplets of the solution and injected them into the separation chamber. Adjustments to this temperature can be made by altering either the gas flow rates or the distance between the substrate and nozzle [96]. A straightforward relationship between the process parameters and the rate of film formation were established by Tricoli et al. [98] in the case of SnO2 films deposited on substrates at temperatures 323 to 723 K in order to study the particle size distribution and deposition dynamics.
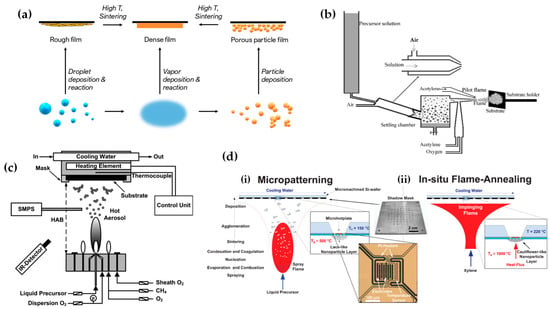
Figure 12.
(a) Schematic illustration of the creation of porous or solid films through the flame deposition of droplets, vapors, or particles. (b) Illustrative diagram of the flame spray pyrolysis deposition setup; the embedded image displays an external mix atomizer for film deposition. Reprinted from [96], with permission from Elsevier. (c) FSP burner combined with a temperature-regulated substrate holder designed for nanofilm creation and deposition. Reproduced with permission from ref. [98] Copyright 2012 Wiley-VCH. (d) Diagrammatic representation: (i) Flame-made nanoparticle layers, exhibiting a high porosity of 98%, are methodically deposited onto a silicon wafer using a shadow mask. Subsequently, (ii) these layers undergo in situ mechanical stabilization via an impinging xylene flame devoid of particles. Reproduced with permission from ref. [99] Copyright 2008 Wiley-VCH.
Incorporating flame-based aerosol techniques to industrial-scale semiconductor device engineering poses new challenges. The mechanical integrity of nanostructured layers hinges on the substrate temperature at the time of deposition. While mechanically robust layers can be achieved at elevated temperatures (850 °C), these temperatures do not align with complementary metal oxide semiconductor (CMOS) substrates that host circuit components, as they cannot withstand temperatures exceeding 400 °C. Thus, Tricoli and his colleagues introduced a strategy for stabilizing deposits thermally by using an in situ rapid flame treatment on nanoparticle micropatterns deposited at low temperatures [99]. This technique can be applied while keeping the substrate at low temperatures, and the original crystallite size remains unchanged. The synthesis of metal oxide nanoparticles occurs through the dispersion and ignition of a precursor spray solution of the target material—in [98], it was SnO2. Particle growth progresses through condensation, surface growth, coagulation, and sintering, producing nanocrystalline material (as seen in Figure 12d(i)). Factors like high-temperature particle residence time (HTPRT), metal concentration, and droplet dispersion during FSP influence the particle size [2]. Nanostructured particles then adhere to the substrate in situ, usually forming a highly porous layer. However, due to the substrate’s low temperature (150 °C), this layer has limited mechanical resilience. To enhance its stability, a second phase involves subjecting to thermal curing in FSP, e.g., an “impinging” step, for 30 s using a particle-free xylene flame, as depicted in Figure 12d(ii). This treatment profoundly alters the layer’s texture, enhancing its adhesion to the substrate. Patterns of nanoparticles, with sizes as small as 100 µm, were created by positioning a shadow mask, designed with a series of circular openings, against a Si-wafer (coated with a thin layer of silicon nitride), as shown in Figure 12d [99].
2.5. Scale-Up FSP
Several of the methods used for nanoparticle synthesis are not scalable, i.e., to transcend laboratory production toward industrial scale for large-volume production. These facts are against the actual implementation of real-life applications, hindering the connection of lab research to market-level production. When more complex nanoparticles require time-consuming synthesis protocols with complex processes, these factors lead to very high prices per kilo of particles [100].
Gas-phase synthesis has already shown much promise for the industrial production of nanoparticles. So far, single-metal nanomaterials are produced through gas-phase processes [27]. Gas-phase synthesis includes many essential commodity products that have been widely produced for many years, with some of the most widespread nanomaterials for the industry, such as carbon black by the company Cabot [101] as a reinforcing agent, P25 (TiO2) by Evonik Industries [102] renowned for its photocatalytic properties, pigmentary titania by the companies DuPont, Cristal, and Ishihara, fumed silica (SiO2) by Cabot and Evonik, as well as ceramic-based nanoparticles with the application of flame aerosol processes. The production of flame-made nanoparticles generates millions of tons with a valuation reaching $15 billion/year [38].
The successful utilization of aerosol-made nanomaterials in the market indicates that the industrial-scale manufacturing of gas phase will expand further with future applications, using more complex particles and multicomponent particles that cannot be easily produced at industrial scale with other methods [1]. Utilizing the advantages of FSP, several start-up companies have grown to produce various particles with controlled characteristics to fill the demand for niche markets [103]. Today, Hemotune AG (Schlieren, Switzerland) [104] produces polymer-decorated iron nanoparticles with carbon encapsulation for the purpose of blood purification. Examples are Anavo Medical (Zurich, Switzerland), for bioactive hybrid metal oxides, and Avantama AG (Stäfa, Switzerland), for the production of several metal oxides [103]. Such start-up companies include Turbobeads AG (Zurich, Switzerland) [105], which creates amine-functionalized cobalt carbon-coated nanoparticles. HeiQ Inc. (Schlieren, Switzerland) [106], which recently had an IPO with a valuation of 127 M£, produces nanosilver by FSP, the third most market-demanded nanomaterial, following carbon black and fumed oxides [107].
FSP synthesis can be implemented by laboratory FSP with production of 10 g h−1 to establish the synthesis optimization protocol and the initial exploration for complex particles and industrial-scale FSP with production rates at kg h−1 [108]. With lab-scale FSP, many particles reach 10 g h−1. Examples include HfO2 (5 nm, 89 SSA) at 15 g h−1 [109] and CeO2 (8 nm, 101 SSA) at 10 g h−1 [110]. Pratsinis et al. synthesized particles of silica/titania with a production rate of 200 g h−1 [111,112], showing very different results with the change in the fuel flow rate and the oxygen flow rate parameters. In this context, many simple oxides have been synthesized with FSP, such as WO3/TiO2 [113], or more complex nanoparticles, such as La0.6Sr0.4Co0.2Fe0.8O3−δ with production rates as high as 400 g h−1 [114].
Industrial FSP: In Table 5, the industrial-scale FSP publications are presented in chronological order and their synthesis/structural characteristics are listed. The first industrial-scale FSP production was demonstrated for SiO2, by Mueller et al., producing uniform 25 nm particles at 1.1 kg h−1 [115]. The study investigated the primary particle diameter, morphology, and carbon content by HMDSO in EtOH at 1.26 M and 3.0 M, as well as pure HMDSO at 4.7 M. Notably, the average primary particle size of the product was precisely controlled within the range of 10 to 75 nm, irrespective of the precursor concentration. Additionally, it was observed that utilizing air instead of O2 as the dispersion gas resulted in minimal variation in the product particle size. Mueller et al. achieved ZrO2 with a production rate of 0.6 kg h−1 with an average size of 30 nm [116]. The study evaluated zirconium n-propoxide in EtOH at 0.5 M and 1 M concentrations. Primary particle size ranged from 6 to 35 nm, with the crystal structure mainly tetragonal (80–95 wt%). The primary particles showed weak agglomeration, forming loosely agglomerated single crystals. Gröhn et al. produced ZrO2 at 0.5 kg h−1 [117] with increased technological expertise by a three-dimensional computational fluid dynamics model showing the fundamentals of the high-temperature particle residence time (HTPRT) for the scale-up synthesis of nanomaterials (see Figure 13a,b). HTPRT effectively regulated primary particle and agglomerate size, morphology, and ZrO2 crystallinity. Maintaining a constant HTPRT while scaling up the production rate from ∼100 to 500 g h−1 showed no significant alteration in product particle properties. In this context, Meierhofer et al. explored CFD-PBM modeling of ZrO2 FSP synthesis [118] at high productions to discover the attributes of the resulting nanoparticles. Jossen et al. produced yttria-stabilized zirconia (Y2O3/ZrO2) with the same parameters, with only the molarity dropped by half, decreasing the production rate at 0.35 kg h−1 [119]. Homogeneous Y2O3/ZrO2 exhibited an average crystallite and particle diameter ranging from 8 to 31 nm, with yttria content varying between 3 and 10 mol%. Interestingly, the yttria content had no discernible impact on the primary particle and crystal sizes.

Table 5.
Industrial production of nanoparticles by FSP, parameters, production rate, size, and SSA.
Table 5.
Industrial production of nanoparticles by FSP, parameters, production rate, size, and SSA.
| Nanomaterial | Production Rate (kg h−1) | Precursors | Solvents | Molarity (mol L−1) | Precursor (mL min−1) | Oxygen (L min−1) | Size (nm) | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 1.1 | HMDSO | Ethanol | 4.7 | 33.3 | 50 | 26 | 108 | [115] |
| ZrO2 | 0.6 | Zr n-propoxide | Ethanol | 1 | 81 | 50 | 30 | 33 | [116] |
| ZrO2 | 0.5 | Zr 2-thylhexanoate | Xylene | 1 | 64 | 80 | 25 | 42 | [117] |
| Y2O3/ZrO2 | 0.35 | Zr n-propoxide/Y nitrate hydrate | Ethanol | 0.5 | 81 | 50 | 31 | 32 | [119] |
| FePO4 | 0.27 | Fe nitrate/tributyl phosphate | 2-ethylhexanoic acid | – | 20 | 40 | 129 | 108 | [108] |
| ZnO | 3 | Zinc nitrate hexahydrate | Ethanol, methanol, 1-propanol, 1-octanol | 3.3 | 200 | 120 | 30 | 26.2 | [120] |
| Ca2SiO4 (Belite) | 0.03 | TEOS/calcium propionate | Ethanol, methanol, deionized water | 1.1 | 30 | 30 | 54 | 34 | [121] |
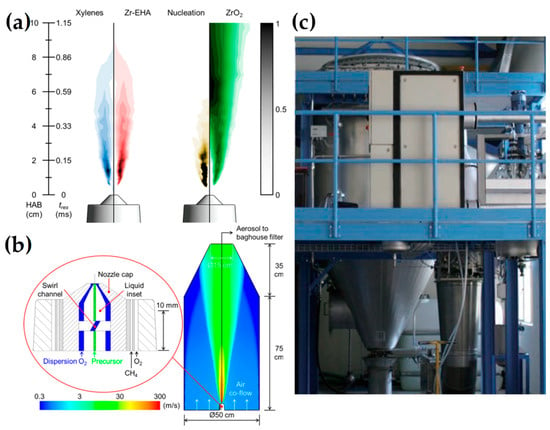
Figure 13.
(a) Predicted normalized gas-phase mass fractions, the ZrO2 formation profile (brown), and volume concentration (green). (b) Schematic of FSP two-phase atomizer geometry. Reprinted (adapted) with permission from [117]. Copyright 2014 American Chemical Society. (c) A flame spray pyrolysis (FSP) pilot plant is designed to produce multiple kg h−1 of nanoparticles, incorporating a baghouse filter for nanoparticle collection with an approximate filtration area of 50 m2. Reprinted from [108].
Wegner et al. produced FePO4 nanoparticles with 0.27 kg h−1 with a mean size of 129 nm (see Figure 13c) [108]. Additionally, a cost analysis for the scale-up FSP was calculated with the pilot plant production costs for simple oxides projected to be less than 100 EUR per kilogram. Among the cost components, raw materials constitute the most significant cost factor in this estimation.
Hembram et al. manufactured ZnO nanorods with the highest production rate so far, at 3 kg h−1. The resulting nanorods had a length of 30 nm and a mean aspect ratio of the rods at 2.3 [120]. Numerous nanorods self-aligned by creating junctions at the basal planes and some were further assembled into tetrapods. The nanorod aspect ratio was controllable through adjustments in the concentration of Zn ions in the initial precursor solution, its delivery rate, and the oxygen flow into the reactor. Betancur-Granados et al., with a production of belite, achieved in scale-up conditions with a production of 0.03 kg h−1—the low production is due to the formation of amorphous and other derivatives, such as alite, CaCO3, etc. [121]. The hydraulic samples exhibit a full hydration reaction within a 24 h duration upon contact with water. This substantiates the technique’s potential in developing highly reactive materials suitable for prospective sustainable construction practices.
3. Complex Functional FSP Nanostructures and Nanodevices
The innovative exploitation of FSP’s potential enables the engineering of unconventional materials, such as non-oxides and complex structures or nanodevices, including perovskites, nanosensors, and fuel-cell-materials.
3.1. Engineering of Perovskites by FSP
FSP has been established mainly for its ability to engineer a wide range of single-metal oxides [2]. However, establishing FSP process parameters for producing ABO3 perovskite nanomaterials (see Figure 14a–f and Figure 15) is typically more challenging, e.g., avoiding the formation of the separate oxides AOn and BOm.
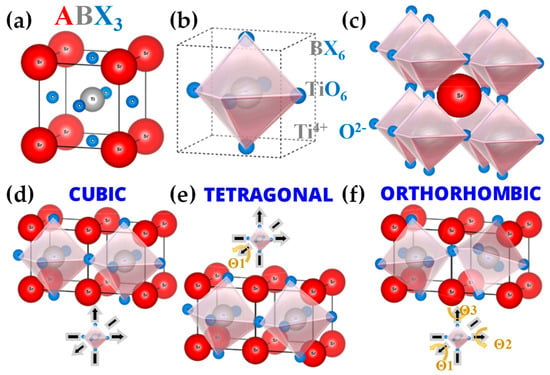
Figure 14.
The ideal cubic perovskite structure exemplified by SrTiO3. (a) The cubic structure with the Ti4+ at the cell center. (b) The octahedral polyhedron structure TiO6. (c) Sr2+ at the cubic center with the octahedral structure surrounding the strontium. Different perovskite structures: (d) the ideal cubic perovskite, axis X, Y, and Z of the octahedral BX6 have a 180-degree separation. (e) Tetragonal perovskite structure, the octahedral BX6 tilted in only one of the three axes at an angle Θ1. (f) The orthorhombic structure, the octahedral, is titled in all three of the axes, in accordance with the angles Θ1, Θ2, and Θ3.
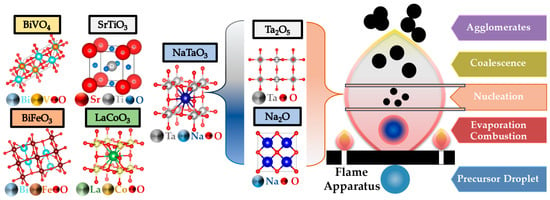
Figure 15.
FSP formation of perovskite structure ABO3 requires avoidance of the formation of the two separate oxides (A-oxide, B-oxide).
The publications regarding perovskite synthesis by FSP (see Figure 15), listed in Table 6, are rather limited in number so far. The first publication was by Kho et al. who demonstrated the synthesis of BiVO4 by FSP [122], where they noticed a bottleneck disadvantage of crucial importance for FSP perovskite formation—that is the short HTPRT, in the order of milliseconds, was inadequate to induce the perovskite phase (see Figure 16a,b). In situ thermal curing, on the filter, was necessary to achieve the desired scheelite BiVO4 phase [122]. The required filter temperature was T > 310 °C up to 360 °C to reach 100% crystallization, resulting in the scheelite-monoclinic phase, in agreement with the Tammann temperature of BiVO4 [109], which occurred at 300 °C. Stathi et al. [123] demonstrated that FSP can engineer W- and Zr-Doped BiVO4 in tandem with control of the BiVO4 lattice oxygen vacancies (VO). W-doping had a minor change on the BiVO4 XRD peaks, although W-doping (10%) caused a major deterioration of the crystallinity. Zr-doping above 1% showed peaks attributed to cubic-ZrO2 particles of 3 nm diameter. Function-wise, the presence of oxygen vacancies in W-BiVO4 and Zr-BiVO4 drastically improved the O2 production efficiency [123].
Psathas et al. [124,125], in their study of the engineering of perovskite BiFeO3 by FSP, concluded that the short HTPRS did not allow for the in situ formation of crystalline BiFeO3. They observed that a very short post-calcination of 5 min at 550 °C allowed for the formation of pure phase BiFeO3. This shows that the FSP-made BiFeO3 material consisted of small nanometric crystallites that were organized in larger BiFeO3 crystalline particles upon a small thermal/calcination boost [124,125]. Additionally, via the same protocol, mullite-type Bi2Fe4O9 was synthesized (see Figure 16c). These BFO materials were employed for the reduction of 4-Nitrophenol to 4-Aminophenol [124] and showed a low-activation energy Ea = 22 kJ mol−1 for BiFeO3, which is comparable to that of noble metal-based catalysts. Highly efficient photocatalytic O2 evolution, as shown in [125], was promoted via the introduction of Fe2+ centers in BiFeO3 via a downshift of the CB and VB edges. FSP-made Bi2Fe4O9 exemplified a highly efficient O2 evolution photocatalyst for the first time [125].
Punginsang et al. [126] reported the engineering of layered perovskite oxide Bi2WO6 consisting of FSP-made orthorhombic phase spherical Bi2WO6 nanoparticles (3–30 nm in diameter) and a very high specific surface area of 197.8 m2g−1. The high thermal stability FSP-Bi2WO6 nanoparticles were applied for gas-sensing measurements and displayed a stable and selective response of 3.72–2000 ppm toward acetone at 350 °C and good selectivity against other gases, surpassing similar materials made by other methods.
Recently, Xiao et al. [127] reported a successful FSP synthesis of LaCo(Fe-dopped) O3. In [127], a LaCo1−xFexO3-based sensing was evaluated as an ammonia sensor, reaching a detection limit of 2 ppm at 475 °C. These effects were corroborated based on the oxygen vacancy and improvement in electrocatalytic performance by doping iron. Moreover, the sensor exhibited good selectivity against other gases and good stability against oxygen and water vapor concentration fluctuation, with long-term stability for 22 days [127].
Perovskite La1−xFeO3−δ sensors (x = 0, 0.02, 0.05, 0.07, and 0.1), with A-site deficiency, were successfully synthesized by FSP [128], with pure orthorhombic phase and size below 10 nm size. The materials were applied for chemo-resistive CO2 sensors, La0.95FeO3−δ was the optimal stoichiometric material for 5–15% CO2 at 425 °C. Due to surface oxygen vacancies attributed to the small amount of Fe4+, this A-site deficiency might be responsible for enhanced sensing performance [128].

Table 6.
The literature summary of characteristics/conditions for FSP-made perovskite-type nanomaterials.
Table 6.
The literature summary of characteristics/conditions for FSP-made perovskite-type nanomaterials.
| Nanomaterial | Precursors | Solvents | Molarity (mol L−1) | Precursor (mL min−1) | Oxygen (L min−1) | Size (nm) | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BiVO4 | Bi acetate/V oxytripropoxide | 2-ethylhexanoic acid/xylene | 0.25 (Bi)/ 0.25 (V) | 10 | 5 | 62–71 | 7–12 | [122] |
| W-, Zr-BiVO4 | Bi(III) nitrate pentahydrate/V(V) oxytripropoxide | Ethoxy triglycol, acetic acid (70/30 v/v)/xylene (Total: 50/50 v/v) | 0.5 (Bi)/ 0.5 (V) | 5 | 5 | 15–22 | 38–49 | [123] |
| BiFeO3 | Bi(III) acetate/ Fe(III) acetylacetonate | 2-Ethylhexanoic acid/xylene (50/50 v/v) | 0.1 (Bi)/ 0.1 (Fe) | 3 | 7 | 48–67 | 11–14 | [125] |
| Bi2WO6 | Bi(III) nitrate pentahydrate/ W(VI) ethoxide | Ethanol, acetic acid (70/30 v/v) | – | 5 | 5 | 198 | 3.3 | [126] |
| LaCoO3 | Co(II) nitrate hexahydrate/Fe(III) nitrate nonahydrate | Methanol | 0.3 (La)/ 0.3 (Co) | – | 5 | 10 | – | [127] |
| LaFeO3 | La nitrate hexahydrate/Fe(III) nitrate nonahydrate | Ethanol | – | – | 5 | 10 | – | [128] |
| SrTi1−xBxO3 | Sr acetate/ tetrabutyl titanate | Acetic acid/ethanol (50/50 v/v) | 0.075 (Sr)/ 0.075 (Ti)/ | 3 | 5 | 19–37 | 31–46 | [129,130,131] |
| SrTiO3/CuO | Sr acetate/Ti(VI) iso-propoxide | Acetic acid/xylene (50/50 v/v) | 0.2 (Sr)/ 0.2 (Ti)/ | 5 | 5 | 45–55 | 32–57 | [58] |
| NaTaO3/ NiO–Pt0 | Na 2-ethylhexanoate/ Ta(V) chloride | Ethanol | 0.05–0.3 (Na)/ 0.05–0.3 (Ta) | 3–9 | 3–9 | 12–34 | 19–84 | [72,132] |
Recently use of FSP to engineer SrTiO3 perovskite (see Figure 14a–f) was established by Yuan et al. [129,130,131] and Psathas et al. [58]. In [129], SrTi1−xBxO3 (B = Co, Fe, Mn, Ni, and Cu) were produced by FSP at different concentrations (10%, 30%, 50% for Co, 10% for everything else). Co cation was shown to be evenly dispersed in the SrTiO3 structure based on EDX mapping, with an increase in dopant and the growing diameter of active Co particles aligning with TEM findings (see Figure 16d–g). As the cobalt concentration increased from 10 to 50 mol%, the size of Co particles progressively expanded, consequently reducing metal dispersion from 6.50% to 2.18%. Substituting B-site in SrTiO3 with varied valence metal cations created vacancies, enhancing CO/CH4 oxidation, with SrTi0.5Co0.5O3 exhibiting the highest activity [129]. In [130], SrTi1−xCuxO3 particles were reported, with different copper concentrations (10%, 15%, 30%), with the copper species either as amorphous species or/and highly dispersed particles below 5 nm, with XRD indicating that copper ions replaced the Ti4+ in the perovskite lattice. Catalytic low-temperature CO oxidation and CH4 combustion at high temperatures were exemplified [130]. In [131], Sr1−xNaxTi1−yByO3 (B = Co, Mn) with x = 0, 0.1 and y = 0, 0.3, 0.5 were produced by FSP [131]. Doping the perovskite with Na and Co resulted in notably larger SSA from 43 to 65 m2 g−1 compared with cobalt doping alone. Sr0.9Na0.1Ti0.5Co0.5O3 demonstrated superior low-temperature reducibility, higher adsorbed oxygen ratio, and well-dispersed Co elements, resulting in outstanding thermodynamic and kinetic activity for formaldehyde oxidation [131].
Recently, Psathas et al. presented the synthesis of La:SrTiO3/CuO particles by DN-FSP, in tandem with La doping [58]. EDX showed that La doping was homogeneous throughout the SrTiO3 matrix, with no secondary phases formed, such as La2O3. Additionally, a pink-hue color is observed in comparison to the white SrTiO3, evidencing a change in the band gap of the SrTiO3. Interestingly, it was found that La doping significantly increased the SSA and pore volume of La:SrTiO3, from 32 to 53 m2/gr, and more significantly, produced a 300% increase in pore volume to 0.39 cm3 g−1.
As discussed in [58], this is a result of the FSP process where the La doping decreases the packing/aggregation of the SrTiO3. La doping consistently boosted SrTiO3 photocatalytic activity, with 0.9% La doping exhibiting a five-fold increase in H2 production to 12 mmol g−1 h−1 compared with 3 mmol g−1 h−1 for pristine SrTiO3 [58].
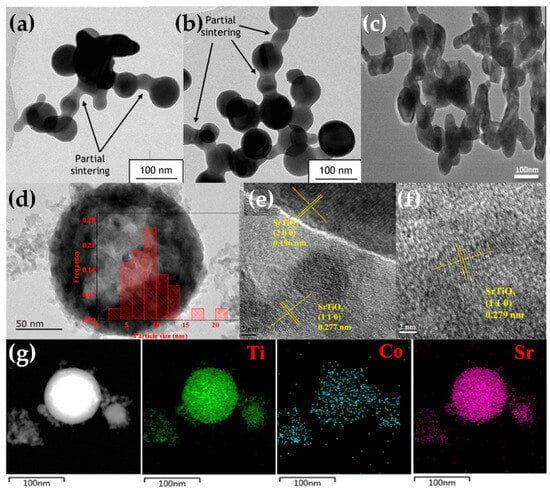
Figure 16.
(a,b) TEM images illustrating necked particles of BiVO4. Reprinted (adapted) with permission from [122]. Copyright 2011 American Chemical Society. (c) TEM images of necked-sintered BiFeO3 particles. Reprinted from [124], with permission from Elsevier. (d–f) HRTEM micrographs of SrTi1−xCoxO3 and the miller planes. (g) EDX element mapping of SrTi1−xCoxO3 for the Ti, Co, and Sr atoms. Reprinted (adapted) with permission from [129]. Copyright 2021 American Chemical Society.
Small-size NaTaO3 was achieved by FSP recently [72,132]. As discussed in [72,132] the inherent need for high-temperature FSP, i.e., HTPRT, required for achieving the ABO3-perovskite formation, poses the challenge of retaining low particle size. As shown in [72], this can be achieved by FSP via diligent control of the temperature profile in the FSP reactor. In this way, highly crystalline small NaTaO3 particles < 15 nm were engineered in one step, outperforming conventional synthesis methods where the previous best size reached 25 nm and typical sizes range around 100 nm [72]. An electron paramagnetic resonance study [132] of these ultrasmall NaTaO3 particles, 12 nm, revealed the significant influence of the nanosize on the life time of photoinduced {h+/e−} pairs. As the NaTaO3 particle size increases, the recombination rate increases and this holds the key to unraveling the fundamental photocatalytic capabilities, e.g., in H2 production [72] of such nanomaterials [132].
3.2. Engineering of Metal Non-Oxides by FSP
The patent of Grass et al. in 2007 marked a pivotal milestone in reducing flame spray pyrolysis (R-FSP) [44]. Since then, FSP technology has undergone a continuous advancement, exploring the engineering of “metal non-oxides” notably encompassing metal sulfides, carbides, halides, phosphates, and carbonates listed in Table 7.
To the utmost extent of our current knowledge, no investigation in regard to the synthesis of nitrides through the FSP methodology has been documented up to the present date. Nitride synthesis via FSP entails a multitude of intricate factors, encompassing nitrogen reactivity, gas chemistry, temperature control, precursor material selection, and prudent safety considerations, collectively rendering it a formidable and demanding process. Kennedy and his colleagues employed the ceramic crucible method to synthesize gallium zinc oxynitrides (GaxZn1−xOyN1−y), with the objective of potentially producing these oxynitrides using the FSP method in forthcoming endeavors [133]. However, a limited body of research exists wherein, in lieu of nitrides, researchers have generated nitrogen (N)-doped titanium dioxide (TiO2) using single-step FSP technology [134,135,136,137,138,139]. Huo et al. [134] and Bi et al. [135] exhibited a novel approach to the synthesis of N-doped TiO2, utilizing a modified FSP reactor. In this method, a spray nozzle was employed to introduce ammonia water, which subsequently underwent vaporization to form H2O vapor and NH3 gas reacting with the TiO2 nanoparticles. Other investigators [136,137,138,139] successfully attained N-doped TiO2 through a straightforward FSP modification, involving the addition of dilute nitric acid to the precursor during the synthesis process, coupled with the introduction of a secondary nitrogen source, specifically urea.
In the aim of carbide synthesis, Herrmann et al. [140] reported the engineering of carbon-encapsulated iron carbide (C/Fe3C) via an R-FSP process. In this case, the spray nozzle was placed in a glove box with a nitrogen atmosphere, which was continuously purged with nitrogen; surrounding the flame allowed for radial inflow of N2 at a flow rate of 25 L min−1 and with a carbon content varying between 1.8 and 8.05 wt%, depending on the acetylene flow rate. Iron exhibits a highly intricate Fe/C phase diagram, featuring numerous intermetallic carbon–iron phases that yield a range of technically valuable metals and steel alloys. By methodically modifying the composition of the flame feed, the authors in [140] were able to monitor the successive reduction of iron oxide to iron and iron carbides (refer to Figure 17).
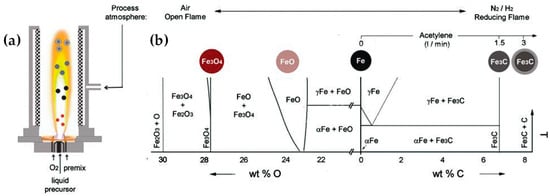
Figure 17.
(a) Reducing FSP configuration for engineering Fe3C or C/Fe3C nanostructures. (b) Controlling the process atmosphere in flame spray synthesis enables the production of diverse iron-based nanoparticles. The resultant composition aligns with the relevant phase diagrams for Fe/O (left side) under oxidizing conditions. Subsequent reduction processes yield iron and iron carbide nanoparticles in accordance with the predictions derived from the Fe/C phase diagram (right side). Reprinted (adapted) with permission from [140]. Copyright 2009, American Chemical Society.
Employing contemporary state-of-the-art methodologies, metal sulfides are typically acquired through diverse chemical processes, including thermolysis, solid-state metathesis, liquid-phase reactions, and thermal decomposition [141]. With respect to flame-made sulfides, a limited number of investigations have been reported in the scientific literature [50,142,143]. Originally, to facilitate the controlled combustion of a ZnS precursor within an environment characterized by oxygen-poor conditions (O2 < 250 ppm), the flame spray nozzle was positioned within a glove box and supplied with nitrogen to establish a reducing atmosphere [50]. Specifically, in [50], Athanassiou et al. mixed ZnO aerosol stream with in situ H2S, which the latter obtained via reductive decomposition of tetrahydrothiophene (THT). Additionally, reducing FSP synthesis was used to investigate the manufacturing of sulfide–oxide PbS−TiO2 heterojunction nanoparticles [142]. Similarly, these investigations were conducted within an enclosed box under a N2 purging environment (as shown in Figure 18a,b). Recently, Pokhrel et al. [143] achieved a significant advancement in this front regarding metal-sulfide nanoparticle synthesis with FSP. As a proof-of-principle [131], MnS, CoS, Cu2S, ZnS, Ag2S, In2S3, SnS, and Bi2S3 are synthesized in an O2-lean and sulfur-rich environment, specifically with metal:sulfur ratios ranging from 1:20 to 1:45 and average primary particle sizes in the range of 10–30 nm (refer to Figure 18d). Explicitly, the researchers mixed all the metal-organic precursors (M = Mn, Co, Cu, Zn, Ag, In, Sn, Bi) with THT directly utilizing enclosed single-droplet (SD) combustion and enclosed reactive spray (RS) FSP configurations (see Figure 18a) [143]. This protocol entailed the need for extreme N2 co-flow, i.e., up to 210 L min−1 of N2 supplied through a quenching ring.
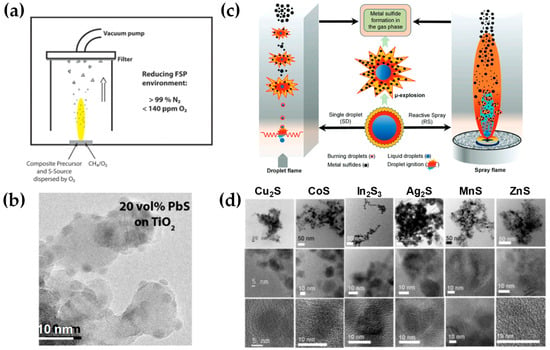
Figure 18.
(a) In a one-step process, a liquid precursor containing lead, titanium, and sulfur is transformed into composite nanoparticles using a high-temperature flame reactor. Precise control of oxygen content enables fine-tuning of the system’s chemistry, facilitating the selective formation of titania support particles (oxides) and lead sulfide quantum dots (sulfides). (b) TEM analysis of a PbS−TiO2 heterojunction. The light contrast TiO2 nanoparticles serve as a support for PbS (darker contrast). Reprinted (adapted) with permission from [142]. Copyright 2012 American Chemical Society. (c) A schematic representation is presented depicting the operation of enclosed single-droplet (SD) combustion and enclosed reactive spray (RS) flame reactors for the production of metal sulfide particles. Emphasis is placed on elucidating the involvement of micro-explosions in the gas-to-particle pathway, observed in both SD and RS configurations. (d) From top to bottom in the column, the images depict the following: an overview of the particles, a high-resolution image of the particles, and a representative single crystalline particle—each of Cu2S, CoS, In2S3, Ag2S, MnS, and ZnS. Reproduced with permission from ref. [143]. Copyright 2023 Wiley-VCH.
In the context of FSP-made halides, Grass and Stark [144] explored FSP for the production of non-oxidic salts, including chlorides in the form of NaCl and fluorides, namely, CaF2, SrF2, BaF2, and Ho-BaF2, using carboxylate precursors and a suitable halide anion source [144]. TEM data showed that the FSP-made BaF2 exhibited well-defined cubic crystallites, whereas SrF2 and CaF2 exhibited slightly irregular spheroidal nanoparticles characterized by dimensions within the range of 10–40 nm [144]. Later, Stepuk et al. [145] reported the synthesis of NaYF4, a sodium yttrium fluoride compound, using a reducing FSP setup (see Figure 19a–c). NaYF4 was doped with rare-earth elements Yb, Tm, or Er for photonic-upconversion applications [136]. The FSP process involved the combustion of precursors within a nitrogen-rich atmosphere (O2 < 100 ppm), with a flow rate of 230 L per hour. TEM analysis evidenced the formation of spherical nanoparticles with sizes 20 to 40 nm (see Figure 19b,c). NaYF4 displayed the capability to undergo a phase transition from cubic to hexagonal upon thermal treatment. The co-doping of Yb-Tm and Yb-Er ion pairs resulted in the generation of blue and green upconversion luminescence, respectively. The aforementioned phosphors are characterized in terms of emission capability, phase purity, and thermal phase evolution [145].
Figure 19.
(a) A diagrammatic representation of the diverse nanoscale structures that can be engineered by the FSP method. (b) TEM depicts the hexagonal FSP-made NaYF4:Yb,Tm nanostructures. (c) XRD data of NaYF4:Yb,Tm nanoparticles obtained under varying fuel/oxygen flow ratios. Reprinted from [145].

Table 7.
The literature summary of FSP characteristics/conditions for multifunctional non-oxide nanostructures.
Table 7.
The literature summary of FSP characteristics/conditions for multifunctional non-oxide nanostructures.
| Nano- Structure | FSP Configuration | Precursor(s) | Solvent(s) | Molarity (mol L−1) | Pilot Flame O2/CH4 (L min−1) | Precursor Flow (mL min−1) | Oxygen Flow (L min−1) | Reducing N2 Flow (L min−1) | Size (nm) | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfides | |||||||||||
| ZnS:Mn2+ | Glove box with N2 atmosphere, two adsorption columns | Zn 2-ethylhexanoate, Mn naphthenate | Tetrahydrofurane, tetrahydrothiophene | 0.25 [S:Zn = 5:1, Mn:Zn = 1/100 at.%] | 2.2/1.2 | 6 | 5 | O2 < 250 ppm | 23 | 33 | [50] |
| PbS–TiO2 | Enclosed box under N2 purge | Pb(II) 2-ethylhexanoate, Ti(IV) isopropoxide, thiophene | Ethylhexanoic acid, THF (2:1) | S/Pb = 2.5 | 2.4/1.13 | 5 | 4–4.5 | >99% N2, O2 < 140 ppm | PbS/TiO2 = 2/10 | 75–85 | [142] |
| MnS | Enclosed reactive spray (large tube) reactor, N2 co-flow through porous ring, additional N2 flow via quenching ring | Mn naphthenate | Tetrahydrothiophene | 0.25 [Mn:S = 1:34] | 2.2/1.2 | 3 | 2.46 | 250 | 13.4 | 62 | [143] |
| CoS | Co naphthenate | Tetrahydrothiophene | 0.25 [Co:S = 1:34] | 2.2/1.2 | 3 | 2.46 | 250 | 13.4 | 10 | [143] | |
| Cu2S | Cu naphthenate or Cu 2-ethylhexanoate | Tetrahydrothiophene | 0.25 [Cu:S = 1:20, 1:41] | 2.2/1.2 | 3 | 2.43–5.61 | 250 | 20.5 | 46 | [143] | |
| ZnS | Zn naphthenate | Tetrahydrothiophene | 0.25 [Zn:S = 1:43] | 2.2/1.2 | 3 | 2.36 | 250 | 5.0 | 64 | [143] | |
| Ag2S | Ag 2-ethoxyhexaonate | Tetrahydrothiophene | 0.25 [Ag:S = 1:45] | 2.2/1.2 | 3 | 2.30 | 250 | 11.6 | [143] | ||
| In2S3 | In acetylacetonate | Tetrahydrothiophene | 0.25 [In:S = 1:43] | 2.2/1.2 | 3 | 2.35 | 250 | 11.6 | 103 | [143] | |
| SnS | Sn 2-ethylhexanoate | Tetrahydrothiophene | 0.25 [Sn:S = 1:42] | 2.2/1.2 | 3 | 2.41, 4.83 | 250 | 11.2 | 106 | [143] | |
| Bi2S3 | Bi neodecanoate | Tetrahydrothiophene | 0.25 [Bi:S = 1:35] | 2.2/1.2 | 3 | 2.45 | 250 | 20.2 | 28 | [143] | |
| Carbides | |||||||||||
| Fe3C | Glove box with radial N2 atmosphere, optional acetylene flow | Carbonyl iron Fe +2-ethylhexanoic acid heating at 140 °C for 24 h→Fe(III)-2-ethylhexanoate | Tetrahydrofuran | 0.5 | 2.2/1.2 | 6 | 5 | 25 | 30 | 30.5 | [140] |
| Halides | |||||||||||
| CaF2 | Open-flame FSP reactor, sheath gas O2 (230 L h−1) through a con-centric sinter metal ring | Ca hydroxide, hexafluorobenzene | 2-ethylhexanoic acid, xylene | 2.4/1.13 | 5 | 5 | – | 14 | 139 | [144] | |
| SrF2 | Sr acetate, hexafluorobenzene | 2-ethylhexanoic acid, xylene | 2.4/1.13 | 5 | 5 | – | 17 | 84 | [144] | ||
| BaF2 | Ba acetate, hexafluorobenzene | 2-ethylhexanoic acid, xylene | 2.4/1.13 | 5 | 5 | – | 34 | 36 | [144] | ||
| Ho–BaF2 | Ba acetate, ho oxide, hexafluorobenzene | 2-ethylhexanoic acid, xylene | 2.4/1.13 | 5 | 5 | – | 26 | 48 | [144] | ||
| NaCl | Sodium hydrogencarbonate, chlorobenzene | 2-ethylhexanoic acid, xylene | 2.4/1.13 | 5 | 5 | – | 92 | 30 | [144] | ||
| NaYF4:Yb (Tm, Er) | Glove box with N2 gas flow | Tm acetate, Y acetate, Yb acetate, Er acetate, sodium bicarbonate | 2-ethylhexanoic acid, xylene | 3, 5, 7, 9 | 7, 5, 3 | O2 < 100 ppm | 20–40 | 29.5–31 | [145] | ||
| Phosphates | |||||||||||
| VOPO4 | The solution was stirred at 60 °C and aged for 1 day | Ammonium vanadate, ammonium dihydrogen phosphate, sucrose | Deionized water, dimethyl-formamide | 0.1 | 3/1 | 5 | 5 | – | 21.7–26.3 | [146] | |
| FePO4 | Open-flame FSP reactor | Fe(III)-acetylacetonate, tri-butylphosphate | Xylene | 0.2 | 2.4/1.13 | 3–7 | 3–8 | – | 30.5, 10.7 | 68.6, 194.7 | [147] |
| FePO4 | Open-flame FSP reactor | Fe(III) nitrate nonahydrate | Denaturized absolute ethanol, tributyl phosphate, 2-ethylhexanoic acid | 0.2 | 2.5/1.25 or 2/3–9 | 2–6 | 6 | – | 10–20 | 104 | [148] |
| Carbon-coated LiFePO4 | Enclosed FSP with quartz tubes, C2H2 and N2 gas flows, sheath O2/N2 mixture (19 L min−1) | Li-acetylacetonate, Fe(III) acetylacetonate, tributyl phosphate | 2-ethylhexanoic acid, toluene, diethylene glycol monobutyl ether, ethanol | 0.24 [Li:Fe:P = 1:1:1] | 2.5/1.25 | 3 | 3 | 20 L min−1 N2, 1 L min−1 C2H2 | 58 | 30 | [149] |
| LiTi2(PO4)3 | Open-flame FSP reactor | Li t-butoxide, Al tri-sec-butoxide, Ti isopropoxide, trimethyl phosphate | 2-methoxyethanol | 2.8 | 5/5.2 | 12.5 | 20 | – | < 50 | [150] | |
| LiGe2(PO4)3 | Open-flame FSP reactor | Li t-butoxide, Al tri-sec-butoxide, Ge ethoxide, trimethyl phosphate | 2-methoxyethanol | 3.2 | 5/5.2 | 12.5 | 20 | – | < 50 | [150] | |
| Biphasic Ca3(PO4)2 | Ultrasonic droplet generator, quartz reactor | Ca Nitrate Tetrahydrate, Diammonium hydrogen phosphate | Ethyl alcohol, distilled water (0.6:0.4) | 0.4 | C3H8 + O2 (5 L min−1) | 5 | 40 | – | 32–38 | [151] | |
| CaP | Open-flame FSP reactor | Ca acetate hydrate, tributyl phosphate | Propionic acid | 0.4 | 2/2 | 2.4 | 9 | – | 23 | 40–50 | [152] |
| CaP:Eu (5 at%) | Open-flame FSP reactor | Ca acetate hydrate, Eu nitrate hexahydrate, tributyl phosphate | 2-ethylhexanoic acid, propionic acid | 0.2, 0.1 | 3.2/1.5 | 8, 3 | 3, 8 | – | 26, 8 | 73, 246 | [153] |
| Carbonates | |||||||||||
| CaCO3 | Open-flame FSP reactor | Ca 2-ethylhexanoate | 2-ethylhexanoic acid, xylene | 0.4 | 3–7 | 3–7 | – | 20–50 | 31.1–102.6 | [154] | |
| BaCO3 | Open-flame FSP reactor | Ba(II) 2-ethylhexanoate | Ethanol | 0.2 | 5 | 5 | – | 50–100 | 20.5 | [155] | |
| Pure metals | |||||||||||
| Bi | Glove box with radial N2 flow, two adsorption columns | Bi(III) 2-ethylhexanoate | Tetrahydrofuran | 2.2/1.2 | 2–6 | 2.5–5 | 25 (O2 < 100 ppm) | 51–127 | [46] | ||
| C/Cu | Glove box with N2 flow | Cu(II)-2- ethylhexanoate | Tetrahydrofuran | 2.2/1.2 | 4.5 | 5 | N2: 1–4 m3/h (O2 < 10 ppm) | 10–20 | 67 | [47] | |
| Fe with Fe3O4 shell | Glove box with N2 flow, supported inverse H2/air diffusion flame | Ferrocene | Xylene, tetrahydrofuran | 0.2–0.8 | 4 | 2.5 | N2: 1.5 m3/h (H2: 0.76 m3/h, air: 0.8 m3/h) | 30–80 | [156] | ||
| Co | Glove box with radial N2 or CO2 flow, two adsorption columns | Co 2-ethylhexanoate | Tetrahydrofuran | 2.2/1.2 | 4.5–6 | 5 | N2 or CO2: 25 (O2 < 100 ppm) | 30 | [57] | ||
Regarding FSP-synthesized phosphates, several investigations have been conducted from the onset of the 21st century to the present day. These studies encompass a broad spectrum of research, examining the properties, applications, and advancements in the realm of phosphates produced via FSP methodology. Kang et al. [157] explored the synthesis of Sr5(PO4)3Cl:Eu2+ phosphor particles through a dual-method approach, employing both conventional spray pyrolysis and flame spray pyrolysis techniques. Subsequently, these synthesized particles underwent post-treatment at a high temperature of 1000 °C over a duration of 3 h within a controlled reducing atmosphere consisting of a gas mixture containing 5% H2/N2. The primary objective of this post-treatment was to activate and optimize the luminescent properties associated with the europium (Eu) component integrated into the phosphor matrix. This process served to enhance the photoluminescence (PL) intensity for light-emitting diode applications [157]. Another work [146] focused on the polymorphic compound VOPO4, known for its exceptional catalytic and electronic properties. For the first time, the utilization of FSP, as an alternative to the conventional hydrothermal synthesis method, is explored to produce various VOPO4 polymorphs [146]. The precursor materials employed in this process consisted of ammonium-based salts, i.e., vanadium and phosphorus, dissolved in an aqueous solution. The resulting FSP products were characterized as hollow, amorphous particles [137] with dimensions 2 to 10 μm and shell thicknesses between 200 and 300 nm (see Figure 20f,g). Last, the study highlighted the size-dependent stability of different VOPO4 polymorphs and examined the influence of distinct precursors in stabilizing the alpha-II and Beta VOPO4 polymorphic forms [146].
Figure 20.
(a) Illustration of an enclosed FSP configuration, comprising a particle formation zone (1), an acetylene carbon black (ACB)-coating region (2), and a quenching zone (3). The precise regulation of O2 stoichiometry within the ACB-coating area is achieved by enclosing the unit with quartz tubes. Subsequently, the coated particles are subjected to cooling using nitrogen (N) at the conclusion of the coating zone to prevent carbon black combustion upon exposure to ambient air during the filtration process. (b) TEM image of as-prepared segregated ACB and LiFePO4 nanostructures. Reprinted from [149], with permission from Elsevier. (c) The XRD pattern of the calcium phosphate (CaP) nanoparticles is presented. Depending on the FSP synthesis conditions employed, the resultant nanoparticles manifest either crystalline or amorphous characteristics. Predominant diffraction peaks are attributed to hydroxyapatite; Ca5(PO4)3OH, though the presence of CaO, is also detected. TEM micrographs of the freshly synthesized (d) CaPL and (e) CaPS materials are provided. The CaPL nanoparticles display a spherical morphology characterized by a loosely agglomerated structure. In contrast, the CaPS particles are evidently fused, with discernible sintered necks. Reprinted from [153]. (f,g) TEM images for as-synthesized vanadium phosphate (VOPO4) particles from (f) sucrose-based solutions and (g) DMF-based solutions. Reprinted from [146].
Nanostructured iron phosphate particles hold appeal for the fortification of staple food products, such as rice and bread, on a large scale [158,159], as well as for their utility in lithium-ion battery materials [160]. Rohner et al. [147] aimed to synthesize FePO4 nanoparticles for bioavailability and assess their toxicity in rats. Amorphous, spherical FePO4 nanopowders were produced via FSP and compared with commercial FePO4 and FeSO4. The FePO4 nanoparticles, both commercial and FSP-produced (mean particle sizes: 30.5 and 10.7 nm), exhibited promising SSA and in vitro solubility, with RBV values close to FeSO4. Importantly, no toxicity indications were observed in histological examinations and thiobarbituric acid reactive substances (TBARS) analysis, suggesting that reducing poorly soluble Fe compounds to the nanoscale could enhance their suitability for human nutrition [147]. Likewise, Rudin and Pratsinis [148] produced FePO4 nanostructured particles FSP, utilizing cost-effective precursor materials. The resulting powders consisted of a fraction of large (mostly > 50 nm) particles made by droplet-to-particle conversion that consisted of maghemite iron oxide (Fe2O3) and a small fraction of the desired amorphous FePO4. The FePO4 powders exhibited excellent solubility in dilute acid, an indicator of relative iron bioavailability [148]. Waser and colleagues [149] employed FSP to synthesize nano-sized spherical LiFePO4 (58 nm)–carbon core-shell (5 nm) particles (see Figure 20b) in a single-step process, achieving a production rate of 7 g per hour, with the intended application for use in Li-ion batteries. A customized FSP apparatus, enclosed within three quartz glass tubes, was employed for this study (as depicted in Figure 20a) [149]. At specific heights, precisely 40 cm and 55 cm above the burner, gaseous C2H2 and N2, respectively, were introduced into the gas stream. This configuration partitioned the reactor into three clearly defined zones: [i] an oxygen-rich core particle formation zone, [ii] an oxygen-deficient region dedicated to C2H2 pyrolysis and carbon coating, and [iii] a designated area for quenching and cooling processes [149]. Li-acetylacetonate, iron(III)-acetylacetonate, and tributylphosphate were combined in a stoichiometric ratio of 1:1:1 (mole ratio Li:Fe:P) and subsequently dissolved within an equimolar mixture of 2-ethylhexanoic acid, toluene, diethylene glycol monobutyl ether, and ethanol. This process yielded a precursor solution with a concentration of 0.24 mol L−1. Additionally as evidenced, the work of Badding et al. [150] pertains to an FSP technique for the production of nanoscale lithium metal phosphate ceramic powders. The precursor solution may incorporate an excess of lithium ranging from 1% to 20% concerning the stoichiometric composition of the ceramic powder. The resulting nanoscale ceramic powders are characterized by nominal compositions denoted as LixAl2−yMy(PO4)3 (<50 nm diameter), with M representing either titanium (Ti) or germanium (Ge), 1 ≤ x ≤ 2 and 1.3 ≤ y ≤ 1.9. Various precursors, such as Li t-butoxide, Al tri-sec-butoxide, Ti isopropoxide, trimethyl phosphate, Li chloride, or Ge ethoxide, were dissolved in 2-methoxyethanol or ethanol to produce the aforementioned lithium metal phosphate nanopowders [150].
Last but not least, it is worth noting that in the past decade and up to the present, various other studies have employed FSP to fabricate nanoscale calcium phosphates (CaP) [161], representing a significant area of scientific investigation and technological development in the field of biomaterials and biomedicine [151,152,153]. Nanometer-sized biphasic calcium phosphate (BCP) powders, featuring various Ca/P molar ratios to achieve specific phase ratios of hydroxyapatite (HA) and beta-tricalcium phosphate (b-TCP), were systematically synthesized using a high-temperature FSP process [151]. These BCP powders displayed spherical morphologies and exhibited narrow size distributions (mean particle size = 38 nm), irrespective of the Ca/P ratios employed. The composition ratio of Ca/P was precisely controlled within the range of 1.500 to 1.723 in the spray solution, allowing for systematic adjustment of the required HA/TCP phase ratios. The FSP configuration comprised an ultrasonic droplet generator and quartz reactor. The precursors employed in this process consisted of calcium nitrate and tetrahydrate diammonium phosphate dissolved in a mixture of ethyl alcohol and distilled water. The degradability of the synthesized BCP powders was studied by the dissolution of calcium ions in a buffer solution under conditions mimicking a human physiological environment [151]. Ataol and colleagues [152] conducted an evaluation of the synthesis of biocompatible nano CaP particles with osteoconductive and osteoinductive properties. These particles were synthesized using an industrially applied aerosol-derived FSP method, with a focus on their potential applications in the biomedical field. The characterization results confirmed that nanometer-sized, amorphous, spherical CaP particles were produced with an average primary particle size of 23 nm. Urine-derived stem cells, resembling mesenchymal stem cells, were exposed to the synthesized amorphous nanoparticles (5–50 µg/mL) with no cytotoxicity. Nanoparticle-treated cells exhibited increased alkaline phosphatase (ALP) activity, indicating osteogenic differentiation, although a slight decline in ALP activity occurred at the highest Ca:P ratios (50 µg/mL) on day 7. These findings suggest that the CaP nanoparticles generated in this study hold promise as potential biomaterials for biomedical applications [152].
Nanoparticles present considerable potential as drug delivery vehicles in the realm of biomedicine, characterized by their substantial surface-to-volume ratio that enables efficient drug loading [162]. In [153], the authors utilized FSP to produce spherical CaP nanoparticles (primary particle diameter of 8 and 26 nm for two samples) with customizable properties (see Figure 20c–e). CaP nanoparticles doped with 5 at% europium (vs. Ca) enabling their detection through luminescence monitoring. These CaP nanoparticles were loaded with model proteins and peptides, and the factors affecting their loading capacity were explored. They also successfully load LL-37, an antimicrobial peptide currently in clinical trials, onto CaP nanoparticles, achieving high loading efficiency and protection against proteolysis in vitro. Importantly, LL-37 maintains its antimicrobial activity against specific pathogens when loaded onto nanoparticles, emphasizing the potential of nanocarriers for optimizing the therapeutic performance of biological drugs [153].
It is noteworthy that a limited body of research, as documented in studies [154,155], has pursued the synthesis of metal carbonates by FSP. Huber and colleagues demonstrated the production of calcium carbonate (CaCO3) nanoparticles of 20–50 nm by FSP [144]. This method involved the combustion of designated calcium-containing precursors, leading to the formation of either amorphous or crystalline calcium carbonate particles, contingent upon the specific spray flow conditions employed. Moreover, Strobel et al. fabricated nanoparticles of bean-like-shaped barium carbonate (BaCO3) with sizes ranging from 50 to 100 nm using FSP (refer to Figure 21). They used barium(II) 2-ethylhexanoate precursor dissolved in ethanol. The key aspect of this preparation process was the rapid quenching, which led to the unique occurrence of pure monoclinic BaCO3 formation.
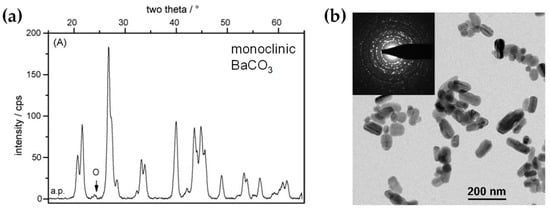
Figure 21.
(a) XRD pattern of the as-synthesized barium carbonate (BaCO3) is presented. Reflections not labeled are attributable to the monoclinic phase of BaCO3. Only minute traces of the orthorhombic phase (O) have been detected. (b) TEM showcasing nanoparticles of BaCO3 synthesized via flame-based methods. The inset provides a representation of the corresponding electron diffraction pattern. Reprinted from [155], with permission from Elsevier.
3.3. Engineering of Metallic Nanoparticles by FSP
Reducing FSP has been shown to allow for the production of metallic nanoparticles (Bi, Cu, Fe, Co). Highly pure metallic bismuth (Bi) nanoparticles [46], with a purity exceeding 98%, were synthesized using a modified flame spray synthesis method conducted in an inert N2 atmosphere, with a radial flow rate of 25 L min−1. This synthesis process involved the oxygen-deficient combustion (O2 < 100 ppm) of a precursor derived from bismuth carboxylates. Scanning electron micrographs revealed the presence of nearly spherical nanoparticles with diameters of 20–80 nm. Copper nanoparticles (C/Cu) featuring an average carbon coating thickness of approximately 1 nm were synthesized by Athanassiou et al. using a reducing FSP method with a N2 atmosphere. TEM images of the C/Cu powder showed that the product consisted of spherical nanoparticles with a diameter of 10–20 nm [47]. Using R-FSP, Li et al. [156] synthesized metallic spherical Fe nanoparticles (size: 30–80 nm) encapsulated by a magnetite Fe3O4 core-shell structure, exhibiting a uniform thickness of 4–6 nm (see Figure 22b). Precursors were based on ferrocene in a solvent mixture xylene and tetrahydrofuran. The ignition of the spray occurred through the utilization of a supported inverse H2/air diffusion flame with specified flow rates (H2: 0.76 m3/h, air: 0.8 m3/h) (see Figure 22a). Additionally, cooling of the spray flame was achieved by introducing 1.5 m3/h of N2. The systematic investigation revealed that controlled nonstoichiometric combustion of the precursor solution leads to morphological and compositional variations in flame-synthesized metallic Fe nanostructures. They proposed a competitive mechanism between in situ flame combustion reduction and oxidation reactions to elucidate the metallic Fe formation [156]. Cobalt nanoparticles [57] were systematically produced through R-FSP conducted under strongly reducing conditions, akin to [46]. A sintered metal tube encompassing the flame facilitated radial inflow of an inert mixing gas (N2 or CO2) at a flow rate of 25 L min−1, ensuring stable combustion. These spherical nanoparticles, with diameters of 20–60 nm, exhibited a metallic face-centered cubic cobalt structure. For further details regarding the chemical synthesis process, please refer to Table 7. Notably, the metal particles were shielded from oxidation by a surface layer measuring less than 1 nm in thickness, primarily composed of cobalt oxide.
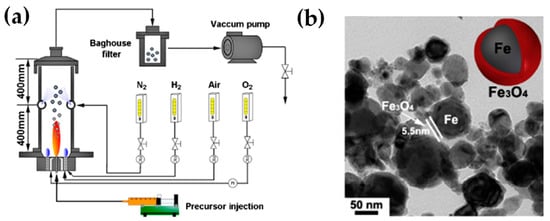
Figure 22.
(a) Illustration depicting the experimental arrangement employed for FSP synthesis of magnetic nanoparticles. Specifically, the reducing flame spray synthesis (RFSP) was executed under a controlled nitrogen atmosphere, with the pilot flame constituted of an O2/H2/air mixture. (b) TEM image of flame-made Fe/Fe3O4 nanostructures. Reprinted from [156], with permission from Elsevier.
3.4. Engineering of Quantum Dots by FSP
Quantum dots (QDs)—with early contributions from pioneering Nobel laureates Ekimov, Brus, and Bawendi [163,164,165]—represent quasi-zero-dimensional (0D) nanomaterials that have garnered considerable interest among researchers [166,167,168,169,170,171]. QDs are semiconductor nanoparticles characterized with dimensions ≪ 10 nanometers. When their dimensions become commensurate with or smaller than the Bohr radius of the respective material, they manifest distinctive characteristics positioned between those of bulk semiconductors and discrete molecules [168,172]. This phenomenon is distinguished by the confinement of excitons within all three spatial dimensions, resulting in the quantization of their energy levels into discrete states [172,173]. A complex challenge concerning the photocatalytic properties of QDs pertains to the differentiation between the advantages arising from increased specific surface areas, stemming directly from reduced particle size, and the emergent properties of the nanolattice, which underpins fundamental solid-state physics [174].
So far, QDs are typically synthesized through [i] top-down syntheses or [ii] bottom-up approaches [170]. For [i], the most commonly used to achieve QDs are electron beam lithography [175,176] and etching processes [177,178] while the wet-chemical (sol-gel [179,180,181], microemulsion [182,183], hot-solution decomposition [165,184]), and vapor-phase methods (molecular beam epitaxy [185], sputtering [186], chemical vapor deposition [187]) are included in [ii]. The aforementioned methods are characterized by extended processing times, multiple steps, and yielding production rates on the order of milligrams per day.
Regarding the FSP production of metal-oxide QDs, there are only a few studies, see Table 8. Mädler et al. [188] reported FSP synthesis of stable zinc oxide quantum dots (ZnO QDs) down to 1.5 nm in diameter (see Figure 23a) stabilized and prevented from growth by adding controlled amounts of silica during spray combustion of Zn/Si precursors [167]. These ZnO QDs exhibited a quantum size effect with a blue shift of light absorption, and their band-gap energy of the ZnO QDs was shown to increase with silica content in the spray and particles consistently [167]. Correspondingly, Riad and colleagues [189] had proposed embedding quantum dots in amorphous matrices such as silica (see Figure 23b). The study demonstrated that FSP can be used to synthesize QDs of many metal oxide semiconducting materials, including TiO2, ZnO, SnO2, and CuO, embedded in a SiO2 matrix [168]. The resulting spherical QDs (mean sizes: 2–8 nm) were found to have a wide range of band gap energies, which could be controlled by adjusting the metal oxide material and the silica content. The SiO2 matrix provided higher mechanical, thermal, and/or chemical stability to the QDs, making them suitable for various applications. Yuan et al. [130] explored the utilization of FSP-made CuO(QD)-SrTiO3 nanocatalysts for the full oxidation of lean CO and CH4. Good performance was achieved due to CuO QDs and metal–support interaction (see Figure 23c). The SrTiO3 perovskite support was discovered to efficiently block CuO quantum dot sintering at high temperatures, resulting in good sintering and water deactivation resistance.

Table 8.
The literature summary of characteristics/conditions for FSP-made quantum dots nanomaterials.
Figure 23.
(a) A HR-TEM image of as-prepared mixed silica-ZnO crystallites. This image prominently displays the crystalline lattice structure of the ZnO component. Additionally, an inset is provided, offering a lower magnification view that elucidates the overall morphology of the powder under investigation. Reprinted from [188], with the permission of AIP Publishing. (b) Combustion flame of the SiO2-metal oxide solution with all the produced colored metal oxide QDs and the TEM image of CuO QDs. Reprinted from [189]. (c) TEM analysis of CuO-SrTiO3 nanostructures, where the CuO QDs are distinctly delineated by dashed yellow circles. Inset: elemental mapping of CuO QDs within a specific region of interest. Reprinted (adapted) with permission from [130]. Copyright 2021 American Chemical Society. (d,e) HR-TEM images and the locally enlarged HR-TEM images of CQDs/TiO2-C. Reprinted from [190], with permission from Elsevier.
Bi et al. [190] fabricated in situ a nano–TiO2 composite material modified by incorporation of internal and external C-species. Specifically, the authors developed a heterostructure composed of carbon quantum dots (CQDs) and TiO2-C through FSP and post-FSP thermal treatment (under Ar/O2 atmosphere) (see Figure 23d,e). During the millisecond-scale reaction process, a portion of the residual carbon resulting from the incomplete combustion of ethanol infiltrates the TiO2 lattice, giving rise to interstitial carbon (Ci) and substituent carbon (Cs). Concurrently, the remaining amorphous CQDs adsorb onto the TiO2 surface. Their investigations, employing in situ temperature-programmed X-ray photoelectron spectroscopy (in situ TPXPS) in combination with various analytical techniques, confirmed the successful fabrication of the CQDs/TiO2-C heterostructure through the flame-based approach, with the atomic ratio of the two carbon species approximating 1:1. This investigation sheds light on the impact of a novel electron transfer pathway established between lattice-bound carbon (C) and CQDs within the CQDs/TiO2–C system during the process of CO2 photoreduction, resulting in a conversion efficiency of 46 μmol g−1 h−1 and nearly 100% selectivity for CO2-to-CO conversion.
3.5. Engineering of Nanoplasmonics by FSP
Nanoplasmonics is a multidisciplinary field of study within the realm of nanophotonics that investigates the interaction between electromagnetic waves and collective oscillations of free electrons, known as surface plasmons, at the interface of metals and dielectric materials [192,193]. In the context of nanoscale dimensions, the phenomenon of localized surface plasmon resonance (LSPR) becomes prominently evident [194,195]. LSPR can be analyzed as comprising both radiative and nonradiative processes: [i] The radiative plasmon decay encompasses the phenomenon of light scattering by the surface of plasmonic particles, thereby giving rise to the distinctive and vibrant colors associated with noble metals. It involves the transfer of energetically “hot” electrons upon irradiation, characterized by substantial kinetic energy, to a compatible acceptor species [196]. Additionally, it engenders the creation of localized amplified electric near-fields, the so-called hot spots owing to the confinement of surface plasmons and incoming photons in the immediate vicinity of the particle [197]. [ii] The nonradiative plasmon decay and the ensuing thermal energy generation may be comprehended as the dissipation of electromagnetic energy into thermal energy. This phenomenon has led to the emergence of a distinct subfield known as thermoplasmonics [198], which harnesses the photothermal characteristics of metallic nanoparticles for various applications. Overall, plasmonics finds diverse applications in various scientific and technological domains, including nanoscale optical waveguiding, sensing, imaging, and enhancing the efficiency of photonic devices, making it a promising area for advancing our understanding of light–matter interactions and facilitating the development of innovative technologies across multiple disciplines. Hereby in this subsection, we shall delineate the research endeavors in which the FSP technique has been successfully employed to engineer plasmonic nanoensembles, with the corresponding characteristics listed in Table 9.
The concept of plasmons was originally postulated in the year 1952 by David Pines and David Bohm [199]. These plasmons were subsequently demonstrated to originate from a Hamiltonian governing the long-range electron–electron correlations [200]. Given that plasmons represent the quantized manifestations of classical plasma oscillations, a substantial portion of their characteristics can be directly deduced through the framework of Maxwell’s equations [201]. Nevertheless, it took approximately half a century for the synthesis of plasmonic nanostructures utilizing the FSP methodology to be achieved. Plasmonic phenomena are principally ascribed to noble metallic elements, notably silver (Ag), gold (Au), and copper (Cu), notwithstanding the potential for plasmonic attributes to manifest in other metallic and material compositions, contingent upon specific environmental conditions. Ag and Au, in particular, display strong plasmonic properties primarily in the visible and near-infrared regions, while copper also exhibits plasmonic behavior, albeit less pronounced, especially in the visible and ultraviolet spectrum.
Several scholarly studies have documented the production of plasmonic materials integrated with support matrices (metal oxides) through the FSP method. The predominant synthesis technique for plasmonic materials involves the use of SiO2 as a core-shell structure (see Figure 24). Silica (SiO2) encapsulation of plasmonic Ag nanoparticles through FSP offers a host of valuable advantages. This method ensures stability by protecting silver nanoparticles from oxidation, while also enhancing their plasmonic properties. Researchers can precisely tailor the nanoparticles’ properties by controlling the thickness of the silica shell. Moreover, the biocompatibility of silica makes these nanoparticles suitable for diverse medical applications. SiO2 shell effectively prevents particle aggregation, ensuring stability in various solvents. Additionally, the silica surface provides a platform for easy functionalization, allowing for targeted interactions. These attributes collectively position them as valuable tools in nanotechnology, materials science, and biotechnology applications (see Figure 25).
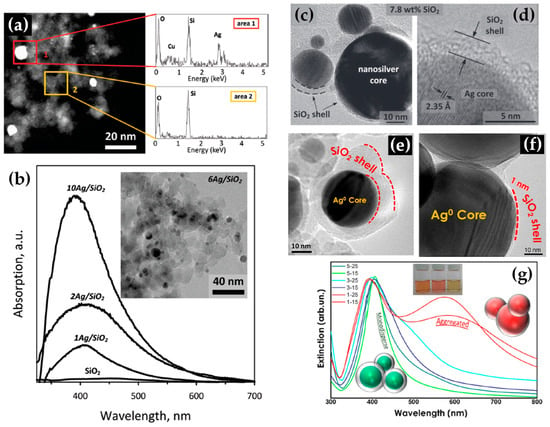
Figure 24.
(a) STEM image of the 2Ag/SiO2 composite material EDX analysis: area 1, containing silver, and area 2, representing pure SiO2. (b) Diffuse reflectance ultraviolet/visible (UV/vis) spectra for various xAg/SiO2 compositions, where x denotes the silver concentration. A consistent plasmon absorption band of Ag metal at 410 nm was observed in all Ag-containing samples. TEM image, shown as an inset, depicted Ag nanoparticles (dark dots) dispersed on nanostructured silica support (gray) in the 6Ag/SiO2 nanostructure. Reprinted (adapted) with permission from [202]. Copyright 2010, American Chemical Society. (c,d) TEM images of the nanosilver coated with 7.8 wt% SiO2 are presented. Reproduced with permission from ref. [78]. Copyright 2010 Wiley-VCH. (e,f) TEM images of (e) 5–25 SiO2@Ag0 NPs (SiO2 thickness = 5 nm), and (f) 1–25 SiO2@Ag0 NPs (SiO2 thickness = 1 nm). (g) UV/vis spectra were recorded for suspensions of SiO2@Ag0 NPs with uniform particle size across three distinct variants: 1–15, 25 (SiO2: 1 nm), 3–15, 25 (SiO2: 3 nm), and 5–15, 25 (SiO2: 5 nm). The inset of the figure presents photos of these particle suspensions. Additionally, schematic representations of the particles are provided to visually convey the influence of shell thickness. Reprinted (adapted) with permission from [80]. Copyright 2019 American Chemical Society.
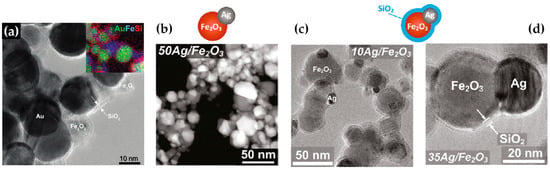
Figure 25.
(a) HR-TEM image depicting nanoparticles consisting of Au/Fe2O3 cores enveloped by amorphous SiO2 shells, measuring 2.6 nm in thickness with the SiO2 content in these shells quantified at 5.7 wt%. Inset: Elemental EDXS mapping for all three elements (Au, Fe, Si) together in a merged image. Reproduced with permission from ref. [203]. Copyright 2014 Wiley-VCH. (b) A HAADF-STEM image, characterized by Z-contrast, displaying the uncoated 50Ag/Fe2O3 sample. In addition, TEM images are presented for (c) the SiO2-coated 10Ag/Fe2O3 sample and (d) the SiO2-coated 35Ag/Fe2O3 sample. Above these visual representations, schematic diagrams are included, illustrating the structural characteristics of both uncoated and SiO2-coated particles. Reprinted (adapted) with permission from [204]. Copyright 2011 American Chemical Society.
In 2004, Johannessen and colleagues [205] synthesized supported noble metals like Au/TiO2 using a quench-cooling device for controlling the residence time at high temperatures in the FSP technique. Hannemann et al. [206] fabricated monometallic Au and bimetallic Au–Ag nanostructures by FSP and characterized their catalytic activity in CO oxidation. The particle size of monometallic Au nanoparticles ranged from 1 to 6 nm, while in the case of bimetallic nanoparticles, the size exhibited a range of 1–10 nm and 25–40 nm [206]. Over the years, a considerable body of research has been dedicated to the synthesis of plasmonic nanoparticles encapsulated within SiO2 and various other matrixes for diverse applications, a trend that continues to be relevant in contemporary studies. Sotiriou and Pratsinis [79] synthesized Ag/SiO2 nanostructures and conducted investigations on the mitigation of nanosilver toxicity, elucidating two mechanisms: (i) the prevention of direct cellular contact with silver and (ii) the inhibition of the release of toxic Ag+ ions. In separate studies [78,202], the same researchers, in collaboration with other colleagues, discriminated between the antibacterial impacts of Ag+ ions and nanosilver particles. They found that smaller Ag nanoparticles (< 10 nm) exhibited a predominant antibacterial effect due to the release of high concentrations of Ag+ ions, whereas larger Ag nanoparticles resulted in antibacterial effects comparable to those of released Ag+ ions and nanosilver particles. All the produced Ag nanoparticles displayed a distinct peak at approximately 400 nm in their UV/visible spectra, which corresponds to the plasmon resonance frequency characteristic of Ag nanoparticles. The precise control over the size of the Ag core and the presence of a SiO2 coating layer are particularly vital properties in numerous applications, notably in the context of bio-labeling [207]. In a related investigation [208], the impact of precursor composition on the characteristics of Ag/SiO2 nanoparticles was examined. Nanosilver synthesized from Ag-acetate and hexamethyldisiloxane (HMDSO) exhibited a unimodal size distribution, while that from Ag-nitrate and HMDSO or tetraethyl orthosilicate precursor solutions displayed a bimodal size distribution. The antibacterial activity against E. coli was evaluated, revealing that Ag-nitrate-derived nanosilver exhibited similar antibacterial effects as Ag-acetate-derived nanosilver, with the fine mode of distribution primarily responsible for bactericidal properties due to the release of silver ions. Furthermore, antibacterial effects correlated best with nanosilver surface area concentration in suspension rather than other magnitudes, suggesting surface area concentrations should be considered in toxicological studies [208].

Table 9.
The literature summary of FSP characteristics/conditions for plasmonic hybrid nanostructures.
Table 9.
The literature summary of FSP characteristics/conditions for plasmonic hybrid nanostructures.
| Nano- Structure | FSP Configuration | Precursor(s) | Solvent(s) | Molarity (mol L−1) | Pilot Flame O2/CH4 (L min−1) | Precursor Flow (mL min−1) | Oxygen Flow (L min−1) | Sheath Oxygen (L min−1) | Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ag/SiO2 | Open-flame FSP reactor, mixed precursors | (a): [Ag nitrate, HMDSO], (b): [Ag acetate, HMDSO], (c): [Ag nitrate, HMDSO] | (a): [Ethanol, diethylene glycolmonobutyl ether], (b): [2-ethylhexanoic acid, toluene], (c): [2-propanol, tetraethyl orthosilicate] | 0.5 | 150 | [202] | ||||
| Core-shell Ag/SiO2 | Enclosed by 2 quartzes tubes of 40 cm + ring deposition with N2 flow (0.8 and 10–30 L min−1) | Ag-acetate, HMDSO | Acetonitrile, 2-ethylhexanoic acid | 3.2/1.5 | 5 | 5 | 40 | Ag: 10–30, SiO2: 3–6 | [79] | |
| Core-shell Ag/SiO2 | Enclosed by quartz tube of 22 cm + ring deposition with N2 flow (0.3–3 and 10–15 L min−1) | Ag-acetate, HMDSO | Acetonitrile, 2-ethylhexanoic acid | 0.3–0.5 SiO2: 8–27 wt% | 5/2.5 | 5–7 | 5 | 5–20 | Ag: 15–25, SiO2: 1–5 | [80] |
| Au/TiO2 | Open-flame FSP reactor, mixed precursors | Ti(IV)-isopropoxide, dimethyl-Au(III) acetylacetonate | Xylene, pyridine (8/2) | 3.1 | 6 | Au: 1–2, TiO2: 9–10 | [209] | |||
| [monometallic Au]–TiO2, Fe2O3/Fe3O4, SiO2 [bimetallic Au/Ag]–TiO2, Fe2O3/Fe3O4, SiO2, Al2O3 | Open-flame FSP reactor, mixed precursors | Dimethyl-Au-(III)-acetylacetonate, silver- (I)-benzoate, Ti(IV)-isopropoxide, Fe(II) naphthenate, tetraethyl ortho-silicate | Xylene, pyridine | 3.5 | 3 | 3 | Au: 1–6, Au/Ag: 1–10, 25–40 | [206] | ||
| Ag/Au–SiO2 | v FSP reactor, mixed precursors | Ag acetate, Au acetate, HMDSO | Acetonitrile, 2-ethylhexanoic acid | 0.15 | 5 | 5 | 4–14 | [210] | ||
| Ag/SiO2 | Closed FSP reaction zone with a H2/air diffusion flame | Ag nitrate, tetraethyl orthosilicate | Ethanol | 3 | 5 | H2/air: 2/25 L min−1 | 30, SiO2: 1–4 | [211] | ||
| TiO2– Ag/TiOx | Open-flame FSP reactor, mixed precursors | Ag acetate, Ti(IV)-isopropoxide | Acetonitrile, 2-ethylhexanoic acid | 0.16 | 3.2/1.5 | 3, 8 | 5 | 20 | 20, Ag: 3–5 | [212] |
| SiO2-coated Ag/Fe2O3 | Enclosed by 30 cm quartz tube + ring deposition with N2 flow (15 L min−1) + 25 cm quartz tube | Ag acetate, Fe(III) acetylace- tonate, HMDSO | Acetonitrile, 2-ethylhexanoic acid | Fe2O3: 0.5, Ag: 0–50 wt% SiO2: 23 wt% | 3.2/1.5 | 5 | 5 | 40 | Fe2O3: 15, Ag: 10–20, SiO2: 1–2 | [204] |
| SiO2-coated Au/Fe2O3 | Enclosed by 30 cm quartz tube + ring deposition with N2 flow (16 L min−1) + 25 cm quartz tube | Au(III) acetate, Fe(III) acetylace- tonate, HMDSO | Acetonitrile, 2-ethylhexanoic acid | Au: 0.25, Fe2O3: 30 at%, SiO2: 0–13 wt% | 3.2/1.5 | 5 | 5 | 40 | Fe2O3: 50–100, Au: 30, SiO2: 2.6 | [203] |
| Ag/Ca3(PO4)2 | Open-flame FSP reactor, mixed precursors | Ag acetate, Ca hydroxide, tributyl phosphate | 2-ethylhexanoic acid, toluene | 0.75 Ca/P: 1.5 Ag: 0–10 wt% | 5 | 5 | 5 | 4 | Ca3(PO4)2: 20–50, Ag: 1–2 | [213] |
Plasmonics give rise to surface-enhanced Raman spectroscopy (SERS) mechanism by facilitating the enhancement of Raman signals. This stems from their ability to create localized electromagnetic field enhancements at the nanoscale, leading to increased scattering cross sections and, thus, contributing significantly to the amplification of the Raman signal of analyte molecules in close proximity [214,215]. In the study of Hu et al. [211], the ultrathin SiO2 shell (1 nm), served a dual purpose by effectively preventing the coalescence of Ag nanoparticle cores at elevated temperatures and functioning as a protective layer for the SERS-active nanostructure. Silica-coated Ag nanoparticles form agglomerates within a large temperature gradient zone, maintaining nanometer-scale gaps between them without direct contact (see Figure 26a). This distinctive feature leads to the creation of numerous Raman hot spots at the interstitial regions among the active Ag core sites, enhancing the overall performance of the SERS substrate. The results [211] demonstrated that the maximum enhancement factor can reach approximately 105, with a detectable concentration as low as 10−10 mol L−1 for rhodamine 6G (R6G) molecules, highlighting the potential of this unique nanostructure for SERS applications (as shown in Figure 26b).
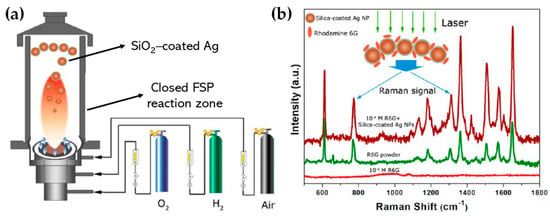
Figure 26.
(a) A schematic figure illustrating the FSP setup employed in the synthesis of silica-coated silver nanoparticles. (b) The Raman spectra analysis encompassed two distinct samples: pure rhodamine R6G powder and R6G molecules at a concentration of 10−6 mol L−1, both with and without the presence of 5 wt% SiO2-coated Ag nanoparticles. The presence of plasmonic particles induces the surface-enhanced Raman scattering (SERS) effect. Reprinted (adapted) with permission from [211]. Copyright 2013, American Chemical Society.
Recently, Moularas et al. [80] engineered core-shell nanoassemblies composed of Ag/SiO2 with diverse SiO2 layer thicknesses (1, 3, and 5 nm (see Figure 24e–g)). These nanostructures were synthesized employing the ring deposition FSP methodology. They investigated the thermoplasmonic heat generation efficiency of these nanostructures [80] and examined the mechanisms underlying plasmon-mediated hot-electron transfer from these plasmonic structures to redox-active metals via hexavalent chromium reduction [81]. In this study, they presented a novel experimental concept and methodology for capturing and quantitatively evaluating plasmon-induced hot electrons produced by core-shell plasmonic nanoaggregates, utilizing EPR spectroscopy [81]. Proton-coupled electron transfer (PCET) reactions, which involve the simultaneous transfer of a proton and an electron, are pivotal in numerous chemical and biological processes. We have [88] introduced a novel phenomenon, plasmon-enhanced PCET, which was achieved using flame-made SiO2-coated Ag nanoparticles functionalized with gallic acid (GA), a natural antioxidant capable of PCET. These GA-functionalized nanoparticles exhibit enhanced plasmonic response in the near-IR range due to GA-induced particle agglomeration. Near-IR laser irradiation induces localized hot spots on the nanoparticles, facilitating PCET by lowering the GA-OH bond dissociation energy through plasmon energy transfer [88].
Nanosilver particles (7–30 nm) immobilized on nanosilica were fabricated by Fujiwara et al. utilizing an open FSP reactor where the two (Ag, SiO2) precursors were mixed together at various precursor feed/dispersion O2 ratios [88]. The presence of Ag2O on the nanosilver surface was confirmed by UV-vis spectroscopy and quantified using thermogravimetric analysis and mass spectrometry. The release of Ag+ ions in deionized water was linked to the dissolution of Ag2O on the nanosilver surface, with rapid pH increase inhibiting further dissolution. However, exposure to CO2 in ambient air led to pH reduction, promoting metallic Ag dissolution and Ag+ ion release. This study investigated Ag+ ion release under different conditions, including CO2 presence, and its relevance to nanosilver’s antibacterial activity [88].
Visible-light active, black TiO2-Ag nanoparticles were synthesized by an FSP setup [205] that promotes the formation of Magnéli Ti4O7 and Ti3O5 nano-spots [205] on their surfaces through the mediation of strong metal–support interactions (SMSI) (see Figure 27) [212]. The TiO2 precursor (Ti-isopropoxide) was combined with various Ag precursor (silver acetate) loadings in a common solution (2-ethylhexanoic acid and acetonitrile), which was subsequently introduced into the flame. These “black titania” plasmonic nanoparticles exhibit excellent stability under ambient conditions and exceptional photoactivity under UV and visible light, making them promising for photocatalytic and solar energy applications [212]. Furthermore, Solakidou et al. [68] employing single-nozzle and double-nozzle FSP (reviewed in Section 2.3), synthesized {plasmonic metal–TiO2 nanohybrids}, specifically Au/TiO2 and Ag/TiO2. These nanohybrids, leveraging the LSPR phenomenon and energetic hot electrons, demonstrated exceptional efficiency in H2 production from photocatalytic reactions involving H2O/methanol [68].
Figure 27.
(a) TEM image of 20Ag/TiO2, synthesized under the condition X/Y = 8/5 (precursor feed rate/dispersion gas) accompanied by selected (b) high-resolution images for detailed examination. Notably, disordered titanium oxide, observable on both the nanosilver and TiO2, is highlighted using blue arrows and red dashed lines. (c) An illustrative diagram is included, depicting the formation of titanium suboxide (Magnéli phases) on nanosilver and TiO2, resulting from robust metal–support interactions (SMSI). Reprinted from [212], with permission from Elsevier.
Biodegradable silver carriers in polymer coatings improve antimicrobial surfaces compared with traditional inert glass or silica-based silver agents. The enhanced silver activity on calcium phosphate Ag/Ca3(PO4)2 as synthesized by Loher et al. [213] is linked to microorganism uptake of nutrition minerals, facilitating timely silver release. This effect results in up to a three-fold increase in bacteria-killing efficiency, notably for E. coli, and reduces silver consumption. These silver carriers have potential applications in food and pharmaceutical production, as well as domestic settings, addressing environmental concerns and offering self-sterilizing surfaces. In healthcare, they exhibit high efficacy against clinically important microorganisms, including C. albicans and P. aeruginosa [213].
Hybrid Ag/Fe2O3 nanoparticles with Janus or dumbbell-like morphology were prepared and coated with a nanothin SiO2 layer using scalable flame aerosol technology [204]. This process involved the utilization of silica vapor through ring deposition. The plasmon absorption band of Ag became more pronounced with increasing Ag content and size, and the Fe2O3 component enabled magnetic manipulation in aqueous suspensions. The SiO2 coating significantly reduced the release of Ag+ ions, rendering the nanoparticles biocompatible for bioimaging. SiO2 coating also minimized agglomeration in contrast to uncoated Ag/Fe2O3 particles, which tended to flocculate and settle quickly (see Figure 25b–d). These hybrid {plasmonic-magnetic} nanoparticles were successfully used as bioprobes, labeled and bound to the membranes of tagged Raji and HeLa cells and detected under dark-field illumination. They combine the benefits of Fe2O3 (particle stability), Ag (plasmonic properties), and SiO2 (inert surface) while overcoming their individual limitations [204]. Similarly, employing the same method, Sotiriou et al. [203] developed biocompatible hybrid nanoaggregates (<100 nm) of Au/Fe2O3 nanoparticles with a nanothin SiO2 coating, suitable for in vivo tumor photothermal ablation via near-IR laser irradiation (see Figure 25a). These nanoparticles exhibit controlled interparticle distances, enabling enhanced photothermal effects. They can effectively ablate tumors in vitro and have potential in in vivo applications. Surface biofunctionalization ensures dispersion in aqueous solutions, while the SiO2 shell preserves superparamagnetism and allows for MRI detection [203].
3.6. Nanofilm Engineering by FSP
Nanofilms, also known as thin films or particle films, are precisely engineered ultrathin layers with nanometer-scale thicknesses that display distinct properties and applications. Nanofilms find diverse utilization in various scientific and technological realms, including optics, electronics, and materials science. Their applications encompass optical coatings for enhancing light transmission or reflection, functional layers in electronic devices, protective coatings against corrosion and wear, templates for the fabrication of nanostructures, and can be efficiently utilized in challenging chemical processes of immediate technological importance, such as H2 production and CO2 reduction, to name a few. The precise control over their thickness and composition allows for tailoring the properties of nanofilms to meet specific requirements, making them indispensable in the advancement of nanotechnology and various cutting-edge technologies. Nanofilms are typically fabricated using various synthesis methods: physical vapor deposition (PVD) [216], chemical vapor deposition (CVD) [217], sol-gel deposition [218], layer-by-layer assembly (LbL) [219], spin coating [220], Langmuir–Blodgett (LB) technique [221], and chemical bath deposition (CBD) [222]. Subsequently, we shall revisit some foundational studies and present recent advancements in the fabrication of nanostructured films through the utilization of FSP methodology (parameters specified in Table 10).
In the mid-2000s, Mädler and coauthors [92] synthesized both undoped and Pt-doped tin dioxide nanoparticles in a single-step process. The aerosol generated via the dry FSP approach was directly subjected to in situ thermophoretic deposition onto interdigitated Pt-electrodes, yielding a porous film with a regulated thickness within the sensor’s active region. A consistent tin oxide grain dimension (10 nm) and elevated film porosity (98%) were maintained across all film thicknesses, spanning from 9 to 40 µm, by adjusting deposition durations. Despite varying deposition times, the tin oxide grain size remained at 10 nm with a film porosity of 98%. Platinum doping had no impact on the SnO2 grain size or film structure. These sensors showcased exceptional carbon monoxide (CO) detection capabilities, especially at 350 °C, with high repeatability and sensitivity. In situ platinum doping improved sensor performance, while adjusting film thickness provided a means to modify sensor resistance.
La0.6Sr0.4CoO3−δ (LSC) thin films, fabricated by Karageorgakis et al. [223] on sapphire substrates and ceria-gadolinia oxide (CGO) pellets using flame spray deposition at 200 °C, were initially amorphous and dense (refer to Figure 28f,g). The precursor solution was formulated by dissolving lanthanum nitrate, strontium chloride, and cobalt nitrate in N,N-dimethylformamide, resulting in a concentration of 0.006 M. Post-annealed at temperatures above 600 °C, they exhibited nanocrystalline grains, with sizes from 21.4 5 nm at 700 °C to 124 22 nm at 900 °C. Early annealing stages introduced 10–23% porosity, which reduced upon further thermal treatments. Electrochemical tests underscored the necessity of crystallinity for optimal LSC film performance [223]. Films annealed at 400 °C had high area-specific resistance (ASR) values (5.8 Ω cm2) at 600 °C, whereas those annealed at 700 °C achieved a lower ASR of 0.96 Ω cm2. However, annealing beyond 700 °C raised the ASR due to grain enlargement. Over a 5-day evaluation at 550 °C, ASR degradation was 3.9% for 700 °C-annealed films and 7% for 800 °C-annealed ones, indicating that 700 °C-annealed LSC films hold promise for micro-solid oxide fuel cells (SOFC) applications [223].

Table 10.
The literature summary of FSP characteristics/conditions for aerosol nanofilm deposition.
Table 10.
The literature summary of FSP characteristics/conditions for aerosol nanofilm deposition.
| Nano-Film | FSP Thin Film Configuration | Precursor(s) | Solvent(s) | Molarity (mol L−1) | Pilot Flame O2/CH4 (L min−1) | Precursor Flow (mL min−1) | Oxygen Flow (L min−1) | Remarks | Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt/SnO2 | The open flame FSP reactor was used to directly deposit pa- rticles onto a sensor (alumina) substrate. | Sn(II) 2-ethylhexanoic acid, Pt acetylacetonate | Toluene | 0.5 | 3.2/1.5 | 5 | 5 | Sensor response = 8 for 50 ppm CO at 350 °C | 10 | [92] |
| ZnO, TiO2 | ZnO/TiO2 films are deposited on amorphous silica substrates of 10 mm × 10 mm. | Zn acetate dissolved in deionized water./ Precipitation of hydrous Ti oxide by add- ing ammonia to TiOCl2 solution. The preci- pitate is filtered, washed, mixed with trie-thanolamine, heated at 200 °C for 2 h, and then diluted with deionized water. | 35 g L−1/ 50 g L−1 | C2H2:O2 = (1:1) | 16,000 | Remazol brilliant blue dye is degraded by ZnO and TiO2. Reactivity: ZnO (90% after 60 min) > TiO2. | 100–500 | [96] | ||
| La0.6Sr0.4 CoO3−δ (LSC) | The LSC thin films have been flame spray deposited on sapphire substrates and ceria-gadolinia oxide pellets. | La nitrate, Sr chloride, Co nitrate | N,N-dimethylformamide | 0.006 | 7/2 | 0.5 | 20 | Area-specific resistance (ASR) of 0.96 Ω cm2 at 600 °C. ASR degraded 3.9% in 5 days at 550 °C in the air. | 38 | [223] |
| SnO2, TiO2 | Particles deposited by orthogonal FSP aerosol impingement on a 20 cm temperature-controlled substrate (alumina) holder. | Sn(II)-ethylhexanoate or Ti(VI) isopropoxide | Xylene | 0.1–0.5 | 3.2/1.5 | 5 | 5 | Introduces a theoretical framework for calculating FSP deposition rates based on the thermal gradient (ΔT) between aerosol and substrate interfaces. | SnO2: 10, TiO2: <50 | [98] |
| Ag/PMMA-coated glass substrates | FSP deposition of Ag particles which are sub sequently embedded by spin-coating to form polymer nanocomposite films. | Ag acetate | 2-ethylhexanoic acid and acetonitrile (1:1) | 0.4 | 3.2/1.5 | 3 | 6 | Scalable manufacturing of <1 μm polymeric nanocomposite films with high conductivity (5 × 104 S cm−1), even under repeated bending. | <100 | [224] |
| ZnO ultraporous networks | FSP deposition of ZnO nanoparticle films onto glass substrates with Au interdigitated electrodes. | Zn naphthenate | Xylene | 0.3 | 2/1.2 | 5 | 7 | Photodetector performance achieved the highest reported photo-to-dark-current ratio (3.4 × 105) at very low light intensity (0.1 mW cm−2). | 19 | [93] |
| BiVO4 photoanodes | FSP and thermophoretic deposition of the nanoparticle aerosols directly on FTO glass substrates. | Bi(III) 2-ethylhe- xanoate, vanadyl naphthenate | 2-ethylhexanoic acid and toluene (1:1) | 0.1 | 2/1.8 | 5 | 5 | BiVO4 photoanodes with 46% film porosity and 400 nm thickness, yielding significant photocurrent densities for sulfite (1.5 mA cm−2) and water (1.0 mA cm−2) oxidation. | <400 | [225,226,227] |
| Pt-Ru | Flame aerosol synthesis of Pt-Ru deposited dire-ctly as a thin layer on the gas diffusion layer. | Ru(III) acetylacetonate, Pt(II) acetylacetonate | Isooctane and tetrahydrofuran (4:1) | 0.5 | 2.2 | Pt-Ru anode electrodes for the direct methanol fuel cell (DMFC). Flame-catalyst surpasses 10%Pt–Ru/C E-TEK commercially with 60% higher activity at 0.4 V for methanol oxidation at 90 °C. | 10.3 | [228] | ||
| Core-shell Ag-SiO2 | FSP and thermophoretic deposition of plasmonic Ag-SiO2 on temperature-controlled cover glasses. | Silver acetate (reflex at 110 °C for 1.5 h), tetraethyl orthosilicate | 2-ethylhexanoic acid and acetonitrile | 3.2/1.5 | 5 | 5 | FSP fabrication of SERS sensing substrates. Excellent performance for detecting pesticide residue in orange juices. | 12 | [229] | |
| Ag/TiOx–polymer | Ag/TiOx was FSP and thermophoretically deposited on Si substrates or PDMS polymer layers and mechanically stabilized with ethanol spray annealing. | Silver acetate, Ti(VI) isopropoxide | 2-ethylhexanoic acid and acetonitrile (1:1) | 0.16 | 8 | 5 | The optimized polymer nanocomposite films effectively eradicate biofilms upon short, on-demand visible light exposure of 15–90 min with no cytotoxic effects on mammalian cells. | 190–600 | [230] | |
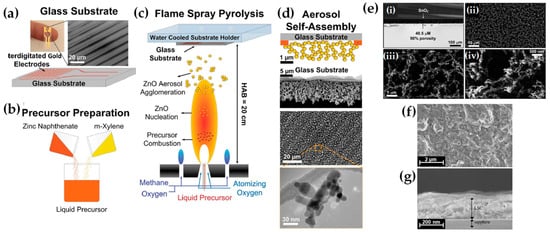
Figure 28.
(a) A photodetector substrate and (b,c) the synthesis via flame spray pyrolysis and subsequent aerosol self-assembly, resulting in (d) ultraporous films composed of electron-depleted ZnO nanoparticles at a 20 cm height above the burner (HAB). These films are uniformly structured and predominantly consist of spherical particles with an average TEM size of 19 nm (d). Reproduced with permission from ref. [93] Copyright 2015 Wiley-VCH. (e) SEM cross-sectional (i) and top views (ii–iv) of a SnO2 nanoparticle film, aerosol-deposited with a precursor concentration of 0.5 mol/L, for a duration of 4 min. Reproduced with permission from ref. [98] Copyright 2012 Wiley-VCH. (f,g) SEM images of an as-deposited LSC thin film produced by flame spray deposition on a sapphire substrate: (f) top view and (g) cross-sectional view. The film was deposited over a duration of 15 min at 200 °C during the flame spray deposition process. Reprinted from [223], with permission from Elsevier.
As discussed in Section 2.5, the deposition of SnO2 and TiO2 nanoparticles from FSP reactors onto temperature-controlled substrates is influenced primarily by the temperature difference (ΔT) between the aerosol and substrate [98]. For SnO2, the deposition rate increased linearly with time and was dominated by thermophoresis up to ΔT of 121 K. This mechanism was linked to the rapid formation of large agglomerates. The primary particle concentration of a SnO2 aerosol aligned with its mobility size distribution, accounting for agglomerate structure and size (see Figure 28e). The study also provided an entrainment rate for surrounding air. Findings suggest that nanoparticle deposition rates for similar aerosols can be predicted using initial precursor concentrations, making the model applicable to various nozzle designs and FSP settings [98].
Polymeric nanocomposite films, characterized by nanoparticle-specific attributes, are gaining attention for potential use in advanced functional materials and miniaturized devices tailored for electronic and biomedical sectors. Blattmann and his colleagues [224] reported the method of flame aerosol deposition of metallic nanosilver onto water-cooled either uncoated or polymer-coated (poly methyl methacrylate, PMMA) glass substrates. Subsequent polymer spin coating resulted in the swift creation of flexible, free-standing, electrically conductive nanocomposite films. The electrical conductivity of these films is ascertained during fabrication and is influenced by the substrate composition and the duration of Ag deposition. Consequently, these researchers have fabricated thin (less than 500 nm) and flexible nanocomposite films with conductivities comparable to metals, such as a value of 5 104 S cm−1, which is maintained even upon repetitive bending [224]. In a recent publication from Nasiri et al. [93], they introduced a sophisticated hierarchical morphology for ultraviolet (UV) photodetectors. This structure offers superior selectivity and has demonstrated an unprecedented high mA photocurrent response even at minimal ultraviolet light intensities, accompanied by nA dark currents. The study elucidates a swift singular-step FSP synthesis and self-assembly process to produce transparent ultraporous films. The FSP system was employed to directly deposit ZnO nanoparticle films onto glass substrates with Au-interdigitated electrodes (see Figure 28a–d) [93]. A solution of 0.3 M zinc naphthenate in xylene was supplied at 5 mL min−1, dispersed using 7 L min−1 O2, and ignited with a premixed O2/CH4 flame. Substrates, situated 20 cm above the burner, were kept below 150 °C using a water-cooled holder. Through optimization of the film structure, an absorption rate exceeding 80% of incoming ultraviolet radiation while maintaining a transmission rate approaching 90% for visible light was achieved. Further examination of the photodetector’s efficacy, especially under minimal light intensity conditions (0.1 mW cm−2), yielded a significant photo-to-dark current ratio of 3.4 105. Such results underscore the potential of this architecture, offering a versatile and scalable technological framework for the swift, cost-efficient production and assimilation of ultraviolet photodetectors in CMOS (complementary metal oxide semiconductor)-compatible mobile apparatuses [93].
Li et al. [229] have presented FSP-based engineering SERS sensing substrates. Plasmonic Ag-SiO2 nanoaggregates were deposited on glass substrates, yielding consistent and efficient SERS sensing films. These substrates exhibited remarkable uniformity, sensitivity, and batch consistency [226]. Compared with other commercial alternatives, these substrates achieved similar performance but at a lower cost, suggesting their broad applicability. Their work’s standout feature was the use of a real-world sample, fresh orange juice, demonstrating SERS’s potential for on-site, label-free detection of contaminants in specific pH conditions using minimal samples [229]. Bletsa et al. [230] synthesized a visible-light-responsive Ag/TiOx coating optimized for application on medical devices, aiming for targeted biofilm eradication. Fabricated via the direct deposition of flame aerosol onto substrates, this methodology facilitated simultaneous nanoparticle generation and film formation (see Figure 29). The outcome is a porous nano-thin suboxide Ag/TiOx particulate layer, which exhibited visible-light photocatalytic activity within, enabling the generation of superoxide radicals adept at biofilm disruption [227]. Various Ag/Ti (Ag acetate/Ti(IV) isopropoxide in a 1:1 mixture of 2-ethylhexanoic acid and acetonitrile) weight concentrations, ranging from 5% to 50%, were produced [230]. The deposition varied in duration from 5 to 60 s, impacting film thickness. These films were mechanically stabilized via in situ annealing with an ethanol spray (as shown in Figure 29a). Notably, the biocompatible surface of the coatings, derived from polydimethylsiloxane (PDMS), exhibited no cytotoxic effects on mammalian cells. Emphasizing the eradication of pre-existing biofilms and evaluating antibiofilm activity on nanoparticle coatings, this study underscores the promise and durability of such coatings for medical device applications [230].
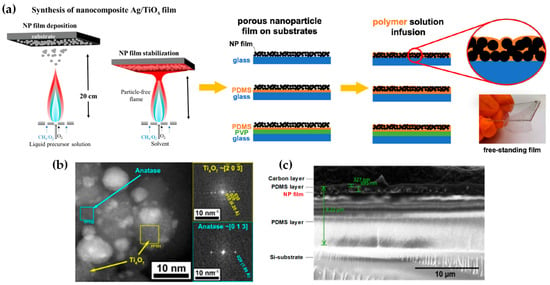
Figure 29.
(a) Using flame aerosol deposition and mechanical stabilization (in situ annealing), nanostructured films are produced in a single step. Nanoparticles formed in the flame are thermophoretically deposited onto substrates like Si, glass, or polymer-coated materials. By infusing a polymer, such as through spin coating, these films gain mechanical stability. Incorporating a sacrificial layer, like polyvinylpyrrolidone (PVP), enables the creation of free-standing polymer nanocomposite films. (b) HRTEM image of Ag/TiOx nanoparticles illustrates the pronounced crystallinity of both Ag and TiOx within the synthesized nanoparticles. (c) Cross-sectional scanning electron microscopy (SEM) representation of the Ag/TiOx polymer nanocomposite film with a deposition time (td) of 15 s and a composition of 50% Ag/Ti. Reprinted from [230].
3.7. FSP Nanostructures for Sensing Applications
In the realm of nanotechnology and sensor technology, extensive reviews have been conducted to assess the efficacy of flame-made particles as viable materials for sensor applications. Recent scientific literature has featured comprehensive reviews aiming to assess the potential of FSP in this context. Kemmler and colleagues [231], in particular, have embarked upon an investigation to evaluate the utility of FSP as an inherently more efficient approach for sensor development. FSP has been employed to synthesize a diverse array of semiconducting metal oxide (SMOX) materials, along with the deposition of sensitive gas films onto substrates. Additionally, Tricoli et al. [232] conducted a comprehensive investigation into the methodologies utilized in gas sensor fabrication, including FSP and other relevant techniques. Righettoni and coauthors [233] offer an evaluation of the capabilities of metal oxide chemi-resistive gas sensors concerning their utility in breath analysis and monitoring. Simultaneously, Güntner et al. [234] delineate the prevailing obstacles and strategic approaches related to breath sensors for health monitoring. A concise review authored by Sheng et al. [235] presents a catalog of catalysts and sensor materials synthesized through flame-based methods. Ultimately, the most recent review by Pokhrel and Mädler [40], encompassing the period until 2020, highlights significant instances of FSP nanostructures deployed in sensor applications. Hereinunder in the present compilation, we discuss an up-to-date record of studies conducted subsequent after 2020 (characteristics cataloged in Table 11), thereby elucidating the latest advances and research in the domain of FSP-based sensors.
Flame-synthesized AgOx-doped SnO2 nanoparticles were assessed for formaldehyde (HCHO) detection [236]. Following FSP, both undoped and AgOx-doped SnO2 nanostructures, ranging from 0.1 to 1 wt%, were homogenously dispersed within an organic paste comprising ethyl cellulose and α-terpineol. This dispersion process was undertaken with the intention of fabricating the sensing films. An optimal 0.2 wt% Ag content yielded a strong response to HCHO, with high selectivity, stability, and minimal sensitivity to environmental conditions (refer to Figure 30a). The improved performance is attributed to p-n heterojunctions and catalytic effects between AgOx and SnO2 [236].
Figure 30.
(a) HCHO-sensing models of SnO2 nanoparticles with AgOx-doping at a moderate content (0.2 wt%), and the response histogram of the AgOx-doped SnO2 sensors with different Ag contents (S-0 to S-1Ag) for toxic gases (NH3 and NO), flammable gases (C2H2, C2H4, H2 and CH4), and VOCs (C3H6O, C6H6, C2H5OH, HCHO, CH3OH, C7H8, and C8H10) at 350 °C. Reprinted from [236], with permission from Elsevier. (b) H2-sensing models of PtOx-loaded Zn2SnO4 nanoparticles with optimum Pt contents, and the sensor response to 10,000 ppm H2 of 0–3 wt% PtOx-loaded Zn2SnO4 (S-0 to S-3Pt) as a function of operating temperature in the range of 200–400 °C. Reprinted from [237], with permission from Elsevier.
Kaewsiri et al. [237] synthesized PtOx-loaded Zn2SnO4 nanoparticles with varying Pt concentrations (0–3 wt%) for hydrogen (H2) sensing using single-nozzle FSP. The structural analysis confirmed the uniform attachment of 1–3 nm PtOx nanoparticles to 5–15 nm cubic Zn2SnO4 nanoparticles. Sensing layers were produced via spin coating and evaluated for their response to environmental gases and VOCs at 200–400 °C under dry ambient conditions. The sensor with an optimal 2 wt% Pt content exhibited remarkable H2 selectivity, with a response of 1500.4 and a fast response time of ∼3.4 s to 10,000 ppm H2 at 350 °C (see Figure 30b). This sensor showed low sensitivity to humidity, high stability, and reproducibility. These outcomes were attributed to the PtOx-Zn2SnO4 heterointerface influence on spillover mechanisms [237].
Detection of ammonia (NH3) is crucial for the optimal modulation of the selective catalytic reduction (SCR) system. In the study of Xiao and colleagues [127], an NH3 sensor was developed using a LaCo1–xFexO3 sensing electrode, yttria-stabilized zirconia (YSZ) electrolyte, and a platinum (Pt) reference electrode. The LaCo1–xFexO3 materials were synthesized via FSP, with Fe substitution at the B-site to enhance sensing efficacy. Within a concentration range of 20–70 ppm at 475 °C, the sensor with the LaCo0.9Fe0.1O3 electrode showcased the highest sensitivity to ammonia compared with other variants. The sensor demonstrated a segmented linear relationship between response value changes and logarithmic ammonia concentration, with sensitivities of 17.52 and 87.22 mV/decade within specified ranges. The exemplary sensing attributes are attributed to oxygen vacancies and the electrocatalytic efficiency of the perovskite electrode. The sensor also exhibited robust selectivity against various gases and maintained stability amid environmental fluctuations. A 22-day test revealed minimal response variance, and the study also touched upon the sensor’s underlying mixed potential sensing mechanism [127]. Moreover, nanoparticles of SnO2 doped with 0.1–2 wt% Nb were synthesized for the first time using FSP [238]. Structural analyses, including XRD and electron microscopy, confirmed Nb5+ integration within the lattice of nanocrystalline tetragonal SnO2 particles (5–15 nm). Sensing layers, created through spin coating, were evaluated against 0.05–1 vol% acetylene (C2H2) from 200–400 °C in the air. Optimal performance was noted with 0.5 wt% Nb, yielding a sensor response of ∼776 and a rapid 1.1-s response time at 350 °C. This Nb-doped SnO2 sensor displayed minimal humidity interference, prolonged stability, and heightened C2H2 selectivity against various compounds. The results underscore the Nb dopant’s catalytic and electronic contributions, positioning the Nb-enhanced SnO2 sensor as a viable candidate for C2H2-sensing applications [238].

Table 11.
The literature summary of FSP characteristics/conditions and sensing data for FSP-made gas/VOC sensors, based on research post-2020.
Table 11.
The literature summary of FSP characteristics/conditions and sensing data for FSP-made gas/VOC sensors, based on research post-2020.
| Nano- Device | FSP Setup, Sensor Fabrication | Precursor(s) | Solvent(s) | Molarity (mol L−1) | Precursor Flow (mL min−1) | Oxygen Flow (L min−1) | Sensing Performances | Size (nm) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas, VOC | Conc. (ppm)/temp. | Response | |||||||||
| AgOx–doped SnO2 | c FSP reactor, spin coating | Sn(II) 2-ethylhexa-noate, silver nitrate | Xylene, acetonitrile | 0.5 | 5 | 5 | HCHO | 100, 200, 2000/350 °C | 67,107,495 | AgOx: <3, SnO2: 5–20 | [236] |
| PtOx–Zn2SnO4 | Open flame FSP reactor, powder pasting, spin coating | Zn(II) acetylacetonate, Sn(II)2-ethylhexanoate/Pt(II) acetylacetonate | Methanol/xylene | 0.5 | 5 | H2 | 150, 1000, 10,000/350 °C | 30.1, 216.4, 1500.4 | PtOx: 1–3, Zn2SnO4: 30–40 | [237] | |
| Er-doped SnO2 | Open flame FSP reactor, powder pasting, spin coating | Sn(II) 2-ethylhexa-noate, Er(III) acetylacetonate hydrate | Ethanol | 0.5 | C2H4O | 30, 10, 5, 1, 0.05/350 °C | 347, 95, 46, 7.6, 1.3 | 5–20 | [239] | ||
| LaCo1–xFexO3 | Enclosed-flame FSP reactor (pilot flame = CH4: 1.25, O2: 3.2, Ar: 5.0 L min−1), powder pasting, spin coating | La nitrate, Co nitrate, Fe nitrate | Methanol | 0.3 | 5 | NH3 | 20–70/475 °C | Sensitivity: 87.22 mV/decade | 10 | [127] | |
| Nb-doped SnO2 | Open flame FSP reactor, powder pasting, spin coating | Sn(II) 2-ethylhexa-noate, Nb(V) ethoxide | Absolute ethanol | 0.5 | 5 | 5 | C2H2 | 1000/350 °C | 776 | 5–15 | [238] |
| Graphene/Rh–doped SnO2 | Open flame FSP reactor, electrolytic exfoliation, powder pasting, spin coating | Rh(III) acetylacetonate, Sn(II) 2-ethylhexanoate | Xylene | H2S | 10/350 °C | 439 | 5–20 | [240] | |||
| Ga2O3/Nb | Open flame FSP reactor with chamber, post-annealing | Potassium acetylacetonate, gallium nitrate in dichloromethane/Nb(V) 2-ethylhexanoate | Toluene | 0.2 | 3 | 1.5 | C3H6O, H2, CH4 | 20/470 °C, 20/500 °C, 10,000/500 °C | 9.8, 2.5, 4 | <3 | [241] |
| La2O3–WO3 | Open flame FSP reactor, powder pasting, spin coating | Ammonium (meta) tungstate hydrate, La(III) nitrate hydrate | Diethylene glycol monobutyl ether, ethanol | 0.02 | 5 | 5 | NO2 | 5, 1, 0.05/ 150 °C | 7213.6, 1045.3, 15.2 | La2O3: 1–2, WO3: 5–20 | [242] |
| La1–xFeO3–δ with A-site deficiency | Enclosed-flame FSP reactor (pilot flame = CH4: 1.25, O2: 3.2, Ar: 5.0 L min−1), powder pasting, screen-printing | La nitrate, Fe nitrate | Absolute ethyl alcohol | 5 | CO2 | 10%/425 °C | 3.38 | <10 | [128] | ||
| Zn2SnO4 | Open flame FSP reactor, powder pasting, spin coating | Zn(II) acetylacetonate, Sn(II) 2-ethylhexanoate | Methanol, xylene | 0.5 | 5 | 5 | HCOOH | 1000, 50, 20/300 °C | 1829, 41.65, 10 | 5–25 | [243] |
The same research team [240] synthesized Rh-doped SnO2/electrochemically exfoliated graphene hybrid materials via flame processes and conducted a comprehensive study on their gas sensing capabilities, particularly toward hydrogen sulfide (H2S). Structural analyses showcased an enhanced surface area due to the integration of graphene sheets on Rh-substituted SnO2 nanoparticles. In systematic evaluations against various gases from 200 to 400 °C in diverse humidity levels (20–80% RH), it was discerned that 0.5 wt% Rh doping substantially improved the H2S sensing of SnO2 nanoparticles. Incorporating an optimal 0.5 wt% graphene further optimized this sensing capability. Specifically, the 0.5 wt% graphene-loaded 0.5 wt% Rh-doped SnO2 sensor yielded peak responses of ∼439 and a swift 6.5-s response to 10 ppm H2S at 350 °C, with pronounced selectivity against other gases. This enhanced H2S detection was attributed to the combined effects of catalytic Rh dopants and the active interfaces between graphene and Rh-doped SnO2 [240]. Utilizing the FSP method, ultrafine β-Ga2O3 nanomaterials with a grain size of 6–12 nm and a surface area above 100 m2/g are produced [241]. Introducing Nb(V) during synthesis results in its integration into the β-Ga2O3 lattice but annealing above 800 °C forms a separate GaNbO4 phase. The incorporation of Nb(V) into β-Ga2O3 results in charge compensation within the cationic sublattice, manifesting through a Ga(III) to Ga(I) electronic transition. Despite these alterations, Nb(V) doping does not significantly enhance the gas-sensing properties of β-Ga2O3. The best sensor response is observed with 1 mol% Nb(V) doped Ga2O3 after annealing at 900 °C for 24 h. These samples excel in gas and VOC detection (H2, CH4, acetone) and offer improved long-term performance [241].
Recently, La2O3-loaded WO3 nanoparticle films, produced via FSP by Siriwalai et al. [242] using spin-coating techniques, were optimized and analyzed for NO2 sensing. Characterization techniques confirmed the presence of 1–2 nm La2O3 nanoparticles on 5–20 nm WO3 nanoparticles. For NO2 detection, the optimal performance was achieved with La concentrations up to 0.2 wt% and three spin-coating cycles. This configuration yielded a response of approximately 7213.6 to 5000 ppb NO2 with a 31.8 s response time at 150 °C. The sensor also showed high NO2 selectivity against other gases and volatile organic compounds, with minimal humidity interference (refer to Figure 31) [242]. Jiao et al. [128] synthesized A-site deficient perovskite nanomaterials, La1–xFeO3–δ (0 ≤ x ≤ 0.1), using the FSP method and employed them in chemo-resistive CO2 sensors. XRD, TEM, and XPS confirmed the material’s structure and indicated enhanced CO2 sensing due to surface oxygen vacancies and Fe4+ ions. Among tested materials, La0.95FeO3–δ was optimal for detecting 5–15% CO2 at 425 °C, showing a response of 3.38 to 10% CO2. This sensor effectively distinguished CO2 from common vehicular emission gases but showed sensitivity to water vapor. Consequently, La0.95FeO3–δ emerges as a promising candidate for CO2 monitoring in vehicle exhaust [128].
Figure 31.
(a) Schematic diagrams for FSP synthesis of La2O3-loaded WO3 nanoparticles. (b) Areal-view SEM images of S-2La (two spin-coating cycles). (c) Sensor responses toward 5000 ppb NO2 of 0–2 wt% La2O3-loaded WO3 two-cycle spin-coated films (S-0 to S-2La) in terms of temperature (25–350 °C). Reprinted (adapted) with permission from [242]. Copyright 2023, American Chemical Society.
3.8. FSP Nanostructures for Electrocatalytic and Energy Conversion Applications
In the field of electrocatalysis technology, comprehensive evaluations have assessed the effectiveness of particles produced by FSP for electrocatalytic applications. Electrocatalysis involves the facilitation of electrochemical reactions via a catalyst affixed to an electrode, effectively lowering the activation energy barrier. The process encompasses the adsorption of reactants onto the electrode-bound catalyst, followed by an electron exchange during which the reactant is either oxidized or reduced. Subsequent desorption of the reaction product from the catalyst surface allows the transformed species to enter the bulk solution. This sequence enhances the reaction kinetics, with the rate-limiting step being a primary determinant of overall efficiency. Electrocatalysts are selected for their efficiency in specific electron transfer processes, optimizing the conversion of reactants to desired products. In photoelectrochemical (PEC) catalysis (see Figure 32a), light-excited semiconductors produce electron–hole pairs that drive redox reactions at electrode interfaces, aided by electrocatalysts, which lower activation energies and improve reaction kinetics, enabling efficient light-to-chemical energy conversion.
Recent review articles focus on exploring the prospects of flame spray pyrolysis (FSP) in this area. Debecker et al. [244] provide a comprehensive examination of how aerosol processing technologies are revolutionizing the preparation and application of heterogeneous catalysts in various chemical reactions, aiming at both current and future innovations. Similarly, Sheng and colleagues [235] highlight FSP as a key technology for the economical and scalable production of catalytic nanomaterials. Chen and collaborators [94] present FSP as a crucial technique for fabricating nanostructured films, emphasizing its role in advancing sustainable energy technologies, particularly in the context of water catalysis for hydrogen fuel production. In our introductory section, we referenced Tran-Phu et al. [42] who underscore the role of FSP in developing effective (photo) electrocatalysts for sustainable energy storage systems without CO2 emissions. Concurrently, John and Tricoli [43] delve into the advancements and future potential of flame-assisted nanofabrication in the context of device integration and industrial applications.
Some studies have selected flame-made cobalt oxide for electrocatalytic processes because it is considered a very promising material for electrochemical reactions. This is due to its high catalytic activity, reasonable electrical conductivity, chemical stability in various environments, high surface area, and versatile morphology. In the study conducted by our laboratory, Belles and coauthors [245] developed flame-made Co3O4/CoO nanocatalysts for oxygen reduction reaction (ORR) electrodes. In an acidic environment, an electrode composed of 5.2% Pt and 4.8% Co3O4 exhibited optimal ORR efficiency, achieving a maximum current density of 8.31 mA/cm2 and a half-wave potential of 0.66 V. Conversely, in an alkaline setting, an electrode with 0.4% Pt and 9.6% CoO/Co3O4 demonstrated enhanced performance (Jmax = 3.5 mA/cm2, E1/2 = 0.08 V). Pozio et al. [246] and Tran-Phu et al. [247] utilized FSP to manufacture Co3O4 spinels for use in the oxygen evolution reaction (OER). In addition, Liu and colleagues [248] investigated the electrocatalytic capabilities of Co3O4 nano-islands, which were directly synthesized and deposited using FSP, as OER catalysts for hydrogen generation on FTO glass substrates.
Tran-Phu and colleagues [227] explored the photooxidation of BiVO4 photoanodes with porosities from 12% to 18%, finding that 46% porosity offered the best photocurrent density for sulfite and water oxidation due to optimal charge transport and reaction surface area (Figure 32c–f). Daiyan et al. [249] revealed that subjecting flame-synthesized CuO nanomaterials to mild plasma treatment introduced oxygen vacancies/defects. These defects were closely linked to enhanced performance and endurance in nitrogen oxide reduction reactions (NOxRR), resulting in an ammonium (NH4+) production rate of 520 μmol cm−2 h−1 at a cell voltage of 2.2 V in a flow electrolyzer (see Figure 32b). Bismuth (Bi) catalysts are among the best performing candidates for electrochemical reduction of CO2 (CO2RR) to formate products [250,251]. Tran-Phu et al. [251] produced flame-made fractals Bi2O3 on carbon fibers, which demonstrated superior catalytic activity for CO2RR to formate, with a high mass-specific formate partial current density of −52.2 mA mg−1 at −1.2 V (Figure 32g–i).
FSP-produced materials have primarily been utilized as electrodes in fuel cells, batteries, supercapacitors, and dye-sensitized solar cells for energy conversion. Their high-quality metal oxide semiconductors, characterized by purity and crystallinity from the flame method, exhibit desirable chemical stability and electronic traits beneficial for various energy devices. Subsequent paragraphs will offer a succinct overview of notable flame-generated material compositions and device structures.
Figure 32.
(a) Schematic representation of a photoelectrochemical (PEC) cell’s fundamental mechanism, featuring an n-type semiconductor photoanode for oxygen evolution and a platinum sheet photocathode for hydrogen generation in water splitting. (b) Optical (i) and SEM (ii–iv) imaging of flame-made BiVO4 films on FTO substrates demonstrate variations based on differing HAB settings, with cross sections at (ii) 15 cm, (iii) 10 cm, and (iv) 6 cm HAB corresponding to 60, 20, and 5 s deposition times, ensuring uniform absorbance in each film. (c–f) BiVO4 photoanode PEC metrics were assessed in relation to (c) porosity, (d) thickness during oxidation in air-saturated 1 M KB (pH 9.3) with 0.2 M Na2SO3 as a hole scavenger under FTO-side (solid), and BiVO4-side (dashed) illumination. (e) PEC responses in 1 M KB (pH 9.3) were measured with (solid) and without (dashed) FeOOH/NiOOH electrocatalyst modification under FTO-side illumination. The SEM inset of (e) displays the morphology post-FeOOH/post FeOOH/NiOOH application on a 12% porosity sample. Dark current densities were logged for the 12% porosity sample with (dark dot) and without (yellow dot) electrocatalyst overlay. Measurements were taken via voltammetry at 0.010 V s−1 ascending potential in 1 sun equivalent light (AM 1.5 G, 100 mW cm−2). (f) Longevity trials were conducted on FeOOH/NiOOH-modified samples in 1 M KB (pH 9). Reproduced with permission from ref. [227]. Copyright 2019 Wiley-VCH. (g–i) CO2RR efficacy of f-Bi2O3 catalysts was evaluated in CO2-saturated 0.1 M KHCO3. (g) f-Bi2O3 exhibited specific voltammetry profiles at 5 mV s−1, with comparative data for filter-collected Bi2O3. (h) The current density for formate production on f-Bi2O3 and Bi2O3 varied with potential during CO2RR. (i) The Faradaic efficiency for formate generation during CO2RR correlated with aerosol deposition duration, with measurements taken at −1.2 V vs. RHE. Reproduced with permission from ref. [251]. Copyright 2019 Wiley-VCH.
A fuel cell is an energy conversion device that transforms the chemical energy of a fuel, typically hydrogen, and an oxidant, usually oxygen from the air, into electricity through an electrochemical reaction, bypassing the traditional combustion process [252]. The basic components of a fuel cell include two electrodes—an anode and a cathode—and an intervening electrolyte (refer to Figure 33a). At the anode, H2 fuel undergoes a chemical reaction that separates it into positively charged H2 ions (protons) and negatively charged electrons. The electrolyte allows only the protons to pass through to the cathode, while the electrons are directed through an external circuit, generating an electric current. On reaching the cathode, the electrons, returning from the external circuit, combine with the protons that have passed through the electrolyte and with O2 from the air. This reaction produces water and heat as the only byproducts, making fuel cells an environmentally friendly technology.
Seo et al. [253] used FSP to engineer Ce1–xGdxO2–x/2 nanoparticles for fuel cell electrolytes, pelletizing and sintering them at 1400 °C for 3 h. They observed that higher temperatures improved ionic conductivity due to increased oxide ion mobility. Their FSP-derived nanoparticles demonstrated the highest conductivity, attributed to larger lattice constants. Lee and colleagues [254] found that incorporating catalysts into fuel cell electrodes, such as FSP-synthesized carbon-supported Pt-Ru (refer to Figure 33d), not only lowers operating temperature but also mitigates CO poisoning. Their study revealed that these flame-made materials exhibited superior methanol oxidation and CO stripping capabilities (see Figure 33b,c), along with competitive electrochemical activity, outperforming commercial catalysts of the same composition in fuel cell performance [254].
Figure 33.
(a) Simplified fuel cell’s schematic representation. (b) Methanol oxidation and (c) CO stripping processes utilizing carbon-supported Pt-Ru catalyst produced via FSP in contrast to the performance of standard E-TEK catalysts. (d) Schematic figure depicting the apparatus used for the flame aerosol synthesis of Pt-Ru/C catalysts, with an accompanying TEM image of the synthesized Pt-Ru/C catalysts in the inset. Reprinted from [254], with permission from Elsevier. (e) Schematic of a Li-ion battery illustrating its constituent components. Reprinted from [255]. (f) The LTO battery cell fabrication involves setting KaCu substrates in an FSP reactor for LTO deposition, followed by compression and transfer to a glove box for sputtering LiPON, lithium, and copper, leading to the final cell assembly. (g,h) Nyquist diagrams for battery cells featuring fully lithiated rock-salt-type Li7Ti5O12 (g) and completely delithiated (initial state) spinel Li4Ti5O12 (h) thin films. (i) Morphological analysis of compressed LTO thin films includes (i) photographs, (ii) SEM imaging on KaCu substrates, (iii) 2D LSM color and laser imaging, and (iv) 3D surface profiling. Reprinted from [256], with permission from Elsevier.
The growing importance of electric energy storage in batteries is driven by the rising use of portable devices and electric vehicles, and the need to store renewable energy. Among different technologies, rechargeable Li-ion batteries stand out for their high energy density and lifespan [257]. These batteries consist of an anode, cathode, and a separator soaked in an electrolyte, with Li-ions moving between the anode and cathode during charge and discharge cycles (Figure 33e). Traditionally made through solid-state or sol-gel methods, Li-ion batteries often face challenges like lengthy production times, multiple steps, and impurity presence. Therefore, numerous studies are focusing on enhancing the fabrication of materials and assembly of electrodes, crucial for battery performance.
Gockeln et al. [256] utilized FSP for the direct fabrication of Li4Ti4O12 (LTO) batteries on flexible polyimide foil (refer to Figure 33f–i). They noticed a potential plateau deviation and shortening at higher current densities, linked to cell polarization under these conditions. Additionally, they explored the performance of in situ-coated nano LTO/C batteries made via a combination of FSP and pressure-based lamination. They observed a stable voltage plateau at discharge rates up to 1 °C, suggesting enhanced charge/discharge reaction kinetics due to improved electronic conductivity in the microstructure.
4. Concluding Remarks—Future Perspectives
Flame spray pyrolysis (FSP) is a highly potent technology for the synthesis of advanced nanostructures. While FSP has achieved significant milestones, both in terms of material variety and process optimization, the journey toward its full potential is ongoing. So far, FSP has produced more than 500 different materials, and it is widely used in academia (more than 30 groups working with FSP), adapted by industry (officially Johnson Matthey has announced the use of an FSP facility), and the largest public reactor installed in Spain (http://www.advance-fsp.eu/, accessed on 5 November 2023) producing several kilograms/day. The future prospect lies in designing such high-enthalpy spray combinations using economic metal salts (nitrates, chlorides, acetates) that react with the solvent producing in situ metal alkoxides opening the door toward easily available wide range of inexpensive precursors.
Diligent control of FSP parameters allows for targeted manipulation of nanoparticle characteristics, which is essential for catalyst optimization. These parameters, including flame temperature and precursor feed rate, significantly affect the size and shape of nanoparticles, thereby directly impacting their catalytic activity. Smaller particles, due to their greater surface areas, exhibit higher activity because of the increased number of active sites. FSP also facilitates the synthesis of tailored composite or doped nanoparticles, offering precise control over their composition and dopant distribution, which enhances activity and selectivity. The high temperature conditions in FSP enable the production of high-purity, crystalline nanoparticles, crucial for ensuring catalyst stability and conductivity. Additionally, surface properties, which are vital in electrocatalysis, can be modified through the adjustment of FSP’s quenching rate and atmosphere, influencing surface defects and functionalities. Finally, managing nanoparticle agglomeration through FSP is key; reducing agglomeration helps maintain an effective surface area, thereby improving electrocatalyst performance.
Incorporating automation and advanced process control through the utilization of artificial intelligence (AI) and machine learning promises enhanced consistency and quality in nanoparticle synthesis. These technologies, in conjunction with sophisticated sensors, enable real-time data acquisition and dynamic optimization of the FSP process. Concurrently, the development of in situ characterization techniques during FSP synthesis offers deeper insights into nanostructure formation mechanisms, thereby facilitating a more exacting command over the synthesis process.
Despite this broad range of benefit and potential, the use of FSP synthesis for the integration of nanocomponents in devices still faces some challenges. Among those, the large amount of nanomaterial produced in the aerosol phase, the need to manage large gas volumes, and high temperatures make the integration of flame reactors in clean rooms and standard nanofabrication facilities difficult. The intrinsic very high porosity of aerosol self-assembled nanoparticle films at low temperature makes them fragile and prevents the use of such films in a liquid environment. Sufficient in situ sintering of the layers during deposition is not always possible for materials having low sintering rates and for substrates not able to withstand sufficiently high temperatures.
Innovations are required in the design of flame-assisted synthesis to overcome these challenges and establish it as a standard fabrication route for the integration of nanomaterials in a variety of devices and applications. As a forward-looking summary, several focal points emerge:
- (a)
- Multifunctionality Enhancement: As the title of this review suggests, multifunctionality is at the heart of FSP’s premise. Future advancements should emphasize developing processes that can fabricate nanostructures with an even broader range of functionalities, expanding their utility across diverse sectors. There is growing interest in the development of hybrid versions of FSP that incorporate additional physical processes, such as electric fields or ultrasound [258,259]. The integration of an electric field into the FSP process, for instance, could offer enhanced control over particle formation. By applying an electric field, it is possible to influence the charge distribution within the flame, potentially leading to more uniform particle sizes and shapes.
- (b)
- Technological Synergies: Harnessing the potential of complementary technologies, like AI and real-time monitoring tools, will trigger the era of “smart” FSP [260]. This would not only ensure the efficient production of desired nanostructures but might also open doors to materials previously deemed unattainable.
- (c)
- Economic Precursor Development: As FSP broadens its reach, the use of economical and readily available precursors will be essential. Research geared toward identifying and harnessing such materials will significantly reduce production costs, making FSP-synthesized nanoparticles more accessible.
- (d)
- Challenge Mitigation: While the review refers to the advancements, it is vital not to overlook the inherent challenges of FSP, be it in scalability, integration with standard fabrication environments, or ensuring the robustness of the nanostructures. Innovations addressing these challenges head-on will be instrumental in FSP’s widespread adoption.
- (e)
- Expanding Application Horizons: While FSP-derived nanoparticles have found their place in various applications, constant research is required to explore untapped potentials. Fields such as renewable energy, biomedicine, and advanced electronics may witness revolutionary products birthed from FSP advancements. Integrating biological components, such as enzymes or other organic molecules, into the FSP process might enable the synthesis of bio-functionalized nanoparticles. These could have specific applications in targeted drug delivery, biosensors, or bio-catalysis.
- (f)
- Environmental and Safety Concerns: As with all industrial processes, the environmental impact of FSP, along with safety concerns, will need continuous evaluation. Future iterations of FSP should aim for greener processes, ensuring sustainability.
In summary, FSP technology is poised to play a pivotal role in the next wave of nanotechnological innovations.
Author Contributions
Writing—original draft preparation, C.D., P.P. and M.S.; writing—review and editing, C.D., P.P. and M.S.; supervision, Y.D.; project administration, Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meierhofer, F.; Fritsching, U. Synthesis of Metal Oxide Nanoparticles in Flame Sprays: Review on Process Technology, Modeling, and Diagnostics. Energy Fuels 2021, 35, 5495–5537. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.D. Special Report. Chem. Eng. News Arch. 1984, 62, 22–29. [Google Scholar] [CrossRef]
- Liu, S.; Mohammadi, M.M.; Swihart, M.T. Fundamentals and Recent Applications of Catalyst Synthesis Using Flame Aerosol Technology. Chem. Eng. J. 2021, 405, 126958. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition (ALD): From Precursors to Thin Film Structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, J.; Berndt, C.C.; Tikkanen, J.; Wang, J.Y.; King, A.H.; Herman, H. Nanomaterial Powders and Deposits Prepared by Flame Spray Processing of Liquid Precursors. Nanostructured Mater. 1997, 8, 61–74. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z.J.M.H. The Evolution of ‘Sol–Gel’ Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal Technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Hess, D.W. Plasma-enhanced CVD: Oxides, Nitrides, Transition Metals, and Transition Metal Silicides. J. Vac. Sci. Technol. A 1984, 2, 244–252. [Google Scholar] [CrossRef]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of Plasma-Enhanced Chemical Vapor Deposition as a Method for Thin-Film Fabrication with Biological Applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part J. 2017, 34, 80–90. [Google Scholar] [CrossRef]
- Jansson, U.; Lewin, E. Sputter Deposition of Transition-Metal Carbide Films—A Critical Review from a Chemical Perspective. Thin Solid Films 2013, 536, 1–24. [Google Scholar] [CrossRef]
- Thornton, J.A. The Microstructure of Sputter-deposited Coatings. J. Vac. Sci. Technol. A 1986, 4, 3059–3065. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave Assisted Synthesis—A Critical Technology Overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis—A Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Swihart, M.T. Vapor-Phase Production of Nanomaterials. Chem. Soc. Rev. 2021, 50, 7132–7249. [Google Scholar] [CrossRef]
- Rsanchez. Technology Readiness Assessment Guide—DOE Directives, Guidance, and Delegations. Available online: https://www.directives.doe.gov/directives-documents/400-series/0413.3-EGuide-04a (accessed on 25 October 2023).
- Workie, A.B.; Ningsih, H.S.; Shih, S.-J. An Comprehensive Review on the Spray Pyrolysis Technique: Historical Context, Operational Factors, Classifications, and Product Applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Teoh, W. A Perspective on the Flame Spray Synthesis of Photocatalyst Nanoparticles. Materials 2013, 6, 3194–3212. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Pratsinis, S.E. Flame Aerosol Synthesis of Smart Nanostructured Materials. J. Mater. Chem. 2007, 17, 4743. [Google Scholar] [CrossRef]
- CRC. Handbook of Chemistry and Physics, 95th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-0867-2. [Google Scholar]
- Sokolowski, M.; Sokolowska, A.; Michalski, A.; Gokieli, B. The “in-Flame-Reaction” Method for Al2O3 Aerosol Formation. J. Aerosol Sci. 1977, 8, 219–230. [Google Scholar] [CrossRef]
- Bickmore, C.R.; Waldner, K.F.; Treadwell, D.R.; Laine, R.M. Ultrafine Spinel Powders by Flame Spray Pyrolysis of a Magnesium Aluminum Double Alkoxide. J. Am. Ceram. Soc. 1996, 79, 1419–1423. [Google Scholar] [CrossRef]
- Tikkanen, J.; Gross, K.A.; Berndt, C.C.; Pitkänen, V.; Keskinen, J.; Raghu, S.; Rajala, M.; Karthikeyan, J. Characteristics of the Liquid Flame Spray Process. Surf. Coat. Technol. 1997, 90, 210–216. [Google Scholar] [CrossRef]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled Synthesis of Nanostructured Particles by Flame Spray Pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Mädler, L. Liquid-Fed Aerosol Reactors for One-Step Synthesis of Nano-Structured Particles. KONA Powder Part. J. 2004, 22, 107–120. [Google Scholar] [CrossRef]
- Camenzind, A.; Caseri, W.R.; Pratsinis, S.E. Flame-Made Nanoparticles for Nanocomposites. Nano Today 2010, 5, 48–65. [Google Scholar] [CrossRef]
- Sotiriou, G.A. Biomedical Applications of Multifunctional Plasmonic Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2013, 5, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of Catalytic Materials in Flames: Opportunities and Challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, Y.; Biswas, P.; Tse, S.D. Flame Aerosol Synthesis of Nanostructured Materials and Functional Devices: Processing, Modeling, and Diagnostics. Prog. Energy Combust. Sci. 2016, 55, 1–59. [Google Scholar] [CrossRef]
- Schneider, F.; Suleiman, S.; Menser, J.; Borukhovich, E.; Wlokas, I.; Kempf, A.; Wiggers, H.; Schulz, C. SpraySyn—A Standardized Burner Configuration for Nanoparticle Synthesis in Spray Flames. Rev. Sci. Instrum. 2019, 90, 085108. [Google Scholar] [CrossRef]
- Pokhrel, S.; Mädler, L. Flame-Made Particles for Sensors, Catalysis, and Energy Storage Applications. Energy Fuels 2020, 34, 13209–13224. [Google Scholar] [CrossRef]
- Venkatesan, S.; Mitzel, J.; Wegner, K.; Costa, R.; Gazdzicki, P.; Friedrich, K.A. Nanomaterials and Films for Polymer Electrolyte Membrane Fuel Cells and Solid Oxide Cells by Flame Spray Pyrolysis. Renew. Sustain. Energy Rev. 2022, 158, 112080. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Daiyan, R.; Ta, X.M.C.; Amal, R.; Tricoli, A. From Stochastic Self-Assembly of Nanoparticles to Nanostructured (Photo)Electrocatalysts for Renewable Power-to-X Applications via Scalable Flame Synthesis. Adv. Funct. Mater. 2022, 32, 2110020. [Google Scholar] [CrossRef]
- John, A.T.; Tricoli, A. Flame Assisted Synthesis of Nanostructures for Device Applications. Adv. Phys. X 2022, 7, 1997153. [Google Scholar] [CrossRef]
- Grass, R.N.; Stark, W.J.; Athanassiou, E.-K. Reducing Flame Spray Pyrolysis Method for the Production of Metal, Non-Oxidic, Ceramic and Reduced Metal Oxide Powders and Nano-Powders, European Patent Office. European Patent EP1760043A1, 7 March 2007. [Google Scholar]
- Grass, R.N.; Albrecht, T.F.; Krumeich, F.; Stark, W.J. Large-Scale Preparation of Ceria/Bismuth Metal-Matrix Nano-Composites with a Hardness Comparable to Steel. J. Mater. Chem. 2007, 17, 1485. [Google Scholar] [CrossRef]
- Grass, R.N.; Stark, W.J. Flame Spray Synthesis under a Non-Oxidizing Atmosphere: Preparation of Metallic Bismuth Nanoparticles and Nanocrystalline Bulk Bismuth Metal. J. Nanopart. Res. 2006, 8, 729–736. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Large-Scale Production of Carbon-Coated Copper Nanoparticles for Sensor Applications. Nanotechnology 2006, 17, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently Functionalized Cobalt Nanoparticles as a Platform for Magnetic Separations in Organic Synthesis. Angew. Chem. Int. Ed. 2007, 46, 4909–4912. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, E.K.; Grass, R.N.; Osterwalder, N.; Stark, W.J. Preparation of Homogeneous, Bulk Nanocrystalline Ni/Mo Alloys with Tripled Vickers Hardness Using Flame-Made Metal Nanoparticles. Chem. Mater. 2007, 19, 4847–4854. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. One-Step Large Scale Gas Phase Synthesis of Mn2+ Doped ZnS Nanoparticles in Reducing Flames. Nanotechnology 2010, 21, 215603. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Pratsinis, S.E. Direct Synthesis of Maghemite, Magnetite and Wustite Nanoparticles by Flame Spray Pyrolysis. Adv. Powder Technol. 2009, 20, 190–194. [Google Scholar] [CrossRef]
- Kumfer, B.M.; Shinoda, K.; Jeyadevan, B.; Kennedy, I.M. Gas-Phase Flame Synthesis and Properties of Magnetic Iron Oxide Nanoparticles with Reduced Oxidation State. J. Aerosol Sci. 2010, 41, 257–265. [Google Scholar] [CrossRef]
- Kumfer, B.M.; Skeen, S.A.; Axelbaum, R.L. Soot Inception Limits in Laminar Diffusion Flames with Application to Oxy–Fuel Combustion. Combust. Flame 2008, 154, 546–556. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Mantzanis, A.; Zindrou, A.; Smykala, S.; Solakidou, M. Control of Monomeric Vo’s versus Vo Clusters in ZrO2−x for Solar-Light H2 Production from H2O at High-Yield (millimoles gr−1 h−1). Sci. Rep. 2022, 12, 15132. [Google Scholar] [CrossRef] [PubMed]
- Zindrou, A.; Belles, L.; Solakidou, M.; Boukos, N.; Deligiannakis, Y. Non-Graphitized Carbon/Cu2O/Cu0 Nanohybrids with Improved Stability and Enhanced Photocatalytic H2 Production. Sci. Rep. 2023, 13, 13999. [Google Scholar] [CrossRef] [PubMed]
- Zindrou, A.; Deligiannakis, Y. Quantitative In Situ Monitoring of Cu-Atom Release by Cu2O Nanocatalysts under Photocatalytic CO2 Reduction Conditions: New Insights into the Photocorrosion Mechanism. Nanomaterials 2023, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Grass, R.N.; Stark, W.J. Gas Phase Synthesis of Fcc-Cobalt Nanoparticles. J. Mater. Chem. 2006, 16, 1825. [Google Scholar] [CrossRef]
- Psathas, P.; Zindrou, A.; Papachristodoulou, C.; Boukos, N.; Deligiannakis, Y. In Tandem Control of La-Doping and CuO-Heterojunction on SrTiO3 Perovskite by Double-Nozzle Flame Spray Pyrolysis: Selective H2 vs. CH4 Photocatalytic Production from H2O/CH3OH. Nanomaterials 2023, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Mädler, L.; Piacentini, M.; Maciejewski, M.; Baiker, A.; Pratsinis, S.E. Two-Nozzle Flame Synthesis of Pt/Ba/Al2O3 for NOx Storage. Chem. Mater. 2006, 18, 2532–2537. [Google Scholar] [CrossRef]
- Minnermann, M.; Grossmann, H.K.; Pokhrel, S.; Thiel, K.; Hagelin-Weaver, H.; Bäumer, M.; Mädler, L. Double Flame Spray Pyrolysis as a Novel Technique to Synthesize Alumina-Supported Cobalt Fischer–Tropsch Catalysts. Catal. Today 2013, 214, 90–99. [Google Scholar] [CrossRef]
- Høj, M.; Pham, D.K.; Brorson, M.; Mädler, L.; Jensen, A.D.; Grunwaldt, J.-D. Two-Nozzle Flame Spray Pyrolysis (FSP) Synthesis of CoMo/Al2O3 Hydrotreating Catalysts. Catal. Lett. 2013, 143, 386–394. [Google Scholar] [CrossRef]
- Schubert, M.; Pokhrel, S.; Thomé, A.; Zielasek, V.; Gesing, T.M.; Roessner, F.; Mädler, L.; Bäumer, M. Highly Active Co–Al2O3 -Based Catalysts for CO2 Methanation with Very Low Platinum Promotion Prepared by Double Flame Spray Pyrolysis. Catal. Sci. Technol. 2016, 6, 7449–7460. [Google Scholar] [CrossRef]
- Horlyck, J.; Pokhrel, S.; Lovell, E.; Bedford, N.M.; Mädler, L.; Amal, R.; Scott, J. Unifying Double Flame Spray Pyrolysis with Lanthanum Doping to Restrict Cobalt–Aluminate Formation in Co/Al2O3 Catalysts for the Dry Reforming of Methane. Catal. Sci. Technol. 2019, 9, 4970–4980. [Google Scholar] [CrossRef]
- Stahl, J.; Ilsemann, J.; Pokhrel, S.; Schowalter, M.; Tessarek, C.; Rosenauer, A.; Eickhoff, M.; Bäumer, M.; Mädler, L. Comparing Co-catalytic Effects of ZrOx, SmOx, and Pt on COx Methanation over Co-based Catalysts Prepared by Double Flame Spray Pyrolysis. ChemCatChem 2021, 13, 2815–2831. [Google Scholar] [CrossRef]
- Gäßler, M.; Stahl, J.; Schowalter, M.; Pokhrel, S.; Rosenauer, A.; Mädler, L.; Güttel, R. The Impact of Support Material of Cobalt-Based Catalysts Prepared by Double Flame Spray Pyrolysis on CO2 Methanation Dynamics. ChemCatChem 2022, 14, e202200286. [Google Scholar] [CrossRef]
- Henning, D.F.; Merkl, P.; Yun, C.; Iovino, F.; Xie, L.; Mouzourakis, E.; Moularas, C.; Deligiannakis, Y.; Henriques-Normark, B.; Leifer, K.; et al. Luminescent CeO2:Eu3+ Nanocrystals for Robust in Situ H2O2 Real-Time Detection in Bacterial Cell Cultures. Biosens. Bioelectron. 2019, 132, 286–293. [Google Scholar] [CrossRef]
- Grossmann, H.K.; Grieb, T.; Meierhofer, F.; Hodapp, M.J.; Noriler, D.; Gröhn, A.; Meier, H.F.; Fritsching, U.; Wegner, K.; Mädler, L. Nanoscale Mixing during Double-Flame Spray Synthesis of Heterostructured Nanoparticles. J. Nanopart. Res. 2015, 17, 174. [Google Scholar] [CrossRef]
- Solakidou, M.; Georgiou, Y.; Deligiannakis, Y. Double-Nozzle Flame Spray Pyrolysis as a Potent Technology to Engineer Noble Metal-TiO2 Nanophotocatalysts for Efficient H2 Production. Energies 2021, 14, 817. [Google Scholar] [CrossRef]
- Tada, S.; Larmier, K.; Büchel, R.; Copéret, C. Methanol Synthesis via CO2 Hydrogenation over CuO–ZrO2 Prepared by Two-Nozzle Flame Spray Pyrolysis. Catal. Sci. Technol. 2018, 8, 2056–2060. [Google Scholar] [CrossRef]
- Li, H.; Erinmwingbovo, C.; Birkenstock, J.; Schowalter, M.; Rosenauer, A.; La Mantia, F.; Mädler, L.; Pokhrel, S. Double Flame-Fabricated High-Performance AlPO4/LiMn2O4 Cathode Material for Li-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4428–4443. [Google Scholar] [CrossRef]
- Lovell, E.C.; Großman, H.; Horlyck, J.; Scott, J.; Mädler, L.; Amal, R. Asymmetrical Double Flame Spray Pyrolysis-Designed SiO2/Ce0.7Zr0.3O2 for the Dry Reforming of Methane. ACS Appl. Mater. Interfaces 2019, 11, 25766–25777. [Google Scholar] [CrossRef]
- Psathas, P.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Highly Crystalline Nanosized NaTaO3/NiO Heterojunctions Engineered by Double-Nozzle Flame Spray Pyrolysis for Solar-to-H2 Conversion: Toward Industrial-Scale Synthesis. ACS Appl. Nano Mater. 2023, 6, 2658–2671. [Google Scholar] [CrossRef]
- Gockeln, M.; Pokhrel, S.; Meierhofer, F.; Glenneberg, J.; Schowalter, M.; Rosenauer, A.; Fritsching, U.; Busse, M.; Mädler, L.; Kun, R. Fabrication and Performance of Li4Ti5O12/C Li-Ion Battery Electrodes Using Combined Double Flame Spray Pyrolysis and Pressure-Based Lamination Technique. J. Power Sources 2018, 374, 97–106. [Google Scholar] [CrossRef]
- Hansen, J.P.; Jensen, J.R.; Livbjerg, H.; Johannessen, T. Synthesis of ZnO Particles in a Quench-Cooled Flame Reactor. AIChE J. 2001, 47, 2413–2418. [Google Scholar] [CrossRef]
- Teleki, A.; Heine, M.C.; Krumeich, F.; Akhtar, M.K.; Pratsinis, S.E. In Situ Coating of Flame-Made TiO2 Particles with Nanothin SiO2 Films. Langmuir 2008, 24, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Teleki, A.; Pratsinis, S.E.; Wegner, K.; Jossen, R.; Krumeich, F. Flame-Coating of Titania Particles with Silica. J. Mater. Res. 2005, 20, 1336–1347. [Google Scholar] [CrossRef]
- Teleki, A.; Buesser, B.; Heine, M.C.; Krumeich, F.; Akhtar, M.K.; Pratsinis, S.E. Role of Gas−Aerosol Mixing during in Situ Coating of Flame-Made Titania Particles. Ind. Eng. Chem. Res. 2009, 48, 85–92. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Sannomiya, T.; Teleki, A.; Krumeich, F.; Vörös, J.; Pratsinis, S.E. Non-Toxic Dry-Coated Nanosilver for Plasmonic Biosensors. Adv. Funct. Mater. 2010, 20, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.; Gass, S.; Pratsinis, S.E. Hermetically Coated Nanosilver: No Ag+ Ion Leaching. MRS Proc. 2012, 1386, mrsf11-1386-d09-24. [Google Scholar] [CrossRef]
- Moularas, C.; Georgiou, Y.; Adamska, K.; Deligiannakis, Y. Thermoplasmonic Heat Generation Efficiency by Nonmonodisperse Core–Shell Ag0@SiO2 Nanoparticle Ensemble. J. Phys. Chem. C 2019, 123, 22499–22510. [Google Scholar] [CrossRef]
- Moularas, C.; Dimitriou, C.; Georgiou, Y.; Evangelakis, G.; Boukos, N.; Deligiannakis, Y. Electron Paramagnetic Resonance Quantifies Hot-Electron Transfer from Plasmonic Ag@SiO2 to Cr6+/Cr5+/Cr3+. J. Phys. Chem. C 2023, 127, 2045–2057. [Google Scholar] [CrossRef]
- Fragou, F.; Stathi, P.; Deligiannakis, Y.; Louloudi, M. Safe-by-Design Flame Spray Pyrolysis of SiO2 Nanostructures for Minimizing Acute Toxicity. ACS Appl. Nano Mater. 2022, 5, 8184–8195. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Huo, J.; Jiang, H.; Li, C.; Huang, G. Stable Core Shell Co3Fe7–CoFe2O4 Nanoparticles Synthesized via Flame Spray Pyrolysis Approach. Ind. Eng. Chem. Res. 2012, 51, 11157–11162. [Google Scholar] [CrossRef]
- Jeanne-Rose, V.; Samain, H.; Deligiannakis, Y.; Louloudi, M. Metal Oxide Particles Coated with a Rare-Earth Oxide and Process for Preparing Same by Flame Spray Pyrolysis. U.S. Patent 17/789,501, 9 February 2023. [Google Scholar]
- Zhou, G.; Zhang, Y.; Zhao, X.; Gui, Y.; Wang, X.; Li, L.; Chen, T.; Huang, Z.; Lin, H. FSP Synthesized Core-Shell CuOx@SiO2 Catalyst with Excellent Thermal Stability for Catalytic Combustion of Ammonia. Fuel 2023, 334, 126824. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Zhao, X.; Wang, H.; Li, S. Dual Liquid/Vapor-Fed Flame Synthesis for the Effective Preparation of SiO2@YAlO3:Nd3+ Nanophosphors. Proc. Combust. Inst. 2021, 38, 1299–1307. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Schneider, M.; Pratsinis, S.E. Green, Silica-Coated Monoclinic Y2O3:Tb3+ Nanophosphors: Flame Synthesis and Characterization. J. Phys. Chem. C 2012, 116, 4493–4499. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-Driven Plasmon Enhanced Proton-Coupled Electron Transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Sahm, T.; Rong, W.; Barsan, N.; Madler, L.; Weimar, U. Sensing of CH4, CO and Ethanol with in Situ Nanoparticle Aerosol-Fabricated Multilayer Sensors. Sens. Actuators B Chem. 2007, 127, 63–68. [Google Scholar] [CrossRef]
- Sahm, T.; Rong, W.; Bârsan, N.; Mädler, L.; Friedlander, S.K.; Weimar, U. Formation of Multilayer Films for Gas Sensing by in Situ Thermophoretic Deposition of Nanoparticles from Aerosol Phase. J. Mater. Res. 2007, 22, 850–857. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Tsikourkitoudi, V.; Stathi, P.; Wegner, K.; Papavasiliou, J.; Louloudi, M. PdO/Pd0/TiO2 Nanocatalysts Engineered by Flame Spray Pyrolysis: Study of the Synergy of PdO/Pd0 on H2 Production by HCOOH Dehydrogenation and the Deactivation Mechanism. Energy Fuels 2020, 34, 15026–15038. [Google Scholar] [CrossRef]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct Formation of Highly Porous Gas-Sensing Films by in Situ Thermophoretic Deposition of Flame-Made Pt/SnO2 Nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Nasiri, N.; Bo, R.; Wang, F.; Fu, L.; Tricoli, A. Ultraporous Electron-Depleted ZnO Nanoparticle Networks for Highly Sensitive Portable Visible-Blind UV Photodetectors. Adv. Mater. 2015, 27, 4336–4343. [Google Scholar] [CrossRef]
- Chen, H.; Mulmudi, H.K.; Tricoli, A. Flame Spray Pyrolysis for the One-Step Fabrication of Transition Metal Oxide Films: Recent Progress in Electrochemical and Photoelectrochemical Water Splitting. Chin. Chem. Lett. 2020, 31, 601–604. [Google Scholar] [CrossRef]
- Mane, R.S.; Lokhande, C.D. Chemical Deposition Method for Metal Chalcogenide Thin Films. Mater. Chem. Phys. 2000, 65, 1–31. [Google Scholar] [CrossRef]
- Kavitha, R.; Meghani, S.; Jayaram, V. Synthesis of Titania Films by Combustion Flame Spray Pyrolysis Technique and Its Characterization for Photocatalysis. Mater. Sci. Eng. B 2007, 139, 134–140. [Google Scholar] [CrossRef]
- Kavitha, R.; Hegde, S.R.; Jayaram, V. Oxide Films by Combustion Pyrolysis of Solution Precursors. Mater. Sci. Eng. A 2003, 359, 18–23. [Google Scholar] [CrossRef]
- Tricoli, A.; Elmøe, T.D. Flame Spray Pyrolysis Synthesis and Aerosol Deposition of Nanoparticle Films. AIChE J. 2012, 58, 3578–3588. [Google Scholar] [CrossRef]
- Tricoli, A.; Graf, M.; Mayer, F.; Kuühne, S.; Hierlemann, A.; Pratsinis, S.E. Micropatterning Layers by Flame Aerosol Deposition-Annealing. Adv. Mater. 2008, 20, 3005–3010. [Google Scholar] [CrossRef]
- Salameh, S.; Gómez-Hernández, J.; Goulas, A.; Van Bui, H.; van Ommen, J.R. Advances in Scalable Gas-Phase Manufacturing and Processing of Nanostructured Solids: A Review. Particuology 2017, 30, 15–39. [Google Scholar] [CrossRef]
- Available online: https://www.Cabotcorp.Com/Solutions#products-Plus (accessed on 5 November 2023).
- Available online: https://Corporate.Evonik.Com/En/Products-and-Solutions/Markets (accessed on 5 November 2023).
- Kelesidis, G.A.; Pratsinis, S.E. A Perspective on Gas-Phase Synthesis of Nanomaterials: Process Design, Impact and Outlook. Chem. Eng. J. 2021, 421, 129884. [Google Scholar] [CrossRef]
- Available online: https://www.Hemotune.Ch/Technology (accessed on 5 November 2023).
- Available online: https://www.Turbobeads.Com (accessed on 5 November 2023).
- Available online: https://www.Heiq.Com/Products/ (accessed on 5 November 2023).
- Sotiris, E.P.; Height, M. Antimicrobial and Antifungal Powders Made by Flame Spray Pyrolysis. U.S. Patent Application 11/884,039, 10 December 2008. [Google Scholar]
- Wegner, K.; Schimmöller, B.; Thiebaut, B.; Fernandez, C.; Rao, T.N. Pilot Plants for Industrial Nanoparticle Production by Flame Spray Pyrolysis. KONA 2011, 29, 251–265. [Google Scholar] [CrossRef]
- Gerken, L.R.H.; Neuer, A.L.; Gschwend, P.M.; Keevend, K.; Gogos, A.; Anthis, A.H.C.; Aengenheister, L.; Pratsinis, S.E.; Plasswilm, L.; Herrmann, I.K. Scalable Synthesis of Ultrasmall Metal Oxide Radio-Enhancers Outperforming Gold. Chem. Mater. 2021, 33, 3098–3112. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Flame-Made Ceria Nanoparticles. J. Mater. Res. 2002, 17, 1356–1362. [Google Scholar] [CrossRef]
- Wegner, K.; Pratsinis, S.E. Gas-Phase Synthesis of Nanoparticles: Scale-up and Design of Flame Reactors. Powder Technol. 2005, 150, 117–122. [Google Scholar] [CrossRef]
- Wegner, K.; Pratsinis, S.E. Scale-up of Nanoparticle Synthesis in Diffusion Flame Reactors. Chem. Eng. Sci. 2003, 58, 4581–4589. [Google Scholar] [CrossRef]
- Akurati, K.K.; Vital, A.; Dellemann, J.-P.; Michalow, K.; Graule, T.; Ferri, D.; Baiker, A. Flame-Made WO3/TiO2 Nanoparticles: Relation between Surface Acidity, Structure and Photocatalytic Activity. Appl. Catal. B Environ. 2008, 79, 53–62. [Google Scholar] [CrossRef]
- Heel, A.; Holtappels, P.; Hug, P.; Graule, T. Flame Spray Synthesis of Nanoscale La0.6Sr0.4Co0.2Fe0.8O3−δ and Ba0.5Sr0.5Co0.8Fe0.2O3−δ as Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. Fuel Cells 2010, 10, 419–432. [Google Scholar] [CrossRef]
- Mueller, R.; Mädler, L.; Pratsinis, S.E. Nanoparticle Synthesis at High Production Rates by Flame Spray Pyrolysis. Chem. Eng. Sci. 2003, 58, 1969–1976. [Google Scholar] [CrossRef]
- Mueller, R.; Jossen, R.; Pratsinis, S.E.; Watson, M.; Akhtar, M.K. Zirconia Nanoparticles Made in Spray Flames at High Production Rates. J. Am. Ceram. Soc. 2004, 87, 197–202. [Google Scholar] [CrossRef]
- Gröhn, A.J.; Pratsinis, S.E.; Sánchez-Ferrer, A.; Mezzenga, R.; Wegner, K. Scale-up of Nanoparticle Synthesis by Flame Spray Pyrolysis: The High-Temperature Particle Residence Time. Ind. Eng. Chem. Res. 2014, 53, 10734–10742. [Google Scholar] [CrossRef]
- Meierhofer, F.; Mädler, L.; Fritsching, U. Nanoparticle Evolution in Flame Spray Pyrolysis—Process Design via Experimental and Computational Analysis. AIChE J. 2020, 66, e16885. [Google Scholar] [CrossRef]
- Jossen, R.; Mueller, R.; Pratsinis, S.E.; Watson, M.; Akhtar, M.K. Morphology and Composition of Spray-Flame-Made Yttria-Stabilized Zirconia Nanoparticles. Nanotechnology 2005, 16, S609–S617. [Google Scholar] [CrossRef]
- Hembram, K.; Sivaprakasam, D.; Rao, T.N.; Wegner, K. Large-Scale Manufacture of ZnO Nanorods by Flame Spray Pyrolysis. J. Nanopart. Res. 2013, 15, 1461. [Google Scholar] [CrossRef]
- Betancur-Granados, N.; Pöllmann, H.; Restrepo-Baena, O.J.; Tobón, J.I. Nanosized Belite Phases Obtained by Flame Spray Pyrolysis: Assessment of Process Conditions on the Mineralogy and Reactivity. Cem. Concr. Res. 2023, 164, 107062. [Google Scholar] [CrossRef]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame Preparation of Visible-Light-Responsive BiVO4 Oxygen Evolution Photocatalysts with Subsequent Activation via Aqueous Route. ACS Appl. Mater. Interfaces 2011, 3, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-Doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Psathas, P.; Georgiou, Y.; Moularas, C.; Armatas, G.S.; Deligiannakis, Y. Controlled-Phase Synthesis of Bi2Fe4O9 & BiFeO3 by Flame Spray Pyrolysis and Their Evaluation as Non-Noble Metal Catalysts for Efficient Reduction of 4-Nitrophenol. Powder Technol. 2020, 368, 268–277. [Google Scholar] [CrossRef]
- Psathas, P.; Solakidou, M.; Mantzanis, A.; Deligiannakis, Y. Flame Spray Pyrolysis Engineering of Nanosized Mullite-Bi2Fe4O9 and Perovskite-BiFeO3 as Highly Efficient Photocatalysts for O2 Production from H2O Splitting. Energies 2021, 14, 5235. [Google Scholar] [CrossRef]
- Punginsang, M.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Ultrafine Bi2WO6 Nanoparticles Prepared by Flame Spray Pyrolysis for Selective Acetone Gas-Sensing. Mater. Sci. Semicond. Process. 2019, 90, 263–275. [Google Scholar] [CrossRef]
- Xiao, B.; Jiao, A.; Zhang, Y.; Yang, L.; Zhao, X.; Wu, C.; Guo, D.; Zhan, R.; Lin, H. Mixed Potential Type Ammonia Sensor Using Fe-Substituted LaCoO3 Sensing Electrode Prepared by Flame Spray Pyrolysis. Sens. Actuators B Chem. 2021, 346, 130470. [Google Scholar] [CrossRef]
- Jiao, A.; Zhang, Y.; Yang, L.; Zhao, X.; Wu, C.; Chen, T.; Zhan, R.; Huang, Z.; Lin, H. Enhanced CO2 Response of La1−xFeO3−δ Perovskites with A-Site Deficiency Synthesized by Flame Spray Pyrolysis. Ceram. Int. 2023, 49, 591–599. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Zheng, C.; Zhao, H. Deep Insight into the Mechanism of Catalytic Combustion of CO and CH4 over SrTi1− xBxO3 (B = Co, Fe, Mn, Ni, and Cu) Perovskite via Flame Spray Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 52571–52587. [Google Scholar] [CrossRef]
- Yuan, X.; Meng, L.; Xu, Z.; Zheng, C.; Zhao, H. CuO Quantum Dots Supported by SrTiO3 Perovskite Using the Flame Spray Pyrolysis Method: Enhanced Activity and Excellent Thermal Resistance for Catalytic Combustion of CO and CH4. Environ. Sci. Technol. 2021, 55, 14080–14086. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, C.; Zhang, T.; Li, L.; Yang, Q.; Zhao, H. Sodium Doped SrTi1−xBxO3 (B = Mn, Co) for Formaldehyde Catalytic Oxidation: Flame Spray Pyrolysis Fabrication and Reaction Mechanism Elaboration. Fuel Process. Technol. 2023, 247, 107763. [Google Scholar] [CrossRef]
- Moularas, C.; Psathas, P.; Deligiannakis, Y. Electron Paramagnetic Resonance Study of Photo-Induced Hole/Electron Pairs in NaTaO3 Nanoparticles. Chem. Phys. Lett. 2021, 782, 139031. [Google Scholar] [CrossRef]
- Kennedy, A.E.; Meekins, B.H. Combustion Synthesis and Photoelectrochemical Characterization of Gallium Zinc Oxynitrides. J. Mater. Res. 2018, 33, 3971–3978. [Google Scholar] [CrossRef]
- Huo, J.; Hu, Y.; Jiang, H.; Hou, X.; Li, C. Continuous Flame Synthesis of near Surface Nitrogen Doped TiO2 for Dye-Sensitized Solar Cells. Chem. Eng. J. 2014, 258, 163–170. [Google Scholar] [CrossRef]
- Bi, W.; Hu, Y.; Jiang, H.; Yu, H.; Li, W.; Li, C. In-Situ Synthesized Surface N-Doped Pt/TiO2 via Flame Spray Pyrolysis with Enhanced Thermal Stability for CO Catalytic Oxidation. Appl. Surf. Sci. 2019, 481, 360–368. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel Continuous Single-Step Synthesis of Nitrogen-Modified TiO2 by Flame Spray Pyrolysis for Photocatalytic Degradation of Phenol in Visible Light. J. Mater. Sci. Technol. 2018, 34, 1494–1502. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel One-Step Synthesis of Nitrogen-Doped TiO2 by Flame Aerosol Technique for Visible-Light Photocatalysis: Effect of Synthesis Parameters and Secondary Nitrogen (N) Source. Chem. Eng. J. 2018, 350, 324–334. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Damma, D.; Inturi, S.N.R. Single-Step Rapid Aerosol Synthesis of N-Doped TiO2 for Enhanced Visible Light Photocatalytic Activity. Catal. Commun. 2018, 113, 1–5. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Boningari, T.; Inturi, S.N.R. Single-Step Synthesis of N-Doped TiO2 by Flame Aerosol Method and the Effect of Synthesis Parameters. Aerosol Sci. Technol. 2018, 52, 913–922. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Grass, R.N.; Mazunin, D.; Stark, W.J. Synthesis and Covalent Surface Functionalization of Nonoxidic Iron Core−Shell Nanomagnets. Chem. Mater. 2009, 21, 3275–3281. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Groeneveld, J.D.; Pokhrel, S.; Mädler, L. Metal Sulfide Nanoparticles: Precursor Chemistry. Chem. Eur. J. 2021, 27, 6390–6406. [Google Scholar] [CrossRef] [PubMed]
- Bubenhofer, S.B.; Schumacher, C.M.; Koehler, F.M.; Luechinger, N.A.; Grass, R.N.; Stark, W.J. Large-Scale Synthesis of PbS–TiO2 Heterojunction Nanoparticles in a Single Step for Solar Cell Application. J. Phys. Chem. C 2012, 116, 16264–16270. [Google Scholar] [CrossRef]
- Pokhrel, S.; Stahl, J.; Groeneveld, J.D.; Schowalter, M.; Rosenauer, A.; Birkenstock, J.; Mädler, L. Flame Aerosol Synthesis of Metal Sulfides at High Temperature in Oxygen-Lean Atmosphere. Adv. Mater. 2023, 35, 2211104. [Google Scholar] [CrossRef] [PubMed]
- Grass, R.N.; Stark, W.J. Flame Synthesis of Calcium-, Strontium-, Barium Fluoride Nanoparticles and Sodium Chloride. Chem. Commun. 2005, 13, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Stepuk, A.; Krämer, K.W.; Stark, W.J. Flame Synthesis of Complex Fluoride-Based Nanoparticles as Upconversion Phosphors. KONA Powder Part. J. 2013, 30, 267–275. [Google Scholar] [CrossRef][Green Version]
- Jodhani, G.; Mikaeili, F.; Gouma, P.I. Flame Spray Synthesis of VOPO4 Polymorphs. Front. Mater. 2019, 6, 254. [Google Scholar] [CrossRef]
- Rohner, F.; Ernst, F.O.; Arnold, M.; Hilbe, M.; Biebinger, R.; Ehrensperger, F.; Pratsinis, S.E.; Langhans, W.; Hurrell, R.F.; Zimmermann, M.B. Synthesis, Characterization, and Bioavailability in Rats of Ferric Phosphate Nanoparticles. J. Nutr. 2007, 137, 614–619. [Google Scholar] [CrossRef]
- Rudin, T.; Pratsinis, S.E. Homogeneous Iron Phosphate Nanoparticles by Combustion of Sprays. Ind. Eng. Chem. Res. 2012, 51, 7891–7900. [Google Scholar] [CrossRef][Green Version]
- Waser, O.; Büchel, R.; Hintennach, A.; Novák, P.; Pratsinis, S.E. Continuous Flame Aerosol Synthesis of Carbon-Coated Nano-LiFePO4 for Li-Ion Batteries. J. Aerosol Sci. 2011, 42, 657–667. [Google Scholar] [CrossRef]
- Badding, M.E.; Brown, J.L.; Fekety, C.R.; Song, Z. Corning Inc, Flame Spray Pyrolysis Method for Forming Nanoscale Lithium Metal Phosphate Powders. U.S. Patent 8,821,771, 3 April 2014. [Google Scholar]
- Cho, J.S.; Ko, Y.N.; Koo, H.Y.; Kang, Y.C. Synthesis of Nano-Sized Biphasic Calcium Phosphate Ceramics with Spherical Shape by Flame Spray Pyrolysis. J. Mater. Sci. Mater. Med. 2010, 21, 1143–1149. [Google Scholar] [CrossRef]
- Ataol, S.; Tezcaner, A.; Duygulu, O.; Keskin, D.; Machin, N.E. Synthesis and Characterization of Nanosized Calcium Phosphates by Flame Spray Pyrolysis, and Their Effect on Osteogenic Differentiation of Stem Cells. J. Nanopart. Res. 2015, 17, 95. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Karlsson, J.; Merkl, P.; Loh, E.; Henriques-Normark, B.; Sotiriou, G.A. Flame-Made Calcium Phosphate Nanoparticles with High Drug Loading for Delivery of Biologics. Molecules 2020, 25, 1747. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Stark, W.J.; Loher, S.; Maciejewski, M.; Krumeich, F.; Baiker, A. Flame Synthesis of Calcium Carbonate Nanoparticles. Chem. Commun. 2005, 5, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.; Maciejewski, M.; Pratsinis, S.E.; Baiker, A. Unprecedented Formation of Metastable Monoclinic BaCO3 Nanoparticles. Thermochim. Acta 2006, 445, 23–26. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Huang, G.; Li, C. Metallic Iron Nanoparticles: Flame Synthesis, Characterization and Magnetic Properties. Particuology 2013, 11, 460–467. [Google Scholar] [CrossRef]
- Kang, Y.C.; Sohn, J.R.; Yoon, H.S.; Jung, K.Y.; Park, H.D. Improved Photoluminescence of Sr5(PO4)3Cl:Eu2+ Phosphor Particles Prepared by Flame Spray Pyrolysis. J. Electrochem. Soc. 2003, 150, H38. [Google Scholar] [CrossRef]
- Hilty, F.M.; Teleki, A.; Krumeich, F.; Büchel, R.; Hurrell, R.F.; Pratsinis, S.E.; Zimmermann, M.B. Development and Optimization of Iron- and Zinc-Containing Nanostructured Powders for Nutritional Applications. Nanotechnology 2009, 20, 475101. [Google Scholar] [CrossRef]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 978-92-4-159401-1. [Google Scholar]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for Lithium Battery Cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Stark, W.; Pratsinis, S.; Maciejewski, M.; Loher, S.; Baiker, A. Flame Synthesis of Metal Salt Nanoparticles, in Particular Calcium and Phosphate Comprising Nanoparticles. U.S. Patent 12/928,185, 17 June 2014. [Google Scholar]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Ekimov, A.; Onushchehko, A. Quantum Size Effect in Three-Dimensional Microscopic Semiconductor Crystals. ZhETF Pis Ma Redaktsiiu 1981, 34, 363. [Google Scholar]
- Rossetti, R.; Brus, L. Electron-Hole Recombination Emission as a Probe of Surface Chemistry in Aqueous Cadmium Sulfide Colloids. J. Phys. Chem. 1982, 86, 4470–4472. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and Characterization of Nearly Monodisperse CdE (E = Sulfur, Selenium, Tellurium) Semiconductor Nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Patial, S.; Sonu; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; Chaukura, N.; Selvasembian, R. A Review on Carbon Quantum Dots Modified G-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment. Appl. Sci. 2022, 12, 11286. [Google Scholar] [CrossRef]
- Jang, E.; Jang, H. Review: Quantum Dot Light-Emitting Diodes. Chem. Rev. 2023, 123, 4663–4692. [Google Scholar] [CrossRef] [PubMed]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Edvinsson, T. Optical Quantum Confinement and Photocatalytic Properties in Two-, One- and Zero-Dimensional Nanostructures. R. Soc. Open Sci. 2018, 5, 180387. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum Dot-Decorated Semiconductor Micro- and Nanoparticles: A Review of Their Synthesis, Characterization and Application in Photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef]
- Kaxiras, E.; Joannopoulos, J.D. Quantum Theory of Materials, 2nd ed.; Cambridge University Press: Cambridge, NY, USA, 2019; ISBN 978-0-521-11711-1. [Google Scholar]
- Dieleman, C.D.; Ding, W.; Wu, L.; Thakur, N.; Bespalov, I.; Daiber, B.; Ekinci, Y.; Castellanos, S.; Ehrler, B. Universal Direct Patterning of Colloidal Quantum Dots by (Extreme) Ultraviolet and Electron Beam Lithography. Nanoscale 2020, 12, 11306–11316. [Google Scholar] [CrossRef] [PubMed]
- Palankar, R.; Medvedev, N.; Rong, A.; Delcea, M. Fabrication of Quantum Dot Microarrays Using Electron Beam Lithography for Applications in Analyte Sensing and Cellular Dynamics. ACS Nano 2013, 7, 4617–4628. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Shi, L.-X.; Du, K.; Qin, Y.; Qu, S.-J.; Xia, D.-Q.; Zhou, Z.; Huang, Z.-G.; Ding, S.-N. Copper-Ion-Assisted Precipitation Etching Method for the Luminescent Enhanced Assembling of Sulfur Quantum Dots. ACS Omega 2020, 5, 5407–5411. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Hu, E.L.; Wilkinson, C.D.W. Reactive Ion Etched II-VI Quantum Dots: Dependence of Etched Profile on Pattern Geometry. Jpn. J. Appl. Phys. 1993, 32, 6233. [Google Scholar] [CrossRef]
- Arachchige, I.U.; Brock, S.L. Sol–Gel Methods for the Assembly of Metal Chalcogenide Quantum Dots. Acc. Chem. Res. 2007, 40, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Spanhel, L.; Anderson, M.A. Semiconductor Clusters in the Sol-Gel Process: Quantized Aggregation, Gelation, and Crystal Growth in Concentrated Zinc Oxide Colloids. J. Am. Chem. Soc. 1991, 113, 2826–2833. [Google Scholar] [CrossRef]
- Lin, K.-F.; Cheng, H.-M.; Hsu, H.-C.; Lin, L.-J.; Hsieh, W.-F. Band Gap Variation of Size-Controlled ZnO Quantum Dots Synthesized by Sol–Gel Method. Chem. Phys. Lett. 2005, 409, 208–211. [Google Scholar] [CrossRef]
- Darbandi, M.; Thomann, R.; Nann, T. Single Quantum Dots in Silica Spheres by Microemulsion Synthesis. Chem. Mater. 2005, 17, 5720–5725. [Google Scholar] [CrossRef]
- Koole, R.; van Schooneveld, M.M.; Hilhorst, J.; de Mello Donegá, C.; Hart, D.C.; van Blaaderen, A.; Vanmaekelbergh, D.; Meijerink, A. On the Incorporation Mechanism of Hydrophobic Quantum Dots in Silica Spheres by a Reverse Microemulsion Method. Chem. Mater. 2008, 20, 2503–2512. [Google Scholar] [CrossRef]
- Wenger, W.N.; Bates, F.S.; Aydil, E.S. Functionalization of Cadmium Selenide Quantum Dots with Poly(Ethylene Glycol): Ligand Exchange, Surface Coverage, and Dispersion Stability. Langmuir 2017, 33, 8239–8245. [Google Scholar] [CrossRef]
- Xin, S.H.; Wang, P.D.; Yin, A.; Kim, C.; Dobrowolska, M.; Merz, J.L.; Furdyna, J.K. Formation of Self-assembling CdSe Quantum Dots on ZnSe by Molecular Beam Epitaxy. Appl. Phys. Lett. 1996, 69, 3884–3886. [Google Scholar] [CrossRef]
- Facsko, S.; Dekorsy, T.; Koerdt, C.; Trappe, C.; Kurz, H.; Vogt, A.; Hartnagel, H.L. Formation of Ordered Nanoscale Semiconductor Dots by Ion Sputtering. Science 1999, 285, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Oshinowo, J.; Nishioka, M.; Ishida, S.; Arakawa, Y. Highly Uniform InGaAs/GaAs Quantum Dots (~15 nm) by Metalorganic Chemical Vapor Deposition. Appl. Phys. Lett. 1994, 65, 1421–1423. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Rapid Synthesis of Stable ZnO Quantum Dots. J. Appl. Phys. 2002, 92, 6537–6540. [Google Scholar] [CrossRef]
- Riad, K.B.; Hoa, S.V.; Wood-Adams, P.M. Metal Oxide Quantum Dots Embedded in Silica Matrices Made by Flame Spray Pyrolysis. ACS Omega 2021, 6, 11411–11417. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Hu, Y.; Jiang, H.; Lei, J.; Wan, X.; Zhang, L.; Li, C. Flame Process Constructing CQDs/TiO2-C Heterostructure with Novel Electron Transfer Channel between Internal and External Carbon Species. Combust. Flame 2021, 228, 163–172. [Google Scholar] [CrossRef]
- Teck, M.; Murshed, M.M.; Schowalter, M.; Lefeld, N.; Grossmann, H.K.; Grieb, T.; Hartmann, T.; Robben, L.; Rosenauer, A.; Mädler, L.; et al. Structural and Spectroscopic Comparison between Polycrystalline, Nanocrystalline and Quantum Dot Visible Light Photo-Catalyst Bi2WO6. J. Solid State Chem. 2017, 254, 82–89. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; ISBN 978-0-387-33150-8. [Google Scholar]
- Kooyman, R.P.H.; Corn, R.M.; Wark, A.; Lee, H.J.; Gedig, E.; Engbers, G.; Walstrom, L.; de Mol, N.J.; Hall, D.R.; Yager, P.; et al. Handbook of Surface Plasmon Resonance; The Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-267-8. [Google Scholar]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 1995; Volume 25, ISBN 978-3-642-08191-0. [Google Scholar]
- Absorption and Scattering of Light by Small Particles|Wiley. Available online: https://www.wiley.com/en-us/Absorption+and+Scattering+of+Light+by+Small+Particles-p-9780471293408 (accessed on 3 October 2023).
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-Induced Hot Carrier Science and Technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.-S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using Metallic Nanostructures as Nano-Sources of Heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Pines, D.; Bohm, D. A Collective Description of Electron Interactions: II. Collective vs Individual Particle Aspects of the Interactions. Phys. Rev. 1952, 85, 338–353. [Google Scholar] [CrossRef]
- Bohm, D.; Pines, D. A Collective Description of Electron Interactions: III. Coulomb Interactions in a Degenerate Electron Gas. Phys. Rev. 1953, 92, 609–625. [Google Scholar] [CrossRef]
- Jackson, J.D. Classical Electrodynamics, 3rd ed.; Wiley: New York, NY, USA, 1998; ISBN 978-0-471-30932-1. [Google Scholar]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial Activity of Nanosilver Ions and Particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Starsich, F.; Dasargyri, A.; Wurnig, M.C.; Krumeich, F.; Boss, A.; Leroux, J.-C.; Pratsinis, S.E. Photothermal Killing of Cancer Cells by the Controlled Plasmonic Coupling of Silica-Coated Au/Fe2O3 Nanoaggregates. Adv. Funct. Mater. 2014, 24, 2818–2827. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.-Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, Silica-Coated, Janus-Like Plasmonic-Magnetic Nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, T.; Jensen, J.R.; Mosleh, M.; Johansen, J.; Quaade, U.; Livbjerg, H. Flame Synthesis of Nanoparticles: Applications in Catalysis and Product/Process Engineering. Chem. Eng. Res. Des. 2004, 82, 1444–1452. [Google Scholar] [CrossRef]
- Hannemann, S.; Grunwaldt, J.-D.; Krumeich, F.; Kappen, P.; Baiker, A. Electron Microscopy and EXAFS Studies on Oxide-Supported Gold–Silver Nanoparticles Prepared by Flame Spray Pyrolysis. Appl. Surf. Sci. 2006, 252, 7862–7873. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Pratsinis, S.E. Composite Nanosilver Structures Suitable for Plasmonic Biosensors. MRS Online Proc. Libr. 2012, 1416, 25–30. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Teleki, A.; Camenzind, A.; Krumeich, F.; Meyer, A.; Panke, S.; Pratsinis, S.E. Nanosilver on Nanostructured Silica: Antibacterial Activity and Ag Surface Area. Chem. Eng. J. 2011, 170, 547–554. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic Hydrogen Production over Flame Spray Pyrolysis-Synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Etterlin, G.D.; Spyrogianni, A.; Krumeich, F.; Leroux, J.-C.; Pratsinis, S.E. Plasmonic Biocompatible Silver–Gold Alloyed Nanoparticles. Chem. Commun. 2014, 50, 13559–13562. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shi, Y.; Jiang, H.; Huang, G.; Li, C. Scalable Preparation of Ultrathin Silica-Coated Ag Nanoparticles for SERS Application. ACS Appl. Mater. Interfaces 2013, 5, 10643–10649. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Deligiannakis, Y.; Skoutelis, C.G.; Pratsinis, S.E. Visible-Light Active Black TiO2-Ag/TiOx Particles. Appl. Catal. B Environ. 2014, 154–155, 9–15. [Google Scholar] [CrossRef]
- Loher, S.; Schneider, O.D.; Maienfisch, T.; Bokorny, S.; Stark, W.J. Micro-Organism-Triggered Release of Silver Nanoparticles from Biodegradable Oxide Carriers Allows Preparation of Self-Sterilizing Polymer Surfaces. Small 2008, 4, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Ru, E.C.L.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-444-54725-5. [Google Scholar]
- Xu, Y.; Zhang, Y.; Li, C.; Ye, Z.; Bell, S.E.J. SERS as a Probe of Surface Chemistry Enabled by Surface-Accessible Plasmonic Nanomaterials. Acc. Chem. Res. 2023, 56, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Mahan, J.E. Physical Vapor Deposition of Thin Films, 1st ed.; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-33001-1. [Google Scholar]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.-Y.; et al. Synthesis of Few-Layer Hexagonal Boron Nitride Thin Film by Chemical Vapor Deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Znaidi, L. Sol–Gel-Deposited ZnO Thin Films: A Review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Jang, W.-S.; Rawson, I.; Grunlan, J.C. Layer-by-Layer Assembly of Thin Film Oxygen Barrier. Thin Solid Films 2008, 516, 4819–4825. [Google Scholar] [CrossRef]
- Sahu, N.; Parija, B.; Panigrahi, S. Fundamental Understanding and Modeling of Spin Coating Process: A Review. Indian J. Phys. 2009, 83, 493–502. [Google Scholar] [CrossRef]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction, Illustrated ed.; Cambridge University Press: Cambridge, NY, USA, 1996; ISBN 978-0-521-42450-9. [Google Scholar]
- Nair, P.K.; Nair, M.T.S.; García, V.M.; Arenas, O.L.; Peña, A.C.Y.; Ayala, I.T.; Gomezdaza, O.; Sánchez, A.; Campos, J.; Hu, H.; et al. Semiconductor Thin Films by Chemical Bath Deposition for Solar Energy Related Applications. Sol. Energy Mater. Sol. Cells 1998, 52, 313–344. [Google Scholar] [CrossRef]
- Karageorgakis, N.I.; Heel, A.; Bieberle-Hütter, A.; Rupp, J.L.M.; Graule, T.; Gauckler, L.J. Flame Spray Deposition of La0.6Sr0.4CoO3−δ Thin Films: Microstructural Characterization, Electrochemical Performance and Degradation. J. Power Sources 2010, 195, 8152–8161. [Google Scholar] [CrossRef]
- Blattmann, C.O.; Sotiriou, G.A.; Pratsinis, S.E. Rapid Synthesis of Flexible Conductive Polymer Nanocomposite Films. Nanotechnology 2015, 26, 125601. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Nasiri, N.; Chen, H.; Wallerand, A.S.; Righettoni, M. Ultra-Rapid Synthesis of Highly Porous and Robust Hierarchical ZnO Films for Dye Sensitized Solar Cells. Sol. Energy 2016, 136, 553–559. [Google Scholar] [CrossRef]
- Chen, H.; Bo, R.; Tran-Phu, T.; Liu, G.; Tricoli, A. One-Step Rapid and Scalable Flame Synthesis of Efficient WO3 Photoanodes for Water Splitting. ChemPlusChem 2018, 83, 569–576. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Chen, H.; Bo, R.; Bernardo, I.D.; Fusco, Z.; Simonov, A.N.; Tricoli, A. High-Temperature One-Step Synthesis of Efficient Nanostructured Bismuth Vanadate Photoanodes for Water Oxidation. Energy Technol. 2019, 7, 1801052. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bischoff, H.; Chorkendorff, I.; Johannessen, T. Mixed Phase Pt-Ru Catalyst for Direct Methanol Fuel Cell Anode by Flame Aerosol Synthesis. J. Electrochem. Soc. 2005, 152, A2357. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Dumont, E.; Slipets, R.; Thersleff, T.; Boisen, A.; Sotiriou, G.A. Democratizing Robust SERS Nano-Sensors for Food Safety Diagnostics. Chem. Eng. J. 2023, 470, 144023. [Google Scholar] [CrossRef]
- Bletsa, E.; Merkl, P.; Thersleff, T.; Normark, S.; Henriques-Normark, B.; Sotiriou, G.A. Highly Durable Photocatalytic Titanium Suboxide–Polymer Nanocomposite Films with Visible Light-Triggered Antibiofilm Activity. Chem. Eng. J. 2023, 454, 139971. [Google Scholar] [CrossRef]
- Kemmler, J.A.; Pokhrel, S.; Mädler, L.; Weimar, U.; Barsan, N. Flame Spray Pyrolysis for Sensing at the Nanoscale. Nanotechnology 2013, 24, 442001. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and Application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath Sensors for Health Monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Kraft, M.; Xu, R. Emerging Applications of Nanocatalysts Synthesized by Flame Aerosol Processes. Curr. Opin. Chem. Eng. 2018, 20, 39–49. [Google Scholar] [CrossRef]
- Khamfoo, K.; Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Formaldehyde Sensor Based on FSP-Made AgOx-Doped SnO2 Nanoparticulate Sensing Films. Sens. Actuators B Chem. 2020, 309, 127705. [Google Scholar] [CrossRef]
- Kaewsiri, D.; Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Flame-Spray-Made PtOx-Functionalized Zn2SnO4 Spinel Nanostructures for Conductometric H2 Detection. Sens. Actuators B Chem. 2020, 316, 128132. [Google Scholar] [CrossRef]
- Khamfoo, K.; Wisitsoraat, A.; Punginsang, M.; Tuantranont, A.; Liewhiran, C. Selectivity towards Acetylene Gas of Flame-Spray-Made Nb-Substituted SnO2 Particulate Thick Films. Sens. Actuators B Chem. 2021, 349, 130808. [Google Scholar] [CrossRef]
- Inyawilert, K.; Sukee, A.; Siriwalai, M.; Wisitsoraat, A.; Sukunta, J.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Effect of Er Doping on Flame-Made SnO2 Nanoparticles to Ethylene Oxide Sensing. Sens. Actuators B Chem. 2021, 328, 129022. [Google Scholar] [CrossRef]
- Inyawilert, K.; Punginsang, M.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Graphene/Rh-Doped SnO2 Nanocomposites Synthesized by Electrochemical Exfoliation and Flame Spray Pyrolysis for H2S Sensing. J. Alloys Compd. 2022, 916, 165431. [Google Scholar] [CrossRef]
- Andreev, M.; Topchiy, M.; Asachenko, A.; Beltiukov, A.; Amelichev, V.; Sagitova, A.; Maksimov, S.; Smirnov, A.; Rumyantseva, M.; Krivetskiy, V. Electrical and Gas Sensor Properties of Nb(V) Doped Nanocrystalline β-Ga2O3. Materials 2022, 15, 8916. [Google Scholar] [CrossRef]
- Siriwalai, M.; Punginsang, M.; Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Flame-Spray-Synthesized La2O3-Loaded WO3 Nanoparticle Films for NO2 Sensing. ACS Appl. Nano Mater. 2023, 6, 995–1008. [Google Scholar] [CrossRef]
- Punginsang, M.; Inyawilert, K.; Siriwalai, M.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Comparative Study on Formic Acid Sensing Properties of Flame-Made Zn2SnO4 Nanoparticles and Its Parent Metal Oxides. Phys. Chem. Chem. Phys. 2023, 25, 15407–15421. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Bras, S.L.; Boissière, C.; Chaumonnot, A.; Sanchez, C. Aerosol Processing: A Wind of Innovation in the Field of Advanced Heterogeneous Catalysts. Chem. Soc. Rev. 2018, 47, 4112–4155. [Google Scholar] [CrossRef] [PubMed]
- Belles, L.; Moularas, C.; Smykała, S.; Deligiannakis, Y. Flame Spray Pyrolysis Co3O4/CoO as Highly-Efficient Nanocatalyst for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Pozio, A.; Bozza, F.; Lisi, N.; Chierchia, R.; Migliorini, F.; Dondè, R.; De Iuliis, S. Cobalt Oxide Synthesis via Flame Spray Pyrolysis as Anode Electrocatalyst for Alkaline Membrane Water Electrolyzer. Materials 2022, 15, 4626. [Google Scholar] [CrossRef] [PubMed]
- Tran-Phu, T.; Daiyan, R.; Leverett, J.; Fusco, Z.; Tadich, A.; Bernardo, I.D.; Kiy, A.; Truong, T.N.; Zhang, Q.; Chen, H.; et al. Understanding the Activity and Stability of Flame-Made Co3O4 Spinels: A Route towards the Scalable Production of Highly Performing OER Electrocatalysts. Chem. Eng. J. 2022, 429, 132180. [Google Scholar] [CrossRef]
- Liu, G.; Karuturi, S.K.; Simonov, A.N.; Fekete, M.; Chen, H.; Nasiri, N.; Le, N.H.; Reddy Narangari, P.; Lysevych, M.; Gengenbach, T.R.; et al. Robust Sub-Monolayers of Co3O4 Nano-Islands: A Highly Transparent Morphology for Efficient Water Oxidation Catalysis. Adv. Energy Mater. 2016, 6, 1600697. [Google Scholar] [CrossRef]
- Daiyan, R.; Tran-Phu, T.; Kumar, P.; Iputera, K.; Tong, Z.; Leverett, J.; Khan, M.H.A.; Esmailpour, A.A.; Jalili, A.; Lim, M.; et al. Nitrate Reduction to Ammonium: From CuO Defect Engineering to Waste NOx-to-NH3 Economic Feasibility. Energy Environ. Sci. 2021, 14, 3588–3598. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Daiyan, R.; Fusco, Z.; Ma, Z.; Rahim, L.R.A.; Kiy, A.; Kluth, P.; Guo, X.; Zhu, Y.; Chen, H.; et al. Multifunctional Nanostructures of Au–Bi2O3 Fractals for CO2 Reduction and Optical Sensing. J. Mater. Chem. A 2020, 8, 11233–11245. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Daiyan, R.; Fusco, Z.; Ma, Z.; Amal, R.; Tricoli, A. Nanostructured β-Bi2O3 Fractals on Carbon Fibers for Highly Selective CO2 Electroreduction to Formate. Adv. Funct. Mater. 2020, 30, 1906478. [Google Scholar] [CrossRef]
- Sharaf, O.Z.; Orhan, M.F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Seo, D.J.; Ryu, K.O.; Park, S.B.; Kim, K.Y.; Song, R.-H. Synthesis and Properties of Ce1−xGdxO2−x/2 Solid Solution Prepared by Flame Spray Pyrolysis. Mater. Res. Bull. 2006, 41, 359–366. [Google Scholar] [CrossRef]
- Lee, H.; Kim, T.J.; Li, C.; Choi, I.D.; Kim, Y.T.; Coker, Z.; Choi, T.-Y.; Lee, D. Flame Aerosol Synthesis of Carbon-Supported Pt–Ru Catalysts for a Fuel Cell Electrode. Int. J. Hydrogen Energy 2014, 39, 14416–14420. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis. Energies 2023, 16, 1365. [Google Scholar] [CrossRef]
- Gockeln, M.; Glenneberg, J.; Busse, M.; Pokhrel, S.; Mädler, L.; Kun, R. Flame Aerosol Deposited Li4Ti5O12 Layers for Flexible, Thin Film All-Solid-State Li-Ion Batteries. Nano Energy 2018, 49, 564–573. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Jung, D.S.; Park, S.B.; Kang, Y.C. Design of Particles by Spray Pyrolysis and Recent Progress in Its Application. Korean J. Chem. Eng. 2010, 27, 1621–1645. [Google Scholar] [CrossRef]
- Kammler, H.K.; Mädler, L.; Pratsinis, S.E. Flame Synthesis of Nanoparticles. Chem. Eng. Technol. 2001, 24, 583–596. [Google Scholar] [CrossRef]
- Pan, J.; Libera, J.A.; Paulson, N.H.; Stan, M. Flame Stability Analysis of Flame Spray Pyrolysis by Artificial Intelligence. Int. J. Adv. Manuf. Technol. 2021, 114, 2215–2228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).